Abstract
Enhanced nitric oxide (NO) production is known to activate silent information regulator 1 (SIRT1), which is a histone deacetylase that regulates PGC-1α, a regulator of mitochondrial biogenesis and coactivator of transcription factors impacting energy homeostasis. Since phosphodiesterase-5 inhibitors potentiate NO signaling, we hypothesized that chronic treatment with phosphodiesterase-5 inhibitor tadalafil would activate SIRT1-PGC-1α signaling and protect against metabolic stress-induced mitochondrial dysfunction in diabetic hearts. Diabetic db/db mice (n = 32/group; 40 wk old) were randomized to receive DMSO (10%, 0.2 ml ip) or tadalafil (1 mg/kg ip in 10% DMSO) for 8 wk. Wild-type C57BL mice served as nondiabetic controls. The hearts were excised and homogenized to study SIRT1 activity and downstream protein targets. Mitochondrial function was determined by measuring oxidative phosphorylation (OXPHOS), and reactive oxygen species generation was studied in isolated mitochondria. Tadalafil-treated diabetic mice demonstrated significantly improved left ventricular function, which is associated with increased cardiac SIRT1 activity. Tadalafil also enhanced plasma NO oxidation levels, myocardial SIRT1, PGC-1α expression, and phosphorylation of eNOS, Akt, and AMPK in the diabetic hearts. OXPHOS with the complex I substrate glutamate was decreased by 50% in diabetic hearts compared with the nondiabetic controls. Tadalafil protected OXPHOS with an improved glutamate state 3 respiration rates. The increased reactive oxygen species production from complex I was significantly decreased by tadalafil treatment. In conclusion, chronic treatment with tadalafil activates NO-induced SIRT1-PGC-1α signaling and attenuates mitochondrial dysfunction in type 2 diabetic hearts.
Keywords: SIRT1, tadalafil, type 2 diabetes, nitric oxide, OXPHOS, cardioprotection
silent information regulator 1 (SIRT1) is a member of a large family of class III histone deacetylases that gained initial attention as a mediator of life span in many model organisms (4, 12, 17). SIRT1 is expressed ubiquitously, and it can deacetylate a number of substrates regulating a wide variety of cellular processes such as apoptosis/cell survival and repair, gene transcription, metabolic and oxidative stress response, and aging (1, 6, 32, 48). More recent studies showed that SIRT1 can impact a wide range of proteins such as p53, forkhead box subgroup O (FOXO), PGC-1α, endothelial nitric oxide (NO) synthase (eNOS), Ku70, NF-κB, peroxisome proliferator-activated receptor-γ (PPAR-γ), and p300 (25, 34, 38, 40, 42, 49, 55, 59) that are known to play pathogenic roles in metabolic diseases, cancer, muscle differentiation, insulin resistance, diabetic nephropathy, and heart failure (1, 19, 53). Consequently, identification of selective novel small molecules that can regulate SIRT1 activity may have a huge impact in the treatment/management of these diseases.
Recent reports indicate that NO could modulate the activation and expression of SIRT1 (35, 39, 41). SIRT1 also plays an essential role in regulating endothelial NO and promoting endothelium-dependent vasodilation by targeting eNOS (35). Mice treated with low doses of red wine, the predominant source of resveratrol and a putative SIRT1 activator, showed coordinated increases in SIRT1 and eNOS expression (37). Inhibition of SIRT1 in the arterial endothelium inhibits endothelium-dependent vasodilation and decreases bioavailability of NO. Moreover, the calorie restriction delays endothelial cellular senescence against oxidative stress through eNOS-dependent production of NO and increase in myocardial SIRT1 expression (21, 39, 47, 57). Downregulation of the NO signaling and SIRT1 has been implicated in the pathogenesis of diabetes-induced cardiovascular complications (33–35). Mild to moderate increase (2- to 7.5-folds) of SIRT1 expression in heart attenuates age-dependent induction of hypertrophy, apoptosis/fibrosis, and consequent left ventricular (LV) dysfunction (4, 36). SIRT1 is also known to regulate the activity of PGC-1α, a central factor in controlling energy state, mitochondrial function, and contractility in cardiac muscle (39). It has been shown that enhanced oxidative stress along with decreased SIRT1 expression and activation of SIRT1 enhanced cellular resistance to oxidative stress by scavenging reactive oxygen species (ROS) and upregulation of antioxidant enzymes in the diabetic hearts (33).
Thus, because of the critical role of NO in SIRT1 expression, we considered phosphodiesterase-5 (PDE5) inhibitors as potential small molecule modulators of SIRT1. This is because PDE5 inhibition with sildenafil triggers a signaling cascade that results in eNOS/inducible NO synthase (iNOS) upregulation (15, 43), resulting in enhanced activation of cGMP-dependent protein kinase (PKG) and protection against ischemia-reperfusion injury (14, 43, 45). The chronic treatment with tadalafil ameliorated circulating inflammatory cytokines and chemokines while improving fasting glucose levels and reducing infarct size following ischemia-reperfusion injury in diabetic hearts (56). Moreover, tadalafil reversed detrimental remodeling of myocardial proteins involved in cytoskeletal rearrangement and redox regulation in diabetic mice (28). Tadalafil treatment in db/db mice attenuated ROS generation and preserved the loss of mitochondrial membrane potential in cardiomyocytes following simulated ischemia-reoxygenation injury in vitro and attenuated myocardial oxidative stress including in vivo lipid peroxidation (26). Based on this background information, we hypothesized that chronic treatment with tadalafil would activate myocardial SIRT1 and protect against metabolic stress-induced cardiac dysfunction in db/db mice. Since activation of SIRT1 decreases oxidative stress via its regulatory effects on mitochondrial function (1), we further determined mitochondrial function as well as the regulation of downstream signaling proteins that might play a role in tadalafil-induced NO-SIRT1 signaling in the heart following tadalafil treatment in db/db mice.
METHODS
Animals.
Adult male leptin receptor null, homozygous diabetic db/db (BKS.Cg-Dock7m+/+Leprdb/J strain) mice and nondiabetic control mice (C57BLKS/J background) were purchased from Jackson Laboratories (Bar Harbor, ME) at a 8 wk of age and housed within School of Medicine Animal Facility, Virginia Commonwealth University, with free access to food and water until 40 wk of age. All animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by the National Institutes of Health (No. 85-23, Revised 1996). The animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Protocol for animal experiments.
The 40-wk-old db/db mice (n = 32/group) were randomized to receive daily intraperitoneal injections of DMSO (10% DMSO in 0.9% NaCl, 0.2 ml) or tadalafil (1 mg/kg in 10% DMSO) for 8 wk. The tadalafil dose (1 mg/kg) was chosen based on interspecies dose extrapolation scaling that would result in plasma concentrations equivalent to those found in humans receiving an oral dose of 20 mg/day. Each animal had specific metabolic parameters monitored during the treatment period, including weekly fasting blood glucose levels and body weight. LV function was assessed by echocardiography before and after the treatments. Upon completion of the 8 wk therapy, the mice were euthanized, anesthetized with pentobarbital sodium (70 mg/kg ip), and blood samples were taken by cardiac puncture (via thoracotomy) for assessment of biochemical parameters. The hearts were collected for further experimental analysis.
Glucose measurement.
Blood glucose concentrations were obtained with a handheld glucometer (Lifescan, Milpitas, CA) using 5 μl of blood obtained from tail vein following 12 h of fasting. The blood was applied directly to glucose test strips to measure glucose levels on a weekly basis.
Measurement of plasma triglycerides and insulin.
After the animals were euthanized, blood was collected into heparinized tubes and centrifuged at 1,000 g for 10 min at 4°C to collect plasma, which was stored at −80°C. The plasma samples were assayed for triglycerides using commercially available colorimetric assay kit (Cayman Chemicals, Ann Arbor, MI). Insulin concentrations were determined by ELISA method following the manufacturer's instructions (Crystal Chem, Downers Grove, IL).
Measurement of plasma nitrate and nitrite.
The blood samples were collected from the mice (n = 6 per group) and centrifuged to obtain the supernatant plasma. Plasma samples were subsequently centrifuged using Amicon Ultra-4 centrifugal filter devices at 7,500 g in 4°C to eliminate large molecules (molecular weight > 30 kDa) from the plasma. The plasma nitrate and nitrite were measured with a SIEVERS NO analyzer (model 280NOA) as previously described (58, 61). The reducing agents used were either vanadium (III) chloride (VCl3) in 1 M HCl (for nitrate) or 1% sodium iodide (NaI) in glacial acetic acid (for nitrite). Five milliliters of a reagent plus 100 μl of 1:30 diluted anti-foaming agent were loaded into the purge vessel for analysis. These reducing agents converted nitrite and nitrate, respectively, to gaseous NO at 90°C, which was quantified by the analyzer.
Echocardiographic assessment of ventricular contractile function.
Echocardiography was performed using the Vevo770 imaging system (VisualSonics, Toronto, Canada) as previously described (44). In brief, under light anesthesia (pentobarbital sodium, 30 mg/kg ip), mice were placed in the supine position. A 30-MHz probe was used to obtain M-mode from parasternal short-axis view at the level of the papillary muscles according to the American Society of Echocardiography recommendation. LV end-systolic and end-diastolic diameters (i.e., LVESD and LVEDD, respectively) were measured. LV fractional shortening (FS) was subsequently calculated as follows: FS = (LVEDD − LVESD)/LVEDD × 100. The ejection fraction was calculated with the Teichholz formula.
Protein purification of SIRT1 for deacetylase activity analysis.
The samples were prepared according to a previously described method (18). In brief, the frozen heart samples were ground with a mortar and pestle in liquid nitrogen and mechanically homogenized in a lysis buffer containing 10 mM Tris·HCl (pH 7.4), 0.5% Nonidet P-40, 250 mM sucrose, 0.1 mM EGTA, 10 mM NaCl, 15 mM MgCl2, 1 mM PMSF, 1 mM Na3VO4, and 1 mM NaF. The tissue homogenates (without protease inhibitors) were spun through 4 ml of 30% sucrose, 10 mM Tris·HCl (pH 7.5), 10 mM NaCl, and 3 mM MgCl2 at 1,300 g for 10 min at 4°C; the pellet was washed with cold 10 mM Tris·HCl (pH 7.5) and 10 mM NaCl. The nuclei were suspended in 100 μl of extraction buffer containing 50 mM HEPES-KOH (pH 7.5), 420 mM NaCl, 0.5 mM EDTA-Na2, 0.1 mM EGTA, and glycerol 10%, sonicated for 30 s and kept on ice for 30 min. After centrifugation at 13,000 rpm for 10 min, an aliquot of the supernatant (crude extract nuclear) was used to determine protein concentration using a Bio-Rad assay (18).
Immunoprecipitation.
The samples were immunoprecipitated with SIRT1 antibody according to the manufacturer's instructions. SIRT1 primary antibody (1 μg, Cyclex) was incubated with 250 μg of protein in extraction buffer (total volume 200 μl) overnight at 4°C. Protein A agarose beads were then incubated with the mixture overnight at 4°C. After centrifugation for 30 s at 4°C, the pellet was washed three times with 250 μl of 1× Cell Lysis Buffer (Cyclex) and 250 μl of Sir2 assay buffer consisting of 50 mM Tris·HCl (pH 8.8), 4 mM MgCl2, and 0.5 mM DTT. The pellet was then suspended in 100 μl of Sir2 assay buffer and kept frozen at −20°C until use.
SIRT1 deacetylase activity assay.
SIRT1 deacetylase activity was evaluated in the whole heart lysates from the mice according to the manufacturer's protocol using a deacetylase fluorometric assay kit (Sir2 Assay Kit, CycLex, Ina, Nagano, Japan). The final reaction mixture (100 μl) contained 50 mM Tris·HCl (pH 8.8), 4 mM MgCl2, 0.5 mM DTT, 0.25 mA/ml lysyl endopeptidase, 1 μM trichostatin A, 200 μM NAD, and 10 μl of crude extract nuclear sample. The fluorescence intensity at 440 nm (excitation, 340 nm) was measured every 5 min for a total of 30 min immediately after the addition of fluoro substrate peptide, and then once immediately after the addition of stop solution. Resveratrol is a putative activator of SIRT1 that has been shown to be cardioprotective by our group and others.
Therefore, we used resveratrol as a positive control for SIRT1 activation (9, 22, 46). Sirtinol (60 μM) was used as the inhibitor of SIRT1, and trichostatin A was used as an inhibitor of class I and class II histone deacetylases (2). All measurements were performed in duplicate, and the results are reported as arbitrary units of relative fluorescence.
Western blot analysis.
The heart samples were collected, and the proteins were extracted in a buffer containing (in mM) 50 potassium phosphate, 1 EDTA, 1 EGTA, 0.2 PMSF, 5 β-glycerophosphate, 2 NaF, 2 Na3VO4, 10 β-mercaptoethanol, 1 μg/ml pepstatin, and 0.5 μg/ml leupeptin (pH 7.0) with a tissue homogenizer. The homogenate was centrifuged at 10,000 g for 15 min under 4°C, and the supernatant was recovered. Fifty milligrams of protein from each sample was separated by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membrane was incubated with primary antibodies at a dilution of 1:1,000 for each of the respective proteins, i.e., phosphorylated (p)eNOS, eNOS (Cell Signaling Technology,), SIRT1, PGC-1α, pAkt, Akt, pAMPK, AMPK (Santa Cruz Biotechnology). The membrane was then washed and incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution, 1 h at room temperature). Detection of the signals was performed using LumiPhos reagent (Pierce), and chemical luminescence was detected using X-omat Kodak film. The densitometric quantification was performed with Bioquant image analysis software.
Isolation of mitochondria.
A single combined population of cardiac mitochondria was isolated from the excised hearts as previously described (52). Briefly, the washed heart tissue was dried, weighed, and then thoroughly minced in a glass beaker. The cardiac tissue was next homogenized in 3 ml of CP1 buffer, consisting of 100 mM KCl, 50 mM MOPS, 5 mM MgSO4, 1 mM EGTA, and 1 mM ATP (pH 7.4), using a polytron tissue blender (Kinematica, Bohemia, NY) for 2.5 s at a rheostat setting of 10,000 rpm. The homogenate was centrifuged at 6,000 g for 10 min at 4°C, and the supernatant was saved as a crude cytosol for further purification. The homogenate pellet was resuspended in 3 ml of CP1 buffer and incubated with 5 mg/g trypsin (Sigma) at 4°C for 15 min with stirring. Three milliliters of CP2 buffer (CP1 buffer containing 0.2% bovine serum albumin) was next added to decrease the trypsin activity. Digested tissue was then homogenized with a tight Teflon pestle/glass tube homogenizer (Fisher Scientific) set at a steady stirring speed of 600 rpm. The undigested tissue and heavier cell fractions were separated by centrifugation at low speed 500 g for 10 min at 4°C. The supernatant containing the mitochondria was centrifuged at 3,000 g for 10 min at 4°C. The mitochondrial pellet was washed with 2 ml of buffer consisting of 100 mM KCl, 50 mM MOPS, 0.5 mM EGTA (KME; pH 7.4) and centrifuged at 3,000 g for 10 min. Lastly, the mitochondria pellet was resuspended in 70 μl of KME. The crude cytosolic fraction was further purified by ultra-centrifugation at 100,000 g for 30 min at 4°C. The protein concentration of the mitochondria and the cytosols was measured by Lowry method (33) using bovine serum albumin as a standard and sodium deoxycholate as the detergent to solubilize the mitochondria.
Measurement of mitochondrial oxidative phosphorylation and enzyme activities.
Oxidative phosphorylation in the isolated mitochondria was studied as previously described (31) using glutamate + malate (complex I), succinate with rotenone (complex II), or tetramethylphenylenediamine (TMPD) with rotenone (complex IV) as substrates to localize defects within the electron transport chain. Enzyme activities in detergent solubilized mitochondria were measured spectrophotometrically at 30°C as previously described (10, 11, 31). Rotenone-sensitive complex I (NADH-ubiquinone oxidoreductase), NADH-ferricyanide reductase, theonyltrifluoroacetone sensitive complex II, antimycin A sensitive complex III (ubiquinol-cytochrome c oxidoreductase), complex IV (cytochrome c oxidase), and citrate synthase activities were measured. Frozen-thawed mitochondria were solubilized in methylsulfonylmethane-EDTA buffer containing 0.5% cholate (pH 7.4) 220 mM mannitol, 70 mM sucrose, 5 mM MOPS, and 2 mM EDTA. Calculated enzyme activities were expressed as nanomoles per minute per milligram of mitochondrial protein or 1/min/mg in the case of complex IV.
Detection of H2O2 production.
Mitochondrial H2O2 was measured by the enzymatic oxidation of the fluorogenic indicator 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red; Invitrogen, Carlsbad, CA) in the presence of horseradish peroxidase (Sigma-Aldrich, Saint Louis, MO), which produced the fluorescence product resorufin (11). Isolated mouse mitochondria (30 μg) were incubated in buffer consisting of 120 mM KCl, 5 mM KH2PO4, 1 mM EGTA, and chelex- treated buffer, containing 25 μM Amplex Red and 0.25 U/ml horseradish peroxidase. Glutamate and succinate were used as substrates along with their respective inhibitors rotenone (5 μM) and antimycin A (10 μM) to generate maximal complex I-dependent and complex III-dependent H2O2 production.
Statistical analysis.
Data were represented as means ± SE. Statistical analysis was performed using one-way ANOVA with subsequent Student-Newman-Keuls post hoc test for pairwise comparison. All analysis was performed using SigmaStat software (SigmaStat for Windows, version 1.0, Jandel). P < 0.05 was considered statistically significant.
RESULTS
Effect of tadalafil on metabolic characteristics.
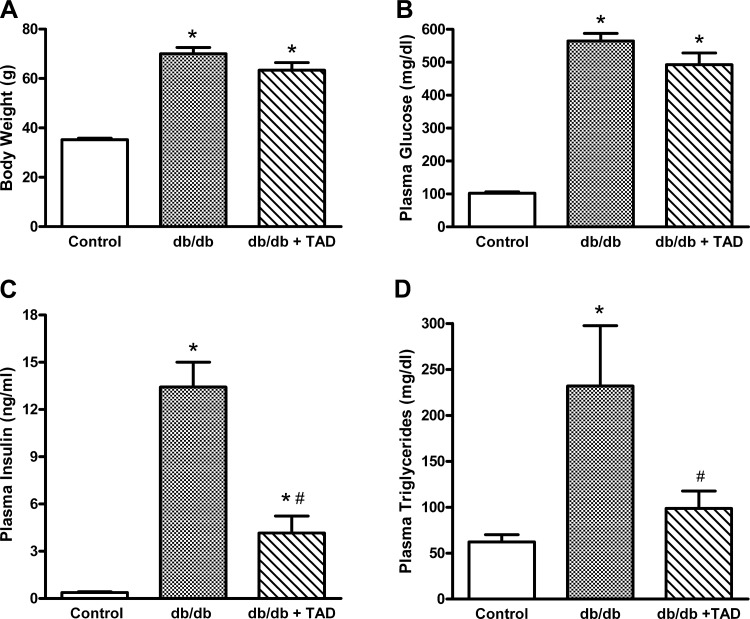
The body weight, plasma glucose, insulin, and triglycerides were significantly higher in 40-wk-old db/db mice compared with their nondiabetic controls (Fig. 1). Following 8 wk, tadalafil-treated mice demonstrated a slight trend toward decreased body weight and fasting glucose levels compared with the untreated db/db mice (Fig. 1, A and B). The tadalafil-treated db/db mice showed significant decrease in fasting plasma insulin levels compared with the untreated db/db mice (4.15 ± 1.08 vs. 13.43 ± 1.57, P < 0.05, n = 6/group; Fig. 1C). Similarly, there was dramatic decrease in the fasting triglycerides in tadalafil-treated db/db mice (231.8 ± 65.7 vs. 98.7 ± 18.7, P < 0.05, n = 6/group; Fig. 1D).
Fig. 1.
Effect of tadalafil (Tad) on metabolic status. Body weight (A), plasma glucose (B), plasma insulin (C), and plasma triglycerides (D) are shown. Data are expressed as means ± SE; n = 6/group. *P < 0.05 vs. control; #P < 0.05 vs. diabetic db/db mice.
Tadalafil treatment enhances plasma of nitrate and NO oxidation levels.
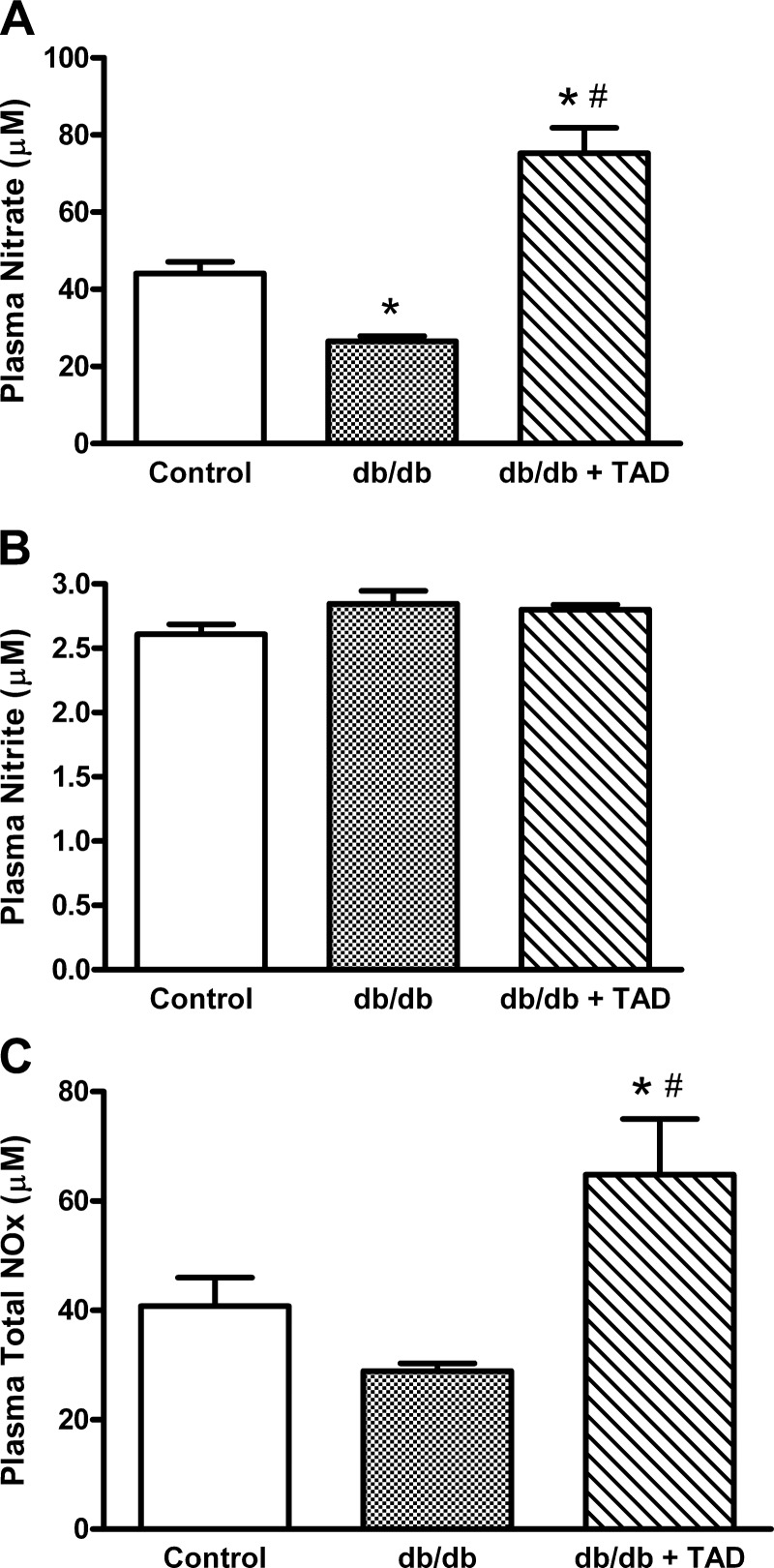
The db/db mice showed a significant reduction in the nitrate levels compared with nondiabetic controls (25.7 ± 1.4 vs. 41.9 ± 4.2, P < 0.05, n = 6/group; Fig. 2A), whereas the nitrite levels remained unchanged (Fig. 2B). Tadalafil treatment significantly enhanced plasma nitrate in db/db mice compared with both the control group and untreated db/db groups (Fig. 2A). In contrast, nitrite levels did not increase in tadalafil-treated db/db mice (Fig. 2B), suggesting a possible impeded nitrate-to-nitrite conversion. Overall, NO oxidation products (NOx) were significantly increased in tadalafil-treated db/db mice compared with the control and untreated db/db groups (Fig. 2C).
Fig. 2.
Effect of tadalafil on plasma nitrate, nitrite, and total nitric oxide oxidation products (NOx). Assessment of plasma levels of nitrate (A), nitrite (B), and total NOx (C). Data are expressed as means ± SE; n = 6/group. *P < 0.05 vs. control; #P < 0.05 vs. db/db.
Tadalafil attenuates LV contractile dysfunction.
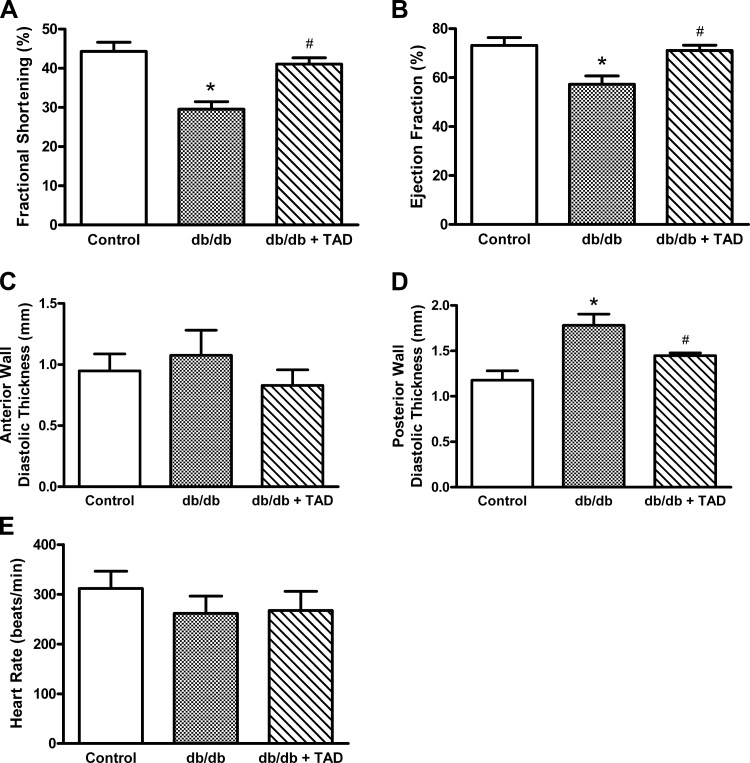
LV ejection fraction and FS decreased significantly in db/db group compared with the nondiabetic control group (Fig. 3, A and B). The db/db mice also exhibited LV posterior wall thickening (Fig. 3D), indicating diabetes-induced cardiomyopathy. Tadalafil treatment significantly preserved ejection fraction and FS (Fig. 3, A and B) and prevented ventricular wall thickening in db/db mice (Fig. 3D). There was no significant difference in heart rate among the three groups of lightly anesthetized mice (Fig. 3E).
Fig. 3.
Echocardiographic assessment of cardiac contractile function. The averaged data of left ventricular fractional shortening (A), ejection fraction (B), left ventricular anterior (C) and posterior (D) wall thickness, and heart rate (E) are presented as means ± SE; n = 6/group. *P < 0.05 vs. control; #P < 0.05 vs. db/db.
Tadalafil enhances phosphorylation of eNOS.
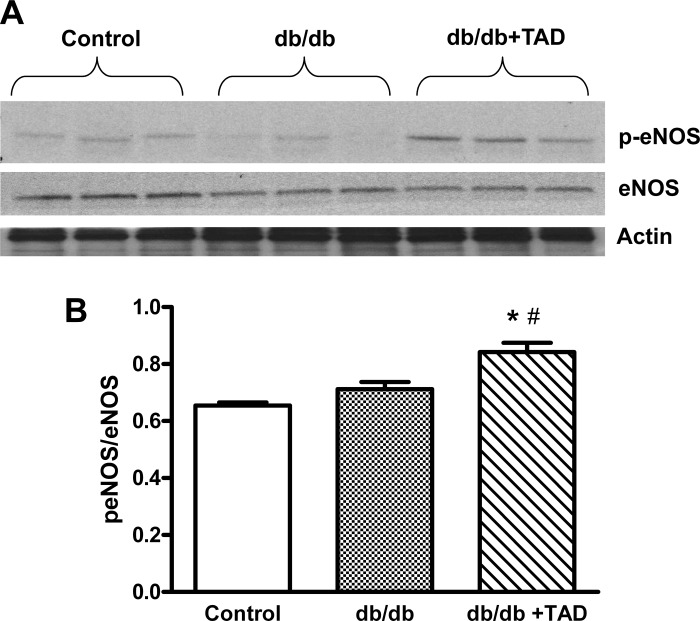
Western blot analysis showed that tadalafil treatment significantly increased phosphorylation levels of eNOS at Ser1177 site in the cardiac tissue of db/db mice (P < 0.05; Fig. 4, A and B).
Fig. 4.
Effect of tadalafil on total and phosphorylated endothelial nitric oxide synthase NOS (peNOS). A: Western blots showing myocardial expression of total and phosphorylated eNOS at Ser1177 site and the loading control actin. B: densitometric quantification of peNOS expression normalized against total eNOS (means ± SE; n = 3/group). *P < 0.05 vs. control; #P < 0.05 vs. db/db.
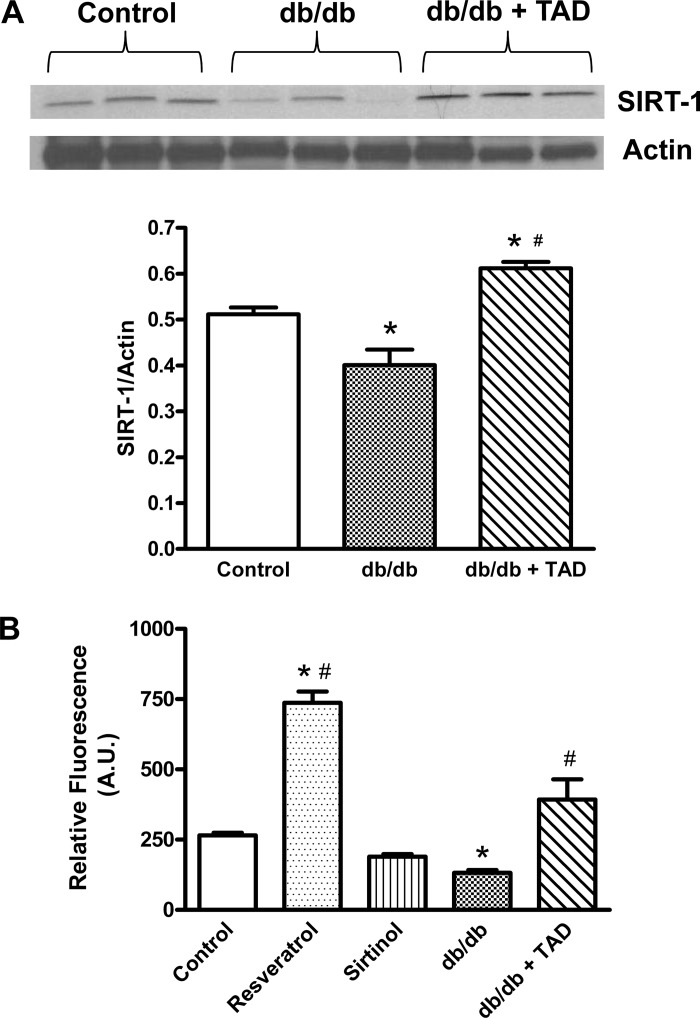
Tadalafil upregulates SIRT1.
Quantitative analysis of SIRT1 levels (normalized with β-actin) showed significant reduction of SIRT1 expression in diabetic mice (Fig. 5A). To assess how the protein levels relate to SIRT1 activity in diabetic hearts, the endogenous SIRT1 was immunoprecipitated using equal amounts of protein, and deacetylase activity was measured. Resveratrol- and sirtinol-treated nondiabetic mice were considered as positive and negative controls, respectively. As shown in Fig. 5B, the SIRT1 activity was significantly reduced by 39.4% in db/db mice relative to the nondiabetic controls. Tadalafil treatment not only preserved the decline in SIRT1 activity in db/db mice but also stimulated it by 56.2% compared with the nondiabetic controls.
Fig. 5.
Effect of tadalafil on myocardial silent information regulator 1 (SIRT1). Myocardial SIRT1 expression (A) and SIRT1 activity (B) in db/db mice are shown. Data are expressed as means ± SE; n = 6/group. AU, arbitrary units. *P < 0.05 vs. control; #P < 0.05 vs. db/db.
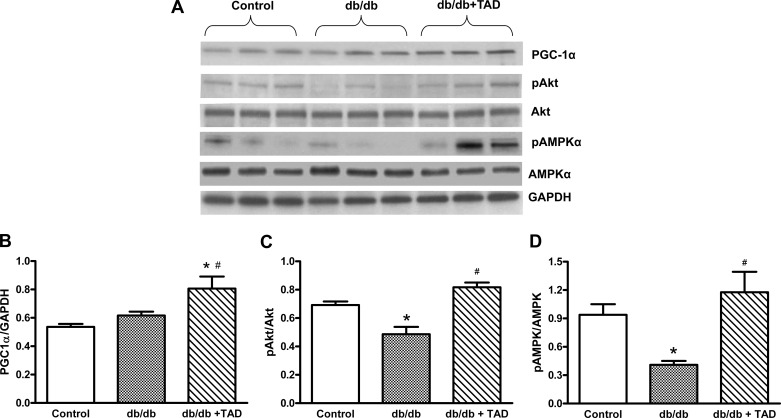
Effect of tadalafil on PGC-1α expression and phosphorylation of Akt and AMPK.
Western blot analysis showed that tadalafil treatment significantly increased PGC-1α expression in db/db mice (P < 0.05; Fig. 6, A and B). The phosphorylated forms of Akt and AMPK in db/db mice hearts were significantly lower than in nondiabetic control mice. Chronic treatment with tadalafil significantly enhanced pAkt and pAMPK in db/db mice (Fig. 6, C and D).
Fig. 6.
Effect of tadalafil on PGC-1α and phosphorylation of AMPK (pAMPK) and Akt (pAkt). Western blots showing the myocardial expression of PGC-1α, pAkt, Akt, pAMPK, AMPK, and representative GAPDH band (A) are shown. Densitometric quantification of PGC-1α expression normalized against GAPDH (B), pAkt normalized against Akt (C), pAMPK normalized against AMPK (D). Data represent means ± SE; n = 6/group. *P < 0.05 vs. control; #P < 0.05 vs. db/db.
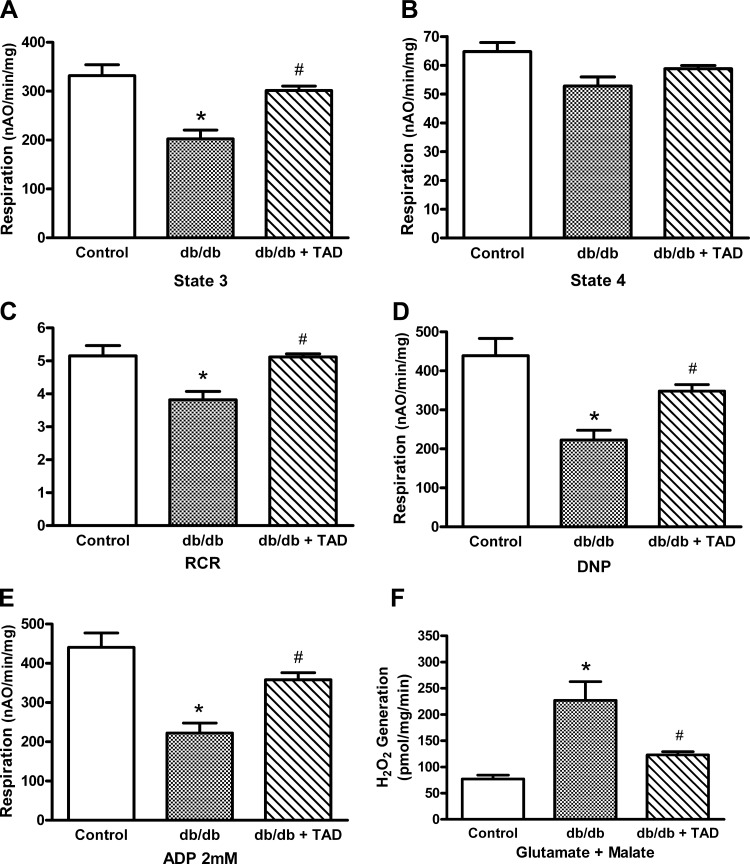
Tadalafil treatment preserves oxidative phosphorylation in cardiac mitochondria.
The mitochondria isolated from the db/db mice showed a decrease in ADP-stimulated respiration (state 3) and ADP-limited respiration (state 4) compared with those from control mice using glutamate and malate as complex I substrates (Fig. 7, A and B). The maximal rate of oxidative phosphorylation (measured by adding saturating 2 mM ADP) decreased in db/db hearts compared with the control (Fig. 7E). The dinitrophenol-uncoupled respiration was also reduced in db/db mice (Fig. 7D), localizing the defect to the electron transport chain. In contrast to glutamate + malate-dependent respiration, succinate and TMPD/ascorbate-dependent rates were similar in both db/db and control mice. Treatment with tadalafil in db/db mice protected complex I as demonstrated by improved state 3 respiration with glutamate + malate as a substrate compared with untreated group. Moreover, tadalafil improved the maximal state 3 respiration compared with the nontreated db/db mice (Fig. 7E). In contrast, succinate and TMPD/ascorbate-dependent rates were unaffected by tadalafil treatment (i.e., control: 2,063 ± 205; db/db, 1808 ± 191; db/db + tadalafil 1,903 ± 52 nAO·min−1·mg−1, means ± SE). Mitochondrial protein yields and ADP-to-O ratios were similar among the three groups (data not shown).
Fig. 7.
Effect of tadalafil on mitochondrial function and reactive oxygen species production. Glutamate + malate-dependent integrated respiration of mitochondria isolated from hearts of control and db/db diabetic mice with or without tadalafil treatment. ADP-stimulated respiration (A), ADP-limited respiration (B), respiratory control ratio (RCR; C), rate of uncoupled respiration (D), maximum rates of state 3 respiration were measured with 2 mM ADP (E), and the maximal capacity of complex I to generate reactive oxygen species (F) are shown. DNP, dinitrophenol. Results are means ± SE; n = 4–6 per group. *P < 0.05 vs. control; #P < 0.05 vs. db/db.
Tadalafil decreases H2O2 generation from the mitochondria.
The maximal capacity of H2O2 produced by complex I was measured using glutamate and malate as a substrate and rotenone as the inhibitor. As shown in Fig. 7F, mitochondrial H2O2 increased in db/db mice compared with nondiabetic control mice. Treatment with tadalafil significantly decreased H2O2 release from complex I (Fig. 7F). There was no difference in H2O2 production between the three groups when succinate and rotenone were used as substrate.
Activities of electron-transport chain and citrate synthase enzyme.
Activities of complex I (NADH-ubiquinone oxidoreductase), complex II (succinate-ubiquinone oxidoreductase), complex III (ubiquinol-cytochrome-c oxidoreductase), and complex IV (cytochrome-c oxidase) were not significantly different in db/db mice compared with the control C57BL6 mice (Table 1). Activity of citrate synthase, a marker enzyme for the mitochondrial matrix, was comparable in all the groups that depict the relative purity of the mitochondrial isolation (Table 1). These data indicate that the decrease in integrated respiration observed with complex I substrates in diabetic mice was not due to decreased content of electron transport complexes.
Table 1.
Electron transport chain-related enzyme activities in one population mitochondria isolated from the control or diabetic hearts with or without tadalafil treatment
| Mitochondria | Complex 1 | NFR | Complex II | Complex III | Complex IV | CS |
|---|---|---|---|---|---|---|
| Control | 741 ± 72 | 2,252 ± 365 | 51 ± 21 | 7,475 ± 902 | 197 ± 17 | 2,635 ± 136 |
| db/db | 553 ± 83 | 2,664 ± 328 | 41 ± 12 | 6,610 ± 926 | 205 ± 60 | 2,921 ± 346 |
| db/db + Tad | 532 ± 18 | 2,765 ± 140 | 42 ± 12 | 5,443 ± 614 | 135 ± 9 | 2,591 ± 217 |
Values are means ± SE (in nmol·min−1·mg protein−1); n = 4–6/group.
Complex I, NADH: decylubiquinone oxidoreductase; NFR, NADH: ferricyanide oxidoreductase; Complex II, succinate-ubiquinone oxidoreductase; Complex III, ubiquinol-cytochrome c oxidoreductase; Complex IV, cytochrome-c oxidase; CS, citrate synthase; db/db, diabetic heart; db/db + Tad, diabetic heart with tadalafil treatment.
DISCUSSION
In the present study, we have shown the potential role of NO-SIRT1-PGC1α signaling cascade in tadalafil-induced cardioprotection in type 2 diabetes. Chronic treatment with tadalafil improved cardiac function as indicated by significantly preserved ejection fraction and FS in the middle-aged db/db mice, which was associated with enhanced expression and activity of SIRT1. The tadalafil-treated group also showed an increase in plasma nitrate/total NOx levels. Diabetes is known to cause endothelial and cardiac dysfunction primarily due to impaired NO bioavailability. Variations at the eNOS gene are known to influence energy expenditure, severity of glucose intolerance, risk of developing type II diabetes, and associated cardiovascular complications (50). Consistent with this, the type 2 diabetic patients have impaired NO synthesis and decreased expression of eNOS and iNOS (54). In this context, tadalafil treatment improved bioavailability of NO as evidenced by enhanced circulating NOx levels (Fig. 2C) and improved cardiac function in diabetic mice (Fig. 5). Moreover, the favorable effects on metabolic status of db/db mice such as a trend toward decreased body weights, blood glucose, and significant improvement in hyperinsulinemia and hypertriglyceridemia suggest possible use of tadalafil in diabetes-induced cardiovascular abnormalities.
Our results also provide first evidence that chronic tadalafil treatment enhances the cardiac expression of PGC-1α in conjunction with increased SIRT1 expression and activity. PGC-1α is a transcriptional coactivator downstream of SIRT1 signaling, which is known to play a key role in regulating genes involved in myocardial fuel metabolism and cardiac function. Recent studies have shown SIRT1 to function together with PGC-1α in promoting adaptation to calorie restriction, a process well known to increases SIRT1 activity (42). PGC-1α can be activated by AMPK or SIRT1, the 2 principal cellular metabolic sensors, via direct phosphorylation or deacetylation, respectively. Both AMPK and SIRT1 could regulate the activity of PGC-1α in controlling energy state, mitochondrial biogenesis, and contractile function in cardiac muscle. Therefore, the metabolic sensors (AMPK, SIRT1) and PGC-1α likely form a vital regulatory network for cellular metabolic balance. The increased expression and activity of SIRT1, pAMPK, and PGC-1α in the db/db mice receiving chronic tadalafil treatment (Fig. 6) suggest its favorable effects in diabetic hearts, which manifest pathological consequences induced by a misbalance of energy homeostasis.
We also postulate that tadalafil activates PGC-1α, a coactivator of transcription factor PPAR-γ, which may form complexes with retinoid-X receptors and bind to promoter regions and activation of several genes involved in fatty acid oxidation and in turn may reduce circulating and cardiac lipids (free fatty acid and triglycerides). In addition, the tadalafil-enhanced eNOS and SIRT1 activity can improve insulin sensitivity in type 2 diabetes. A number of studies also suggest that SIRT1 has a role in the regulation of glucose metabolism and insulin secretion (30, 36, 51). SIRT1 also regulates p53, FOXO, PGC-1α, eNOS, Ku70, NF-κB, PPAR-γ, and p300, all of which have roles in regulating gene expression in β-cells. Gene knockdown or inhibition of SIRT1 can induce insulin resistance in cells and tissues (51). Resveratrol, a putative SIRT1 activator, enhanced insulin sensitivity in vitro in a SIRT1-dependent manner and attenuated high-fat-diet-induced insulin resistance in vivo (3). Furthermore, SIRT1 deacetylates IRS-2 and promotes insulin-induced IRS phosphorylation (60). Suppression of SIRT1 activity also selectively inhibits insulin-induced tyrosine phosphorylation of IRS-1 and insulin responses, suggesting a major role of SIRT1 in insulin signaling. Taken together, our current finding on tadalafil-induced SIRT1 activation and improvement of insulin sensitivity has significant implications toward alleviating insulin resistance in type 2 diabetes.
Oxidative stress is a major cause of reduced endothelial NO bioavailability in diabetes and is involved in the pathogenesis and progression of diabetic tissue damage. Activation of SIRT1 is known to decrease oxidative stress via its regulatory effects on mitochondrial function (1). A recent study from our laboratory demonstrated that tadalafil attenuates oxidative stress in diabetic hearts (26). However, whether activation of SIRT1 by tadalafil had any effects on mitochondrial function and ROS generation in diabetic hearts is unknown. Hence, we determined the functionality of the mitochondria in the hearts by measuring its oxidative phosphorylation using substrates that selectively donate electrons to specific complexes of the electron transport chain. Our results indicated that mitochondrial respiratory function through complex I was decreased in diabetic mice by ∼50% (control, 441 ± 43 nAO·min−1·mg−1 protein; and db/db, 222 ± 28, P < 0.05, Fig. 6E). Treatment of diabetic mice with tadalafil protected oxidative phosphorylation with an improved rates of oxidative phosphorylation through complex I (358 ± 20 vs. 222 ± 28, P < 0.05, Fig. 7E) compared with the nontreated db/db mice. A previous study demonstrated that Fidarestat, a novel inhibitor of aldose reductase, protected against cardiomyocyte dysfunction through a Sir2-dependent pathway while attenuating intracellular superoxide formation in db/db mice (16). Our study is in agreement with this report that indicated enhanced oxidative stress along with decreased SIRT1 expression in db/db mouse hearts (16). Remarkably, the increased production of ROS from complex 1 in diabetic mice was decreased by tadalafil treatment. Since activation of SIRT1 is known to decrease ROS levels, it is possible that tadalafil attenuated ROS generation and mitochondrial dysfunction in diabetic hearts, at least in part, via SIRT1 activation.
Our previous studies demonstrated that tadalafil treatment prevented detrimental remodeling of extracellular matrix proteins like CGRP-3, collagen type VI α1 in db/db mice (28). The changes in extracellular matrix proteins have been connected to altered mitochondrial function through altered mechanosignal transduction produced by changes in intermediate filament protein abundance (23, 56). We showed that tadalafil downregulated mitochondrial elongation factor thermo unstable in diabetic hearts (28). The activation of elongation factor thermo unstable by phosphorylation leads to inactivation of mitochondrial protein synthesis with consequent loss of mitochondrial subunits essential for function of complexes I and IV (7).
The present study demonstrated that tadalafil preserved mitochondrial function in diabetic hearts, which further elucidates its protective effects against detrimental remodeling of cardiac proteins. Mitochondrial dysfunction is present in the db/db mouse heart manifest by decreased rates of oxidative phosphorylation, increased production of ROS, and increased fatty acid-driven uncoupling of mitochondrial respiration detected in permeabilized cardiac fibers (8). The mitochondrial dysfunction in the db/db mouse was localized to the subsarcolemmal population of mitochondria with minimal decreases in respiration present in the interfibrillar population of mitochondria (13). The present study isolated a combined population of cardiac mitochondria to assess the potential effect of tadalafil on the global mitochondrial population. The defects in oxidative phosphorylation previously observed in respiration with fatty acid and NADH-dependent substrates were shown in the present study to be selective for complex I, with respiration through the distal electron transport chain unaltered. Decreases in enzyme activity of complex I, III, and IV previously observed only in subsarcolemmal and reflected in decreased content of particular component subunits (13) were not evident in the combined population of mitochondria used in the current study. Thus the decrease in integrated respiration previously observed with both fatty acid and carbohydrate-linked substrates (8, 13) was localized to the impact of complex I. Importantly, this complex I-based defect in integrated respiration in the total mitochondrial population was abrogated by tadalafil treatment.
Mitochondria in the db/db mouse are an increased source of ROS, measured as H2O2, in part due to reverse electron flow in complex I (8). In the present study, forward flow through complex I also increased production of H2O2 in mitochondria from db/db mice that was markedly attenuated by treatment with tadalafil. The fatty acid-enhanced uncoupling of mitochondrial respiration in the db/db heart (8) was not observed since fatty acid substrates were not used.
Increased oxidative stress in diabetes reduces NO bioavailability and induces detrimental changes in the signaling molecules of the myocardium. Our previous studies (15, 43) have shown that PDE5 inhibitor sildenafil significantly enhances NO production via augmented levels of mRNA and/or protein expression of eNOS and iNOS in intact heart or isolated cardiomyocytes from nondiabetic mice. Our present data (Fig. 4) demonstrated that chronic treatment with a long-acting PDE5 inhibitor tadalafil in db/db diabetic mice led a significant enhancement in phosphorylation of eNOS at Ser1177 site, which could increase NO production (29). We also further illustrate that db/db mouse hearts exhibited decreased expression of pAkt, which is a protein downstream of NO-SIRT1 signaling cascade (5). Tadalafil treatment restored the depletion of pAMPK and pAkt in the diabetic hearts. These observations confirm the role of AMPK and Akt in mediating cardioprotective effects in diabetic hearts as previously reported (24). Tadalafil treatment also enhanced cGMP and PKG levels in mouse models of cardiac injury, suggesting a critical role of NO-cGMP-PKG signaling pathway in cardioprotection (27, 45). A recent report from our laboratory has shown that tadalafil treatment significantly increased PKG activity in cardiomyocytes isolated from diabetic mice (56). Our data suggest the pivotal role of NO-dependent SIRT1 signaling along with enhanced expression downstream targets such as PGC-1α and pAkt. Taken together, our results provide an interesting paradigm where tadalafil enhances mitochondrial function via activation of AMPK and SIRT1 and their downstream targets, including PGC-1α in the heart. We propose that PDE5 inhibition with tadalafil may activate a signaling cascade that involves increased NO production, SIRT1 activation, enhanced phosphorylation of key survival kinases, Akt and AMPK, and improved mitochondrial respiratory function, which ultimately lead to the observed cardioprotection in type 2 diabetic hearts.
Nevertheless, the current study has several limitations. First, the cause-and-effect relationship between the tadalafil-enhanced cardiac NO production and the observed cardioprotective effects by this PDE5 inhibitor was not experimentally confirmed. This is mainly due to apparent inability to use a pan NOS inhibitor (i.e., Nω-nitro-l-arginine methyl ester). The 8-wk chronic treatment with Nω-nitro-l-arginine methyl ester in the 40-wk-old db/db mice would likely lead to severe side effects such as hypertension and renal failure, which will confound the interpretation of results. In addition, we did not directly measure ATP production in the limited amount of tissues samples from the chronically treated db/db mice. However, the phosphorylating rate of state 3 respiration (in nAO·min−1·mg−1 protein) was increased by chronic tadalafil treatment (Fig. 7A), which would correlate with improved mitochondrial generation of ATP since the ADP-to-O ratio was similar in db/db mice with and without tadalafil treatment (data not shown). Furthermore, mitochondrial content within tissue, estimated by mitochondrial protein yield was also similar (data not shown). Thus, with a similar mitochondrial content by protein, similar coupling of respiration, yet an ∼1/3 increase in phosphorylating respiration (state 3), the capacity for mitochondrial production of ATP is clearly improved by tadalafil. The tissue content of ATP, obviously integrating production largely from mitochondria, as well as from other sources versus consumption, will be of interest for future studies.
Clinical perspective.
Cardiovascular diseases and associated risks such as diabetes are a major cause of morbidity and mortality. Mounting scientific evidence suggests that switching gears toward survival and stress-resistant factors such as sirtuins may lead to the discovery of optimal therapeutic interventions that induce protection against heart diseases and other metabolic disorders. Hence, there is an intense search for SIRT1 activators. The present study elucidates the mechanistic insights of cardioprotection by tadalafil in a diabetic model and provides valuable novel information on the modulation of SIRT1 by chronic treatment with PDE5 inhibitor tadalafil. PDE-5 inhibitors, which could act as SIRT1 activators, represent a highly safe and attractive class of compounds that are already Food and Drug Administration approved for erectile dysfunction and pulmonary hypertension. Furthermore, human data showing improvement in diabetic cardiomyopathy following chronic treatment with PDE5 inhibitor sildenafil (20) underscore the potential of tadalafil as a therapeutic modality for type 2 diabetes-related cardiac and other complications.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-51045, HL-79424, and HL-118808 (to R. C. Kukreja) and Department of Veterans Affairs Merit Review Award (to E. J. Lesnefsky). S. Koka was supported by postdoctoral fellowship 11POST7400028 from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.K., E.J.L., and R.C.K. conception and design of research; S.K., H.S.A., and L.X. performed experiments; S.K., H.S.A., and L.X. analyzed data; S.K., L.X., E.J.L., and R.C.K. interpreted results of experiments; S.K., H.S.A., and L.X. prepared figures; S.K. drafted manuscript; S.K., H.S.A., L.X., E.J.L., and R.C.K. approved final version of manuscript; L.X., E.J.L., and R.C.K. edited and revised manuscript.
REFERENCES
- 1.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res 95: 971–980, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci 69: 2245–2260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem 73: 417–435, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 115: 3213–3223, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De JR, Beemster P, De Jong JW. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol 465: 115–123, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294: C460–C466, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associatedwith alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299: H529–H540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281: 38644–38652, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280: 12944–12955, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dong F, Ren J. Fidarestat improves cardiomyocyte contractile function in db/db diabetic obese mice through a histone deacetylase Sir2-dependent mechanism. J Hypertens 25: 2138–2147, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292: 288–290, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 11: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell 12: 51–62, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, Naro F, Morano S, Fedele F, Lenzi A. Chronic inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation 125: 2323–2333, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Matsui-Hirai H, Miyazaki-Akita A, Fukatsu A, Funami J, Ding QF, Kamalanathan S, Hattori Y, Ignarro LJ, Iguchi A. Endothelial cellular senescence is inhibited by nitric oxide: implications in atherosclerosis associated with menopause and diabetes. Proc Natl Acad Sci USA 103: 17018–17023, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res 47: 549–555, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet 35: 367–371, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kewalramani G, An D, Kim MS, Ghosh S, Qi D, Abrahani A, Pulinilkunnil T, Sharma V, Wambolt RB, Allard MF, Innis SM, Rodrigues B. AMPK control of myocardial fatty acid metabolism fluctuates with the intensity of insulin-deficient diabetes. J Mol Cell Cardiol 42: 333–342, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab 2: 153–163, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Koka S, Das A, Salloum FN, Kukreja RC. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med 60: 80–88, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther 334: 1023–1030, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koka S, Xi L, Kukreja RC. Chronic treatment with long acting phosphodiesterase-5 inhibitor tadalafil alters proteomic changes associated with cytoskeletal rearrangement and redox regulation in Type 2 diabetic hearts. Basic Res Cardiol 107: 249, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Kukreja RC, Xi L. eNOS phosphorylation: a pivotal molecular switch in vasodilation and cardioprotection? J Mol Cell Cardiol 42: 280–282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 273: H1544–H1554, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289: 2126–2128, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 34.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2: 105–117, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Napoli C, Balestrieri ML, Sica V, Lerman LO, Crimi E, De RG, Schiano C, Servillo L, D'Armiento FP. Beneficial effects of low doses of red wine consumption on perturbed shear stress-induced atherogenesis. Heart Vessels 23: 124–133, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol 28: 1634–1639, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado DO, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potente M, Dimmeler S. NO targets SIRT1: a novel signaling network in endothelial senescence. Arterioscler Thromb Vasc Biol 28: 1577–1579, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–597, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–H1406, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120: S31–S36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalwala M, Zhu SG, Das A, Salloum FN, Xi L, Kukreja RC. Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS One 9: e86977, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 295: H2348–H2355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126: 987–1002, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science 273: 59–63, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suganthalakshmi B, Anand R, Kim R, Mahalakshmi R, Karthikprakash S, Namperumalsamy P, Sundaresan P. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis 12: 336–341, 2006 [PubMed] [Google Scholar]
- 51.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6: 307–319, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem 286: 29610–29620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tikoo K, Tripathi DN, Kabra DG, Sharma V, Gaikwad AB. Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett 581: 1071–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Torres SH, De Sanctis JB, de LB, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 181: 419–427, 2004 [DOI] [PubMed] [Google Scholar]
- 55.van der Horst A, Tertoolen LG, Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J Biol Chem 279: 28873–28879, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC. Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS One 7: e45243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res 87: 540–542, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation 99: 2157–2163, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 282: 34356–34364, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol 57: 2181–2189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]