Abstract
Background
Our previous studies demonstrated that C-reactive protein (CRP) acts as an inflammatory factor to induce endothelial dysfunction and hypertension in rats. The anti-inflammatory effects of statins suggest that they may attenuate CRP-induced endothelial dysfunction and hypertension in Sprague Dawley (SD) rats.
Methods
Male SD rats were injected with an adeno-associated virus (AAV) to induce overexpression of human CRP (AAV-hCRP) or GFP control (AAV-GFP). Two months after injection, rats were administered rosuvastatin by daily oral gavage (10 mg/kg) for two additional months. Blood pressure was monitored, serum hCRP concentrations were assessed by ELISA, and vascular levels of endothelial nitric oxide synthase (eNOS), PI3K/Akt, Rho kinase, angiotensin type 1 (AT1) receptor, MAPK, SOD-1, and NADPH oxidase was determined by immunoblotting.
Results
Rosuvastatin administration attenuated the increased blood pressure and loss of vascular eNOS expression in AAV-hCRP-treated rats. Rosuvastatin also activated PI3K/Akt, inhibited Rho kinase activity, and downregulated the AT1 receptor expression in aorta. Rosuvastatin reduced production of ROS through downregulation of NADPH oxidase subunit p22 phox and gp91 phox, and upregulation of SOD-1 expression.
Conclusions
Rosuvastatin attenuated the increase in blood pressure in AAV-hCRP-treated rats through endothelial protection and antioxidant effects. Our data reveals a novel mechanism through which statins may lower blood pressure and suggests the potential use of statins in the treatment of hypertension.
Keywords: C-reactive protein, rosuvastatin, hypertension, myosin binding subunit, reactive oxygen species
Introduction
Inflammatory processes are important participants in the pathophysiology of hypertension (1, 2). C-reactive protein (CRP) is an acute-phase reactant which has traditionally been viewed and used as a systemic marker of inflammation. Numerous epidemiological studies have demonstrated that minor elevations in CRP levels are associated with increased risk of hypertension (3–5). CRP was shown to impair eNOS expression and activity in cultured endothelial cells (6, 7). Furthermore, CRP upregulated angiotensin II type 1 (AT1) receptors in vascular smooth muscle cells (8). Vongpatanasin and co-workers demonstrated that transgenic mice overexpressing rabbit CRP have systolic hypertension via downregulation of vascular angiotensin II type 2 (AT2) receptors (9). More recently, we also found that recombinant adeno-associated virus delivery of human C-reactive protein increased systolic blood pressure up to 20 mmHg in rats through downregulation of endothelial NO synthase (eNOS) and AT2 receptor expression, and upregulation of (AT1) and endothelin A (ETA) receptors (10). Together, these results suggested that CRP is not only a marker of inflammation but also an important participant in the development of hypertension.
There is accumulating evidence that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, or statins, exert numerous beneficial effects independent of their action on blood lipids (11). The beneficial cardiovascular mechanisms of statins include modulation of endothelial function (12, 13), anti-inflammatory actions (14), and antioxidant properties (15). In the past few years, the effects of statins on blood pressure have been widely investigated. After 30 days of atorvastatin treatment, blood pressure in spontaneously hypertensive rats (SHR) was reduced up to 20 mmHg through downregulation of AT1 receptor mRNA expression and reduced production of reactive oxygen species (ROS) (16). Similarly, 12 weeks of rosuvastatin treatment decreased blood pressure by more than 20 mmHg in SHR (17).
Recently, the UCSD Statin Study conducted a large double-blind, placebo-controlled clinical trial with equal allocation to 20 mg simvastatin, 40 mg pravastatin or placebo for 6 months. Statins modestly but significantly reduced systolic blood pressure by 2.2 mm Hg and diastolic blood pressure by 2.4 mmHg relative to placebo (18). These findings suggest that statin treatment could decrease blood pressure, though others have failed to reproduce this hypotensive effect (19). Several clinical trials, including the JUPITER study (20), have shown that statin therapy could significantly reduce CRP levels and improve clinical outcomes independent of effects on LDL cholesterol (14). Hence, the purpose of the present study was to investigate whether the rosuvastatin exerts antihypertensive effects and improves endothelial function and vascular oxidative stress in a rat model of CRP-overexpression-induced hypertension.
Materials and methods
Preparation of recombinant adeno-associated virus
Type-8 double-stranded recombinant adeno-associated virus vectors (dsAAV8) containing either human CRP (AAV-hCRP) or GFP (AAV-GFP) cDNAs were prepared by triple plasmid co-transfection in HEK293 cells as described (10, 21).
Animals
Male SD rats weighing 200–250g were obtained from the Institutional Animal Research Committee of Tongji Medical College and treated in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Thirty-six rats were randomized to three treatment groups (12 animals per group): saline control; AAV-GFP control; and AAV-hCRP. Animals received a single intravenous injection of either saline or AAV (1×1012 virions per rat), respectively. Two months after AAV injection, half of the rats of each group began rosuvastatin (Crestor, AstraZeneca, Macclesfield, Cheshire, England) treatment at a dose of 10 mg/kg of body weight per day by oral gavage. Thus, there were six treatment groups, with six rats per group as follows: control; control + rosuvastatin; AAV-GFP; AAV-GFP + rosuvastatin; AAV-hCRP; and AAV-hCRP + rosuvastatin. All animals were housed in a temperature and humidity controlled facility with twelve hour light/dark cycles and allowed free access to normal rat chow and water ad libitum. Rats were sacrificed four months after injection after performing hemodynamic measurements.
Measurement of blood pressure and hemodynamics
Systolic blood pressure was measured each month by the photoelectric tail-cuff method (Powerlab NIBP system, Australian) in conscious animals. At the end of the experiment, heart rate, maximum left ventricular pressure, cardiac output (CO), rate of contraction (dP/dt max) and inter-arterial blood pressure were measured invasively using a Millar microtransducer catheter (SPR-838, Millar Instruments; Houston, TX) as described previously (10).
Analytical methods
Blood was collected from the tail vein at two and four months after AAV injection. Serum hCRP was assessed by ELISA (BioCheck Inc, Foster City, CA, USA). Serum nitric oxide (NO) concentrations were measured using a nitric oxide assay kit (Cayman. Inc, Ann Arbor, MI, USA). Serum insulin was measured by ELISA (Millipore. Inc, Billerica, MA, USA). Other plasma parameters including total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides were assessed using an automatic analyzer (Roche) in the clinical laboratories of Tongji Hospital.
Immunoblotting
Frozen tissues were sonicated in RIPA lysis buffer and protein concentrations were determined by the Bradford method. Extracts were resolved by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Antibodies to hCRP, eNOS, p-Akt, total Akt, PI3K, p-MBS, total MBS, AT1, AT2, p22 phox, gp91 phox, p40 phox, p47, p67 phox, SOD-1, p-p38, total p38, p-ERK1/2, total ERK1/2, p-JNK, total JNK, p-c-Jun, total c-Jun and β-actin were purchased from Santa Cruz Biotechnology or Cell Signaling Technology. Proteins were visualized by immunoblotting with enhanced chemiluminescence (Pierce, Inc.) as previously described (22).
Measurement of Superoxide Release
Aortas were cut into 5-mm segments and placed in Krebs-HEPES buffer. Samples were transferred into scintillation vials that contained 2 ml of Krebs-HEPES buffer with 5 mmol/L lucigenin. Chemiluminescence was assessed in a scintillation counter (Berthold Lumat LB 9501). Superoxide release was expressed as relative chemiluminescence per milligram of aortic tissue as previously described (16).
Statistical analysis
Data are presented as mean ± SEM obtained in 3–12 independent analyses. Comparisons among groups were made by one-way ANOVA, followed by a Scheffe F-test. P<0.05 was used to indicate statistical significance.
Results
Effect of AAV-hCRP treatment on CRP expression
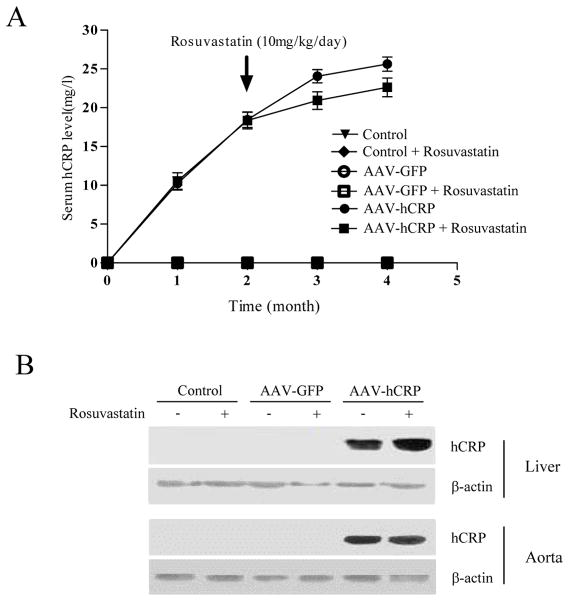
Serum hCRP levels were increased beginning one month after AAV-hCRP injection, and reached a peak at four months after injection. Rosuvastatin treatment did not significantly alter serum hCRP levels (Figure 1A). Immunoblots confirmed that rAAV-hCRP injection induced significant increases in hCRP expression in liver and aortic tissue after four months (Figure 1B). Thus, a single injection of AAV-hCRP in rats induced significant and prolonged increases in circulating hCRP levels and hCRP tissue expression.
Figure 1.
AAV-mediated expression of hCRP. (A) Serum concentrations of hCRP at different times after injection of AAV vectors and rosuvastatin treatment. (B) Representative immunoblot of hCRP expression in liver and aorta four months after gene delivery.
Physiologic parameters of animals
Plasma concentrations of total, HDL and LDL cholesterol, and triglycerides four months after AAV-hCRP injection and two months after rosuvastatin treatment are shown in Table 1. All measured parameters were within the normal range in the control group. Neither rosuvastatin treatment nor AAV-hCRP injection significantly altered plasma concentrations of cholesterol or triglycerides. Other physiologic parameters, including body weight, heart rate, fasting plasma glucose and fasting plasma insulin levels, were not significantly different between treatment groups (Supplemental Table S1A and S1B).
Table 1.
Lipid profile in control and rosuvastatin-treated rats four months after AAV treatment.
| Cholesterol (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | Triglyceride (mg/dl) | |
|---|---|---|---|---|
| Control | 42.8 ± 2.0 | 18.0 ± 1.4 | 19.6 ± 1.8 | 28.0 ± 1.7 |
| Control + Rosuvastatin | 41.8 ± 1.4 | 18.4 ± 1.7 | 18.6 ± 0.9 | 27.2 ± 2.3 |
| AAV-GFP | 42.0 ± 2.3 | 19.0 ± 1.5 | 19.0 ± 1.3 | 27.0 ± 2.4 |
| AAV-GFP + Rosuvastatin | 42.2 ± 2.7 | 19.2 ± 2.1 | 19.6 ± 1.4 | 27.6 ± 2.4 |
| AAV-hCRP | 42.4 ± 3.2 | 17.0 ± 1.5 | 18.2 ± 1.0 | 29.0 ± 1.9 |
| AAV-hCRP + Rosuvastatin | 41.6 ± 2.1 | 16.6 ± 2.1 | 17.6 ± 1.5 | 28.4 ± 1.5 |
Plasma concentrations of total, LDL and HDL cholesterol, and triglycerides were determined four months after AAV-hCRP injection and two months after rosuvastatin treatment. Data are expressed as mean ± SEM (n=6 per group). There were no significant differences between the treatment groups.
Effect of rosuvastatin on CRP-induced increases in systolic blood pressure
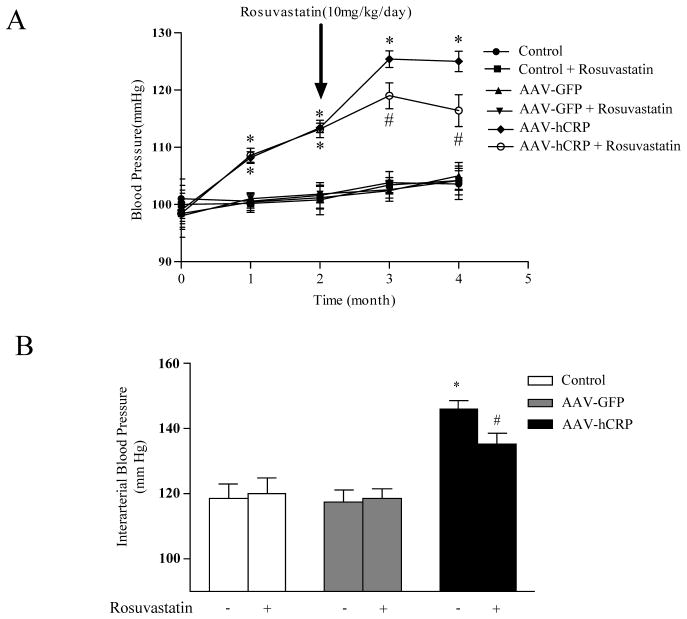
Animals that received AAV-hCRP showed a significant increase in systolic blood pressure at one and two months after injection, an effect that became more pronounced (approximately 20 mmHg higher than control) at three and four months after injection (Figure 2A). The increased blood pressure in AAV-hCRP treated rats was significantly attenuated by rosuvastatin treatment (9 mmHg lower than AAV-hCRP group, p<0.05, Figure 2A). Intra-arterial blood pressure measurements were in agreement with non-invasive blood pressure measurements and confirmed a significant increase following AAV-hCRP injection and an attenuation in animals treated with rosuvastatin (p<0.05, Figure 2B). Therefore, rosuvastatin modestly, but significantly, lowered systolic blood pressure induced by CRP overexpression in rats. Maximum left ventricular pressure, cardiac output (CO), and dP/dt max were not significantly altered after rosuvastatin treatment (Supplemental Table S2)
Figure 2.
Effect of hCRP and rosuvastatin on systolic blood pressure. (A) Systolic blood pressure was monitored from the beginning of the study using the tail-cuff method. Rats injected with AAV-hCRP had significantly increased blood pressure starting one month after gene delivery (p<0.05). Blood pressure was reduced by rosuvastatin treatment (p<0.05). (B) Intra-arterial pressure was measured at the end of the experiment using the Millar catheter. Results were consistent with non-invasive blood pressure measurements (p<0.05). Data represent mean ± SEM. * p<0.05 vs. control group. # p<0.05 vs. AAV-hCRP group.
Effect of rosuvastatin on vascular endothelial signaling
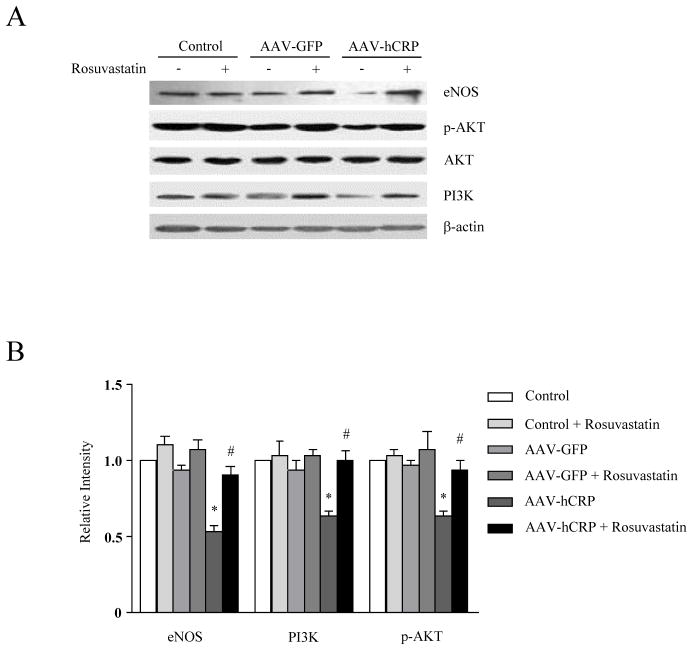
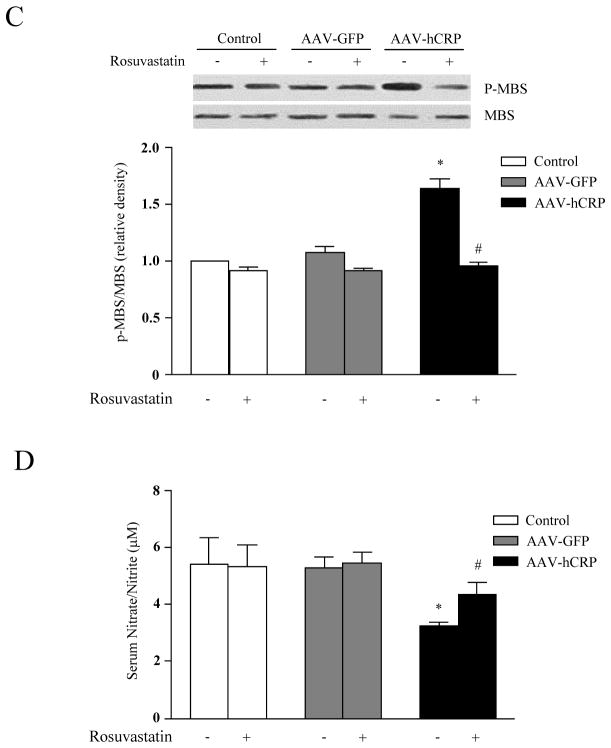
rAAV-hCRP injection significantly reduced expression of eNOS in aorta (p<0.05, Figure 3A and 3B), consistent with our previous report (10). PI3K protein expression and Akt phosphorylation were also significantly reduced by AAV-hCRP injection. Interestingly, rosuvastatin treatment restored eNOS, PI3K and phosphorylated Akt to normal levels (p<0.05, Figure 3A and 3B). Phosphorylation of myosin binding subunit (MBS) is a surrogate for Rho kinase (ROCK) activation, which contributes to arterial smooth muscle constriction. Interestingly, we found that MBS phosphorylation was increased by AAV-hCRP injection, an effect that was normalized by treatment with rosuvastatin (p<0.05, Figure 3C). We also measured serum nitrate/nitrite levels using a nitric oxide assay kit. Similar to effects on eNOS expression, rosuvastatin treatment attenuated the loss of serum nitrate/nitrite observed with AAV-hCRP injection (p<0.05, Figure 3D). Together, these results suggest that rosuvastatin treatment improved endothelial dysfunction in AAV-hCRP injected rats via restoration of normal eNOS, PI3K/Akt, and ROCK pathway activation.
Figure 3.
Effect of hCRP and rosuvastatin on endothelial function. (A) Representative immunoblot analysis for eNOS, PI3K and Akt. (B) Densitometric quantification shows eNOS expression, PI3K expression and Akt phosphorylation were suppressed by AAV-hCRP but normalized by rosuvastatin treatment (p<0.05); (C) Immunoblot and relative densitometric analysis (Control group = 1) show that myosin binding substrate (MBS) phosphorylation was increased by AAV-hCRP injection, but normalized by rosuvastatin treatment (p<0.05). (D) AAV-hCRP injection induced a decrease in serum nitrate/nitrite concentration, an effect that was attenuated by rosuvastatin treatment (p<0.05). Data represent mean ± SEM. * p<0.05 vs. control group. # p<0.05 vs. AAV-hCRP group.
Effect of rosuvastatin on vascular NADPH oxidase expression and ROS production
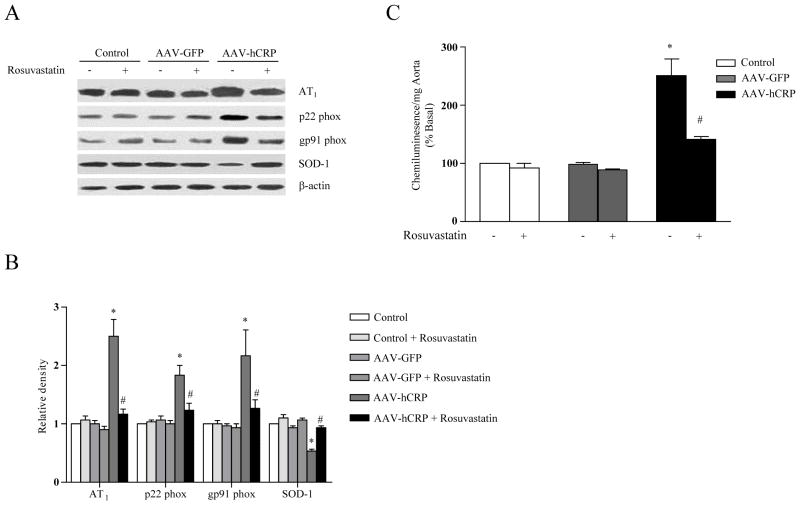
Similar to our previous studies, vascular AT1 receptor expression was upregulated in AAV-hCRP-injected rats (p<0.05, Figure 4A and 4B). Rosuvastatin treatment significantly attenuated the rise in vascular AT1 receptor expression (p<0.05, Figure 4A and B). Vascular ROS production is mediated, at least in part, by the AT1 receptor. Therefore, we investigated whether CRP or rosuvastatin influenced vascular NADPH oxidase subunit expression and/or vascular ROS production. AAV-hCRP injection markedly induced expression of NADPH oxidase p22 phox and gp91 phox subunits, an effect that was reversed by rosuvastatin treatment (p<0.05, Figure 4A and 4B). Expression of AT2, p40 phox, p47 phox and p67 phox were not significantly altered by AAV-hCRP injection or rosuvastatin treatment (Supplementary Figure S1). Levels of SOD-1, an important antioxidant enzyme, were diminished by AAV-hCRP injection, an effect that was normalized by rosuvastatin treatment (p<0.05, Figure 4A and 4B). Vascular production of ROS was assessed by a lucigenin chemiluminescence assay in isolated aortic segments. AAV-hCRP injection increased vascular production of superoxide by 2–3 fold, an effect that was significantly attenuated by rosuvastatin treatment (p<0.05, Figure 4C). Together, these results suggest that CRP overexpression upregulated AT1 receptor, p22 phox and gp91 phox expression which led to increased vascular ROS production. CRP overexpression also may have led to increased ROS through downregulation of the antioxidant enzyme SOD-1. Rosuvastatin attenuated the reduction in SOD-1 and restored ROS levels to normal.
Figure 4.
Effect of hCRP and rosuvastatin on vascular expression of AT1 receptors, NAD(P)H oxidase subunits and SOD-1. (A) Representative immunoblot analysis for AT1 receptor, p22 phox, gp91 phox and SOD-1 expression in vasculature. (B) Densitometric analysis (Control = 1) of immunoblots shows that CRP-induced increases in AT1 receptor, p22 phox and gp91phox expression were abolished by rosuvastatin treatment (p<0.05). SOD-1 was downregulated by AAV-hCRP injection, but normalized by rosuvastatin treatment (p<0.05). (C) Superoxide release is expressed as relative chemiluminescence per milligram of aortic tissue (Control = 100) (p<0.05). Data represent mean ± SEM. * p<0.05 vs. control group. # p<0.05 vs. AAV-hCRP group.
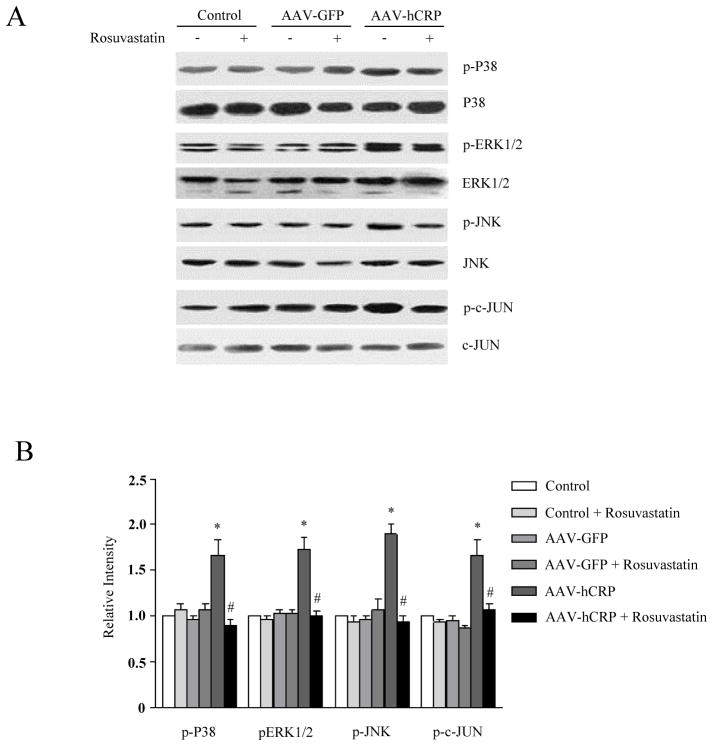
Effect of rosuvastatin on CRP–induced JNK and MAPK activation
CRP has been shown to activate c-Jun N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) in vascular smooth muscle cells and endothelial cells (23–25). To investigate whether rosuvastatin modifies these signaling pathways in the vasculature, we evaluated extracellular signal–regulated kinase (ERK) 1/2, p38 MAPK and JNK/c-JUN activation. AAV-hCRP injection significantly induced ERK1/2, JNK/c-JUN and p38 MAPK phosphorylation in aorta (p<0.05, Figure 5A and 5B). The effects of CRP overexpression on ERK1/2 and JNK/c-JUN and p38 MAPK phosphorylation were significantly attenuated by rosuvastatin treatment (Figure 5A and B).
Figure 5.
Effect of hCRP and rosuvastatin on vascular MAPK activation. (A) Representative immunoblot of ERK1/2, p38 MAPK and JNK/c-JUN. (B) Vascular p38 MAPK, ERK1/2 and JNK/c-JUN phosphorylation were increased in AAV-hCRP injected rats, an effect that was attenuated by rosuvastatin (p<0.05). Results are expressed as the ratio of phospho-ERK1/2, p38, JNK and c-JUN to total ERK1/2, p38, JNK and c-JUN, respectively. Data represent mean ± SEM. * p< 0.05 vs. control group. # p<0.05 vs. AAV-hCRP group.
Discussion
AAV delivery of hCRP resulted in long term, stable hCRP expression, induced markers of vascular dysfunction, and increased systolic blood pressure consistent with previous studies (9, 10). The present study demonstrates that rosuvastatin treatment significantly attenuates the hypertension and dysregulation of vascular signaling induced by CRP.
Our initial hypothesis was that rosuvastatin treatment might lower blood pressure by reducing high serum CRP level. Previous studies show that CRP is mainly secreted from the liver into the systemic circulation, and suggest that statins may reduce CRP levels by decreasing CRP excretion from hepatocytes (26). However, AAV-hCRP induced prolonged and sustained gene expression, which was not reduced by rosuvastatin. Systolic blood pressure was increased after AAV-CRP administration. While not lowing CRP levels significantly, rosuvastatin decreased blood pressure significantly. In contrast, blood pressure in control and AAV-GFP groups were not elevated after AAV-hCRP injection or altered by rosuvastatin treatment. Our results are not consistent with some previous reports (16, 17), which may be due to differences in statistical power, experimental conditions or animal models used in these studies.
The partial rescue of CRP-induced hypertension by rosuvastatin was accompanied by a restoration of normal vascular signaling. CRP induced several changes indicative of endothelial dysfunction. AAV-hCRP induced a loss of eNOS expression and activity, likely through reduced PI3K and Akt activation, and increased Rho kinase activation. CRP also upregulated AT1 receptor expression and NADPH oxidase subunit expression, while downregulating SOD-1, and led to an increase in vascular ROS production. Rosuvastatin treatment produced a nearly complete reversal of these CRP-induced signaling changes. Thus, statin therapy may reduce hypertension through a variety of signaling mechanisms which reverse vascular dysfunction.
Endothelial-derived NO is an important regulator of blood pressure (27). Our previous studies suggested that CRP-induced hypertension may be due to impairment of eNOS expression and activity (10). However, rosuvastatin previously failed to decrease blood pressure in L-NAME treated-rats (17). In this study, rosuvastatin therapy improved eNOS activity and nitric oxide production, which is likely a primary cause of the blood pressure reduction. Statins can affect the expression and activity of eNOS downstream of PI3K/Akt and ROCK activation. ROCK pathway activation is increased in endothelial dysfunction and implicated in decreased NO production (28). In this study, rosuvastatin was able to reverse the loss of PI3K expression and Akt activity, and the increase in ROCK activation seen in AAV-hCRP treated rats. By preventing the dysregulation of these pathways, rosuvastatin may maintain normal eNOS signaling in the vasculature.
Rho kinases are also recognized as major regulators of cell contraction, via MBS phosphorylation and inhibition of myosin light chain phosphatase activity (29). Statins have been shown to be efficient inhibitors of ROCKs (13) and thus may present a novel therapeutic target in hypertension (30). CRP has been shown to activate Rho/Rho-kinase signaling in endothelial cells (31), as it did in aortas from our AAV-hCRP-treated rats. However, after two months of rosuvastatin therapy, ROCK activation was restored to normal levels in CRP-treated rat aorta, which would decrease vascular constriction and help reduce hypertension.
The relationship between oxidative stress and hypertension has been established in prior studies (32). In vascular smooth muscle cells, CRP has been shown to increase AT1 receptor expression and AT1-mediated production of ROS (8). We previously found that vascular AT1 receptor expression upregulation and AT2 receptor downregulation may partly contribute to CRP-induced hypertension (10). The activation of vascular NADPH oxidase is dependent on the assembly of its membrane-bound (gp91 phox and p22 phox) and cytoplasmic (p40 phox, p47 phox and p67 phox) subunits (33). Consistent with previous reports (REF), CRP upregulated AT1 receptor expression and vascular ROS production. Increased ROS production correlated with increased p22 phox and gp91 phox protein expression. Induction of AT1 receptor, p22 phox, gp91 phox, and ROS was attenuated by rosuvastatin treatment. These results are similar to those observed using atorastatin therapy in the spontaneous hypertensive rat (SHR) model, with several notable differences (16). Rosuvastatin did not significantly affect AT2 receptor expression while atorvastatin blocked p22 phox, but not gp91 phox upregulation. In addition to excess ROS generation, decreased antioxidant defense mechanisms contribute to oxidative stress in hypertension. Superoxide dismutases represent a major antioxidant defense system in the vasculature. The Cu/Zn cytosolic SOD (SOD-1) is highly expressed in the endothelium (34). SOD-1 was diminished by CRP but restored by rosuvastatin, in agreement with previous observations in bovine aortic endothelial cells (35). Thus, rosuvastatin therapy may have lowered blood pressure through increases in antioxidant defenses and diminished NADPH oxidase expression.
Extracellular signal–regulated kinase (ERK) 1/2, p38 MAPK and c-Jun N-terminal kinase (JNK) are activated by CRP in vascular endothelial cells and vascular smooth muscle cells (23–25). ERK1/2, p38 MAPK and JNK have been shown to promote vascular smooth muscle contraction (36–38). In the present study, we found increased ERK1/2, JNK and p38 MAPK activation in arterial tissues of CRP-treated rats. Rosuvastatin therapy reversed ERK1/2, JNK and p38 MAPK activation in vasculature, which may also have contributed to reduced hypertension.
In conclusion, these results demonstrate that rosuvastatin attenuates CRP-induced hypertension through improvement of vascular endothelial function and reduction of oxidative stress. These results also support the potential for the use of statins as an additive therapy for the treatment of resistant hypertension associated with increased CRP.
Supplementary Material
Acknowledgments
This work was supported by “973” project (No. 2007CB512004), Wuhan City grants and Hubei Province project on risk prediction of Cardiovascular Diseases and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025043).
References
- 1.Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA. 2003;290:3000–2. doi: 10.1001/jama.290.22.3000. [DOI] [PubMed] [Google Scholar]
- 2.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–8. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 3.Lakoski SG, Cushman M, Palmas W, Blumenthal R, D’Agostino RB, Jr, Herrington DM. The relationship between blood pressure and C-reactive protein in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2005;46:1869–74. doi: 10.1016/j.jacc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 5.Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, et al. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003;16:429–33. doi: 10.1016/s0895-7061(03)00566-1. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz R, Osborne-Lawrence S, Hahner L, Gibson LL, Gormley AK, Vongpatanasin W, et al. C-reactive protein downregulates endothelial NO synthase and attenuates reendothelialization in vivo in mice. Circ Res. 2007;100:1452–9. doi: 10.1161/01.RES.0000267745.03488.47. [DOI] [PubMed] [Google Scholar]
- 7.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 8.Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107:1783–90. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- 9.Vongpatanasin W, Thomas GD, Schwartz R, Cassis LA, Osborne-Lawrence S, Hahner L, et al. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115:1020–8. doi: 10.1161/CIRCULATIONAHA.106.664854. [DOI] [PubMed] [Google Scholar]
- 10.Guan H, Wang P, Hui R, Edin ML, Zeldin DC, Wang DW. Adeno-associated virus-mediated human C-reactive protein gene delivery causes endothelial dysfunction and hypertension in rats. Clin Chem. 2009;55:274–84. doi: 10.1373/clinchem.2008.115857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auer J, Berent R, Weber T, Eber B. Clinical significance of pleiotropic effects of statins: lipid reduction and beyond. Curr Med Chem. 2002;9:1831–50. doi: 10.2174/0929867023369024. [DOI] [PubMed] [Google Scholar]
- 12.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–35. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 13.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–36. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 15.Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–5. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 16.Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–7. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 17.Susic D, Varagic J, Ahn J, Slama M, Frohlich ED. Beneficial pleiotropic vascular effects of rosuvastatin in two hypertensive models. J Am Coll Cardiol. 2003;42:1091–7. doi: 10.1016/s0735-1097(03)00926-4. [DOI] [PubMed] [Google Scholar]
- 18.Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med. 2008;168:721–7. doi: 10.1001/archinte.168.7.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams B, Lacy PS, Cruickshank JK, Collier D, Hughes AD, Stanton A, et al. Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation-Lipid-Lowering Arm (CAFE-LLA) Study. Circulation. 2009;119:53–61. doi: 10.1161/CIRCULATIONAHA.108.785915. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–32. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–15. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 23.Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc Res. 2003;58:186–95. doi: 10.1016/s0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- 24.Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 26.Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, Mach F. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–6. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 27.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 28.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–34. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 29.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 30.Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;15:1062–4. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 31.Nakakuki T, Ito M, Iwasaki H, Kureishi Y, Okamoto R, Moriki N, et al. Rho/Rho-kinase pathway contributes to C-reactive protein-induced plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:2088–93. doi: 10.1161/01.ATV.0000183607.50230.9f. [DOI] [PubMed] [Google Scholar]
- 32.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 (Suppl 2):S170–80. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 33.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–97. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 34.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–73. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 35.Desjardins F, Sekkali B, Verreth W, Pelat M, De Keyzer D, Mertens A, et al. Rosuvastatin increases vascular endothelial PPARgamma expression and corrects blood pressure variability in obese dyslipidaemic mice. Eur Heart J. 2008;29:128–37. doi: 10.1093/eurheartj/ehm540. [DOI] [PubMed] [Google Scholar]
- 36.Lee YR, Lee CK, Park HJ, Kim H, Kim J, Lee KS, et al. c-Jun N-terminal kinase contributes to norepinephrine-induced contraction through phosphorylation of caldesmon in rat aortic smooth muscle. J Pharmacol Sci. 2006;100:119–25. doi: 10.1254/jphs.fp0050777. [DOI] [PubMed] [Google Scholar]
- 37.Touyz RM, El Mabrouk M, He G, Wu XH, Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res. 1999;84:505–15. doi: 10.1161/01.res.84.5.505. [DOI] [PubMed] [Google Scholar]
- 38.Kim B, Kim J, Bae YM, Cho SI, Kwon SC, Jung JY, et al. p38 mitogen-activated protein kinase contributes to the diminished aortic contraction by endothelin-1 in DOCA-salt hypertensive rats. Hypertension. 2004;43:1086–91. doi: 10.1161/01.HYP.0000125995.85427.fd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.