Abstract
Redox networks in the cell integrate signaling pathways that control metabolism, energetics, cell survival, and death. The physiological second messengers that modulate these pathways include nitric oxide, hydrogen peroxide, and electrophiles. Electrophiles are produced in the cell via both enzymatic and nonenzymatic lipid peroxidation and are also relatively abundant constituents of the diet. These compounds bind covalently to families of cysteine-containing, redox-sensing proteins that constitute the electrophile-responsive proteome, the subproteomes of which are found in localized intracellular domains. These include those proteins controlling responses to oxidative stress in the cytosol—notably the Keap1-Nrf2 pathway, the autophagy-lysosomal pathway, and proteins in other compartments including mitochondria and endoplasmic reticulum. The signaling pathways through which electro-philes function have unique characteristics that could be exploited for novel therapeutic interventions; however, development of such therapeutic strategies has been challenging due to a lack of basic understanding of the mechanisms controlling this form of redox signaling. In this review, we discuss current knowledge of the basic mechanisms of thiol-electrophile signaling and its potential impact on the translation of this important field of redox biology to the clinic. Emerging understanding of thiolelectrophile interactions and redox signaling suggests replacement of the oxidative stress hypothesis with a new redox biology paradigm, which provides an exciting and influential framework for guiding translational research.
Keywords: Electrophiles, Keap1, Nrf2, Bioenergetics
Introduction
In the field of free radical biology, the “oxidative stress paradigm” has been the central dogma that has provided the framework for understanding the mechanisms leading to the development of novel therapeutics. It is an attractive concept that simply postulates that there is a balance between free radicals or oxidants [commonly called reactive oxygen species (ROS) or reactive species] with antioxidants in normal physiology. Pathology occurs when reactive species are produced in excess of the endogenous antioxidants, and this leads to indiscriminate damage to cellular macromolecules (proteins, lipids, and DNA) and kills cells [1]. Interestingly, much of the evidence for this process occurring in health and disease is derived from the oxidative modifications of proteins by products of lipid peroxidation—the reactive lipid species [2–6]. Accordingly, the development of therapeutics initially focused on developing compounds that could terminate the lipid peroxidation chain reaction such as α-tocopherol or dietary-derived polyphenolics [7].
The oxidative stress paradigm resulted in the widespread notion that supplementation of dietary antioxidants that target lipid peroxidation will prevent many human diseases. Over time, the mechanistic basis of the concept was largely forgotten and instead of the oxidative stress hypothesis becoming more precise in terms of molecular targets and mechanism, it became diffuse and nonspecific. This has unfortunately resulted in the widely held belief that all ROS are extremely reactive and share common biophysical properties and that all antioxidants are then also capable of scavenging any reactive species irrespective of the biochemical mechanism. The antioxidants which have achieved most attention in this respect are those that intercept lipid radicals and include α-tocopherol (vitamin E), β-carotene, ascorbic acid (vitamin C), and the numerous natural polyphenolic compounds present in the diet [8–10]. However, despite excellent animal model studies, basic research, and epidemiological data that collectively show that oxidative protein modifications by reactive lipid species are increased in many chronic diseases, controlled clinical trials with lipid radical scavenging antioxidants have not yielded the anticipated benefits [6,11–19].
It is now clear that several critical predictions of the oxidative stress paradigm are not supported by experiment. Using advanced mass spectrometry techniques, it has become possible to measure both the frequency of modification of biomolecules by reactive species and their levels in vivo. In direct contrast to the predictions from the oxidative stress paradigm in oxidant-dependent pathologies, the relative levels of protein modification are extremely low, and antioxidants are still abundantly present in the cells and tissues [20,21]. In addition, the hypothesis predicts that exogenous oxidants should contribute to pathology. This is indeed the case, but the levels of exogenous oxidants needed to place the system out of balance in vitro and in vivo are orders of magnitude higher than the levels that can ever be produced in biology in either health or disease.
At the inception of the oxidative stress hypothesis, the concept that endogenous molecules such as nitric oxide or hydrogen peroxide played a role in cell signaling had not been developed. It is now clear that not only do low levels (typically 10–100 nM) of these compounds play a role in cell signaling, but, as with other signaling pathways, control is exerted in specific domains which are not in redox equilibrium with the rest of the cell. We have proposed that endogenous antioxidants serve as redox insulators of these cell signaling domains [22]. Because exogenous signaling molecules such as hydrogen peroxide must break down the redox insulation before an effect can be observed, high nonphysiological concentrations are often needed. Thus, the idea that “free radicals are bad and antioxidants are good” is clearly undergoing a critical and high-profile reappraisal [23]. As the field of redox biology has developed, it has become apparent that the major predictions of the oxidative stress paradigm do not effectively explain the biological actions of reactive species and are not supported by experimental evidence. In this review, we propose that the oxidative stress hypothesis has reached the limits of its utility and should be replaced with the “redox biology paradigm” in which antioxidants play the primary role of modulating the complex networks controlling cell signaling and metabolism.
While it is possible that the modifications of proteins by reactive species are an unimportant epiphenomenon, it is clear that this is not the case; reactive species (including nitric oxide, hydrogen peroxide, and reactive lipid species) are known to act as cell signaling molecules, supporting the need for a reevaluation of the oxidative stress paradigm [22,24–28]. With the discovery that nitric oxide is a signaling molecule, the field is now embracing the paradigm that reactive species play an essential role in biology and that antioxidants serve a regulatory, not a protective, function. An important example in the field has been the realization that one class of reaction products from both enzymatic and nonenzymatic lipid peroxidation is electrophilic and can selectively modify families of cysteine-containing proteins, or electrophile-responsive proteomes, so modulating cell function [22,29]. That these products are derived from lipid radical targets of α-tocopherol, vitamin C, and β-carotene likely explains the tight biological control of levels of these molecules in human subjects and the marginal beneficial effects of supplementation [30]. In this context, the role of radical scavenging antioxidants such as vitamin E is to control the domain and levels of reactive lipid species for normal redox cell signaling. The impact of these new concepts on the development of redox therapeutics is now emerging and is discussed below.
Electrophile signaling as the master regulator of cellular antioxidant regulation
Cells have developed intricate mechanisms by which they sense and adapt to oxidants and electrophiles that are either endogenous or environmental in origin. There are several stress-responsive signaling pathways that are activated by endogenously produced electrophiles or xenobiotics [22,29,31–34]. As with other signaling pathways, this is not a nonspecific or random process but a controlled mechanism involving the posttranslational modification of redox-active cysteines in key signaling proteins. The concept of redox signaling first identified reactive oxygen and nitrogen species as molecules capable of modifying reactive cysteines, thus eliciting downstream signaling responses [35]. However, it has become evident that electrophiles may also react with cysteine moieties via Michael addition, thereby expanding the repertoire of reactive species having distinct signaling functions. In the cellular milieu, electrophiles are produced by oxidation and nitration of unsaturated fatty acids resulting in a formation of a host of reactive species such as aldehydes, α-β-unsaturated carbonyls, and nitroalkenes that react with cysteines in a reversible or irreversible manner [22,29,32].
Keap1-Nrf2 pathway
One of the best characterized signaling pathways activated by electrophiles is the Keap1-Nrf2-antioxidant response element (ARE) pathway (Fig. 1), which orchestrates cellular responses to oxidant and electrophile stress [22,31,36,37]. Under basal conditions, Keap1 tethers Nrf2, directing it to ubiquitination and degradation by cullin-3-dependent proteasome. During electrophile stress, Keap1 can no longer support its function as a substrate adaptor in the ubiquitin ligase complex, leading to stabilization of Nrf2. The key signaling protein transducing the effects of electrophiles is Keap1, and it has an exceptionally high number of thiol residues: a total of 25 in mouse and 27 in human. These can be extensively modified in vitro by a variety of electrophiles.
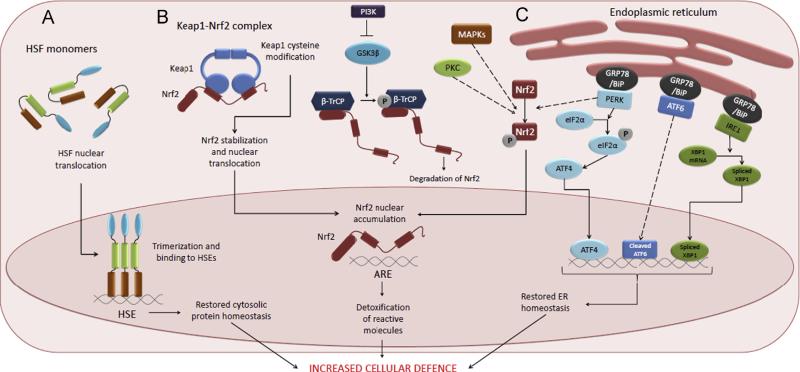
Fig. 1.
Stress signaling pathways activated by electrophiles. (A) Heat shock response (HSR). Upon activation, heat shock factors (HSFs), primarily HSF1, translocate to the nucleus, trimerize and bind to heat shock elements (HSEs) and drive the expression of target genes, such as heat shock proteins. (B) Keap1-Nrf2 pathway. During electrophile stress, Keap1 is no longer able to deliver Nrf2 to proteasomal degradation, and the stabilized Nrf2 protein translocates to the nucleus, binds to antioxidant response elements (AREs), and regulates target genes. Keap1-independent mechanisms for Nrf2 nuclear accumulation include phosphorylation of Nrf2 by PKC, PERK, or MAP Kinases. In addition, PI3K may regulate Nrf2 via indirect mechanism by inhibiting GSK-3β activity. Active GSK-3β phosphorylates Nrf2 Neh6 domain binding protein β-TrCP, which results in Cul1-dependent ubiquitination and degradation of Nrf2. (C) Unfolded protein response (UPR). Accumulation of misfolded proteins in the ER activates the three arms of the UPR, resulting in activation of three transcription factors that regulate the expression of UPR target genes. PERK kinase can also phosphorylate and activate Nrf2.
While high enough concentrations of reactive electrophiles are able to adduct most of the cysteine residues in recombinant Keap1, lower concentrations yield distinct patterns of modification [38,39]. It has therefore been postulated that each electrophile covalently binds to a specific set of cysteine residues within a proteome (i.e., the “cysteine code”) and can be classified according to this feature [40]. It has also been proposed that Keap1 perceives endogenous stress via three phylogenetically conserved sensors that are responsive to nitric oxide, zinc, and alkenals and that xenobiotics exploit this feature to sense endogenous redox homeostasis [41]. Both hypotheses postulate that Keap1 can sense very different stress stimuli, but the outcome is the same—stabilization of Nrf2 and induction of target genes that include antioxidant and phase II enzymes as well as other genes promoting cell survival. In addition, to direct interactions with Keap1 many of the kinases and phosphatases, which also modulate this pathway, possess redox-active cysteine residues which are also potentially targets of electrophiles [42]. Furthermore, recent findings have revealed alternative pathways that can degrade Nrf2. Nrf2 can bind β-transducin repeat-containing protein (β-TrCP) via the Neh6 domain, which can be phosphorylated by glycogen synthase kinase 3β (GSK-3β), leading to cullin 1 (Cul1)-dependent ubiquitination and degradation of Nrf2 [43–45]. PI3K kinase is postulated to regulate the activity of GSK-3β and thus downregulate Nrf2, suggesting that the PI3K-pathway inhibits the activity of GSK-3β, leading to increase in Nrf2 [43].
Heat shock response (HSR)
In addition to the Keap1-Nrf2 pathway, other stress signaling pathways are activated by electrophiles [29]. The most notable of these is the heat shock response pathway (HSR, Fig. 1), which is a transcriptional response to a wide array of acute and chronic stress conditions including heat, electrophiles, and other reactive species produced during inflammation [32]. HSR is regulated by heat shock transcription factors, primarily by heat shock factor 1 (HSF1). On activation, HSF1 undergoes multistep processing involving post-translational modifications, nuclear enrichment, trimerization, and binding to heat shock elements (HSEs), resulting in the transcription of a large family of heat shock genes [32,46]. The initial trigger causing HSR by electrophiles is not completely understood, but the most likely candidates mediating the effects of electrophiles are chaperone proteins HSP70 and HSP90, which repress HSF1 activation through binding and sequestration under normal conditions [46]. Both chaperones are known to bind electrophiles such as 4-hydroxynonenal (HNE) [47]. Recently, two redox-active cysteine residues have been identified in yeast HSP70 analog Ssa1 [48]. Mutation of these rendered cells insensitive to Hsf1 activation by thiol-active compounds but not by heat shock, indicating that Ssa1 is a bona fide sensor of HSR by electrophiles in yeast. Inasmuch as HSR is an important protective pathway in proteotoxic stress, especially in neurodegeneration, its modulation by low molecular weight activators is of high clinical interest. Intriguingly, many of the novel potent HSR activators identified by high-throughput chemical screening are electrophiles that can simultaneously activate the Keap1-Nrf2 pathway, indicating that these two stress response pathways act in concert [49,50].
Unfolded protein response (UPR)
The third stress signaling pathway known to be activated by electrophiles is the endoplasmic reticulum (ER) stress response (ESR, Fig. 1) [32]. ESR is triggered by accumulation of misfolded or unfolded proteins, and is also called the unfolded protein response [51]. UPR modifies gene expression and protein translation to restore ER protein folding and homeostasis. Three distinct UPR signaling pathways have been identified, each transmitted by a transmembrane signaling protein residing in the ER: activating transcription factor-6 (ATF6), inositol requiring protein-1 (IRE1), and PERK. In addition, an ER-resident chaperone protein 78-kDa glucose-regulated protein (GRP78, also called BiP) is involved in the signaling, as it forms complexes with the three UPR signaling proteins [51]. Several mediators of the UPR can be triggered by electrophilic lipids such as 4-HNE, oxononenal, acrolein, and cyclopentenone prostaglandins as well as core aldehydes, such as POVPC and PEIPC, which derive from the oxidation of phosphatidylcholine [32,52]. The mechanism by which these electrophilic lipids activate UPR is not known. However, the likely target(s) are chaperones in the ER: fluorescently labeled 4-HNE and cyclopentenone prostaglandins accumulate there [15,16,53], and HNE was shown to promote the carbonylation of Grp78 and protein disulfide isomerase [52]. Hence, we speculate that direct modification of protein chaperones in the ER results in loss of protein folding capacity, which is consistent with previous studies showing that HNE inhibits the protein folding function of chaperone proteins [54].
Redox regulation of autophagy
Although it has been demonstrated that the Keap1-Nrf2 pathway can regulate proteasomal activity, evidence to support the concept that it also regulates the autophagy system is now emerging [55] (Fig. 2).
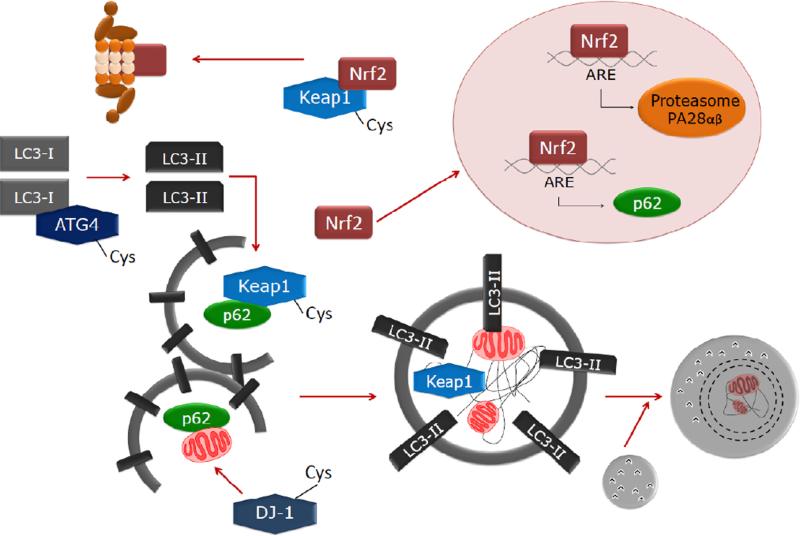
Fig. 2.
Redox regulation of autophagy. The Keap1-Nrf2 pathway is not only regulated by the proteasomes but also by autophagic activity. The cysteine-rich protein Keap1 serves as a redox sensor and can bind to p62 and be degraded by autophagic activity. Undegraded Keap1 complexes with Nrf2 and mediates Nrf2 ubiquitination and degradation by the proteasomes. In response to oxidants, cysteine modification on Keap1 releases Nrf2 by inhibiting its ubiquitination. Keap1-free Nrf2 is stabilized and transported to the nucleus where it activates transcription of antioxidant genes, including HO-1 and NQO1, proteasomal PA28αβ, thereby increases proteasomal degradation of oxidatively damaged proteins as well as p62, which plays an important role in autophagy by interacting with both ubiquitinated proteins and microtubule-associated protein light chain 3 (LC3), thereby facilitates autophagy-lysosomal degradation of proteins and dysfunctional organelles such as the mitochondria. Some of the autophagy proteins are also susceptible to cysteine modification, examples include ATG4 which is a cysteine protease involved in pro-LC3 cleavage, LC3I to LC3II conversion by lipidation, and LC3II to LC3I conversion by delipidation. DJ-1 is a cysteine protease playing a role in mitochondrial quality control and DJ-1 mutant mice or human Parkinson's disease patients accumulate abnormal mitochondria.
Autophagy is a complex pathway for protein and organelle turnover which comprises a lysosomal-mediated degradation process that involves 430 proteins. It involves the formation of double membrane intracellular vesicles, recognition of specific proteins or organelles, encircling these proteins and organelles into autophagosomes, followed by fusion with lysosomes and degradation of proteins and organelles [56–58]. Autophagy has been noted to play a role in cell survival primarily during nutrient deprivation to conserve energy and in response to stress to clear damaged and toxic intracellular components. Autophagic activities thus need to be highly regulated to sense intracellular stress, through mechanisms involving cellular redox signaling [59,60].
One reported example of such regulation is based on studies of ATG4 (AuTophaGy). ATG4 is a cysteine protease important for cleavage of pro-LC3 to produce LC3-I, and delipidation of autophagosomal outer membrane LC3-II for recycling [61,62]. ATG4 can be modified by hydrogen peroxide on cysteine 81 (C81), and this modification inhibits its delipidation activity. In addition to ATG4, DJ-1 also has redox-sensitive cysteines, and it offers a direct link among redox signaling, autophagy, and bioenergetic dysfunction because it regulates mitochondrial dynamics and mitophagy [63–65]. The LC3 conjugation enzymes ATG7 and ATG3, and lysosomal cysteine proteases, depend on cysteines for their activities; however how they are modified by cellular redox status is unknown [66]. Loss of function due to thiol oxidation is likely detrimental since loss of function of ATG3 or ATG7 has been shown to result in mitochondrial dysfunction in T cells and β cells, respectively [67–69]. Future studies to determine whether ATG4, DJ-1, ATG3, ATG7 or other autophagy proteins are modified at cysteine residues in vivo in different cells and in response to different redox signals, and whether their oxidation attenuates autophagy and cell survival are needed.
In addition to direct regulation of autophagy proteins, hydrogen peroxide exposure has been shown to induce cytoplasmic ataxia-telangiectasia mutate (ATM) phosphorylation, which in turn activates the LKB1-AMPK pathway and inactivates mTOR, leading to autophagy activation [70]. The targets of hydrogen peroxide in this scenario have not been identified. Redox modification of the Nrf2-Keap1 pathway has been shown to play an important role in transcriptional regulation through the antioxidant response element of p62/SQSTM1—an important ubiquitin and LC3-binding protein involved in autophagy [71] (Fig. 2).
Autophagic regulation of redox signaling
The Keap1-Nrf2-p62 interaction in autophagy regulation and redox signaling is complex. In addition to being regulated by Nrf2 at the level of transcription, p62 also binds Keap1 and sends Keap1 to be degraded by autophagy, further promoting Nrf2 transcriptional activity [71–73] (Fig. 2). In further support of a complex interaction between these systems, liver injury was severe in the Atg7::Keap1::Alb double knockout, but partially attenuated in the Atg7::Keap1::p62 triple knockout mice, and prevented in the Atg7::Keap1::Nrf2 triple mutant mice. Taken together, these data suggest that Nrf2 promotes liver injury in the absence of autophagy, and p62 contributes to damage by promoting Nrf2 accumulation. Furthermore, Atg7 or p62 liver knockout mice accumulate Keap1 protein [73]. In HepG2 cells, Keap1 levels are highly sensitive to starvation but insensitive to the proteasome inhibitor MG132, suggesting that autophagy plays an important role in constitutive Keap1 degradation [73].
In addition to regulation of Nrf2-mediated redox signaling, autophagy can also regulate cellular redox status by removing damaged mitochondria. Dysfunctional mitochondria may generate more reactive species due to mtDNA mutations, loss of membrane potential, or damaged respiratory chain proteins or enzymes in the matrix [57,60,74].
Bioenergetics and metabolism: Integration with redox signaling
The generation and metabolism of oxidants and electrophiles are tightly linked with the bioenergetic status of the cell. There appears to be three levels of regulation that coordinate intracellular oxidant/antioxidant status with bioenergetics. These can be grouped into the following redox networks in the cell.
Pyridine nucleotides and energy-redox balance
Pyridine nucleotides and the balance between their oxidized and reduced forms play critical roles in the cell. For example, NADH is well known to carry electrons to the electron transport chain for the generation of ATP [75]. Changes in the reduced and oxidized states of these pyridine nucleotides are sensed by several oxidant and antioxidant enzymes (Fig. 3).
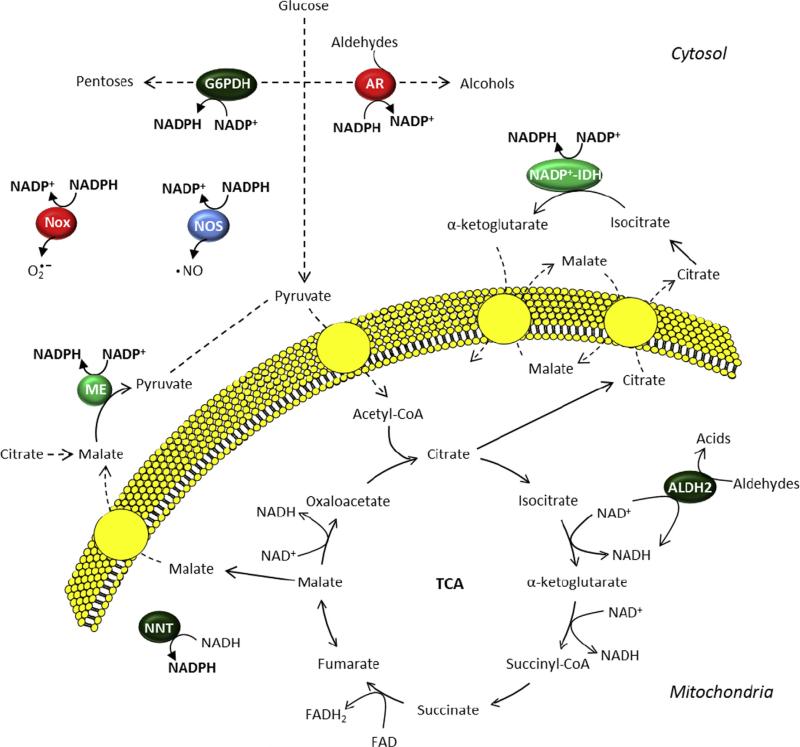
Fig. 3.
Coordination of intracellular redox status with intermediary metabolic pathways. Pyridine nucleotide balance is influenced by glucose-metabolizing enzymes such as glucose-6-phosphate dehydrogenase (G6PDH) and aldose reductase (AR). AR also utilizes NADPH in the reduction of aldehydes to their corresponding alcohols. Other cytosolic proteins that regulate NADPH levels include NADPH oxidases (Nox) and nitric oxide synthases (NOS). In the mitochondrion, the tricarboxylic acid cycle (TCA) as well as metabolism of aldehydes by aldehyde dehydrogenase 2 (ALDH2) can contribute to the production of NADH, which is not only used for energy transfer but can be converted to NADPH through the actions of nicotinamide nucleotide transhydrogenase (NNT). The cytosolic enzymes malic enzyme (ME) and NADPþ-dependent isocitrate dehydrogenase (NADPþ-IDH) utilize metabolic intermediates created in the TCA cycle to produce NADPH.
Through the actions of nicotinamide nucleotide transhydrogenase (NNT), NADH levels influence the phosphorylated pyridine nucleotide (NADPþ/NADPH) pool [76], which is important in regulating glutathione and used as cofactors in free radical generating enzymes [such as NADPH oxidase (NOX) and nitric oxide synthase (NOS)]. In addition to NNT, mitochondria also regulate NADPH levels through the actions of the NADPþ-dependent enzymes isocitrate dehydrogenase and malic enzyme [77]. Together, these mitochondrial enzymes act as tethers between energy metabolism and the redox state of the cell: NNT couples its transhydrogenase activity to the proton motive force and the latter enzymes link their NADPH-producing capacity with their ability to oxidize metabolic substrates. While NNT is primarily a mitochondrial enzyme, cytosolic enzymes important in intermediary metabolism also regulate NADPH. The most important of these is glucose-6-phosphate dehydrogenase (G6PDH), which is the first step in the pentose phosphate pathway and has the capacity to generate relatively large amounts of NADPH [78,79]. Aldose reductase (AR) is another glucose-utilizing enzyme that has opposite effects on the NADPH pool: it can reduce sugars as well as lipid-derived aldehydes to their respective alcohols in an NADPH-dependent manner [80,81]. Collectively, these pathways of intermediary metabolism necessarily modulate the antioxidant capacity of the cell by regulating NADPH levels.
In addition, dehydrogenase enzymes that convert reactive lipid aldehydes to nonreactive carboxylates commonly utilize NADþ as a cofactor. Because the NADþ/NADH ratio is a function of the energetic state of the mitochondrion, the detoxification capacity of such enzymes is then envisaged to be fundamentally linked with the energy status of the cell. This is perhaps best illustrated by the aldehyde dehydrogenase (ALDH) class of enzymes, with particular emphasis on mitochondria-localized ALDH2. In isolated rat liver mitochondria, it was shown that the ability of this enzyme to oxidize acetaldehyde was stimulated when ADP was provided. This then suggests that aldehydes themselves may be substrates for mitochondrial respiration and that they could contribute to ATP generation. Indeed, the ADP/O ratio of acetaldehyde-fueled respiration was found to be 2.6, which is similar to that with glutamate added as substrate [82]. The linkage between the detoxification and respiratory activities of the enzyme is further evinced by the fact that inhibition of mitochondrial activity with rotenone and antimycin A abolishes acetaldehyde oxidation and that addition of other respiratory substrates such as succinate (which would favor a more reduced pyridine nucleotide pool) decreases aldehyde detoxification activity [82,83]. Hence, alde-hyde oxidation in mitochondria is dependent upon NADþ levels and the energy state of the organelle [84], which predicts that pathological conditions that perturb energy and NADþ metabolism will impact the detoxification of reactive species. This appears to be the case, especially in the context of tissue ischemia. In the heart, ischemia was found to decrease the NADþ/NADH ratio from 11 (under aerobic conditions) to 0.4 (during ischemia), which contributed to a diminished ability to detoxify HNE to its corresponding acid and resulted in accumulation of mitochondrial protein-HNE adducts [85].
Oxidative posttranslational modifications of metabolic proteins
The second level of regulation involves the direct modification of proteins involved in intermediary metabolism (Fig. 4).
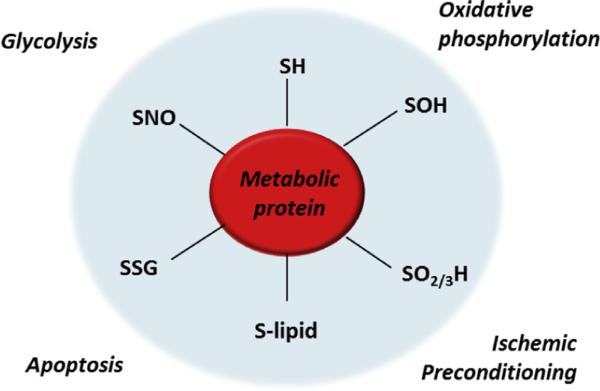
Fig. 4.
Oxidative modification of metabolic proteins. The cysteine side chains of multiple metabolic proteins are modified by reactive species. Modifications such as sulfenic acid (-SOH), sulfinic and sulfonic acids (-SO2/3H), electrophile adducts (e.g., by reactive lipids; -S-lipid), glutathiolation (-SSG), and S-nitrosation (-SNO) can regulate protein activity and promote adaptive responses in metabolism that can regulate cytoprotection or promote cell death.
Modifications such as S-glutathiolation have been shown to have the capacity of modulating glycolytic flux as well as mitochondrial metabolism [86], and several key glucose-handling and mitochondrial proteins can be modified by reactive oxygen, nitrogen, and lipid species. Key enzymes in glucose metabolism pathways modified by reactive species include glyceraldehyde-3-phosphate dehydrogenase (GAPDH), AR, and G6PDH. All of these enzymes have been shown to have reactive cysteines in the active site or vicinal thiols that can modulate catalytic activity. For example, Cys152 of GAPDH can be modified by oxidants such as hydrogen peroxide [87–90], nitric oxide [91,92], and nitroalkene derivatives [93]. Interestingly, the activity of AR, which converts glucose to sorbitol and also has the capacity to reduce reactive aldehydes to their corresponding alcohols, is tightly controlled by oxidants: oxidation of Cys298 to a sulfenic acid results in robust activation of the enzyme [94,95], while protein glutathiolation at the same site inhibits its catalytic function [96]. Thus, this represents a redox switch that can be turned on and off by different oxidative posttranslational modifications, the addition and removal of which are controlled by enzymes such as GSTs and glutaredoxins [97]. Interestingly, the NADPH-producing enzyme, G6PDH, is also regulated by oxidation [98–102], suggesting an integrated mode of feedback to the redox state of the cell. Hence, it appears that many important nodes of glucose metabolism are regulated by redox-active cysteines or nucleophiles, which are subject to modification by a number of reactive species. This would appear to be important in cellular decisions directing the flux of glucose, which is not only critical for the generation of ATP but for the synthesis of nucleotides and phospholipids as well [103].
Mitochondria are subject to even more complex regulation by oxidants and electrophiles. The activity of several electron transport chain complexes is modulated by posttranslational modifications such as S-nitros(yl)ation, S-glutathio(ny)lation, and/or electrophile additions. In particular, complex I, the entry point of electrons derived from NADH-producing reactions, is modified by nitric oxide (or its derivatives) [104–108] and glutathione [109– 113]. Inhibition of complex I through S-nitrosation was shown to be protective in the context of myocardial ischemia-reperfusion injury [114–116], and this modification appears to increase super-oxide production from the complex [106,117]. Nitrotyrosine modification has also been demonstrated on the complex [118,119], as has glutathione modification, which appears to be evolved in this context via thiyl radical formation [113]. Similarly, other electron transport complexes (e.g., complexes II and V) as well as proteins in the mitochondrial matrix (e.g., α-ketoglutarate dehydrogenase, isocitrate dehydrogenase) have been shown to be modified by reactive species [120–132], with the modifications, in most cases, affecting protein catalytic activity. Interestingly, recent studies suggest that S-nitrosation has remarkable effects on the activity of specific pathways of mitochondrial metabolism. For example, the enzyme very long chain acyl-coenzyme A dehydrogenase was shown to be S-nitrosated in an endothelial NOS-dependent manner, which increases the catalytic activity of the enzyme [133] and would be thought to increase the oxidation of long chain fats in the mitochondrion. The idea that specific modifications participate in metabolic fuel selection in tissues is thus an exciting prospect for future studies.
Oxidative modifications have also been shown to be critical in the regulation of proteins involved in mitochondrial metabolic control and cell death pathways. The adenine nucleotide transporter (ANT), for example, has been shown to be modified at sites critical for its function. Cys57, which is located on the matrix face of the cyclophilin D binding site, has been shown to be glutathiolated in vivo in the hearts of inducible NOS transgenic mice [134] and alkylated by nitro-oleate [135]. Further studies are required to develop a more complete model of the role of such modifications in controlling respiratory activity and protecting the mitochondrion from irreversible damage during conditions of high oxidant generation.
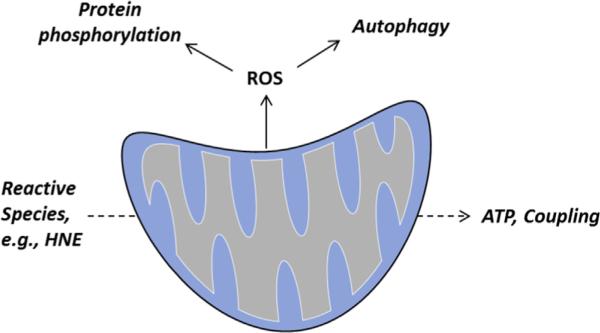
Redox signaling and bioenergetic responses to reactive species
The integration of metabolic responses with adaptation to rapidly changing redox environments is extremely important in acute pathological states such as ischemia-reperfusion injury (Fig. 5). Conditions of ischemia result in a buildup of NADH that, upon reperfusion, creates a burst of free radicals from electron transport chain complexes. This can further propagate lipid radical reactions that produce electrophile species. Attempts to model this condition in multiple cell types have yielded surprising results.
Fig. 5.
Redox signaling and bioenergetic responses to reactive species. The response of the mitochondrion to reactive species can influence cell signaling pathways or activate pathways of protein degradation, such as autophagy. Reactive species such as HNE can regulate ATP production and turnover. In addition, conditions such as ischemia result in mitochondrial H2O2 generation which participates in redox signaling, leading to adaptive responses to stress.
Neonatal and adult cardiomyocytes treated with HNE show remarkable increases in both glycolytic and mitochondrial activity [136,137], which appears to be driven both by increases in ATP demand and proton leak. Other cell types such as smooth muscle cells show an opposite effect, where oxygen consumption is decreased by exposure to HNE [137]. NO and derivative species elicit even more complex bioenergetic responses. Exposure of cells to NO species could have multiple effects, including direct inhibition of cytochrome oxidase; nitrosation, oxidation, or glutathiolation of thiol residues; and nitration of tyrosines. Hence, species such as peroxynitrite and S-nitrosothiols are predicted to have differential effects on mitochondrial respiration and glycolysis [25,138,139]. In most cases, cells with higher glycolytic or mitochondrial reserve capacities appear more resistant to reactive species-induced mitochondrial dysfunction [137,140]. It is hypothesized that a higher reserve capacity could bestow the cell with a better capacity to maintain critical ATP-dependent processes during stress, such as ATP-dependent proteolysis [141,142].
Redox stress can elicit signaling from mitochondria to adapt to environmental conditions or pathological stressors. Most commonly, ROS such as superoxide and H2O2 are suggested to be important for regulating the redox status of the cell and to help maintain viability or trigger cell death [143]. The concept of ROS-mediated signaling requires careful consideration for spatial regulation of redox-sensitive signaling pathways, gradients between subcellular compartments, and cause-and-effect relationships. Significant levels of superoxide production have been shown to occur from pyruvate dehydrogenase, oxoglutarate dehydrogenase, complex I, electron transfer flavoprotein ubiquinone oxidoreductase, and glycerol-3-phosphate dehydrogenase [144]. The generation of superoxide, in most cases, leads to the production of H2O2, which is more stable and able to diffuse across biological membranes. These characteristics impart the molecule with an ability to propagate signal transduction. For example, growth factor-mediated signaling requires H2O2 [145,146], and H2O2 has been shown to be critical in protein phosphorylation-mediated signaling, as it oxidizes critical cysteine residues of protein phosphatases [147]. Retrograde, superoxide/hydrogen peroxide-dependent signaling from mitochondria has also been shown to be important to quality control processes in the cell, such as autophagy [59]. Not surprisingly, this third level regulation is highly linked with pyridine nucleotides and oxidative modifications. Bioenergetic responses to stress may be initiated by changes in pyridine nucleotides and substrate availability, and oxidative modifications such as protein glutathiolation of respiratory complexes regulate mitochondria-derived superoxide or hydrogen peroxide, which could direct cell signaling.
Therapeutic applications
The notion that multiple antioxidant and cytoprotective genes could be induced by a single molecule via activation of stress signaling pathways has attracted considerable attention in drug development, as this approach is potentially attractive for many disease processes in which oxidative stress and inflammation play a role [148,149]. The first molecules that were tested were derived from plants. The most commonly used of these is sulforaphane, which is usually administered in the form of broccoli extract. Sulforaphane was first used for chemoprevention and its primary mode of action is the activation of the Keap1-Nrf2 pathway, although it has other signaling effects such as the inhibition of NF-κB [150]. Other indications have followed, and the NIH clinical trial registry ClinicalTrials.gov now lists a total of 26 trials for conditions ranging from chronic obstructive pulmonary disease (COPD) to sickle cell disease.
Apart from sulforaphane, several other Nrf2 activating drugs have entered clinical development. Bardoxolone methyl, a triterpenoid and potent Nrf2 activator, was acquired from Reata by Abbott for clinical development [151]. However, while a Phase II study (BEAM) for chronic kidney disease in type II diabetics showed an improvement in kidney function assessed by glomerular filtration rate, the Phase III study was terminated due to a higher rate of cardiovascular events than with placebo, which prompted termination of the trial [152]. Subsequently, all other clinical trials with the compound were halted. However, dimethyl fumarate, another Nrf2 activator that was initially developed as an oral treatment for psoriasis in 1950s, was proven to be effective in multiple sclerosis (MS) in two Phase III clinical trials where the number of relapses and lesions detected by MRI and the rate of disability progression were decreased, without any serious adverse effects [153,154]. The drug is now approved by FDA and European Medicines Agency (EMA) for relapsing forms of MS. Of note, in preclinical animal studies, dimethyl fumarate has been shown to protect against cardiac ischemia-perfusion injury via activation of Nrf2, suggesting that other indications may soon follow [155].
In addition fatty acid-derived electrophilic compounds such as cyclopentenone prostaglandins and nitroalkenes have been tested in preclinical studies and likely possess convergent mechanisms of action [156]. For example, 15d-PGJ2 has been shown to attenuate inflammation via the Keap1-Nrf2 pathway and other redox-sensitive pathways in a number of models of inflammation [157–159]. Electrophilic neurite outgrowth-promoting prostaglandin (NEPP) compounds have been developed based on the chemical structures of cyclopentenone prostaglandins [160], and one of these, NEPP11, was found to be protective against ischemia-reperfusion injury in mice via Nrf2 activation [161]. There is a wealth of preclinical evidence of the protective effects of nitroalkenes, OA-NO2 in particular, in various animal models of metabolic and inflammatory disorders [29]. These are now in clinical development for the treatment of diseases associated with kidney injury, inflammation, and metabolic disorders, the initial clinical target being contrast imaging, dye-induced nephropathy.
While the success of dimethyl fumarate has increased enthusiasm for the use of Nrf2 activating drugs for various degenerative diseases, there are issues that warrant attention. The mode of action of the majority of Nrf2 activating agents developed to date is via covalent adduction of Keap1 thiol residues, and practically all have other targets as well. An important aspect of electrophilic signaling is that the covalent modification of a signaling molecule can accumulate slowly over time with a progressive activation of a signaling pathway [162]. This has important implications in the pharmacological application of electrophiles because the cellular response to electrophiles is typically biphasic, with activation of protective signaling pathways occurring at low concentrations and promotion of ER stress and cell death as exposure increases [34,39,140,163]. The mitochondria of highly energetic tissues such as the heart and kidney are particularly vulnerable with extended exposures to electrophiles and thus are susceptible to electrophile-dependent toxicity. This phenomenon may well explain the cardiotoxic effects of bardoxolone methyl [152]. Furthermore, the detoxification pathways for electrophiles vary in human populations, suggesting, as with other therapies, the need for a personalized medicine approach [164–166]. Classical pharmacological approaches for determining the bioavailability and pharmacokinetics of electro-philes are hampered by their reactivity with nucleophilic residues, particularly cysteine. Since with some electrophiles these covalent adducts are reversible in the presence of other nucleophiles there is potential for a reservoir of electrophilic therapeutics to accumulate during long-term dosing. As noted above, the cell signaling effects of electrophiles also accumulate over time and this can greatly enhance their potency [162]. Clearly, a new approach to the pharmacology of electrophiles will be needed to understand how these molecules interact with both normal and diseased cells. At a minimum monitoring the extent of thiol modification over time may be necessary to achieve an effective therapeutic index.
Underlying the potential toxicity of electrophiles is the fact that the “cysteine code” and the resulting electrophile-responsive proteome are distinct for each electrophile [33,167]. This is potentially important in dictating off-target effects, as exemplified by differences in side effects between bardoxolone methyl and dimethyl fumarate. Another important factor to take into consideration is the possibility of adverse effects of Nrf2 activation. Although Nrf2 is used for chemoprevention, constitutively over-active Nrf2 is common in certain cancers, promoting cancer cell proliferation and chemoresistance [31]. Conceptually, an interesting idea is emerging in which the consequence of hyperactivation of the Nrf2 system is a “reductive stress” with attendant unique redox-related pathologies [168]. While it has been argued that high levels of Nrf2 activity present in cancer cells where the Keap1-Nrf2 system is dysregulated are not attainable by Nrf2 activating drugs, only long-term use of these drugs will reveal whether their use is associated with a higher incidence of cancer. In addition to cancer cell growth promoting effects, Nrf2 has metabolic effects that are still somewhat ill-characterized and controversial. Paradoxically, the loss of Nrf2 in hypercholesterolemic, ApoE-deficient mice protects against atherosclerosis [169–171], yet its absence in bone marrow-derived cells is proatherogenic [172,173], suggesting a link between Nrf2 and atherosclerosis that is cell-type specific. In addition, Nrf2 affects liver and adipose tissue lipid and glucose metabolism and thus Nrf2 activation may have unfavorable systemic metabolic effects [174].
Summary
Our understanding of the redox biology of the cell has matured since the first simple concepts underlying the oxidative stress paradigm. The translational application of the key idea that supplementation with oxidant scavenging will prevent or reverse pathologies associated with oxidative stress has not been successful. However, in the course of these studies a new perspective has emerged. The central idea is that redox-active mediators such as nitric oxide, hydrogen peroxide, and lipid electrophiles act as site-specific mediators of cell signaling: protein cysteine residues are the sensors or receptors of these different redox mediators and the “traditional” antioxidants such as glutathione and α-tocopherol serve the essential function of insulating distinct redox signaling domains in the cell from cross-talk. Interestingly, an important implication of these ideas is that targeting of redox therapeutics will dramatically alter their pharmacological effects. Pathology is associated with dysregulation of these pathways and this can affect metabolism, autophagy, cell growth, and repair. The most successful translational exploitation of these pathways to date has been the selective activation of the Keap1-Nrf2 system. As with all new therapeutics the application of selective modulators of this pathway is revealing how much we have yet to learn about this growing field of redox biology.
Acknowledgments
The authors are grateful to the following agencies for support of their research programs: Emil Aaltonen Foundation and Orion-Farmos Foundation (EK), the Academy of Finland and Sigrid Juselius Foundation (ALL), NIHR01-NS064090, a VA merit award (to J.Z.), and NIH GM103492 (B.G.H.).
Abbreviations
- ALDH2
aldehyde dehydrogenase 2
- ANT
adenine nucleotide transportor
- AR
aldose reductase
- AMPK
AMP-activated protein kinase
- ATF6
activating transcription factor 6
- ATG
AuTophaGy
- ATM
ataxia-telangiectasia mutate
- COPD
chronic obstructive pulmonary disease
- EMA
European Medicines Agency
- ER
endoplasmic reticulum
- ESR
electrophilic stress response
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- G6PDH
glucose-6-phosphate dehydrogenase
- GRP78/BiP
78-kDa glucose-regulated protein
- GST
gluthione-S-transferase
- HNE
4-hydroxynonenal
- HSE
heat shock element
- HSF1
heat shock factor-1
- HSP
heat shock protein
- HSR
heat shock response
- IRE1
inositol-requiring protein-1
- ME
malic enzyme
- mTOR
mammalian target of rapamycin
- MS
multiple sclerosis
- NNT
nicotinamide nucleotide transhydrogenase
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- LKB1
liver kinase B1
- LC3
microtubule-associated protein light chain 3
- p62/SQSTM1
sequestosome-1
- PERK
double-stranded RNA-dependent protein kinase (PKR)-like ER kinase
- ROS
reactive oxygen species
- UPR
unfolded protein response
References
- 1.Sies H. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 2.Niki E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012;586:3767–3770. doi: 10.1016/j.febslet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34:171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 4.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta. 2014;1840:809–817. doi: 10.1016/j.bbagen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Davies SS, Roberts LJ., 2nd F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 7.Niki E. Assessment of antioxidant capacity of natural products. Curr. Pharm Biotechnol. 2010;11:801–809. doi: 10.2174/138920110793262097. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi N, Niki E. Phenolic antioxidants: a rationale for design and evaluation of novel antioxidant drug for atherosclerosis. Free Radic. Biol. Med. 2000;28:1538–1546. doi: 10.1016/s0891-5849(00)00256-2. [DOI] [PubMed] [Google Scholar]
- 9.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niki E, Traber MG. A history of vitamin E. Ann. Nutr. Metab. 2012;61:207–212. doi: 10.1159/000343106. [DOI] [PubMed] [Google Scholar]
- 11.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traber MG. Does vitamin E decrease heart attack risk? Summary and implications with respect to dietary recommendations. J. Nutr. 2001;131:395S–-397S. doi: 10.1093/jn/131.2.395S. [DOI] [PubMed] [Google Scholar]
- 13.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 14.Roberts LJ, 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 2007;43:1388–-1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HY, Caballero B, Chang S, Alberg AJ, Semba RD, Schneyer CR, Wilson RF, Cheng TY, Vassy J, Prokopowicz G, Barnes GJ, 2nd, Bass EB. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann. Intern. Med. 2006;145:372–385. doi: 10.7326/0003-4819-145-5-200609050-00135. [DOI] [PubMed] [Google Scholar]
- 16.Gey KF. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br. Med. Bull. 1993;49:679–699. doi: 10.1093/oxfordjournals.bmb.a072640. [DOI] [PubMed] [Google Scholar]
- 17.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 2006;84:1200–1207. doi: 10.1093/ajcn/84.5.1200. [DOI] [PubMed] [Google Scholar]
- 18.Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, Tognoni G, Valagussa F. Vitamin E increases the risk of developing heart failure after myocardial infarction: results from the GISSI-Prevenzione trial. J. Cardiovasc. Med. (Hagerstown) 2006;7:347–350. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- 19.Traber MG, Frei B, Beckman JS. Vitamin E revisited: do new data validate benefits for chronic disease prevention? Curr. Opin. Lipidol. 2008;19:30–38. doi: 10.1097/MOL.0b013e3282f2dab6. [DOI] [PubMed] [Google Scholar]
- 20.Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 21.Terentis AC, Thomas SR, Burr JA, Liebler DC, Stocker R. Vitamin E oxidation in human atherosclerotic lesions. Circ. Res. 2002;90:333–339. doi: 10.1161/hh0302.104454. [DOI] [PubMed] [Google Scholar]
- 22.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3:120144. doi: 10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawa T, Arimoto H, Akaike T. Regulation of redox signaling involving chemical conjugation of protein thiols by nitric oxide and electrophiles. Bioconjugate Chem. 2010;21:1121–1129. doi: 10.1021/bc900396u. [DOI] [PubMed] [Google Scholar]
- 25.Hill BG, Dranka BP, Bailey SM, Lancaster JR, Jr, Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010;285:19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 28.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006;147(Suppl. 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traber MG. Mechanisms for the prevention of vitamin E excess. J. Lipid Res. 2013;54:2295–2306. doi: 10.1194/jlr.R032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kansanen E, Jyrkkanen HK, Levonen AL. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012;52:973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 33.Higdon AN, Landar A, Barnes S, Darley-Usmar VM. The electrophile responsive proteome: integrating proteomics and lipidomics with cellular function. Antioxid. Redox Signal. 2012 doi: 10.1089/ars.2012.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higdon AN, Benavides GA, Chacko BK, Ouyang X, Johnson MS, Landar A, Zhang J, Darley-Usmar VM. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. physiol. Heart Circ. Physiol. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem. Sci. 2002;27:489–492. doi: 10.1016/s0968-0004(02)02191-6. [DOI] [PubMed] [Google Scholar]
- 36.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 37.Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J. Biol. Chem. 2005;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- 38.Kansanen E, Kivela AM, Levonen AL. Regulation of Nrf2-dependent gene expression by 15-deoxy-delta12,14-prostaglandin J2. Free Radic. Biol. Med. 2009;47:1310–1317. doi: 10.1016/j.freeradbiomed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 39.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickinson DA, Levonen AL, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, Forman HJ. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 45.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H, Garcia-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell. Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell. Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem. Res. Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Gibney PA, West JD, Morano KA. The yeast Hsp70 Ssa1 is a sensor for activation of the heat shock response by thiol-reactive compounds. Mol. Biol. Cell. 2012;23:3290–3298. doi: 10.1091/mbc.E12-06-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calamini B, Silva MC, Madoux F, Hutt DM, Khanna S, Chalfant MA, Saldanha SA, Hodder P, Tait BD, Garza D, Balch WE, Morimoto RI. Small-molecule proteostasis regulators for protein conformational diseases. Nat. Chem. Biol. 2012;8:185–196. doi: 10.1038/nchembio.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santagata S, Xu YM, Wijeratne EM, Kontnik R, Rooney C, Perley CC, Kwon H, Clardy J, Kesari S, Whitesell L, Lindquist S, Gunatilaka AA. Using the heat-shock response to discover anticancer compounds that target protein homeostasis. ACS Chem. Biol. 2012;7:340–349. doi: 10.1021/cb200353m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell. Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 52.Haberzettl P, Hill BG. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013;1:56–64. doi: 10.1016/j.redox.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi S, Odani N, Tomokiyo K, Furuta K, Suzuki M, Ichikawa A, Negishi M. Localization of a cyclopentenone prostaglandin to the endoplasmic reticulum and induction of BiP mRNA. Biochem. J. 1998;335(Pt 1):35–42. doi: 10.1042/bj3350035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 2005;18:1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- 55.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell T, Chacko B, Ballinger SW, Bailey SM, Zhang J, Darley-Usmar V. Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem. Soc. Trans. 2013;41:127–133. doi: 10.1042/BST20120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 2013;63:207–-221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013;1:19–23. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyama A, Saito Y, Yamanaka K, Hayashi K, Hamakubo T, Noguchi N. Oxidation of DJ-1 induced by 6-hydroxydopamine decreasing intracellular glutathione. PLoS One. 2011;6:e27883. doi: 10.1371/journal.pone.0027883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Jia W, He YW. Temporal regulation of intracellular organelle homeostasis in T lymphocytes by autophagy. J. Immunol. 2011;186:5313–5322. doi: 10.4049/jimmunol.1002404. [DOI] [PubMed] [Google Scholar]
- 70.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 73.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA. 2012;109:13561–-13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakamura M, Bhatnagar A, Sadoshima J. Overview of pyridine nucleotides review series. Circ. Res. 2012;111:604–610. doi: 10.1161/CIRCRESAHA.111.247924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gameiro PA, Laviolette LA, Kelleher JK, Iliopoulos O, Stephanopoulos G. Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J. Biol. Chem. 2013;288:12967–12977. doi: 10.1074/jbc.M112.396796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HC, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013;63:446–456. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 78.Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994;8:174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- 79.Hecker PA, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H491–H500. doi: 10.1152/ajpheart.00721.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petrash JM. All in the family: aldose reductase and closely related aldo-keto reductases. Cell. Mol. Life Sci. 2004;61:737–749. doi: 10.1007/s00018-003-3402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del Corso A, Cappiello M, Mura U. From a dull enzyme to something else: facts and perspectives regarding aldose reductase. Curr. Med. Chem. 2008;15:1452–1461. doi: 10.2174/092986708784638870. [DOI] [PubMed] [Google Scholar]
- 82.Hasumura Y, Teschke R, Lieber CS. Characteristics of acetaldehyde oxidation in rat liver mitochondria. J. Biol. Chem. 1976;251:4908–4913. [PubMed] [Google Scholar]
- 83.Cinti DL, Keyes SR, Lemelin MA, Denk H, Schenkman JB. Biochemical properties of rat liver mitochondrial aldehyde dehydrogenase with respect to oxidation of formaldehyde. J. Biol. Chem. 1976;251:1571–1577. [PubMed] [Google Scholar]
- 84.Lee IY, Chance B. Regulatory factors of acetaldehyde metabolism in isolated rat liver mitochondria. Adv. Exp. Med. Biol. 1977;85A:203–224. doi: 10.1007/978-1-4899-5181-6_14. [DOI] [PubMed] [Google Scholar]
- 85.Hill BG, Awe SO, Vladykovskaya E, Ahmed Y, Liu SQ, Bhatnagar A, Srivastava S. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem. J. 2009;417:513–524. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill BG, Higdon AN, Dranka BP, Darley-Usmar VM. Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation. Biochim. Biophys. Acta. 2010;1797:285–295. doi: 10.1016/j.bbabio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brodie AE, Reed DJ. Reversible oxidation of glyceraldehyde 3-phosphate dehydrogenase thiols in human lung carcinoma cells by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1987;148:120–125. doi: 10.1016/0006-291x(87)91084-9. [DOI] [PubMed] [Google Scholar]
- 88.Schuppe-Koistinen I, Moldeus P, Bergman T, Cotgreave IA. S-Thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment. Eur. J. Biochem. 1994;221:1033–1037. doi: 10.1111/j.1432-1033.1994.tb18821.x. [DOI] [PubMed] [Google Scholar]
- 89.Shenton D, Grant CM. Protein S-thiolation targets glycolysis and protein synthesis in response to oxidative stress in the yeast Saccharomyces cerevisiae. Biochem. J. 2003;374:513–519. doi: 10.1042/BJ20030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang NR, Yim SH, Kim YM, Jeong J, Song EJ, Lee Y, Lee JH, Choi S, Lee KJ. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem. J. 2009;423:253–264. doi: 10.1042/BJ20090854. [DOI] [PubMed] [Google Scholar]
- 91.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-Nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochim. Biophys. Acta. 2006;1762:502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaiserova K, Srivastava S, Hoetker JD, Awe SO, Tang XL, Cai J, Bhatnagar A. Redox activation of aldose reductase in the ischemic heart. J. Biol. Chem. 2006;281:15110–15120. doi: 10.1074/jbc.M600837200. [DOI] [PubMed] [Google Scholar]
- 95.Kaiserova K, Tang XL, Srivastava S, Bhatnagar A. Role of nitric oxide in regulating aldose reductase activation in the ischemic heart. J. Biol. Chem. 2008;283:9101–9112. doi: 10.1074/jbc.M709671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wetzelberger K, Baba SP, Thirunavukkarasu M, Ho YS, Maulik N, Barski OA, Conklin DJ, Bhatnagar A. Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J. Biol. Chem. 2010;285:26135–26148. doi: 10.1074/jbc.M110.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hill BG, Bhatnagar A. Protein S-glutathiolation: redox-sensitive regulation of protein function. J. Mol. Cell. Cardiol. 2012;52:559–567. doi: 10.1016/j.yjmcc.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida A. Change of activity and substrate specificity of human glucose 6-phosphate dehydrogenase by oxidation. Arch. Biochem. Biophys. 1973;159:82–88. doi: 10.1016/0003-9861(73)90431-1. [DOI] [PubMed] [Google Scholar]
- 99.Szweda LI, Stadtman ER. Iron-catalyzed oxidative modification of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides. Structural and functional changes. J. Biol. Chem. 1992;267:3096–3100. [PubMed] [Google Scholar]
- 100.Szweda LI, Stadtman ER. Oxidative modification of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides by an iron(II)-citrate complex. Arch. Biochem. Biophys. 1993;301:391–395. doi: 10.1006/abbi.1993.1161. [DOI] [PubMed] [Google Scholar]
- 101.Szweda LI, Uchida K, Tsai L, Stadtman ER. Inactivation of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Selective modification of an active-site lysine. J. Biol. Chem. 1993;268:3342–3347. [PubMed] [Google Scholar]
- 102.Ciolino HP, Levine RL. Modification of proteins in endothelial cell death during oxidative stress. Free Radic. Biol. Med. 1997;22:1277–1282. doi: 10.1016/s0891-5849(96)00495-9. [DOI] [PubMed] [Google Scholar]
- 103.Newsholme EA, Board M. Application of metabolic-control logic to fuel utilization and its significance in tumor cells. Adv. Enzyme Regul. 1991;31:225–246. doi: 10.1016/0065-2571(91)90015-e. [DOI] [PubMed] [Google Scholar]
- 104.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta. 2004;1658:44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem. J. 2006;394:627–-634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 107.Galkin A, Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J. Biol. Chem. 2007;282:37448–37453. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 108.Hill BG, Darley-Usmar VM. S-Nitrosation and thiol switching in the mitochondrion: a new paradigm for cardioprotection in ischaemic preconditioning. Biochem. J. 2008;412:e11–e13. doi: 10.1042/BJ20080716. [DOI] [PubMed] [Google Scholar]
- 109.Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 110.Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J. Biol. Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 111.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–5765. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J. Biol. Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kang PT, Zhang L, Chen CL, Chen J, Green KB, Chen YR. Protein thiyl radical mediates S-glutathionylation of complex I. Free Radic. Biol. Med. 2012;53:962–973. doi: 10.1016/j.freeradbiomed.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc. Natl. Acad. USA. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borutaite V, Brown GC. S-Nitrosothiol inhibition of mitochondrial complex I causes a reversible increase in mitochondrial hydrogen peroxide production. Biochim. Biophys. Acta. 2006;1757:562–566. doi: 10.1016/j.bbabio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 118.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J. Biol. Chem. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 119.Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome Res. 2009;8:3222–3238. doi: 10.1021/pr900039c. [DOI] [PubMed] [Google Scholar]
- 120.Stachowiak O, Dolder M, Wallimann T, Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. J. Biol. Chem. 1998;273:16694–16699. doi: 10.1074/jbc.273.27.16694. [DOI] [PubMed] [Google Scholar]
- 121.Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc. Natl. Acad. USA. 1999;96:6689–6693. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. J. Biol. Chem. 2003;278:45154–45159. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
- 123.Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J. Biol. Chem. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 124.Venkatraman A, Landar A, Davis AJ, Ulasova E, Page G, Murphy MP, Darley-Usmar V, Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G521–G527. doi: 10.1152/ajpgi.00399.2003. [DOI] [PubMed] [Google Scholar]
- 125.Benderdour M, Charron G, Comte B, Ayoub R, Beaudry D, Foisy S, Deblois D, Des Rosiers C. Decreased cardiac mitochondrial NADP+-isocitrate dehydrogenase activity and expression: a marker of oxidative stress in hypertrophy development. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2122–H2131. doi: 10.1152/ajpheart.00378.2004. [DOI] [PubMed] [Google Scholar]
- 126.Yarian CS, Rebrin I, Sohal RS, Aconitase ATP. synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem. Biophys. Res. Commun. 2005;330:151–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bulteau AL, Lundberg KC, Ikeda-Saito M, Isaya G, Szweda LI. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc. Natl. Acad. USA. 2005;102:5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim J, Rodriguez ME, Guo M, Kenney ME, Oleinick NL, Anderson VE. Oxidative modification of cytochrome c by singlet oxygen. Free Radic. Biol. Med. 2008;44:1700–1711. doi: 10.1016/j.freeradbiomed.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen CL, Chen J, Rawale S, Varadharaj S, Kaumaya PP, Zweier JL, Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J. Biol. Chem. 2008;283:27991–28003. doi: 10.1074/jbc.M802691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murray CI, Kane LA, Uhrigshardt H, Wang SB, Van Eyk JE. Site-mapping of in vitro S-nitrosation in cardiac mitochondria: implications for cardioprotection. Mol. Cell. Proteomics. 2011;10(M110):004721. doi: 10.1074/mcp.M110.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Curtis JM, Hahn WS, Stone MD, Inda JJ, Droullard DJ, Kuzmicic JP, Donoghue MA, Long EK, Armien AG, Lavandero S, Arriaga E, Griffin TJ, Bernlohr DA. Protein carbonylation and adipocyte mitochondrial function. J. Biol. Chem. 2012;287:32967–32980. doi: 10.1074/jbc.M112.400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McLain AL, Cormier PJ, Kinter M, Szweda LI. Glutathionylation of alpha-ketoglutarate dehydrogenase: the chemical nature and relative susceptibility of the cofactor lipoic acid to modification. Free Radic. Biol. Med. 2013;61C:161–169. doi: 10.1016/j.freeradbiomed.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci. Signal. 2013;6:rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: an intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- 135.Nadtochiy SM, Zhu Q, Urciuoli W, Rafikov R, Black SM, Brookes PS. Nitroalkenes confer acute cardioprotection via adenine nucleotide translocase 1. J. Biol. Chem. 2012;287:3573–3580. doi: 10.1074/jbc.M111.298406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem. Biol. Interact. 2011;191:288–295. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Diers AR, Broniowska KA, Hogg N. Nitrosative stress and redox-cycling agents synergize to cause mitochondrial dysfunction and cell death in endothelial cells. Redox Biol. 2013;1:1–7. doi: 10.1016/j.redox.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sansbury BE, Riggs DW, Brainard RE, Salabei JK, Jones SP, Hill BG. Responses of hypertrophied myocytes to reactive species: implications for glycolysis and electrophile metabolism. Biochem. J. 2011;435:519–528. doi: 10.1042/BJ20101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 142.Livnat-Levanon N, Glickman MH. Ubiquitin-proteasome system and mitochondria—reciprocity. Biochim. Biophys. Acta. 2011;1809:80–87. doi: 10.1016/j.bbagrm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 143.Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic. Biol. Med. 2001;30:1008–1018. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- 144.Brand MD. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 146.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 147.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 148.Yates MS, Kensler TW. Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect. 2007;20:109–117. doi: 10.1358/dnp.2007.20.2.108343. [DOI] [PubMed] [Google Scholar]
- 149.Groeger AL, Freeman BA. Signaling actions of electrophiles: anti-inflammatory therapeutic candidates. Mol. Interv. 2010;10:39–50. doi: 10.1124/mi.10.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW, Talalay P. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top. Curr. Chem. 2013;329:163–177. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Crunkhorn S. Deal watch: Abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat. Rev. Drug Discov. 2012;11:96. doi: 10.1038/nrd3655. [DOI] [PubMed] [Google Scholar]
- 152.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 154.Kappos L, Gold R, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Sarda SP, Agarwal S, Zhang A, Sheikh SI, Seidman E, Dawson KT. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: The DEFINE study. Mult. Scler. 2013 doi: 10.1177/1352458513507817. [DOI] [PubMed] [Google Scholar]
- 155.Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, Isackson H, Yavari A, Stottrup NB, Contractor H, Cahill TJ, Sahgal N, Ball DR, Birkler RI, Hargreaves I, Tennant DA, Land J, Lygate CA, Johannsen M, Kharbanda RK, Neubauer S, Redwood C, de Cabo R, Ahmet I, Talan M, Gunther UL, Robinson AJ, Viant MR, Pollard PJ, Tyler DJ, Watkins H. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, Ohashi K, Kawakami K, Kumagai Y, Freeman BA, Yamamoto M, Kobayashi M. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16:46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(delta)12-14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. FASEB J. 2003;17:2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- 158.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-delta(12,14)-prostaglandin J(2). Mol. Cell. Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin. Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 160.Satoh T, Furuta K, Tomokiyo K, Namura S, Nakatsuka D, Sugie Y, Ishikawa Y, Hatanaka H, Suzuki M, Watanabe Y. Neurotrophic actions of novel compounds designed from cyclopentenone prostaglandins. J. Neurochem. 2001;77:50–62. doi: 10.1046/j.1471-4159.2001.t01-1-00229.x. [DOI] [PubMed] [Google Scholar]
- 161.Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc. Natl. Acad. Sci. USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oh JY, Giles N, Landar A, Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. Biochem. J. 2008;411:297–306. doi: 10.1042/bj20071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-delta(12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 164.Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, Krishnamoorthy R. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- 165.Rahman SH, Ibrahim K, Larvin M, Kingsnorth A, McMahon MJ. Association of antioxidant enzyme gene polymorphisms and glutathione status with severe acute pancreatitis. Gastroenterology. 2004;126:1312–1322. doi: 10.1053/j.gastro.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 166.Koide S, Kugiyama K, Sugiyama S, Nakamura S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J. Am. Coll. Cardiol. 2003;41:539–545. doi: 10.1016/s0735-1097(02)02866-8. [DOI] [PubMed] [Google Scholar]
- 167.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann. N. Y. Acad. Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 168.Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid. Redox Signal. 2011;14:957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur. J. Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 172.Ruotsalainen AK, Inkala M, Partanen ME, Lappalainen JP, Kansanen E, Makinen PI, Heinonen SE, Laitinen HM, Heikkila J, Vatanen T, Horkko S, Yamamoto M, Yla-Herttuala S, Jauhiainen M, Levonen AL. The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovas. Res. 2013;98:107–115. doi: 10.1093/cvr/cvt008. [DOI] [PubMed] [Google Scholar]
- 173.Collins AR, Gupte AA, Ji R, Ramirez MR, Minze LJ, Liu JZ, Arredondo M, Ren Y, Deng T, Wang J, Lyon CJ, Hsueh WA. Myeloid deletion of nuclear factor erythroid 2-related factor 2 increases atherosclerosis and liver injury. Arterioscler. Thromb. Vasc. Biol. 2012;32:2839–2846. doi: 10.1161/ATVBAHA.112.300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chartoumpekis DV, Kensler TW. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr. Diabetes Rev. 2013;9:137–145. doi: 10.2174/1573399811309020005. [DOI] [PMC free article] [PubMed] [Google Scholar]