Abstract
Epithelial injury is often detected in lung allografts, however, its relation to rejection pathogenesis is unknown. We hypothesized that sterile epithelial injury can lead to alloimmune activation in the lung. We performed adoptive transfer of mismatched splenocytes into recombinant activating gene 1 (Rag1)-deficient mice to induce an alloimmune status and then exposed these mice to naphthalene to induce sterile epithelial injury. We evaluated lungs for presence of alloimmune lung injury, endoplasmic reticulum (ER) stress, and hyaluronan expression, examined the effect of ER stress induction on hyaluronan expression and lymphocyte trapping by bronchial epithelia in vitro, and examined airways from patients with bronchiolitis obliterans syndrome and normal controls histologically. We found that Rag1-deficient mice that received mismatched splenocytes and naphthalene injection displayed bronchial epithelial ER stress, peribronchial hyaluronan expression, and lymphocytic bronchitis. Bronchial epithelial ER stress led to the expression of lymphocyte-trapping hyaluronan cables in vitro. Blockade of hyaluronan binding ameliorated naphthalene-induced lymphocytic bronchitis. ER stress was present histologically in >40% of bronchial epithelia of BOS patients and associated with subepithelial hyaluronan deposition. We conclude that sterile bronchial epithelial injury in the context of alloimmunity can lead to sustained ER stress and promote allograft rejection through hyaluronan expression.
Keywords: endoplasmic reticulum stress, lung rejection
lung transplantation is the only viable therapeutic option for many terminal lung diseases, but long-term survival remains disappointingly low compared with other solid organ transplants. Chronic airway rejection [bronchiolitis obliterans syndrome (BOS)] is the major reason for graft failure and patient morbidity and mortality. Many risk factors for BOS have been identified; however, the unifying principle may be that the lung, among transplanted solid organs, is uniquely exposed to the external environment.
There is increasing evidence for the presence of epithelial injury in lung transplantation. Even before the onset of clinical BOS, allograft airway epithelia demonstrate senescence (21) and epithelial-to-mesenchymal transition (31). Epithelial injury commonly results in accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER), leading to so-called ER stress (16). ER stress can be caused by environmental exposure (32), is found in chronic obstructive pulmonary disease airways (18), and can lead to a fibrotic response in human idiopathic pulmonary fibrosis (12). We therefore investigated whether epithelial injury can lead to alloimmune activation in the lung, and whether ER stress can be found in transplanted lungs, and be involved in the pathogenesis of airway rejection.
ER stress can lead to epithelial expression of hyaluronan (13, 17), and bronchoalveolar lavage hyaluronan is increased in lung transplant rejection (23, 29). Hyaluronan is ubiquitously present in the extracellular matrix, and in tissue injury short-fragment hyaluronan promotes inflammation (28) and activates innate immunity (9) that in turn can lead to alloimmune lung injury (6). Hyaluronan can also directly activate alloimmunity in transplantation (29). We therefore investigated the role of hyaluronan in epithelial injury-induced alloimmune activation.
In this work, we report that sterile epithelial injury, in the presence of alloimmunity, can lead to the recruitment of lymphocytes to the airways and the development of lymphocytic bronchiolitis. We demonstrate that naphthalene-induced bronchial epithelial injury is associated with ER stress, and hyaluronan expression. Hyaluronan in turn is critical in retention of lymphocytes to the subepithelial space, since pharmacological hyaluronan binding blockade ameliorates lymphocytic bronchiolitis in this model. In vitro, induction of ER stress in bronchial epithelia induces hyaluronan expression, and entrapment of lymphocytes in hyaluronan cable-like structures. Furthermore, we demonstrate a significant degree of ER stress in bronchial epithelia in human BOS, associated with increased deposition of hyaluronan in the subepithelial space. In aggregate, our results suggest that bronchial epithelial injury may be a proximal cause of alloimmune activation in lung transplant rejection, and that this may be at least partly due to ER stress-induced hyaluronan expression.
METHODS
Mice.
All experiments were approved by the Institutional Animal Care and Use Committee of the National Institute of Environmental Health Sciences. Male mice were used for all studies. Eight-week-old C57BL/6J, C3Hb/FeJ, and B6.129S7-recombinant activating gene (Rag) 1tm1Mom/J mice were purchased from the Jackson Laboratories (Bar Harbor, ME). All mice were fed normal chow and water ad libitum and were housed in apathogenic, quarantine conditions.
Adoptive transfer.
Eight-week-old C57BL/6J or C3Hb/FeJ mice were killed, and the spleens were removed and ground over a 40-μm strainer, washed, and resuspended in RPMI 1640 with 10% FBS. Five million live splenocytes (by trypan blue assay) in 400 μl media were injected retroorbitally in isoflurane-anesthetized recipient B6.129S7-Rag1tm1Mom/J mice. Mice were allowed to recover for 7 days and then underwent exposures as described below. Mice receiving C57BL/6J splenocytes were designated isoimmune adoptive transfer recipients (iso-AT), and mice receiving C3Hb/Fe splenocytes were alloimmune AT (allo-AT).
Naphthalene exposure.
Approximately 9- to 10-wk-old mice received 275 mg/kg naphthalene in corn oil (both from Sigma Aldrich, St. Louis, MO) via intraperitoneal injection. Control mice received an equal volume of corn oil only. Mice were killed by CO2 asphyxiation at days 7, 14, and 21 after naphthalene. Some mice received three repeated doses of naphthalene at days 0, 21, and 42, and were killed at day 63. Other mice received custom-made hyaluronan-binding peptide pep-1 (19) in 2% DMSO/PBS, scrambled control, or vehicle at 0.8 mg/mouse, at days 1, 3, 7, and 10 after single naphthalene dosing, and were killed at day 14.
Histology.
Mouse lungs were lavaged with 3 ml ice-cold saline, and cell-free supernatant was stored at −80. The left lungs were inflated with 10% buffered formalin. CD3+ cells were stained with rabbit anti-mouse CD3 polyclonal antibody, ab5690 (Abcam, Cambridge, MA). Myofibroblasts were detected with rabbit anti-mouse α-smooth muscle actin polyclonal antibody, ab5694 (Abcam). Hyaluronan was stained with biotinylated HABP (Seikagaku, East Falmouth, MA), and inter-α-inhibitor with rabbit anti-human (cross-reacting with mouse) polyclonal antibody (DakoCytomation, Carpinteria, CA). For the quantification of positive staining for ER stress markers, a blinded reviewer counted all airway epithelial cells (visible by DAPI staining) in a captured image, as well as all positively stained cells (for ER stress markers), and calculated the percentage of positive cells.
RT-PCR.
Total RNA was extracted from whole lung homogenates or BEAS-2B cells using a Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). RNA samples were treated with RNase-free DNase I (Ambion, Austin, TX) to clear DNA contamination. cDNA was synthesized using Invitrogen SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) or the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions. Quantitative polymerase chain reaction was performed using ABI SDS 7500 and SYBR Green Reagent (Applied Biosystems). Primers were produced by IDT (Coralville, IA), and their sequences are in Table 1.
Table 1.
List of primers used in this study
| Primer | |
|---|---|
| mCHOP | |
| Forward | CATACACCACCACACCTGAAAG |
| Reverse | CCGTTTCCTAGTTCTTCCTTGC |
| mATF4 | |
| Forward | CACGAAATCCAGCAGCAGTGT |
| Reverse | GGCTGCAAGAATGTAAAGGGG |
| mHAS1 | |
| Forward | GCCCTCCTCCTTCCTTCGT |
| Reverse | GTATAGCCACTCTCGGAAGTAAGATTTG |
| mHAS2 | |
| Forward | TCATGGGTAACCAATGCAGTTTT |
| Reverse | TTTAGTTGCATAGCCCAGACTCAA |
| mHAS3 | |
| Forward | CCTATGAATCAGTGGTCACAGGTTT |
| Reverse | TGCGGCCACGGTAGAAAA |
| mGAPDH | |
| Forward | ATCATCTCCGC CCCTTCTG |
| Reverse | GGTCATGAGCCCT TCCACAAT |
| mCol3α1 | |
| Forward | CCCTGGTCCACAAGGATTACA |
| Reverse | CTCCAGGTGCACCAGAATCA |
| mTnc | |
| Forward | TAGATGTTCCAAAGAGCCAGCAA |
| Reverse | CGTAAGTCCTTGGGTGCATCA |
| m18S | |
| Forward | CGGCTACCACATCCAAGGAA |
| Reverse | GCTGGAATTACCGCGGCT |
| mTGF-β1 | |
| Forward | CAAGTGGACATCAACGGTGAGG |
| Reverse | TGGCCATGAGAAGCAGGAAAGG |
| hHAS1 | |
| Forward | GTGAGTGGCTGTACAACGCG |
| Reverse | AGAGGGACGTAGTTAGCGGC |
| hHAS2 | |
| Forward | CGCAACACGTAACGCAATTGG |
| Reverse | CCACAGATGAGGCTGGGTCAAG |
| hHAS3 | |
| Forward | GGCGATTCGGTGGACTACATCC |
| Reverse | ACGCTGCTCAGGAAGGAAATCC |
| h18S | |
| Forward | CGGCTACCACATCCAAGGAA |
| Reverse | GCTGGAATTACCGCGGCT |
m, Mouse; h, human; HAS, hyaluronan synthase; GAPDH, glucose-a-phosphate dehydrogenase; Col3α1, collagen 3, α−chain 1; Tnc, tenascin C; TGF-β1, transforming growth factor-β1.
Collagen quantification.
Twenty-five milligrams of lung tissue were homogenized in RIPA buffer, and the homogenate was assayed for total collagen using the Sircol assay (Biocolor; Carrickfergus, County Antrim, UK) per the manufacturer's instructions. Results were expressed as microgram collagen per 100 mg lung tissue.
Flow cytometric analysis.
Lung tissue was placed in 60-mm tissue culture dishes containing 0.5 ml EDTA-free HBSS, minced, collagenase A, collagenase XI, hyaluronidase, and DNase I were added (all from Sigma-Aldrich), and the tissue was incubated at 37°C for 35 min. Digestion was stopped with 1 ml of cold 120 mM EDTA/PBS, and the minced tissue was strained and centrifuged for 5 min at 450 g. Cells were resuspended in FACS buffer, Histopaque 1083 (Sigma-Aldrich) was layered under the cells, and the cells were centrifuged at 450 g for 20 min. Cell layers were collected carefully and washed with and resuspended in FACS buffer. Antibodies used in tagging are as follows (all purchased from eBioscience, San Diego, CA, except for QDot from Invitrogen): primary binding antibodies, biotin anti-mouse/human CD45R (B220) (0.125 μg/106 cells) clone RA3-6B2, secondary antibody Qdot585-streptavidin (concentration 1:50), FITC anti-mouse Pan-NK cells (0.25 μg/106 cells) clone DX5, Pacific blue anti-mouse CD3 (0.5 μg/106 cells) clone 17A2, APC anti-mouse CD4 (0.125 μg/106 cells) clone RM4–5, APC-alexa fluor 750 anti-mouse CD8a (ly-2) (0.5 μg/106 cells) clone 53–6.7, PE-anti-mouse CD69 (0.25 μg/106 cells) clone H1.2F3, PE-Cy5 anti-mouse/human CD44 (0.125 μg/106 cells) clone IM7; isotype controls, rat IgG2Bk-PE-cy5, rat IgG2b-pacific blue, rat IgMk-fitc, rat IgG2a, k-apc-alexa fluor750, rat IgG2a-APC, rat IgG2a-biotin, and armenian hamster IgG-PE. Cells were analyzed by flow cytometry on a LSRII (BD Biosciences, San Jose, CA) equipped with FACSDiva software. Cells (10,000) were examined for each sample. A lymphocyte gate was initially set on a forward-scatter vs. side-scatter dot plot. The percent of CD4- and CD8-positive cells was determined from the CD3-positive population. Gates were set for the positive cells based on isotype controls.
In vitro ER stress induction.
BEAS-2B cells from Lonza (Walkersville, MD) were grown in BEGM until confluent. Cells were treated with 5 μg/ml tunicamycin (Sigma Aldrich) in 2% DMSO for 48 h and assayed for HAS expression and hyaluronan deposition over several time points. RT-PCR and PCR assays demonstrated persistent elevation of ER stress markers C/EBP homology protein (CHOP), BiP, and alternative splicing of XBP1 starting at least 6 h after the beginning of exposure and persisting through 48 h after cessation of exposure (data not shown). For the lymphocyte adhesion assay, 54,000 BEAS-2B cells/well were exposed to 5 μg/ml tunicamycin or vehicle (DMSO) for 24 h, washed repeatedly, and incubated at 4°C for 30 min. Human CD3+ lymphocytes were obtained from healthy volunteers via magnetic bead isolation per the manufacturer's instructions (Miltenyi Biotec, Auburn, CA), washed, and resuspended in ice-cold RPMI at 4 million/ml. One million CD3+ cells were added to each BEAS-2B well and incubated at 4°C for 30 min. In one tunicamycin group, 50 μl of Streptomyces hyaluronidase (Sigma) were added at 5.2 mg/ml concentration, and wells were incubated another 5 min at room temperature. Wells were washed very carefully with ice-cold BEGM five times, fixed in 4% methanol, stained for CD3, and imaged in an inverted Zeiss NLO 510 inverted fluorescent microscope. CD3+ cells per high-power field were counted.
MRC5 coculture with ER-stressed BEAS-2B cells.
BEAS-2B cells were plated at 45,000 cells/cm2 and exposed to tunicamycin as described above, except the exposure duration was 24 h and the cells were grown in Transwell plates (Corning, Tewksbury, MA). MRC-5 lung embryonic fibroblasts (American Type Culture Collection, Manassas, VA) were simultaneously plated at 20,000 cells/cm2 in Tranwell plates and grown per the supplier's instructions. After tunicamycin exposure for 24 h, the BEAS-2B cells were washed, and the inserts were placed over the MRC-5 cell wells. The cells were kept another 24 h in BEBM, which we previously verified is not toxic for MRC-5 cells, and then assayed for HAS gene expression.
Human tissue.
BOS lungs were explanted from patients at the time of retransplantation. All subjects had BOS3 rejection. Control lungs were rejected or trimmed tissues from transplantation grafts. All tissue was recovered from so-called pathology waste and was therefore exempt from Institutional Review Board approval.
Statistics.
Results are expressed as averages ± SE. Statistical analysis was performed using Student's t-test or ANOVA with post hoc correction (Bonferroni or Tukey Honest Significant Difference test) as appropriate.
RESULTS
Naphthalene-induced epithelial injury leads to lymphocytic bronchitis in rag1−/− mice that received alloimmune splenocyte adoptive transfer.
We first examined whether sterile epithelial injury could induce an alloimmune response in mouse airways. To avoid lung epithelial injury through irradiation or ischemia-reperfusion, while still having an alloimmune reactive status in mouse lungs, we used an AT model and injected isolated splenocytes from either fully MHC-mismatched (C3Hb/FeJ, H2k) or MHC-matched (C57BL/6J, H2b) mice into rag1-deficient mice (fully backcrossed to C57BL/6J, H2b). Engrafted splenocytes were detectable 7 days after AT (data not shown), at which point mice received naphthalene by intraperitoneal injection. Seven days after naphthalene, there was clear evidence of bronchial injury in exposed mice (Fig. 1), but no immune cell infiltration. However, at 2 and 3 wk after naphthalene, most airways showed inflammatory cell infiltration (Fig. 1, C and D, and Fig. 2, A–C). Most of these cells were lymphocytes, as evidenced by CD3 staining (Fig. 2, E and F). Using a semiquantitative score (6) we found that naphthalene-exposed allo-AT mice had persistently higher peribronchial infiltration than corn oil-treated allo-AT mice, although the degree of perivascular infiltration was similar (Fig. 2G). There was no significant change in the degree of infiltration when we performed up to three repeated naphthalene injections at 3-wk intervals (data not shown). allo-AT mice had significantly more CD69+ cells and significantly decreased NK1.1+ cells, CD4+ cells, and CD4-to-CD8 ratio compared with iso-AT mice (Table 2). C57Bl/6 mice, rag1-deficient mice, and rag1-deficient mice that received C57Bl/6 splenocytes (iso-AT) had no appreciable peribronchial infiltration up to 3 wk after naphthalene exposure (data not shown). There was no significant increase in collagen deposition after single naphthalene exposure by Masson-trichrome staining (data not shown) or by collagen III expression (Table 3), although there was an increase in TGF-β1 at 7 days after injury (Table 3) and an increase in α-smooth muscle actin staining 21 days after naphthalene exposure (Fig. 3), consistent with airway myocyte changes. However, after repeated naphthalene exposure, there was an increase in peribronchial collagen deposition (Fig. 2D) that was associated with increased collagen content in the lung (211.7 ± 9.3 μg/100 mg lung tissue for naphthalene-treated mice vs. 140.0 ± 12.3 μg/100 mg lung tissue for corn oil-treated mice, P < 0.001) and expression of collagen III (ΔΔCt = 374 ± 128% for naphthalene-treated mice vs. 105 ± 26% for corn oil-treated mice, P < 0.01). There was also a pronounced, sustained increase in tenascin C expression even after single exposure (Table 3), suggesting persistence of epithelial injury (27).
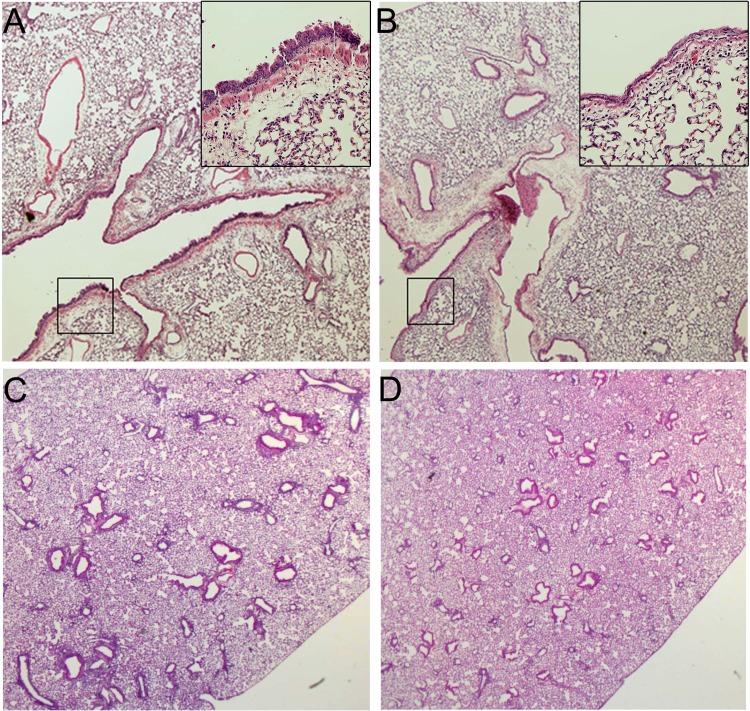
Fig. 1.
Histological evaluation of alloimmune adoptive transfer (allo-AT) lungs after naphthalene exposures. A and B: one week after exposure, there is evidence of epithelial injury in naphthalene-treated mice (A, inset: higher-magnification image of boxed-in area) but not corn oil-treated mice (B). C and D: 3 wk after exposure, naphthalene-treated mice show peribronchial infiltration (C), and corn oil-treated mice show infiltration around vessels (D). Magnification: A and B, ×4 (insets: ×20); C and D, ×2, HE staining.
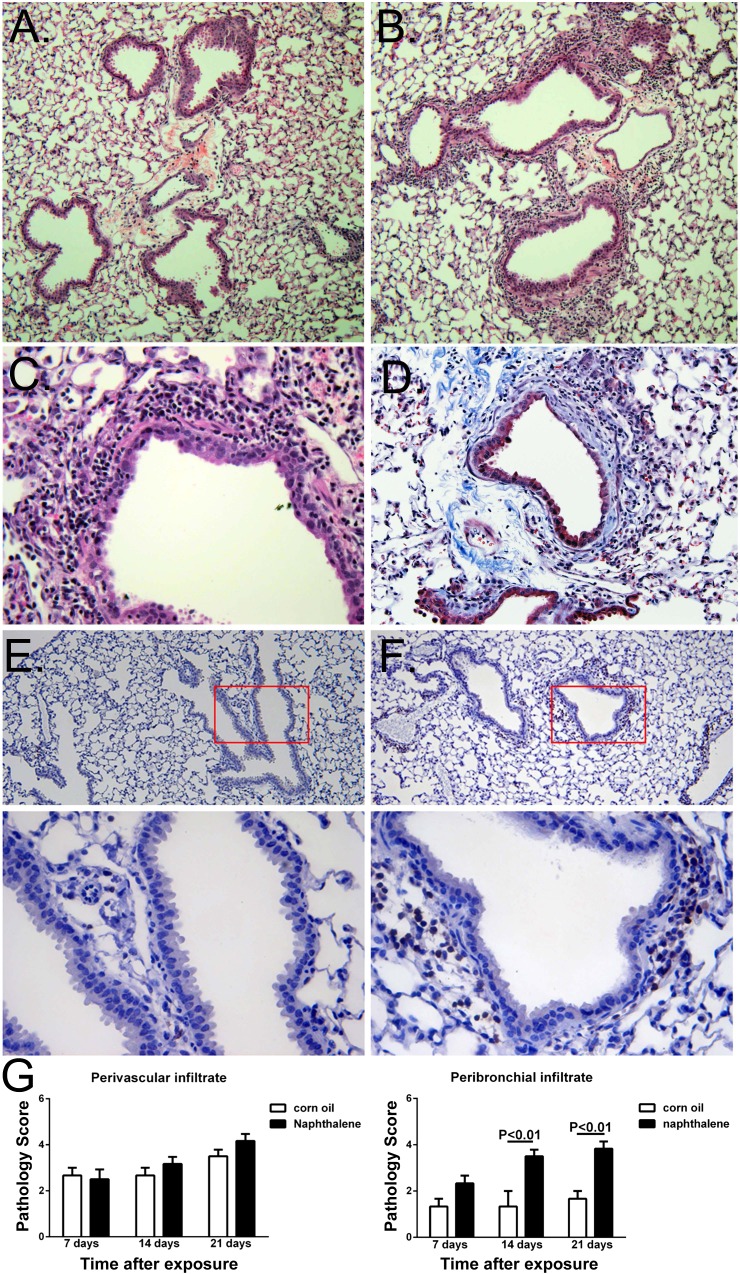
Fig. 2.
Histological evaluation of allo-AT lungs after naphthalene exposures. A and B: 3 wk after exposure, corn oil-treated mice show infiltration around vessels (A), and naphthalene-treated mice show peribronchial infiltration (B). C: higher-power magnification of a naphthalene-treated allo-AT mouse lung, demonstrating infiltration of epithelia by immune cells, which morphologically appear lymphocytic. D: peribronchial fibrosis in a mouse that received naphthalene two times after adoptive transfer. Immunohistochemically, there is little or no peribronchial staining for CD3 in corn oil-treated mice (E), but clearly visible CD3+ cells in naphthalene-treated mice (F). Panels on bottom show higher-magnification images of boxed-in areas in E and F. G: semiquantitative evaluation of infiltration score over time after exposure. There is similar and not progressive perivascular infiltration between naphthalene- and corn oil-treated mice (left), but statistically significant and sustained increase in peribronchial CD3 infiltration in naphthalene-treated mice only (right). N = 12–20 mice/group. Magnification: A and B, ×10; C, ×40, HE staining; D, ×20, Masson-Trichrome staining; E and F, ×4 (top) and ×20 (bottom), CD3 staining.
Table 2.
Flow cytometric analysis of lung lysates for relevant lymphocyte fractions
| allo-AT | iso-AT | P Value | |
|---|---|---|---|
| B220 | 25 ± 19 | 16 ± 8 | 0.3 |
| NK1.1 | 12 ± 7 | 37 ± 9 | 0.0005* |
| CD69 | 14 ± 5 | 8 ± 2 | 0.02* |
| CD4 | 34 ± 8 | 47 ± 5 | 0.006* |
| CD8 | 37 ± 9 | 34 ± 8 | 0.5 |
| CD4/CD8 | 0.80 ± 0.20 | 1.43 ± 0.33 | 0.005* |
AT, adoptive transfer. t-Test of 3 separate experiments with a total of 18–24 mice/group.
Significant P values.
Table 3.
Real-time RT-PCR expression of tenascin C, TGF-β1, and collagen III in naphthalene-treated AT mice
|
Week 1 |
Week 2 |
Week 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tenascin C | TGF-β1 | Collagen III | Tenascin C | TGF-β1 | Collagen III | Tenascin C | TGF-β1 | Collagen III | |
| Naphthalene | 201 ± 28 | 363 ± 115 | 116 ± 27 | 385 ± 175 | 132 ± 24 | 149 ± 56 | 254 ± 69 | 76 ± 39 | 86 ± 63 |
| Corn oil | 101 ± 19 | 102 ± 24 | 103 ± 19 | 100 ± 3 | 110 ± 51 | 100 ± 11 | 104 ± 4 | 116 ± 82 | 105 ± 40 |
| P < 0.01 | P < 0.01 | NS | P < 0.05 | NS | NS | P < 0.01 | NS | NS | |
Corn oil-treated mice serve as the reference control group (the average ΔΔCt for corn oil mice is arbitrarily set as 100% expression, and individual expression levels for naphthalene and corn oil mice are calculated in reference to this average). N = 4 mice/group. NS, not significant.
Fig. 3.
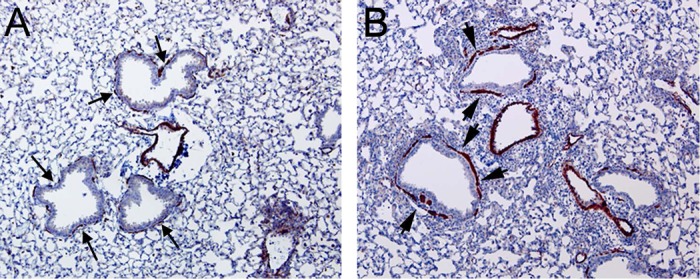
α-Smooth muscle actin (α-SMA) staining around airways is sparse and discontinuous in corn-oil-treated mice (A, arrows) but much more pronounced and expanded in naphthalene-treated mice (B, arrowheads). Magnification: ×4.
Naphthalene-induced injury leads to ER stress in bronchial epithelia.
Naphthalene induces bronchial club cell (Clara cell) epithelial injury by the formation of toxic metabolites that bind to cytoplasmic proteins (4, 5). Because protein adducts have been associated with ER stress (11), we asked whether ER stress could be detected in the airways of naphthalene-treated allo-AT mice. We found evidence or ER stress by positive staining for CHOP and activating transcription factor (ATF)-6 in a significant number of allo-AT mouse epithelia by immunohistochemistry (Fig. 4A) as well as quantitative RT-PCR for CHOP and ATF-4 (Fig. 4B) at 21 days after naphthalene, in contrast to corn oil-treated allo-AT mice and to naphthalene-treated mice without adoptive transfer, suggesting that allo-AT promotes sustained epithelial injury after the initial insult.
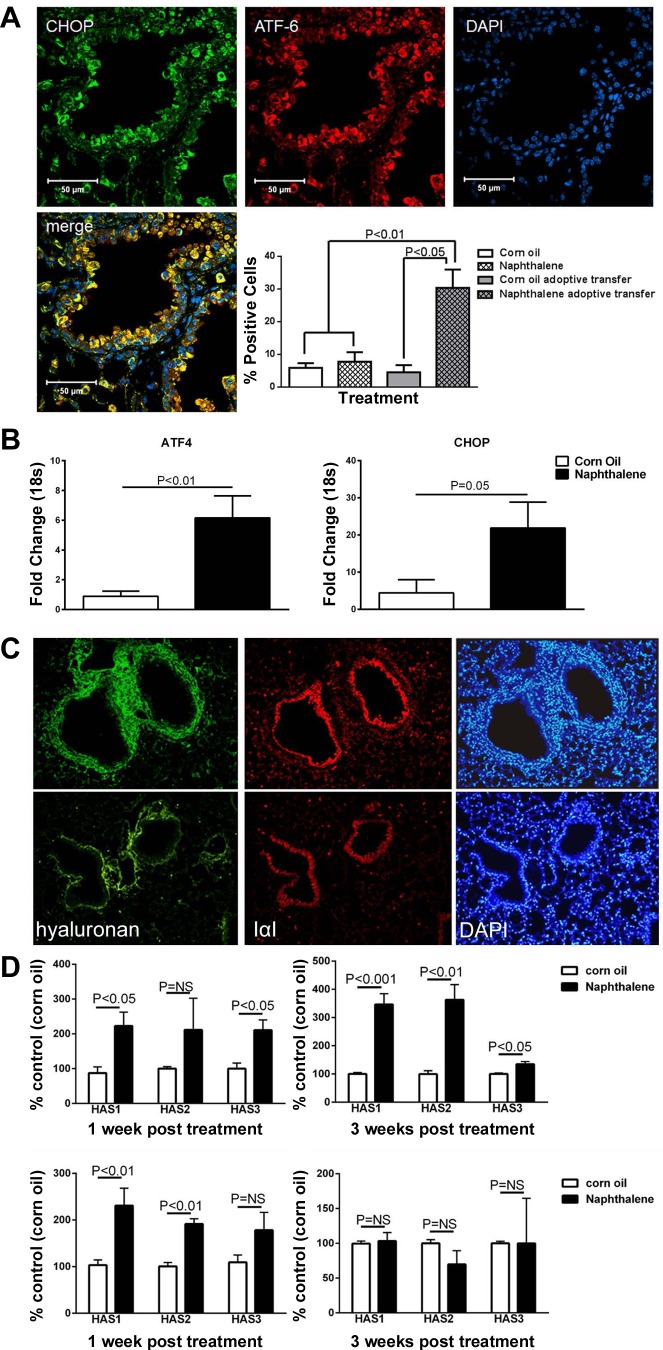
Fig. 4.
A: immunohistochemical staining of allo-AT lungs for endoplasmic reticulum (ER) stress with C/EBP homology protein (CHOP, green) and activating transcription factor (ATF)-6 (red). There is significant (but not universal) positive staining in naphthalene-treated mice that is statistically more than in corn oil-treated AT-mice and non-AT naphthalene-treated mice. N = 12–20 mice/group. Magnification: ×20. B: quantitative RT-PCR shows statistically significant upregulation of ER stress genes ATF-4 and CHOP. N = 15–20 mice/group. C: hyaluronan expression in allo-AT mouse lungs. Naphthalene-treated allo-AT lungs demonstrate significant deposition of hyaluronan and its binding partner IαI (top). Note the nuclear density in DAPI staining that colocalizes with the hyaluronan area. There is no increase of hyaluronan expression over baseline in corn oil-treated lungs (bottom). Magnification: ×10. D: allo-AT mice demonstrate a sustained increased in mRNA expression of all three hyaluronan synthases (HAS) over 3 wk (top), whereas iso-AT mouse lungs demonstrate an increase in HAS expression after naphthalene expression, but only in the 1st wk after exposure (bottom). N = 4/group.
Naphthalene-induced injury and ER stress induce hyaluronan expression in vivo.
Because ER stress has been associated with hyaluronan expression, we asked whether there was increased hyaluronan expression in our naphthalene/allo-AT model. Indeed, we found increased deposition of hyaluronan at 14 and 21 days after naphthalene exposure, in contrast to the control corn oil/adoptive transfer mice (Fig. 4C) and naphthalene/iso-AT mice (data not shown). We then quantified the expression of hyaluronan synthase (HAS) genes by RT-PCR. One week after naphthalene injury, all HAS genes were upregulated in lung lysates, regardless of allo-AT or iso-AT status. In contrast, 3 wk after naphthalene, only allo-AT mice retained a significant upregulation of HAS genes, particularly HAS1 and HAS2 (Fig. 4D).
ER stress in bronchial epithelia induces hyaluronan cables that trap lymphocytes.
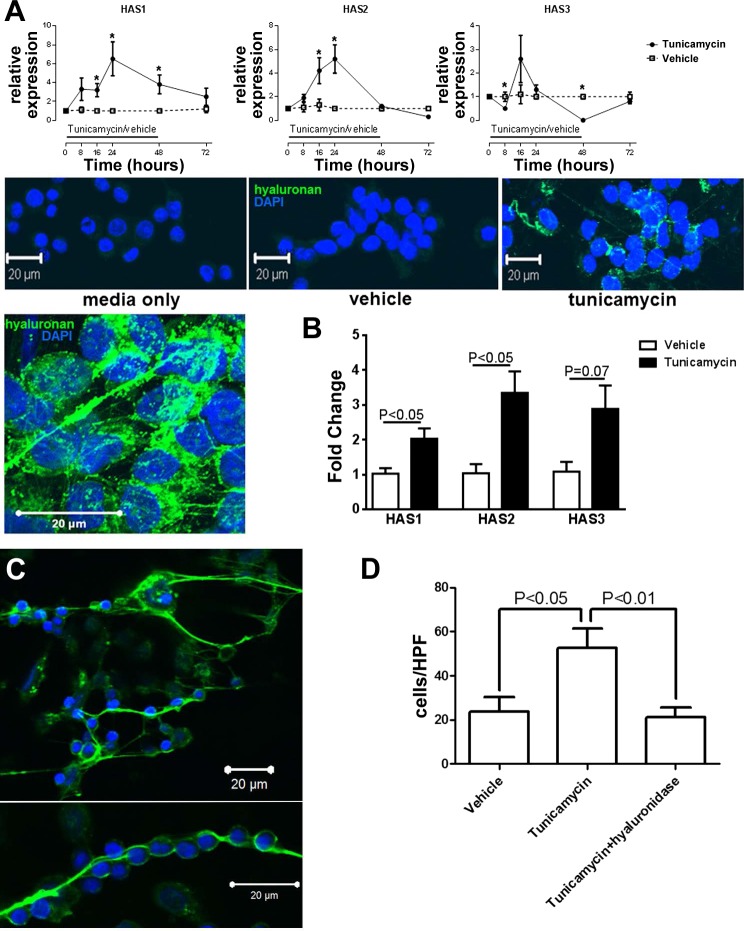
We then examined whether hyaluronan expression by ER-stressed bronchial epithelia may contribute to the pathogenesis of lymphocytic bronchiolitis. Epithelial ER stress has been associated with the formation of hyaluronan cable-like structures that can trap immune cells (13, 17). We therefore treated human bronchial epithelial BEAS-2B cells with tunicamycin, which induces ER stress, and observed significant upregulation of HAS1 and HAS2, as well as deposition of hyaluronan, which in part appeared in cable-like structures (Fig. 5A). Because epithelial cells are not the main source of extracellular hyaluronan in the subepithelial space, we also investigated whether ER-stressed epithelia can induce expression of HAS in fibroblasts. We found that human embryonic fibroblasts express more HAS1 and HAS2 and show a trend toward higher HS3 expression after a 24-h coculture with BEAS-2B cells that had been previously exposed to 24 h of tunicamycin (Fig. 5B). We then asked whether the epithelial hyaluronan cables were lymphocyte adhesive. We observed that human CD3+ lymphocytes were being trapped by hyaluronan cables in vitro, an effect that was abolished after treatment with hyaluronidase [Fig. 5C and Supplemental movie 1 (Supplemental data for this article may be found on the American Journal of Physiology: Lung Cellular and Molecular Physiology website.)].
Fig. 5.
A: in vitro ER stress induction in BEAS-2B cells leads to upregulation of HAS1 and HAS2 (top), and deposition of hyaluronan (in green, bottom), which appears to partly form cable-like structures. *P < 0.05 compared with control-treated cells. Eight experimental repeats per exposure. B: coculture with ER-stressed BEAS-2B cells (previously exposed to tunicamycin for 24 h) leads to upregulation of HAS enzymes in MRC-5 cells. C: hyaluronan cables (green) trap lymphocytes (visible from blue nuclear DAPI staining). The lymphocytes can be found on net-like hyaluronan structures (top) or form beads-on-a-string structures. D: quantification of trapped lymphocytes shows the positive effect of tunicamycin, which is abolished by treatment with hyaluronidase. Eight experimental repeats per exposure.
Pep-1 treatment in allo-AT mice ameliorates lymphocytic bronchiolitis.
Because in vitro removal of hyaluronan reduces the lymphocytic entrapment by ER-stressed epithelia, we examined whether neutralization of hyaluronan binding could reduce the degree of lymphocytic bronchiolitis in vivo. We exposed our allo-AT mice to naphthalene and then treated them with pep-1, a known inhibitor of hyaluronan binding (19), its scrambled control peptide, or vehicle. When we evaluated the degree of lymphocytic infiltration in the lung, we saw no difference in perivascular infiltration, but a significant decrease in lymphocytic bronchitis (Fig. 6). There was no difference in ER stress by pep-1 treatment, a finding that was expected since hyaluronan expression is downstream of ER stress.
Fig. 6.
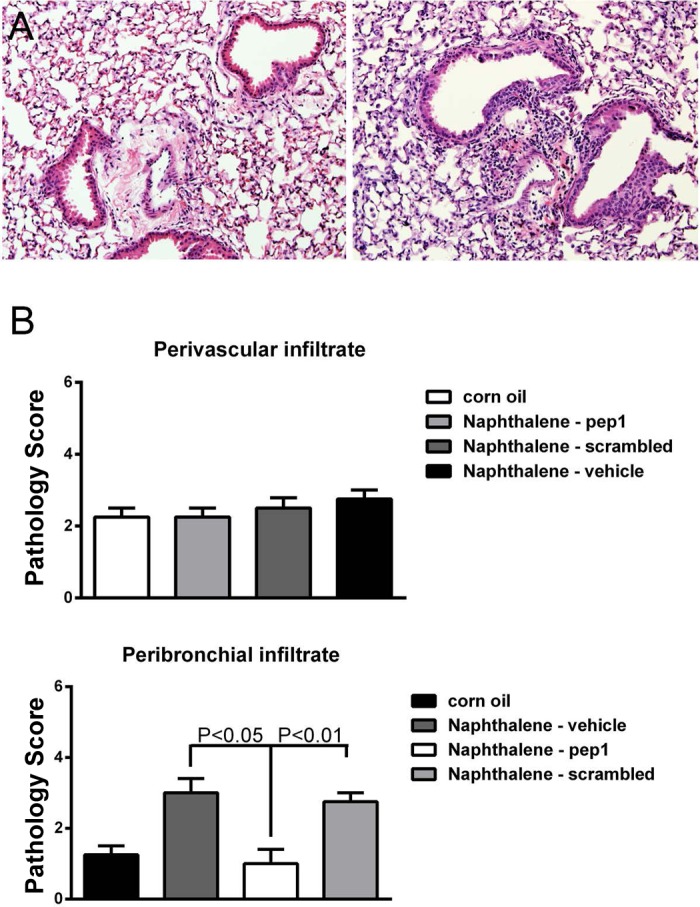
A: hyaluronan binding blockade ameliorated lymphocytic bronchitis in the naphthalene model. Pep-1-treated mice had significant reduction in peribronchial inflammation (left) compared with scrambled peptide-treated mice (right). B: quantification of inflammatory score. There is no difference in perivascular immune infiltrates by treatment, but pep-1 reduces the peribronchial infiltration score. N = 6–8/group.
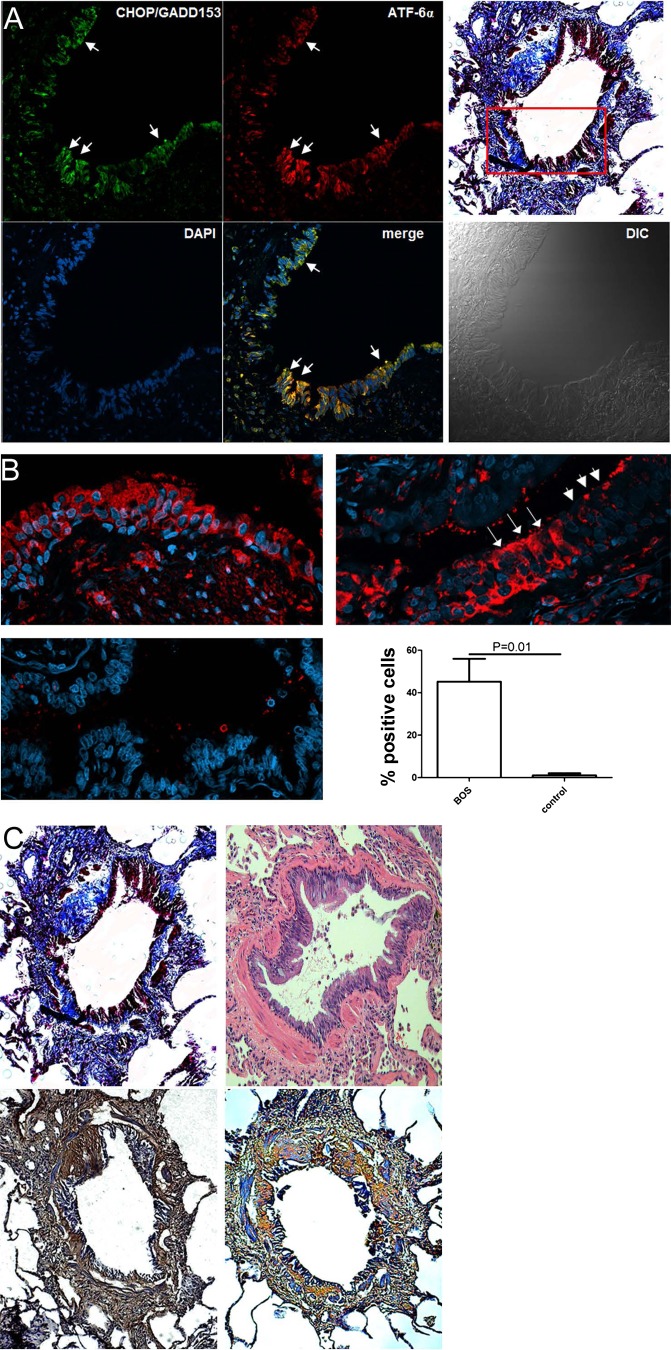
ER stress is present in human airway rejection, and is associated with hyaluronan expression.
We then detected the presence of ER stress in human lung transplant rejection (Fig. 7A). We stained sections from six patients with BOS and four healthy controls for ATF-6 and CHOP, and quantified the number of positive epithelia in the airways. ER stress was prominently present in affected airways in BOS patients (Fig. 7B). In some cases, positive staining occurred in most cells in an airway, and, in others, skip lesions were observed. The percentage of positive cells ranged from 18 to 80% in BOS patients and from 0 to 4% in controls, with an average ± SD of 45 ± 26 and 1 ± 2%, respectively (Fig. 7B). The remodeled subepithelial space stained strongly positive for hyaluronan and its binding partner inter-α-inhibitor (Fig. 7C).
Fig. 7.
A: evidence for ER stress in an airway that is affected by bronchiolitis obliterans. The immunofluorescent staining depicts the boxed-in area of the airway highlighted in Masson-Trichrome staining. B: quantification of ER stress in bronchiolitis obliterans-afflicted airways. A: some airways demonstrate almost continuous positive staining for CHOP (top left, in red). Other airways show skip-pattern lesions (top right). Negative control (rejected transplant lung) shows no staining (bottom left). Magnification: ×40. Quantification shows a significant difference in CHOP/ATF6-positive staining (bottom right). N = 4–6/group. C: consecutive sections of a scarified airway (Masson-trichrome and hematoxylin-eosin, top) stain strongly positive for hyaluronan (bottom left, brown) and IαI (bottom right, orange) in the subepithelial area and scar tissue. Magnification: ×10.
DISCUSSION
Chronic allograft rejection remains the main limitation to survival in lung transplant recipients. Lung allografts are unique in their continuous exposure to environmental injury, which triggers immune responses that could promote the disproportionately high lung transplant rejection rates. A major pathway for the initiation of the alloimmune response may be epithelial injury from inhaled environmental toxicants.
Increasing evidence suggests that epithelial injury is a hallmark of lung allograft dysfunction. Senescence and epithelial-to-mesenchymal transition have been observed in airway epithelia from rejected lung allografts (1, 21, 31), and can contribute to a defective healing response in epithelial injury. Furthermore, significant club cell injury was recently demonstrated in BOS (10). Club cells are metabolically active and involved in xenobiotic metabolism through cytochrome p450, which may make them especially vulnerable to environmental injury (2). Naphthalene specifically injures club cells through p450-mediated toxic metabolites that form protein adducts with cellular proteins and induce oxidative damage (4). We now show that naphthalene-induced sterile club cell injury is sufficient for the induction of an alloimmune response against the lung. Club cells participate in immune regulation as well as epithelial regeneration after airway injury (7, 27), and may therefore play a central role in the pathogenesis of chronic airway rejection, which combines both immune and remodeling components. Chronic club cell injury induces lymphocytic infiltration of the lungs, which is mediated through the IFNγ system (24). Furthermore, prolonged club cell injury leads to sustained tenascin C expression (27), which may contribute to the pathogenesis of airway remodeling and has been associated with the onset of BOS (20). Interestingly, in our model, we see a very similar phenotype: sustained lymphocytic infiltration and induction of tenascin C. Previously published models of chronic club cell injury have used transgenic expression of toxins as the inciting mechanism. In our model, the presence of activated immune cells may perpetuate epithelial injury, thus creating a vicious cycle of injury and alloimmune activation. Consistent with previous reports on the role of lymphocytes in transplant rejection, we found increased numbers of activated lymphocytes (CD69+), and reduced CD4+ cells as well as CD4+-to-CD8+ ratio in the allo-AT mice, consistent with the presence of alloactive immune cells. The observed reduction of NK1.1 cells would also suggest a regulatory role for this cell type in our model, as described recently (30).
Our results support that hyaluronan plays an important role in the pathogenesis of lymphocytic retention in the airways. Hyaluronan is a ubiquitous extracellular matrix component and has multifaceted effects on the response to lung injury. Hyaluronan can activate innate immunity, dendritic cells, and alloimmunity (9, 29), and increased levels have been detected in BOS-affected lungs (23, 29). Epithelial injury also has been linked to hyaluronan expression. Epithelial injury can induce the secretion by epithelial and mesenchymal cells of cable-like structures that are able to trap immune cells (13, 17). We demonstrate for the first time that a similar mechanism may contribute to the perpetuation of lymphocytic infiltration in alloimmune lung injury. We show sustained hyaluronan synthesis and deposition after club cell injury, demonstrate in vitro that epithelial hyaluronan can trap lymphocytes, and finally show that neutralization of hyaluronan binding can ameliorate lymphocytic bronchitis in our model. Hyaluronan binding seems to be important for the entrapment of lymphocytes in the vicinity of injured epithelia. For example, hyaluronan deposition has been associated with lymphocytic infiltration in allergic asthma (3). Activated lymphocytes can interact with airway smooth muscle cells and induce cell proliferation and airway remodeling (22), a process that involves the hyaluronan receptor CD44 (15). We therefore speculate that binding to the hyaluronan matrix is critical for the interactions between epithelial, immune, and structural cells that ultimately lead to airway fibrosis and rejection.
Although by no means the only mechanism, our results suggest that epithelial ER stress may be a crucial link between epithelial injury and hyaluronan expression. We demonstrate significant ER stress in our animal model and in human BOS that was associated with subepithelial hyaluronan deposition. ER stress is being increasingly recognized in chronic airway disease (for excellent reviews, see Refs. 25 and 33). Furthermore, both in animal models of airway disease and human samples, airway ER stress affects a substantial number of imaged airway cells (8, 26). Thus, our results are in agreement with emerging evidence that ER stress may be widespread in chronic obstructive lung disease situations. Although our results suggest that ER stress leads to hyaluronan deposition by injured epithelia, we do not believe that this is the only mechanism for the observed hyaluronan expression. Mesenchymal cells are avid producers of hyaluronan, and are very likely to significantly contribute to its production. We show that tunicamycin-treated BEAS-2B cells induced hyaluronan in cocultured fibroblasts in vitro. Thus, our results suggest that epithelial ER stress contributes to hyaluronan expression both directly and indirectly. Furthermore, epithelial ER stress greatly enhances the fibrotic response to other stimuli (14). Thus, the presence of ER stress may be a crucial component of BOS pathogenesis, both by promoting lymphocytic bronchiolitis and supporting the fibrotic response to injury.
No single animal model can accurately replicate human airway rejection, and our model necessarily shares this weakness. In particular, our model does not include ischemic time and is rather an in situ graft-vs.-host response than a host-vs.-allograft injury as observed in human lung transplantation. However, we developed this model to specifically allow us to control the time and occurrence of epithelial injury, something that would not have been possible in an orthotopic or heterotopic lung transplant model, or a bone marrow transplant model. Furthermore, bronchiolitis obliterans is also observed in human bone marrow transplantation, suggesting that the alloimmune response mechanisms triggered by epithelial injury are shared. We thus believe that our model provides useful insight into the role of epithelial injury in the onset of alloimmune response against the airway.
In summary, our data show for the first time the presence of ER stress in human lung rejection, and suggest that sterile club cell injury promotes the development of lymphocytic bronchiolitis, partly through subepithelial hyaluronan deposition.
GRANTS
Research was supported in part by the Division of Intramural Research of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences. J. W. Hollingsworth appreciates support provided by the NIH, including Grants ES-016126 and ES-020350. S. M. Palmer receives support by the NIH, including Grant HL-091140.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.P.S., C.S., Q.C., R.L.H., C.D.B., J.W.H., I.P.N., S.M.P., and S.G. performed experiments; V.P.S., C.S., Q.C., R.L.H., C.D.B., J.W.H., and S.G. analyzed data; V.P.S., C.S., Q.C., R.L.H., C.D.B., J.W.H., I.P.N., S.M.P., and S.G. approved final version of manuscript; S.G. conception and design of research; S.G. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Lori Snyder for assistance with flow cytometry, Laura Miller Degraff and Ligon Perrow for animal husbandry, and Jeff Tucker for assistance with confocal microscopy.
Present addresses: R. L. Heise, Virginia Commonwealth University, Richmond, VA; I. P. Neuringer, Massachusetts General Hospital, Boston, MA.
REFERENCES
- 1.Borthwick LA, Parker SM, Brougham KA, Johnson GE, Gorowiec MR, Ward C, Lordan JL, Corris PA, Kirby JA, Fisher AJ. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax 64: 770–777, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, Plopper C. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol 47: 74–81, 1995. [PubMed] [Google Scholar]
- 3.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology 23: 43–58, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chichester CH, Buckpitt AR, Chang A, Plopper CG. Metabolism and cytotoxicity of naphthalene and its metabolites in isolated murine Clara cells. Mol Pharmacol 45: 664–672, 1994. [PubMed] [Google Scholar]
- 5.Cho M, Chichester C, Morin D, Plopper C, Buckpitt A. Covalent interactions of reactive naphthalene metabolites with proteins. J Pharmacol Exp Ther 269: 881–889, 1994. [PubMed] [Google Scholar]
- 6.Garantziotis S, Palmer SM, Snyder LD, Ganous T, Chen BJ, Wang T, Cook DN, Schwartz DA. Alloimmune lung injury induced by local innate immune activation through inhaled lipopolysaccharide. Transplantation 84: 1012–1019, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol Lung Cell Mol Physiol 275: L924–L930, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME, Anathy V. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis (Abstract). Respir Res 14: 141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Kelly FL, Kennedy VE, Jain R, Sindhwani NS, Finlen Copeland CA, Snyder LD, Eu JP, Meltzer EB, Brockway BL, Pavlisko E, Stripp BR, Palmer SM. Epithelial clara cell injury occurs in bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant 12: 3076–3084, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitaguchi Y, Taraseviciene-Stewart L, Hanaoka M, Natarajan R, Kraskauskas D, Voelkel NF. Acrolein induces endoplasmic reticulum stress and causes airspace enlargement. PloS one 7: e38038, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer ME, Erzurum SC, Mukhopadhyay D, Vasanji A, Drazba J, Wang A, Fulop C, Hascall VC. Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J Biol Chem 283: 26283–26296, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108: 10562–10567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazaar AL, Albelda SM, Pilewski JM, Brennan B, Pure E, Panettieri RA., Jr T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med 180: 807–816, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Ann Rev Pathol 3: 399–425, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 278: 47223–47231, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med 180: 1196–1207, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med 192: 769–779, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paivaniemi OE, Maasilta PK, Alho HS, Vainikka TL, Salminen US. Epithelial tenascin predicts obliterative airway disease. J Heart Lung Transplant 27: 400–407, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Parker SM, Goriwiec MR, Borthwick LA, Johnson G, Ward C, Lordan JL, Corris PA, Saretzki GC, Fisher AJ. Airway epithelial cell senescence in the lung allograft. Am J Transplant 8: 1544–1549, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Barbon D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest 115: 1580–1589, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao PN, Zeevi A, Snyder J, Spichty K, Habrat T, Warty V, Dauber J, Paradis I, Duncan S, Pham S. Monitoring of acute lung rejection and infection by bronchoalveolar lavage and plasma levels of hyaluronic acid in clinical lung transplantation. J Heart Lung Transplant 13: 958–962, 1994. [PubMed] [Google Scholar]
- 24.Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol 287: L1256–L1265, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro CM, O'Neal WK. Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med 12: 872–882, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem 280: 17798–17806, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol 40: 633–642, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 6: 2622–2635, 2006. [DOI] [PubMed] [Google Scholar]
- 30.van der Touw W, Burrell B, Lal G, Bromberg JS. NK cells are required for costimulatory blockade induced tolerance to vascularized allografts. Transplantation 94: 575–584, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward C, Forrest IA, Murphy DM, Johnson GE, Robertson H, Cawston TE, Fisher AJ, Dark JH, Lordan JL, Kirby JA, Corris PA. Phenotype of airway epithelial cells suggests epithelial to mesenchymal cell transition in clinically stable lung transplant recipients. Thorax 60: 865–871, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watterson TL, Hamilton B, Martin R, Coulombe RA., Jr Urban particulate matter causes ER stress and the unfolded protein response in human lung cells. Toxicol Sci 112: 111–122, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Wei J, Rahman S, Ayaub EA, Dickhout JG, Ask K. Protein misfolding and endoplasmic reticulum stress in chronic lung disease. Chest 143: 1098–1105, 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.