Abstract
Autonomic and endocrine profiles of chronic hypertension and heart failure resemble those of acute dehydration. Importantly, all of these conditions are associated with exaggerated sympathetic nerve activity (SNA) driven by glutamatergic activation of the hypothalamic paraventricular nucleus (PVN). Here, studies sought to gain insight into mechanisms of disease by determining the role of PVN ionotropic glutamate receptors in supporting SNA and mean arterial pressure (MAP) during dehydration and by elucidating mechanisms regulating receptor activity. Blockade of PVN N-methyl-d-aspartate (NMDA) receptors reduced (P < 0.01) renal SNA and MAP in urethane-chloralose-anesthetized dehydrated (DH) (48 h water deprivation) rats, but had no effect in euhydrated (EH) controls. Blockade of PVN α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors had no effect in either group. NMDA in PVN caused dose-dependent increases of renal SNA and MAP in both groups, but the maximum agonist evoked response (Emax) of the renal SNA response was greater (P < 0.05) in DH rats. The latter was not explained by increased PVN expression of NMDA receptor NR1 subunit protein, increased PVN neuronal excitability, or decreased brain water content. Interestingly, PVN injection of the pan-specific excitatory amino acid transporter (EAAT) inhibitor dl-threo-β-benzyloxyaspartic acid produced smaller sympathoexcitatory and pressor responses in DH rats, which was associated with reduced glial expression of EAAT2 in PVN. Like chronic hypertension and heart failure, dehydration increases excitatory NMDA receptor tone in PVN. Reduced glial-mediated glutamate uptake was identified as a key contributing factor. Defective glutamate uptake in PVN could therefore be an important, but as yet unexplored, mechanism driving sympathetic hyperactivity in chronic cardiovascular diseases.
Keywords: sympathetic, PVN, glutamate, blood pressure
throughout evolution, dehydration (i.e., water deprivation) has been among the most frequently encountered, and potentially life threatening, challenges to homeostasis. Extreme dehydration can lead to neurological complications including confusion, neurosensory disturbances, and seizures (33). In rats, acute dehydration is characterized by increased renin-angiotensin system activity (46), elevated plasma vasopressin (5), increased sympathetic nerve activity (SNA), and elevated arterial blood pressure (ABP) (4, 13, 51). This neurohumoral and cardiovascular profile mimics that occurring in certain chronic diseases, including obesity and certain forms of arterial hypertension (1, 20, 38). Thus understanding acute neural circuitry and cellular adaptive responses to acute dehydration could aid in elucidation of mechanisms contributing to disease-associated chronic autonomic and cardiovascular disturbances.
Activation of the hypothalamic paraventricular nucleus (PVN) contributes to numerous sympathoexcitatory states (24, 29, 44, 48), both acute and chronic. The PVN receives synaptic input from a variety of brain regions including the forebrain lamina terminalis (40, 47), where neurons sense and respond to changes in plasma osmolality, angiotensin II, and other physiological stimuli (12, 34, 40, 41, 47, 53). Parvocellular PVN neurons are functionally diverse, but a major subgroup forms neural pathways targeting major autonomic control regions such as the rostral ventrolateral medulla (RVLM) and spinal intermediolateral cell column. When activated, sympathetic regulatory PVN neurons increase SNA and raise ABP (10, 11, 24, 36, 37). Previous studies indicate that PVN neuronal activation supports SNA and ABP during 48 h dehydration as c-fos expression is elevated (23, 45) and acute neuronal inhibition decreases renal and lumbar SNA as well as ABP (43, 44).
There is abundant evidence that glutamatergic inputs to PVN elevate SNA and ABP (6, 9, 19, 27). For example, bilateral blockade of PVN ionotropic glutamate receptors with kynurenic acid significantly decreases MAP in dehydrated rats (18). Moreover, PVN N-methyl-d-aspartate (NMDA) receptors specifically are implicated in multiple animal models of heightened sympathetic outflow, including models of neurogenic hypertension (28) and heart failure (29). Furthermore, patch-clamp studies demonstrate that glutamate receptors drive spiking of parvocellular neurons (3) and that a tonic NMDA current contributes significantly to net inward current at resting membrane potential (17). In this study, we hypothesized that increased PVN NMDA receptor activation supports SNA and ABP in rats dehydrated by water deprivation for 48 h.
In support of our hypothesis, initial experiments revealed that activity of PVN NMDA receptors, but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, plays a major role in maintaining SNA and ABP during dehydration. We therefore next investigated mechanisms contributing to increased PVN NMDA receptor “tone,” including increased expression of NMDA receptors, elevated PVN neuronal excitability, brain dehydration, and blunted glutamate uptake. These possible mechanisms are consistent with evidence from studies where dehydration was induced by salt loading, which increased glutamatergic synaptic activity, increased glutamate receptor density, and enhanced glutamate receptor-effector coupling in PVN (14). By contrast, we recently reported that glutamate uptake into PVN synaptosomes from 48-h water-deprived rats is increased compared with euhydrated controls (25). Taken together, available evidence led us to hypothesize that homeostatic activation of SNA during dehydration critically depends on an increase of PVN glutamatergic tone mediated by increased NMDA receptor expression and elevated excitability of local sympathetic control neurons, both of which overcome greater transporter-mediated clearance of glutamate.
METHODS
Animals
Male Sprague-Dawley rats (200–300 g, Charles River Laboratory, Wilmington, MA) were housed in a temperature-controlled room (22–23°C) with a 14:10 h light-dark cycle. Tap water and laboratory chow were available ad libitum unless otherwise specified. All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and were performed in strict adherence to procedures set forth in the United States National Institutes of Health Guide to the Care and Use of Laboratory Animals.
In Vivo Experimental Procedures
Rats were assigned to one of two groups: dehydrated (DH) and euhydrated (EH). DH rats were deprived of water, but not food, for 48 h before experiments. EH rats served as controls and had continuous access to both food and water ad libitum. On the day of experiments, rats were anesthetized with an intraperitoneal injection of a mixture of urethane (750 mg/kg) and α-chloralose (75 mg/kg), implanted with catheters in the femoral artery and vein (PE-50 tubing) for recording ABP and administration of drugs, respectively, and prepared for recordings of renal SNA as described previously (2, 39, 43). Rats were artificially ventilated with oxygen-enriched room air and end-tidal CO2 was maintained between 4% and 5%. Adequate depth of anesthesia was monitored by lack of a limb withdrawal reflex in response to a noxious pinching of the foot. Rats were then paralyzed with gallamine triethiodide (20 mg/ml, 0.25 ml/h iv), and adequacy of anesthesia was determined thereafter by lack of a pressor or sympathoexcitatory response to a foot pinch. Supplemental anesthesia (10% of initial dose) was given as needed. Body temperature was maintained at 37 ± 1°C. Recorded variables (see above) were allowed to stabilize for 30 min after surgery before initiating experimental protocols.
PVN microinjections were performed as previously described by our laboratory (2, 9, 39, 43, 44). Injection sites were marked with 0.2% rhodamine beads added to microinjected solutions. At the conclusion of experiments, brains were removed and postfixed in 4% paraformaldehyde for 3–6 days and sectioned at 50 μm on a sliding microtome.
Hematology
Plasma osmolality, hematocrit, and plasma protein were determined in a subset of animals as previously described (21, 41, 43). Blood samples (500 μl) were taken and centrifuged (10,000 g, 60 s). Plasma osmolality was measured using a freezing point depression osmometer (model 3320, Advanced Instruments, Norwood, MA). Hematocrit was determined from capillary tubes using a Lancer microhematocrit reader. Plasma protein concentration was determined by refractometry.
Effects of Glutamate-Receptor Blockade
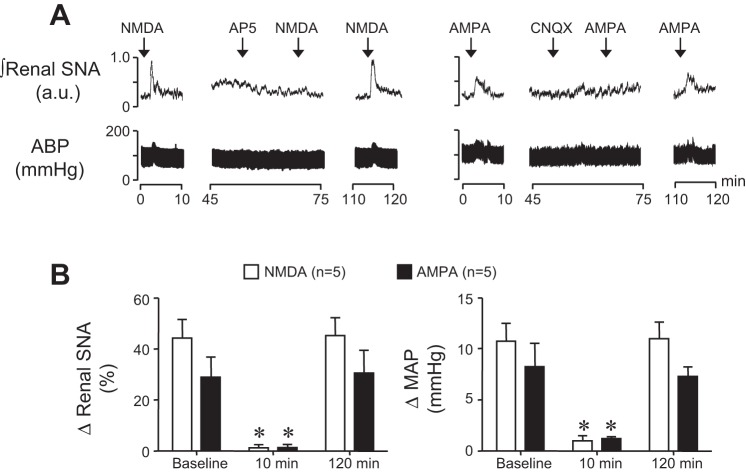
To determine the contribution of PVN NMDA and non-NMDA receptors during dehydration, rats were prepared as described above. After baseline renal SNA, ABP, and heart rate were recorded for 10 min, the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (AP5, Sigma; 3 nmol/50 nl) or the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Sigma; 125 nmol/50 nl) was bilaterally microinjected into the PVN. Variables were then recorded for 60 min. In separate experiments (see Fig. 1), it was determined that a 200 pmol/50 nl dose of AP5 and a 12.5 nmol/50 nl dose of CNQX effectively abolished responses to EC50 doses of NMDA and AMPA, respectively. The duration of AP5 and CNQX efficacy was determined by injecting NMDA and AMPA 10 and 120 min following microinjection of antagonists.
Fig. 1.
A: representative examples of renal sympathetic nerve activity (SNA) and arterial blood pressure (ABP) responses to paraventricular nucleus (PVN) injection of N-methyl-d-aspartate (NMDA) (left) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (right) at baseline, 10 min post-, and 120 min postmicroinjection of (2R)-amino-5-phosphonopentanoate (AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), respectively. B: summary data of peak changes of renal SNA and mean arterial pressure (MAP) in response to PVN NMDA (n = 5) and AMPA (n = 5) at baseline, 10 min post-, and 120 min postmicroinjection of AP5 and CNQX, respectively. Note that AP5 and CNQX nearly abolished responses to their respective receptor agonists NMDA and AMPA at 10 min. Responses recovered after 120 min. *P < 0.05 vs. baseline response.
NMDA Dose-Response Curves
To determine the impact of dehydration on responses to NMDA, dose-response curves were generated in DH and EH rats prepared as described above. After a 5-min baseline recording, graded doses of NMDA (50, 100, 200, and 400 pmol/50 nl) were unilaterally microinjected into the PVN in random sequence. Each injection was made over a period of 20 s, and injections were separated by an interval of at least 20 min to prevent receptor desensitization and ensure that recorded variables had returned to baseline.
Brain Water Content
To determine effects of 48 h dehydration on brain water content, rats were anesthetized with 5% isoflurane and brains quickly removed. Individual brains were weighed, placed in a desiccator, and weighed again each day for a total of 13 days until all water had been removed. Water content was calculated as the difference between brain weight before and after desiccation.
Protein Expression in PVN
Tissue collection.
Rats were deeply anesthetized with 5% isoflurane, and brains were quickly removed, placed in isopentane on dry ice, and cut using a chilled brain matrix. Sections (0.5 mm thick) of interest were removed and, using an 18-gauge needle, the PVN was removed and frozen in liquid nitrogen. Samples were immersed in RIPA buffer (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% sodium deoxycholate) to which 2% sodium dodecyl sulfate (SDS) was added along with 1 mM freshly prepared protease inhibitor phenyl methylsulfonyl fluoride (PMSF). Samples were stored at −20°C until use.
Western blot analysis.
PVN samples were thawed and protein concentration of each sample was determined using Pierce BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer's directions. Each sample of 25 μg was mixed with an equal volume of 2× loading buffer (100 mM Tris·HCl, pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol, 2% β-mercaptoethanol) and incubated at 95°C for 15 min before use. Samples were separated by SDS-PAGE (8% gel) and then transferred to a polyvinylidene membrane (Bio-Rad) by electrophoresis at constant 300 V for 45 min. The membrane was incubated in blocking buffer (7% nonfat milk in TBS) for 1 h at room temperature and incubated overnight at 4°C with a monoclonal rabbit NMDAR1 antibody (1:600, Millipore) or a polyclonal rabbit excitatory amino acid transporter (EAAT) 2 antibody (1:600, Santa Cruz). After overnight incubation, membranes were washed (3×, 5 min) in TBS containing 0.05% Tween-20, incubated with goat anti-rabbit IgG (1:1,000, Santa Cruz Biotechnology) or donkey anti-rabbit IgG (1:4,000, GE Healthcare) conjugated to horseradish peroxidase at room temperature for 1 h, incubated in ECL-plus for 5 min (GE Healthcare), and exposed to X-ray film. As a loading control, the endogenous α-tubulin was detected by mouse monoclonal α-tubulin antibody (1:600, Santa Cruz Biotechnology). NMDAR1 and EAAT2 band intensity for each sample was quantified using NIH ImageJ (http://rsbweb.nih.gov/ij) and normalized to the respective α-tubulin band intensity.
Electrophysiological Experiments in Brain Slices
Retrograde labeling of PVN-RVLM neurons.
PVN neurons were retrogradely labeled by microinjecting (100 nl) undiluted rhodamine-containing microspheres (Lumafluor, Naples, FL) into the ipsilateral RVLM of isoflurane (3% in O2)-anesthetized rats as previously described (7, 8). Briefly, rats were placed in a stereotaxic frame, and a small craniotomy was performed to remove bone overlying the cerebellum. A glass micropipette was lowered into the pressor region of the RVLM based on the following coordinates referenced to bregma: −12.7 mm caudal, 8.9 mm ventral, and 1.8 mm lateral. The location of the tracer was verified postmortem in histological sections through the RVLM.
Preparation of brain slices.
Rats were anesthetized with isoflurane (3% in O2) and rapidly decapitated. Brains were removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM) 144 NaCl, 2.5 KCl, 1 NaH2PO4, 2.5 CaCl2, 10 HEPES, and 10 d-glucose. Osmolality and pH were adjusted to 295–300 mosmol/kg H2O and 7.4, respectively. Coronal slices through the PVN were cut to a thickness of 300 μm on a vibrating microtome (Leica VT 1000S; Leica, Nussloch, Germany). Slices were incubated at room temperature (24–26°C) for ≥1 h in continuously gassed aCSF. For recording, slices were transferred to a glass-bottomed recording chamber and continuously perfused with room temperature aCSF at 2–3 ml/min. Slices were visualized through an upright microscope (E600FN, Nikon) equipped with DIC optics, epi-fluorescence, infrared (IR) filter, and an IR-sensitive video camera (Roper Scientific Photometrics CoolSnap ES, Tucsan, AZ). Retrogradely labeled PVN-RVLM neurons were identified under epi-fluorescence and differential interference contract (DIC) optics before patching.
Electrophysiology.
Patch electrodes were pulled (Flaming/Brown P-97, Sutter Instrument, Novato, CA) from borosilicate glass capillaries and polished to a tip resistance of 4–7 MΩ when filled with pipette solution containing (in mM) 130 K-gluconate, 10 HEPES, 5 EGTA, 1.0 MgCl2, 1.0 NaCl, 2.0 Na2ATP, and 0.5 Na2GTP (osmolality: 280–285 mosmol/kg H2O; pH 7.2). Whole cell current-clamp recordings were made using an Axopatch 200B amplifier and pCLAMP software (v10.0, Axon Instruments, Union City, CA). After a gigahom seal and the whole cell configuration were achieved, cell capacitance, access resistance, and resting membrane potential (Vm) were recorded until stable. Signals were filtered at 1 kHz, digitized at 10 kHz (Digidata 1322A, Axon Instruments), and saved for offline analysis.
Neuronal excitability was tested in current-clamp mode by adjusting Vm to −80 mV by injection of negative current as previously described (8). Current pulses were delivered in increment steps of +50 pA, each for a duration of 800 ms.
Blockade of PVN Glutamate Transporters
To determine the significance of PVN glutamate uptake, rats were prepared as described above. The glutamate transport blocker dl-threo-β-benzyloxyaspartic acid (TBOA, 250 pmol/50 nl; Tocris) was bilaterally microinjected into the PVN of DH and EH rats. The contribution of NMDA receptors to the sympathoexcitatory response to glutamate transport blockade was determined by bilateral microinjection of the NMDA receptor antagonist AP5 (3 nmol/50 nl) 10 min before TBOA microinjection.
Data Analysis
All data are expressed as means ± SE. Changes in integrated SNA were calculated by subtracting background noise following hexamethonium administration (30 mg/kg iv). For all variables, 2-min segments at each time point were compared with three 2-min baseline period measurements. Statistical tests were performed using Prism software (v5.0, Graphpad), and data were analyzed by one- or two-way ANOVA. Post hoc tests were performed with independent or paired t-tests, with a layered Bonferroni correction. Differences between means were considered significant at P > 0.05.
RESULTS
NMDA Receptor Blockade in PVN Decreases Renal SNA and MAP in Dehydrated Rats
As expected, 48 h dehydration significantly (P < 0.05) elevated plasma osmolality, hematocrit, and plasma protein (Table 1). To determine the contribution of PVN NMDA and AMPA receptor activity in support of renal SNA and ABP in DH rats, we first established working doses of the receptor antagonists AP5 and CNQX that effectively blocked responses to their respective receptor agonists NMDA and AMPA. Figure 1A shows a representative response to NMDA (200 pmol/50 nl) before and after AP5 (3 nmol/50 nl) and AMPA (12.5 pmol/50 nl) before and after CNQX (125 pmol/50 nl). Note that 10 min after AP5 and CNQX injections, responses to NMDA and AMPA, respectively, were significantly blunted but recovered within 120 min. Summary results (n = 5/group) are shown in Fig. 1B.
Table 1.
Baseline hematological values
| Group | n | Posm, mosmol/kg H2O | Hematocrit, % | Plasma Protein, g/dl |
|---|---|---|---|---|
| Euhydrated (EH) | 8 | 303 ± 4 | 53 ± 1 | 5.2 ± 0.1 |
| Dehydrated (DH) | 8 | 318 ± 2* | 60 ± 1* | 6.5 ± 0.1* |
Values are means ± SE; n, number of rats.
Posm, plasma osmolality.
P < 0.05 vs. EH
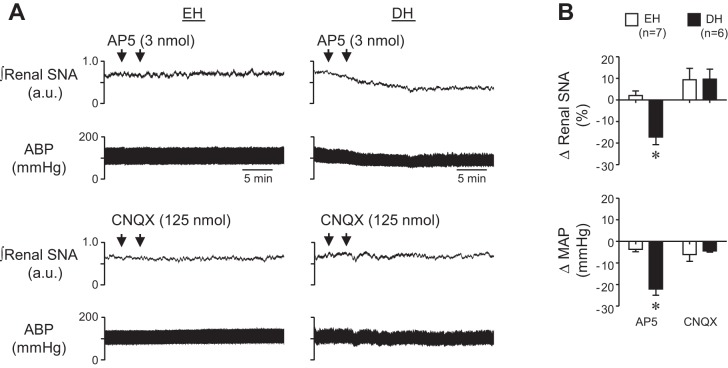
Figure 2A shows representative responses to AP5 and CNQX microinjection into the PVN of an EH (left) and DH (right) rat. Peak changes are summarized in Fig. 2B. Blockade of PVN NMDA receptors with AP5 significantly reduced renal SNA and resting MAP in DH rats. In contrast, AP5 had no effect in EH controls and microinjection of CNQX had no effect in either group. Recorded variables returned to baseline within 60 min after AP5 injection into the PVN of DH rats. These data are consistent with evidence that dehydration strongly activates forebrain glutamatergic inputs to PVN that drive heightened neuronal discharge to increase SNA.
Fig. 2.
A: representative examples of renal SNA and ABP responses to PVN microinjection of AP5 (top) and CNQX (bottom) in euhydrated (EH) and dehydrated (DH) rats. B: summary data of peak changes of renal SNA and MAP after bilateral microinjection of AP5 or CNQX into PVN of EH (n = 7) and DH (n = 6) rats. Whereas AMPA receptor blockade had no effect in either EH or DH rats, blockade of PVN NMDA receptors significantly reduced ongoing renal SNA and MAP in DH rats only. *P < 0.05 vs. EH.
PVN NMDA Dose-Response Curve is Shifted Upward in DH Rats
Because blockade of NMDA receptors, but not AMPA receptors, decreased renal SNA and MAP in DH rats, additional experiments were performed to investigate the underlying mechanisms. Experiments first determined whether dehydration increased the response to NMDA receptor activation. All doses of PVN NMDA increased renal SNA and MAP in DH rats and EH controls (Fig. 3A). Lower concentrations of NMDA (50 and 100 pmol/50 nl) elicited similar elevations in renal SNA and MAP between groups. In contrast, both 200 and 400 pmol/50 nl doses produced significantly greater increases in renal SNA in DH than EH rats, whereas changes in MAP were similar across groups (Fig. 3B). A sigmoidal variable slope function fit to NMDA dose-response data revealed that whereas the EC50 was similar in EH and DH rats, the maximum agonist evoked response (Emax) response was significantly greater in the DH group. In addition to increasing glutamatergic input to PVN, these findings raise the possibility that dehydration increases responsiveness to NMDA.
Fig. 3.
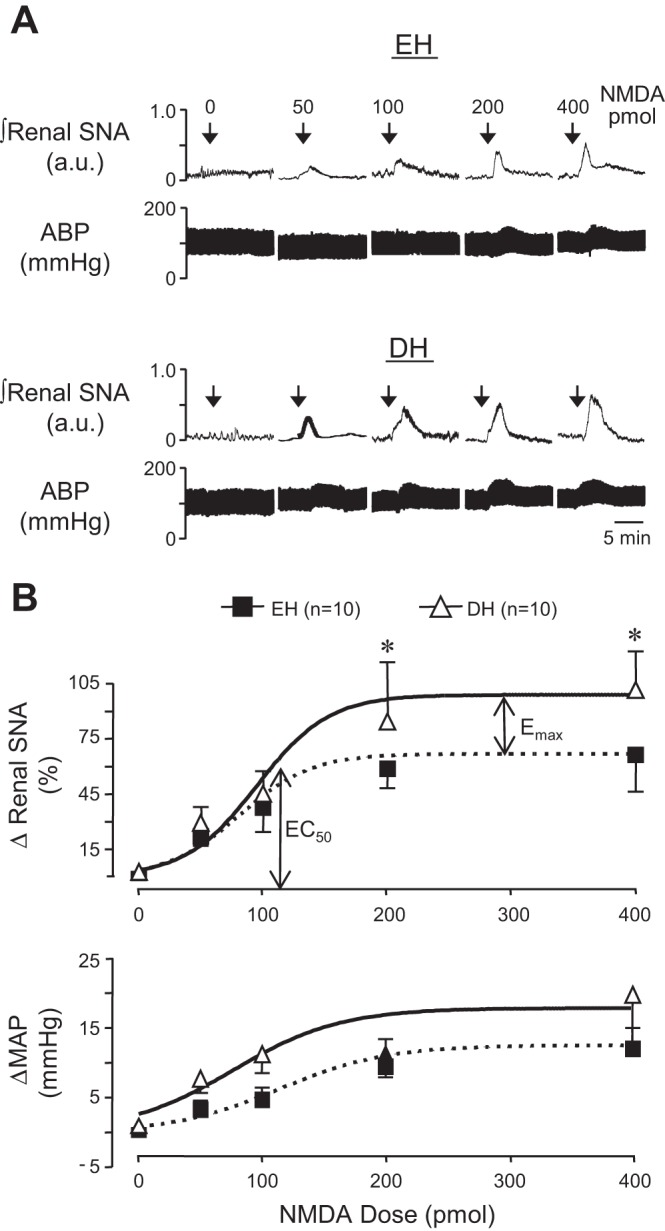
A: representative examples of renal SNA and ABP responses to PVN microinjection of graded doses of NMDA in an EH (top) and DH (bottom) rat. B: summary data illustrating peak changes of renal SNA and MAP in response to graded doses of NMDA microinjected into the PVN of control and DH rats. Data were fitted with a sigmoidal variable slope dose-response function to illustrate that, whereas the EC50 dose elicited similar responses across groups, the maximum agonist evoked response (Emax) response of renal SNA was significantly increased in DH compared with EH rats. *P < 0.05 vs. EH.
Dehydration Does Not Change NMDA Receptor NR1 Subunit Expression in the PVN
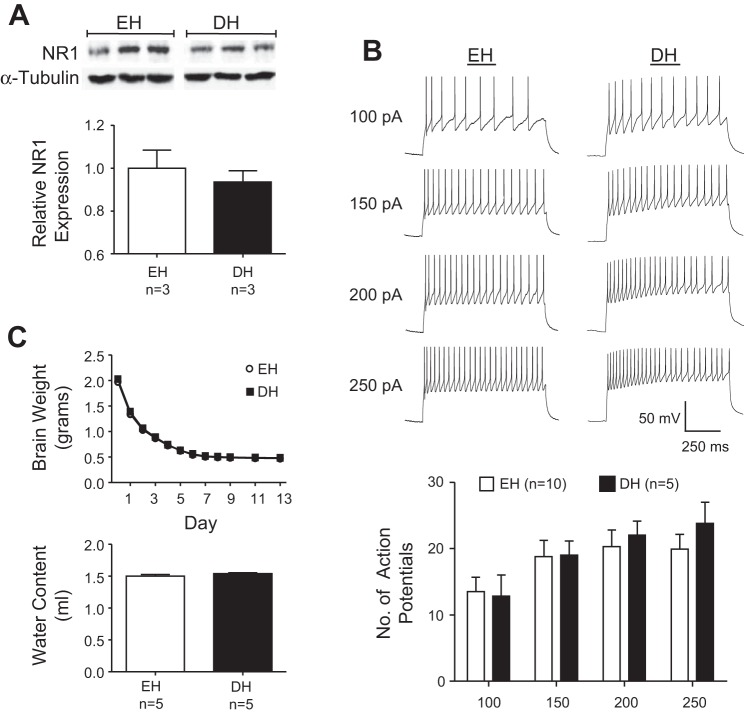
To determine factors that promote NMDA responsiveness during dehydration, we next investigated if expression of NMDA receptors in PVN was increased in DH rats. As shown in Fig. 4A, Western blot analysis revealed NMDA receptor NR1 subunit protein was present in the PVN of both EH and DH rats, but there was no difference in expression across groups (P = 0.4). Thus increased NMDA receptor tone and maximal renal SNA responses to NMDA in the PVN of DH rats do not appear to reflect increased receptor expression.
Fig. 4.
A: expression of NMDA receptor NR1 subunit protein in PVN samples measured by Western blot analysis. Mean band densities of NR1 relative to α-tubulin were not significantly different between DH and EH rats. B: representative traces of action potentials evoked by 100, 150, 200, and 250 pA current injections in a PVN-rostral ventrolateral medulla (RVLM) neuron from a control and DH rat (top). Summary of the number of action potentials evoked by graded current injections in neurons from control and DH rats (bottom). There was no difference of excitability among PVN-RVLM neurons from EH and DH rats at any level of current injection. C: weight of brains from control and DH rats. Initial weights were not significantly different between groups and remained similar through 13 days of dehydration (top). By day 13, brain weights were no longer changing and brain water content (difference between brain weight before and after desiccation) was not different between EH and DH rats (bottom).
Excitability of PVN-RVLM Neurons is Similar in DH and EH Rats
Whole cell patch-clamp recordings were performed on retrogradely labeled PVN-RVLM neurons from DH and EH rats. Retrograde tracer was confined to the RVLM, which is located ventral to the nucleus ambiguus and 200- to 250-μm posterior to the caudal pole of the facial nucleus. All neurons tested lacked spontaneous discharge, but depolarizing current injections consistently evoked repetitive action potential firing. Figure 4B shows representative responses (top) and summary data (bottom) indicating that the number of action potentials evoked by each level of current injection was similar among neurons from the DH (n = 5 neurons, n = 4 rats) and EH (n = 10 neurons, n = 9 rats) groups. These data suggest that greater effects of AP5 and NMDA in the PVN of DH rats do not reflect increased intrinsic excitability of PVN-RVLM neurons.
Dehydration Does Not Affect Brain Water Content
As noted in Table 1, dehydration significantly increased body fluid osmolality and decreased systemic volume. It was previously unknown if water content of the brain is similarly depleted. If so, then heighted responses to AP5 and NMDA in DH rats might reflect increased extracellular concentration of endogenous glutamate and of exogenous NMDA in the PVN due to reduced interstitial volume. We measured the weight of brains from DH and EH rats over 13 days of desiccation. Brain weights were similar on day 0 when initially removed and weights remained equivalent as water evaporated over 13 days (Fig. 4C, top). Brain water content, calculated as the difference between brain weight before and after desiccation, was similar between the two groups (Fig. 4C, bottom, P > 0.2). These data indicate that extracellular volume of PVN was likely similar in DH and EH rats such that similar levels of synaptic glutamate release and similar doses of NMDA injected into PVN would likely produce similar interstitial concentrations in DH and EH rats. Thus extracellular brain dehydration is not likely to contribute significantly to exaggerated responses to AP5 and NMDA in DH rats.
Sympathoexcitation by Glutamate Transporter Blockade in PVN is Blunted in DH Rats
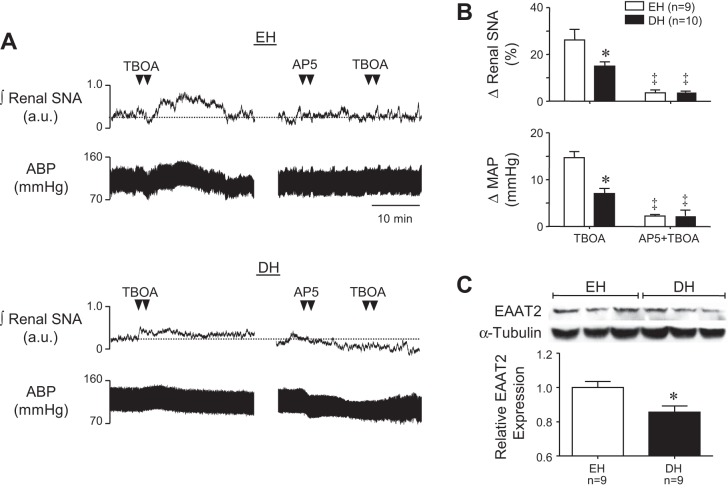
To investigate the possibility that greater sympathoinhibitory and depressor responses to PVN AP5 in DH rats could be explained by reduced uptake of endogenously released glutamate, we next compared sympathoexcitatory responses to PVN glutamate transport blockade in DH and EH rats. Figure 5A shows representative responses to PVN microinjection of TBOA (250 pmol/50 nl) (left) and to AP5 followed by TBOA (right) in an EH (top) and DH (bottom) rat. Summary data are shown in Fig. 5B. TBOA increased renal SNA (top) and MAP (bottom) in both groups, reflecting effects of endogenously released glutamate in the PVN. Responses to TBOA were significantly greater in EH rats (P < 0.05). Prior microinjection of AP5 decreased renal SNA and MAP in the DH rats and effectively prevented sympathoexcitatory and pressor responses to subsequent injection of TBOA in both groups.
Fig. 5.
A: representative examples of renal SNA and ABP responses to PVN microinjection of the nonspecific glutamate transporter inhibitor dl-threo-β-benzyloxyaspartic acid (TBOA) (left) and AP5+TBOA (right) in an EH and DH rat. B: summary of peak changes of renal SNA (top) and MAP (bottom) in response to PVN microinjection of TBOA (250 pmol/50 nl) and TBOA+AP5 in EH and DH rats. C: protein expression of excitatory amino acid transporter-2 (EAAT2) in PVN samples measured by Western blot analysis. Mean data of band densities of EAAT2 relative to α-tubulin was significantly decreased in DH rats. *P < 0.05 vs. EH. ‡P < 0.05 vs. TBOA alone.
PVN Expression of EAAT2 is Reduced in DH Rats
Given that responses to PVN TBOA were blunted in DH rats together with the recent finding that specific blockade of the dominant glial expressed transporter EAAT2 had a blunted effect on nearby vasopressin-releasing magnocellular neurons during dehydration (17), we examined PVN expression of EAAT2 in EH and DH rats. EAAT2 protein was present in PVN samples from both groups, but expression was significantly less (P < 0.05) in samples from DH rats (Fig. 5C). Samples were run in triplicate and no differences were seen between runs (data not shown). These findings suggest that reduced uptake of glutamate in the PVN of DH rats could contribute both to heightened glutamatergic support of SNA and MAP and to exaggerated sympathoexcitatory and pressor responses to exogenously delivered NMDA.
Histology
Injection sites were marked with rhodamine beads. Sites were located within the area of the PVN previously indicated by our laboratory (2, 39, 40). This included the region of the dorsal cap and both magnocellular and parvocellular areas (Fig. 6). The spread of rhodamine beads did not enter the third ventricle or areas surrounding the PVN. Injections of AP5, NMDA, and TBOA were similar in EH and DH rats.
Fig. 6.
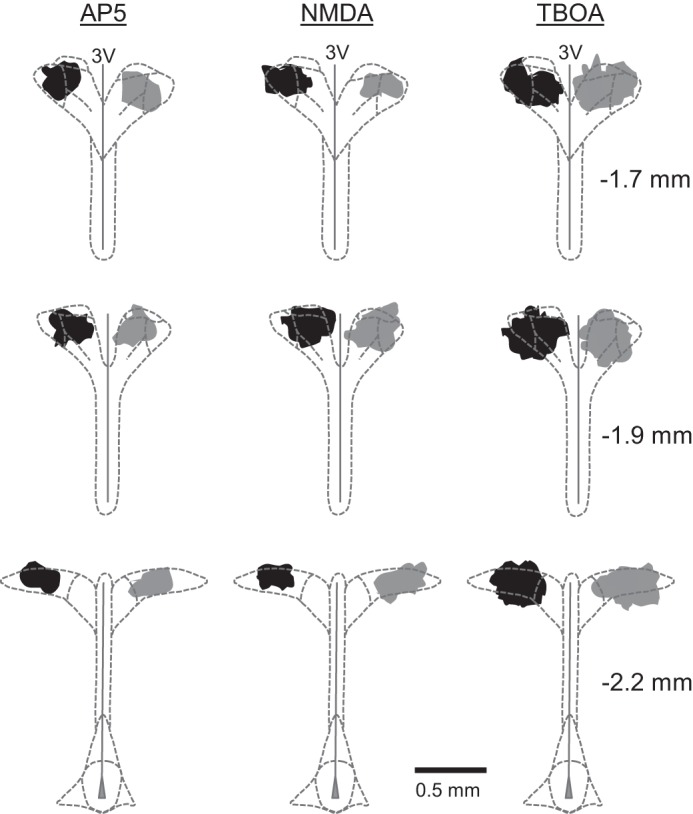
Injection sites were located within the area of the PVN in DH (black) and EH (gray) rats. Shaded regions represent the farthest distribution of fluorescent microspheres among all rats comprising each group. Sites of AP5 (n = 6–7/group), NMDA (n = 10/group), and TBOA (n = 9–10/group) injections were consistently made near the center of PVN. Coinjected fluorescent beads extended in the rostral-caudal plane to include most of the parvocellular and magnocellular subregions. Stereotaxic coordinates between the right most panels are referenced to bregma. 3V; third cerebral ventricle.
DISCUSSION
Similar to dehydration, SNA in diseases such as hypertension, heart failure, and obesity depends on PVN neuronal activity (18, 30, 43, 44, 48). The present study used acute dehydration to reveal new features of neurotransmitter dynamics that underlie recruitment of PVN in support of ongoing SNA. We determined that: 1) activation of PVN NMDA, but not AMPA, receptors drives SNA and supports ABP during dehydration; 2) the Emax renal SNA response to graded doses of NMDA delivered to PVN is increased in DH rats; 3) expression of NMDA receptor NR1 subunit protein in PVN is unaffected by dehydration; 4) excitability of PVN-RVLM neurons is unaltered in DH rats; 5) brain water content is equivalent in DH and EH rats; 6) glutamate transporter blockade produces a blunted sympathoexcitatory response in DH rats; and 7) dehydration reduces PVN expression of the dominant glial glutamate transporter EAAT2. Taken together, results suggest that sympathetic activation during acute dehydration results from glutamatergic activation of NMDA receptors in the PVN and that postsynaptic PVN neuronal activation is enhanced by reduced glutamate clearance due to concurrent loss of transporter expression.
A caveat to this interpretation is that experiments were performed under anesthesia, which might have differentially impacted neuronal function and synaptic transmission in euhydrated versus dehydrated rats. It seems unlikely, however, that anesthesia significantly influenced experimental outcomes. This is the case because the present findings are consistent with evidence from c-fos staining (42) and NMDA microinjection (32) studies in conscious euhydrated and dehydrated rats. Of note, the present studies were performed in rats anesthetized with a urethane-chloralose cocktail. Initial preliminary experiments performed with rats continuously anesthetized with isoflurane revealed that MAP in dehydrated rats was not well maintained during the experimental period. More importantly, PVN inhibition with muscimol and blockade of NMDA receptors with AP5 both failed to reduce SNA and MAP in isoflurane-anesthetized euhydrated and dehydrated rats. Collectively, these findings indicate that isoflurane anesthesia does not preserve PVN neuronal activation during dehydration. Moreover, responses under isoflurane anesthesia are inconsistent with evidence of dehydration-induced PVN activation in conscious rats. Importantly, this interpretation is consistent with recent literature evidence that isoflurane, over a similar experimental time frame, induces significant internalization of NMDA receptor subunit proteins (especially the NR2B subunit) (15) and causes significant inhibition of transmitter release from glutamatergic nerve terminals (54).
In 2007, Freeman and Brooks demonstrated that ionotropic glutamate receptors in the PVN support ABP during 48 h dehydration (18). Here, we show that renal SNA and ABP are maintained specifically by PVN NMDA receptors. The fall of MAP evoked by PVN AP5 in our study (∼25 mmHg) is comparable to that reported by Freeman and Brooks. Based on this, we conclude that acute dehydration works largely through an NMDA receptor-dependent mechanism and does not depend on AMPA receptor activation to increase PVN-driven SNA to support ABP. Additionally, a recent report by our laboratory found that increased SNA during dehydration is driven by a noncardiac and nonrespiratory rhythmic component (21). Additional studies will be needed to determine whether the increase of “tonic” SNA is mediated by ongoing tonic activation of PVN NMDA receptors. This will be important because, as discussed below, studies in brain slices have determined that nearby magnocellular neurons express a tonic NMDA inward current that is increased by dehydration (17). Whether or not such a current is expressed by presympathetic neurons and is subject to positive modulation by dehydration remains to be determined.
The origin(s) of dehydration-driven glutamatergic input to PVN have not been conclusively identified. However, a recent study clearly revealed that glutamatergic inputs originate in circumventricular organs of the lamina terminalis (31) and acute dehydration increases c-fos expression in this region (22). Accordingly, dehydration elevates body fluid osmolality and circulating angiotensin II, factors sensed by circumventricular organ neurons, specifically those in the organum vasculosum of the lamina terminalis (OVLT) and subfornical organ (SFO) (34, 49). Because the OVLT and SFO lack a blood-brain barrier, humoral factors can exit the blood stream and drive neurons that project to the hypothalamus (34). OVLT and SFO neurons project to the median preoptic area as well as directly to PVN making them likely brain structures involved in dehydration (40, 49). Further studies are required to examine the role of circumventricular organs in driving SNA and ABP during dehydration and whether glutamatergic pathways are activated from those brain regions to PVN. Identifying and fully characterizing factors that activate neurons that project to PVN is an important step toward unraveling the full complexity of sympathetic control during homeostatic challenges and in chronic diseases (35, 50).
We originally hypothesized that PVN glutamatergic tone would be elevated during dehydration because of greater NMDA receptor expression and elevated PVN-RVLM neuronal excitability in the face of greater synaptic glutamate transport. This was based on previous evidence of elevated PVN NMDA receptor expression driving exaggerated SNA in heart failure (29), increased excitability of PVN-RVLM neurons in neurogenic ANG II-salt hypertensive rats (8), and increased synaptosomal glutamate transport in dehydrated rats (25). However, our findings did not support this hypothesis.
An important caveat to consider is that our hypothesis largely assumed that increased levels of glutamate in the PVN during dehydration would result from increased synaptic release. However, we tested the alternative possibility that brain dehydration might reduce interstitial volume and thereby increase glutamate concentration without requiring increased synaptic release. Whereas this possibility remains to be fully tested, it appears unlikely as brain water content was identical in euhydrated and dehydrated rats. Thus, at least over a period of 48 h, homeostatic control of osmolyte fluxes in the brain appear to fully protect it from dehydration (52).
Contrary to our hypothesis, NMDA receptor NR1 subunit expression was unchanged between groups. This does not rule out increased expression of other NMDA receptor subunits such as NR2A and NR2B, which have been reported to be upregulated in spontaneously hypertensive rats (55). It is important to note that with no change of NR1 expression, increased expression of other subunits would likely change the subunit composition of NMDA receptors rather than increase the total number since the NR1 subunit is required for glutamate binding and channel gating. Increased NR2B subunit expression could be a promising avenue for future study as NR2B subunit containing NMDA receptors have increased affinity for glutamate, slow desensitization kinetics and traffic to both postsynaptic and extrasynaptic sites (16, 26). The latter point is noteworthy in that a recent study reported that nearby magnocellular neurosecretory neurons from dehydrated rats have an enlarged tonic NMDA current (17). Alternatively, 48 h dehydration could modify covalent modifications of NR subunits such as tyrosine phosphorylation, which is reported to significantly grade gating and conductance properties of NMDA receptors. Finally, it is possible that dehydration could increase membrane localization of NMDA receptors such that even without a net increase of NR1 subunit expression, greater membrane localization of receptors could have contributed to greater NMDA receptor tone and exaggerated NMDA responses in dehydrated rats.
A further blow to our hypothesis was that excitability of PVN-RVLM neurons was not different between control and DH rats. It is possible that a different subpopulation of PVN neurons could have heightened excitability and contributes to the exaggerated SNA response in DH rats. However, evidence indicates that axons of many PVN neurons branch soon after exiting the PVN and collateral branches target the RVLM and spinal IML (and possibly elsewhere) (10, 37, 45). Thus labeled PVN-RVLM neurons likely represent a large fraction of sympathetic regulatory PVN neurons. It is also possible that dehydration increased excitability of a fraction of PVN-RVLM neurons. This being the case, the number of neurons recorded might have been too small to reveal their existence.
In this study, we are the first to report on the effect of glutamate transport blockade in the PVN in a state of elevated SNA and ABP in vivo. Our findings are most consistent with the view that acute dehydration causes an overabundance of glutamate release from PVN synapses which is not fully compensated for by activation of glutamate transporters. Although this is not a direct measure of glutamate uptake, our findings suggest that reduced glutamate uptake by membrane transporters might provide a means for enhancing effects of glutamatergic input during dehydration. Thus EAATs represent a novel and potentially important target for lowering SNA and ABP in states of sympathoexcitation. It is also possible that glutamate transport may be altered at other brain structures which could contribute to the neurological deficits associated with more extreme bouts of dehydration (52).
Here, the competitive nonspecific EAAT blocker TBOA, which inhibits EAAT1-3, was used to assess the role of glutamate transport in PVN during the acute homeostatic challenge and sympathoexcitatory state of dehydration. Our study does not allow us to determine the relative contribution of each specific EAAT, but based on previous studies, we hypothesized that EAAT2 would play a significant role. A 2011 study by Fleming et al. (17) examined the effect of specific EAAT2 blockade on hypothalamic supraoptic nucleus (SON) magnocellular neurons during 48 h dehydration. Similar to our findings with TBOA, they reported that dihydrokainic acid, an EAAT2-specific inhibitor, increased the firing rate of SON neurons in euhydrated but had virtually no effect in dehydrated animals. They concluded that excitatory currents were increased in SON magnocellular neurons from dehydrated rats because of reduced EAAT2 buffering of synaptically released glutamate (17). Our study further indicates a significant role for EAAT2 as PVN expression was decreased in DH rats, providing a likely explanation for the blunted sympathoexcitatory response to PVN glutamate transport blockade with TBOA in DH rats. Efforts to quantify expression of EAAT1,3,4 were unsuccessful due to poor specificity of available antibodies. Additional in vivo and in vitro studies are necessary to quantify the extent to which activity of PVN-RVLM (and other sympathetic neurons) is elevated during acute dehydration and the role played by reduced EAAT2 activity in supporting heightened discharge. Such experiments are important because PVN tissue punches include functionally heterogeneous neurons. Consequently, the net reduction of EAAT2 expression could reflect a complex pattern of regional variation across the nucleus and might not be specific to sympathetic subregions.
Upon initial inspection our findings appear to conflict with a study recently published by our laboratory in collaboration with others (25). In that study, we reported that 48 h dehydration significantly increased PVN synaptosomal glutamate uptake. There are multiple possibilities for the disparate findings. First, measurements in the original study were made from synaptosomes prepared from PVN tissue punches and not from live animals. Trafficking and membrane localization of transporters could be different as could the presence of endogenous factors that modulate transporter function. Second, it is unclear from our study what the “ceiling” might be for renal SNA during 48 h dehydration. Because of this, it is possible that dehydration might increase glutamate uptake and that TBOA did allow glutamate levels to rise as much in DH rats as EH rats. However, if renal SNA was already near its maximum, transport blockade and the ensuing increase of extracellular glutamate might not have evoked a further increase. This possibility cannot be entirely ruled out, but is perhaps unlikely given that PVN injections of NMDA evoked exaggerated, not dampened, sympathoexcitatory responses in dehydrated rats.
Perspectives and Significance
The present study revealed that PVN NMDA receptors support SNA and ABP during acute dehydration. This increase in functional glutamatergic “tone” likely reflects increased glutamatergic input together with reduced uptake by excitatory amino acid transporters. Although we originally hypothesized that PVN-RVLM neuronal excitability would be elevated and NMDA receptor expression would be increased in dehydrated rats, we found both to be unaltered. These findings highlight a potential new avenue of study as the role of glutamate transporters has not been examined previously in a whole animal model of sympathoexcitation. Future studies will be needed to fully understand how changes in glutamate clearance kinetics contribute to other states where PVN activation drives sympathetic hyperactivity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-102310 and HL-088052 (to G. M. Toney) and T32 HL-07446 (to M. E. Bardgett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.E.B., Q.-H.C., and G.M.T. conception and design of research; M.E.B., Q.-H.C., Q.G., A.S.C., and M.A.A. performed experiments; M.E.B. and Q.-H.C. analyzed data; M.E.B. interpreted results of experiments; M.E.B. prepared figures; M.E.B. drafted manuscript; M.E.B. and G.M.T. edited and revised manuscript; M.E.B., Q.-H.C., Q.G., A.S.C., M.A.A., and G.M.T. approved final version of manuscript.
REFERENCES
- 1.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bardgett ME, Holbein WW, Herrera-Rosales M, Toney GM. Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-α. Hypertension 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 77: 3396–3400, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Brooks VL, Qi Y, O'Donaughy TL. Increased osmolality of conscious water-deprived rats supports arterial pressure and sympathetic activity via a brain action. Am J Physiol Regul Integr Comp Physiol 288: R1248–R1255, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Burnier M, Biollaz J, Brunner DB, Brunner HR. Blood pressure maintenance in awake dehydrated rats: renin, vasopressin, and sympathetic activity. Am J Physiol Heart Circ Physiol 245: H203–H209, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Busnardo C, Tavares RF, Correa FM. Role of N-methyl-d-aspartate and non-N-methyl-d-aspartate receptors in the cardiovascular effects of l-glutamate microinjection into the hypothalamic paraventricular nucleus of unanesthetized rats. J Neurosci Res 87: 2066–2077, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen QH, Andrade MA, Calderon AS, Toney GM. Hypertension induced by angiotensin II and a high salt diet involves reduced SK current and increased excitability of RVLM projecting PVN neurons. J Neurophysiol 104: 2329–2337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension 42: 725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collister JP, Hendel MD. Chronic effects of angiotensin II and AT1 receptor antagonists in subfornical organ-lesioned rats. Clin Exp Pharmacol Physiol 32: 462–466, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Colombari DS, Colombari E, Freiria-Oliveira AH, Antunes VR, Yao ST, Hindmarch C, Ferguson AV, Fry M, Murphy D, Paton JF. Switching control of sympathetic activity from forebrain to hindbrain in chronic dehydration. J Physiol 589: 4457–4471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology 145: 5141–5149, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Wu X, Zhang G, Xu Z, Zhang Y, Gautam V, Kovacs DM, Wu A, Yue Y, Xie Z. Isoflurane facilitates synaptic NMDA receptor endocytosis in mice primary neurons. Current Molec Med 13: 488–498, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol 563: 345–358, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol 589: 3929–3941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman KL, Brooks VL. AT1 and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol 292: R1675–R1682, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gabor A, Leenen FH. Cardiovascular effects of angiotensin II and glutamate in the PVN of Dahl salt-sensitive rats. Brain Res 1447: 28–37, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Holbein WW, Toney GM. Sympathetic network drive during water deprivation does not increase respiratory or cardiac rhythmic sympathetic nerve activity. J Appl Physiol (1985) 114: 1689–1696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji LL, Fleming T, Penny ML, Toney GM, Cunningham JT. Effects of water deprivation and rehydration on c-Fos and FosB staining in the rat supraoptic nucleus and lamina terminalis region. Am J Physiol Regul Integr Comp Physiol 288: R311–R321, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ji LL, Gottlieb HB, Penny ML, Fleming T, Toney GM, Cunningham JT. Differential effects of water deprivation and rehydration on Fos and FosB/DeltaFosB staining in the rat brainstem. Exp Neurol 203: 445–456, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiologica Scand 177: 7–15, 2003 [DOI] [PubMed] [Google Scholar]
- 25.King TS, Toney GM, Tian PY, Javors MA. Dehydration increases sodium-dependent glutamate uptake by hypothalamic paraventricular nucleus synaptosomes. Neuro Endocrinol Lett 32: 763–768, 2011 [PMC free article] [PubMed] [Google Scholar]
- 26.Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron 18: 493–503, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637–1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302: R424–R432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins-Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM. Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Frontiers Physiol 3: 490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maughan RJ. Impact of mild dehydration on wellness and on exercise performance. Euro J Clin Nutr 57, Suppl 2: S19–S23, 2003 [DOI] [PubMed] [Google Scholar]
- 34.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci 19: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin II-salt hypertension in the rat. Exp Physiol 97: 80–88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter JP, Brody MJ. Neural projections from paraventricular nucleus that subserve vasomotor functions. Am J Physiol Regul Integr Comp Physiol 248: R271–R281, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 43: 169–175, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 305: H1172–H1780, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol 586: 5231–5245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol 293: R2279–R2289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol 286: R719–R725, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stocker SD, Stricker EM, Sved AF. Arterial baroreceptors mediate the inhibitory effect of acute increases in arterial blood pressure on thirst. Am J Physiol Regul Integr Comp Physiol 282: R1718–R1729, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568: 599–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res 543: 296–300, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veitenheimer BJ, Engeland WC, Guzman PA, Fink GD, Osborn JW. Effect of global and regional sympathetic blockade on arterial pressure during water deprivation in conscious rats. Am J Physiol Heart Circ Physiol 303: H1022–H1034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verbalis JG. Control of brain volume during hypoosmolality and hyperosmolality. Adv Exp Med Biol 576: 113–129; discussion 361–113, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Vieira AA, Nahey DB, Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ANG II in conscious rats. Am J Physiol Regul Integr Comp Physiol 299: R1564–R1571, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Westphalen RI, Desai KM, Hemmings HC., Jr. Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth 110: 592–599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye ZY, Li L, Li DP, Pan HL. Casein kinase 2-mediated synaptic GluN2A up-regulation increases N-methyl-d-aspartate receptor activity and excitability of hypothalamic neurons in hypertension. J Biol Chem 287: 17438–17446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]