Abstract
A better understanding of the pathogenesis and the resolution of the acute respiratory distress syndrome (ARDS) is needed. Although some progress has been made with the use of protein biomarkers and candidate gene studies in understanding the pathobiology of ARDS, we propose that new studies that measure the chemical breakdown products of cellular metabolism (metabolomics) may provide new insights into ARDS, in part because metabolomics targets a later point in the genomics cascade than is possible with studies of DNA, RNA, and protein biomarkers. Technological advances have made large-scale metabolomic profiling increasingly feasible. Metabolomic approaches have already achieved novel insights in nonpulmonary diseases such as diabetes mellitus and malignancy, as well as in sepsis, a major risk factor for developing ARDS. Metabolomic profiling is a promising approach to identify novel pathways in both patients at risk for developing ARDS as well as in the early phase of established ARDS.
Keywords: acute lung injury, pulmonary edema, genomics, acute respiratory failure
a major limitation to the development of new therapies for the acute respiratory distress syndrome (ARDS) is the substantial heterogeneity of the syndrome. Even the clinical risk factors for ARDS (pneumonia, trauma, sepsis, aspiration) are highly variable. For the field to advance, a better understanding of the biological pathways that contribute to the pathogenesis and resolution of ARDS is needed. In this Perspectives article, we make the case for using metabolomics as a promising genomic technique for identifying novel biology in ARDS (20). We review the recent application of this technology to multiple disease phenotypes [including cancer, diabetes mellitus (DM), and more recently, sepsis and sepsis-related mortality], in each case identifying novel biological pathways. Finally, we review early work in ARDS and identify several promising metabolomic analyses that could further our understanding of ARDS pathogenesis and move the field forward.
Moving from Genetics to Genomics in ARDS
The genomic revolution has made possible rapid, transformative increases in knowledge in certain diseases. In autoimmune diseases such as Crohn's disease and DM, dozens to hundreds of novel, highly replicated loci have been identified using genomewide association studies (GWASs) of single nucleotide polymorphisms (SNPs) (2, 9, 28). Such GWAS successes led to major advances in our understanding of the biology in those diseases, including unanticipated disease mechanisms, an improved understanding of the intermediate phenotypes and disease epidemiology, and identification of novel therapeutic and pharmacogenetic targets (4).
In contrast, genomics has offered relatively modest gains in our understanding of ARDS. Candidate gene studies have identified variants in 25 genes associated with ARDS (12), including genes involved in regulating inflammation, coagulation, endothelial cell function, and apoptosis (21–23). The hypothesis-free GWAS approach has played only a minor role in ARDS, with one publication to date that demonstrated a replicated association with a SNP near PPFIA1 in trauma-associated ARDS (5).
There are likely several reasons for the limited yield of GWAS in ARDS to date (14). First and foremost, ARDS is a syndrome, a common end point for multiple heterogeneous pathophysiological cascades. Developing ARDS requires a precipitating environmental event (developing an ARDS risk factor such as sepsis, pneumonia, or pancreatitis, for example) before a risk allele can exert its influence, limiting the power of studies. Many controls in ARDS genetic studies are healthy or convenience controls; although they may well carry the risk alleles, they have not experienced a sufficient environmental stressor and thus are included as controls, biasing those studies toward the null. Finally, the sample sizes required to identify risk loci of modest risk typically identified in GWAS studies are often dauntingly high (10,000 subjects and more). Given heterogeneity in ARDS recruitment and enrollment strategies, such numbers cannot be achieved by pooling established ARDS trial databases.
Thus we propose that a more distal point in the genomics cascade may represent a more promising target of research (13, 34). In contrast to SNP or most epigenetic marks, which are present at birth, plasma gene expression, proteins, and metabolites can acutely change in the setting of an environmental perturbation (Fig. 1). Studies of gene expression using microarrays and targeted proteomics analyses have already identified important and unexpected biology in other lung diseases (10, 27, 29, 33) and even ARDS (1, 7, 15, 26, 36, 38). In contrast, metabolomics, the study of the chemical products of cellular metabolism, has been less well studied to date, largely because of technical limitations in profiling of highly disparate chemical compounds that have only very recently been overcome (6).
Fig. 1.
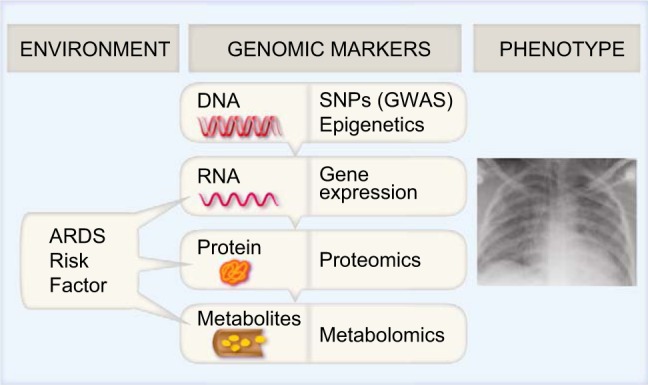
In response to environmental influences [development of a risk factor for acute respiratory distress syndrome (ARDS)], plasma levels of mRNA, proteins, and metabolites change. Metabolomics, a later point in the genomic cascade, offers a snapshot of the processes occurring at the time ARDS develops. SNPs, single nucleotide polymorphisms; GWAS, genomewide association study.
Metabolomics is particularly promising in ARDS for several reasons. First, as the end point of the genomics cascade, metabolic changes are highly dynamic and offer insight into chemical processes occurring at any given time. In contrast to gene expression and plasma proteins, which can exist in various splice variants and isoforms, the total number of plasma metabolites is more circumscribed. Currently, there are 4,513 metabolites identified in human blood; however, because the human metabolome is not complete, this is likely an underestimate (39). Thus comprehensive metabolomic analyses are subject to a less stringent multiple testing penalty than the other genomic markers, which often measure >20,000 to 1,000,000 tests in a single experiment. Metabolomics technology including liquid and gas chromatography and mass spectroscopy has markedly advanced in recent years, allowing high-resolution screening of the human metabolome, a process that was not feasible even 10 years ago (6). Although not nearly as developed or inexpensive as SNP genotyping or sequencing, technological advances have reduced the cost of conducting metabolomics analyses, making large-scale analyses feasible.
Metabolomic Profiling Techniques and Early Applications
A full discussion of the technical aspects of metabolomic profiling is beyond the scope of this Perspectives article but is well described elsewhere (8, 17). Briefly, metabolic profiling is usually performed by utilizing a combination of gas and lipid chromatography to separate metabolites followed by quantification using mass spectroscopy or nuclear magnetic resonance mass spectroscopy techniques. Metabolites are small molecules including lipids, nucleotides, amino acids, carbohydrates, and even drug metabolites; because of their very different chemical and physical properties, a single method for surveying them all is not feasible, and thus most analyses utilize multiple methodologies.
Profiling strategies can be either untargeted (designed to measure all metabolites in a given sample) or targeted (designed to identify a fixed subset of metabolites of interest). Both methods have their strengths. The major advantage of untargeted profiling is the lack of bias and broader ability to discover novel, unanticipated metabolites. Limitations to unbiased profiling, however, include time required to definitively follow up identified metabolites and lack of absolute quantification of metabolites. Even in untargeted profiling, resolution of metabolites based on hydrophilicity and size may vary depending on strategy used. Conversely, targeted metabolomic profiling identifies only a subset of metabolites (often on the order of 100–500), but using internal calibration standards does allow absolute quantification and high confidence in the individual metabolites attained.
Several high-profile studies have recently applied targeted metabolomics profiling to complex trait phenotypes. Rhee et al. (30) examined more than 100 plasma lipid metabolites in a cohort of 189 subjects who developed DM over 12 years of follow-up and compared them with 189 controls. They found that lipids with lower carbon number were associated with increased risk of DM, whereas those with a higher carbon number were protective. Jain et al. (16) performed metabolomic profiling of cancer cell line media and identified increased glycine consumption in multiple fast-growing tumors. A targeted gene expression study showed that expression of glycine pathway genes varies with cancer mortality. Recently, Wang et al. (37) evaluated 70 lipid metabolites in 188 individuals who developed DM during 1 year of follow-up and compared them with 188 propensity-matched controls. The metabolite most strongly associated with development of DM was 2-aminoapidic acid (2AAA). In functional follow-up of this finding, mice whose diets were supplemented with 2AAA produced more insulin and had lower fasting plasma glucose levels; application of 2AAA to human pancreatic islet cell lines similarly increased insulin production. These results highlight the potential for metabolomic profiling to identify both novel biomarkers of disease or disease severity, and even disease-modulating strategies.
Metabolomics Applied to Sepsis Mortality
Several groups including our own have performed targeted metabolomic profiling in sepsis, focusing particularly on metabolic changes associated with mortality (Table 1). The study of metabolomics in critically ill subjects is particularly compelling because lactate, a metabolite of anaerobic glycolysis, is the most widely used biomarker in sepsis and is highly associated with mortality and response to therapies. Mickiewicz et al. (24) performed metabolomic profiling of 58 metabolites in the serum of 60 pediatric patients with septic shock, of whom 10 died. Using supervised analysis, they identified models that separated survivors with high fidelity.
Table 1.
Published metabolomic studies in sepsis mortality
| Cases | Controls | Control Population | Metabolites Profiled | Analytic Strategy | Metabolites in Final Model | Reference |
|---|---|---|---|---|---|---|
| 10 | 10 | Septic shock survivors | 58 | PCA & PLS | 11 metabolites (not identified) | 25 |
| Discovery: 31 | Discovery: 119 | Surviving subjects with SIRS or sepsis | >300 | Support Vector Machine | cis-4-decenoyl-carnitine, 2-methyl-butyroylcarnitine, butyroylcarnitine, hexanoylcarnitine, lactate, (also included age and hematocrit) | 18* |
| Validation 1: 33 | Validation 1:67 | |||||
| Validation 2: 25 | Validation 2: 65 | |||||
| Discovery: 30 | Discovery: 60 | Surviving subjects with SIRS or sepsis | 167 | Bayesian network | γ-glutamylphenyl-alanine, -γ-glutamyl-tyrosine, 1-arachi-donoyl-GPC(20:4), taurochenodeoxycholate, 3-(4-hydroxy-phenyl) lactate, sucrose, kynurenine | 31* |
| Validation: 34 | Validation: 115 |
PCA and PLS, principal components analysis and partial least squares regression techniques, respectively; SIRS, systemic inflammatory response syndrome.
The Langley et al. and Rogers et al. analyses use highly overlapping datasets.
More extensive targeted metabolomic profiling of >300 metabolites was independently performed in two adult populations at high risk for death: 149 patients with infections and presumed sepsis participating in the Community Acquired Pneumonia & Sepsis Outcomes Diagnostics (CAPSOD) cohort, and 90 critically ill intensive care unit patients in the Registry of Critical Illness (RoCI) (18). Despite substantial phenotypic differences in the two populations (e.g., higher mortality, more cancer, more immunosuppression, less renal failure, in the RoCI cohort), metabolic changes in patients who died were widespread and very similar. In both populations, most metabolites associated with mortality were upregulated (i.e., higher in patients who died). Among the 167 metabolites tested in both populations, 57 metabolites were associated with mortality in RoCI; more than 50% (31) were also associated with mortality in CAPSOD.
In contrast to these remarkably similar results in individual metabolites, network models created from these datasets to predict death were not the same. Langley et al. (18) used Support Vector Machine analysis to create a signature of five metabolites and two clinical variables associated with death in CAPSOD and validated the importance of the model in RoCI. Our group (31) used Bayesian modeling to examine the metabolome of the same two adult cohorts and identified a model associated with death in both cohorts, but selected seven metabolites completely nonoverlapping with those identified by Langley et al. The results of these different models in these two studies likely have several explanations. First, many metabolites are highly correlated, meaning that a reductive process may select one or the other metabolite to incorporate in a predictive model by chance alone. Most importantly, analytic methodology in metabolomics is a rapidly evolving field and the optimal statistical methods for adequately handling highly correlated data and known chemical relationships have not been determined (17).
Thus these results show the promise of metabolomics to identify novel biology across clinically heterogeneous populations but reinforce that it is premature to focus on a single pathway or network in sepsis mortality at this time. Although the final network models were distinct in the above analyses, a common finding across all three sepsis cohorts is that subjects who progress to die have widespread metabolic derangements, and most metabolites are higher in nonsurvivors (18, 24, 31).
Metabolomic Investigations in ARDS
Several small metabolomics analyses have already been performed in established ARDS, measuring various potentially relevant fluids: exhaled breath, bronchoalveolar lavage (BAL), and plasma (Table 2). The earliest work, published in 1998, examined nine metabolites in the exhaled breath of 37 surgical intensive care unit patients and identified marginally lower levels of isoprene in the 19 subjects with ARDS (32). Evans et al. (11) performed untargeted metabolomics profiling of BAL from 18 subjects with established ARDS (39% with sepsis-associated ARDS) and compared it to BAL from 8 healthy controls. They identified widespread metabolomic differences in ARDS subjects, including several that mirror the sepsis work, with higher levels of many amino acids and products of glycolysis and lower levels of many lipid intermediates in ARDS subjects. Stringer et al. (35) identified 40 metabolites in plasma from 13 subjects with sepsis-induced ARDS vs. 6 controls and found 4 metabolites that differed between the two groups.
Table 2.
Published metabolomic studies in established ARDS
| Sample Type | Cases | Controls | Control Population | Metabolites Profiled | Disease-Associated Metabolites | Reference |
|---|---|---|---|---|---|---|
| Exhaled breath | 19 | 18 | SICU control | 9 | ↓ isoprene | 32 |
| BAL | 18 | 8 | Healthy | >500 (untargeted) | >20, all classes | 11 |
| Plasma | 13 | 6 | Healthy | 40 | 4 differ, ↓ sphingomyelin | 35 |
ARDS, acute respiratory distress syndroms; SICU, surgical intensive care unit; BAL, bronchoalveolar lavage; ↓, decreased.
These first studies are so small (total N < 60 ARDS cases) and the specific metabolites profiled overlap so little that this data cannot be used to identify the optimal sample to study in ARDS (BAL or plasma or even lung tissue itself) or offer even preliminary clues about pathway similarities in these disparate sample types. Our experience in studying the metabolome of critically ill patients suggests there is substantial interindividual variability in the majority of metabolites, suggesting that fairly large numbers of samples will be needed to identify disease-associated variants. Although studying the metabolic changes in the lung itself would be compelling, we anticipate that most early human studies will focus on more readily available sample types that can be obtained in larger numbers of patients: BAL, plasma, and urine.
The Future of Metabolomic Studies in ARDS
These preliminary studies in ARDS show the promise of metabolomics for advancing our understanding of disease pathogenesis. We anticipate that the next 5 years will bring multiple advances in the field. First, given rapid improvements in metabolomics profiling technology, leading to both a substantial decline in cost and higher throughput of targeted assays, it will be possible to greatly enhance sample size (and thus power) to identify novel pathways. Simultaneously, analyses can quantify a much larger number of metabolites, making it possible to study upward of 400 metabolites across diverse metabolic classes (lipids, peptides, or carbohydrates, for example) with a small quantity of plasma. Examining the metabolome of various biological fluids simultaneously, including BAL, plasma, and urine, may provide important insights into the biology of ARDS. For example, does the metabolome in the BAL of a sepsis-induced ARDS patient mirror that of plasma, or are independent metabolomics processes at work within the lung? Studies of patients at high risk for ARDS are critical, because the profile in the early phase of acute lung injury may be different from that in established ARDS and thus may provide new insights into the pathogenesis of ARDS (19). Equally importantly, biomarkers at the early stage could be particularly useful for identifying high-risk patients to enroll in ARDS clinical trials, such as the newly formed NHLBI-sponsored Prevention and Early Treatment of Acute Lung Injury (PETAL) network. Identifying metabolites that could serve not only as a biomarker but as a potential therapeutic target (as suggested by 2AAA in DM) in ARDS would be an added advance. Animal models of ARDS may further clarify the functional importance of metabolic changes; such work has already begun in proteomics (3). Finally, network analyses that integrate data across multiple 'omics platforms (proteomics, gene expression, and metabolomics) may provide additional insights into disease pathogenesis.
In summary, metabolomics has the potential to advance our understanding of ARDS biology. Unlike GWASs, which require very large, homogeneous samples and have not been of major value in ARDS, metabolomics targets a much later point in the genomics cascade (Fig. 1). Promising preliminary data in multiple complex trait diseases including sepsis and several small ARDS studies coupled with rapidly advancing technology suggest that large-scale metabolomics analyses in patients at risk for ARDS or with early ARDS may provide new insights into pathogenesis or prognosis.
GRANTS
This work was supported by the Parker B. Francis Family Foundation (A. J. Rogers) and NIH/NHLBI R37HL51856 (M. A. Matthay).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.J.R. prepared figures; A.J.R. and M.A.M. drafted manuscript; A.J.R. and M.A.M. edited and revised manuscript; A.J.R. and M.A.M. approved final version of manuscript.
REFERENCES
- 1.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med 187: 736–742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, NIDDK IBD Genetics Consortium, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBD Consortium, Wellcome Trust Case Control Consortium, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 40: 955–962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhargava M, Dey S, Becker T, Steinbach M, Wu B, Lee SM, Higgins L, Kumar V, Bitterman PB, Ingbar DH, Wendt CH. Protein expression profile of rat type two alveolar epithelial cells during hyperoxic stress and recovery. Am J Physiol Lung Cell Mol Physiol 305: L604–L614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billings LK, Florez JC. The genetics of type 2 diabetes: what have we learned from GWAS? Ann NY Acad Sci 1212: 59–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie JD, Wurfel MM, Feng R, O'Keefe GE, Bradfield J, Ware LB, Christiani DC, Calfee CS, Cohen MJ, Matthay M, Meyer NJ, Kim C, Li M, Akey J, Barnes KC, Sevransky J, Lanken PN, May AK, Aplenc R, Maloney JP, Hakonarson H; Trauma ALI SNP Consortium (TASC) investigators. Genome wide association identifies PPFIA1 as a candidate gene for acute lung injury risk following major trauma. PLoS One 7: e28268, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev 26: 51–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185: 1225–1234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn WB, Ellis DI. Metabolomics: current analytical platforms and methodologies. TrAC Trends Anal Chem 24: 285–294, 2005 [Google Scholar]
- 9.Ellinghaus D, Zhang H, Zeissig S, Lipinski S, Till A, Jiang T, Stade B, Bromberg Y, Ellinghaus E, Keller A, Rivas MA, Skieceviciene J, Doncheva NT, Liu X, Liu Q, Jiang F, Forster M, Mayr G, Albrecht M, Hasler R, Boehm BO, Goodall J, Berzuini CR, Lee J, Andersen V, Vogel U, Kupcinskas L, Kayser M, Krawczak M, Nikolaus S, Weersma RK, Ponsioen CY, Sans M, Wijmenga C, Strachan DP, McArdle WL, Vermeire S, Rutgeerts P, Sanderson JD, Mathew CG, Vatn MH, Wang J, Nothen MM, Duerr RH, Buning C, Brand S, Glas J, Winkelmann J, Illig T, Latiano A, Annese V, Halfvarson J, D'Amato M, Daly MJ, Nothnagel M, Karlsen TH, Subramani S, Rosenstiel P, Schreiber S, Parkes M, Franke A. Association between variants of PRDM1 and NDP52 and Crohn's disease, based on exome sequencing and functional studies. Gastroenterology 145: 339–347, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esther CR, Jr, Olsen BM, Lin FC, Fine J, Boucher RC. Exhaled breath condensate adenosine tracks lung function changes in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L504–L509, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans CR, Karnovsky A, Kovach MA, Standiford TJ, Burant CF, Stringer KA. Untargeted LC-MS metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J Proteome Res 13: 640–649, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Barnes KC. Recent advances in genetic predisposition to clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol 296: L713–L725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerszten RE, Wang TJ. The search for new cardiovascular biomarkers. Nature 451: 949–952, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Gong MN. Gene association studies in acute lung injury: replication and future direction. Am J Physiol Lung Cell Mol Physiol 296: L711–L712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howrylak JA, Dolinay T, Lucht L, Wang Z, Christiani DC, Sethi JM, Xing EP, Donahoe MP, Choi AM. Discovery of the gene signature for acute lung injury in patients with sepsis. Physiol Genomics 37: 133–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 336: 1040–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korman A, Oh A, Raskind A, Banks D. Statistical methods in metabolomics. In: Evolutionary Genomics: Statistical and Computational Methods, edited by Anisimova M. New York: Humana, 2012, p. 381–413 [DOI] [PubMed] [Google Scholar]
- 18.Langley RJ, Tsalik EL, Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, Freeman DH, Wang M, You J, Wulff J, Thompson JW, Moseley MA, Reisinger S, Edmonds BT, Grinnell B, Nelson DR, Dinwiddie DL, Miller NA, Saunders CJ, Soden SS, Rogers AJ, Gazourian L, Fredenburgh LE, Massaro AF, Baron RM, Choi AM, Corey GR, Ginsburg GS, Cairns CB, Otero RM, Fowler VG, Jr, Rivers EP, Woods CW, Kingsmore SF. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 5: 195ra195, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation*. Crit Care Med 41: 1929–1937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer NJ, Christie JD. von Willebrand factor and angiopoietin-2: toward an acute lung injury endothelial endophenotype? Crit Care Med 40: 1966–1967, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer NJ, Daye ZJ, Rushefski M, Aplenc R, Lanken PN, Shashaty MG, Christie JD, Feng R. SNP-set analysis replicates acute lung injury genetic risk factors. BMC Med Genet 13: 52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M, Aplenc R, Abramova H, Atochina-Vasserman EN, Beers MF, Calfee CS, Cohen MJ, Pittet JF, Christiani DC, O'Keefe GE, Ware LB, May AK, Wurfel MM, Hakonarson H, Christie JD. ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med 183: 1344–1353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mickiewicz B, Vogel HJ, Wong HR, Winston BW. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am J Respir Crit Care Med 187: 967–976, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mickiewicz B, Vogel HJ, Wong HR, Winston BW. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am J Respir Crit Care Med 187: 967–976, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller EJ, Cohen AB, Nagao S, Griffith D, Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M, Christophers E, Matthay MA. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis 146: 427–432, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, Heinzmann A, Simma B, Frischer T, Willis-Owen SA, Wong KC, Illig T, Vogelberg C, Weiland SK, von Mutius E, Abecasis GR, Farrall M, Gut IG, Lathrop GM, Cookson WO. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448: 470–473, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Muller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stancakova A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutskov K, Langford C, Leander K, Lindholm E, Lobbens S, Mannisto S, Mirza G, Muhleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurethsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvanen AC, Eriksson JG, Peltonen L, Nothen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L, Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network-Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium. Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njolstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyovalti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jockel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI, DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 44: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 101: 14895–14900, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O'Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121: 1402–1411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers AJ, McGeachie M, Baron RM, Gazourian L, Haspel JA, Nakahira K, Fredenburgh LE, Hunninghake GM, Raby BA, Matthay MA, Otero RM, Fowler VG, Rivers EP, Woods CW, Kingsmore SF, Langley R, Choi AM. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS One 9: e87538, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert JK, Muller WP, Benzing A, Geiger K. Application of a new method for analysis of exhaled gas in critically ill patients. Intensive Care Med 24: 415–421, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364: 1503–1512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am J Respir Crit Care Med 184: 647–655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stringer KA, Serkova NJ, Karnovsky A, Guire K, Paine R, 3rd, Standiford TJ. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma 1H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am J Physiol Lung Cell Mol Physiol 300: L4–L11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng S, Rhee EP, Sinha S, McCabe E, Fox CS, O'Donnell CJ, Ho JE, Florez JC, Magnusson M, Pierce KA, Souza AL, Yu Y, Carter C, Light PE, Melander O, Clish CB, Gerszten RE. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 123: 4309–4317, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, May AK, Calfee CS, Matthay MA. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care 17: R253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res 41: D801–807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


