Abstract
Free vascular endothelial growth factor (VEGF) is undetectable in plasma during human pregnancy. However, studies examining pregnant rats have reported both low (8–29 pg/ml) and high (527–1,030 pg/ml) free VEGF. These discrepancies cast uncertainty over the use of rat models to study angiogenic factors in pregnancy and preeclampsia. This study investigates methodological factors that may explain these discrepancies. Plasma VEGF in nonpregnant, day 7 pregnant, and day 19 pregnant rats was measured using rat and mouse ELISAs (R&D Systems). The rat ELISA detected VEGF in plasma from nonpregnant rats but not in plasma from day 19 pregnant rats. The mouse ELISA detected higher VEGF concentrations than the rat ELISA in every sample tested. This discrepancy was greater in day 19 pregnant rats (median: 2,273 vs. 0 pg/ml) than in nonpregnant (97 vs. 20 pg/ml) and day 7 pregnant (66 vs. 2 pg/ml) rats. Recovery of recombinant rat VEGF (rrVEGF) spiked into plasma from nonpregnant and day 7 pregnant rats was high for the rat ELISA (82–105%) but low for the mouse ELISA (17–22%). The rat ELISA did not recover rrVEGF in plasma from day 19 pregnant rats, suggesting that this ELISA measures free VEGF. The use of the rat versus mouse ELISA likely explains the differences in reported VEGF concentrations in pregnant rats. While the rat ELISA appears to measure free VEGF, plasma concentrations in nonpregnant and pregnant rats are below the assay sensitivity limit. As most previous studies of pregnant rats used the mouse VEGF ELISA, these data should be interpreted cautiously.
Keywords: vascular endothelial growth factor, preeclampsia, pregnancy, sFLT-1, heparin
free vascular endothelial growth factor (VEGF) is undetectable in plasma from most women in late gestation (28, 31), likely due to rapid binding with its soluble receptor fms-like tyrosine kinase-1 (sFLT-1) (6). In contrast, studies in pregnant Sprague-Dawley rats suggest two paradoxically opposite profiles for free VEGF concentrations in late gestation. Some studies have observed low free VEGF concentrations (8–29 pg/ml) (2, 24), similar to human pregnancy. Others report very high free VEGF concentrations (527–1,404 pg/ml) (3, 4, 10, 12, 15) in late-pregnant rats. These discrepancies are important because rat models are often used to study the hypothesis that sFlt-1 released by the placenta contributes to the pathophysiology of preeclampsia (23). This hypothesis has been the subject of intense research for over a decade. sFLT-1 acts as an anti-angiogenic factor by binding the pro-angiogenic VEGF and placental growth factor (PGF) as a nonsignaling decoy (23).
The two opposite profiles of free VEGF concentrations reported in pregnant Sprague-Dawley rats present a quandary for researchers. If free VEGF concentrations in rat pregnancy mirror the pattern observed in human pregnancy, this would give investigators confidence when extrapolating their results to human pregnancy and in using rat models of preeclampsia to study angiogenic dysregulation. However, opposite profiles would suggest that VEGF bioavailability is regulated very differently in rat and human pregnancies. This result would question whether rat models of preeclampsia should be used to study VEGF and related pro- and anti-angiogenic factors in human pregnancy.
The reason for the opposite profiles of free VEGF reported in pregnant rats (i.e., those that were not exposed to interventions used to model preeclampsia) is not known. All studies used commercially available VEGF ELISA kits from R&D Systems. Differences in free VEGF levels do not appear to be explained by differences in the gestational day of measurement, animal supplier, or surgery in the days before euthanasia. Multiple laboratories have reported each profile, and the profiles are consistent within laboratories that have performed these measurements in multiple studies (5, 7, 8, 12–14, 16–18). Our objectives were to investigate possible methodological factors that may contribute to this discrepancy and to accurately quantify plasma VEGF in late-pregnant Sprague-Dawley rats.
MATERIALS AND METHODS
All protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee and followed National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Nonpregnant or timed pregnant female Sprague-Dawley rats (CD IGS stain code 001) from Charles River were received on day 4 or day 12 of gestation. Rats had access to food and water ad libitum and were housed on a 12:12 h dark-light cycle at a constant temperature of 22°C. On day 7 or day 19 of gestation, animals were anesthetized and maintained with 2% isoflurane. A midline laparotomy was made in each rat to expose the abdominal inferior vena cava. The inferior vena cava was cannulated with a 23-gauge needle and whole blood was collected. The animal was euthanized after blood collection. Unless otherwise specified, the needle and syringe were coated with EDTA solution (1 mg EDTA/10 μl phosphate-buffered saline), and the needle was removed before transferring the blood into centrifuge tubes to minimize red cell lysis due to shear stress.
Sample processing.
Blood from day 7 and day 19 pregnant rats (n = 4/group) was drawn into an EDTA-coated syringe and then divided into 1-ml aliquots. Red cells in the first aliquot were subjected to shear stress by transferring the blood into a centrifuge tube without removing the 23-gauge needle. The needle was then removed before transferring the remaining blood into the centrifuge tubes. The sheared aliquot was centrifuged immediately. The nonsheared aliquots were centrifuged immediately or after sitting for 2 or 6 h at room temperature. All samples were centrifuged at 2,000 g for 20 min at 4°C. Plasma was frozen at −80°C.
ELISAs.
Samples were assayed in duplicate using commercially available Quantikine VEGF ELISAs with capture antibodies directed against rat VEGF (R&D Systems RRV00) or mouse VEGF (R&D Systems MMV00). These ELISAs will be referred to as either the rat VEGF ELISA or mouse VEGF ELISA for the remainder of the paper. Previous investigators have interpreted these ELISAs as measuring free (5, 7, 14) or total (20) VEGF; however, R&D Systems does not specify what the kits measure. Therefore, we have simply referred to these measurements as VEGF in the results and discussion. Samples for the sample-processing experiments were assayed on the rat VEGF ELISA. Eighteen previous studies used the mouse ELISA to measure VEGF in samples from pregnant rats (4, 5, 7, 8, 10–18, 21, 25) after an early paper reported validating this ELISA with spike-recovery experiments in rat plasma (15). Two studies used the rat ELISA (2, 24). One sample (centrifuged immediately) from each rat was assayed on the rat and mouse VEGF ELISAs to compare the performance of these two kits. ELISAs were performed in accordance with the instructions, between June and November 2013. For the mouse VEGF ELISA, samples were diluted 1:5 (nonpregnant and day 7 pregnant) or 1:10 (day 19 pregnant) in accordance with kit instructions. The minimum detectable concentration of mouse VEGF that the mouse ELISA can detect is <3.0 pg/ml. Samples did not require dilution for the rat VEGF ELISA. The minimum detectable concentration of rat VEGF that the rat ELISA can detect ranges between 3.4 and 25.0 pg/ml (mean 8.4 pg/ml); therefore, the manufacturer reports a sensitivity of 25 pg/ml for the rat ELISA (http://www.rndsystems.com/Products/RRV00). Both ELISAs are validated for use with EDTA and heparin plasma.
Spike recovery.
Plasma from nonpregnant, day 7 and day 19 pregnant rats (n = 3/group) was analyzed using both the rat and mouse VEGF ELISAs. Samples were tested with and without a spike of recombinant rat VEGF (rrVEGF, 564-RV-010, R&D Systems), which was calculated to increase VEGF concentrations by 980 pg/ml. A control well of spiked assay diluent was run on each ELISA. For each rat, neat and spiked samples were prepared using a single aliquot of plasma. Each spiked plasma sample was prepared in a single tube and incubated for 30 min at room temperature before analysis. The mouse ELISA instructions require a fivefold dilution for plasma samples. Spiked samples were loaded directly onto the rat ELISA platform or pipetted into a separate tube for dilution before loading onto the mouse ELISA.
Heparin.
Heparin decreases free VEGF in nonpregnant humans by increasing sFLT-1 concentrations (20, 29). Other studies have suggested that the mouse VEGF ELISA measures total VEGF, whereas the human VEGF ELISA only recognizes free VEGF that is not bound to sFLT-1 (20). We therefore examined the effect of a heparin bolus on plasma VEGF in pregnant rats measured using both the rat and mouse VEGF ELISAs (see ELISAs). Heparin was administered to day 7 and day 19 pregnant rats at euthanasia (3 rats/group). The inferior vena cava was cannulated with a 23-gauge needle and 1 ml of blood was drawn. The needle and syringe were coated with EDTA solution (1 mg EDTA/10 μl phosphate-buffered saline). After hemostasis was obtained by applying manual pressure to the inferior vena cava, 100 USP units of sodium heparin (Novaplus, Irving, TX) were injected into the inferior vena cava. Manual pressure was again applied to obtain hemostasis. Two minutes after heparin administration, the remaining blood was drawn into a syringe not coated with EDTA. Samples were centrifuged at 2,000 g for 20 min at 4°C. Plasma was frozen at −80°C. Pre- and postheparin samples from each rat were then assayed using the rat and mouse VEGF ELISAs.
Statistical analysis.
Data are expressed as median values with ranges. Differences between paired values measured using the rat and mouse ELISAs (VEGF concentrations, percent recovery of spiked rrVEGF), as well as differences in VEGF concentrations following heparin administration, were calculated and presented also. Nonpregnant, day 7, and day 19 pregnant rats were compared using the Kruskal-Wallis test, followed by the Wilcoxon-Mann Whitney test for post hoc comparisons (9). Paired samples comparisons were done using Wilcoxon signed-rank test where sample sizes were appropriate (n > 5/group) (22). Differences were considered statistically significant if P < 0.05. Statistical analysis was performed using SPSS statistical software (SPSS for Windows, release 21.0, SPSS, Chicago, IL).
RESULTS
Sample processing (rat VEGF ELISA).
We found no evidence that sample processing could explain the large variations in VEGF concentrations reported in the literature. Allowing the blood sample to sit at room temperature before centrifugation did not cause large changes in the VEGF concentrations detected by the rat VEGF ELISA. Plasma VEGF in all conditions was very low for day 7 pregnant rats [n = 4; processed immediately (median, range): 0, 0–2 pg/ml; 2-h incubation at room temperature: 1, 0–3 pg/ml; 6-h incubation at room temperature: 4, 0–8 pg/ml], and undetectable in samples from day 19 pregnant rats (n = 4). Shearing red cells by transferring blood into the centrifuge tube without removing the needle from the syringe also did not cause large changes in VEGF concentration. VEGF concentrations in sheared and nonsheared samples were very low for day 7 pregnant rats [n = 3; no shear (median, range): 0, 0–2 pg/ml; sheared: 4, 0–6 pg/ml], and undetectable in samples from day 19 pregnant rats (n = 3).
VEGF concentrations and rat versus mouse VEGF ELISA.
VEGF levels measured in rat plasma using the rat ELISA were low in nonpregnant rats (median, range: 20, 15–21 pg/ml, Fig. 1). Low or undetectable VEGF concentrations were observed on day 7 of gestation (2, 0–29 pg/ml). No VEGF was detectable in plasma collected from pregnant rats on day 19 of gestation. VEGF concentrations in day 19 pregnant rats were significantly lower than those observed in nonpregnant (P = 0.003) and day 7 pregnant rats (P = 0.025). VEGF concentrations were below the manufacturer's recommended sensitivity limit for the assay (25 pg/ml) in all but one sample.
Fig. 1.
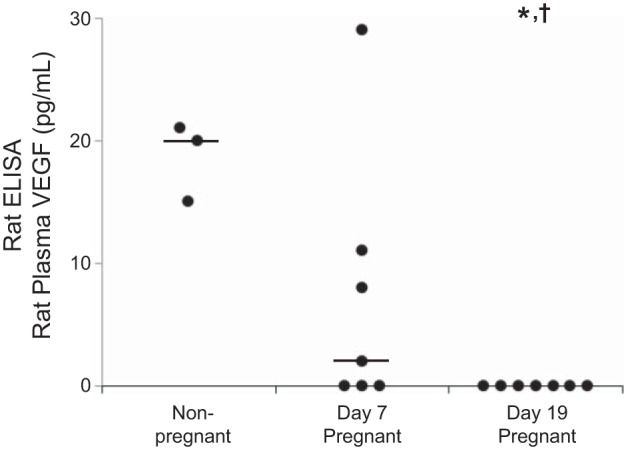
Plasma vascular endothelial growth factor (VEGF) in nonpregnant and pregnant rats measured by the rat ELISA. Circles show values for individual rats. Lines show the group median (0 pg/ml for day 19 pregnant rats). Significant difference from: nonpregnant, *P < 0.05; day 7 pregnant, †P < 0.05.
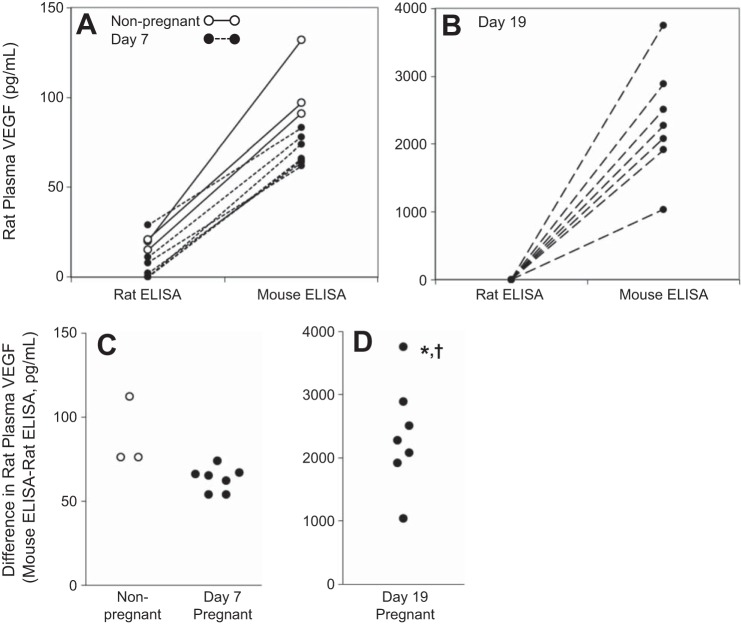
When compared with the rat ELISA, the mouse ELISA detected higher VEGF concentrations in every rat sample tested (P < 0.001). However, the effect was most pronounced in the day 19 pregnant rats (median: 0 vs. 2,273 pg/ml, Fig. 2) compared with the nonpregnant (20 vs. 97 pg/ml) and day 7 pregnant rats (2 vs. 66 pg/ml). The difference between ELISAs was significantly greater in the day 19 pregnant rats than in the nonpregnant (P = 0.016) and day 7 pregnant (P = 0.002) rats. The difference in the measured VEGF concentrations between the rat and mouse ELISAs was also significantly greater in the samples from the nonpregnant rats compared with the day 7 pregnant rats (P = 0.016).
Fig. 2.
Rat plasma VEGF measured by the rat and mouse ELISAs. Lines represent the same sample, run on the rat and mouse ELISAs, in nonpregnant (A, n = 3), day 7 pregnant (A, n = 7), and day 19 pregnant (B, n = 7) rats. The difference between the mouse and rat ELISAs (C, D) was most pronounced in day 19 pregnant rats (note the difference in scale from A and B). The panels are designed to show paired data; hence some overlapping data points are obscured in A and B. Figure 1 shows VEGF concentrations measured on the rat ELISA with no overlapping data points. Significant difference from: nonpregnant rats, *P < 0.5; day 7 pregnant rats †P < 0.05.
Spike-recovery of rrVEGF.
If an assay measures free VEGF, then recovery of spiked VEGF should be high in samples with low sFlt-1 levels (assay diluent, nonpregnant) but low in conditions with high sFlt-1 levels (late pregnant) (6). With the use of the rat VEGF ELISA, recovery of a 980 pg/ml spike of rrVEGF ranged between 82% and 105% when the spike was added to assay diluent or to plasma from nonpregnant or day 7 pregnant rats (Fig. 3). No VEGF was recovered from the plasma on the day 19 pregnant rats. Recovery of rrVEGF in the mouse ELISA ranged between 17% and 22% when the spike was added to the assay diluent or plasma from nonpregnant or day 7 pregnant rats. The recovery in plasma from day 19 pregnant rats was low and may have been more variable (−2% to 20%).
Fig. 3.
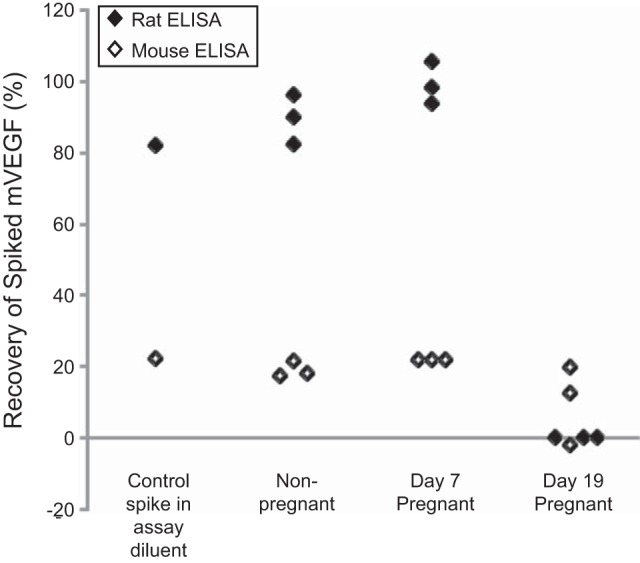
Recovery of spiked recombinant rat VEGF (rrVEGF) in rat plasma using the rat and mouse VEGF ELISAs. Percent recovery of a 980 pg/ml spike of rrVEGF is shown for the same samples run on the rat and mouse ELISAs. Percent recovery differed significantly between the rat and mouse ELISAs (P = 0.04). A single aliquot from each rat was used to prepare neat and spiked plasma samples, which were then run on the rat and mouse ELISAs at the same time. Spiked plasma was prepared in a single tube; then loaded directly onto the rat ELISA or pipetted into a second tube for dilution with assay diluent before loading onto the mouse ELISA. To reduce the complexity of the figure, lines were not used to show pairing.
Effect of a heparin bolus.
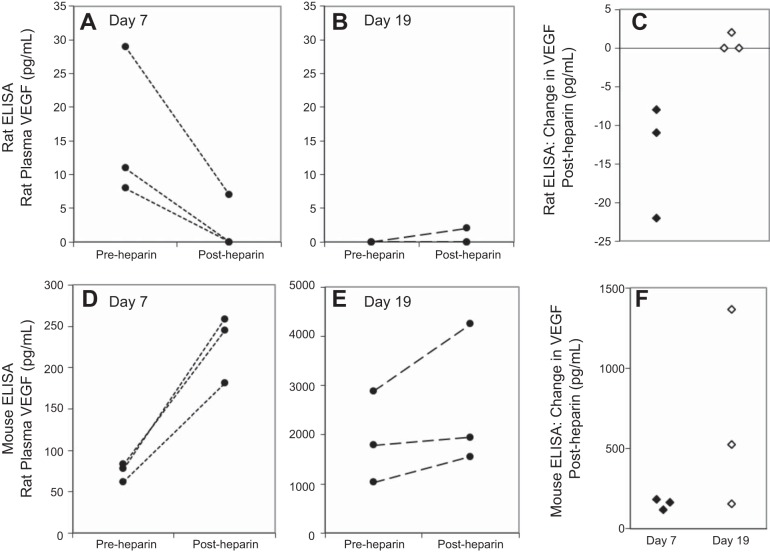
Free VEGF concentrations are expected to decrease after heparin administration, as heparin releases sFlt-1 (20). Data from the rat ELISA were consistent with this expected pattern. VEGF decreased after heparin administration in each of the day 7 pregnant rats. All day 7 rats tested in this experiment had detectable VEGF concentrations preheparin administration (Fig. 4). In the day 19 pregnant rats, plasma VEGF was undetectable before heparin administration. VEGF remained undetectable postheparin in two rats and increased to 2 pg/ml in the third rat. When compared with the rat ELISA, the mouse ELISA detected very different responses to heparin (Fig. 4, P = 0.028 for a main effect of ELISA in pooled data from day 7 and day 19 pregnant rats). Plasma VEGF increased postheparin in all samples from day 7 and day 19 pregnant rats (P = 0.028 for pooled data).
Fig. 4.
Effect of a heparin bolus on rat plasma VEGF measured by the rat and mouse ELISAs. Lines represent samples from the same rat collected immediately before and 2 min postheparin administration run on the rat (A, B) and mouse (D, E) ELISAs. Postheparin changes in VEGF differed between the rat and mouse ELISAs (C vs. F; P = 0.028). There were 3 day 7 pregnant rats and 3 day 19 pregnant rats. Two day 19 pregnant rats had pre- and postheparin concentrations of 0 pg/ml on the rat ELISA. These overlapping data points are not visible in B.
DISCUSSION
Our objectives were to investigate methodological factors that could account for the large discrepancies in reported plasma free VEGF concentrations in pregnant rats and to accurately quantify free VEGF levels in Sprague-Dawley rats in late gestation. We observed that the two opposite patterns of plasma free VEGF concentrations reported in pregnant rats are likely explained by the use of the rat versus mouse ELISAs from R&D Systems. The mouse ELISA detects very high VEGF concentrations, whereas the rat ELISA detects no free VEGF in plasma from late-pregnant rats. When measured using the rat VEGF ELISA, plasma VEGF concentrations in nonpregnant and pregnant rats appear consistent with reported free VEGF concentrations in humans. Spike recovery and heparin administration experiments support the hypothesis that the rat ELISA measures free VEGF in rat plasma, whereas the mouse ELISA gives very different results. However, plasma VEGF concentrations in both nonpregnant and pregnant rats were close to or below the sensitivity limit of the rat ELISA. This suggests that more sensitive methods are needed to accurately quantify free VEGF in rat plasma. Further studies are required to determine what the mouse VEGF ELISA measures in rat plasma, especially in late gestation, given the poor recovery of spiked rrVEGF. Fifteen of eighteen previous studies used the mouse ELISA to measure VEGF in samples from pregnant rats (4, 5, 7, 8, 10–18, 21, 25). Data from these studies should be interpreted with caution. Studies should also determine whether the mouse sFlt-1 and placental growth factor 2 (PlGF-2) ELISAs (R&D Systems) accurately quantify rat sFlt-1 and PlGF-2. Investigators routinely use these ELISAs in rat studies, as R&D Systems does not offer rat ELISAs for these proteins.
The higher plasma VEGF concentrations detected by the mouse ELISA suggest that the mouse ELISA should have higher recovery rates of spiked rrVEGF. However, we observed the opposite result. Recovery was 17–22% when rrVEGF was spiked into assay diluent or into plasma from nonpregnant or pregnant rats. This is consistent with the manufacturer's reported recovery rate of 25% for a 1,000 pg/ml spike of rrVEGF on the mouse ELISA. There are two potential explanations for these findings. The combination high plasma VEGF, but low rrVEGF recovery rates, suggests that the mouse ELISA capture antibody cross-reacts with an unknown substance in rat plasma that is elevated in late gestation. Both ELISAs are reported to detect VEGF-164, which shares 97% amino acid sequence homology between the mouse and rat. However, the mouse ELISA has a polyclonal capture antibody (http://www.rndsystems.com/pdf/mmv00.pdf), whereas the rat ELISA has a monoclonal capture antibody (http://www.rndsystems.com/pdf/RRV00.pdf). Cross-reactivity is often a greater concern with polyclonal antibodies. However, it is also possible that the mouse ELISA may detect a small portion of VEGF bound to sFlt-1. The level of recombinant mouse sFlt-1 that interferes with the assay is an order of magnitude higher in the mouse ELISA (>2,500 pg/ml) compared with the rat ELISA (>200 pg/ml). Interference with rat sFlt-1 has not been tested, as R&D Systems does not sell recombinant rat sFlt-1.
Our experiments cannot determine whether the high rat plasma VEGF concentrations measured by the mouse ELISA are due to cross-reactivity or detection of a small portion of VEGF bound to sFlt-1. Both conditions which increase sFlt-1 (pregnancy and heparin administration) may also increase the concentration of total VEGF and other substances that may interfere with VEGF measurement. Heparin releases many other substances into the circulation; therefore, cross-reactivity is possible. However, several VEGF isoforms have heparin-binding domains and would likely be released by heparin administration. This has not yet been confirmed in humans. The large amounts of sFLT-1 released by heparin would bind any VEGF, making it undetectable using the human VEGF ELISA. Human PlGF-2 and PlGF-4 both have heparin-binding domains, and free PlGF increases following a heparin bolus (29). This increase in free PGF is likely detectable because sFlt-1 binds VEGF with a greater affinity than PGF. Extensive testing would be required to determine whether the high rat plasma VEGF concentrations measured by the mouse ELISA are due to cross-reactivity or to detection of some VEGF bound to sFlt-1. If cross-reactivity were excluded, additional tests would be needed to determine whether the portion of total rat VEGF that the mouse ELISA detected was consistent across different conditions. Without such testing, rat VEGF concentrations measured by the mouse VEGF ELISA are nearly impossible to interpret. The mouse ELISA does not measure total VEGF in rat samples, as the ELISA detected less than 25% of spiked rrVEGF.
Rat plasma VEGF levels are similar to those in late human pregnancy when measured using the rat ELISA. VEGF was detectable in nonpregnant rats but fell below detectable concentrations by day 19 of pregnancy. Free plasma VEGF concentrations are ∼100 pg/ml in nonpregnant women (28) and fall to very low or undetectable levels during pregnancy (28, 31). Spike recovery experiments using the rat ELISA demonstrated 82–105% recovery when rrVEGF was spiked into the assay diluent and into plasma from the nonpregnant or day 7 pregnant rats. However, no rrVEGF was recovered from day 19 pregnant rat plasma. Previous investigators using the R&D Systems human VEGF ELISA observed similar results. The average recovery of spiked VEGF was 86% in 10 samples from nonpregnant women (6). However, no VEGF was recovered in 9 of 12 samples from pregnant women (8–32 wk gestation) (6). Recovery of spiked VEGF in the three remaining pregnant women did not exceed 25% (6). The authors subsequently determined that the human ELISA recognizes free VEGF but not VEGF bound to sFLT-1 (6). The difference in recovery of rrVEGF between the nonpregnant and day 19 pregnant rats suggests that the rrVEGF binds to sFlt-1, and the rat ELISA does not recognize VEGF bound to sFlt-1.
Although our data suggest that the rat kit measures free VEGF, they also show that more sensitive techniques are needed to accurately quantify free VEGF in rat plasma. The manufacturer reports that the sensitivity of the rat ELISA is variable (range: 3.4 to 25.0 pg/ml, mean: 8.4 pg/ml) and lists the sensitivity limit of the kit as 25.0 pg/ml. Blank wells on each rat ELISA plate used for this experiment yielded concentrations of less than 4.5 pg/ml; hence, it is likely that the plates we used were more sensitive than 25 pg/ml. However, only one of the samples that we tested had a VEGF concentration that exceeded 25 pg/ml. All samples tested were below the lowest standard (31.25 pg/ml).
We also examined the effect of a heparin bolus on plasma VEGF in pregnant rats, measured using both the rat and mouse VEGF ELISAs. In nonpregnant humans, heparin decreases free VEGF by releasing sFLT-1 bound to heparin sulfate proteoglycans in the vascular glycocalyx (20, 29). The rat VEGF ELISA results were consistent with this, supporting the hypothesis that the rat ELISA measures free VEGF. VEGF levels decreased after heparin administration in all day 7 pregnant rats with detectable preheparin VEGF concentrations. In the day 19 pregnant rats, heparin had no effect as free VEGF was undetectable at baseline. In contrast, the mouse ELISA detected increases in plasma VEGF concentrations following heparin administration in every rat sample tested. This provides further evidence that the mouse ELISA does not measure free VEGF in rat plasma.
The use of different ELISAs likely explains the discrepancies among previous studies. Most studies do not clearly state which specific kit they used. We contacted study authors to confirm this information. R&D released the mouse VEGF ELISA in November 1996. The rat VEGF ELISA was released in June 2005 (personal communication, R&D Systems) and was not available for the early studies. Consequently, fifteen of eighteen rat studies used the mouse VEGF ELISA (4, 5, 7, 8, 10–18, 21, 25) after an early paper reported validating this ELISA with spike-recovery experiments in rat plasma (15). As shown in Table 1, papers using the mouse ELISA have reported high plasma VEGF concentrations in late-pregnant rats. Two studies that used the rat ELISA reported low plasma VEGF concentrations (2, 24). The third study that used the rat ELISA reported high VEGF concentrations in serum (3). However, VEGF concentrations in serum are higher than those in plasma as VEGF is released from platelets and other blood components during clotting (19).
Table 1.
VEGF Concentrations in late pregnant Sprague-Dawley rats in the control group
| Study | Day of Gestation | Rat VEGF, pg/ml | ELISA§ | Sample |
|---|---|---|---|---|
| Gilbert et al., 2007 (15) | 19 | 833 ± 37‡ | Mouse | EDTA plasma |
| Bridges et al., 2009 (8) | 18 | 780 ± 48† | Mouse | EDTA plasma |
| Gilbert et al., 2010 (18) | 19 | 1,404 ± 90‡ | Mouse | EDTA plasma |
| George et al., 2011 (10) | 19 | 1,030 ± 88† | Mouse | Plasma |
| George et al., 2011 (12) | 19 | 527 ± 86† | Mouse | Plasma |
| Banek et al., 2012 (4) | 19 | 795 ± 72† | Mouse | Plasma |
| Gilbert et al., 2012 (16) | 19 | 850 ± 50‡ | Mouse | EDTA plasma |
| Gilbert et al., 2012 (17) | 19 | 830 ± 70‡ | Mouse | EDTA plasma |
| Banek et al., 2013 (5) | 19 | 915 ± 65‡ | Mouse | Plasma |
| Bauer et al., 2013 (7) | 19 | 925 ± 45‡ | Mouse | EDTA plasma |
| George et al., 2013 (13) | 19 | 287 ± 23‡ | Mouse | Plasma |
| George et al., 2013 (14) | 19 | 1,017 ± 95† | Mouse | Plasma |
| Lillegard et al., 2013 (21) | 19 | 1,004 ± 100‡ | Mouse | EDTA plasma |
| Agunanne et al., 2010 (2) | 17–20 | 29 ± 2* | Rat | Plasma |
| Ramesar et al., 2011 (24) | 20 | 8 ± 1† | Rat | Heparin plasma |
| Bahtiyar et al., 2007 (3) | 21 | 579 ± 14† | Rat | Serum# |
Values are means ± SD.
Mean ± SE or
mean and SE estimated from a figure using Plot Digitizer 2.6.3.
Confirmed with study authors.
VEGF concentrations in serum are higher than those in plasma as VEGF is released from platelets and other blood components during clotting (19). Two additional studies that used the mouse ELISA to measure vascular endothelial growth factor (VEGF) in rat samples were not listed. One study measured VEGF in media from rat placental villous explants (11). The second compared plasma VEGF in lean wild-type Wistar Hannover rats and obese melanocortin-4-receptor heterozygous rats on day 19 of gestation (25). The mean plasma VEGF concentrations in the lean rats was approximately 2,865 pg/ml (25).
Comparisons of VEGF values across studies are complicated by the fact that experiments were conducted over 8 years, and companies do not always inform investigators of kit modifications. Reagents, antibody binding, and other aspects of commercial ELISA performance are often considered proprietary. Commercial ELISAs are attractive because they save researchers time and prevent confusion due to differences between in-house ELISAs. However, the absence of standardized notification procedures for kit modifications detracts from these perceived advantages. sFLT-1 concentrations measured by the R&D Systems human sFLT-1 ELISA dropped substantially in approximately 2007, suggesting that the kit formulations and reagents had changed. Companies should be encouraged to list the date of each change in kit inserts, as well as any known effects on measured concentrations. This would help researchers to compare studies over time. Researchers should repeat validation experiments regularly, especially if they use kits for nonstandard purposes. Manuscript methods sections should include the years in which the ELISAs were run.
Failed attempts to reproduce many basic science experiments and the inability to translate animal experiments into human clinical trials are a growing concern among scientists, academic journals, and funding agencies (1, 27). Proposed solutions often focus on issues such as blinding, randomization, power calculations, and statistical analyses (go.nature.com/oloeip). These solutions are attractive because they apply to many types of studies. However, this study highlights the crucial role of methodological factors and emphasizes the importance of specific reporting of materials and protocols. Most studies investigating VEGF in pregnant rodent models do not clearly state whether they used the rat or mouse ELISA. Recommendations to remove word limits for methods sections (27) should encourage more detailed reporting. This should make it easier, faster, and less expensive to identify methodological differences that contribute to disparate results.
Numerous studies of rat models of preeclampsia have also used mouse ELISAs from R&D Systems to measure rat sFlt-1 and PlGF, because R&D Systems does not offer rat kits for sFlt-1 and PlGF. The current study questions the use of mouse ELISAs for rat samples, even when there is a high degree of homology between proteins. The reported mean sFlt-1 concentrations measured by the R&D Systems mouse sFlt-1 ELISA range between 16 and 2,779 pg/ml for late-pregnant control rats (2, 4). In addition to being highly variable, these concentrations are well below those observed in late-pregnant mice [40,000 pg/ml (30)] using the same ELISA. These large differences suggest that either sFlt-1 concentrations in pregnant rats are much lower than those in mice, or that the mouse ELISA does not adequately recognize rat sFlt-1. Almost all of the published data on angiogenic balance in rat models of preeclampsia are based on mouse ELISAs. These data should be interpreted with caution.
Limitations.
This study has three important limitations. First, we focused on discrepancies in VEGF; hence, sFlt-1 values were not investigated for the reasons described above. Second, other substances that bind VEGF, such as α2 macroglobulin (19), may also interfere with VEGF ELISAs (26). We focused on perturbations that decrease free VEGF by increasing sFlt-1 concentrations. However, it is possible that the rat ELISA detects VEGF bound to substances other than sFlt-1. Third, a recent paper recommends that samples be collected in CTAD (sodium citrate, theophylline, adenosine, and dipyridamole) (31). This prevents platelet activation and the release of angiogenic factors from peripheral blood mononuclear cells and other blood components (31). Although our samples were collected in EDTA, they were centrifuged within 5 min of collection. Plasma VEGF concentrations measured using the rat ELISA did not increase when the blood samples sat for 6 h at room temperature.
Perspectives and Significance
The use of the rat versus mouse ELISA from R&D Systems likely explains the differences in reported plasma VEGF concentrations in pregnant rats. While the rat ELISA appears to measure free VEGF, VEGF concentrations in rat plasma are close to or below the assay sensitivity limit. The rat ELISA may be more useful for other types of rat samples that have higher VEGF concentrations. Investigators should not use the mouse ELISA to measure rat VEGF. Further testing is needed to determine what the mouse VEGF ELISA measures in rat samples, especially in late gestation. The mouse ELISA either cross-reacts with an unknown substance that is elevated in plasma from late-pregnant rats or measures a small portion of VEGF bound to sFlt-1. The mouse ELISA does not measure total VEGF in rat samples, as the ELISA recovered less than 25% of spiked rrVEGF. Most previous studies have used the mouse ELISA to measure free VEGF in pregnant rats. These data should be interpreted with caution. Researchers who use ELISAs should report which specific ELISA they used and the dates on which the ELISA was run. Investigators should repeat validation experiments for commercial ELISAs regularly, especially if they are using the ELISA for a purpose not specified by the manufacturer. Studies should also determine whether the mouse sFlt-1 and PlGF-2 ELISAs (R&D Systems) accurately quantify rat sFlt-1 and PlGF-2, as investigators routinely use these kits in rat studies.
GRANTS
The project described was supported by Award Number P-50 AG44170 (V. D. Garovic, V. M. Miller) from the National Institute on Aging. Tracey Weissgerber was supported by the Office of Women's Health Research (Building Interdisciplinary Careers in Women's Health award K12HD065987).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.L.W., A.M., B.E.K., K.A.B., and V.D.G. conception and design of research; T.L.W., A.M., B.E.K., and K.A.B. performed experiments; T.L.W., A.M., and N.M. analyzed data; T.L.W., S.R.H., W.M.W., V.M.M., and V.D.G. interpreted results of experiments; T.L.W. prepared figures; T.L.W. drafted manuscript; T.L.W., A.M., B.E.K., K.A.B., S.R.H., W.M.W., N.M., V.M.M., and V.D.G. edited and revised manuscript; T.L.W., A.M., B.E.K., K.A.B., S.R.H., W.M.W., N.M., V.M.M., and V.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Eric George for help in determining that the use of the rat versus mouse ELISA was the source of the discrepancy in the literature.
REFERENCES
- 1.Annonymous. Reproducing our irreproducibility. Nature 496: 398, 2013 [Google Scholar]
- 2.Agunanne EE, Uddin MN, Horvat D, Puschett JB. Contribution of angiogenic factors in a rat model of pre-eclampsia. Am J Nephrol 32: 332–339, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bahtiyar MO, Buhimschi C, Ravishankar V, Copel J, Norwitz E, Julien S, Guller S, Buhimschi IA. Contrasting effects of chronic hypoxia and nitric oxide synthase inhibition on circulating angiogenic factors in a rat model of growth restriction. Am J Obstet Gynecol 196: 72 e71–e76, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Banek CT, Bauer AJ, Gingery A, Gilbert JS. Timing of ischemic insult alters fetal growth trajectory, maternal angiogenic balance, and markers of renal oxidative stress in the pregnant rat. Am J Physiol Regul Integr Comp Physiol 303: R658–R664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banek CT, Bauer AJ, Needham KM, Dreyer HC, Gilbert JS. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol 304: H1159–H1165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks RE, Forbes MA, Searles J, Pappin D, Canas B, Rahman D, Kaufmann S, Walters CE, Jackson A, Eves P, Linton G, Keen J, Walker JJ, Selby PJ. Evidence for the existence of a novel pregnancy-associated soluble variant of the vascular endothelial growth factor receptor, Flt-1. Mol Hum Reprod 4: 377–386, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension 61: 1103–1110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22: 564–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel W. Biostatistics: A Foundation for Analysis in the Health Sciences. New York: Wiley, 2009 [Google Scholar]
- 10.George EM, Arany M, Cockrell K, Storm MV, Stec DE, Granger JP. Induction of heme oxygenase-1 attenuates sFlt-1-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 301: R1495–R1500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George EM, Cockrell K, Adair TH, Granger JP. Regulation of sFlt-1 and VEGF secretion by adenosine under hypoxic conditions in rat placental villous explants. Am J Physiol Regul Integr Comp Physiol 299: R1629–R1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George EM, Hosick PA, Stec DE, Granger JP. Heme oxygenase inhibition increases blood pressure in pregnant rats. Am J Hypertens 26: 924–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol 305: R397–R403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 303: R520–R526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K. Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat. Hypertension 60: 1545–1551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem 47: 617–623, 2001 [PubMed] [Google Scholar]
- 20.Kapur NK, Shenoy C, Yunis AA, Mohammad NN, Wilson S, Paruchuri V, Mackey EE, Qiao X, Shah A, Esposito ML, Karas RH, Jaffe IZ. Distinct effects of unfractionated heparin versus bivalirudin on circulating angiogenic peptides. PLos One 7: e34344, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol 56: 91–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann P. Introductory Statistics. New York: Wiley, 2009 [Google Scholar]
- 23.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol 157: 136–140, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 1: e00081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tissot van Patot MC, Leadbetter G, Keyes LE, Bendrick-Peart J, Beckey VE, Christians U, Hackett P. Greater free plasma VEGF and lower soluble VEGF receptor-1 in acute mountain sickness. J Appl Physiol (1985) 98: 1626–1629, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Wadman M. NIH mulls rules for validating key results. Nature 500: 14–16, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Weissgerber TL, Davies GA, Roberts JM. Modification of angiogenic factors by regular and acute exercise during pregnancy. J Appl Physiol 108: 1217–1223, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Weissgerber TL, Rajakumar A, Myerski AC, Edmunds LR, Powers RW, Roberts JM, Gandley RE, Hubel CA. Vascular pool of releasable soluble VEGF receptor-1 (sFLT1) in women with previous preeclampsia and uncomplicated pregnancy. J Clin Endocrinol Metab: jc20133277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, Davisson RL. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 57: 94–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamudio S, Kovalenko O, Echalar L, Torricos T, Al-Khan A, Alvarez M, Illsley NP. Evidence for extraplacental sources of circulating angiogenic growth effectors in human pregnancy. Placenta 34: 1170–1176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]