Abstract
Inhibitor of differentiation proteins (Id1, 2, 3 and 4) are dominant negative regulators of basic helix loop helix transcription factors and play dominant roles in cancer cells, spanning several molecular pathways including senescence, invasion, metastasis, proliferation and apoptosis. In contrast to high Id1, Id2 and Id3 expression, the expression of Id4 is epigenetically silenced in prostate cancer. In the present study we demonstrated a novel role of Id4, that of promotion of cellular senescence in prostate cancer cells. Materials and Methods: Id4 was ectopically expressed in DU145 cells (DU145+Id4). The cells treated with Doxorubicin (0–500 nm) or vehicle control were analyzed for apoptosis, senescence (SA-beta Galactosidase), and expression of CDKN1A (p21), CDKN1B(p27), CDKN2A (p16), E2F1, vimentin and E-cadherin by immuno-histochemistry and/or Western blot. Results: In the present study we demonstrated that Id4 promotes cellular senescence in prostate cancer cell line DU145. Ectopic overexpression of Id4 in androgen receptor-negative DU145 prostate cancer cells resulted in increased expression of p16, p21, p27, E-cadherin and vimentin but down-regulated E2F1 expression. Id4 also potentiated the effect of doxorubicin induced senescence and apoptosis. Conclusion: The absence of functional p16, pRB and p53 in DU145 suggests that Id4 could alter additional molecular pathways such as those involving E2F1 to promote senescence and increased sensitivity to doxorubicin-induced apoptosis. The results of the present study support the role of Id4 as a tumor suppressor in prostate cancer.
Keywords: Id4, senescence, E2F1, apoptosis, DU145
Inhibitor of differentiation proteins 1, 2, 3 and 4 (Id1–4), are dominant negative regulators of the basic helix-loop helix (bHLH) family of transcription factors (1). Id proteins lack the basic domain hence can form transcriptionally inactive heterodimers with bHLH proteins. In doing so, Id proteins can alter the transcription of genes that are dependent on functional dimerization between bHLH proteins. It is generally believed that Id proteins act as transcriptional regulators that inhibit differentiation and promote proliferation (2).
Recently Id4 has emerged as a potential tumor suppressor in prostate, colon, and pancreatic cancer that is down-regulated through promoter hypermethylation during cancer progression (3, 4). Id4 has been also shown to induce several other tumor suppressor genes (e.g. E-cadherin, p21), which play crucial roles in the induction of cellular senescence (5). We have previously, shown that overexpression of Id4 in DU145 prostate cancer cell line promotes apoptosis and attenuates cell cycle through increased CDKN1A expression (5). Although, this study was designed to investigate the mechanism by which Id4 promotes cell-cycle arrest, we intriguingly observed that Id4 promotes senescence, a significant tumor suppressor pathway. Our results suggest that Id4 down-regulates E2F1, a critical facilitator of G1/S transition and S-phase and a critical barrier for induction of senescence (6). In cancer cells, E2F1 is required for cell-cycle progression through interaction with the retinoblastoma (RB) protein (7).
Senescence is typically regulated through the control of several G1 checkpoint proteins including p16, p21and pRB (8). DU145 prostate cancer cells have a de-regulated G1 checkpoint due to mutations within the p16 (9) and pRB (10) regulatory proteins hindering the ability of these cells to readily undergo senescence. In-spite of the mutations in p16 and pRb, Id4 is still capable of inducing senescence in DU145 indicating a strong regulatory control over cell cycle fate by Id4. In addition to mutations in p16 and pRb, the tumor suppressor and pro-apoptotic p53 is also mutated in DU145 cells. Our results suggest that the induction of senescence by Id4 is downstream of the p16/Rb and p53. Since most anticancer agents such as doxorubicin act in stimulating intracellular mechanisms for cellular senescence and apoptosis, physiological determinants required for the sensitivity and efficacy of the anticancer drugs such as expression of Id4, could be an important consideration for developing therapeutic regimens.
Materials and Methods
Cell line and treatment
The DU145 prostate cancer cell line was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) and cultured as per ATCC recommendations. Human Id4 was over-expressed in DU145 cells as previously described (5). Cells were treated with 0–500 nm of doxorubicin (DOX) for 24, 48 and/or 72 h.
Quantitative senescence associated (SA)-β-galactosidase assay
The cells were cultured in six-well plates with respective media. The cells were then treated with DOX at 60–70% confluency and stained by senescence associated-β-galactosidase (SA-βgal) staining kit (Cell signaling, Danvers, MA, USA) as per manufacturer’s instructions. At least 15 representative fields were randomly selected for the quantitation of the percentage of SA-βgal-positive cells. The images were captured in both phase contrast and bright field to better visualize cellular details.
Western blot analysis
Total cell protein was prepared from cultured prostate cancer cells using M-PER (Thermo Scientific, Waltham, MA, USA). 30ug of total protein was size fractionated on 4–20% SDS-polyacrylamide gel and subsequently blotted onto a nitrocellulose membrane (Whatman, Piscataway, NJ, USA). The blotted nitrocellulose membrane was subjected to western blot analysis using specific antibodies (Cell signaling). After washing with 1× PBS, 0.5% Tween20, the membranes were incubated with horseradish peroxidase (HRP) coupled secondary antibody against rabbit or mouse IgG and visualized using the Super Signal West Dura Extended Duration Substrate (Thermo Scientific) on Fuji Film LAS-3000 Imager.
Apoptosis assay
Quantitation of apoptosis was performed by Alexa Fluor 488 Annexin V/Dead cell apoptosis kit (Invitrogen) as per manufacturer’s instructions. The cells in suspension were used for flow Cytometric analysis with Accuri C6 flow cytometer. An aliquot of the stained cells was deposited on the slides and mounted using glycerol and visualized in Zeiss fluorescence microscope through Axiovision software.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA (2 µg) was reverse transcribed in a final volume of 25 µl as per standard protocols (11). Reverse-transcribed RNA from cells was used for PCR using gene specific primers as described previously (12). The following PCR primers were used for E2F1: Forward 5’-ACT CCT CGC AGA TCG TCA TCA TCT and reverse 5’-GGA CGT TGG TGA TGT CAT AGA TGC G.
Immunocytochemistry
Cells were grown on glass chamber slides to 75% confluency. The slides were then washed with PBS (3×) and fixed in ice cold methanol for 10 min at room temperature and stored at −20°C until further use. Before use, the slides were equilibrated at room temperature, washed with PBS three times for 5 min, blocked with 1% BSA in PBST for 30 min at room temp and Incubated overnight (4°C) with primary a antibody (1% Bovine Serum Albumin in phosphate bufferd saline with 0.05% Tween 20 PBST). The slides were then washed in PBS and incubated with secondary antibody with fluorochrome conjugated to DyLight in 1% BSA for 1hr at room temp in dark. The slides were subsequently washed again and stained in DAPI (1 µg/ml) for 1 min and mounted with glycerol. Images were acquired by Zeiss fluorescence microscope through Axiovision software.
Statistical analysis
The Student’s t-test was used to evaluating statistical differences between groups. A p-value of <0.05 was considered as statistically significant.
Results
Id4-transfected DU145 cells undergo senescence
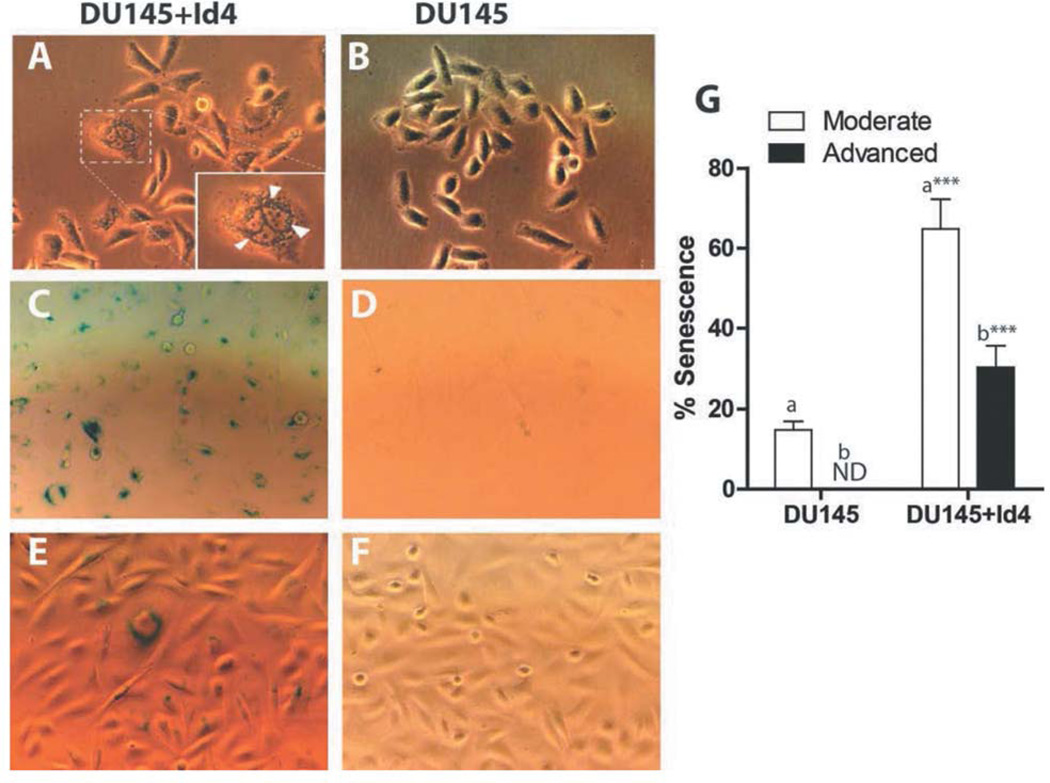
Previously we have reported that Id4 acts as a tumor suppressor in prostate cancer by inhibiting proliferation through increased expression of cyclin-dependent kinase inhibitors (5). Enlarged morphology and accumulation of cytoplasmic aggregates (Figure 1A, arrowheads) were observed in DU145+Id4 cells as compared to DU145 cells (Figure 1B), indicative of senescence (13, 14). An increase in senescence associated beta-galactosidase (SA-βgal) staining (Figure 1C and E) suggested that ectopic expression of Id4 in DU145 cells promotes senescence at a higher frequency than in un-transfected DU145 cells (Figure 1D and F). The results summarized in Figure 1G demonstrate a significant increase in the number of cells with SA-βgal staining in DU145+Id4. The intensity SA-βgal staining was graded as no staining, intermediate and strong. The cells with intermediate staining were considered as moderately senescent (moderate), and those with strong staining were classified as advanced senescent (63.8±7.56% moderate and 30.2±5.41% advanced), as compared to DU145 cells (14.88+2.14% moderate). DU145 cells did not demonstrate advanced SA-βgal staining.
Figure 1.
Id4 promotes senescence in the DU145 prostate cancer cell line. Cells (DU145 and DU145+Id4) cells were stained with SA-b-galactosidase. The blue nuclei due to SA-b-galactosidase staining were counted in 15 randomly-selected fields and are expressed as mean+SEM (panel G). The inset in panel A shows the granular structures (arrowheads). The flattened nuclei with intense blue staining were classified as cells with advanced senescence and smaller light blue nuclei were counted as cells with moderate senescence. ***p<0.001, t-test performed for columns “a” and “b”.
Increased CDKNI (p16, p21 and p27) expression in senescent DU145+Id4 cells
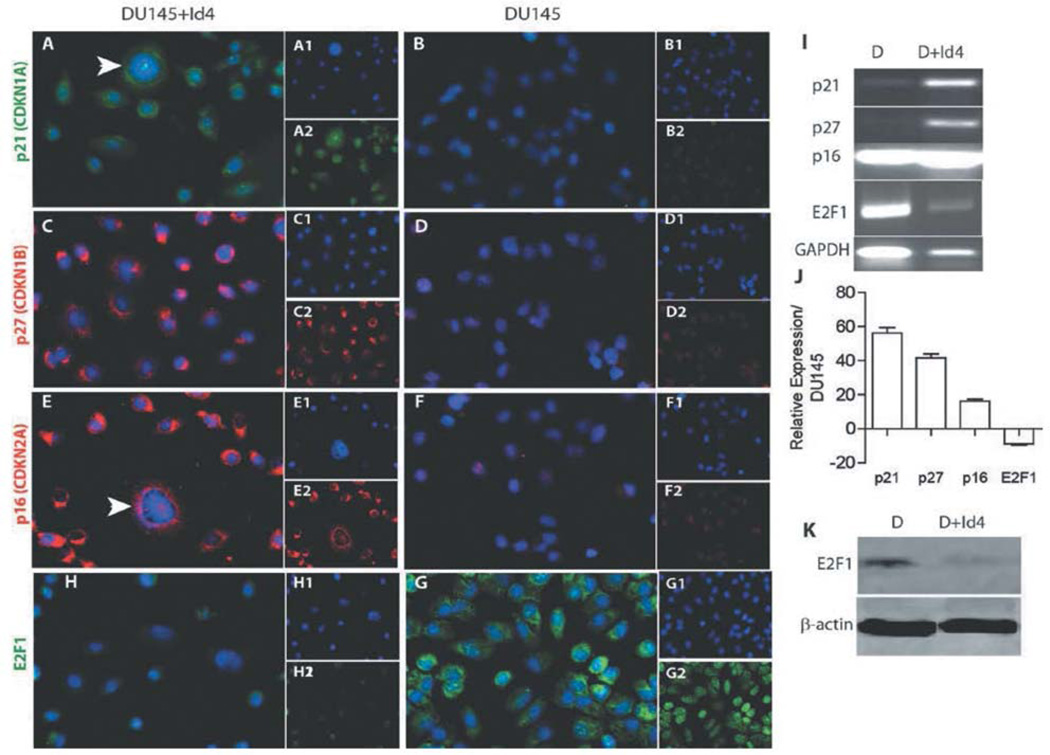
Several tumor suppressor genes are capable of inducing senescence through inhibition of the cell cycle by inducing a G1 arrest via either p21 (15), p27 (16) or p16 and subsequently inhibiting the CDK2-dependent phosphorylation of the RB protein. However, DU145 cells have a highly de-regulated cell cycle with mutations in p16 and Rb genes, the key regulators of the senescent pathway (6). Immunocytochemical analysis shown in Figure 2 clearly indicate that Id4 up-regulates G1 cell-cycle regulators p21 (Figure 2A, I and J) and p27 (Figure 2C, I and J), as compared to DU145 cells (Figure 2B, D, I and J, respectively). We also observed an increased p16 expression at protein (Figure 2E) and transcript levels (Figure 2I and J) in DU145+Id4 cells, compared to DU145 cells (Figure 2F, I and J) but its functional relevance in DU145 cells with respect to senescence remains obscure. The cellular localization studies indicated that CDKNI expression was clearly partitioned between the cytoplasm and nucleus. We speculate that in the absence of functional Rb, the primary phosphorylation target of CDK2, p21 and p27 could activate/alternate Rb-independent cell-cycle regulatory pathways such as those involving E2F1. p21 is directly associated to E2F1 (17) and suppresses its transcriptional activity and/or represses Myc-dependent transcription (18).
Figure 2.
Id4-dependent senescence is associated with increased expression of E2F1 and cyclin-dependent kinase inhibitors p16, p21 and p27. Immunocytochemical (ICC) localization of CDKNIs p21 (panels A and B, green), p27 panels (panels C and D, red), p16 (panels E and F, red) and E2F1 (panels H and G, green) in DU145+Id4 (A, C, E and H) and DU145 cells (B, D, F and G). The insets in each panel are individual channels showing the protein-specific staining and DAPI (blue, nuclei). Panel I is the RT-PCR analysis of p21, p27, p16 and E2F1 gene expression in DU145 and DU145+Id4 cells. GAPDH was used as a loading control. Panel J is RT-PCR analysis of the genes in panel I. Data are expressed as fold change compared to DU145, set to 1 and normalized to GAPDH. Panel K: Western blot analysis of E2F1 expression in DU145 and DU145+Id4 cells. The antibody used is the same as the one used for ICC (Panels H and G). Representative data from at least 3 different experiments are shown.
Senescence in DU145+Id4 cells is associated with decreased E2F1 expression
Restraining E2F1 either at the transcriptional or post-transcriptional level, independently of Rb can block cell cycle in DU145 cells (6). Interestingly, E2F1 expression was significantly down-regulated in DU145+Id4 cells compared to DU145, both at transcriptional and protein levels (Figures 2H, I, J and K). These cellular localization studies provided compelling evidence that decreased E2F1 could be associated with senescence in DU145+Id4 cells.
Id4 sensitizes prostate cancer cells to doxorubicin-induced senescence
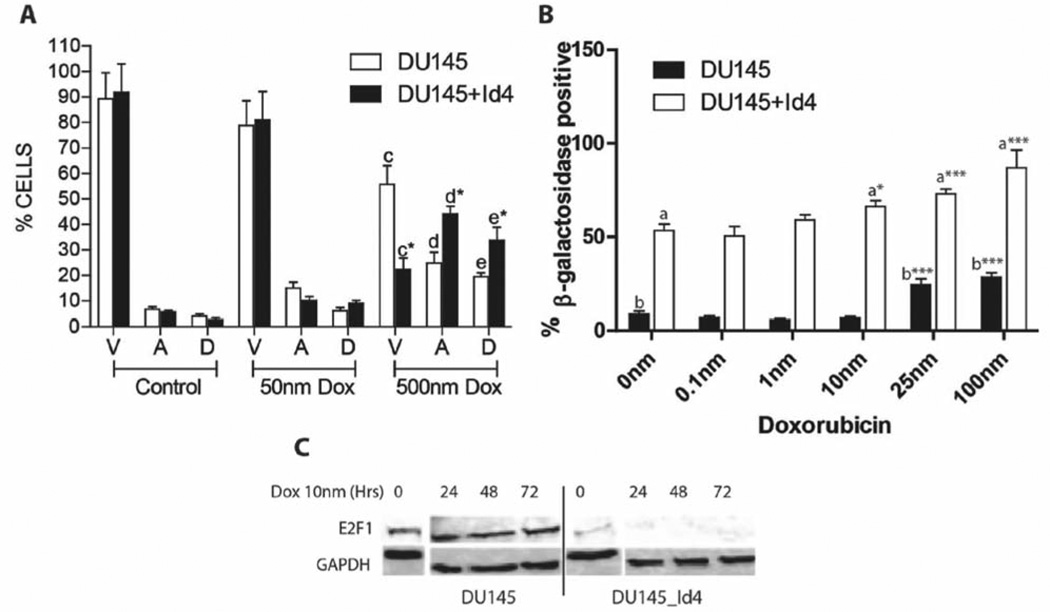
Senescence in cancer cells can be readily induced by treatment with chemotherapeutic agents, radiation or genetic/chemical manipulations that promote differentiation (19). The ability of Id4 to promote senescence in prostate cancer cells prompted us to investigate whether Id4 can potentiate senescence in response to chemotherapeutic agents such as doxorubicin. DU145 cells were exposed to increasing concentrations of doxorubicin (0–100 nM more than 72 h), known to induce senescence within these cells (20, 21). A significant decrease in viable cells or increase in apoptotic cells between DU145 and DU145+Id4 cells was not observed at any of the doxorubicin concentrations used. Data from 50 nm for 24 h treatment are shown in Figure 3A. Interestingly, when the cells were exposed to 500 nm of doxorubicin for 24 h, significantly larger populations of DU145+Id4 cells were apoptotic with a corresponding decrease in viable cells as compared to DU145 cells (Figure 3A). These results suggested that Id4 also promotes increased sensitivity to doxorubicin-induced cell death. The cells exposed to increasing concentrations of doxorubicin from 0.1–100 nM, demonstrated that the number of senescent cells in DU145+Id4 was significantly higher at all concentrations of doxorubicin as compared to DU145. At 100 nM doxorubicin concentration, 28% (±2.38%) of DU145 cells were positive for SA-βgal compared to 87% (±9.6%, p<0.001) of DU145+Id4 cells (Figure 3B). At 10 nm doxorubicin, the number of DU145+Id4 senescent cells was significantly higher as compared to 0 nm, whereas a significant increase in DU145 senescent cells was only achieved at 25 nm suggesting that Id4 increases sensitivity to doxorubicin-induced senescence (Figure 3B). We next investigated the expression of E2F1 in DU145 and DU145+Id4 cells treated with 10 nm doxorubicin for 0, 24, 48 and 72 h (Figure 3C). At 10 nm, a significant increase in senescence was not observed in DU145 cells. The basal (0 h) E2F1 expression was significantly lower in DU145+Id4 cells as compared to DU145 cells whereas E2F1 expression was almost undetectable in DU145+Id4 cells at subsequent time points. Interestingly, a decrease in E2F1 expression was not observed in DU145 cells that were consistent with the lack of senescence in these cells. These results suggest that increased senescence observed in DU145+Id4 in absence of doxorubicin could be due to lower E2F1, further supporting our earlier observations (Figure 2). Thus, ectopic expression of Id4 in DU145 cells increases the susceptibility of these cells to undergo senescence in spite of non-functional senescence mediators (mutant p16, pRB and p53) suggesting the activation of an atypical senescent pathway, such as loss of E2F1 expression.
Figure 3.
Effect of doxorubicin on apoptosis and senescence in DU145 and DU145+Id4 prostate cancer cell lines. A: Flow cytometric data show the effect of 0 (control), 50 and 500 nm doxorubicin treatment (24 h) on apoptosis in DU145 and DU145+Id4 cells after AnnexinV – Alexa488 and PI staining. The viable (V), apoptotic (A) and dead (D) cells are represented (mean±SEM of three experiments in duplicate). Apoptosis in DU145 and DU145+Id4 cells treated with 50 nm doxorubicin was not statistically different, whereas a significant increase in apoptosis was observed in both cells types with 500 nm doxorubicin. *p<0.001, Student’s t-test. B. DU145 and DU145+Id4 cells were treated with 0–100 nm doxorubicin and stained with SA-b-galactosidase. The blue nuclei due to SA-b-galactosidase staining were counted in 15 randomly-selected fields and are expressed as mean±SEM. The blue SA-b-galactosidase-positive nuclei were counted and expressed as mean+SEM. ***p<0.001, t-test for columns “a” and “b”. C: Western blot analysis of E2F1 expression in DU145 and DU145+Id4 cells treated with 10 nm doxorubicin for 0, 24, 48 and 72 h.
Id4-dependent senescence is associated with altered vimentin and E-cadherin expression
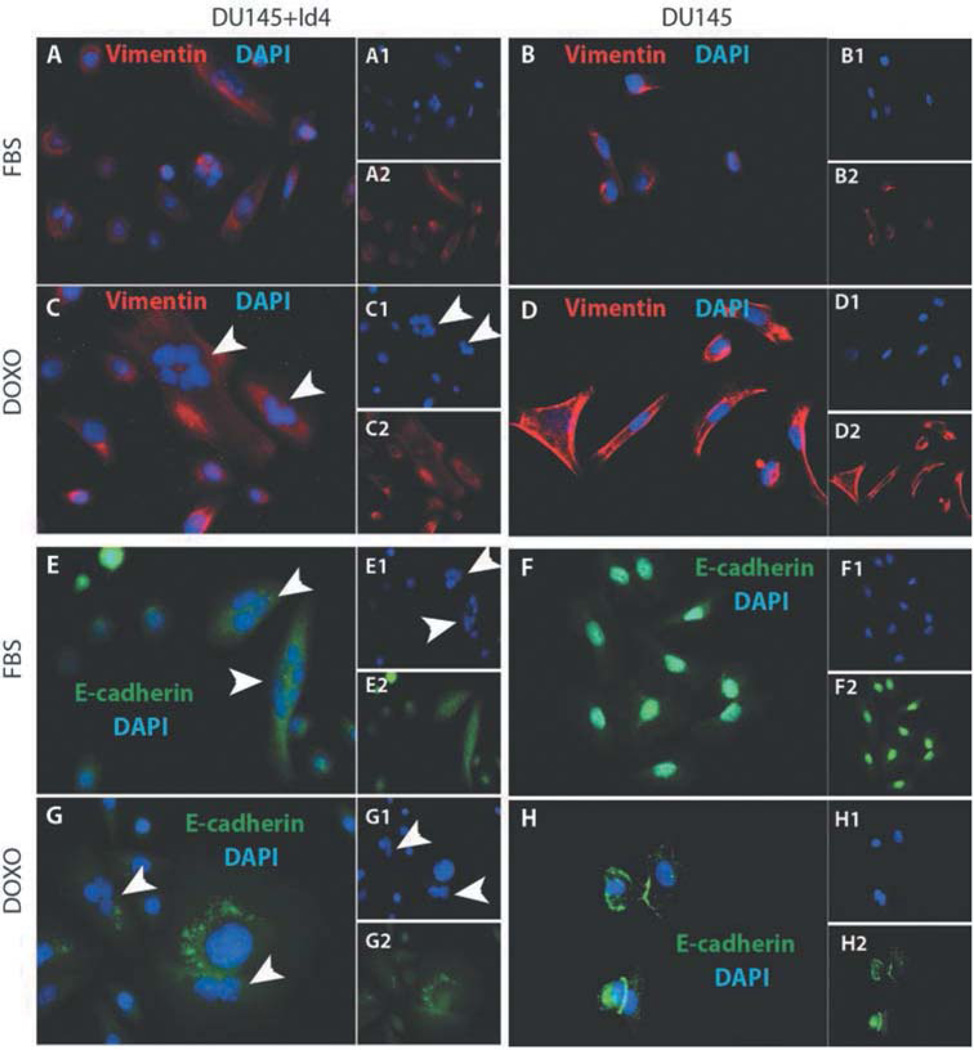
Increased vimentin expression is associated with a senescent phenotype (22). Low vimentin expression was observed in DU145 cells alone (Figure 4B), but a significant increase was observed after treatment of cells with doxorubicin (Figure 4D). Although increased vimentin levels were also observed in DU145+Id4 and DU145+Id4+doxorubicin cells (Figure 4A and C, respectively), as compared to DU145 cells alone, the strikingly clear discrete bundles observed in DU145+doxorubicin cells were not apparent. Weak-to-marginal vimentin bundles were observed in DU145+Id4 and DU145+Id4+doxorubicin cells that could possibly be due to the large size of the DU145+Id4 cells as compared to DU145 cells. These results suggest that vimentin re-organization in response to doxorubicin in DU145 is distinct from DIU145+Id4 cells, although increased senescence was observed in both cell types. The results also suggest that overexpressing Id4 in DU145 cells increased vimentin expression
Figure 4.
Id4 and doxorubicin-dependent senescence is associated with increased expression and/or cellular distribution of vimentin and E-cadherin. Immunocytochemical (ICC) localization of vimentin (panels A–D, red) and E-cadherin (panels E–H, green) in DU145+Id4 (A, C, E and G) and DU145 cells (B, D, F and H) in the absence (A, B, E and F) or presence (C, D, G and H) of 50 nm doxorubicin. The insets in each panel are individual channels showing the protein-specific staining and DAPI (blue, nuclei). Representative data from at least 3 different experiments are presented.
E-cadherin expression is also altered in DU145+Id4 cells undergoing morphological transformation to a senescent phenotype. Cytoplasmic accumulation of E-cadherin expression (Figure 4E and G) in DU145+Id4 was observed as compared to mainly nuclear in DU145 cells (Figure 4F and H). Nuclear E-cadherin is a tumorigenic-associated function of E-cadherin in several types of cancers (23), as compared to cytoplasmic localization involved in maintaining cell-cell interactions in normal cells. In the presence of 50 nm doxorubicin, DU145 cells regain the cytoplasmic expression profile of E-cadherin (Figure 4G). However no large accumulation of E-cadherin expression in the cytoplasm was observed in DU145 cells in response to 50 nM doxorubicin exposure (Figure 4H), as compared to the one observed in the DU145+Id4 cells (Figure 4G).
One of the hallmarks of senescence is the formation of multi-nucleated cells due to mitotic catastrophe and is indicative of replicative senescence (24). The most distinguishing features of DU145+Id4 cells was the frequent presence of multi-nucleated cells, possibly due to impaired cytokinesis (Figure 4C, E and G, arrowheads). The multinucleated cells were not observed in DU145 cultures even after doxorubicin-induced senescence (Figure 4D and H) suggesting that multi-nucleation is a specific response to Id4-dependent pathways in DU145+Id4 cells. Although the exact molecular mechanism of multi-nucleation in DU145+Id4 cells was not investigated, however a disturbed cell cycle due to the loss of critical cell-cycle regulatory genes such as p16, Rb, p53 and E2F1 and/or over-production of centrosomes could result in this phenotype.
Discussion
The ability of cancer cells to undergo cellular senescence is a major roadblock in the transformation of normal cells to cells expressing the cancer phenotype. Therefore, an inherent ability of cancer cells to regulate the cell cycle through induction of senescence poses a possible paradigm shift in cancer biology and proposes a mechanism of cellular reprogramming of cancer cells. On this regard, the cellular reprogramming of DU145 cancer cells by Id4 is a major observation. The expression of key senescence-associated markers in DU145+Id4 such as SA-gal and vimentin strongly supports the role of Id4 in promoting senescence. These observations gain additional significance given that Id4 promotes senescence even in the absence of key senescent regulatory proteins (e.g. p16, pRB and p53). Thus Id4-alone has the capacity to induce senescence in transformed neoplastic cells such as DU145, that lack senescence-associated tumor suppressors. The similarity between the expression of these senescent markers and doxorubicin-induced senescence in DU145 cells further supports the role of Id4 in promoting senescence in the absence of classical senescent regulatory proteins (e.g. p16, pRB and p53). In fact potentiation of Id4-dependent senescence in the presence of doxorubicin suggests similar pathways leading to senescence: for example, increased vimentin expression associated with bundle formation and re-organization of E-cadherin. The clear nuclear-to-cytoplasmic shuttling of E-cadherin in DU145+Id4 cells, therefore, not only supports the role of Id4 as a tumor suppressor but also suggests that this shuttling mechanism is involved in senescence, a novel observation.
Low-dose doxorubicin induced senescence involves cytostasis, which permanently disables the proliferative capacity of cells without inducing cell death (19). We speculate that Id4-alone could induce mitotic catastrophe in rapidly proliferating DU145 cells, as seen by the presence of multi-nucleated cells, not observed in doxorubicin-induced DU145 senescent cells as part of the Id4-dependent tumor suppressor mechanism. Multi-nucleated cells have been observed in doxorubicin-induced senescence (25) but the lack of such a process in our system could be due to the length of treatment (72 h) that limited the formation and subsequent detection of multi-nucleated cells.
It is apparent that the role of Id4 in cell-cycle regulation is more divergent than other Id proteins. Id1 has been shown to down-regulate several cell cycle regulatory proteins including p16, and p21 in cancer cells to promote cell proliferation, metastasis and invasion (26, 27). However, in contrast to Id1, the ectopic expression of Id4 reverses the pro-oncogenic cell cycle profile associated with Id1 and leads to the up-regulation of p21 and p27 (12). Increased expression of both these cyclin-dependent kinase inhibitors alone can also promote senescence (16, 28).
As the cell cycle in cancer cells is highly de-regulated, the alternated mechanisms as opposed to de-regulated classical senescent pathways may play a crucial role of cancer cell reprogramming. Inhibition of E2F1 activity is an often-overlooked aspect of p16/pRB senescence induction in replicating cells and points to E2F1 as a viable target in cancer cell senescence induction. Overexpression of E2F1 has been linked to induction of p53-independent apoptosis in several types of cancer but has also been linked to an increase in proliferation (29). Increasing evidence suggests the essential role of E2F1 in G1-S phase transition (30). Therefore, E2F1 down-regulation may be an alternative mechanism of a senescence in p53, p16 and Rb-mutant cancer cells such as DU145. In DU145+Id4 cells, E2F1 expression is down-regulated at the mRNA level by Id4 suggesting that Id4 possesses transcriptional control. These studies suggest that the loss in Id4 expression may be essential to transformation of cancer cells. Indeed, prostatic intra-epithelial neoplasia has been observed in Id4−/− mice prostates.
In conclusion, we convincingly demonstrate that Id4-alone is capable of inducing senescence in prostate cancer cell line DU145 that has by-passed many major tumor suppressor blocks (e.g. lack functional p16, pRb and p53), possibly through an E2F1-dependent mechanism. Thus, Id4 expression could be a major facilitator of therapy induced senescence (e.g. with Doxorubicin) and apoptosis, that needs to be investigated in additional cell lines. The Id4-dependent senescence may promote a persistent terminal growth arrest characterized by increased expression of CDKNIs p21 and p27. Thus Id4 expression in pre-malignant lesions and cancers may have favorable implications with regard to tumor biology and prognosis.
Acknowledgements
The research was supported by NIH/NCI CA128914 to JC. Support for core facilities and additional resources were funded in part by NIH/NCRR/RCMI G12RR03062.
References
- 1.Murre C, Bain G, van Dijk MA, Engel I, Furnari BA, Massari ME, Matthews JR, Quong MW, Rivera RR, Stuiver MH. Structure and function of helix-loop-helix proteins. Biochim Biophys Acta. 1994;1218:129–135. doi: 10.1016/0167-4781(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 2.Yokota Y, Mori S. Role of Id family proteins in growth control. J Cell Physiol. 2002;190:21–28. doi: 10.1002/jcp.10042. [DOI] [PubMed] [Google Scholar]
- 3.Umetani N, Mori T, Koyanagi K, Shinozaki M, Kim J, Giuliano AE, Hoon DSB. Aberrant hypermethylation of ID4 gene promoter region increases risk of lymph node metastasis in T1 breast cancer. Oncogene. 2005;24:4721–4727. doi: 10.1038/sj.onc.1208538. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Chinaranagari S, Patel D, Carey J, Chaudhary J. Epigenetic inactivation of inhibitor of differentiation 4 (Id4) correlates with prostate cancer. Cancer medicine. 2012;1:176–186. doi: 10.1002/cam4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey JP, Asirvatham AJ, Galm O, Ghogomu TA, Chaudhary J. Inhibitor of differentiation 4 (Id4) is a potential tumor suppressor in prostate cancer. BMC Cancer. 2009;9:173. doi: 10.1186/1471-2407-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park C, Lee I, Kang WK. E2F-1 is a critical modulator of cellular senescence in human cancer. Int J Mol Med. 2006;17:715–720. [PubMed] [Google Scholar]
- 7.Nevins JR. The Rb/E2F pathway and cancer. Human molecular genetics. 2001;10:699–703. doi: 10.1093/hmg/10.7.699. [DOI] [PubMed] [Google Scholar]
- 8.Lanigan F, Geraghty JG, Bracken AP, et al. Transcriptional regulation of cellular senescence. Oncogene. 2011;30:2901–2911. doi: 10.1038/onc.2011.34. [DOI] [PubMed] [Google Scholar]
- 9.Tamimi Y, Bringuier PP, Smit F, van Bokhoven A, Debruyne FM, Schalken JA. p16 mutations/deletions are not frequent events in prostate cancer. British journal of cancer. 1996;74:120–122. doi: 10.1038/bjc.1996.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bookstein R, Rio P, Madreperla SA, Hong F, Allred C, Grizzle WE, Lee WH. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci USA. 1990;87:7762–7766. doi: 10.1073/pnas.87.19.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asirvatham AJ, Schmidt MA, Chaudhary J. Non-redundant inhibitor of differentiation (Id) gene expression and function in human prostate epithelial cells. The Prostate. 2006;66:921–935. doi: 10.1002/pros.20366. [DOI] [PubMed] [Google Scholar]
- 12.Carey JPW, Asirvatham AJ, Galm O, Ghogomu TA, Chaudhary J. Inhibitor of differentiation 4 (Id4) is a potential tumor suppressor in prostate cancer. BMC Cancer. 2009;9:173–173. doi: 10.1186/1471-2407-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz ML, Robison WG, Herrmann RK, Groome AB, Bieri JG. Lipofuscin accumulation resulting from senescence and vitamin E deficiency: spectral properties and tissue distribution. Mech Ageing Dev. 1984;25:149–159. doi: 10.1016/0047-6374(84)90137-4. [DOI] [PubMed] [Google Scholar]
- 14.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia L, Li H, Sun Y. Induction of p21-dependent senescence by an NAE inhibitor, MLN4924, as a mechanism of growth suppression. Neoplasia. 2011;13:561–569. doi: 10.1593/neo.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder PK, Grisanzio C, O’Connell F, Barry M, Brito JM, Xu Q, Guney I, Berger R, Herman P, Bikoff R, Fedele G, Baek WK, Wang S, Ellwood-Yen K, Wu H, Sawyers CL, Signoretti S, Hahn WC, Loda M, Sellers WR. A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell. 2008;14:146–155. doi: 10.1016/j.ccr.2008.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delavaine L, La Thangue NB. Control of E2F activity by p21Waf1/Cip1. Oncogene. 1999;18:5381–5392. doi: 10.1038/sj.onc.1202923. [DOI] [PubMed] [Google Scholar]
- 18.Kitaura H, Shinshi M, Uchikoshi Y, Ono T, Iguchi-Ariga SM, Ariga H. Reciprocal regulation via protein-protein interaction between c-Myc and p21(cip1/waf1/sdi1) in DNA replication and transcription. J Biol Chem. 2000;275:10477–10483. doi: 10.1074/jbc.275.14.10477. [DOI] [PubMed] [Google Scholar]
- 19.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. Journal of the National Cancer Institute. 2010;102:1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewald JA, Peters N, Desotelle JA, Hoffmann FM, Jarrard DF. A high-throughput method to identify novel senescence-inducing compounds. Journal of biomolecular screening. 2009;14:853–858. doi: 10.1177/1087057109340314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio K, Inoue A, Qiao S, Kondo H, Mimura A. Senescence and cytoskeleton: overproduction of vimentin induces senescent-like morphology in human fibroblasts. Histochem Cell Biol. 2001;116:321–327. doi: 10.1007/s004180100325. [DOI] [PubMed] [Google Scholar]
- 23.Elston MS, Gill AJ, Conaglen JV, Clarkson A, Cook RJ, Little NS, Robinson BG, Clifton-Bligh RJ, McDonald KL. Nuclear accumulation of e-cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J Clin Endocrinol Metab. 2009;94:1436–1442. doi: 10.1210/jc.2008-2075. [DOI] [PubMed] [Google Scholar]
- 24.Vergel M, Marin JJ, Estevez P, Carnero A. Cellular senescence as a target in cancer control. Journal of aging research. 2010;2011:1–12. doi: 10.4061/2011/725365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sliwinska MA, Mosieniak G, Wolanin K, Babik A, Piwocka K, Magalska A, Szczepanowska J, Fronk J, Sikora E. Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mech Ageing Dev. 2009;130:24–32. doi: 10.1016/j.mad.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Ling MT, Wang X, Ouyang XS, Xu K, Tsao SW, Wong YC. Id-1 expression promotes cell survival through activation of NF-kappaB signalling pathway in prostate cancer cells. Oncogene. 2003;22:4498–4508. doi: 10.1038/sj.onc.1206693. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Patel D, Chaudhary J. Id1 and Id3 expression is associated with increasing grade of prostate cancer: Id3 preferentially regulates CDKN1B. Cancer medicine. 2012;1:187–197. doi: 10.1002/cam4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999;18:4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Wingate H, Swisher SG, Keyomarsi K, Hunt KK. Absence of pRb facilitates E2F1-induced apoptosis in breast cancer cells. Cell Cycle. 2010;9:1122–1130. doi: 10.4161/cc.9.6.10990. [DOI] [PubMed] [Google Scholar]
- 30.Dimri GP, Campisi J. Molecular and cell biology of replicative senescence. Cold Spring Harb Symp Quant Biol. 1994;59:67–73. doi: 10.1101/sqb.1994.059.01.010. [DOI] [PubMed] [Google Scholar]