Abstract
Large conductance Ca2+-activated K+ channels (BKCa) contribute to negative feedback regulation of smooth muscle cell (SMC) tone. However, the effects of aging on BKCa function are unclear. We tested the hypothesis that aging alters SMC BKCa function in superior epigastric arteries (SEAs) by using perforated patch recording of enzymatically isolated SMCs from 3- to 4-mo-old male C57BL/6 mice (Young) and 24- to 26-mo-old male C57BL/6 mice (Old). SMC capacitance from Young (15.7 ± 0.4 pF; n = 110) was less than Old (17.9 ± 0.5 pF; n = 104) (P < 0.05). SMCs displayed spontaneous transient outward currents (STOCs) at membrane potentials more positive than −30 mV; depolarization increased STOC amplitude and frequency (P < 0.05; n = 19–24). STOC frequency in Young (2.2 ± 0.6 Hz) was less than Old (4.2 ± 0.7 Hz) at −10 mV (P < 0.05, n = 27–30), with no difference in amplitude (1.0 ± 0.1 vs. 0.9 ± 0.1 pA/pF, respectively). At +30 mV, STOC amplitude in Young (3.2 ± 0.3 pA/pF) was less than Old (5.0 ± 0.5 pA/pF; P < 0.05, n = 61–67) with no difference in frequency (3.9 ± 0.4 vs. 3.2 ± 0.3 Hz, respectively). BKCa blockers (1 μM paxilline, 100 nM iberiotoxin, 1 mM tetraethylammonium) or a ryanodine receptor antagonist (100 μM tetracaine) inhibited STOCs (n ≥ 6; P < 0.05 each). Western blots revealed increased expression of BKCa α-subunit protein in Old. Pressure myography revealed no effect of age on SEA maximal diameter, myogenic tone, or paxilline-induced constriction (n = 10–12; P > 0.05). Enhanced functional expression of SMC BKCa-dependent STOCs in Old may represent an adaptation of resistance arteries to maintain functional integrity.
Keywords: aging, resistance arteries, potassium channels, vascular smooth muscle, myogenic tone
large conductance calcium -activated K+ channels (BKCa) contribute to the negative feedback regulation of vascular smooth muscle tone (37). BKCa are activated by membrane depolarization and increases in subsarcolemmal Ca2+ (36, 37), and limit smooth muscle contraction elicited by vasoconstrictors or increases in transmural pressure by attenuating depolarization (20, 37). However, the impact of aging on the function and expression of these ion channels in vascular smooth muscle cells (SMCs) of resistance microvessels remains unclear.
Three different patterns of aging-related changes in expression and function of BKCa have appeared in the literature. Studies of SMCs from rat coronary arteries have consistently shown a decrease in both the expression and function of BKCa with aging (1, 31, 38, 46). In contrast, despite an age-associated decrease in the expression of BKCa α-subunits, BKCa function, as measured by pressure myography, appeared to be enhanced in arterioles from soleus muscle or unchanged in arterioles from gastrocnemius muscle in aged rats (26). Finally, in rat cerebral arteries, BKCa expression and function were unaffected by aging (39). Collectively, these data suggest that there are regional differences in the effect of aging on BKCa expression and function.
In skeletal muscle, blood flow is controlled by changes in tone of both feed arteries and arteriolar networks that they supply (45). However, as shown in resistance networks of the mouse and hamster cremaster muscles, the regulation of BKCa in arterioles differs from that in feed arteries (49, 50), and it is unknown how aging affects BKCa function in SMCs of skeletal muscle feed arteries. A recent study of endothelial tubes freshly isolated from murine superior epigastric arteries (SEAs) suggests that aging increased the function of endothelial KCa (4). Therefore, in the present study, we tested the hypothesis that aging would alter BKCa expression and/or function in SMCs of SEAs, feed arteries that supply skeletal muscles of the anterior abdominal wall, by using patch clamp electrophysiology on freshly isolated SMCs and pressure myography of isolated SEAs. Our results from SEA SMCs of male C57BL/6 mice indicate that 1) aging increases mean whole-cell current and membrane conductance due to increased membrane capacitance, 2) SMCs display BKCa- and ryanodine receptor (RyR)-dependent spontaneous transient outward currents (STOCS), 3) aging increases the amplitude of STOCs at positive membrane potentials while shifting the voltage-frequency relationship to more negative potentials, 4) aging is associated with an increase in the expression of the BKCa α-subunit protein, and 5) irrespective of age, the activity of BKCa appears solely as STOCS. Our data show further that although BKCa contribute negative-feedback regulation of myogenic tone in SEAs, aging has little effect on their integrated function.
METHODS
Solutions.
Physiological salt solution (PSS) contained (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose [pH 7.4, 295 mosmol (kg/H2O)]. Ca2+-free physiological salt solution (Ca2+-free PSS) contained (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 10 HEPES, and 10 glucose [pH 7.4, 295 mosmol (kg/H2O)]. Dissection solution consisted of Ca2+-free PSS containing 10 μM sodium nitroprusside, 10 μM diltiazem, and 1% bovine serum albumin (BSA; Affymetrix: USB, Cleveland, OH). Pipette solution contained (in mM) 100 K-aspartate, 43 KCl, 1 MgCl2, 10 HEPES, 1 EGTA, and 10 glucose [pH 7, 295 mosmol (kg/H2O)]. Stock solution of amphotericin B was made daily at a concentration of 3 mg/50 μl DMSO, and 2 to 3 μl of this stock solution were added to 1 ml of pipette solution to allow whole-cell current recording in the perforated-patch configuration (see Whole-cell current recording).
Animal care and use and microdissection.
All experiments were approved by, and conducted in accordance with the guidelines of, the Institutional Animal Care and Use Committees at Michigan State University and the University of Missouri and were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (2011). Mice were housed on a 12-h:12-h light/dark cycle at ∼23°C with fresh water and food available ad libitum. Male C57BL/6 mice (20–30 g; National Institute on Aging colonies at Charles River Laboratories, Wilmington, MA) were studied at 3 to 4 mo (Young) and 24 to 26 mo (Old). For patch clamp and pressure myography experiments, mice were euthanized by CO2 asphyxiation followed by cervical dislocation. After the abdomen was shaved, a ventral midline incision was made in the skin from the sternum to the pubis to expose the entire abdominal wall and the proximal ends of the SEAs. The abdominal wall containing SEAs was removed and placed in 4°C dissection solution. The inclusion of sodium nitroprusside and diltiazem in this solution helped to maintain SMCs in a relaxed state during dissection and subsequent enzymatic isolation; their actions are reversible and without subsequent effect on ionic currents and vascular reactivity (23). Individual SEAs were hand-dissected while viewing through a stereomicroscope as described (49), cleaned of connective tissue and fat, and cut into segments 400–800 μm long.
For RT-PCR measurements and Western blots, mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (60 mg/kg) and SEAs were isolated as described above. The anesthetized mice were euthanized by intracardiac injection of an overdose of pentobarbital followed by cervical dislocation.
Enzymatic isolation of SMCs for patch clamp recording.
SMCs were enzymatically isolated as described (23). Identical protocols were used to isolate SMCs from SEAs removed from Young and Old. SEA segments were pooled into 1 ml of dissociation solution (dissection solution to which 100 μM CaCl2 was added; see Solutions) containing 1.24 mg/ml papain and 1 mg/ml dithioerythritol. After 35 min incubation at 37°C, segments were transferred into 1 ml of dissociation solution containing 1.5 mg/ml collagenase, 93 Units/ml elastase (EMD Millipore: Calbiochem, San Diego, CA), and 1 mg/ml soybean trypsin inhibitor, and further incubated for 19 min at 37°C. Afterwards, this solution was replaced with 4 ml of ice-cold dissociation solution and incubated for 10 min at room temperature (20–23°C) to allow vessel segments and dissociated cells to settle. The supernatant was then replaced with 1 ml dissociation solution, and vessel segments were triturated using a 1 ml Eppendorf-style pipette to release SMCs. The 1 ml of dissociation solution containing the dissociated SMCs was kept at room temperature for up to 4 h.
Whole-cell current recording.
All recordings were conducted at room temperature. A 100–200 μl aliquot of dissociation solution containing SMCs was pipetted into a recording chamber mounted on the stage of an inverted microscope. Cells were allowed to settle and attach to the bottom of the chamber for 5 min before superfusion with PSS. Pipettes were pulled from borosilicate glass tubes (no. 7052; Garner Glass, Claremont, CA) and fire polished; tip resistances were 2.5–4 MΩ when filled with pipette solution (see Solutions). High resistance, GΩ seals were established by application of light suction, and whole-cell currents were recorded in the perforated patch configuration by using Amphotericin B (see Solutions). Mean access resistance was similar in SMCs from Young (16.55 ± 0.28 MΩ; n = 102 cells from 42 mice) and Old (16.84 ± 0.25 MΩ; n = 93 cells from 39 mice) (P > 0.05). Currents were acquired using a MultiClamp 700B amplifier controlled by a Digidata 1440A data-acquisition system that was interfaced to a personal computer running pCLAMP version 10.2 software (all from Molecular Devices, Sunnyvale, CA). Cell capacitance was determined by application of 10 mV depolarizing pulses from a holding potential of −60 mV using the membrane test utility in pClamp 10.2. For determination of current (I)-voltage (V) relationships, cells were held at −60 mV and then stepped to test potentials from −90 to +60 mV in 10-mV increments for 400 ms at each potential. Currents elicited by these voltage steps were filtered at 5 kHz, sampled at 5 kHz, and digitized. The currents recorded during the last 200 ms of the steps were averaged, normalized to cell capacitance, and used for comparison of I–V relationships and assessment of experimental interventions. Blocker-sensitive current densities (in pA/pF) were obtained by subtraction of current densities in the presence of a blocker from their respective control current densities.
Vessel cannulation and pressure myography.
Isolated SEAs were cannulated at room temperature with glass micropipettes and tied in place with 11-0 nylon suture. The vessels were tested for leaks by pressurization to 80 cmH2O with no flow through the lumen while immersed in Dissection Solution to obtain maximal diameter and adjust their length to approximate that in situ. If the vessels were leak-free, then transmural pressure was reduced to 20 cmH2O and they were superfused with Ca2+-containing PSS, warmed to 37°C and internal diameter measured (9, 22, 49, 50). Only leak-free vessels were studied; no other selection criteria were applied. After a 30-min equilibration period, pressure was increased to 80 cmH2O. Vessels were allowed to develop myogenic tone, and the responses to phenylephrine (10 μM) and paxilline (1 μM) were assessed. At the end of experiments, vessels were superfused with Ca2+-free PSS and cooled to room temperature to define maximal passive diameters.
Real-time PCR.
SMCs used for real-time (RT)-PCR were isolated as described (16). Briefly, freshly dissected SEAs were cannulated with glass micropipettes and blood was flushed from their lumens with dissection solution. Blood-free segments (1 to 2 mm) were then incubated in 1 ml of dissociation solution containing 0.62 mg/ml papain, 1.5 mg/ml collagenase, and 1 mg/ml dithioerythritol and incubated for 30 min at 34°C. Vessel segments were then transferred to enzyme-free dissociation solution at room temperature, and SMCs isolated by gentle trituration of the segments through the heat-polished tip of a micropipette (I.D. ∼100–120 μm). Approximately 500 single, dissociated SMCs were aspirated into the micropipette and transferred to a vial containing 200 μl lysis buffer (RNAqueous Micro Kit; Ambion, Austin, TX).
Total RNA was extracted using the RNAqueous Micro Kit following the manufacturer's instructions. RNA integrity was verified electrophoretically by SYBR safe gel staining (Invitrogen, Carlsbad, CA) and by OD 260 nm/OD 280 nm absorption ratio >1.95 using a Nanodrop 1000 (Thermo Fisher Scientific, Wilmington, DE). Reverse transcription was performed using a high capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA), along with RNase-Free DNase Set (5Prime, Gaithersburg, PA) following the manufacturers′ instructions. Taqman primer assays specific to mouse Glucuronidase-β (Gusb; NM_010368.1, Mm00446953_m1), BKCa α-subunit (BKα; NM_010610.2, Mm00516078_m1), and BKCa β1-subunit (BKβ1; NM_031169.4, Mm00466621_m1) were purchased from Applied Biosystems (Foster City, CA). Duplicate cDNA (2.5 ng) samples were used as templates to perform RT-PCR for 40 cycles on an Applied Biosystems 7900 Fast Real Time PCR System, and the results were averaged. Taqman Fast Advanced Master Mix (Applied Biosystems) was used under the following temperature conditions as set by the manufacturer: 50°C for 2 min, 95°C for 20 s, 95°C for 1 s, and 60°C for 20 s. A minus RT reaction was also performed to ensure no genomic DNA contamination. Linearity and efficiency (E) of amplification for each gene product were verified by creating standard curves, plotting the critical threshold (CT) versus log of the dilution of cDNA. Efficiency-normalized relative abundance (40) was determined for BKα and BKβ1 using Gusb as the reference transcript and was computed as (EGusbCT/EtargetCT) as described (49).
Western blot analysis of BKCa α-subunit protein expression.
For protein extraction, bilateral SEAs from an individual mouse were dissected, flushed to remove residual blood, and pooled. Each pair of arteries was homogenized using a precooled pestle and mortar in 25 μl radioimmunoprecipitation assay solution plus 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Each homogenate was transferred to a precooled microcentrifuge tube, incubated on ice for 30 min, and then sonicated for 45 s. Finally, cellular debris was removed by centrifugation at 8,000 g for 5 min at 4°C. The supernatant was removed from each sample and used to determine the total protein concentration using the BCA protein Assay kit (Thermo Scientific) and a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Rockford, IL). Equal amounts of total protein (2 μg) were dissolved in freshly prepared 2× Laemmli buffer containing β-mercaptoethanol (5% vol/vol) and left at room temperature for 20 min.
To evaluate the relative expression of BKCa α-subunits in SEAs from Old vs. Young, protein extracts were separated by SDS-PAGE on 10% TGX Precast Gels (Bio-Rad, Hercules, CA) and then transferred onto polyvinylidene difluoride membranes overnight at 4°C in an electric field of 25 volts. Each gel/blot contained samples from 2 to 3 Young and 2 to 3 Old along with an arbitrary SEA sample from an aged mouse (calibrator) that was run on each gel/blot to account for variability between experiments. The blots were probed with a mouse anti-BKCa antibody (clone L6/60, 1:500; NeuroMab, Davis, CA) and a mouse monoclonal anti-α smooth muscle actin primary antibody (Sigma-Aldrich). The membranes were incubated overnight at 4°C in TBST (in mM) of 137 NaCl, 3 KCl, 20 Tris·HCl, and 0.1% TWEEN 20 containing the primary antibodies. Finally, specific binding was visualized using anti-mouse IgG (whole molecule)-peroxidase conjugated secondary antibody (1:5,000; Sigma-Aldrich). Bound antibodies for α-BKCa were detected using SuperSignal West Dura ECL Chemiluminescent Substrate (34075; Thermo Scientific), and SuperSignal West Pico ECL Chemiluminescent Substrate (34080; Thermo Scientific) was used for α-actin. Images were collected using a ChemiDoc XRS+ System (Bio-Rad) and analyzed by Image Lab software (Bio-Rad). The ratio of band intensities for α-BKCa to α-actin for each sample was normalized to the α-BKCa-to-α-actin intensity ratio for the calibrator included in each experiment.
Drugs and chemicals.
All compounds were purchased from Sigma-Aldrich (St. Louis, MO) unless noted otherwise. Other than amphotericin (see Solutions, above) and paxilline, drugs were dissolved in distilled water as concentrated stock solutions and were frozen at −20°C until used. Paxilline was dissolved in DMSO. On the day of experiments, stock solutions were thawed and then added to PSS to yield the concentrations indicated in the text.
Data analysis and statistics.
Summary data are expressed as means ± SE. Statistical significance was determined using Student's paired and unpaired t-tests, Welch's corrected t-tests to account for differences in sample variances, or two-way analyses of variance followed by Bonferroni t-tests for post hoc comparisons of means using Prism Software 5.0 (Graphpad Software; San Diego, CA). Prism 5.0 also was used to perform linear and nonlinear regression analyses and to statistically compare fitted lines and curves. The number of observations refers to the number of SMCs or SEA preparations studied from at least 3 animals, with no more than 4 cells studied from any one animal. A value of P < 0.05 was considered statistically significant.
RESULTS
Aging increases mean whole-cell currents and cell capacitance.
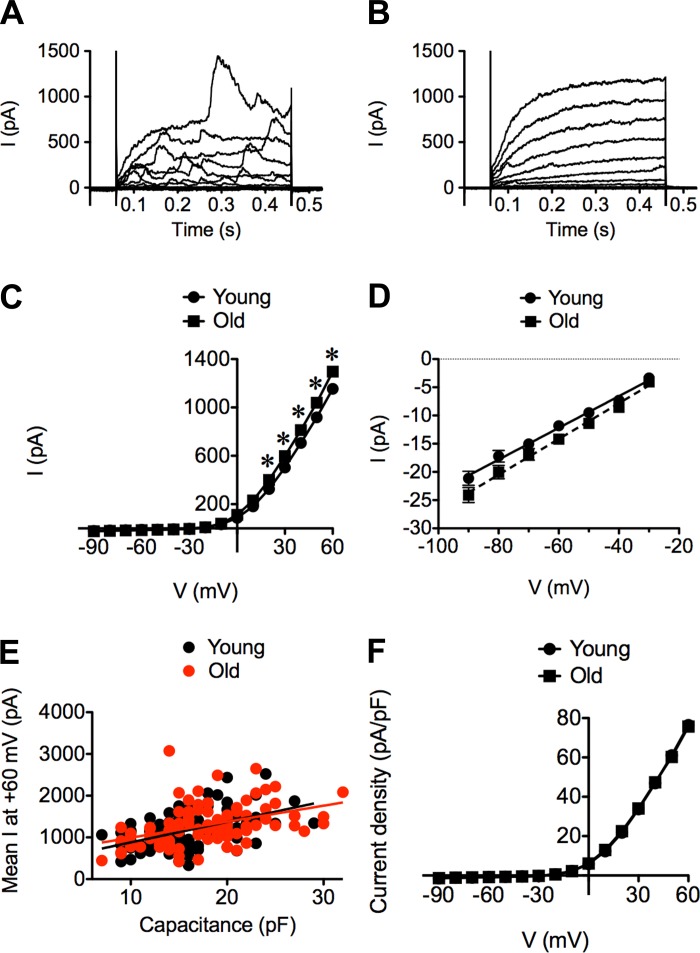
SMCs displayed large (>1 nA at +60 mV), outwardly rectified, whole-cell currents (Fig. 1). The majority of cells studied in both Young (79%, n = 102 cells from 42 mice) and Old (84%, n = 93 cells from 39 mice; P > 0.05 vs. Young) displayed noisy currents (Fig. 1A). The remaining cells displayed currents as shown in Fig. 1B. Thus the presence or absence of “noisy” outward currents was independent of age. However, outward currents recorded at positive membrane potentials (0 to +60 mV) in SMCs from Old were significantly larger than those recorded in SMCs from Young (Fig. 1C). The magnitude of inward currents at negative membrane potentials (−90 to −30 mV) also was significantly greater in Old versus Young (Fig. 1D; P < 0.05). Whole-cell conductance, computed from the linear portion of the I–V relationship between −90 and −30 mV, was significantly greater in Old (317 ± 11 pS) vs. Young (281 ± 10 pS) (P < 0.05; Fig. 1D). Due to cell-to-cell variability in all currents recorded, relatively large sample sizes (n > 20 cells) were required to reliably define differences between Young and Old.
Fig. 1.
Characterization of whole-cell currents recorded from superior epigastric arteries (SEA) smooth muscle cells (SMCs) from 3- to 4-mo-old male C57BL/6 mice (Young) and 24- to 26-mo-old male C57BL/6 mice (Old). A and B: superimposed families of whole-cell currents recorded from SMCs elicited by voltage-step pulses from a holding potential of −60 mV to test potentials from −90 to +60 mV (10-mV increments). A: typical recording in a SMC from Young with “noisy” outward currents. B: typical recording in a SMC from Young with less “noisy” outward currents. Similar current morphologies were present in SMCs from Old (not shown). C: mean current-voltage (I–V) relationship during the last 200 ms at each voltage step for SMCs from Young (n = 102 cells from 42 mice) and Old (n = 93 cells from 39 mice). Outward currents recorded at positive potentials in Old were consistently larger than those recorded in Young. *P < 0.05, Old vs. Young. D: linear component of I–V plots recorded at negative membrane potentials depicted in C were analyzed by linear regression to obtain the mean whole-cell conductance of each group (note difference in ordinate scales). E: mean whole-cell current recorded at +60 mV plotted against the capacitance of the corresponding cell. Data from Young and Old displayed linear relationships with similar slopes. F: expressing the data in C as current densities (in pA/pF) eliminated the difference between the I–V plots for SMCs from Young vs. Old (P > 0.05), suggesting that the differences observed in C and D were due to the greater capacitance of cells from Old vs. Young.
The increase in whole-cell currents measured in SMCs from Old (Fig. 1C) may be due to an increase in cell size, as previously reported for SMCs isolated from murine mesenteric arteries (11). Consistent with this hypothesis, the capacitance of SMCs from Old (17.9 ± 0.5 pF; n = 104 cells from 43 mice) was significantly greater than that measured in SMCs from Young (15.7 ± 0.4 pF; n = 110 cells from 46 mice; P < 0.05). Furthermore, peak whole-cell currents at +60 mV were proportional to cell capacitance in both age groups (Fig. 1E), and the slopes of the respective regression lines were not significantly different (Young = 49 ± 8 pA/pF; Old = 38 ± 9 pA/pF; P > 0.05). Normalization of whole-cell currents shown in Fig. 1C to cell capacitance eliminated statistical differences between the age groups at all membrane potentials (Fig. 1F; P > 0.05). Thus, although aging was associated with an increase in cell capacitance, whole-cell mean current densities (in pA/pF) were unaffected.
STOCs are present in SEA SMCs.
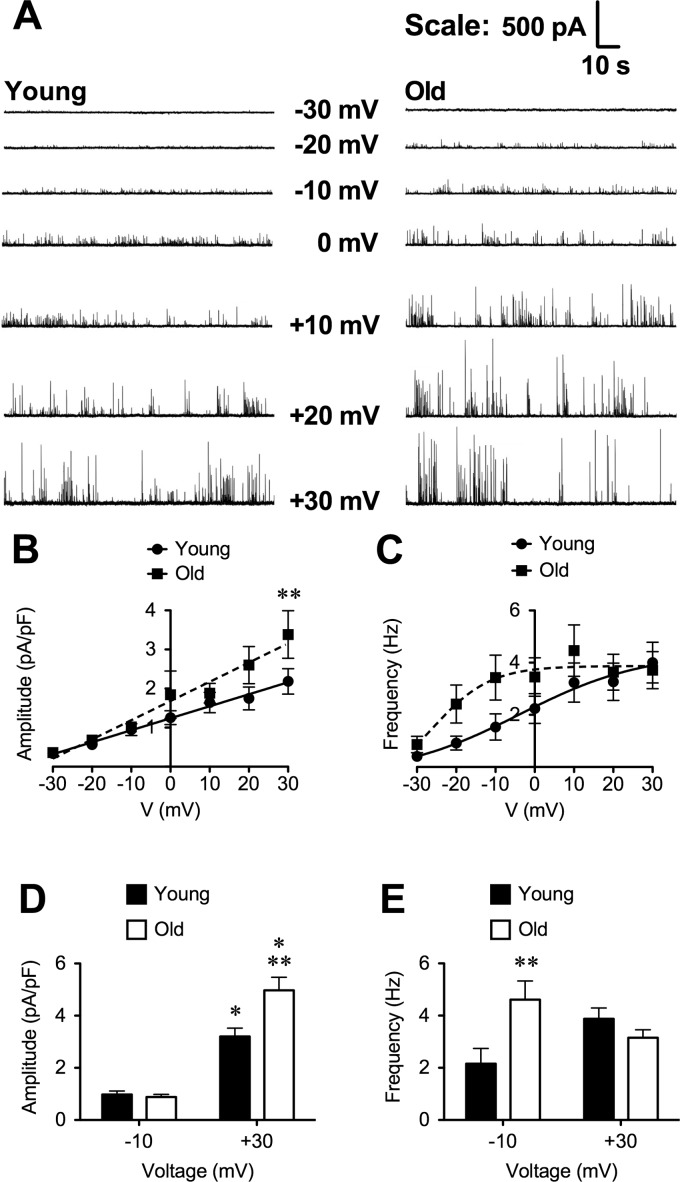
We hypothesized that the noisy variation in outward currents observed in recordings from most SMCs (Fig. 1A) was due to the presence of STOCs (5, 36). Therefore, we acquired gap-free recordings (2–5 min, 5-kHz sampling frequency) of whole-cell currents from SMCs clamped at designated membrane potentials between −30 and +30 mV in 10-mV increments, with the order randomized between cells. Figure 2A shows typical recordings of STOCs in SMCs from Young and Old. As expected, the amplitude of STOCs increased with depolarization, and STOC occurrence increased in frequency at more positive membrane potentials in SMCs from both Young and Old (Fig. 2). The amplitude of STOCs was greater in Old such that the slope of the relationship between voltage and STOC amplitude was significantly greater in Old (0.049 ± 0.004 pA·pF−1·mV−1) versus Young (0.031 ± 0.001 pA·pF−1·mV−1; P < 0.05; Fig. 2B). Aging also was associated with an apparent leftward-shift in the STOC frequency-voltage relationship (Fig. 2C). However, the variability of responses was such that no significant differences between Young and Old could be detected between parameters estimated from Boltzman fits to the data from respective age groups (i.e., the 95% confidence intervals for the parameters overlapped), despite detecting a significant difference between the overall fits to the two data sets (P = 0.039). Analysis of the data shown in Fig. 2C by two-way ANOVA confirmed significant effects of age and voltage on STOC frequency (P < 0.05) with no interaction (P > 0.05). However, subsequent comparison of means using Bonferroni-corrected t-tests did not reveal differences in the means at each voltage, likely due to the low statistical power of this multiple comparison method.
Fig. 2.
Voltage dependence of spontaneous transient outward currents (STOCs) in SEA SMCs. A: representative 2-min gap-free recording of STOCs in SEA SMCs from Young (left) and Old (right) as indicated. Holding potentials (in mV) are indicated with corresponding trace. Note increased STOC amplitude and frequency as cells were depolarized, with greater increases in amplitude for Old. B and C: summary means ± SE (n = 24 cells from 10 mice for Young; n = 19 cells from 10 mice for Old) for amplitude (B) and frequency (C) of STOCs recorded at designated membrane potentials. STOC amplitude increased linearly with depolarization, with a greater slope for Old vs. Young. STOC frequency tended to be higher at negative membrane potentials (−10 and −20 mV). D and E: means ± SE (n = 27–67 cells from 11–28 mice for Young and n = 31–61 cells from 14–30 mice for Old) for STOC amplitude (D) and frequency (E) recorded at −10 and +30 mV. *P < 0.05, −10 vs. +30 mV at the same age; **P < 0.05, Old vs. Young, at the same voltage.
A larger sample size of SMCs supported the findings suggested by the data shown in Fig. 2, B and C. At −10 mV (n = 27 cells from 11 mice for Young and n = 31 cells from 14 mice for Old), there was no significant difference in STOC amplitude between Young and Old (Fig. 2D; P > 0.05). However, STOC frequency (Fig. 2E) was significantly higher in Old versus Young (P < 0.05) at this voltage. At +30 mV (n = 67 cells from 28 mice for Young and n = 61 cells from 30 mice for Old), STOC amplitude was greater in SMCs from Old versus Young (Fig. 2D; P < 0.05). However, STOC frequency did not differ between SMCs from Old versus Young at this positive membrane potential (Fig. 2E). In SMCs from Young and Old, STOC amplitude was significantly (P < 0.05) higher at +30 mV than at −10 mV (Fig. 2D). No significant effect of voltage on STOC frequency was detected in this group of cells (P > 0.05; Fig. 2E).
BKCa and RyR underlie STOCS in SEA SMCs.
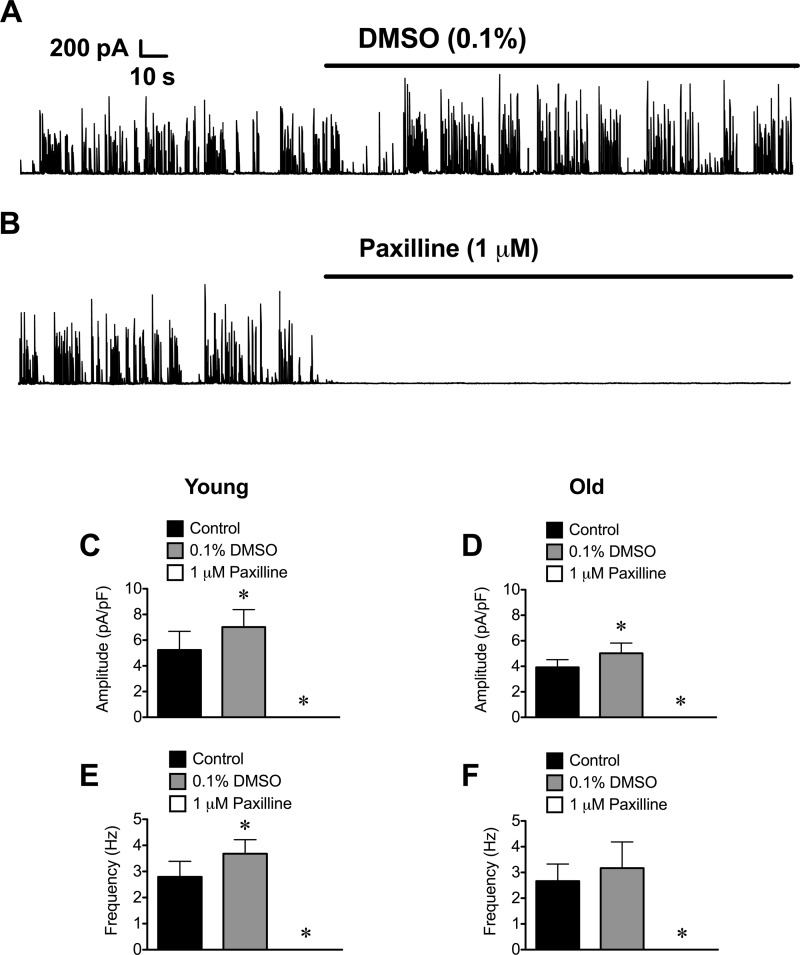
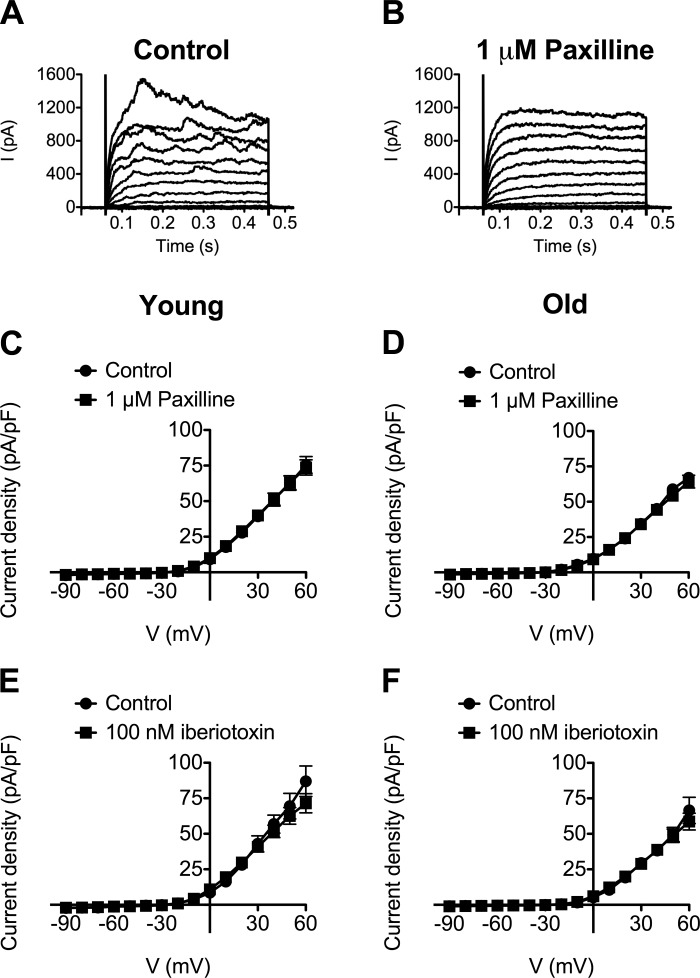
In SMCs from cerebral arteries, STOCs are generated by activation of BKCa following Ca2+ release events (Ca2+ sparks) from a cluster of RyR located near the plasma membrane (36). To test whether this model applied to STOCs recorded in SEA SMCs, we investigated the effect of several known BKCa blockers on STOCs recorded at +30 mV. We found that STOCs in SMCs of both Young and Old were abolished by the indole alkaloid paxilline (1 μM; Fig. 3) (27), with little or no recovery after washout (data not shown). This was not a vehicle effect, because 0.1% DMSO alone produced a significant increase in both the amplitude and frequency of STOCs in SMCs from Young (Fig. 3, A, C, and E; P < 0.05). In SMCs from Old, 0.1% DMSO also significantly increased STOC amplitude (Fig. 3D; P < 0.05), with no significant effect on STOC frequency. From these data we conclude that STOC inhibition was due to the actions of paxilline and not to an effect of DMSO.
Fig. 3.
Blockade of large conductance Ca2+-activated K+ channels (BKCa) with paxilline abolishes STOCs in SEA SMCs. A and B: representative gap-free recordings of STOCs in a SMC from Young held at +30 mV. A: exposure to 0.1% DMSO (vehicle for paxilline) increased STOC amplitude and frequency as shown. B: subsequent exposure to paxilline (1 μM) in 0.1% DMSO abolished STOCs. C–F: data are mean ± SE amplitude (C and D) and frequency (E and F) of STOCs recorded in SMCs from Young (C and E; n = 6 cells from 3 mice) or Old (D and F; n = 6 cells from 3 mice) before application of DMSO (Control), after application of 0.1% DMSO, and after application of 1 μM paxilline in 0.1% DMSO. *P < 0.05 compared with Control.
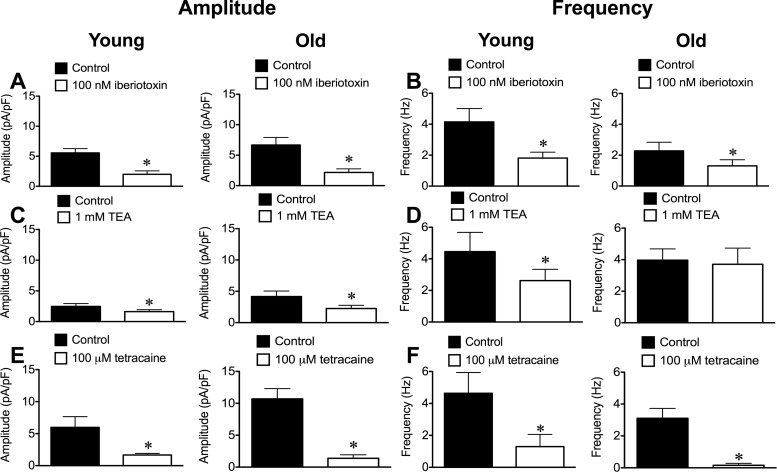
The scorpion venom peptide iberiotoxin (100 nM) (14) significantly (P < 0.05) inhibited STOC amplitude and frequency in SEA SMCs from both Young and Old (Fig. 4, A and B). The quaternary ammonium compound tetraethylammonium (TEA; 1 mM) (29) also reduced STOC amplitude significantly in Young and Old (Fig. 4C), but only inhibited the frequency of STOCs in SMCs from Young (Fig. 4D). Unlike the irreversible effect of paxilline, STOC activity recovered after washout of either iberiotoxin or TEA (data not shown).
Fig. 4.
Inhibition of STOCs in SEA SMCs from Young and Old. BKCa blockers iberiotoxin and tetraethylammonium (TEA) and ryanodine receptor (RyR) antagonist tetracaine inhibited STOCs recorded at +30 mV in SEA SMCs from Young and Old. Data are means ± SE for the amplitude (A, C, and E) and frequency (B, D, and F) of STOCs. A and B: 100 nM iberiotoxin (n = 8 cells from 3 mice for Young and n = 10 cells from 4 mice for Old). C and D: 1 mM TEA (n = 15 cells from 6 mice for Young and n = 12 cells from 6 mice for Old). E and F: 100 μM tetracaine (n = 6 cells from 3 mice for Young and n = 8 cells from 3 mice for Old). *P < 0.05 vs. Control.
To test the role of RyR in STOCs, we also assessed the effects of the RyR antagonist tetracaine (33, 41, 47, 49, 50) on STOC amplitude and frequency. Tetracaine (100 μM) significantly (P < 0.05) and reversibly inhibited both STOC amplitude and frequency in SMCs from both Young (Fig. 4E) and Old (Fig. 4F). Thus, irrespective of age, STOCs in SEA SMCs likely arise from Ca2+ events through RyR and subsequent activation of BKCa.
BKCa do not contribute to mean whole-cell currents in SEA SMCs.
To examine whether BKCa contribute to the mean current-voltage relationship, we repeated the protocols represented in Figs. 1 and 2 in the presence of the BKCa blockers. Neither paxilline (1 μM) nor iberiotoxin (100 nM) significantly affected the mean current-voltage relationship elicited by 10-mV voltage steps between −90 and +60 mV in SMCs from Young or Old (Fig. 5). However, the “noise” of the currents at positive holding potentials was attenuated (Fig. 5B), consistent with the inhibition of STOCs. As observed in gap-free recordings, the effects of paxilline appeared to be irreversible (data not shown). In contrast, current “noise” returned after washout of iberiotoxin (data not shown). Consistent with the lack of significant effect of paxilline and iberiotoxin on whole-cell currents during voltage-step protocols, we also found that these BKCa antagonists had no significant effect on the baseline currents onto which STOCs were superimposed during gap-free recordings. Thus, at +30 mV, baseline current before paxilline was 1.8 ± 0.2 vs. 1.7 ± 0.1 pA/pF in the presence of paxilline (n = 5, P > 0.05) in SMCs from Old. In SMCs from Young, baseline current before paxilline was 1.7 ± 0.2 pA/pF vs. 1.5 ± 0.1 pA/pF in the presence of paxilline (n = 6, P > 0.05). Similarly, baseline current at +30 mV was 1.8 ± 0.2 pA/pF before iberiotoxin vs. 1.7 ± 0.2 pA/pF in the presence of iberiotoxin (n = 10, P > 0.05) in SMCs from Old. In SMCs from Young, baseline current was 1.9 ± 0.2 pA/pF before iberiotoxin vs. 1.8 ± 0.3 pA/pF in the presence of iberiotoxin (n = 8, P > 0.05).
Fig. 5.
Neither paxilline nor iberiotoxin alters mean whole-cell current densities of SEA SMCs. A: representative superimposed families of whole-cell currents recorded from a holding potential of −60 mV stepping, in 10-mV increments, from −90 to +60 mV in a SMC from Old in the presence of 0.1% DMSO (Control). B: exposure to 1 μM paxilline in 0.1% DMSO reduces current noise without affecting mean currents. Data in C–F are mean whole-cell current densities ± SE (in pA/pF) recorded during the last 200 ms of voltage steps. C and D: lack of effect of 1 μM paxilline in SMCs from Young (C; n = 14 cells from 7 mice) or Old (D; n = 14 cells from 5 mice). E and F: lack of effect of 100 nM iberiotoxin in SMCs from Young (E; n = 8 cells from 4 mice) or Old (F; n = 8 cells from 3 mice).
KV channels contribute to mean whole-cell currents in SEA SMCs.
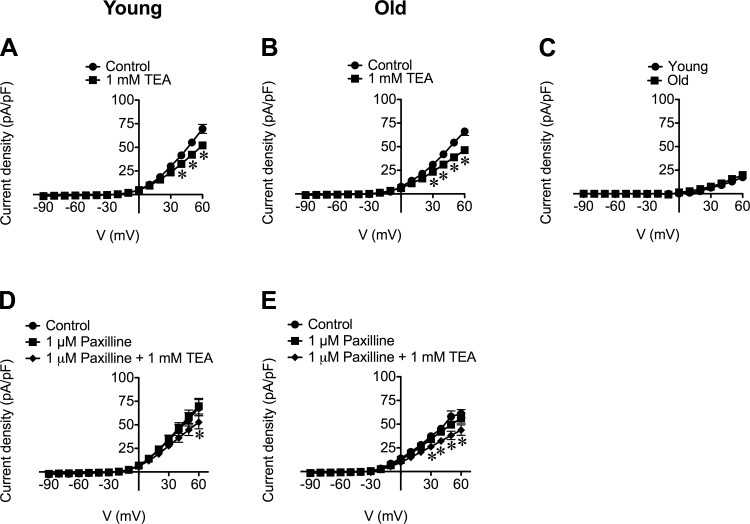
Contrary to the lack of effect of paxilline or iberiotoxin, TEA (1 mM) significantly (P < 0.05) attenuated whole-cell currents in SMCs from both Young and Old at positive holding potentials (Fig. 6, A and B). The TEA-sensitive currents recorded in SMCs from Young and Old were not significantly different (Fig. 6C). This effect of TEA (1 mM) on whole-cell currents was also observed in SMCs pretreated with paxilline (1 μM) (Fig. 6, D and E), suggesting that TEA (1 mM) blocked K+ channels in addition to BKCa. Indeed, the difference in effect between TEA compared with paxilline or iberiotoxin could be explained by blockade of voltage-dependent K+ (KV) channels by TEA, which inhibits some KV channels at 1 mM (32). Consistent with its effects on whole-cell currents recorded during voltage step protocols, TEA (1 mM) significantly reduced baseline currents observed during gap-free recordings: at +30 mV, TEA reduced the baseline current from 1.7 ± 03 pA/pF to 1.4 ± 0.2 pA/pF in SMCs from Old (n = 12, P < 0.05) and from 1.6 ± 0.2 pA/pF to 1.1 ± 0.2 pA/pF in SMCs from Young (n = 15, P < 0.05).
Fig. 6.
TEA inhibits whole-cell current densities of SEA SMCs irrespective of paxilline. Data are mean current densities ± SE (in pA/pF) in the absence (Control) or presence of TEA (1 mM), as indicated. A and B: TEA inhibits whole-cell current density at positive membrane potentials in SMCs from Young (A; n = 32 cells from 14 mice) or Old (B; n = 30 cells from 14 mice). C: TEA-sensitive currents in SMCs from Young were not different from those recorded in SMCs from Old. D and E: TEA inhibits whole-cell current densities in the presence of paxilline in SMCs from Young (D; n = 7 from 4 mice) and Old (E; n = 8 from 3 mice), respectively. *P < 0.05 compared with Control.
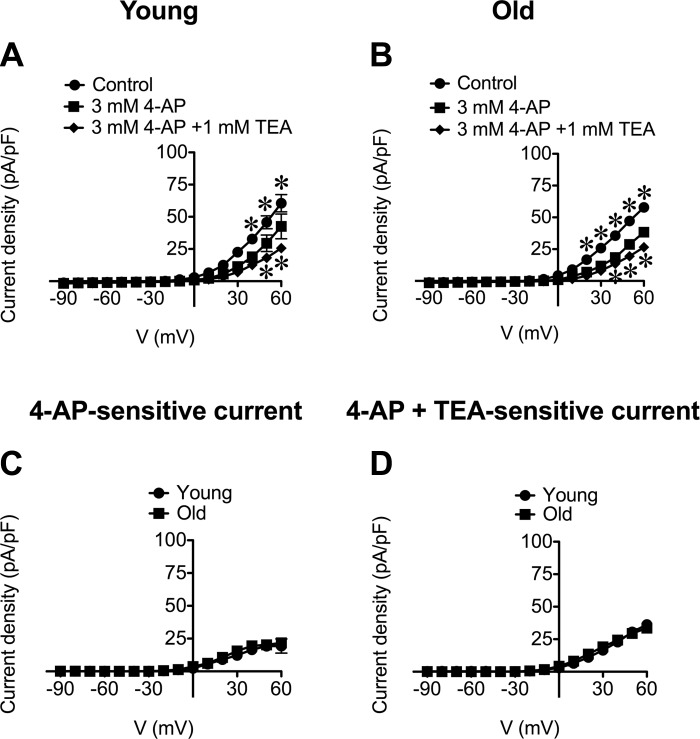
To assess the contribution of KV channels to whole-cell currents in SEA SMCs, we examined the effect of the KV channel inhibitor 4-aminopyridine (4-AP; 3 mM) (32). We found that 4-AP significantly (P < 0.05) inhibited outward currents at positive potentials in SMCs from both Young and Old (Fig. 7, A and B). The magnitudes of the 4-AP-sensitive currents were not different between cells from respective age groups (Fig. 7C). Addition of TEA (1 mM), in the presence of 4-AP (3 mM) produced significant (P < 0.05) additional block of outward currents in SMCs from both Young and Old (Fig. 7, A and B). The total current sensitive to the combination of these blockers was not different between age groups (Fig. 7D) and their cumulative effect suggest that they are each inhibiting distinct ion channels. Washout of 4-AP led to recovery of currents in SMCs from both Young and Old (data not shown). Application of 3 mM 4-AP also significantly (P < 0.05) inhibited outward currents in SMCs pretreated with 1 μM paxilline (data not shown; n = 7 cells from 3 Young and n = 6 cells from 3 Old), further confirming that 4-AP acts on channels other than BKCa.
Fig. 7.
4-Aminopyridine (4-AP) and TEA have cumulative effects on whole-cell currents in SEA SMCs. A and B: data are mean current densities ± SE (in pA/pF) in the absence (Control) or presence of 4-AP (3 mM) or 4-AP + TEA (1 mM) in SMCs from Young (A; n = 17 cells from 6 mice) and Old (B; n = 16 cells from 8 mice). 4-AP (3 mM) inhibited outward currents at positive membrane potentials in SMCs from Young (A) and Old (B) (top, *P < 0.05, Control vs. 4-AP). Subsequent addition of TEA (1 mM) caused further inhibition of current density in SMCs from both age groups (bottom, *P < 0.05, 4-AP vs. 4-AP + TEA). C and D: 4-AP-sensitive currents (C) and 4-AP + TEA-sensitive currents (D) were not different between SMCs from Young vs. Old (P > 0.05).
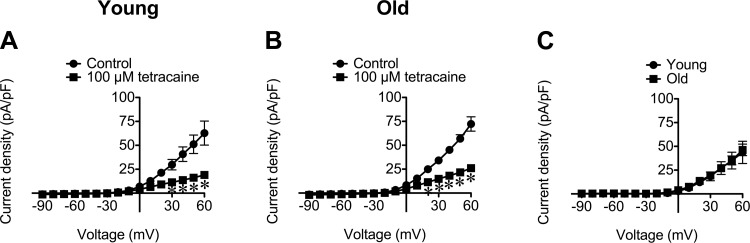
The effects of tetracaine on whole-cell currents also were assessed because this local anesthetic not only blocks RyR but also has been shown to block KV channels in dorsal root ganglion neurons (28). We found, at the same concentration used to inhibit STOCs (Fig. 4, E and F), that tetracaine (100 μM) significantly (P < 0.05) inhibited whole-cell currents in SMCs from both age groups (Fig. 8, A and B), and this effect was reversible upon washout (data not shown). The tetracaine-sensitive currents detected in SMCs from both age groups were not significantly different (Fig. 8C), indicating a lack of effect of aging on the ion channels inhibited by tetracaine. Consistent with its effect on whole-cell currents recorded during voltage step protocols, tetracaine (100 μM) significantly reduced baseline currents observed during gap-free recordings: at +30 mV, tetracaine (100 μM) reduced the baseline current from 2.5 ± 0.2 pA/pF to 2.0 ± 0.1 pA/pF in SMCs from Young (n = 5, P < 0.05) and from 4.0 ± 0.6 pA/pF to 3.3 ± 0.7 pA/pF in SMCs from Old (n = 8, P < 0.05).
Fig. 8.
Tetracaine inhibits outward currents at positive membrane potentials in SEA SMCs from Young and Old. A and B: data are mean current densities ± SE (in pA/pF) in the absence (Control) and presence of tetracaine (100 μM) in SMCs from Young (A; n = 6 cells from 3 mice) and Old (B; n = 7 cells from 3 mice). *P < 0.05 vs. Control. C: tetracaine-sensitive currents were not different between SMCs from Young vs. Old (P > 0.05).
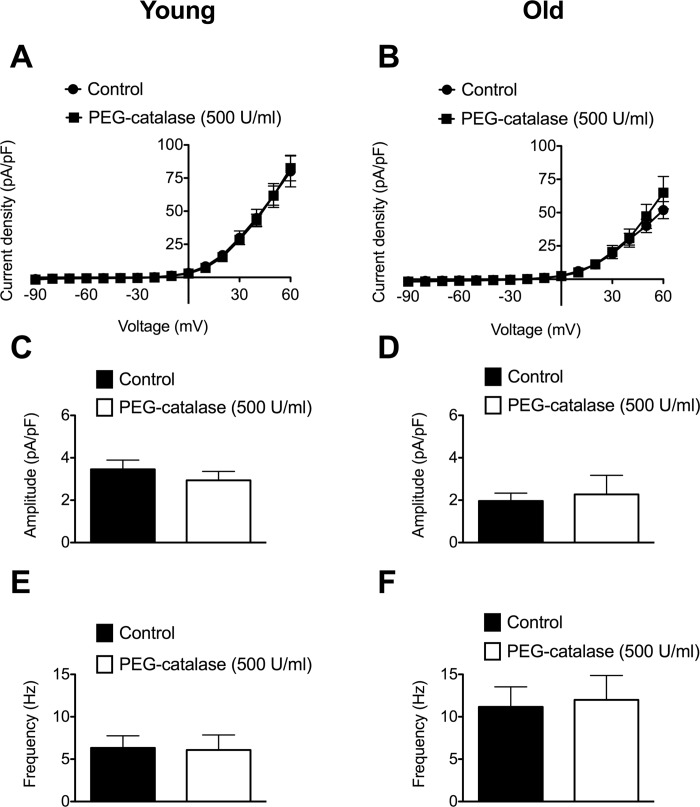
Lack of effect of polyethylene glycol-conjugated catalase on whole-cell currents and STOCs.
Aging has been shown to increase the function of KCa channels expressed in endothelial cells isolated from SEAs, an age-related effect that was eliminated by exposure of the cells to polyethylene glycol-conjugated (PEG) catalase (4). Therefore, we also assessed the effects of PEG-catalase (500 U/ml) on SMC whole-cell currents between −90 and +60 mV, as well as STOC amplitude and frequency assessed at +30 mV. We found that PEG-catalase had no significant effect on whole-cell currents or the properties of STOCs in SMCs isolated from Young or Old (Fig. 9). Consistent with the current densities shown in Fig. 9, A and B, baseline currents during gap-free recordings also were not significantly affected by PEG-catalase: control = 4.4 ± 1.4 pA/pF vs. PEG-catalase = 4.9 ± 1.3 pA/pF in Young (n = 8, P > 0.05) and control = 1.9 ± 0.4 pA/pF vs. PEG-catalase = 2.1 ± 0.4 pA/pF in Old (n = 10, P > 0.05).
Fig. 9.
Lack of effect of polyethylene glycol-conjugated (PEG)-catalase on whole-cell currents and STOCs in SMCs from SEAs. A and B: mean whole-cell current densities ± SE (n = 6 cells from 3 animals for Young and Old) in the absence (Control) and presence of PEG-catalase (500 U/ml). Two-way ANOVA revealed significant effects of voltage on mean current densities (P < 0.05), but no significant effect of PEG-catalase (P > 0.05) in both Young and Old. C–F: mean amplitude (in pA/pF; C and D) or frequency (in Hz; E and F) ± SE (n = 8 cells from 3 mice for Young and 10 cells from 4 mice Old) in the absence (PSS) and presence of PEG-catalase (500 U/ml). Paired t-tests revealed no significant effect of PEG-catalase on amplitude (P > 0.05) or frequency (P > 0.05) of STOCs in Young (C and E) or Old (D and F).
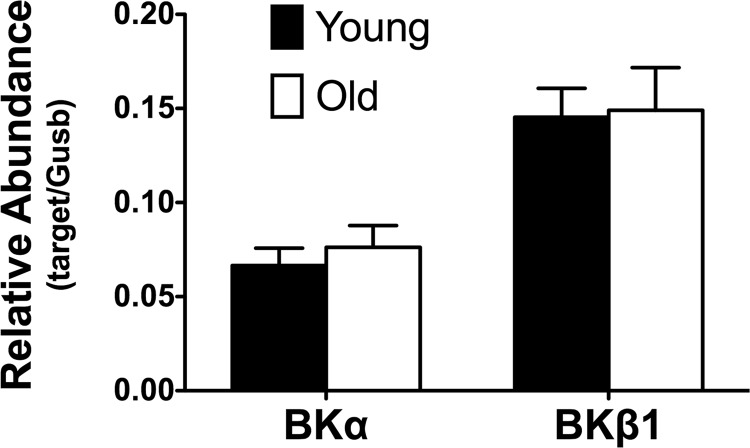
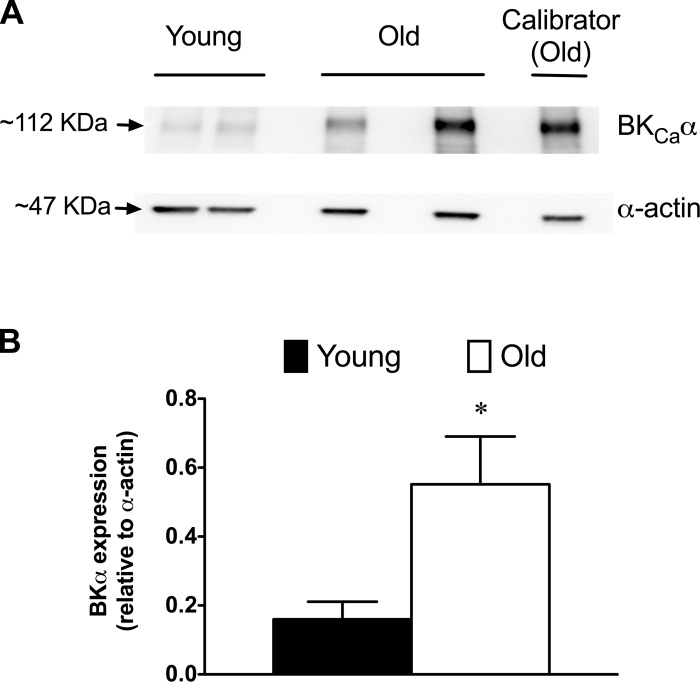
Expression of BKCa subunits in SEA SMCs.
Real-time PCR was used to assess the effects of aging on the expression of BKCa α-and β1-subunit mRNA levels in SMCs isolated from SEAs. We found no significant difference (P > 0.05) in the relative abundance of transcripts for these subunits between SMCs from Young and Old (Fig. 10). However, Western blots of whole-vessel lysates revealed significantly greater expression of BKCa α-subunit protein in SEAs isolated from Old versus Young (Fig. 11).
Fig. 10.
Relative abundance of transcripts for BKCa subunits in SEA SMCs. Data are mean relative abundances ± SE (n = 7 independent samples from 7 mice) for the α- and β1-subunits of BKCa in samples of SEA SMCs, as indicated. There was no significant difference (P > 0.05) in relative abundance of either transcript in Young vs. Old. Gusb, glucuronidase-β.
Fig. 11.
Increased expression of BKCa α-subunit protein in SEA from aged mice. A: typical Western blot results for BKCa α-subunits and α-smooth muscle actin from a representative experiment. Calibrator sample used to normalize data across separate blots is shown for reference. B: quantitation of BKCa α-subunit protein expression relative to α-actin. Data are means ± SE (n = 7 animals/group). *Significantly different from Young, P < 0.05.
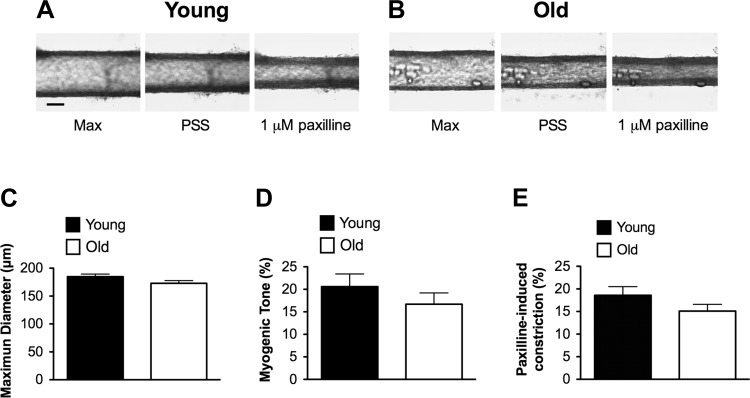
BKCa contribute to the negative feedback regulation of myogenic tone in SEA.
To examine the functional role of BKCa in the regulation of myogenic tone in SEAs, we assessed the effects of paxilline (1 μM) on the diameter of cannulated, pressurized arteries. We found that SEAs from Young and Old had similar maximal diameters (Fig. 12, A-C). When pressurized to 80 cmH2O at 37°C, SEAs developed myogenic tone that was not different between age groups (Fig. 12, A, B, and D). Vasoconstriction induced by paxilline (1 μM) also was not significantly different in SEAs from Young versus Old (Fig. 12, A, B, and E). For reference, paxilline-induced constriction was similar in magnitude to that produced by exposure of SEAs to the α1-adrenergic receptor agonist phenylephrine (10 μM), with no difference between Young and Old in the phenylephrine-induced response (respectively: 20 ± 2% constriction, n = 10 vs. 18 ± 2% constriction, n = 12; P > 0.05). These data indicate that BKCa contribute negative feedback to the regulation of myogenic tone in murine SEAs and that aging has no significant effect on this integrated function in an intact resistance artery.
Fig. 12.
Maximum diameter, myogenic tone, and BKCa function are similar in SEAs from Young and Old. A (scale bar = 100 μm) and B: typical images of SEAs from Young (A) and Old (B) depicting (from left to right in each panel) maximal diameters (Max) at 80 cmH2O in the absence of myogenic tone, development of myogenic tone in PSS containing 1.8 mM Ca2+, and constriction induced by the BKCa blocker paxilline (1 μM). C–E: summary data (means ± SE) for maximum diameters (C), myogenic tone (percent relative to maximum diameter; D), and the response to paxilline (percent constriction; E) for SEAs from Young (n = 10 vessels from 10 mice) and Old (n = 12 vessels from 12 mice). There was no significant difference in maximum diameters of SEAs from Young vs. Old (C; P > 0.05). Similarly, SEAs from Young and Old had no significant difference in the levels of myogenic tone (D; P > 0.05) or constriction induced by paxilline (E; P > 0.05).
DISCUSSION
The major new findings from the present study are: 1) SMCs from a murine skeletal muscle feed artery display BKCa- and RyR-dependent STOCs, 2) aging increased the effect of membrane depolarization on STOC amplitude, 3) aging shifted the effect of membrane depolarization on STOC frequency to more negative membrane potentials, 4) aging was associated with an increase in expression of BKCa α-subunit protein, and 5) aging had no significant effect on the integrated function of SEAs in terms of development of myogenic tone or the contribution of BKCa to the regulation of myogenic tone. We also found that aging was associated with an increase in whole-cell capacitance of SEA SMCs, extending observations from mouse mesenteric arteries (11) to a skeletal muscle feed artery.
SMCs isolated from murine SEAs displayed large, outwardly rectified whole-cell currents at positive membrane potentials (Fig. 1), typical of vascular SMCs (20, 23, 37). The magnitude of the outwardly rectified currents recorded between 0 and +60 mV was significantly greater in cells isolated from Old versus Young mice, as were the small inward currents recorded between −90 and −30 mV, consistent with an increase in SMC membrane conductance. However, this age-associated increase in whole-cell current and membrane conductance was eliminated when the data were normalized to whole-cell capacitance, because cell capacitance was also significantly greater in Old. We interpret this increase in capacitance to indicate an increase in membrane area. Our findings suggest that the functional expression of ion channels responsible for the whole-cell currents is similar per unit area of membrane in SMCs from Young and Old. We speculate that the increase in whole-cell capacitance in SMCs from aged mice results from an increase in SMC size, as has previously been reported in SMCs isolated from mouse mesenteric arteries (11), and suggested in studies of rat mesenteric arteries (8). Thus vascular SMC hypertrophy may be a common response to aging of the arterial system. When taken in light of sustained vasomotor tone and maximal diameters of SEAs with aging, such adaptations in SMCs may serve to maintain functional integrity of the intact vessel. The mechanisms responsible for the age-related increase in SMC membrane capacitance reported here and elsewhere (11) remain to be defined.
Freshly isolated SMCs from SEAs display STOCs.
At membrane potentials more positive than −30 mV, STOCs were observed in SMCs from both Young and Old and were superimposed on more steady, voltage-dependent outward currents (Figs. 1 and 5). Membrane potential of SMCs in pressurized skeletal muscle resistance arteries is ∼−30 mV (13), suggesting that STOCs at this membrane potential are physiologically relevant. These spontaneous events were abolished by paxilline (1 μM; Figs. 3 and 5) and inhibited by iberiotoxin (100 nM; Fig. 4) and TEA (1 mM; Fig. 4), established blockers of BKCa (7, 14, 27). Thus STOCs in SMCs of the SEA are carried by currents through BKCa, consistent with earlier reports (10, 36, 51). STOCs also were inhibited by the RyR blocker tetracaine (100 μM; Fig. 4). These data support the interpretation that RyR also are involved in the generation of STOCs in SMCs of murine SEAs (10, 36, 51).
Surprisingly, neither paxilline (1 μM) nor iberiotoxin (100 nM) significantly inhibited the steady component of the outwardly rectified currents observed in SEA SMCs during voltage-step protocols (Fig. 5). These BKCa antagonists also had no significant effect on the baseline current onto which STOCs were superimposed during gap-free recordings. These findings suggest that within the voltage range studied, and under the conditions of the present study, currents through BKCa are solely expressed as STOCs. This interpretation is consistent with earlier findings in cerebral arteries (36).
The amplitude (in pA/pF) and frequency (in Hz) of STOCs increased as membrane potential was depolarized from −30 to +30 mV (Fig. 2). Given that STOCs arise from currents through BKCa, the voltage-dependence of STOC amplitude is consistent with that of BKCa (7, 21, 44). However, the increase in STOC frequency with depolarization suggests that there is voltage-dependence of discrete events that activate BKCa, for example, Ca2+ sparks through RyR. Previous studies in rat cerebral arteries have shown that depolarization increases the frequency of Ca2+ sparks, which may be triggered by voltage-dependent Ca2+ influx through voltage-gated Ca2+ channels (24). Our data are consistent with this behavior.
Aging increased the effects of depolarization on STOC amplitude (Fig. 2B). At −30 mV, STOC amplitudes were similar in SMCs from young and old mice. However, at +30 mV, STOC amplitude was significantly increased in cells from Old vs. Young (Fig. 2, B and D). With the exception of the data shown in Figs. 3, D and E, and Fig. 9, we observed this age-related increase in STOC amplitude (Figs. 2 and 4). We suggest that the variability and small sample sizes (n = 6 cells from 3 animals per group in Fig. 3 and 8–10 cells from 3 to 4 animals in Fig. 9) explain why this pattern was not observed in the data sets shown in Figs. 3 and 9, where STOC amplitude appeared lower in cells from aged animals. The difference in STOC amplitude shown in Figs. 2 and 4 between age groups might be due, in part, to the increased expression of BKCa α-subunit protein that we observed (Fig. 11). However, this cannot be the sole explanation of the difference, because an increase in density of BKCa expressed per unit membrane capacitance should have been accompanied by a parallel shift upward in the voltage-STOC amplitude relationship, and this predicted shift was not observed. The increased slope of the voltage-STOC amplitude relationship in Fig. 2B could result from a steeper voltage-dependence of BKCa expressed in SMCs from Old, or from an age-related increase in the voltage-dependent amplitude of Ca2+ sparks from which the STOCs likely arise. In rat coronary SMCs, although aging decreased BKCa expression, the voltage- and Ca2+-dependent activation of these channels appeared unchanged (38). The effects of aging on subsarcolemmal Ca2+ signals that affect BKCa activity is not known. Studies in which Ca2+ sparks and STOCs are recorded simultaneously in SEA SMCs from Young and Old are needed to distinguish between age-related changes in the voltage-dependence of BKCa and/or Ca2+ signaling in SEA SMCs.
Aging also tended to shift the voltage-STOC frequency relationship to the left, such that the peak effects of voltage on STOC frequency occurred at more negative membrane potentials in SMCs from Old versus Young mice (Fig. 2C). This conclusion is supported by our observation that STOC frequency was higher in Old compared with Young at −10 mV in a large sample of SMCs (Fig. 2E). The voltage-dependence of Ca2+ sparks (and hence STOCs) may be related to the voltage-dependence of Ca2+ entry through voltage-gated Ca2+ channels (24). Aging is associated with an increase in the current density through these channels in mouse mesenteric artery SMCs (11). Thus the leftward shift in the effects of membrane potential on STOC frequency may be related to altered Ca2+ influx through voltage-gated Ca2+ channels and their subsequent effect on Ca2+ sparks. However, an increase in Ca2+ entry through voltage-gated Ca2+ channels might be predicted to increase myogenic tone in the aged. That we did not observe a difference in myogenic tone in the present study suggests that either the effect of an increase in Ca2+ entry is exactly balanced by an increase in STOCs or that additional compensatory adaptations were present. It is also possible that aging affects the coupling of Ca2+ sparks to BKCa. Again, further studies will be required to investigate these relationships.
In contrast with the lack of effects of paxilline and iberiotoxin on mean whole-cell currents (Fig. 5), TEA (1 mM) and tetracaine (100 μM) significantly attenuated the steady, outwardly rectified currents recorded from SEA SMCs (Figs. 6–8) and reduced the baseline current in gap-free recordings. These inhibitory effects suggest that in SEA SMCs, TEA and tetracaine block KV channels, in addition to blocking BKCa and RyR, respectively. Our observations are consistent with findings in neurons that TEA (1 mM) (32) and tetracaine (100 μM) (28) can inhibit some KV channels. In testing the effect of the known KV channel blocker 4-AP (3 mM), we found that it too significantly attenuated outwardly rectified, whole-cell currents (Fig. 7). These data support our conclusion that KV channels carry the outward currents in SMCs of SEAs. However, the currents inhibited by TEA appeared distinct from those inhibited by 4-AP because their effects were additive (Fig. 7, A and B). These data suggest the presence of multiple KV channel isoforms in SEA SMCs. Further studies will be required to define the molecular identity of the currents inhibited by TEA and tetracaine in these cells. Nevertheless, the inhibition of currents through K+ channels other than BKCa by TEA, and in addition to RyR with tetracaine, indicates limited utility of these two blockers in functional experiments in SEAs. For this reason, we did not investigate the actions of TEA or tetracaine in our pressure myograph studies (Fig. 12).
The TEA-sensitive, 4-AP-sensitive, and tetracaine-sensitive outward currents were similar in SMCs from Young and Old (Figs. 6, 7, and 8). These data collectively suggest that aging does not affect the overall function of the KV channels that underlie respective currents. Such lack of effect of aging on KV channel currents is consistent with findings from pressure-myography studies performed in first-order arterioles from rat gastrocnemius muscle (26), but differ from data obtained in arterioles from rat soleus muscle where KV channel function appeared greater in vessels from aged animals (26). In light of the present data, these collective findings support the hypothesis that there is regional heterogeneity in the adaptation of resistance arteries to aging.
mRNA and protein expression of BKCa subunits.
We found that aging had no significant effect on the expression of mRNA for BKCa subunits in SMCs isolated from SEAs (Fig. 10). In contrast, Western blot analysis of lysates of whole SEAs showed that expression of the BKCa pore-forming α-subunit was significantly increased in Old versus Young (Fig. 11). These data support the often cited lack of correlation between message and protein expression (48). Our finding of increased expression of BKCa α-subunit protein with age differs from results reported for coronary arteries (1, 31, 38, 46) and arterioles from rat soleus and gastrocnemius muscles (26), where decreased protein expression was observed, and from rat cerebral arteries where aging had no effect on BKCa α-subunit protein expression (39). These differences in the effect of aging on protein expression in different vascular beds further indicate that there are important regional differences in the effect of and adaptations to aging in the circulation.
Pressure myography of intact SEAs.
Aging had no significant effect on intact SEAs studied by pressure-myography (Fig. 12). These results are consistent with the lack of effect of aging on first-order arterioles from in rat gracilis muscle (34). Also consistent with studies in aged rats (34), we found that vasoconstriction induced by the α-adrenergic receptor agonist phenylephrine was similar (18–20%; see results) between SEAs from Young versus Old mice. However, in contrast with studies in microvessels of the rat, where aging was associated with decreased myogenic tone (26, 34), we found that myogenic tone was not different between SEAs from Young and Old (Fig. 12D). This difference could mean that there are species (mouse vs. rat), regional (abdominal muscles vs. limb muscles), or vessel order (feed arteries vs. first-order arterioles)-dependent differences in the vascular adaptation to aging.
We interrogated the physiological role played by BKCa in SEA SMCs by examining the effects of paxilline (1 μM) on the diameter of SEAs using pressure-myography. We found that this BKCa antagonist caused significant constriction (Fig. 12E), similar in magnitude to that produced by the α1-adrenergic agonist phenylephrine (10 μM). These data indicate that BKCa contribute significantly to the negative feedback regulation of myogenic tone in murine SEAs, consistent with data reported in other resistance arteries (7, 10, 36, 49, 51). However, our finding that paxilline-induced constriction of SEAs was not significantly affected by aging (Fig. 12E) suggests that any age-related differences in BKCa-dependent STOCs (Fig. 2) do not translate into altered negative feedback regulation of myogenic tone under the conditions of our experiments. It should be noted that STOC amplitude and frequency at −30 mV, likely the membrane potential of SMCs in a pressurized feed artery exhibiting spontaneous myogenic tone (13), were not different between SMCs from Young and Old (Fig. 2, B and C), consistent with the lack of effect of aging on paxilline-induced constriction.
Our observation that paxilline-induced constriction of murine SEA was not significantly affected by aging (Fig. 12E) is consistent with the findings in first-order arterioles from rat gastrocnemius muscle in which iberiotoxin was used to block BKCa (26). Our results differ, however, from data obtained in rat soleus muscle arterioles, where aging was associated with an increase in iberiotoxin-induced constriction (26). This could be related to differences in the muscle fiber type from which the vessels originated (26). The fiber-type composition of mouse abdominal muscles has not been established. However, in rats (17) abdominal muscles are a mixture of type I and type II fibers. We predict a similar fiber-type distribution in murine abdominal muscles based upon complementary fiber type profiles of other skeletal muscles (6). The lack of effect of aging on apparent BKCa function in murine SEAs also contrasts with findings in rat coronary arteries (1, 31), where aging was associated with a decrease in BKCa expression and function. Regional- and/or species-related heterogeneity in response to aging may explain these differences.
Limitations.
We did not explore endothelial cell function in the present study and cannot exclude a modulatory role for endothelial cells in the observed responses. Thus we cannot exclude an effect of aging on endothelial cell function in the present study, as this was not examined. A recent investigation of endothelial cells isolated from SEAs has shown that the resting membrane potential is more negative in cells isolated from Old compared with Young, a difference that could be eliminated by scavenging hydrogen peroxide with catalase (4). In contrast, we found that PEG-catalase had no effect on whole-cell currents or the properties of STOCs in Young or Old. These data suggest that aging may have selective effects on endothelial cells versus SMCs. The lack of effect of aging on myogenic tone in the present study also suggests that the age-related difference in endothelial cell membrane potential and hydrogen peroxide production previously observed (4) did not translate into altered whole-vessel behavior under the conditions of our experiments. In turn, these findings suggest that additional compensatory adaptations to aging may have occurred.
The Western blot experiments that we performed (Fig. 11) do not define the cell-type in which the increased BKCa α-subunit occurred nor whether the detected protein was functionally expressed in the plasma membrane. Although it is likely that much of the BKCa α-subunit that we measured was expressed in SMCs, we cannot exclude expression of this channel in endothelial cells (18, 43) or other cell types associated with SEAs. Further studies will be required to resolve these issues.
Summary and perspective.
Our data support the conclusion that aging is accompanied by an increase in the capacitance and membrane conductance of SMCs in a murine resistance artery. The increase in cell capacitance has functional implications for vasomotor control underlying blood flow regulation. As shown in other resistance arteries, endothelial cells are electrically coupled to the overlying SMCs by myoendothelial gap junctions (13) and may contribute to regulation of vascular tone through ionic currents passed through these heterocellular junctions by affecting smooth muscle membrane potential, e.g., endothelium-dependent hyperpolarization of SMCs producing vasodilation through closure of voltage-gated (L-type) Ca2+ channels (2, 15, 35). With other factors equal, an increase in SMC membrane area would require larger endothelium-derived ionic currents to evoke the same change in SMC membrane potential. The increased requirement for current flow in SMCs from the aged could produce attenuated endothelium-dependent hyperpolarization, contributing to the impaired endothelium-dependent dilation that accompanies aging (25, 30). Increased SMC capacitance also would be expected to attenuate the transmission of electrical signals along the vessel and contribute to impairment of conducted (ascending) vasodilation of feed arteries (19). Longitudinal signaling can be further impaired by greater current leakage along the endothelium, as recently shown in endothelial tubes prepared from SEAs from the same strain of mouse studied here (3, 4). Functionally, impairing the ability of vasodilation to spread along and among branches of the resistance vasculature can explain how blood flow to exercising muscle is restricted with aging (12, 19, 42). Effective vasodilation during blood flow control requires vasomotor tone at rest, which did not differ between Young and Old. Nevertheless, our finding that aging enhanced the functional expression of BKCa-dependent STOCs suggests that this mechanism for modulating myogenic tone in SMCs may represent an adaptive response to maintain their functional integrity. The present study provides new insight into how subtle changes in the electrical properties of individual cells can contribute to alterations in the physiology of vasomotor activity underlying blood flow control.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant RO1-HL-086483. V. Bradley was supported by NHLBI Research Education Grant R25-HL-103156 and the Michigan State University College of Veterinary Medicine Summer Research Program. E. M. Boerman was supported by 5F32 HL-118836. The support of Z. Nourian by Michael A. Hill and NHLBI Grant R01 HL-092241 is gratefully acknowledged.
DISCLAIMER
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.H., E.M.B., Z.N., S.S.S., and W.F.J. conception and design of research; S.H., V.B., E.M.B., Z.N., and W.F.J. performed experiments; S.H., V.B., Z.N., and W.F.J. analyzed data; S.H., V.B., E.M.B., Z.N., S.S.S., and W.F.J. interpreted results of experiments; S.H., V.B., E.M.B., Z.N., and W.F.J. prepared figures; S.H., V.B., E.M.B., Z.N., and W.F.J. drafted manuscript; S.H., E.M.B., Z.N., S.S.S., and W.F.J. edited and revised manuscript; S.H., V.B., E.M.B., Z.N., S.S.S., and W.F.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rebecca Shaw for performing the real-time quantitative PCR to evaluate BKCa subunit transcript levels.
REFERENCES
- 1.Albarwani S, Al-Siyabi S, Baomar H, Hassan MO. Exercise training attenuates ageing-induced BKCa channel downregulation in rat coronary arteries. Exp Physiol 95: 746–755, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiologica 202: 271–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca2+-activated K+ channels. Circ Res 110: 1311–1321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced KCa channel activation. Arterioscler Thromb Vasc Biol 33: 1892–1901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells. J Physiol 381: 385–406, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 7: e35273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Briones AM, Salaices M, Vila E. Mechanisms underlying hypertrophic remodeling and increased stiffness of mesenteric resistance arteries from aged rats. J Gerontol A Biol Sci Med Sci 62: 696–706, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bychkov R, Gollasch M, Ried C, Luft FC, Haller H. Regulation of spontaneous transient outward potassium currents in human coronary arteries. Circulation 95: 503–510, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Corsso CD, Ostrovskaya O, McAllister CE, Murray K, Hatton WJ, Gurney AM, Spencer NJ, Wilson SM. Effects of aging on Ca2+ signaling in murine mesenteric arterial smooth muscle cells. Mech Ageing Dev 127: 315–323, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990 [PubMed] [Google Scholar]
- 15.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation 15: 503–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hijikata T, Wakisaka H, Yohro T. Architectural design, fiber-type composition, and innervation of the rat rectus abdominis muscle. Anat Rec 234: 500–512, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol 299: H1439–H1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588: 2269–2282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson WF. Ion channels and vascular tone. Hypertension 35: 173–178, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson WF, Blair KL. Characterization and function of Ca2+-activated K+ channels in arteriolar muscle cells. Am J Physiol Heart Circ Physiol 274: H27–H34, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol 155: 514–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation 4: 35–50, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol Cell Physiol 274: C1755–C1761, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Jin X, Satoh-Otonashi Y, Zamami Y, Hobara N, Koyama T, Sun P, Li S, Kitamura Y, Kawasaki H. Age-related disappearance of the inhibitory effect of vascular endothelium on agonist-induced vasoconstriction in rat mesenteric vascular beds. J Pharmacol Sci 111: 372–380, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kang LS, Kim S, Dominguez JM, 2nd, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol 107: 389–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB, 3rd, Kaczorowski GJ, Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33: 5819–5828, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Komai H, McDowell TS. Local anesthetic inhibition of voltage-activated potassium currents in rat dorsal root ganglion neurons. Anesthesiology 94: 1089–1095, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Langton PD, Nelson MT, Huang Y, Standen NB. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol Heart Circ Physiol 260: H927–H934, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Long DA, Newaz MA, Prabhakar SS, Price KL, Truong LD, Feng L, Mu W, Oyekan AO, Johnson RJ. Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in aging. Kidney Int 68: 2154–2163, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca2+-activated K+ channels in coronary smooth muscle during aging. Circ Res 88: 210–216, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Mathie A, Wooltorton JRA, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol 30: 13–24, 1998 [DOI] [PubMed] [Google Scholar]
- 33.McCarron JG, Craig JW, Bradley KN, Muir TC. Agonist-induced phasic and tonic responses in smooth muscle are mediated by InsP3. J Cell Sci 115: 2207–2218, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Nausch LWM, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 302: H594–H602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol 559: 849–862, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimaru K, Eghbali M, Stefani E, Toro L. Function and clustered expression of MaxiK channels in cerebral myocytes remain intact with aging. Exp Gerontol 39: 831–839, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizarro G, Csernoch L, Uribe I, Rios E. Differential effects of tetracaine on two kinetic components of calcium release in frog skeletal muscle fibres. J Physiol 457: 525–538, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Riddle MA, Hughes JM, Walker BR. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am J Physiol Cell Physiol 301: C1404–C1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Toro L, Marijic J, Nishimaru K, Tanaka Y, Song M, Stefani E. Aging, ion channel expression, and vascular function. Vascul Pharmacol 38: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Tumelty J, Scholfield N, Stewart M, Curtis T, McGeown G. Ca2+-sparks constitute elementary building blocks for global Ca2+-signals in myocytes of retinal arterioles. Cell Calcium 41: 451–466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Der Kelen K, Beyaert R, Inze D, De Veylder L. Translational control of eukaryotic gene expression. Crit Rev Biochem Mol Biol 44: 143–168, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol 590: 1849–1869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol 587: 3025–3044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]