Abstract
Matrix metalloproteinase-9 (MMP-9) is a matrix-degrading enzyme implicated in many biological processes, including inflammation. It is produced by many cells, including fibroblasts. When cultured in three-dimensional (3D) collagen gels, fibroblasts contract the surrounding matrix, a function that is thought to model the contraction that characterizes both normal wound repair and fibrosis. The current study was designed to evaluate the role of endogenously produced MMP-9 in fibroblast contraction of 3D collagen gels. Fibroblasts from mice lacking expression of MMP-9 and human lung fibroblasts (HFL-1) transfected with MMP-9 small-interfering RNA (siRNA) were used. Fibroblasts were cast into type I collagen gels and floated in culture medium with or without transforming growth factor (TGF)-β1 for 5 days. Gel size was determined daily using an image analysis system. Gels made from MMP-9 siRNA-treated human fibroblasts contracted less than control fibroblasts, as did fibroblasts incubated with a nonspecific MMP inhibitor. Similarly, fibroblasts cultured from MMP-9-deficient mice contracted gels less than did fibroblasts from control mice. Transfection of the MMP-9-deficient murine fibroblasts with a vector expressing murine MMP-9 restored contractile activity to MMP-9-deficient fibroblasts. Inhibition of MMP-9 reduced active TGF-β1 and reduced several TGF-β1-driven responses, including activity of a Smad3 reporter gene and production of fibronectin. Because TGF-β1 also drives fibroblast gel contraction, this suggests the mechanism for MMP-9 regulation of contraction is through the generation of active TGF-β1. This study provides direct evidence that endogenously produced MMP-9 has a role in regulation of tissue contraction of 3D collagen gels mediated by fibroblasts.
Keywords: lung, repair, transforming growth factor-β
the matrix metalloproteinases (MMPs) are a large family of enzymes believed important in tissue remodeling. MMP-9 (gelatinase B) is a member of this gene family that is present at low levels in the healthy lung but is increased in a number of lung diseases, including asthma, pulmonary fibrosis, and chronic obstructive pulmonary disease (COPD) (3, 6, 8, 9, 21, 22, 31, 35, 37, 41). Among its many functions, MMP-9 has been reported to activate latent transforming growth factor (TGF)-β to its active form and to induce TGF-β production in epithelial cells (27, 33, 45). Because TGF-β stimulates fibroblasts, these observations have led to the suggestion that MMP-9 plays an important role in the tissue remodeling that characterizes many lung diseases.
A number of in vitro assays have been used to explore tissue remodeling. Among these, culture of fibroblasts in three-dimensional (3D) collagen gels has been used to study the contraction that characterizes both normal wound healing and the development of fibrotic scar tissue (2, 11). Exogenous stimulation with mediators believed to play an important role in the development of fibrosis, such as TGF-β, has been found to stimulate fibroblast contractility using this system (24, 25). Contraction of 3D collagen gels, however, is an intrinsic property of fibroblasts. Importantly, cells cultured from fibrotic tissues contract more than cells from normal tissues (29), whereas fibroblasts from COPD lung contract less (40). This suggests that endogenous factors can modulate fibroblast contraction and that this could play a role in the pathogenesis of disease.
The current study, therefore, was designed to explore the potential role of endogenous MMP-9 in modulating fibroblast contractility. To accomplish this, two experimental systems were used. Fibroblasts were isolated from the lungs of MMP-9-deficient and control mice, as were normal human lung fibroblasts in which MMP-9 was suppressed with small-interfering RNA (siRNA) or inhibited with nonspecific MMP inhibitors. Results obtained suggest that MMP-9 regulates fibroblast contraction of 3D collagen gels and suggest that this effect is mediated through the generation of active TGF-β1. These effects, moreover, are observed under “baseline” conditions of cell culture.
MATERIALS AND METHODS
Materials and cell culture.
Type I collagen [rat tail tendon collagen (RTTC)] was extracted from rat tail tendons by a previously published method (30). Protein concentration was determined by weighing a lyophilized aliquot from each batch of collagen. The RTTC was stored at 4°C until use. Dulbecco's modified Eagle's medium (DMEM), fetal calf serum (FCS), trypsin/EDTA, penicillin G sodium, and streptomycin were purchased from Invitrogen (Life Technologies, Grand Island, NY). Amphotericin B was purchased from Pharma-Tek (Elmira, NY). Recombinant human TGF-β1, tumor necrosis factor (TNF)-α, IL-1β anti-TGF-β1 antibody, biotinylated anti-TGF-β1 antibody, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from R&D Systems (Minneapolis, MN). Rabbit anti-goat and mouse IgG horseradish peroxidase (HRP) were purchased from Rockland Immunochemicals (Gilbertsville, PA). FITC-conjugated monoclonal anti-pan cytokeratin antibody, anti-vimentin antibody, and propidium iodide were purchased from Sigma (St. Louis, MO). GM-6001 was purchased from Chemicon International (Temecula, CA). Anti-Smad3 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Human fetal lung fibroblasts (HFL-1) were obtained from the American Type Culture Collection (Rockville, MD). Mice deficient in MMP-9 and wild-type (WT) controls were generated, as described previously (42). MMP-9 null 129 SvEv mice (gelatinase B−/−) were generated by targeted mutagenesis and housed with WT littermates (gelatinase B+/+) in a pathogen-free animal facility. Mice were used at 3–4 mo of age. Mouse lung tissues were minced on 60-mm tissue culture dishes (Falcon; Becton-Dickinson Labware, Lincoln Park, NJ) and cultured in DMEM, supplemented with 10% FCS, 50 U/ml penicillin G sodium, 50 μg/ml streptomycin sulfate, and 1 μg/ml amphotericin B for ∼3 wk. Under these conditions, only fibroblasts migrated from the minced tissue and proliferated (38). This is routinely confirmed by vimentin and keratin immunohistochemistry. After trypsinization, cells were replated in a 60-mm dish and cultured another 3 wk with the same medium. Following this, fibroblasts were cultured in 100-mm tissue culture dishes in DMEM with 10% FCS. The fibroblasts were refed three times weekly, and cells between passages 15 to 18 for human and 3 to 8 for murine were used.
Transfection of murine lung fibroblasts with MMP-9.
The pRc/CMV vector (Invitrogen) was used to drive expression of murine MMP-9. The cDNA for murine MMP-9, a gift of Dr. G. Opdenakker, University of Leuven, Belgium, was inserted into the pRc/CMV(Not I-Xba I) vector.
Transfection of pRcCMV/mGelB (MMP-9) was performed as described below. Briefly, murine lung fibroblasts were seeded in six-well plates at 5 × 105 cells/well in DMEM with 10% FCS. Once confluent, transfection with the vector was performed. In one tube, 10 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) were mixed gently with 40 μl of Opti-MEM medium (Invitrogen) and incubated 5 min at room temperature. In another tube, 5 μg of DNA were mixed gently with 50 μl of Opti-MEM medium. The two tubes were then combined, gently mixed, and incubated for 20 min at room temperature. After incubation, DMEM without FCS and antibiotics was added to obtain a final volume sufficient to add 0.5 ml to each well. Cells were washed with sterile PBS two times and incubated with 0.5 ml of the transfection solution for 4 h at 37°C. Media were then changed to DMEM with 10% FCS, and cells were cultured for 20 h before use.
Transfection with siRNA.
siRNA for human MMP-9 and nonspecific siRNA for control were purchased from Dharmacon (SMARTpool, Lafayette, CO). Transfection with siRNA was performed as described previously (16). Briefly, HFL-1 cells were seeded in six-well plates at 1 × 104 cells/cm2 in DMEM with 10% FCS. At 70% confluence, transfection with siRNA was performed. In one tube, 10 μl of Lipofectamine 2000 (Invitrogen) were mixed gently with 250 μl of Opti-MEM medium (Invitrogen) and incubated 5 min at room temperature. In another tube, 200 pmol of each siRNA were mixed gently with 250 μl of Opti-MEM medium. The two tubes were then combined, gently mixed, and incubated for 20 min at room temperature. After incubation, DMEM without FCS and antibiotics was added to obtain a final volume sufficient to add 2 ml (final concentration of siRNAs = 100 nM) for each well. Cells were washed with sterile PBS two times and incubated with 2 ml of the siRNA transfection solution for 6 h at 37°C. Media were then changed to DMEM with 10% FCS and cultured for 18 h before use.
Immunofluorescence staining for pan-cytokeratin and vimentin.
Murine cells were seeded on eight-chamber glass slides (Nunc, Rochester, NY) at 0.5 × 105 cells/well in DMEM with 10% FCS. The next day, cells were fixed in 4% paraformaldehyde in PBS for 10 min, permeabilized with 0.1% Triton X in PBS for 10 min, and incubated with FITC-conjugated monoclonal anti-pan-cytokeratin antibody (1:200 in PBS) or anti-vimentin antibody (1:200 in PBS) for 1 h. After washing with PBS, propidium iodide (1 μg/ml in PBS) was added for 15 min as a counter stain, and the slides were mounted using VectaShield mounting medium.
Immunofluorescence staining for Smad3.
After being transfected with MMP-9 siRNA or control siRNA, HFL-1 cells were seeded on eight-chamber glass slides (Nunc) at 0.5 × 105 cells/well in DMEM with 10% FCS. The next day, after stimulation with 100 pM TGF-β1 for 30 min, cells were fixed in 4% paraformaldehyde in PBS for 10 min, permeabilized with 0.1% Triton X in PBS for 10 min, and incubated with anti-Smad3 antibody (1:50 in PBS) overnight at 4°C, followed by incubation with fluorescein-conjugated secondary antibody (1:1,000 in PBS). After being washed with PBS, cells were incubated with DAPI (1 μg/ml in PBS) for 15 min as a counter stain, then mounted using VectaShield mounting medium. Cellular localization of fluorescence was examined by fluorescence microscopy and photographed.
3D collagen gel culture.
Before preparing collagen gels as described below, fibroblasts were detached by 0.05% trypsin in 0.53 mM EDTA and suspended in 10 ml serum-free DMEM containing soybean trypsin inhibitor. The cell number was then counted with a Coulter Counter. Collagen gels were prepared, as previously described (30), by mixing RTTC, distilled water, 4× DMEM, and cells. The final concentration was 1× DMEM, 0.75 mg/ml of collagen, and fibroblasts were present at 3 × 105 cells/ml for human and 4.5 × 105 cells/ml for murine. Following this, 500 μl of the mixture was cast into each well of a 24-well culture plate (Falcon). The solution was then allowed to polymerize at room temperature, generally completed in 20 min. After polymerization, the gels were either allowed to remain attached to the plates in which they were cast, or, for the gel contraction assay, the gels were gently released from the plates in which they were cast and transferred to 60-mm tissue culture dishes (3 gels in each dish) that contained 5 ml of SF-DMEM for human cells or 0.1% FCS-DMEM for murine lung fibroblasts with or without TGF-β1 (100 pM). We have found murine lung fibroblasts do not contract collagen gels under serum-free conditions. Thus 0.1% FCS was added to the medium in which gels were floated. Our prior work has demonstrated that, with concentrations up to 0.2% FCS, the number of fibroblasts in the collagen gels does not increase (24). The area of each gel was measured daily with an image analyzer (Optomax, Burlington, MA). Data are expressed as the percentage of area compared with the initial gel area. The gels were then incubated at 37°C in a 5% CO2 atmosphere.
Zymography.
Gelatin zymography was performed by modification of a previously published procedure (15). After fibroblasts were cultured in gels, conditioned media (0.5 ml) were precipitated with ethanol and resuspended with 30 μl of ddH2O and subjected to SDS-PAGE under nonreducing conditions in 10% acrylamaide gels containing 0.1% gelatin. After electrophoresis, gels were washed by gentle shaking for 2 h at room temperature in 2.5% (vol/vol) Triton X. The gels were then incubated at 37°C with shaking in the metalloproteinase buffer (0.06 M Tri·HCl, pH 7.5, containing 5 mM CaCl2 and 1 μM ZnCl2) for 18 h at 37°C and subsequently stained with Coomassie blue. Zones of proteolysis appeared as clear bands against a blue background. Supernatants from HT1080 cells were used as positive controls in the zymograms.
Measurement of TGF-β1 and fibronectin by ELISA.
TGF-β1 in the media of collagen gel cultures was determined by ELISA, which was purchased from R&D Systems and could be used for both human and murine TGF-β1 quantification. Briefly, ELISA plates were coated with monoclonal anti-TGF-β1 antibody at 4°C overnight. Plates were washed, and samples or standards of recombinant human TGF-β1 were added in triplicate to individual wells and incubated at room temperature for 2 h. To measure TGF-β1, all samples were assayed both with and without acidification and neutralization to convert the latent form to the active form. To accomplish this, a 500-μl sample was mixed with 100 μl of 1 N HCl and, after 10 min at room temperature, neutralized with 100 μl of 1.2 N NaOH/0.5 M HEPES. After being washed, bound antigen was detected after adding biotinylated anti-TGF-β1 antibody for 1 h at room temperature, followed by another wash, and then streptavidin-HRP conjugate (1:20,000 dilution) was added for 1 h. Bound HRP was then detected with 3,3′,5,5′-tetramethylbenzenzidine. The reaction was stopped with 1 M H2SO4, and absorbance at 450 nm was determined in a BenchMark microplate reader (Bio-Rad, Hercules, CA). The standard curve was linearized and subjected to regression analysis.
Fibronectin in the media of collagen gel culture was determined by ELISA. Briefly, plates were coated with monoclonal anti-fibronectin antibody at 4°C overnight. After being washed three times, standards and samples were added and incubated at room temperature for 2 h. Bound antigen was detected after adding polyclonal anti-human fibronectin antibody (1:2,000 dilution) at room temperature for 1 h followed by HRP-conjugated anti-rabbit IgG antibody (1:10,000 dilution) at room temperature for 1 h. Bound HRP was detected with 0.1 mg/ml O-phenylenediamine. The reaction was stopped with 8 M H2SO4, and the product was quantified at 490 nm with a microplate reader (BenchMark; Bio-Rad).
Reporter gene assay.
After exposure of HLF-1 cells to MMP-9 siRNA or control siRNA for 24 h, cells were transfected with a total of 2 μg/well of CAGA12-Luc (kindly provided by A. Roberts, National Institutes of Health) together with pRL-TK (Renilla for normalization; Promega, Madison, WI) plasmid DNA. After transfection (48 h), media were replaced with serum-free DMEM with or without 100 pM TGF-β1 for 16 h. After this, Luciferase and Renilla activity were determined with the Dual-Luciferase Reporter Assay kit (Promega) and luminometer (MicroLumat Plus-LB96V; EG&G Berthold, Badwildbad, Germany). These values were normalized against Renilla values and presented as fold induction compared with control.
Statistical analysis.
Results are presented as means ± SE. Statistical comparisons of paired data were performed using Student's t-test, whereas multigroup data were analyzed by ANOVA followed by the Tukey's or Bonferroni's posttest using Statview software (Abacus Concepts, Cary, NC). P < 0.05 was considered significant.
RESULTS
Characterization of mouse cells.
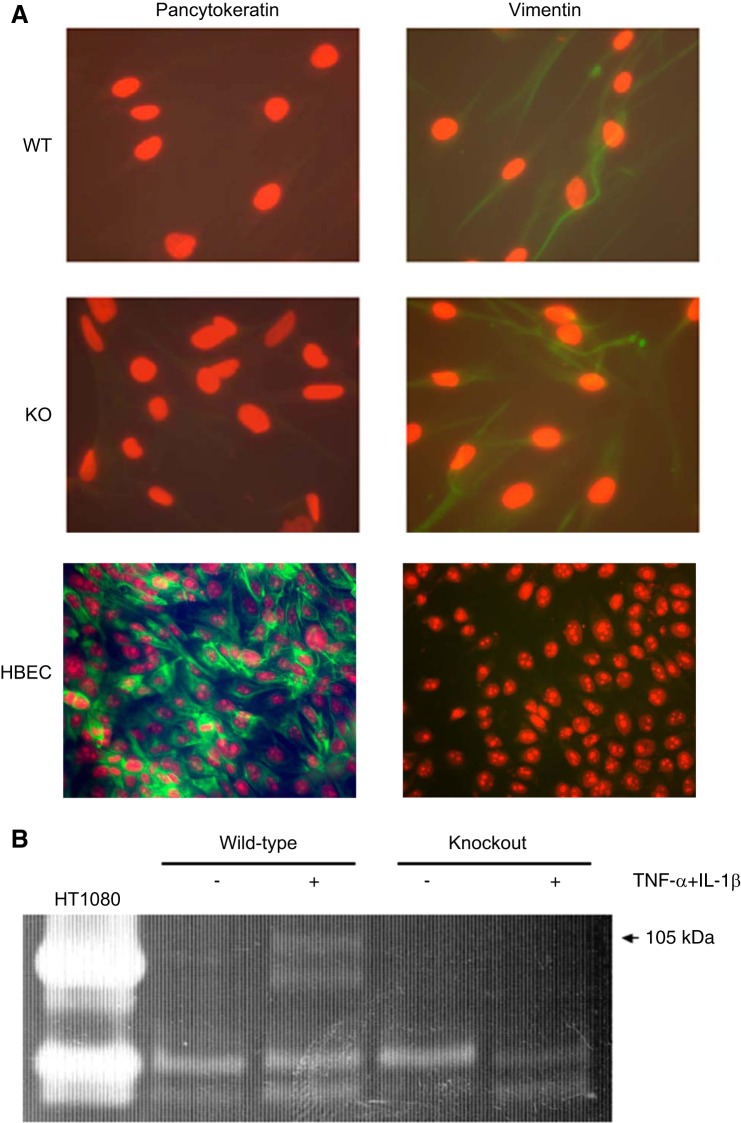
After expansion in culture, fibroblasts from mouse lungs were characterized by immunofluorescence staining for pan-cytokeratin and vimentin. Cells had the characteristic spindle-shaped morphology of fibroblasts. Moreover, as shown in Fig. 1A, both cells from WT and knockout (KO) mice were negative for pan-cytokeratin staining and positive for vimentin. Together these features identify these cells as fibroblasts. To assess production of MMP-9 from murine lung fibroblasts, fibroblasts were cultured in 3D collagen gels for 5 days with or without cytokine stimulation. The conditioned media were then harvested and subjected to gelatin zymography assay (Fig. 1B). As expected, in the WT lung fibroblasts, murine pro-MMP-9 (105 kDa) was released in the culture medium in the presence of TNF-α and IL-1β. In contrast, in the KO cells, murine pro-MMP-9 (105 kDa) was not released, even with cytokine stimulation.
Fig. 1.
Characterization of murine lung fibroblasts. A: vimentin and pan-cytokeratin expression by murine lung fibroblasts from matrix metalloproteinase (MMP)-9-deficient mice. After isolation from murine lung, cells were stained for vimentin and pan-cytokeratin (see materials and methods). Human bronchial epithelial cells (HBEC) were used as positive control for pan-cytokeratin staining. Data presented are one representative of three separate experiments. WT, wild type; KO, knockout. B: gelatin zymography. Fibroblasts were cultured in 3-dimensional (3D) collagen gels for 5 days in the presence or absence of cytokines [10 ng/ml tumor necrosis factor (TNF)-α and 5 ng/ml IL-1β]. Media in which the collagen gels were suspended were harvested after 5 days, and gelatin zymography was performed. Media from HT1080 cells, which secrete copious amounts of human MMP-9, which has an apparent molecular size of 90 kDa, and MMP-2, are included as a positive control. Data presented are one representative of three experiments.
Effect of MMP-9 deficiency on fibroblast-mediated collagen gel contraction.
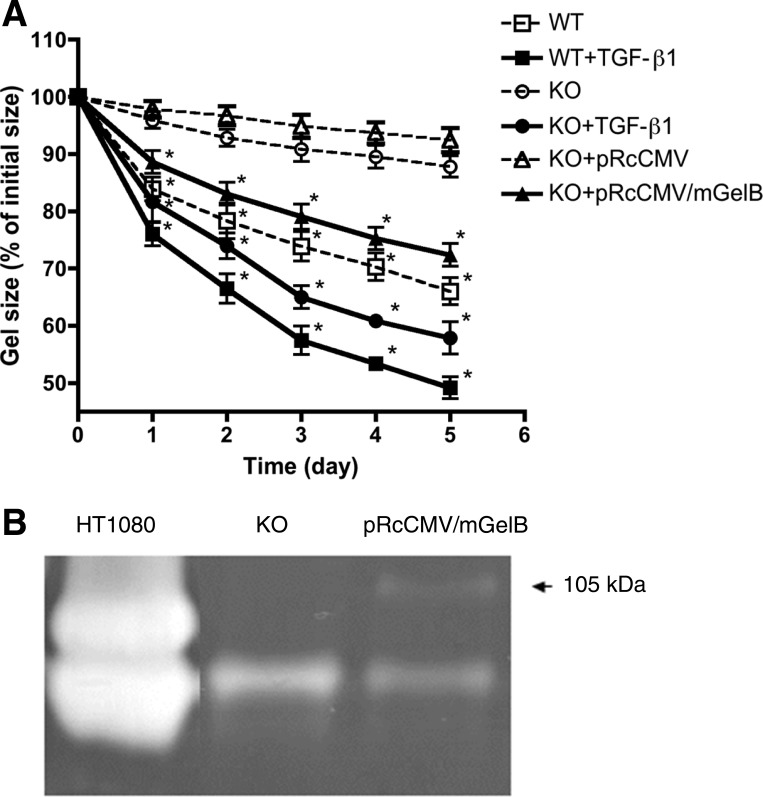
After 5 days, murine WT lung fibroblasts cultured in collagen gels (0.1% FCS-DMEM) had contracted those gels to 69.3 ± 2.4% of their initial area (Fig. 2A). In contrast, murine KO lung fibroblasts contracted the gels significantly less than WT fibroblasts (90.1 ± 1.0%, P < 0.05) after 5 days. Both cell types responded to exogenous TGF-β, indicating that MMP-9 KO cells were capable of contracting (Fig. 2A). In addition, endogenous TGF-β1 from MMP-9 KO murine lung fibroblast was significantly less than that from WT lung fibroblasts (see data below, see Fig. 5A).
Fig. 2.
Effect of murine gelatinase B (mGelB) transfection in MMP-9 KO murine fibroblasts. A: effect on collagen gel contraction. Fibroblast-populated collagen gels were released into 60-mm tissue culture dishes. Gel size was measured daily with an image analyzer. Vertical axis, gel size expressed as % initial size; horizontal axis, time (days of culture); WT, murine fibroblasts from wild-type mice; KO, fibroblasts from MMP-9-deficient mice; KO + pRcCMV, MMP-9-deficient murine fibroblasts transfected with vector only; KO + pRcCMV/mGelB, MMP-9-deficient murine fibroblasts transfected with vector containing mGelB (MMP-9) sequence. B: gelatin zymography. After transfection of expression vector for murine MMP-9 to the murine MMP-9-deficient cells (KO), media were harvested at 24 h, and gelatin zymography was performed. The arrow indicates an apparent molecular size of 105 kDa, consistent with latent murine MMP-9. Media from HT1080 cells, which secrete copious amounts of human MMP-9, which has an apparent molecular size of 90 kDa, and MMP-2 are included as a positive control. *P < 0.05 compared with KO cells. Data are shown as means ± SE. Data presented are from one representative experiment performed in triplicate on three separate occasions.
Fig. 5.
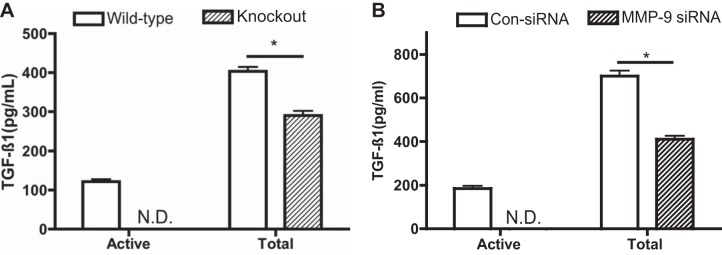
Effect of MMP-9 KO or suppression on transforming growth factor (TGF)-β1 production and activation in the gel culture. A: murine lung fibroblasts. WT and MMP-9 KO murine lung fibroblasts were cast into collagen gels. Gels were released and allowed to contract. After 2 days, culture medium was harvested for TGF-β1 quantification by ELISA. B: HFLs. After transfection of control or MMP-9 specific for siRNA into HFL-1 cells, fibroblast-populated collagen gels were prepared, and the media were harvested on day 2 after the gels were released and cultured in 60-mm dishes. TGF-β1 was measured by ELISA. Vertical axes, TGF-β1 concentration (pg/ml); horizontal axes, active form or total TGF-β1; open bars, WT murine lung fibroblasts (A) or control siRNA-transfected HFL-1 cells (B); hatched bars, MMP-9 KO murine lung fibroblasts (A) or MMP-9 siRNA-transfected HFL-1 cells (B). Data are shown as means ± SE. *P < 0.05; ND, not detectable.
To determine if endogenous MMP-9 could contribute to the reduced gel contraction, MMP-9-deficient cells that were transfected with an MMP-9 expression vector (pRcCMV/mGelB) were assessed. The KO cells released no MMP-9 (molecular mass 105 kDa) but did release MMP-2, which was detected as a band of lower molecular mass. Following transfection with the expression vector, production of MMP-9 was readily detected by gelatin zymography. Interestingly, MMP-2 release was decreased by transfection (Fig. 2B). When assessed in the gel contraction assay, MMP-9 KO cells transfected with the expression vector contracted the gels significantly more than untransfected cells (73.3 ± 2.1%) and were similar to WT cells. In contrast, cells transfected with the control vector were no different from the KO cells (Fig. 2A).
Effect of MMP inhibitor on fibroblast-mediated collagen gel contraction.
To determine the influence of MMPs on contraction of human lung fibroblasts, gels containing HFL-1 fibroblasts were exposed to the nonspecific MMP inhibitor GM-6001. GM-6001 (1 μM) inhibited fibroblast contraction of collagen gels significantly over the period of observation. After 5 days, control HFL-1 gels contracted to 58.0 ± 1.2% of their initial area (Fig. 3). In contrast, gels exposed to GM-6001 contracted significantly less than control (87.7 ± 0.9%, P < 0.05).
Fig. 3.
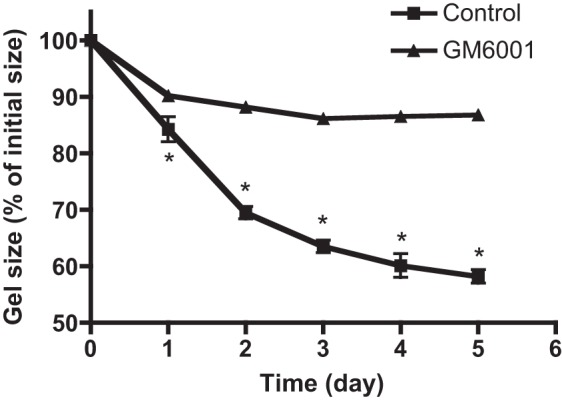
Effect of MMP inhibitor on fibroblast-mediated collagen gel contraction. Human lung fibroblast-populated collagen gels were released in 60-mm tissue culture dishes with or without the MMP inhibitor (GM-6001, 1 μM). Gel size was measured daily with an image analyzer. Vertical axis, gel size expressed as % initial size; horizontal axis, time (days of culture). *P < 0.05 compared with control cells. Data are shown as means ± SE.
Effect of MMP-9 suppression on fibroblast-mediated collagen gel contraction.
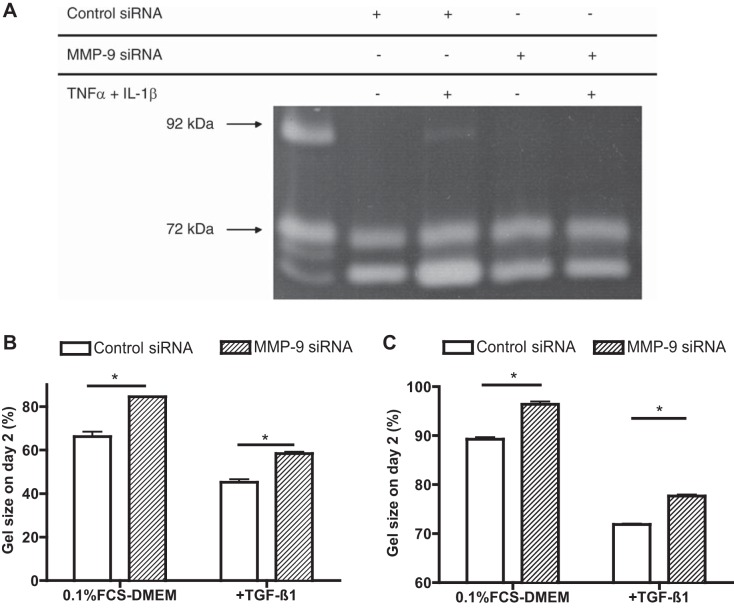
Because GM-6001 is a nonspecific inhibitor, its inhibition of gel contraction supports a role for MMPs but does not address a specific role for MMP-9. Because specific pharmacological inhibitors of MMP-9 were not available to us, to investigate the effect of MMP-9 on human fibroblast-mediated collagen gel contraction, siRNA was used to specifically target MMP-9. Suppression of MMP-9 in HFL-1 cells by siRNA resulted in inhibition of the release of MMP-9 in the surrounding medium. However, this was only detectable in the presence of cytokine stimulation (Fig. 4A). The ability of MMP-9 siRNA-transfected cells to contract collagen gels was significantly less than that of control cells transfected with control siRNA. After 2 days, control siRNA-transfected fibroblast-populated gels were 66.2 ± 2.3% of their initial area. In contrast, gels containing MMP-9 siRNA-transfected cells were 84.5 ± 0.2% of their initial size (P < 0.05). Both control and siRNA-transfected cells, however, increased gel contraction when exposed to exogenous TGF-β1, although the control cells still contracted more (45.2 ± 1.3%) than the siRNA-transfected cells (58.4 ± 0.7%, P < 0.05) (Fig. 4B). Similar to the results observed with human fetal lung fibroblasts, adult human bronchial fibroblasts also contracted less when transfected with siRNA for MMP-9 (Fig. 4C).
Fig. 4.
Suppression of MMP-9 by small-interfering RNA (siRNA) and its effect on collagen gel contraction by fetal lung fibroblasts (HFL-1) or by human bronchial fibroblasts (HBF). A: gelatin zymography. After transfection of HFL-1 cells with MMP-9 siRNA or control siRNA, cells were cast into 3D collagen gel maintained in the presence or absence of cytokines (10 ng/ml TNF-α and 5 ng/ml IL-1β). Media in which the gels were suspended were harvested, and gelatin zymography was performed. Media from HT1080 cells, which secrete copious amounts of human MMP-9, which has an apparent molecular size of 90 kDa, and MMP-2 are included as a positive control. Data presented are from one experiment repeated with similar results on three separate occasions. B and C: effect of MMP-9 depletion on HFL-1 (B) or HBF (C) cell-mediated collagen gel contraction. After treatment with MMP-9 siRNA or control siRNA, HFL-1 or HBF cell-populated collagen gels were released in 60-mm tissue culture dishes with or without TGF-β1 (100 pM). Gel size was measured daily with an image analyzer. Vertical axis, gel size on day 2 expressed as %initial size; horizontal axis, culture medium with or without TGF-β1 (100 pM); open bars, control siRNA-transfected cells; hatched bars, MMP-9 siRNA-transfected cells. Data are shown as means ± SE. Data presented are from one representative experiment performed in triplicate on three separate occasions. *P < 0.05.
Effect of MMP-9 suppression on TGF-β1 release in the gel culture.
To determine the effect of MMP-9 on TGF-β1 release in the 3D collagen gel culture system, release of TGF-β1 in the culture medium by murine lung fibroblasts with MMP-9 deficiency or HFL-1 cells transfected with MMP-9 siRNA or control siRNA was measured. Both total and active TGF-β1 concentration was significantly decreased in the 3D culture of MMP-9 KO murine lung fibroblasts compared with WT murine lung fibroblasts (Fig. 5A, P < 0.05) or MMP-9 siRNA-transfected human lung fibroblasts compared with control siRNA-transfected cells (Fig. 5B, P < 0.05).
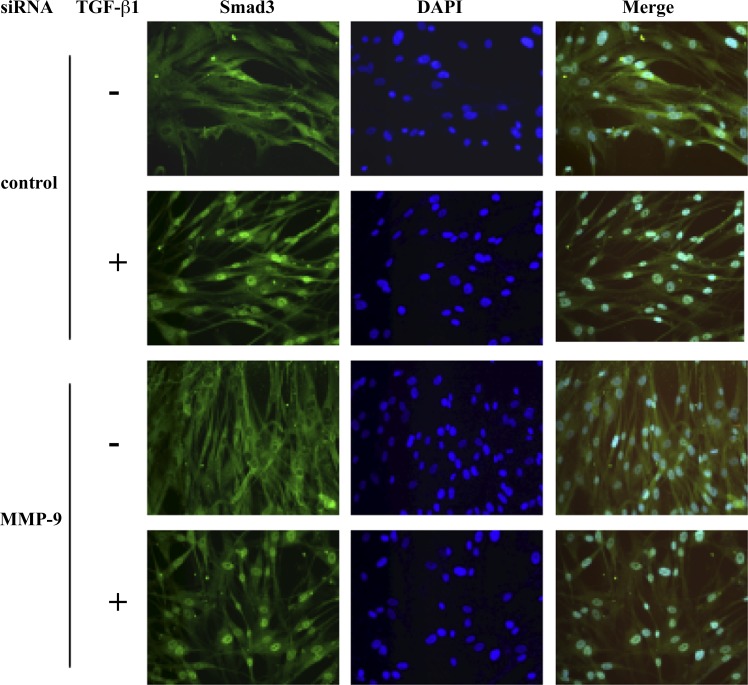
Effect of MMP-9 suppression on subcellular localization of Smad3.
Immunostaining was performed to test whether MMP-9 siRNA could affect nuclear translocation of Smad3 (Fig. 6). Under baseline conditions, neither control siRNA nor MMP-9 siRNA-transfected cells showed Smad3 nuclear localization. Upon TGF-β1 exposure, nuclear translocation and accumulation of Smad3 in both groups were observed, indicating that MMP-9, within the sensitivity of the assay used, did not affect Smad3 nuclear translocation.
Fig. 6.
Subcellular localization of Smad3 in HFL-1 cells. After transfection of MMP-9 siRNA or control siRNA, human lung fibroblasts were stimulated with TGF-β1 for 30 min followed by immunostaining for Smad3. Nuclear counterstaining was performed with DAPI. One representative datum of three experiments is shown.
Effect of MMP-9 suppression on TGF-β signaling.
Because it appeared that MMP-9 suppression by siRNA knock down or gene deficiency was acting, at least in part, through inhibition of the generation of active TGF-β1 under baseline conditions, we used the following two methods to assess the effect of MMP-9 suppression by siRNA on TGF-β1 activity. First, we used the Smad3 reporter construct CAGA12. Incubation of cells with the MMP-9 siRNA significantly reduced CAGA12 reporter activity in baseline cells (Fig. 7A). Second, we assessed the effect of the MMP-9 siRNA on fibroblast production of fibronectin, the production of which is markedly stimulated by active TGF-β. Suppression of MMP-9 by siRNA significantly inhibited baseline fibronectin production (Fig. 7B). The effect of the siRNA for MMP-9 was not due to a generalized inhibition of TGF-β1 signaling, since addition of exogenous TGF-β1 stimulated both fibronectin production and CAGA12 activity (Fig. 7).
Fig. 7.
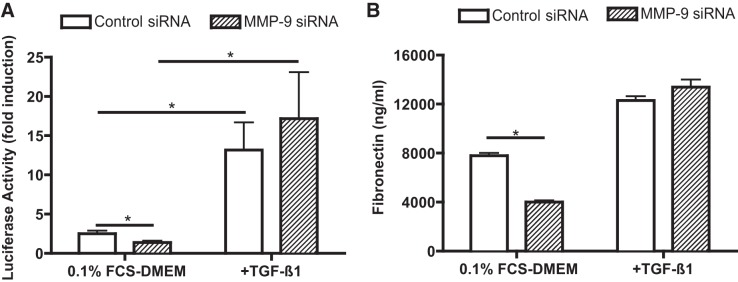
Effect of MMP-9 suppression on transcriptional activity of Smad3. A: effect of MMP-9 depletion on Smad3 reporter activity. HLF-1 cells transfected with MMP-9 siRNA or control siRNA were cotransfected with CAGA12-Luc reporter plasmid together with pRL-TK. The cells were then treated with or without TGF-β1 for 30 min. Vertical axis, Smad3 reporter activity; horizontal axis, cells were treated with or without TGF-β1 (100 pM); open bars, cells transfected with control siRNA and reporter plasmid; hatched bars, cells transfected with MMP-9 siRNA and reporter plasmid. Data are shown as means ± SE. *P < 0.05. B: effect of MMP-9 depletion on fibronection production by HFL-1 cells. After collagen gel contraction assay, the media were harvested on day 2 and used for fibronection quantification by ELISA. Vertical axis, fibronection concentration (ng/ml); horizontal axis, culture medium with or without TGF-β1 (100 pM); open bars, control siRNA-transfected cells; hatched bars, MMP-9 siRNA-transfected cells. *P < 0.05.
DISCUSSION
The current study was designed to evaluate the role of endogenous MMP-9 in modulating fibroblast-mediated contraction of 3D collagen gels. Fibroblasts cultured from the lungs of mice that are genetically deficient in MMP-9 contracted collagen gels significantly less than did fibroblasts from WT mice lungs. Contractility was restored by transfection of the deficient cells with a MMP-9-expressing vector. Reduced contraction of 3D collagen gels was also observed when normal human lung fibroblasts were incubated with the nonspecific MMP inhibitor GM-6001 and when siRNA was used to suppress MMP-9 expression. MMP-9 deficiency or suppression by siRNA was associated with a reduction in the amount of active TGF-β1 detectable in murine or human lung fibroblast cultures, respectively. Consistent with a role for endogenous MMP-9 in activating TGF-β, siRNA for MMP-9 reduced baseline activity of a Smad3-dependent promoter and baseline production of fibronectin. Importantly, the siRNA inhibition did not reduce the ability of exogenous TGF-β1 to stimulate the Smad3 reporter activity or to stimulate fibronectin production. Taken together, these results suggest that MMP-9 participates in regulation of fibroblast contraction of 3D collagen gels and that this is associated with MMP-9 activation of TGF-β1.
MMP-9 is a mixed-function proteinase produced by a large number of cells as a proenzyme with a molecular mass of 92 kDa in human and 105 kDa in mouse (1, 4, 43). Proteolytic cleavage of MMP-9 yields the active enzyme, which, in turn, can degrade a large number of substrates (7, 14, 17, 26). Targeted deletion of MMP-9 in the mouse results in animals that have delayed long bone growth and altered epithelial migration (10, 19, 42, 50). This suggests that MMP-9 plays a role in tissue repair and remodeling. In this context, MMP-9 has been suggested to release or activate a number of cytokines and growth factors, including TGF-β1 (33, 45).
TGF-β1 is a multifunctional cytokine that modulates many functions believed important in tissue repair and remodeling (5, 28). Included among these is the production of extracellular matrix and the production of enzymes that can degrade extracellular matrix and inhibitors of those enzymes (12, 20). TGF-β1 also is a potent stimulator of fibroblast contraction of 3D collagen gels (24, 25), which is believed to be a model of the contraction that characterizes both normal wound healing and fibrotic scar tissue. The current study supports the concept that MMP-9 may contribute to modulation of tissue structure by inducing the formation of active TGF-β1, leading to augmented contractile activity of fibroblasts. TGF-β can be activated by multiple pathways, including both proteolytic cleavage and integrin-mediated conformational changes (36, 39). In this context, fibroblast contraction can activate TGF-β by an αbβ5-integrin-dependent mechanism (47). Our results demonstrate that MMP-9 stimulates contraction and contributes to TGF-β activation. Direct activation of TGF-β MMP-9, which can stimulate contraction, may be one possible pathway (45). Alternatively, MMP-9 could stimulate contraction by an alternate mechanism, and the contraction could lead to TGF-β activation. This raises the possibility that MMP-9 and TGF-β could participate in a contraction-dependent positive feedback loop that regulates fibroblast function. The potential for positive feedback between TGF-β and MMP-9 is further supported, since TGF-β can modulate release of MMP-9 (32, 46).
MMP-9 production by unstimulated fibroblasts is low. MMP-9 was, in fact, undetectable by zymography in our human cell cultures, although a faint band was detectable in normal murine lung fibroblasts. Low levels of MMP-9 were detected in human fibroblasts under basal conditions by ELISA and by Western blot. Interestingly, in other studies, we have shown that MMP-9 release by fibroblasts is greater in the 3D collagen culture system used in the current study than it is in monolayer culture (data not shown). In the presence of inflammatory cytokines such as IL-1β and TNF-α, MMP-9 synthesis was also upregulated. Under baseline conditions, despite its low level of expression, the current study suggests that MMP-9 plays a functional role in our collagen gel contraction assay system. In the presence of IL-1β and TNF-α, although the amount of MMP-9 was increased, there was not further augmentation of collagen gel contraction by fibroblasts, rather, as we have previously shown, collagen gel contraction is significantly inhibited due to stimulation of PGE2 and NO synthesis induced by these cytokines (49).
Consistent with the effect of MMP-9 on gel contraction under baseline conditions, both the genetically deficient murine lung fibroblasts and the siRNA-treated human lung fibroblasts have similar and clearly detectable functional alterations. It is unlikely that the effects of the siRNA were due to nonspecific effects, since the control siRNA was without effect. Off-target effects, however, cannot be entirely excluded. The sensitivity of these measures of TGF-β1 to MMP-9 suppression by siRNA was confirmed by pharmacological inhibition, although the inhibitor used, GM-6001, is not specific for MMP-9. Importantly, very low levels of active TGF-β1 appear to be present within our cultures, and these are suppressed in the absence of MMP-9.
The current study provides direct immunoassay evidence that MMP-9 leads to activation of TGF-β under baseline conditions. This is confirmed using the Smad3 reporter gene construct and fibronectin as markers of TGF-β function. Both of these functional consequences of active TGF-β1 were reduced by suppression of MMP-9. We have previously reported that Smad3, but not Smad2, was required for exogenous TGF-β1 augmentation of collagen gel contraction by human lung fibroblasts (16, 24). In the current study, with endogenous TGF-β1 under baseline conditions, we were unable to demonstrate an alteration in Smad3 nuclear localization due to MMP-9 suppression. However, the failure to detect a difference in Smad3 nuclear localization may be due to the insensitivity of the histochemical assay. Consistent with this, very little Smad3 nuclear localization was observed despite clear activity with the Smad3 reporter. These functional assays provide indirect evidence that MMP-9 by activating TGF-β1 contributes to the “basal” activity of fibroblasts.
Several additional lines of evidence suggest that the effects observed are not due to nonspecific inhibition of MMPs. First, similar results were obtained with both the deficient murine cells and the siRNA-treated human cells. Second, transfection of the murine cells with an MMP-9-expressing vector restored contractility. Third, the addition of exogenous TGF-β1 to both the murine KO and the human siRNA-treated cells resulted in augmented contraction. This indicates that both the murine KO cells and the siRNA-suppressed human cells were capable of contracting and responding to TGF-β1.
TGF-β signals by activating a cell-surface receptor that is a serine kinase. Several signal transduction pathways are activated by TGF-β. Previous results have demonstrated that Smad3 signaling is required for TGF-β1 augmentation of 3D collagen gel contraction (24). TGF-β1 can also signal by additional mechanisms, involving either Smad2 or TAK (34). TGF-β induction of fibronectin, for example, is believed to be a Smad3-independent effect (13). The inhibition of fibronectin production observed with the siRNA for MMP-9, therefore, is consistent with a generalized inhibition of TGF-β1 actions.
The current study evaluated fibroblasts cultured in 3D gels made of native type I collagen maintained in floating culture. This culture system has been widely used to evaluate the contraction of extracellular matrix that characterizes both normal wound healing and fibrosis. Fibroblasts cultured in 3D collagen gels respond to growth factors and produce proteins differently than fibroblasts maintained in monolayer culture (14, 23). Under the conditions used, in the presence of 0.1% FCS, fibroblasts neither undergo apoptosis nor do they proliferate (24). While these cells resemble cells in tissue in a number of ways, baseline culture conditions are certainly not the same as resting conditions in vivo, and the cells may have been activated in some ways. In this regard, both latent (72 kDa) and active (66 kDa) forms of MMP-2 were constitutively released into the surrounding medium when fibroblasts were cultured three dimensionally as detected by zymogram. While the role of MMP-2 remains to be investigated, we have previously reported that collagen is not degraded by MMP-2 under baseline conditions even in the presence of elastase (48). In contrast, the latent form (92 kDa) MMP-9 could be detected by zymogram only in the presence of cytokines such as IL-1β and TNF-α. Furthermore, we have previously reported that latent MMP-9 does not degrade the collagen gel as determined by hydroxylproline measurement (48). Therefore, it is unlikely that MMP-9 would degrade the collagen gels under baseline conditions in the current report.
Fibroblasts are capable of responding to siRNAs, although the effects may be transient. Whereas the time course of siRNA suppression was not assessed in the current study, time-dependent recovery would have minimized the effects observed. The observations made, therefore, support a role for MMP-9 in modulating collagen gel contraction and TGF-β in fibronectin release.
The full functional role of MMP-9 remains to be defined. The current study, however, suggests that MMP-9 may play a key role in modulating tissue reorganization mediated through fibroblast contractile activity and that this effect is due to MMP-9 generation of endogenous active TGF-β1. Consistent with our findings, mice that lack MMP-9 display delayed wound healing (18), and MMP-9 induced cardiac fibroblast migration and collagen and cytokine secretion (44).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-47328 and the Alan A. and Edith L. Wolff Charitable Trust.
DISCLOSURES
In the last three years, SIR has consulted or been a member of an advisory board for Able Associates, Adelphi Research, Almirall/Prescott, APT Pharma/Britnall, Aradigm, AstraZeneca, Boehringer Ingelheim, Chiesi, CommonHealth, Consult Complete, COPDForum, DataMonitor, Decision Resources, Defined Health, Dey, Dunn Group, Eaton Associates, Equinox, Gerson, GlaxoSmithKline, Infomed, KOL Connection, M. Pankove, MedaCorp, MDRx Financial, Mpex, Novartis, Nycomed, Oriel Therapeutics, Otsuka, Pennside Partners, Pfizer (Varenicline), Pharma Ventures, Pharmaxis, Price Waterhouse, Propagate, Pulmatrix, Reckner Associates, Recruiting Resources, Roche, Schlesinger Medical, Scimed, Sudler and Hennessey, TargeGen, Theravance, UBC, Uptake Medical, VantagePoint Mgmt. He has lectured for American Thoracic Society, AstraZeneca, Boehringer Ingelheim, California Allergy Society, Creative Educational Concept, France Foundation, Information TV, Network for Continuing Ed, Novartis, Pfizer, SOMA. He has received industry-sponsored grants from AstraZeneca, Biomarck, Centocor, Mpex, Nabi, Novartis, Otsuka.
AUTHOR CONTRIBUTIONS
Author contributions: T. Kobayashi, X.L., and S.I.R. conception and design of research; T. Kobayashi and H.K. performed experiments; T. Kobayashi, H.K., X.L., and H.S. analyzed data; T. Kobayashi prepared figures; T. Kobayashi drafted manuscript; T. Kobayashi, X.L., and S.I.R. edited and revised manuscript; T. Kobayashi, H.K., X.L., H.S., T. Kohyama, Q.F., F.-Q.W., S.A., X.W., J.J.A., J.M.S., R.M.S., and S.I.R. approved final version of manuscript; X.L., T. Kohyama, and S.I.R. interpreted results of experiments.
ACKNOWLEDGMENTS
We appreciate the helpful discussions with Dr. A. Roberts (National Institutes of Health) and the secretarial support of Lillian Richards.
REFERENCES
- 1.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 28: 12–24, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 76: 1274–1278, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 159: 1985–1991, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197–250, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350–1358, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Choi KH, Lee HB, Jeong MY, Rhee YK, Chung MJ, Kwak YG, Lee YC. The role of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in cryptogenic organizing pneumonia. Chest 121: 1478–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 151: 879–889, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay GA, Russell KJ, McMahon KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax 52: 502–506, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest 78: 687–698, 1998 [PubMed] [Google Scholar]
- 10.Garg P, Jeppsson S, Dalmasso G, Ghaleb AM, McConnell BB, Yang VW, Gewirtz AT, Merlin D, Sitaraman SV. Notch1 regulates the effects of matrix metalloproteinase-9 on colitis-associated cancer in mice. Gastroenterology 141: 1381–1392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124: 401–404, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev 8: 171–179, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 18: 1345–1356, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johanson M, Zhao XR, Huynh-Ba G, Villar CC. Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and inflammation in cyclosporine A-induced gingival enlargement: a pilot in vitro study using a three-dimensional model of the human oral mucosa. J Periodontol 84: 634–640, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218: 325–329, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Liu X, Wen FQ, Fang Q, Abe S, Wang XQ, Hashimoto M, Shen L, Kawasaki S, Kim HJ, Kohyama T, Rennard SI. Smad3 mediates TGF-beta1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun 327: 393–398, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Koenig GC, Rowe RG, Day SM, Sabeh F, Atkinson JJ, Cooke KR, Weiss SJ. MT1-MMP-dependent remodeling of cardiac extracellular matrix structure and function following myocardial infarction. Am J Pathol 180: 1863–1878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 28: 65–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, Rabach ME, Shipley JM, Shapiro SD, Senior RM, Elias JA. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest 110: 463–474, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lemjabbar H, Gosset P, Lamblin C, Tillie I, Hartmann D, Wallaert B, Tonnel AB, Lafuma C. Contribution of 92 kDa gelatinase/type IV collagenase in bronchial inflammation during status asthmaticus. Am J Respir Crit Care Med 159: 1298–1307, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Lemjabbar H, Gosset P, Lechapt-Zalcman E, Franco-Montoya ML, Wallaert B, Harf A, Lafuma C. Overexpression of alveolar macrophage gelatinase B (MMP-9) in patients with idiopathic pulmonary fibrosis: effects of steroid and immunosuppressive treatment. Am J Respir Cell Mol Biol 20: 903–913, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Kohyama T, Wang H, Zhu YK, Wen FQ, Kim HJ, Romberger DJ, Rennard SI. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am J Physiol Lung Cell Mol Physiol 282: L1049–L1056, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Wen FQ, Kobayashi T, Abe S, Fang Q, Piek E, Bottinger EP, Roberts AB, Rennard SI. Smad3 mediates the TGF-beta-induced contraction of type I collagen gels by mouse embryo fibroblasts. Cell Motil Cytoskeleton 54: 248–253, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Liu XD, Umino T, Ertl R, Veys T, Skold CM, Takigawa K, Romberger DJ, Spurzem JR, Zhu YK, Kohyama T, Wang H, Rennard SI. Persistence of TGF-beta1 induction of increased fibroblast contractility. In Vitro Cell Dev Biol Anim 37: 193–201, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 102: 647–655, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 110: 625–632, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J. TGF-beta signal transduction. Annu Rev Biochem 67: 753–791, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Miki H, Mio T, Nagai S, Hoshino Y, Nagao T, Kitaichi M, Izumi T. Fibroblast contractility: usual interstitial pneumonia and nonspecific interstitial pneumonia. Am J Respir Crit Care Med 162: 2259–2264, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim 32: 427–433, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 78: 1077–1087, 1998 [PubMed] [Google Scholar]
- 32.Overall CM, Wrana JL, Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem 264: 1860–1869, 1989 [PubMed] [Google Scholar]
- 33.Perng DW, Chang KT, Su KC, Wu YC, Chen CS, Hsu WH, Tsai CM, Lee YC. Matrix metalloprotease-9 induces transforming growth factor-beta(1) production in airway epithelium via activation of epidermal growth factor receptors. Life Sci 89: 204–212, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Roberts AB, Russo A, Felici A, Flanders KC. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann NY Acad Sci 995: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 117: 684–694, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev 24: 395–402, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 162: 1949–1956, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, Bargar TW, Berro A, Casale TB, Rennard SI. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol 37: 424–430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatler AL, Jenkins G. TGF-beta activation and lung fibrosis. Proc Am Thorac Soc 9: 130–136, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, Mueller KC, Branscheid D, Welker L, Watz H, Magnussen H, Rennard SI. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med 178: 248–260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tonnel AB, Gosset P, Tillie-Leblond I. Characteristics of the Inflammatory response in bronchial lavage fluids from patients with status asthmaticus. Int Arch Allergy Immunol 124: 267–271, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 14: 2123–2133, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Xu F, Chen J, Shen X, Deng Y, Xu L, Yin J, Chen H, Teng F, Liu X, Wu W, Jiang B, Guo DA. Matrix metalloproteinase-9 induces cardiac fibroblast migration, collagen and cytokine secretion: inhibition by salvianolic acid B from Salvia miltiorrhiza. Phytomedicine 19: 13–19, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, Zhang Y, Harris D, Zheng G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J Nephrol 2: 84–89, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem 285: 22382–22393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu YK, Liu X, Ertl RF, Kohyama T, Wen FQ, Wang H, Spurzem JR, Romberger DJ, Rennard SI. Retinoic acid attenuates cytokine-driven fibroblast degradation of extracellular matrix in three-dimensional culture. Am J Respir Cell Mol Biol 25: 620–627, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Zhu YK, Liu XD, Skold MC, Umino T, Wang H, Romberger DJ, Spurzem JR, Kohyama T, Wen FQ, Rennard SI. Cytokine inhibition of fibroblast-induced gel contraction is mediated by PGE(2) and NO acting through separate parallel pathways. Am J Respir Cell Mol Biol 25: 245–253, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 99: 6292–6297, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]