Abstract
We recently found that young cigarette smokers display cutaneous vascular dysfunction relative to nonsmokers, which is partially due to reduced nitric oxide (NO) synthase (NOS)-dependent vasodilation. In this study, we tested the hypothesis that reducing oxidative stress improves NO bioavailability, enhancing cutaneous vascular function in young smokers. Ten healthy young male smokers, who had smoked for 6.3 ± 0.7 yr with an average daily consumption of 9.1 ± 0.7 cigarettes, were tested. Cutaneous vascular conductance (CVC) during local heating to 42°C at a rate of 0.1°C/s was evaluated as laser-Doppler flux divided by mean arterial blood pressure and normalized to maximal CVC, induced by local heating to 44°C plus sodium nitroprusside administration. We evaluated plateau CVC during local heating, which is known to be highly dependent on NO, at four intradermal microdialysis sites with 1) Ringer solution (control); 2) 10 μM 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (tempol), a superoxide dismutase mimetic; 3) 10 mM Nω-nitro-l-arginine (l-NNA), a nonspecific NOS inhibitor; and 4) a combination of 10 μM tempol and 10 mM l-NNA. Tempol increased plateau CVC compared with the Ringer solution site (90.0 ± 2.3 vs. 77.6 ± 3.9%maximum, P = 0.028). Plateau CVC at the combination site (56.8 ± 4.5%maximum) was lower than the Ringer solution site (P < 0.001) and was not different from the l-NNA site (55.1 ± 4.6%maximum, P = 0.978), indicating the tempol effect was exclusively NO dependent. These data suggest that in young smokers, reducing oxidative stress improves cutaneous thermal hyperemia to local heating by enhancing NO production.
Keywords: tobacco, reactive oxygen species, free radicals, microcirculation, skin
almost 6 million people die from tobacco use and exposure each year (53), and the majority of tobacco-related deaths are due to cardiovascular disease (11). Indeed, chronic exposure to cigarette smoking changes the structure and function of human conduit arteries (45). Oxidative stress is suspected to be a major contributor to chronic cigarette smoking-induced vascular alterations, as reducing oxidative stress with antioxidants (e.g., vitamin C) in smokers improves conduit artery vascular function, as evaluated by noninvasive flow-mediated dilation (FMD) (41, 43, 50) or by intra-arterial administration of endothelium-dependent vasodilators, such as ACh (17–19) and bradykinin (17). Furthermore, antioxidant-induced improvements in conduit artery vascular function in smokers are not observed when antioxidants are administered in conjunction with nitric oxide (NO) synthase (NOS) inhibition (31), suggesting that oxidative stress impairs conduit artery function by reducing NO bioavailability.
In addition to human conduit artery function, chronic cigarette smoking impairs function of the human microcirculation, such as the skin (9, 10, 14, 42). Given that microvascular dysfunction is a crucial step in the complications that lead to cardiovascular disease (1, 34, 38), exploring the mechanistic underpinnings of impaired microvascular function in smokers is important; however, few investigators have studied this issue. We (14) recently reported that young smokers have an impaired cutaneous vasodilatory response to administration of ACh compared with nonsmokers, which was partially due to attenuated NOS-dependent vasodilation. Given that oxidative stress reduces NO bioavailability in the conduit arteries of smokers (31), reducing oxidative stress may also improve NOS-dependent vasodilation in the cutaneous microcirculation of smokers, thereby enhancing vascular function. However, this has not been directly tested.
Using the above information as background, we hypothesized that tempol (a superoxide dismutase mimetic) would improve cutaneous vascular function through enhancing NOS-dependent vasodilation in young smokers. As a test of cutaneous vascular function, we evaluated cutaneous thermal hyperemia to local heating to 42°C at a rate of 0.1°C/sec. This test was selected as plateau vasodilation during local heating is predominantly (∼50–70%) mediated by NO (3, 5, 13, 20, 32, 39, 40, 49).
MATERIALS AND METHODS
Subjects.
This study was approved by the Institutional Review Board of The University of Oregon and conformed with guidelines set forth by the Declaration of Helsinki. Verbal and written informed consent were obtained from all subjects before their participation in the study. Smokers were defined as having smoked for at least 1 yr with an average daily cigarette consumption of ≥6 cigarettes/day. We recruited 10 healthy young (19–26 yr of age) smokers who had no history of hypertension, heart disease, diabetes, or autonomic disorders. This is important since advanced age (22, 26, 35, 40), hypertension (46), and disease status (e.g., chronic renal failure, postural tachycardia syndrome) (47, 48) are known to independently impair skin microvascular function. All subjects were not currently taking prescription medications. All subjects abstained from taking over-the-counter medications (including nonsteroidal anti-inflammatory agents and vitamins), alcohol, and caffeine for at least 24 h before the study. They also refrained from heavy exercise the night before the study and cigarette smoking for at least 12 h before the study to avoid any acute effects of cigarette smoking on skin blood flow regulation (9, 28, 52).
Instrumentation.
Upon arrival at the laboratory, subjects voided their bladder, and their body weight and height were measured. Subjects were placed in a semirecumbent position and instrumented with four microdialysis fibers (30-kDa cutoff, 10-mm membrane, MD2000, Bioanalytical Systems, West Lafayette, IN) on the ventral side of the forearm in the dermal layer of the skin. A 25-gauge needle was first inserted into the unanesthetized skin using aseptic techniques with at least 4.0 cm between each site. The entry and exit points were ∼2.5 cm apart. The microdialysis fiber was then threaded through the lumen of the needle, after which the needle was withdrawn leaving the fiber in place. Microdialysis fibers were secured with tape. Lactated Ringer solution was perfused through each microdialysis fiber at a rate of 2.0 μl/min (CMA 1025 microdialysis pump, CMA Microdialysis, Kista, Sweden) until the start of drug infusions (see below).
Experimental protocol.
Once the trauma caused by microdialysis fiber placement had dissipated (∼60–90 min), the experimental protocol began. Microdialysis fibers were randomly assigned to receive 1) lactated Ringer solution (control), 2) 10 μM 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (tempol, EMD Millipore Chemicals, Billerica, MA) to reduce superoxide (O2·−), 3) 10 mM Nω-nitro-l-arginine (l-NNA; Sigma-Aldrich, St. Louis, MO) to nonselectively inhibit NOS and thus NO production, and 4) 10 μM tempol plus 10 mM l-NNA. Drug concentrations were selected as the minimum dose required for maximal effects, as reported in previous studies (36, 37). All pharmacological agents were dissolved in lactated Ringer solution. All drugs were infused continuously at a rate of 2.0 μl/min (CMA 102 Microdialysis Pump, CMA Microdialysis) until the end of local heating to 42°C. To ensure adequate drug effects, all pharmacological agents were perfused for at least 75 min before the start of local heating.
After 75+ min of drug infusion, baseline was recorded for at least 10 min while skin temperature was held constant at 33°C (Skin Heater/Temperature Monitor SHO2, Moor Instruments, Devon, UK). Thereafter, local heating of the skin to 42°C at a rate of 0.1°C/s was applied to all skin sites to induce cutaneous vasodilation. Once skin vasodilation reached a plateau (25–35 min after the initiation of heating), local skin temperature was further elevated to 44°C at a rate of 0.1°C/s with an administration of 56 mM sodium nitroprusside (SNP; Nitropress, Ciba Pharmaceuticals, East Hanover, NJ) at a rate of 2.0 μl/min to achieve maximal vasodilation.
Measurements.
Arterial blood pressure was measured via automated brachial oscillation (Dinamap ProCare 100, GE Medical Systems, Tampa, FL) throughout the protocol. Mean arterial blood pressure (MAP) was calculated as diastolic arterial blood pressure plus one-third pulse pressure. To obtain an index of skin blood flow, cutaneous red blood cell flux was measured with a single-point laser-Doppler flowmetry probe (MoorLab; Moor Instruments) seated in the center of the local heater over each microdialysis fiber. Cutaneous vascular conductance (CVC) was evaluated as cutaneous red blood cell flux divided by MAP. All CVC data were expressed as percentages of maximal CVC to minimize the effect of site-to-site heterogeneity in the level of skin blood flow (38). Data were recorded and stored on a computer using Windaq data-acquisition software (Dataq Instruments, Akron, OH). Figure 1 shows the CVC response to local heating, averaged across all subjects, which was characterized as follows. Baseline CVC was determined by taking an average CVC at least over 3 min before heating. Upon the initiation of local heating, CVC rapidly increased and exhibited an initial peak. After a brief nadir, CVC then gradually increased and reached a stable plateau. The initial peak and nadir CVC were determined by taking averaged CVC over 30 s, and the plateau and maximal CVC were determined from averaged CVC over at least 2 min. We evaluated the difference in plateau CVC between tempol and tempol plus l-NNA sites as an index of NOS-dependent vasodilation with tempol. Similarly, the difference in plateau CVC between Ringer solution and l-NNA sites was evaluated as an index of NOS-dependent vasodilation without tempol.
Fig. 1.
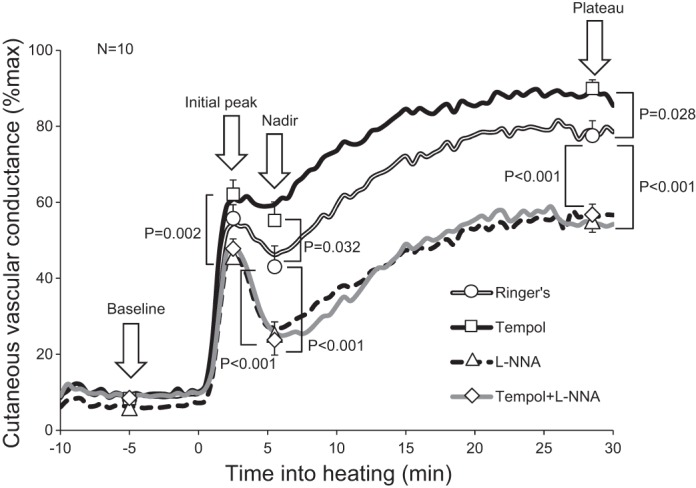
Averaged time-course changes in cutaneous vascular conductance during local heating. Baseline, initial peak, nadir, and plateau cutaneous vascular conductance obtained from individuals were averaged and compared across the four drug sites. Time 0 indicates the initiation of local heating. Tempol, 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl, a superoxide dismutase mimetic; l-NNA, NG-nitro-l-arginine (a nonspecific nitric oxide synthase inhibitor).
Statistical analyses.
Two-way repeated-measures ANOVA was conducted with factors of drug (Ringer solution, tempol, l-NNA, and combination of tempol and l-NNA) and phase of response (baseline, initial peak, nadir, plateau, and maximal periods) for absolute (mV/MAP × 100) and relative (%maximum) CVC. We used two-way ANOVA rather than one-way ANOVA to consider a potential interaction between drug and phase of response. When a significant main effect or interaction was detected, significant differences between paired variables across drug sites were determined by Tukey's honestly significant difference post hoc test. A two-tailed paired t-test was used to compare the difference in plateau CVC between tempol and tempol plus l-NNA sites with the difference in plateau CVC between Ringer solution and l-NNA sites. The level of significance was set at 0.05. Values are presented as means ± SE.
RESULTS
Characteristics of subjects.
Subjects were 22.5 ± 0.7 yr of age, with an average body mass index of 23.8 ± 0.8 kg/m2. They had smoked for 6.3 ± 0.7 yr with an average daily consumption of 9.1 ± 0.7 cigarettes/day. Their systolic blood pressure, diastolic blood pressure, and MAP were 112.9 ± 2.7, 63.9 ± 1.8, and 80.2 ± 1.7 mmHg, respectively. Note that their body mass indexes and arterial blood pressures were within healthy ranges.
Cutaneous variables.
There was an interaction between drug and phase of response on CVC represented as both absolute and %maximum values (both P < 0.001). Plateau CVC at the tempol site was greater than that at the Ringer solution site (Fig. 1). Plateau CVC at the l-NNA site was reduced relative to the Ringer solution site (Fig. 1). Plateau CVC at the site that received combined tempol and l-NNA was lower compared with the Ringer solution site and was not different from the value at the l-NNA site (P = 0.978; Fig. 1). The difference in plateau CVC between the tempol and tempol plus l-NNA sites tended to be higher compared with the difference in plateau CVC between the Ringer solution and l-NNA sites (31.5 ± 4.1 vs. 19.2 ± 6.7%maximum, P = 0.163).
Baseline CVC at the Ringer solution site did not differ from that at the tempol (P = 0.999), l-NNA (P = 0.948), and tempol plus l-NNA (P = 0.999) sites (Fig. 1). Initial peak CVC at the Ringer solution site was not different from that at the tempol (P = 0.455), l-NNA (P = 0.095), and tempol plus l-NNA (P = 0.250) sites (Fig. 1). Absolute maximal CVC (mV/MAP × 100) at the Ringer solution site (246 ± 25) was not different from the tempol (314 ± 52, P = 0.180), l-NNA (277 ± 34, P = 0.790), and tempol plus l-NNA (292 ± 22, P = 0.514) sites. Based on these data, we calculated the minimum sample sizes required to produce a significant level of 0.05 with 80% power, which demonstrated we would need 18 subjects for the difference in baseline CVC between the Ringer solution and l-NNA sites to be significant, 28 subjects for the difference in initial peak CVC between the Ringer solution and tempol sites to be significant, 28 subjects for the difference in absolute maximal CVC between the Ringer solution and tempol sites to be significant, and 23 subjects for the difference in absolute maximal CVC between the Ringer solution and tempol plus l-NNA sites to be significant. Relative to the Ringer solution site, nadir CVC at the tempol site was higher, whereas that at the l-NNA and combination sites was lower (Fig. 1).
DISCUSSION
We are the first to investigate how tempol, a superoxide dismutase mimetic, affects the cutaneous vascular response to local heating in young smokers. We also used l-NNA (NOS inhibitor) to evaluate whether tempol-induced improvements in microvascular function were through improved NO bioavailability. Our main findings were that 1) tempol enhanced the plateau phase of cutaneous vasodilation to local heating to 42°C and 2) the plateau at the combination site (tempol plus l-NNA) was lower than at the Ringer solution site but was comparable to the l-NNA site. These results suggest that in young smokers, reducing oxidative stress in the microvasculature improves cutaneous thermal hyperemia to local heating through NO-dependent mechanisms.
Oxidative stress.
Accumulating evidence supports the concept that smokers have impaired cutaneous vascular function compared with nonsmokers (9, 10, 14, 42). In line with this, plateau CVC in the young smokers of the present study was attenuated compared with that in the young nonsmokers of previous studies in which the same heating protocol was used (Table 1) (3, 5, 16, 21, 33, 49, 54). In the present study, we found that tempol significantly improved plateau CVC compared with the Ringer solution site (Fig. 1), up to a similar level as the plateau in those same studies in nonsmokers (∼90%), suggesting that oxidative stress is a major factor contributing to the impaired cutaneous vascular function in young smokers.
Table 1.
Comparison of averaged plateau cutaneous vascular conductance during local heating at the control site between young smokers and nonsmokers
| Author | Reference | Year | Plateau Cutaneous vascular conductance (%max) | Comment |
|---|---|---|---|---|
| Young smokers | ||||
| Present study | 77 | |||
| Young nonsmokers | ||||
| Hodges and Sparks | 21 | 2014 | 86–90 | At a rate of 0.5°C/10 s |
| Brunt and Minson | 5 | 2012 | 84–88 | |
| Bruning et al. | 3 | 2012 | 95 | |
| Greaney et al. | 16 | 2012 | 93 | |
| Wong and Fieger | 54 | 2010 | 92 | |
| Kellogg et al. | 33 | 2008 | 85 | Local heating to 41.5°C at a rate of 0.6°C/min |
| Stewart et al. | 49 | 2008 | 91 | |
All of the above studies used local heating to 42°C at a rate of 0.1°C/s unless otherwise indicated.
On the other hand, in healthy nonsmokers, antioxidants such as vitamin C do not affect vascular function, as evaluated by FMD or ACh-induced vasodilation in forearm conduit arteries (17, 19, 41, 43) as well as by the cutaneous vasodilatory response during whole body heating at rest (23, 25). More relevant to the present study, Medow et al. (36) showed in healthy young nonsmokers that tempol did not affect plateau CVC during the same local heating protocol as was used in the present study. The lack of an effect of antioxidants in healthy, nonsmoking subjects is not surprising, as healthy humans are expected not to have significant oxidative stress. Medow et al. (36) further showed that tempol restored plateau CVC when oxidative stress was induced in healthy nonsmokers by infusing angiotensin II, thus suggesting an antioxidative role of tempol during local heating. However, it should be considered that tempol may have nonantioxidative effects. For example, tempol-mediated opening of ATP-sensitive K+ channels has been reported in rats with systemic MAP changes (8), and opening of Ca2+-activated K+ (KCa) channels has been reported in rat mesenteric arterial smooth muscles (55). However, these effects are unlikely to have occurred in the present study, which focused on human skin blood flow regulation in smokers as no NO-independent tempol effects were observed, as discussed below.
NOS pathway.
Impaired NOS-dependent cutaneous vasodilation in smokers has been suggested by our previous study, which used ACh administration (14), and by the fact that, in the present study, NOS inhibition reduced plateau CVC during local heating to a lesser extent than what has previously been reported in healthy young nonsmokers (19 vs. 33–72%maximum) (3, 5, 13, 39, 40). Given that oxidative stress generally reduces NO bioavailability, it is plausible that in the present study, plateau CVC was improved with tempol administration by restoring NOS-dependent vasodilation. This notion is strongly supported by our observation that there was no difference in plateau CVC between l-NNA and tempol plus l-NNA sites (Fig. 1). Also, the difference in plateau CVC between the tempol and tempol plus l-NNA sites (an index of NOS-dependent vasodilation with tempol) tended to be higher compared with the difference in plateau CVC between the Ringer solution and l-NNA sites (an index of NOS-dependent vasodilation without tempol) (31.5 ± 4.1 vs. 19.2 ± 6.7%maximum, P = 0.163).
Possible mechanism(s) for how tempol improves NO bioavailability.
Cigarette smoking causes oxidative stress as a direct effect of the compounds within cigarette smoke itself (44). For example, the semiquinone radical in the cigarette tar yields O2·− (44). Additionally, NADPH oxidase, which produces O2·−, is directly activated by both nicotine, as shown in rat pial arterioles (12), and a stable thiol-reactive agent, as indicated in bovine, human, and rat pulmonary arteries (29). O2·− easily binds with NO to produce peroxynitrite, thus reducing NO bioavailability. Additionally, peroxynitrite depletes tetrahydrobiopterin, an essential cofactor for endothelial NOS (eNOS). This results in an increase in uncoupled eNOS, which then procures O2·− instead of NO (51), further reducing NO bioavailability. By removing O2·−, preventing it from binding with NO, and reducing uncoupled eNOS, tempol leads to higher NO bioavailability and thus improved plateau CVC. Moreover, the reaction of O2·− and tempol results in the production of H2O2, which may be another mechanism by which tempol improves plateau CVC. For example, scavenging of H2O2 with ebselen attenuates plateau CVC during local heating (36). Additionally, H2O2 can activate KCa channels, as shown in vascular smooth muscles of pig coronary arteries (2). Vasodilation via KCa channels contributes substantially to the plateau (5).
Limitations.
Tempol scavenges O2·− but not other ROS. Other ROS, such as H2O2 and hypochlorite may reduce NO bioavailability, as previously reported in porcine aortic endothelial cells (30), thus contributing to the attenuated plateau CVC during local heating in the skin of young smokers.
Only male subjects were included in this study. Thus, our conclusions cannot be applied to female subjects. Female sex hormones may be cardioprotective against the effects of chronic smoking, as reflected by the fact that the carotid and femoral artery wall thicknesses are greater (15) and conduit artery FMD is lower (6) in male smokers but not in female smokers compared with former or never smokers. Furthermore, female sex hormones enhance cutaneous thermal hyperemia to local heating (4, 7). Therefore, the effects of chronic cigarette smoking on cutaneous thermal hyperemia may be different between men and women and/or may be modulated by levels of female sex hormones. Further studies are warranted to address these issues.
Absolute maximal CVC at the tempol and tempol plus l-NNA sites tended to be higher relative to that at the Ringer solution site, although it was not significant due to the limited sample size. Reduced maximal cutaneous vasodilatory capacity has been reported in young (14) and older (10) smokers relative to nonsmoking counterparts. Our results suggest this may be due to oxidative stress, but further studies are required to flush that out.
For this study, we chose not to study a subset of nonsmokers, based on the number of studies showing no benefit of antioxidant administration on vascular responses in healthy, young nonsmokers. Although doing so would have allowed us to make comparisons between smokers and nonsmokers, we decided to specifically focus this study on investigating whether tempol would improve cutaneous vascular function in young smokers.
Perspectives.
Microvascular dysfunction may be a crucial step in the complications leading to cardiovascular disease and can be detected in the early stages of disease progression in the cutaneous circulation (24, 27, 38). The present study shows that impaired cutaneous microvascular function in young smokers is caused by oxidative stress in a similar fashion as is observed in aging (25). As such, chronic cigarette smoking has been suggested to cause a premature aging effect. Based on our results, we speculate that reducing oxidative stress in young smokers may potentially reduce the premature aging effect of chronic cigarette smoking on microvascular function, which, in turn, may prevent or delay smoking-related cardiovascular disease and mortality.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-081671. N. Fujii is the recipient of a research fellowship from the Uehara Memorial Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.F., V.E.B., and C.T.M. conception and design of research; N.F. and V.E.B. performed experiments; N.F. analyzed data; N.F., V.E.B., and C.T.M. interpreted results of experiments; N.F. prepared figures; N.F., V.E.B., and C.T.M. drafted manuscript; N.F., V.E.B., and C.T.M. edited and revised manuscript; N.F., V.E.B., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the volunteer subjects in the present study.
REFERENCES
- 1.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart 89: 175–178, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol Heart Circ Physiol 275: H1283–H1289, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol 112: 2019–2026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt VE, Miner JA, Meendering JR, Kaplan PF, Minson CT. 17-β estradiol and progesterone independently augment cutaneous thermal hyperemia but not reactive hyperemia. Microcirculation 18: 347–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol 87: 1719–1723, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293: H3246–H3253, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dalla Vecchia L, Palombo C, Ciardetti M, Porta A, Milani O, Kozakova M, Lucini D, Pagani M. Contrasting effects of acute and chronic cigarette smoking on skin microcirculation in young healthy subjects. J Hypertens 22: 129–135, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Edvinsson ML, Andersson SE, Xu CB, Edvinsson L. Cigarette smoking leads to reduced relaxant responses of the cutaneous microcirculation. Vasc Health Risk Manag 4: 699–704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet 362: 847–852, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Fang Q, Sun H, Arrick DM, Mayhan WG. Inhibition of NADPH oxidase improves impaired reactivity of pial arterioles during chronic exposure to nicotine. J Appl Physiol 100: 631–636, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol 95: 946–954, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Fujii N, Reinke MC, Brunt VE, Minson CT. Impaired acetylcholine-induced cutaneous vasodilation in young smokers: roles of nitric oxide and prostanoids. Am J Physiol Heart Circ Physiol 304: H667–H673, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gariepy J, Denarie N, Chironi G, Salomon J, Levenson J, Simon A. Gender difference in the influence of smoking on arterial wall thickness. Atherosclerosis 153: 139–145, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Greaney JL, Dupont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107: 416–421, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Heitzer T, Herttuala SY, Wild E, Luoma J, Drexler H. Effect of vitamin E on endothelial vasodilator function in patients with hypercholesterolemia, chronic smoking or both. J Am Coll Cardiol 33: 499–505, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges GJ, Sparks PA. Noradrenaline and neuropeptide Y contribute to initial, but not sustained, vasodilatation to local skin warming in humans. Exp Physiol 99: 381–392, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293: H1090–H1096, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ijzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33: 536–542, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ijzerman RG, Serne EH, van Weissenbruch MM, de Jongh RT, Stehouwer CD. Cigarette smoking is associated with an acute impairment of microvascular function in humans. Clin Sci 104: 247–252, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol 24: 1031–1036, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Jaimes EA, Sweeney C, Raij L. Effects of the reactive oxygen species hydrogen peroxide and hypochlorite on endothelial nitric oxide production. Hypertension 38: 877–883, 2001 [PubMed] [Google Scholar]
- 31.Jitsuiki D, Higashi Y, Goto C, Kimura M, Noma K, Hara K, Nakagawa K, Oshima T, Chayama K, Yoshizumi M. Effect of edaravone, a novel free radical scavenger, on endothelium-dependent vasodilation in smokers. Am J Cardiol 94: 1070–1073, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 118: 968–976, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol 79: 297–301, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111: 20–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation 15: 569–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol 109: 1239–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Yasue H. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: effect of vitamin C. Am J Physiol Heart Circ Physiol 273: H1644–H1650, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Pellaton C, Kubli S, Feihl F, Waeber B. Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. Am Heart J 144: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R. Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: studies in cultured cells and smokers. Am J Physiol Heart Circ Physiol 296: H1781–H1792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci 686: 12–27, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol 5: 276–292, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol 287: H2687–H2696, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertension 53: 767–774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 294: H466–H473, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takase B, Etsuda H, Matsushima Y, Ayaori M, Kusano H, Hamabe A, Uehata A, Ohsuzu F, Ishihara M, Kurita A. Effect of chronic oral supplementation with vitamins on the endothelial function in chronic smokers. Angiology 55: 653–660, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner DO, Joyner MJ, Charkoudian N. Nicotine increases initial blood flow responses to local heating of human non-glabrous skin. J Physiol 559: 975–984, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. Global Status Report on Noncommunicable Diseases. 2010. Description of the Global Burden of NCDs, Their Risk Factors and Determinants (online) http://www.who.int/nmh/publications/ncd_report2010/en/ [31 March 2014].
- 54.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu H, Jackson WF, Fink GD, Galligan JJ. Activation of potassium channels by tempol in arterial smooth muscle cells from normotensive and deoxycorticosterone acetate-salt hypertensive rats. Hypertension 48: 1080–1087, 2006 [DOI] [PubMed] [Google Scholar]


