Abstract
Low body weight is associated with increased risk for fractures, whereas higher body weight has been shown to be protective against osteoporosis. This study evaluated whether body weight plays a role regulating bone turnover and mass in normal-weight (body mass index (BMI) <25 kg/m2), overweight (BMI 25–29.9 kg/ m2) and obese (BMI ≥ 30 kg/m2) postmenopausal women who were either receiving hormone replacement therapy [HRT(+)] or not [HRT(−)] (total of six groups). Body weight, BMI, total body bone mineral content (TBBMC), and markers of bone formation (serum osteocalcin) and bone resorption (urinary pyridinoline (PYD) and deoxypyridinoline) were retrospectively analyzed in 210 postmenopausal women. The mean age was 67±6 years, with mean body weight of 70.8±14.2 kg, ranging from 45.0 to 115.5 kg. Body weight was positively correlated with TBBMC (r=0.50, p<0.0001). There was a lower TBBMC and higher bone formation rate in normal-weight than obese HRT(−) women, but in women taking HRT there were no differences between BMI categories. In addition, in normal-weight HRT(−) women only, PYD and body weight showed a negative correlation (r=−0.39, p=0.01). Among normal-weight, but not overweight or obese subjects, we observed higher TBBMC and lower bone turnover in the HRT(+) compared with the HRT(−) group. Regression models explained 36% of the variance in TBBMC, mainly through body weight. Additional models could only explain 11–15% of the variance in bone turnover. Taken together, these data suggest that among normal-weight but not obese postmenopausal women, higher bone turnover is associated with lower bone mass, and that only normal-weight women show a different bone turnover profile with HRT treatment. Body weight should be considered an important factor in bone metabolism with relevant clinical implications.
Keywords: Bone formation, Bone resorption, Bone mass, Body weight, Hormone replacement therapy
Introduction
The positive correlation between body weight and bone mass is well known [1]. Studies have consistently observed an increased risk of osteoporotic fractures among women with lower body weights compared with those who are heavier [2].
The association between bone mass and biochemical markers of bone turnover has received somewhat less attention. High bone turnover has been associated with low bone mass [3, 4]. The value of bone turnover markers in reflecting bone density, however, is not well established. It has been found that bone markers are sensitive predictors of bone mineral density (BMD) changes due to hormone replacement therapy (HRT) and/or Ca supplementation [5, 6] but there is controversy as to whether bone markers can offer practical information for estimating BMD [7].
The relationship between body weight and markers of bone turnover has not been clearly established. It has been observed that markers of bone turnover are higher in leaner compared with heavier postmenopausal women [8], but no studies have included a population of obese women to address how bone turnover differs in subjects with low risk of osteoporosis compared with a higher-risk group (i.e., normal-weight individuals with lower bone mass).
The purpose of this study was to investigate the associations between body weight, markers of bone turnover and bone mass in a population of normal-weight, overweight and obese postmenopausal women. We also examined the impact of HRT among these weight categories. We hypothesized that higher body weights are accompanied by lower bone turnover, explaining at least in part the greater bone mass in obese compared with leaner individuals. Our results show, however, that the higher TBBMC in obese women could not be explained by an altered bone turnover compared with normal-weight women, and that HRT was associated with lower bone turnover only in normal-weight women.
Subjects and methods
Study subjects
Postmenopausal women (at least 3 years since menopause, n=258) recruited from 1995 to 1999 at Rutgers University (NJ), Columbia University (NY) and The University of Georgia (GA) were included in this retrospective study. Women were categorized according to body size, defined by ranges of body mass index (BMI, kg/m2) as indicated by guidelines from the National Institutes of Health [9], into normal-weight (BMI ≤ 25 kg/m2), overweight (BMI between 25.1 and 29.9 kg/m2) and obese (BMI ≥30 kg/m2). Due to large and clinically relevant age differences between groups (original age range 40–80 years), we excluded both extremes of age values (i.e., highest and lowest 10%), leaving a population of 210 women. In addition, the sample was separated into women with or without hormone replacement therapy (HRT(+) and HRT(−), respectively). The subjects signed informed consent and approval was obtained by the Institutional Review Boards of Rutgers University, St Lukes–Roosevelt Hospital, Columbia University, and the University of Georgia.
Bone mass, weight and height
Total body bone mineral content (TBBMC) was measured using dual-energy X-ray absorptiometry (DXA). Lunar (DPX; Lunar, Madison, WI) and Hologic (QDR 100 W; Waltham, MA) instruments were used (coefficients of variation (CV) of 0.9% and 0.6%, respectively). TBBMC measurements from the two machines were standardized as previously validated in our laboratories [10] in men and were based on equations in pre- and postmenopausal women. Body weight and height, measured on a balance scale, were used to calculate BMI (kg/m2).
Bone turnover
Markers of bone resorption (pyridinium crosslinks: pyridinoline (PYD) and deoxypyridinoline (DPD)) were measured in spot urine samples collected after an overnight fast (0900–1000 hours, second void). Urinary PYD and DPD were analyzed by high-performance liquid chromatography after submitting hydrolyzed samples to a prefractionation procedure, as previously described [6]. Peaks were detected by fluorescence and quantitated by external standards (courtesy of S. Robins), and expressed per millimole urinary creatinine (Creat) (#555, Sigma Chemical, St Louis, MO). The interassay coefficients of variation (CVs) for PYD/Creat and DPD/ Creat were 5.5% and 10.0%, respectively. Correction of urinary crosslink excretion for creatinine excretion is necessary to account for the interindividual variability in urine concentration and/or volume, when 24 h urine samples are not available. Although creatinine excretion over 24 h is influenced by lean body mass, it is unlikely to affect spot samples. However, if there was a small effect of muscle mass on creatinine excretion, it could compromise the sensitivity of the crosslinks between different BMI categories. Urinary creatinine excretion was measured (in spot urine samples) in a subset of the data that was available (total of 83 subjects; n=24, 26 and 33 for normal-weight, overweight and obese, respectively). Fasting serum osteocalcin (OC, a marker of bone formation) was determined by radioimmunoassay (RIA; Diagnostic Laboratories Systems, Webster, TX) with intra and interassay CVs <9%.
Statistical analysis
Two-way ANOVA was initially used to analyze the effects of the independent variables body size and HRT on the different dependent variables examined. Due to significant age differences among the different body size groups, age was included as a covariate in the model (ANCOVA). Differences among the various study groups were tested by Tukey’s post-hoc analysis. The associations among the variables studied (weight, height, BMI, markers of bone turnover and TBBMC) were examined using age-adjusted Pearson correlation coefficients (r) in the entire population and by body size and HRT(+)/HRT(−) categories. Stepwise linear regression analysis was used to assess the contribution of independent variables (age, weight, height, bone turnover) to TBBMC, and, in a different model, to markers of bone turnover (TBBMC considered independent variables in addition to others already mentioned). A p value ≤ 0.05 was considered significant. Data are presented as means±SD unless otherwise indicated. All analyses were conducted using the SAS statistical package (SAS Institute, Cary, NC; version 8.0).
Results
The characteristics of the 210 postmenopausal women included in this study are presented in Table 1. The mean age was 67±6 years, and ranged from 55 to 76 years. There were fewer subjects receiving HRT (n=85, 40.5% total) compared with those in the HRT(−) group (n=125, 59.5% total). In an analysis combining HRT(+) and HRT(−) groups, obese women were younger (p<0.05) than both the overweight and normal-weight groups (64±6, 67±5 and 68±5 years for obese, overweight and normal-weight, respectively). Body weights averaged 70.8±14.2 kg and ranged from 45.0 kg to 115.5 kg, while mean BMI was 27.0±5.0 kg/ m2 and ranged from 17.7 kg/m2 to 41.1 kg/m2.
Table 1.
Body weight, bone mass and bone turnover markers in the study population. Values are mean±SD. Values with different superscripts within a row are significantly different (Tukey’s post-hoc test, p<0.05) (TBBMC total body bone mineral content, PYD/ Creat pyridinoline/creatinine, DPD/Creat deoxypyridinoline/creatinine, OC serum osteocalcin)
| No HRT (n=125)
|
HRT (n=85)
|
|||||
|---|---|---|---|---|---|---|
| Normal weight (n=38) | Overweight (n=38) | Obese (n=49) | Normal weight (n=48) | Overweight (n=24) | Obese (n=13) | |
| Weight (kg)§§ | 58.9±4.6a | 72.6±6.4b | 87.8±10.1c | 58.0±5.5a | 69.3±7.5b | 85.6±11.8c |
| Height (m) | 1.62±0.06 | 1.62±0.06 | 1.62±0.07 | 1.62±0.05 | 1.61±0.07 | 1.62±0.0733 |
| BMI (kg/m2)§§ | 22.5±1.6a | 27.7±1.5b | 33.6±3.0c | 22.2±1.7a | 26.6±1.3b | 32.5±3.0c |
| TBBMC (g)*, §§ | 1744±283a | 2007±277b | 2186±309b | 2003±245b | 2007±294b | 2190±431b |
| PYD/Creat (nmol/mmol)† | 30.4±8.2ab | 31.2±13.5ab | 33.6±11.8b | 25.1±12.6a | 23.7±9.2a | 31.5±8.8ab |
| DPD/Creat (nmol/mmol)† | 10.7±3.6a | 9.5±4.3ab | 10.1±4.3ab | 7.9±4.8b | 7.4±3.2b | 9.2±2.9ab |
| OC (nmol/l)§, †† | 1.3±0.5a | 1.1±0.4ac | 0.9±0.3bc | 0.8±0.3b | 0.7±0.3b | 0.7±0.3b |
ANOVA, treatment effect (HRT(+) vs HRT(−)):
p ≤ 0.01,
p<0.0001
ANOVA body size effect (normal weight, overweight or obese):
p<0.05,
p ≤ 0.0001
Two-way ANOVA interaction between treatment and bodysize,
p<0.05
Bone mass, body weight and height
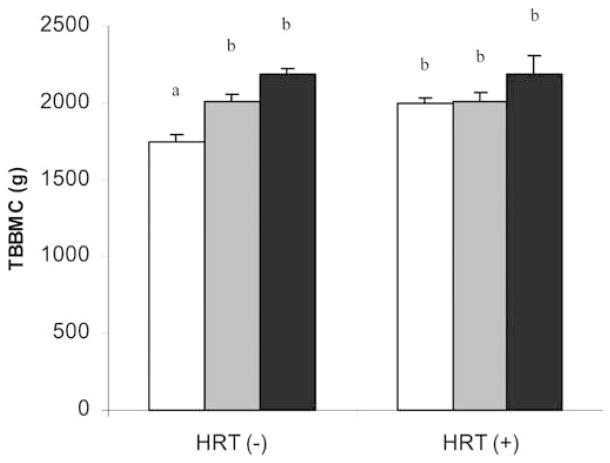
Among women in the HRT(−) group, normal-weight women had lower TBBMC compared with overweight and obese women. No differences in bone mass with BMI category were observed among women receiving HRT (Table 1, Fig. 1). Height did not vary between the BMI and HRT groups (Table 1).
Fig. 1.
Hormone replacement therapy (HRT) status in normal-weight postmenopausal women influences the effect of weight category on total body bone mineral content (TBBMC). Weight categories are defined as normal weight (open columns), overweight (gray columns) and obese (black columns). Each bar represents the mean±SEM of 38 normal-weight, 38 overweight and 49 obese women in the HRT(−) group; and 48 normal-weight, 24 overweight and 13 obese women in the HRT(+) group. Bars labeled with different superscripts are significantly different (two-way ANOVA and Tukey’s post hoc test, p<0.05)
As expected, there was a strong age-adjusted correlation between body weight and TBBMC, that became stronger when women in the HRT(+) group were excluded from the analysis (Table 2). This was not the case among the obese (the association actually became stronger in the HRT(+) group); however, the lower number of subjects in this group may have influenced these results.
Table 2.
Pearson’s age-adjusted correlation coefficients of total body bone mineral content with body weight, body mass index (BMI), pyridinoline/creatinine (PYD/Creat), deoxypyridinoline/creatinine (DPD/Creat) and serum osteocalcin (OC) for the whole group and by body size and HRT categories
| Weight (kg) | Height (m) | BMI (kg/m2) | PYD/Creat (nmol/mmol) | DPD/Creat (nmol/mmol) | OC (ng/ml) | |
|---|---|---|---|---|---|---|
| Entire | ||||||
| All (n=210) | 0.52*** | 0.50*** | 0.31*** | −0.11 | −0.20** | −0.23*** |
| Normal weight (n=86) | 0.42*** | 0.51*** | 0.05 | −0.15 | −0.19† | −0.21* |
| Overweight (n=62) | 0.50*** | 0.54*** | 0.12 | −0.23† | −0.36** | −0.24† |
| Obese(n=62)a | 0.61*** | 0.61*** | 0.21 | −0.17 | −0.16 | −0.19 |
| HRT(−) | ||||||
| All (n=125) | 0.61*** | 0.46*** | 0.41*** | −0.07 | −0.19* | −0.21* |
| Normal weight (n=38) | 0.54*** | 0.67*** | −0.09 | −0.04 | −0.09 | 0.10 |
| Overweight (n=38) | 0.57*** | 0.52*** | 0.19 | −0.22 | −0.39* | −0.20 |
| Obese (n=49) | 0.47*** | 0.57*** | 0.07 | −0.14 | −0.12 | −0.20 |
| HRT(+) | ||||||
| All (n=85) | 0.50*** | 0.57*** | 0.27* | −0.15 | −0.16 | −0.23* |
| Normal weight (n=48) | 0.51*** | 0.50** | 0.21 | −0.09 | −0.05 | −0.16 |
| Overweight (n=24) | 0.40† | 0.55** | −0.09 | −0.28 | −0.37† | −0.43* |
| Obese(n=13)a | 0.96*** | 0.77** | 0.61* | −0.46 | −0.63 | −0.17 |
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001;
trend: p ≤ 0.09
Except for PYD/Creat and DPD/Creat, where n=56 (entire sample) and n=7 [HRT(+)]
Bone formation
Values of serum OC were higher in normal-weight than obese women (p<0.05). However, HRT suppressed OC (1.1±0.5 nmol/l and 0.8±0.3 nmol/l, respectively, p<0.0001), and eliminated the differences between normal-weight and obese subjects (Table 1). A negative association was found between OC and TBBMC, consistently when age-adjusted (Table 2) and not adjusted (Fig. 2A). Serum OC showed a negative correlation with body weight in the HRT(−) group (Fig. 3A).
Fig. 2.
Correlations between total body bone mineral content (TBBMC) and A serum osteocalcin (OC) in 125 HRT(+) and 85 HRT(−) women, and between TBBMC and B urinary pyridinoline (PYD/Creat) and C deoxypyridinoline (DPD/Creat) in 125 HRT(+) and 78 HRT(−) women
Fig. 3.
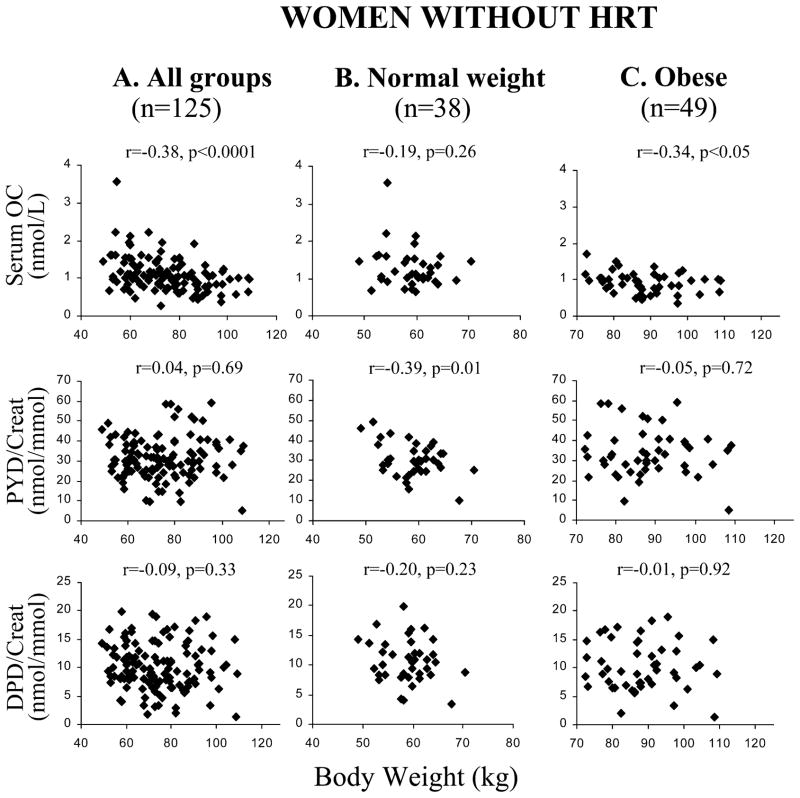
Correlations between the markers of bone turnover serum osteocalcin (OC), pyridinoline (PYD/Creat), deoxypyridinoline (DPD/ Creat)) and body weight in A all postmenopausal women not taking hormone replacement therapy [HRT(−)], and in the B normal-weight HRT(−) and C obese HRT(−) subgroups
Bone resorption
Bone resorption was examined in 203 subjects (7 obese subjects in the HRT(+) group were not available), showing that PYD and DPD excretion was higher in the HRT(−) group (31.9±11.4 nmol/mmol and 10.1± 4.1 nmol/mmol, respectively) compared with women receiving HRT (25.2±11.4 nmol/mmol and 7.9± 4.2 nmol/mmol, p<0.01). Markers of bone resorption did not differ across BMI categories (Table 1) and did not correlate with body weight (Fig. 3). The exception was among normal-weight HRT(−) subjects, who showed a negative correlation between PYD and body weight (r=−0.39, p=0.01; Fig. 3B). Higher DPD excretion was associated with lower TBBMC in the entire sample (Table 2) and in the HRT(−) subgroup (but not the HRT(+) subgroup), whether adjusted for age (Table 2) or not (Fig. 2C).
There were no significant differences in urinary creatinine excretion among groups. Creatinine values were 10.4±4.9 mmol/l, 10.7±5.7 mmol/l and 11.4± 5.0 mmol/l in normal-weight, overweight and obese women, respectively (p=0.7, one-way ANOVA).
Predictors of bone mass and turnover
Among the variables studied (age, weight, height, PYD, DPD and OC), the best predictors for TBBMC in the entire sample were body weight, height, DPD, OC and age, accounting for approximately 49% of the variance in TBBMC (Table 3). We also analyzed predictors of TBBMC separately for the HRT(+) and HRT(−) groups (data not shown). In women without HRT, body weight and height were the best predictors of TBBMC, accounting for approximately 46% and 9% of the variance, respectively. In HRT(+) women, TBBMC was best predicted by height (~31%) and OC (~7%). To determine whether bone turnover could be predicted by the variables studied, we included PYD, DPD and OC as the dependent variables in stepwise regression for the entire population (Table 3). The variables studied accounted for a low percent of the variance in bone turnover markers; approximately 11% for PYD (predictors: OC and body weight and TBBMC), approximately 13% for DPD (predictor: OC), and approximately 15% for OC (predictors: DPD and TBBMC), respectively, indicating that with the parameters assessed in this study, bone turnover is less predictable than bone mass.
Table 3.
Multiple linear stepwiseregression analysis in 210 postmenopausal women(TBBMC total body bone mineral content, PYD/ Creat pyridinoline/creatinine, DPD/Creat deoxypyridinoline/ creatinine, OC serum osteocalcin)
| βcoefficient | p value | r2% | Model r2% | |
|---|---|---|---|---|
| Dependent: TBBMC | ||||
| Body weight | 0.381 | <0.0001 | 30.64 | 48.62 |
| Height | 0.356 | <0.0001 | 12.13 | |
| OC | −0.135 | 0.0159 | 3.35 | |
| DPD/Creat | −0.127 | 0.0222 | 1.39 | |
| Age | −0.113 | 0.0413 | 1.12 | |
| Dependent: PYD/Creat | ||||
| OC | 0.216 | 0.0022 | 5.67 | 10.78 |
| Body weight | 0.261 | 0.0015 | 2.17 | |
| TBBMC | −0.210 | 0.0120 | 2.93 | |
| Dependent: DPD/Creat | ||||
| OC | 0.356 | <0.0001 | 12.67 | 12.67 |
| Dependent: OC | ||||
| DPD/Creat | 0.324 | <0.0001 | 12.67 | 15.45 |
| TBBMC | −0.170 | 0.0118 | 2.77 | |
Discussion
The present study investigated the effect of body weight on bone turnover and TBBMC in a healthy population of postmenopausal women and over a wide range of body weights. Not surprisingly, we found that body weight was a much better predictor of TBBMC than bone turnover. In contrast to our original hypothesis, the higher TBBMC in heavier compared with leaner postmenopausal women could not be explained by lower levels of bone resorption and/or higher bone formation. In addition, we found that HRT was associated with lower bone turnover only in normal-weight women.
Bone turnover accounted for only a small (but significant) fraction of the variance in TBBMC (~5%), which is consistent with the findings of others [11]. A strong association may not be expected since TBBMC is primarily cortical bone and turnover markers are more indicative of cancellous bone activity. Although some investigators have shown no relationship between markers and bone mass [12], others have shown a small but significant correlation between a higher BMC with lower bone turnover [3, 11, 13], consistent with our data. Garnero et al. [14] have also found a direct association between bone markers and fracture risk. Ultimately, a model that includes bone parameters (markers and mass) and clinical factors (such as body weight, height and postmenopausal age) should be developed to assess fracture risk.
To our knowledge, the relationship between body weight and bone turnover in a healthy postmenopausal population has only been assessed in one previous study [8]. Ravn et al. [8] showed that, consistent with our findings, leaner women had higher rates of bone turnover. However, our population differed from this study [8] because we included women later in their menopausal years, who typically have more stable rates of bone turnover and loss, and included obese women. Women with extreme weight differences were included to test the hypothesis that differences in bone turnover could explain the higher measured bone mass in obese compared with normalweight women [1]. In addition, to provide clinically relevant information we used well-defined BMI categories (i.e., normal-weight, overweight and obese) [9]. Nevertheless, when comparing populations of extreme differences in body size, it must be considered that DXA has limitations in measuring bone mass due to artifacts introduced by the composition of both soft tissue and bone marrow [15, 16]. In addition, the instrumentation, scanning conditions and the bone variable used (BMD vs BMC) will affect the accuracy of DXA values. To reduce error in this data set, we used a standardization factor for BMC values [10], and avoided the use of BMD, an areal measurement, which can increase error compared with BMC [15, 17]. Finally, the main purpose of this study was to examine the relationship of body weight and bone turnover, rather than bone mass, to address the physiologic relevance of the well-documented (but controversial) association between body weight and bone mass, and ultimately explain why weight is a major determinant of osteoporosis risk.
Studies of the association of body weight and bone turnover have been performed in more specific populations, such as patients suffering from anorexia nervosa or obesity. Similar to our results, patients with anorexia nervosa show a negative association between BMI and bone resorption [18]. However, in contrast to findings in our leaner healthy women, bone formation is suppressed with the extremely low weights associated with anorexia nervosa [18]. Unlike the severe malnutrition in patients with anorexia nervosa [19], the higher bone formation in our healthy lean women may occur simply due to a coupling with elevated rates of resorption. In obese patients, on the other hand, there are more variable findings showing either elevated [20] or unchanged [21] levels of bone resorption and elevated [20] or lower [21] levels of formation. Different hormonal profiles (e.g., higher levels of bioavailable sex hormones) and the presence of other disorders may have influences on turnover markers that could account for these discrepant findings in obese populations [20, 21]. In addition, the sensitivity of bone markers to measure bone turnover in individuals of any BMI category has been questioned due to their limitations (such as coming from nonskeletal sources), but despite these drawbacks they have been proven useful in both research and many clinical practices [22]. Hence, it is essential to determine whether other factors (such as weight) add to their source of variation.
As expected [4], we observed that those women taking HRT have lower levels of bone turnover. Although estrogen has a well-established effect on the skeleton [23], our findings showed greater bone mass and lower bone turnover in HRT(+) compared with HRT(−) subjects for the normal-weight group, but not the obese group. This indicates that women of lower body weights may be more responsive to HRT compared with obese subjects, whose higher body fat content may be a significant endogenous source of estrogen [24], and whose higher weight-bearing (compared with normal-weight women) is expected to independently promote higher bone mass. Nevertheless, once women with higher endogenous sources of estrogen (i.e., as in obesity) take HRT, these endogenous sources are less likely to be important. In two previous retrospective studies [25, 26], it was found that larger women have a better response to estrogen compared with lean women. However, baseline values (such as the presence of fractures prior to therapy in some subjects) make these data difficult to interpret in at least one of these studies [26]. Bjarnason and Christiansen [27] found that BMI did not influence the response to HRT treatment, whereas a large study evaluating postmenopausal risk factors for hip fracture found that the best protective effect of HRT was among leaner women [28]. Our BMC results using a wider range of body weights are consistent with these findings. We suggest that the greater effects of HRT on bone in leaner women should be considered together with the traditional risks and benefits of HRT [29] when choosing the best treatment for postmenopausal women. These crosssectional observations, however, have to be interpreted with caution, and prospective longitudinal trials are indicated to confirm our findings.
In normal-weight postmenopausal women (BMI <25 kg/m2), an increased bone turnover is indicative of a low bone mass. In addition, only these leaner women show a different bone profile with HRT treatment (i.e., higher TBBMC and lower bone turnover) in this cross-sectional study, suggesting that they could be better candidates for a beneficial bone response to HRT compared with their obese counterparts. Taken together, these data suggest that special attention should be paid to body weight in the prevention and the expected response to osteoporosis treatment.
Acknowledgments
We would like to thank Dr Abbe Herzig, statistical consultant at Rutgers University, for her advice with the study design and analysis. This work was supported by grants from the National Institutes of Health (AG-12161) to S.A.S. and from Dairy Management Inc. to M.A.J.
Contributor Information
M. Cifuentes, Department of Nutritional Sciences, Rutgers University, New Brunswick, New Jersey, USA
M.A. Johnson, Department of Foods and Nutrition, The University of Georgia, Athens, Georgia, USA
R.D. Lewis, Department of Foods and Nutrition, The University of Georgia, Athens, Georgia, USA
S.B. Heymsfield, New York Obesity Research Center and Body Composition Unit, St Luke’s–Roosevelt Hospital Columbia University, New York, New York, USA
H.A. Chowdhury, Department of Nutritional Sciences, Rutgers University, New Brunswick, New Jersey, USA
C.M. Modlesky, Department of Foods and Nutrition, The University of Georgia, Athens, Georgia, USA
S.A. Shapses, Email: Shapses@aesop.rutgers.edu, Department of Nutritional Sciences, Rutgers University, New Brunswick, New Jersey, USA. Rutgers, The State University of New Jersey, 96 Lipman Drive, New Brunswick, NJ 08901-8525, USA, Tel.: +1-732-9329403, Fax: +1-732-9326837
References
- 1.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–9. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 2.Ensrud KE, Lipschutz RC, Cauley JA, Seeley D, Nevitt MC, Scott J, et al. Body size and hip fracture risk in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Am J Med. 1997;103:274–80. doi: 10.1016/s0002-9343(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 3.Ravn P, Fledelius C, Rosenquist C, Overgaard K, Christiansen C. High bone turnover is associated with low bone mass in both pre- and postmenopausal women. Bone. 1996;19:291–8. doi: 10.1016/8756-3282(96)00201-3. [DOI] [PubMed] [Google Scholar]
- 4.Fall PM, Kennedy D, Smith JA, Seibel MJ, Raisz LG. Comparison of serum and urine assays for biochemical markers of bone resorption in postmenopausal women with and without hormone replacement therapy and in men. Osteoporos Int. 2000;11:481–5. doi: 10.1007/s001980070089. [DOI] [PubMed] [Google Scholar]
- 5.Delmas PD, Hardy P, Garnero P, Dain M. Monitoring individual response to hormone replacement therapy with bone markers. Bone. 2000;26:553–60. doi: 10.1016/s8756-3282(00)00271-4. [DOI] [PubMed] [Google Scholar]
- 6.Shapses SA, Robins SP, Schwartz EI, Chowdhury H. Short-term changes in calcium but not protein intake alter the rate of bone resorption in healthy subjects as assessed by urinary pyridinium cross-link excretion. J Nutr. 1995;125:2814–21. doi: 10.1093/jn/125.11.2814. [DOI] [PubMed] [Google Scholar]
- 7.Stepan JJ. Prediction of bone loss in postmenopausal women. Osteoporos Int. 2000;11:S45–54. doi: 10.1007/s001980070005. [DOI] [PubMed] [Google Scholar]
- 8.Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–7. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(Suppl 2):S51–209. [PubMed] [Google Scholar]
- 10.Modlesky CM, Lewis RD, Yetman KA, Rose B, Rosskopf LB, Snow TK, et al. Comparison of body composition and bone mineral measurements from two DXA instruments in young men. Am J Clin Nutr. 1996;64:669–76. doi: 10.1093/ajcn/64.5.669. [DOI] [PubMed] [Google Scholar]
- 11.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–49. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 12.Marcus R, Holloway L, Wells B, Greendale G, James MK, Wasilauskas C, et al. The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: results from the Postmenopausal Estrogen/ Progestin Interventions (PEPI) trial. J Bone Miner Res. 1999;14:1583–95. doi: 10.1359/jbmr.1999.14.9.1583. [DOI] [PubMed] [Google Scholar]
- 13.Chung KW, Kim MR, Yoo SW, Kwon DJ, Lim YT, Kim JH, et al. Can bone turnover markers correlate bone mass at the hip and spine according to menopausal period? Arch Gynecol Obstet. 2000;264:119–23. doi: 10.1007/s004040000093. [DOI] [PubMed] [Google Scholar]
- 14.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Bio-chemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–36. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 15.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–42. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 16.Bolotin HH, Sievanen H. Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral density can seriously mislead diagnostic/prognostic interpretations of patient-specific bone fragility. J Bone Miner Res. 2001;16:799–805. doi: 10.1359/jbmr.2001.16.5.799. [DOI] [PubMed] [Google Scholar]
- 17.Leslie WD, DeVos G, Dupont JO, Peterfy AE. Reproducibility of volume-adjusted bone mineral density of spine and hip from dual X-ray absorptiometry. J Clin Densitom. 2001;4:307–12. doi: 10.1385/jcd:4:4:307. [DOI] [PubMed] [Google Scholar]
- 18.Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K. The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2000;85:200–6. doi: 10.1210/jcem.85.1.6321. [DOI] [PubMed] [Google Scholar]
- 19.Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68:548–54. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 20.Ostrowska Z, Zwirska-Korczala K, Buntner B, Pardela M, Drozdz M. Assessment of bone metabolism in obese women. Endocr Regul. 1998;32:177–181. [PubMed] [Google Scholar]
- 21.Hyldstrup L, Andersen T, McNair P, Breum L, Transbol I. Bone metabolism in obesity: changes related to severe over-weight and dietary weight reduction. Acta Endocrinol (Co-penh) 1993;129:393–8. doi: 10.1530/acta.0.1290393. [DOI] [PubMed] [Google Scholar]
- 22.Miller PD, Baran DT, Bilezikian JP, Greenspan SL, Lindsay R, Riggs BL, et al. Practical clinical application of biochemical markers of bone turnover: consensus of an expert panel. J Clin Densitom. 1999;2:323–42. doi: 10.1385/jcd:2:3:323. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay R. Estrogen deficiency. In: Riggs BL, Melton LJ, editors. Osteoporosis: etiology, diagnosis, and management. Philadelphia: Lippincott-Raven; 1995. pp. 133–60. [Google Scholar]
- 24.Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Davis S, et al. Local estrogen biosynthesis in males and females. Endocr Rel Cancer. 1999;6:131–7. doi: 10.1677/erc.0.0060131. [DOI] [PubMed] [Google Scholar]
- 25.Komulainen M, Kroger H, Tuppurainen MT, Heikkinen AM, Honkanen R, Saarikoski S. Identification of early postmenopausal women with no bone response to HRT: results of a fiveyear clinical trial. Osteoporos Int. 2000;11:211–8. doi: 10.1007/s001980050283. [DOI] [PubMed] [Google Scholar]
- 26.Armamento-Villareal R, Civitelli R. Estrogen action on the bone mass of postmenopausal women is dependent on body mass and initial bone density. J Clin Endocrinol Metab. 1995;80:776–82. doi: 10.1210/jcem.80.3.7883830. [DOI] [PubMed] [Google Scholar]
- 27.Bjarnason NH, Christiansen C. The influence of thinness and smoking on bone loss and response to hormone replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 2000;85:590–6. doi: 10.1210/jcem.85.2.6364. [DOI] [PubMed] [Google Scholar]
- 28.Michaelsson K, Baron JA, Johnell O, Persson I, Ljunghall S. Variation in the efficacy of hormone replacement therapy in the prevention of hip fracture. Swedish Hip Fracture Study Group. Osteoporos Int. 1998;8:540–6. doi: 10.1007/s001980050096. [DOI] [PubMed] [Google Scholar]
- 29.Beral V, Banks E, Reeves G, Appleby P. Use of HRT and the subsequent risk of cancer. J Epidemiol Biostat. 1999;4:191–210. [PubMed] [Google Scholar]