Abstract
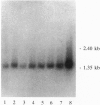
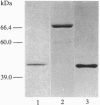
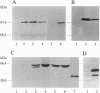
In search of proteins which interact with activated steroid hormone receptors, we screened a human liver lambda gt11 expression library with the glucocorticoid receptor. We identified and cloned a cDNA sequence of 1322 bp that encodes a protein of 274 aa. This protein consists predominantly of hydrophilic amino acids and contains a putative bipartite nuclear localization signal. The in vitro translated receptor-associating protein runs in SDS/polyacrylamide gels with an apparent molecular mass of 46 kDa. By use of the bacterially expressed fusion protein with glutathione S-transferase we have found that interaction is not limited to the glucocorticoid receptor but included other nuclear receptors--most notably, the estrogen and thyroid receptors. Binding also occurs with the glucocorticoid receptor complexed with the antiglucocorticoid RU 38486, with the estrogen receptor complexed with the antiestrogen 4-hydroxytamoxifen or ICI 164,384, and even with receptors not complexed with ligand. Association with steroid hormone receptors depends on prior receptor activation--i.e., release from heat shock proteins. The sequence identified here appears to be a general partner protein for nuclear hormone receptors, with the gene being expressed in a variety of mammalian tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Lutz Y., Bellocq J. P., Chenard-Neu M. P., Rouyer N., Metzger D. Production and characterization of monoclonal antibodies recognising defined regions of the human oestrogen receptor. Hybridoma. 1993 Aug;12(4):391–405. doi: 10.1089/hyb.1993.12.391. [DOI] [PubMed] [Google Scholar]
- Amero S. A., Kretsinger R. H., Moncrief N. D., Yamamoto K. R., Pearson W. R. The origin of nuclear receptor proteins: a single precursor distinct from other transcription factors. Mol Endocrinol. 1992 Jan;6(1):3–7. doi: 10.1210/mend.6.1.1738368. [DOI] [PubMed] [Google Scholar]
- Baniahmad C., Nawaz Z., Baniahmad A., Gleeson M. A., Tsai M. J., O'Malley B. W. Enhancement of human estrogen receptor activity by SPT6: a potential coactivator. Mol Endocrinol. 1995 Jan;9(1):34–43. doi: 10.1210/mend.9.1.7760849. [DOI] [PubMed] [Google Scholar]
- Carpenter T. A., Everett J. R., Hall L. D., Harper G. P., Hodgson R. J., James M. F., Watson P. J. High-resolution magnetic resonance imaging of arthritic pathology in the rat knee. Skeletal Radiol. 1994 Aug;23(6):429–437. doi: 10.1007/BF00204603. [DOI] [PubMed] [Google Scholar]
- Cavaillès V., Dauvois S., Danielian P. S., Parker M. G. Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10009–10013. doi: 10.1073/pnas.91.21.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman F. C., Koenig R. J., Perdew G. H., Massa E., Pratt W. B. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J Biol Chem. 1990 Mar 5;265(7):3615–3618. [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Gagne D., Pons M., Philibert D. RU 38486: a potent antiglucocorticoid in vitro and in vivo. J Steroid Biochem. 1985 Sep;23(3):247–251. doi: 10.1016/0022-4731(85)90401-7. [DOI] [PubMed] [Google Scholar]
- Gehring U., Mugele K., Arndt H., Busch W. Subunit dissociation and activation of wild-type and mutant glucocorticoid receptors. Mol Cell Endocrinol. 1987 Sep;53(1-2):33–44. doi: 10.1016/0303-7207(87)90189-4. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., Tjian R. TBP-TAF complexes: selectivity factors for eukaryotic transcription. Curr Opin Cell Biol. 1994 Jun;6(3):403–409. doi: 10.1016/0955-0674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi S., Marden E., Martin G., MacKay H., Abbondanza C., Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994 Jun 3;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Jacq X., Brou C., Lutz Y., Davidson I., Chambon P., Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994 Oct 7;79(1):107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Choi H. S., Gyuris J., Brent R., Moore D. D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995 Feb;9(2):243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Goldman M. E. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem. 1994 Apr 22;269(16):11945–11949. [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Muchardt C., Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993 Nov;12(11):4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas T. C., Gametchu B., Watson C. S. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995 Mar;9(5):404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Parker M. G. Steroid and related receptors. Curr Opin Cell Biol. 1993 Jun;5(3):499–504. doi: 10.1016/0955-0674(93)90016-j. [DOI] [PubMed] [Google Scholar]
- Pasqualini J. R., Sumida C., Giambiagi N. Pharmacodynamic and biological effects of anti-estrogens in different models. J Steroid Biochem. 1988 Oct;31(4B):613–643. doi: 10.1016/0022-4731(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Petty K. J. Tissue- and cell-specific distribution of proteins that interact with the human thyroid hormone receptor-beta. Mol Cell Endocrinol. 1995 Feb 27;108(1-2):131–142. doi: 10.1016/0303-7207(94)03466-7. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Welsh M. J. Chaperone functions of the heat shock proteins associated with steroid receptors. Semin Cell Biol. 1994 Apr;5(2):83–93. doi: 10.1006/scel.1994.1012. [DOI] [PubMed] [Google Scholar]
- Prins G. S., Birch L., Greene G. L. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991 Dec;129(6):3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Robbins J., Dilworth S. M., Laskey R. A., Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991 Feb 8;64(3):615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Sensel M. G., Binder R., Lazier C. B., Williams D. L. Reactivation of apolipoprotein II gene transcription by cycloheximide reveals two steps in the deactivation of estrogen receptor-mediated transcription. Mol Cell Biol. 1994 Mar;14(3):1733–1742. doi: 10.1128/mcb.14.3.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., Sato T., Krajewski S., Kochel K., Irie S., Millan J. A., Reed J. C. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995 Jan 27;80(2):279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E., Bowler J. Biology and mode of action of pure antioestrogens. J Steroid Biochem. 1988;30(1-6):141–147. doi: 10.1016/0022-4731(88)90086-6. [DOI] [PubMed] [Google Scholar]
- Westphal H. M., Moldenhauer G., Beato M. Monoclonal antibodies to the rat liver glucocorticoid receptor. EMBO J. 1982;1(11):1467–1471. doi: 10.1002/j.1460-2075.1982.tb01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen P. M., Wilcox E. C., Chin W. W. Steroid hormone receptors selectively affect transcriptional activation but not basal repression by thyroid hormone receptors. Endocrinology. 1995 Feb;136(2):440–445. doi: 10.1210/endo.136.2.7835274. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S. K., Peterson C. L., Herskowitz I., Yamamoto K. R. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science. 1992 Dec 4;258(5088):1598–1604. doi: 10.1126/science.1360703. [DOI] [PubMed] [Google Scholar]
- Zeiner M., Gehring U. Cloning of 5' cDNA regions by inverse PCR. Biotechniques. 1994 Dec;17(6):1050-2, 1054. [PubMed] [Google Scholar]
- Zeiner M., Gehring U. Expression screening for interacting proteins using immunochemical detection. Nucleic Acids Res. 1994 Aug 11;22(15):3255–3256. doi: 10.1093/nar/22.15.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner M., Gehring U. Glucocorticoid receptor expression during differentiation of human promyeloic leukemia cells. Cancer Res. 1993 Aug 1;53(15):3513–3517. [PubMed] [Google Scholar]