Abstract
Background
Miscarriage occurs in 10% to 15% of pregnancies. The traditional treatment, after miscarriage, has been to perform surgery to remove any remaining pregnancy tissues in the uterus. However, it has been suggested that drug-based medical treatments, or expectant care (no treatment), may also be effective, safe and acceptable.
Objectives
To assess the effectiveness, safety and acceptability of any medical treatment for early incomplete miscarriage (before 24 weeks).
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (September 2009) and reference lists of retrieved papers. We updated this search on 23 July 2012 and added the results to the awaiting classification section of the review.
Selection criteria
Randomised controlled trials comparing medical treatment with expectant care or surgery. Quasi-randomised trials were excluded.
Data collection and analysis
Two authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. Data entry was checked.
Main results
Fifteen studies (2750 women) were included, there were no studies on women over 13 weeks’ gestation. Studies addressed a number of comparisons and data are therefore limited.
Three trials compared misoprostol treatment (all vaginally administered) with expectant care. There was no significant difference in complete miscarriage (average risk ratio (RR) 1.23, 95% confidence interval (CI) 0.72 to 2.10; two studies, 150 women), or in the need for surgical evacuation (average RR 0.62, 95% CI 0.17 to 2.26; two studies, 308 women). There were few data on ‘deaths or serious complications’.
Nine studies involving 1766 women addressed the comparison of misoprostol (four oral, four vaginal, one vaginal + oral) with surgical evacuation. There was no statistically significant difference in complete miscarriage (average RR 0.96, 95% CI 0.92 to 1.00, eight studies, 1377 women) with success rate high for both methods. Overall, there were fewer surgical evacuations with misoprostol (average RR 0.07, 95% CI 0.03 to 0.18; eight studies, 1538 women) but more unplanned procedures (average RR 6.32, 95% CI 2.90 to 13.77; six studies, 1158 women). There were few data on ‘deaths or serious complications’.
Limited evidence suggests that women generally seem satisfied with their care. Long-term follow up from one included study identified no difference in subsequent fertility between the three approaches.
Authors’ conclusions
The available evidence suggests that medical treatment, with misoprostol, and expectant care are both acceptable alternatives to routine surgical evacuation given the availability of health service resources to support all three approaches. Women experiencing miscarriage at less than 13 weeks should be offered an informed choice.
[Note: the 34 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Medical Subject Headings (MeSH): Abortifacient Agents, Nonsteroidal [administration & dosage]; Abortion, Incomplete [*therapy]; Misoprostol [administration & dosage]; Pregnancy Trimester, First; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
Miscarriage is generally defined as the spontaneous loss of a pregnancy prior to 24 weeks’ gestation, that is, before the fetus is usually viable outside the uterus (Shiers 2003). The clinical signs of miscarriage are vaginal bleeding usually with abdominal pain and cramping. If the pregnancy has been expelled, the miscarriage is termed ‘complete’ or ‘incomplete’ depending on whether or not tissues are retained in the uterus. If a woman bleeds but her cervix is closed, this is described as a ‘threatened miscarriage’ as it is often possible for the pregnancy to continue and not to miscarry (RCOG 2006; Shiers 2003); if the pregnancy is in the uterus but the cervix is open, this is described as an ‘inevitable miscarriage’, i.e. it will not usually be possible to save the pregnancy and fetus. The now widespread use of ultrasound in early pregnancy, either for specific reasons (e.g. bleeding) or as a routine procedure, reveals pregnancies which are destined to miscarry inevitably, because they are ‘non-viable’ (Sawyer 2007; Weeks 2001). Non-viable pregnancies are either a ‘missed miscarriage’ if an embryo or fetus is present but is dead, or an ‘anembryonic pregnancy’ if no embryo has developed within the gestation sac.
Regardless of the type of miscarriage, the overall incidence is considered to be between 10% and 15%, although the real incidence may be greater (Shiers 2003). Most miscarriages occur within the first 12 weeks of pregnancy and are called ‘early miscarriage’, with those occurring after 13 weeks being known as ‘late miscarriage’. The cause of miscarriage is generally unknown, but most are likely to be due to chromosomal abnormalities. The risk of miscarriage has been reported to be higher in older women, and where there are structural abnormalities of the genital tract, infection and maternal complications such as diabetes, renal disease and thyroid dysfunction. Also, some environmental factors have been linked with miscarriage including alcohol and smoking (Shiers 2003). Miscarriage can sometimes lead to haemorrhage and infection, and it can be an important cause of morbidity, and even mortality, particularly in low-income countries (Lewis 2007).
Women experiencing miscarriage may be overwhelmed by the symptoms and also quite distressed (Shiers 2003). Psychological problems can follow a miscarriage, and these can include loss of self-esteem resulting from the woman’s feeling of inability to rely on her body to give birth (Swanson 1999). Emotional responses described include those of emptiness, guilt and failure (Swanson 1999). There can also be depression, anxiety, grief and anger (Klier 2002; Thapar 1992). A number of other consequences, including sleep disturbance, social withdrawal, anger and marital disturbance, may occur following miscarriage (Lok 2007). Fathers can also be affected emotionally (Klier 2002).
Description of the intervention
Traditionally, all pregnancies that had miscarried were considered by clinicians as potentially incomplete. Therefore, surgical curettage was performed routinely to remove any retained products of conception. If no tissue was obtained, then a retrospective diagnosis of complete miscarriage was made. Surgical curettage was the ‘gold standard management’ for miscarriage for many years (Ankum 2001) because it is quickly performed and it is possible to remove completely any retained products of conception. Histological examination of the removed tissues also allowed exclusion of trophoblastic disease, e.g. hydatidiform mole - although this is quite rare. New clinical approaches have evolved to try to minimise unnecessary surgical interventions whilst aiming to maintain low rates of morbidity and mortality from miscarriage. These approaches have included ultrasound imaging to diagnose complete miscarriage and thus avoid treatment, or more conservative treatments of incomplete miscarriage such as drug (medical) treatment or no active treatment (expectant management) (Ankum 2001; Luise 2002). Various types of medical treatment could be suitable as alternatives to routine surgical treatment for miscarriage and these include the use of prostaglandins, or other uterotonic (uterus-contracting) drugs or anti-hormone therapy.
How the intervention might work
a) Prostaglandins, e.g. misoprostol, prostaglandin F2alpha
Misoprostol is a synthetic prostaglandin E1 analogue and is marketed for the prevention and treatment of peptic ulcers. Recognised as a potent method for pregnancy termination (Costa 1993; Norman 1991), it is inexpensive, stable at room temperature and has few systemic effects, although vomiting, diarrhoea, hypertension and even potential teratogenicity (causing fetal malformation) when misoprostol fails to induce the abortion, have been reported (Fonseca 1991).
Misoprostol has been shown to be an effective myometrial stimulant of the pregnant uterus, selectively binding to EP-2/EP-3 prostanoid receptors and stimulating contractions which push the products or pregnancy out. It is rapidly absorbed orally and vaginally. Vaginally absorbed serum levels are more prolonged and vaginal misoprostol may have locally mediated effects (Zieman 1997). Misoprostol could be especially useful in low-income countries, where transport and storage facilities are inadequate and the availability of uterotonic agents and blood is limited. Its use in obstetrics and gynaecology has been explored, especially to induce first and second trimester abortion (Costa 1993; Norman 1991), for the induction of labour (Alfirevic 2006; Hofmeyr 2003) and for the prevention of postpartum haemorrhage (Gülmezoglu 2007). The stimulatory actions of misoprostol on the early pregnancy uterus could, in theory, help to expel retained tissue from the uterus after miscarriage, and provide an attractive medical alternative to surgical treatment of incomplete miscarriage (Chung 1995). It is important to distinguish between the use of misoprostol for incomplete miscarriage and its use for termination of viable pregnancies.
b) Other uterotonics, e.g. ergometrine, oxytocin
Ergometrine (extracted from the rye fungus, ergot) will promote contraction of involuntary muscles throughout the body (Hawk 1985; Kawarabayashi 1990), and oxytocin promotes strong rhythmic contractions of the uterus (Arthur 2007; Mota-Rojas 2007). Both drugs could potentially have a role in expelling tissue after miscarriage.
c) Progesterone antagonist
A number of progesterone antagonists are now available and these drugs will interfere with the production or functioning, or both, of progesterone. The progesterone antagonist mifepristone has an established role in the termination of first and second trimester pregnancy (Jain 2002) and may be also be effective in promoting expulsion of retained products of conception following miscarriage (Tang 2006b).
Why it is important to do this review
Bleeding in early pregnancy is the most common reason for women to present to the gynaecology emergency department and in many of these women miscarriage will be diagnosed (Ramphal 2006). It is now clear that routine surgical evacuation of the uterus following miscarriage may not be indicated, and can pose a risk of infection, haemorrhage, cervical damage, and uterine perforation that may not be justified, as well as exposing the woman to the risks of anaesthesia (Harris 2007). In order to optimise clinical management of this common condition, it is important to establish whether the use of medical treatment (drugs), or expectant management (no routine treatment) may offer a safer alternative for women with incomplete miscarriage and whether there are specific circumstances where one type of treatment plan is superior to others.
We initially aimed to systematically review medical treatments for both non-viable pregnancies and incomplete miscarriages combined. On further reflection, this seemed illogical. Non-viable pregnancies contain viable trophoblast (placental) tissue, which produces hormones, which may in theory make these pregnancies more susceptible to anti-hormone therapy and more resistant to uterotonic (stimulating uterine contractions) therapy than pregnancies in which (incomplete) miscarriage has already taken place. Therefore, this review will focus on the management of incomplete miscarriage. Another review has covered non-viable pregnancies, ‘Medical treatment of early fetal death (less than 24 weeks)’ (Neilson 2006).
Other relevant Cochrane reviews on the treatment of miscarriage include, ‘Uterine muscle relaxant drugs for threatened miscarriage’ (Lede 2005), ‘Progestogen for treating threatened miscarriage’ (Wahabi 2007), ‘Surgical procedures to evacuate incomplete miscarriage’ (Forna 2001) and ‘Expectant care versus surgical treatment for miscarriage’ (Nanda 2006). There are also a series of Cochrane reviews on the possible prevention of miscarriage (Aleman 2005; Bamigboye 2003; Empson 2005; Haas 2008; Kaandorp 2009; Porter 2006; Rumbold 2005). In addition, there are Cochrane reviews on medical and surgical interventions for induced abortions (Dodd 2004; Kulier 2004; Lohr 2008; Medema 2005; Say 2002).
OBJECTIVES
To assess, from clinical trials, the effectiveness and safety of different medical managements for incomplete miscarriage, in terms of success, death or serious complications, additional unplanned surgical evacuation, blood transfusion, haemorrhage, blood loss, anaemia, days of bleeding, pain relief, pelvic infection, cervical damage, digestive disorders, hypertensive disorders, duration of stay in hospital, psychological effects, subsequent fertility, women’s views of treatment options, and costs.
METHODS
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs). Quasi-RCTs were excluded.
Types of participants
Participants were women being treated for spontaneous miscarriage (pregnancy loss at less than 24 weeks), either where there was ultrasound evidence of retained tissue (incomplete miscarriage) or where the diagnosis had been made on clinical grounds alone and where there would be uncertainty whether the miscarriage was complete or incomplete. In communities in which termination of pregnancy was illegal or unavailable, this could have included women who had undergone unsafe abortion.
Women with non-viable pregnancies (i.e. where the embryo or fetus had died in utero, but in whom miscarriage had not yet occurred) were excluded as they are covered by another Cochrane review (Neilson 2006).
Studies on induced abortion of a live fetus and for fetal anomaly were also excluded as these are covered in other Cochrane reviews (Dodd 2004; Kulier 2004; Lohr 2008; Medema 2005; Say 2002).
Types of interventions
We considered trials if they compared medical treatment of incomplete miscarriage with other methods (e.g. expectant management, placebo or any other intervention including surgical evacuation, either curettage or vacuum aspiration). Comparisons between different routes of administration of medical treatment (e.g. oral versus vaginal), or between different drugs or doses of drug, or duration or timing of treatment, were also included if data existed.
Types of outcome measures
Primary outcomes
Complete miscarriage (diagnosis of complete miscarriage based on findings at surgery and/or ultrasound examination after a specific period and/or cessation of symptoms and signs);
surgical evacuation;
death or serious complications (e.g. uterine rupture, haemorrhage, sepsis, coagulopathy, uterine perforation, hysterectomy, organ failure, intensive care unit admission).
Secondary outcomes
Unplanned surgical intervention (i.e. a second evacuation in the surgical group but a first evacuation in the medical or expectant group);
blood transfusion;
haemorrhage (blood loss greater than 500 ml, or as defined by trial authors);
blood loss;
anaemia (Hb less than 10 g/dl, or as defined by trial authors);
days of bleeding;
pain relief;
pelvic infection;
cervical damage;
digestive disorders (nausea or vomiting or diarrhoea);
hypertensive disorders;
duration of stay in hospital;
psychological effects;
subsequent fertility;
women’s views/acceptability of method;
death;
serious morbidity;
pathology of fetal/placental tissue;
costs.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (September 2009). We updated this search on 23 July 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists at the end of papers for further studies. We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Each potential study was assessed for inclusion by at least two review authors independently (JP Neilson (JPN), G Gyte (GG), L Dou (LD)). We resolved any disagreement through discussion, or if required we consulted a third author.
Data extraction and management
We designed a form to extract data. Two review authors (GG, LD) extracted the data using the agreed form. We resolved discrepancies through discussion, or if required we consulted a third author. Data were entered into Review Manager software (RevMan 2008), (all or a subsample) (GG), and checked for accuracy (LD). When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (GG, LD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Any disagreement was resolved by discussion or by involving a third author.
1) Sequence generation (checking for possible selection bias)
We have described for each included study, the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it would produce comparable groups.
We have assessed the methods as:
adequate (e.g. random-number table; computer random-number generator);
inadequate (odd or even date of birth; hospital or clinic record number); or
unclear.
2) Allocation concealment (checking for possible selection bias)
We have described for each included study, the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We have assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively-numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes; alternation; date of birth);
unclear.
3) Blinding (checking for possible performance bias)
We have described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We have also provided any information relating to whether the intended blinding was effective. Where blinding was not possible or inadequate, we have discussed whether the lack of blinding was likely to have influenced outcome assessment and introduced bias.
We have assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate inadequate or unclear for outcome assessors.
4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers (compared with the total randomised participants), reasons for attrition/exclusion where reported, and any re-inclusions in analyses which we undertook.
We have assessed the methods as:
adequate (e.g. where there were no missing data or where reasons for missing data are balanced across groups);
inadequate (e.g. where missing data are likely to be related to outcomes or are not balanced across groups);
unclear (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to be made).
We have discussed whether missing data greater than 20% might impact on outcomes, acknowledging that with long-term follow up, complete data are difficult to attain.
5) Selective reporting bias
We have described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We have assessed the methods as:
adequate (it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
6) Other sources of bias
We have described for each included study any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data-dependent process? Was there extreme baseline imbalance? Had the study been claimed to be fraudulent?
We have assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
7) Overall risk of bias
We have made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above, we have assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We were to explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis) but there were insufficient data available.
Measures of treatment effect
We have carried out statistical analysis using the Review Manager software (RevMan 2008).
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way in the trials. We have used the standardised mean difference to combine trials that measured the same outcome, but with different methods.
Unit of analysis issues
Cluster-randomised trials
There were no cluster-randomised trials identified, but if any are undertaken in the future and require to be included in a review update, then we will include them in the analyses along with individually randomised trials. Their sample sizes will be adjusted using the methods described in Gates 2005; Higgins 2008 using an estimate of the intracluster correlation co-efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, this will be reported and sensitivity analyses conducted to investigate the effect of variation in the ICC. If we identify both cluster-randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta-analysis. Therefore, the meta-analysis will be performed in two parts as well.
Dealing with missing data
For included studies, levels of attrition were noted. We have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we have carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we have attempted to include all participants randomised in the analyses, and we have analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes were known to be missing (‘available case’ analysis).
Assessment of heterogeneity
We have assessed statistical heterogeneity in each meta-analysis using the T2 (tau-squared), I2 and Chi2 statistics. We have regarded heterogeneity as substantial if T2 was greater than zero and either I2 was greater than 30% or there was a low P-value (less than 0.10) in the Chi2 test for heterogeneity. Where there is heterogeneity and random-effects was used, we have reported the average risk ratio, or average mean difference or average standard mean difference.
Assessment of reporting biases
Had there been 10 or more studies in a meta-analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually, and would have used formal tests for funnel plot asymmetry. For continuous outcomes, we would have used the test proposed by Eggar 1997, and for dichotomous outcomes, we would have used the tests proposed by Harbord 2006. If asymmetry had been detected by any of these tests or had been suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we aimed to contact study authors to ask them to provide missing outcome data. However, this proved difficult and where missing data were thought to introduce serious bias, the impact of including such studies in the overall assessment of results was to be explored by a sensitivity analysis. However, we felt we had insufficient information to make these assessments. None of the studies reported only significant findings.
Intention-to-treat analysis
We have analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Incomplete outcome data (attrition and exclusions)
See Assessment of risk of bias in included studies and Assessment of reporting biases sections above.
Selective outcome reporting bias
See Assessment of risk of bias in included studies and Assessment of reporting biases sections above.
Data synthesis
We have carried out statistical analysis using the Review Manager software (RevMan 2008). We have used fixed-effect meta-analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we have used random-effects analysis to produce an overall summary, if this was considered clinically meaningful. We felt it appropriate to use random-effects where we pooled data from different routes of misoprostol administration (Comparison 2). If an average treatment effect across trials was not clinically meaningful we have not combined heterogeneous trials. If we used random-effects analyses, the results have been presented as the average treatment effect and its 95% confidence interval.
Subgroup analysis and investigation of heterogeneity
We have conducted planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001.
We carried out the following subgroup analyses.
Women less than 13 weeks’ gestation.
Women between 13 and 23 weeks’ gestation.
Gestation not specified.
Sensitivity analysis
We were to carry out sensitivity analysis to explore the effect of trial quality for important outcomes in the review, but there were insufficient data for these assessments. Where there was risk of bias associated with a particular aspect of study quality (e.g. inadequate sequence generation, inadequate allocation concealment and incomplete outcome data not addressed) this was to be explored by sensitivity analysis. Again, there were insufficient data.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
One hundred and sixty-four reports were identified in the search which covered medical interventions for miscarriage before 24 weeks’ gestation, both for women with incomplete miscarriage and women with intrauterine fetal death. (Thirty reports from an updated search on 23 July 2012 have been added to Studies awaiting classification.)
Fifteen studies involving 2750 women were included (Bique 2007; Blanchard 2004; Blohm 2005; Clevin 2001; Dao 2007; Moodliar 2005; Ngoc 2005; Niinimaki 2006; Pang 2001; Sahin 2001; Shelley 2005; Shwekerela 2007; Trinder 2006; Weeks 2005; Zhang 2005), a further four are awaiting classification (Diop 2009;Jabir 2009; Shaikh 2008; Yu 2000) and one is an ongoing trial (Unkels 2008). The remaining reports were excluded (reasons are given in table of Characteristics of excluded studies).
Included studies
Twelve of the 15 included studies involved only women with incomplete miscarriage (Bique 2007; Blanchard 2004; Blohm 2005; Clevin 2001; Dao 2007; Moodliar 2005; Ngoc 2005; Pang 2001; Sahin 2001; Shelley 2005; Shwekerela 2007; Weeks 2005). Three studies included both women with incomplete miscarriage and women with an intrauterine fetal death (Niinimaki 2006; Trinder 2006; Zhang 2005). One of these studies reported the findings for incomplete miscarriage separately from those for intrauterine fetal death (Trinder 2006) and for the other two studies, the authors kindly sent us the separated data (Niinimaki 2006; Zhang 2005). There are a further 10 studies that looked at both women with incomplete miscarriage and women with intrauterine fetal death, and we have tried to contact these authors for the separated data but as yet have been unsuccessful. We have therefore excluded these studies from this review. We have added some recently published studies to the section Characteristics of studies awaiting classification.
Therefore, 15 studies, with a total of 2750 women, and involving 70 meta-analyses, are included. All the studies addressed medical treatment for incomplete miscarriage before 13 weeks and we found no studies addressing this question for women between 13 and 23 weeks’ gestation.
Ten of the studies used ultrasound to confirm the diagnosis (Blanchard 2004; Blohm 2005; Clevin 2001; Dao 2007; Moodliar 2005; Ngoc 2005; Niinimaki 2006; Pang 2001; Shelley 2005; Zhang 2005). The other five studies used clinical assessment for the diagnosis (Bique 2007; Shelley 2005; Shwekerela 2007; Trinder 2006; Weeks 2005). Studies assessed complete miscarriage either by ultrasound or clinical assessment and at times that varied from three days to eight weeks. We have included the specific information in the Characteristics of included studies and also at the beginning of the results section for each comparison.
Excluded studies
The excluded studies are listed in the reference section under excluded studies. The table Characteristics of excluded studies states the reasons for exclusion from this review. These reasons mainly include: study not randomised; study including women with intrauterine fetal death only; studies including women having termination of pregnancy. We have also excluded studies where we have been unable to contact the authors for data separated by incomplete miscarriage and intrauterine fetal death (Bagratee 2004; Demetroulis 2001; Hinshaw 1997; Johnson 1997; Louey 2000; Machtinger 2004; Ngai 2001; Nielsen 1999; Shaikh 2008). Where authors have kindly responded but have been unable to supply their data separated by incomplete miscarriage and intrauterine fetal death, we have also needed to exclude these studies (Chung 1999).
Risk of bias in included studies
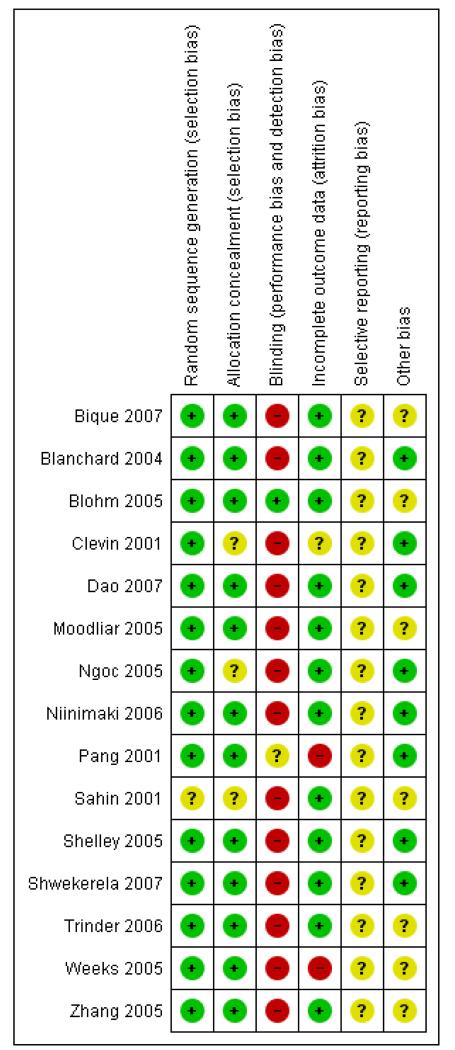
Overall, the quality of studies was good, although in most studies it was not possible to blind participants and clinicians. It was unclear whether any of the studies were free of selective reporting bias as we did not assess the trial protocols.
Allocation
Studies where group allocation was not random were excluded. The random sequence generation was adequate in all studies except one (Sahin 2001) where it was unclear. Allocation concealment was adequate in all studies except three (Clevin 2001; Ngoc 2005; Sahin 2001) where it was unclear.
Blinding
Blinding was adequate in only one study (Blohm 2005) and unclear in one study (Pang 2001), and for the remainder it was inadequate. However, for many studies it was considered not possible to blind, especially where medical treatment was being compared with surgery.
Incomplete outcome data
Loss to follow up and exclusions after randomisation were low in all studies except three. One where it was considered unclear (Clevin 2001) and two where it was considered high (Pang 2001; Weeks 2005). In the Pang study (Pang 2001) it appeared that intention-to-treat analysis was not used and the data could not be re-included. In the Weeks study (Weeks 2005), there was complete follow up at six days but by two weeks there was a 33% loss to follow up in the misoprostol group and 45% in the group having surgery. This was explained by women not returning from their communities for follow up .
Selective reporting
It was unclear to us whether any of the studies were free of selective reporting bias as we were unable to assess the protocols for the studies.
Other potential sources of bias
Seven out of the 15 studies appeared to be free of other sources of bias (Blanchard 2004; Clevin 2001; Dao 2007; Ngoc 2005; Pang 2001; Shelley 2005; Shwekerela 2007) and for the remained it was unclear.
Effects of interventions
All the 15 studies were assessing the medical treatment of incomplete miscarriage for women at less than 13 weeks’ gestation. There were no studies involving women between 13 and 23 weeks’ gestation and none where gestation was not specified.
For the comparisons of misoprostol (by any route of administration versus expectant care or versus surgery), we have used random-effects meta-analyses because of the clinical heterogeneity around route of administration. For other meta-analyses we have used the fixed-effect model except where significant heterogeneity was indicated (see Assessment of heterogeneity above).
1. Misoprostol versus expectant care (three studies, 335 women, Analyses 1.1 to 1.26
For women less than 13 weeks’ gestation
Three studies involving 335 women addressed this comparison for women with incomplete miscarriage (Blohm 2005; Shelley 2005; Trinder 2006). There were two further studies that involved both women with incomplete miscarriage and women with intrauterine fetal deaths, but to date we have been unable to obtain the data separated by incomplete miscarriage and intrauterine fetal death for these studies (Bagratee 2004; Ngai 2001).
Diagnosis of incomplete miscarriage and assessment of complete miscarriage after treatment were made using clinical judgement in two studies (Shelley 2005; Trinder 2006) and using ultrasound in one study (Blohm 2005). Assessment of the outcome of complete miscarriage were made at differing times in the three studies: Blohm 2005 assessed at one week and Shelley 2005 at 10 to 14 days. Trinder 2006 assessed at eight weeks and so these data have not been included. There was as assessment at two weeks, but the findings were not reported separately for women with incomplete miscarriage and women with intrauterine fetal death. We have written to the authors to seek these data.
All the studies looked at vaginal misoprostol compared with expectant care (Blohm 2005; Shelley 2005; Trinder 2006). There were no studies assessing other routes of administration.
The studies are of good quality overall. However, blinding of participants and clinicians was only used in one (Blohm 2005) and not the other two (Shelley 2005; Trinder 2006).
We have chosen to use random-effects meta-analyses for all the outcomes in this comparison as we believe there is clinical heterogeneity as we are pooling differing routes of administration (vaginal, oral, rectal and sublingual). We are, therefore, reporting the average risk ratio or mean difference. Although there are currently only data from studies using vaginal misoprostol, we believe other studies will be undertaken in the future and will be added at future updates of this review. The individual routes of administration of misoprostol are assessed for effectiveness below in Comparisons 3 to Comparison 6.
Primary outcomes
Complete miscarriage
Only two of the three studies assessed this outcome (Blohm 2005; Shelley 2005), with the primary outcome for the third study (Trinder 2006) being infection at 14 days.
There was no difference identified in complete miscarriage between misoprostol and expectant care (average risk ratio (RR) 1.23, 95% confidence interval (CI) 0.72 to 2.10; two studies, 150 women, [T2 = 0.12; Chi2 P = 0.02; I2 = 81%], Analysis 1.1). In terms of clinical impact, the success rate with misoprostol ranged from 80% to 81% and for expectant care from 52% to 85%. The heterogeneity may result from the different times at which complete miscarriage was assessed with expectant care. One study assessed at one week and found a success rate of 52% (Blohm 2005) and the other study assessed at two weeks and found a success rate of 85% (Shelley 2005).
Surgical evacuation
There was also no difference identified in surgical evacuation between misoprostol and expectant care (average RR 0.62, 95% CI 0.17 to 2.26; two studies, 308 women, [T2 = 0.78; Chi2 P = 0.003; I2 = 89%], Analysis 1.2).
Death or serious complication
The outcome of death or serious complication showed no difference either (RR 2.91, 95% CI 0.12 to 70.05; one study, 126 women, Analysis 1.3) though the review is underpowered to assess this outcome.
Secondary outcomes
Unplanned surgical intervention
There was no difference identified in unplanned surgical intervention between misoprostol and expectant care (average RR 0.62, 95% CI 0.17 to 2.26; two studies, 308 women, [T2 = 0.78; Chi2 P = 0.003; I2 = 89%], Analysis 1.4).
Blood transfusion
There was no difference identified in blood transfusion (average RR 3.07, 95% CI 0.13 to 74.28; three studies, 332 women, [though only one study estimable], Analysis 1.5),
Pain relief
There was no difference identified in pain relief (average RR 1.12, 95% CI 0.67 to 1.88; two studies, 308 women, random-effects [T2 = 0.10; Chi2 P = 0.08; I2 = 67%], Analysis 1.10).
Pelvic infection
There was no difference identified in pelvic infection (average RR 2.42, 95% CI 0.59 to 9.98; three studies, 333 women, [T2 = 0.00; Chi2 P = 0.43, I2 = 0%], Analysis 1.11).
Women’s views
There was no information reported on women’s views.
Other outcomes
The other pre-specified secondary outcomes were not assessed in these studies.
2. Misoprostol versus surgery (nine studies, 1449 women, Analyses 3.1 to 3.26)
For women less than 13 weeks’ gestation
Nine studies involving 1499 women addressed this comparison for women with incomplete miscarriage at less than 13 weeks’ gestation (Bique 2007; Dao 2007; Moodliar 2005; Sahin 2001; Shelley 2005; Shwekerela 2007; Trinder 2006; Weeks 2005; Zhang 2005). The included studies were of good quality overall (Figure 1), with all having adequate sequence generation and concealment allocation although for Sahin 2001 it was unclear. Blinding was not possible in any of the studies when comparing medical treatment with surgery. Only one study had incomplete data and these related to the study being undertaken on rural Uganda where women in the community did not return for follow-up checks (Weeks 2005). We were unclear about the possibility of selective reporting bias as we did not assess any of the study protocols. Three of the nine studies appeared to be free of other biases (Dao 2007; Shelley 2005; Shwekerela 2007).
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Diagnosis of incomplete miscarriage and assessment of complete miscarriage after treatment was made using clinical judgement in four studies (Bique 2007; Shelley 2005; Shwekerela 2007; Weeks 2005) and using ultrasound in four studies (Dao 2007; Moodliar 2005; Sahin 2001; Zhang 2005). Assessment of the outcome of complete miscarriage was made at differing times in the studies: five studies assessed at one week (Bique 2007; Dao 2007;Shwekerela 2007; Weeks 2005; Zhang 2005) and three studies assessed around 10 to 14 days (Moodliar 2005; Sahin 2001; Shelley 2005). Trinder 2006 assessed at eight weeks and so these data have not been included. There was as assessment at two weeks in this study, but the findings were not reported separately for women with incomplete miscarriage and women with intrauterine fetal death. We have written to the authors to seek these data.
We have chosen to use random-effects meta-analyses for all the outcomes in this comparison as we believe there is clinical heterogeneity as we are pooling differing routes of administration (vaginal, oral, vaginal + oral, rectal and sublingual). Although there are currently only data from studies using vaginal misoprostol, we believe other studies will be undertaken in the future and will be added at future updates of this review. The individual routes of administration of misoprostol are assessed for effectiveness compared with surgery below in Comparisons 7 to Comparison 11.
Primary outcomes
Complete miscarriage
There was no difference identified in complete miscarriage with misoprostol compared with surgery (average RR 0.96, 95% CI 0.92 to 1.00, eight studies; 1377 women, [T2 = 0.00; Chi2 P = 0.002, I2 = 68%], Analysis 2.1). However, from the clinical perspective the success rate was very good for both misoprostol and surgery. Misoprostol achieving between 80% and 99% success across studies and surgery achieving between 91% and 100% success across studies.
Surgical evacuation
There were fewer surgical evacuations with misoprostol (average RR 0.07, 95% CI 0.03 to 0.18; eight studies, 1538 women, [T2 = 1.36; Chi2 P < 0.00001; I2 = 91%], Analysis 2.2).
Death or serious complication
There was no difference identified between misoprostol and surgery (average RR 1.00, 95% CI 0.04 to 22.64; two studies, 132 women but only one study was estimable (Analysis 2.3)).
Secondary outcomes
Unplanned surgical intervention
There were more unplanned surgery with misoprostol (average RR 6.32, 95% CI 2.90 to 13.77; six studies, 1158 women, Analysis 2.4).
Blood transfusion
There was no difference identified for the number of blood transfusions undertaken between misoprostol and surgery (average RR 1.73, 95% CI 0.19 to 16.08; four studies, 430 women, [T2 = 0.00; Chi2 P = 0.62; I2 = 0%], Analysis 2.5).
Anaemia
There was no difference identified in anaemia identified (RR 1.71, 95% CI 0.24 to 12.24; one study, 36 women, Analysis 2.8).
Days of bleeding
There were more days of bleeding with misoprostol than with surgery (average mean difference (MD) 2.12, 95% CI 1.18 to 3.07; three studies, 211 women, Analysis 2.9). This difference was also considered clinically significant.
Pain relief
There was no difference identified with the use of pain relief between women who had misoprostol and women who had surgery (average RR 1.48, 95% CI 0.67 to 3.25; four studies, 525 women, [T2 = 0.50; Chi2 P < 0.00001; I2 = 90%], Analysis 2.10).
Pelvic infection
There was no difference identified in the incidence of pelvic infection between women who had misoprostol and those who had surgery (average RR 0.70, 95% CI 0.25 to 1.99; seven studies, 907 women, Analysis 2.11).
Cervical damage
There was no statistically significant difference identified in cervical damage although only one study (Weeks 2005) assessed this outcome (RR 0.07, 95% CI 0.00 to 1.25; one study, 189 women, Analysis 2.12).
Women’s views/satisfaction
There was no difference identified in women’s satisfaction between misoprostol and surgery when expressed by whether they were satisfied or not (average RR 0.99, 95% CI 0.98 to 1.01; four studies, 1134 women, Analysis 2.18). Women were very satisfied overall, and satisfaction with misoprostol ranged from 91% to 99% across studies and satisfaction with surgery ranged from 95 to 100%.
When assessed using visual analogue scales, there were were more women satisfied with surgery (average standardised mean difference (SMD) 1.01, 95% CI 0.01 to 2.00; two studies, 131 women, random-effects [T2 = 0.41; Chi2 P = 0.03; I2 = 78%], Analysis 2.19) but the difference was small and probably not clinically significant. Taken with the findings above, it appears that overall most women are satisfied with the treatment they received.
Digestive disorders
More women had nausea with misoprostol compared with surgery (average RR 3.18, 95% CI 1.78 to 5.70; six studies, 1115 women, [T2 = 0.10; Chi2 P = 0.29; I2 = 19%], Analysis 2.24). This is likely to be clinically significant.
More women had vomiting with misoprostol compared with surgery (average RR 2.25, 95% CI 1.14 to 4.43; five studies, 1090 women, [T2 = 0.00; Chi2 P = 0.46; I2 = 0%], Analysis 2.25). This may be less clinically significant than the nausea.
There was no difference identified in the incidence of diarrhoea (average RR 4.25, 95% CI 0.76 to 23.73; three studies, 437 women, [T2 = 0.00; Chi2 P = 0.97; I2 = 0%], Analysis 2.26).
Other secondary outcomes
Other secondary outcomes were either not assessed in the included studies or there are data from just one study from which it is hard to draw conclusions. See other graphs in this comparison.
3. Vaginal misoprostol versus expectant care (three studies, 335 women, Analyses 3.1 to 3.26)
For women less than 13 weeks’ gestation
Three studies involving 335 women addressed this comparison for women with incomplete miscarriage (Blohm 2005; Shelley 2005; Trinder 2006). There were two further studies that involved both women with incomplete miscarriage and women with intrauterine fetal deaths, but to date we have been unable to obtain the data separated by incomplete miscarriage and intrauterine fetal death for these studies (Bagratee 2004; Ngai 2001).
The studies are of good quality overall. However, blinding of participants and clinicians was only used in one (Blohm 2005) and not the other two (Shelley 2005; Trinder 2006).
Diagnosis of incomplete miscarriage and assessment of complete miscarriage after treatment was made using clinical judgement in two studies (Shelley 2005; Trinder 2006) and using ultrasound in one study (Blohm 2005). Assessment of the outcome of complete miscarriage was made at differing times in the three studies: Blohm 2005 assessed at one week; Shelley 2005 at 10 to 14 days and Trinder 2006 at eight weeks although there was as assessment at two weeks, findings were not reported separately for women with incomplete miscarriage and women with intrauterine fetal death. We have written to the authors to see if they have earlier data for incomplete miscarriage.
Primary outcomes
Complete miscarriage
Only two of the three studies assessed this outcome (Blohm 2005; Shelley 2005), with the primary outcome for the third study (Trinder 2006) being infection at 14 days.
There was no difference identified in complete miscarriage between vaginal misoprostol and expectant care (average RR 1.23, 95% CI 0.72 to 2.10; two studies, 150 women, random-effects [T2 = 0.12; Chi2 P = 0.02; I2 = 81%], Analysis 3.1.1). From the clinical perspective, the success rate with vaginal misoprostol ranged from 80% to 81% and for expectant care from 52% to 85%. The heterogeneity may result from the different times at which complete miscarriage was assessed with expectant care. One study assessed at one week and found a success rate of 52% (Blohm 2005) and the other study assessed at 10 to 14 days and found a success rate of 85% (Shelley 2005).
Surgical evacuation
There was also no difference identified in surgical evacuation between vaginal misoprostol and expectant care (average RR 0.62, 95% CI 0.17 to 2.26; two studies, 308 women, random-effects [T2 = 0.78; Chi2 P = 0.003;I2 = 89%], Analysis 3.2.1).
Death or serious complication
The outcome of death or serious complication showed no difference (RR 2.91, 95% CI 0.12 to 70.05; one study, 126 women, Analysis 3.3.1) though the review is underpowered to assess this outcome.
Secondary outcomes
Unplanned surgical intervention
There was no difference identified in unplanned surgical interventions between vaginal misoprostol and expectant care (average RR 0.62, 95% CI 0.17 to 2.26; two studies, 308 women, random-effects [T2 = 0.78; Chi2 P = 0.003; I2 = 89%], Analysis 3.4.1).
Blood transfusion
There was no difference identified in blood transfusion (RR 3.07, 95% CI 0.13 to 74.28; three studies, 332 women [though only one study was estimable], Analysis 3.5.1),
Pain relief
There was no difference identified in pain relief (average RR 1.12, 95% CI 0.67 to 1.88; two studies, 308 women, random-effects [T2 = 0.10; Chi2 P = 0.08; I2 = 67%], Analysis 3.10.1).
Pelvic infection
There was no difference identified in pelvic infection (RR 2.81, 95% CI 0.77 to 10.33; three studies, 333 women, Analysis 3.11.1).
Women’s views
There was no information reported on women’s views.
Other outcomes
The other pre-specified secondary outcomes were not assessed in these studies.
4. Oral misoprostol versus expectant care (no studies)
There were no studies that addressed this comparison.
5. Rectal misoprostol versus expectant care (no studies)
There were no studies that addressed this comparison.
6. Sublingual misoprostol versus expectant care (no studies)
There were no studies that addressed this comparison.
7. Vaginal misoprostol versus surgery (four studies, 339 women, Analyses 7.1 to 7.26)
For women less than 13 weeks’ gestation
Four studies involving 339 women addressed this comparison for women incomplete miscarriage (Moodliar 2005; Shelley 2005; Trinder 2006;.Zhang 2005). Two further studies involved both women with incomplete miscarriage and women with intrauterine fetal deaths, but to date we have been unable to obtain the data separated by incomplete miscarriage and intrauterine fetal death for these studies so these studies have been excluded (Demetroulis 2001; Louey 2000).
The studies were of good quality overall. However, the nature of the intervention and comparison meant it was not possible to blind participants or clinicians, and it was mostly unclear whether the studies had selective reporting bias, or other biases.
Diagnosis of incomplete miscarriage and assessment of complete miscarriage after treatment was made using clinical judgement in two studies (Shelley 2005; Trinder 2006) and using ultrasound in two studies (Moodliar 2005; Zhang 2005). Assessment of the outcome of complete miscarriage was made at differing times in the studies: Zhang 2005 assessed at three days; Shelley 2005 at 10 to 14 days; Moodliar 2005 at two weeks and Trinder 2006 at eight weeks although there was as assessment at two weeks, findings were not reported separately for women with incomplete miscarriage and women with intrauterine fetal death. We have written to the authors to seek these data.
Primary outcomes
Complete miscarriage
Fewer women had complete miscarriage with vaginal misoprostol compared with surgery (RR 0.90, 95% CI 0.82 to 0.99; three studies, 154 women, Analysis 7.1.1). However, from the clinical perspective the success rate was high in both groups, vaginal misoprostol ranged from 80% to 91% and for surgery from 89% to 100%.
Surgical evacuation
Fewer women had surgical evacuation with vaginal misoprostol compared with women who were given surgery straight away (average RR 0.18, 95% CI 0.08 to 0.44; three studies; 315 women, random-effects [T2 = 0.46; Chi2 P = 0.008; I2 = 79%], Analysis 7.2.1). This finding was not perhaps surprising as the comparison group was surgical intervention, but it is an important outcome to assess as clinical management would be to use surgery if misoprostol failed. This reduction in the use of surgery with vaginal misoprostol helps to confirm the success of this intervention. The reasons for the heterogeneity were unclear.
Death or serious complication
There was no difference identified in the composite outcome of death or serious complications (RR 1.00, 95% CI 0.04 to 22.64; two studies, 132 women, [though only one was estimable], Analysis 7.3.1) though the review is underpowered to assess this outcome.
Secondary outcomes
Unplanned surgical intervention
In the vaginal misoprostol group, there was a higher incidence of unplanned surgical intervention (average RR 5.56, 95% CI 1.11 to 27.90; three studies, 315 women, [T2 = 0.96; Chi2 P = 0.15; I2 = 47%], Analysis 7.4.1). Again this finding is unsurprising as surgery is the comparative intervention and one would anticipate that few additional operations would be required if surgery was successful.
Blood transfusion
There was no difference identified in blood transfusions (RR 1.82, 95% CI 0.21 to 15.70; three studies, 241 women, Analysis 7.5.1).
Anaemia
There was no difference identified in anaemia (RR 1.71, 95% CI 0.24 to 12.24; one study, 36 women, Analysis 7.8.1).
Days of bleeding
Women treated with vaginal misoprostol had more days of bleeding than women treated with surgery (MD 2.76, 95% CI 1.55 to 3.97; two studies, 131 women, Analysis 7.9.1).
Pain relief
Women treated with vaginal misoprostol used more pain relief than women treated with surgery (RR 1.75, 95% CI 1.21 to 2.54; three studies, 313 women, Analysis 7.10.1).
Pelvic infection
There was no difference identified in pelvic infection (RR 1.27, 95% CI 0.37 to 4.42; four studies, 338 women, Analysis 7.11.1).
Women’s views/satisfaction
Women were more satisfied with surgery (average SMD 1.01, 95% CI 0.01 to 2.00; two studies, 131 women, random-effects [T2 = 0.41; Chi2 P = 0.03; I2 = 78%], Analysis 7.19.1) but the difference was small and based on just two small studies. Reasons for the heterogeneity were unclear.
Digestive disorders
There was no difference identified in nausea (RR 1.37, 95% CI 0.58 to 3.22; three studies, 156 women, Analysis 7.24.1), vomiting (RR 1.48, 95% CI 0.25 to 8.93; two studies, 131 women, Analysis 7.25.1) or diarrhoea (RR 4.30, 95% CI 0.52 to 35.36; two studies, 131 women, Analysis 7.26.1).
Other outcomes
The other pre-specified secondary outcomes were not assessed in these studies.
8. Oral misoprostol versus surgery (four studies, 1347 women, Analyses 8.1 to 8.26)
For women less than 13 weeks’ gestation
Four studies involving 1347 women addressed this comparison for women with incomplete miscarriage (Bique 2007; Dao 2007; Shwekerela 2007; Weeks 2005). We identified a further study involving both women with incomplete miscarriage and women with intrauterine fetal deaths but the authors, although they were able to supply additional data, were unable to separate outcomes by women with incomplete miscarriage and women with intrauterine death and so we excluded this study (Chung 1999).
The included studies were of good quality overall, with all having adequate sequence generation and concealment allocation. Blinding was not possible when comparing medical treatment with surgery. Three of the studies had little loss to follow up and exclusions after randomisation (Bique 2007; Dao 2007; Shwekerela 2007). However, one study, though it had no loss to follow up at six days, had considerable loss to follow up at one to two weeks (33% in the misoprostol group and 45% in the group having surgery) which was not similar between the groups (Weeks 2005). This seemed to arise from women returning home to their communities and not coming back for follow-up appointments, and this was fully discussed by the authors (Weeks 2005). Sensitivity analysis was not undertaken because outcomes at six days appeared not subject to bias.
Diagnosis of incomplete miscarriage and assessment of complete miscarriage after treatment was made using clinical judgement in three studies (Bique 2007; Shwekerela 2007; Weeks 2005) and using ultrasound in one study (Dao 2007). Assessment of the outcome of complete miscarriage was made at seven days in all four studies (Bique 2007; Dao 2007; Shwekerela 2007; Weeks 2005).
Primary outcomes
Complete miscarriage
There was no difference identified in the number of complete miscarriages with oral misoprostol compared with surgery (RR 0.97, 95% CI 0.93 to 1.02; four studies, 1143 women, Analysis 8.1.1). In addition, in terms of clinical impact, the success rate was high in both groups, for oral misoprostol it ranged from 91% to 99% and surgery ranged from 91% to 100%.
Surgical evacuation
Fewer women had surgical evacuation with oral misoprostol (average RR 0.05, 95% CI 0.02 to 0.10; four studies, 1143 women, random-effects [T2 = 0.33. Chi2 P = 0.03; I2 = 68%], Analysis 8.2.1). The reasons for the heterogeneity were unclear.
Death or serious complication
There were no data on this outcome.
Secondary outcomes
Unplanned surgical intervention
There were more women needing unplanned surgical intervention in the oral misoprostol group (RR 7.07, 95% CI 2.34 to 21.30; three studies, 843 women, Analysis 8.4.1).
Blood transfusion
It was not possible to produce a risk ratio with these data (Analysis 8.5.1).
Pain relief
There was less pain relief required with oral misoprostol than with surgery (RR 0.85, 95% CI 0.77 to 0.92; one study, 212 women, Analysis 8.10.1) but the difference was small and most women used pain relief whether they had misoprostol or surgery.
Pelvic infection
There was no difference identified in pelvic infection (RR 0.26, 95% CI 0.03 to 2.41; two studies, 489 women, Analysis 8.11.1).
Cervical damage
There was no difference identified in cervical damage (RR 0.07, 95% CI 0.00 to 1.25; one study, 189 women, Analysis 8.12.1).
Women’s views
There was no difference identified in women’s satisfaction (RR 0.99, 95% CI 0.97 to 1.01; four studies, 1134 women, Analysis 8.18.1).
Digestive disorders
There was more nausea (RR 4.77, 95% CI 2.68 to 8.49; three studies, 959 women, Analysis 8.24.1) and vomiting (RR 2.59, 95% CI 1.29 to 5.21; three studies, 959 women, Analysis 8.25.1) with oral misoprostol compared with surgery, but there was no difference identified in diarrhoea (RR 4.63, 95% CI 0.22 to 95.55; one study, 306 women, Analysis 8.26.1).
Other outcomes
The other pre-specified secondary outcomes were not assessed in these studies.
9. Vaginal plus oral misoprostol versus surgery (one study, 80 women, Analyses 9.1 to 9.26)
For women less than 13 weeks’ gestation
One study involving 80 women assessed this comparison (Sahin 2001).
The study was of low quality with uncertainty around sequence generation, allocation concealment, selective reporting bias and other potential biases and it was not possible to blind participants and clinicians.
Assessment of incomplete miscarriage was undertaken using ultrasound and assessment of outcomes was undertaken at 10 days.
Primary outcomes
Complete miscarriage
There was no difference identified in incomplete miscarriage (RR 0.95, 95% CI 0.87 to 1.04; one study, 80 women, Analysis 9.1.1). In clinical terms though, with success in this one study was 95% with the medical treatment and 100% with surgery.
Surgical evacuation
There was less surgical evacuation with misoprostol than with surgery (RR 0.04, 95% CI 0.01 to 0.18; one study, 80 women, Analysis 9.2.1).
Death or serious complication
Not reported.
Secondary outcomes
Days of bleeding
There was no difference identified in the number of days of bleeding (RR1.55, 95% CI 0.58 to 2.52; one study, 80 women, Analysis 9.9.1).
Pelvic infection
There was no difference identified in pelvic infection (RR 0.50, 95% CI 0.05 to 5.30; one study, 80 women, Analysis 9.11.1).
Women’s views
There was no information reported on women’s views
Other outcomes
The other pre-specified secondary outcomes were not assessed in this study.
10. Rectal misoprostol versus surgery (no studies)
There were no studies that addressed this comparison.
11. Sublingual misoprostol versus surgery (one ongoing study)
There is one ongoing study addressing this comparison (Unkels 2008).
12. Vaginal misoprostol versus oral misoprostol (one study, 201 women, Analyses 12.1 to 12.26)
For women less than 13 weeks’ gestation
One study involving 201 women addressed this comparison for women incomplete miscarriage (Pang 2001). One further study involved both women with incomplete miscarriage and women with intrauterine fetal deaths, but to date we have been unable to obtain the data separated by incomplete miscarriage and intrauterine fetal death for this study so it has been excluded from this review (Machtinger 2004).
The study quality (Pang 2001) was good in terms of there having adequate sequence generation, concealment allocation and appeared to be free of other potential sources of bias, however it was not clear whether participants, clinicians and assessors were blinded to the intervention given.
Assessment of incomplete miscarriage was undertaken using ultrasound and assessment of outcomes was undertaken at one day after treatment.
Primary outcomes
Complete miscarriage
There was no difference identified in complete miscarriage (RR 0.94, 95% CI 0.76 to 1.16; one study, 198 women, Analysis 12.1.1), with the success rate being 61% with vaginal misoprostol and 65% with oral misoprostol, both assessed on day one.
Surgical evacuation
There was no difference identified in surgical evacuation (RR 1.11, 95% CI 0.77 to 1.60; one study, 198 women, Analysis 12.2.1).
Death or serious complications
Not reported.
Secondary outcomes
Unplanned surgical intervention
There was no difference identified in unplanned surgical intervention (RR 0.36, 95% CI 0.01 to 8.80; one study, 186 women, Analysis 12.4.1).
Pain relief
There was also no difference identified in pain relief (RR 1.43, 95% CI 0.93 to 2.17; one study, 186 women, Analysis 12.10.1).
Digestive disorders
There were no differences identified in nausea (RR 0.63, 95% CI 0.26 to 1.54; one study involving 198 women, Analysis 12.24.1) and vomiting (RR 0.36, 95% CI 0.07 to 1.75; one study, 198 women, Analysis 12.25.1).
There was a reduction in diarrhoea for women using vaginal misoprostol compared with oral misoprostol (RR 0.21, 95% CI 0.12 to 0.36; one study, 198 women, Analysis 12.26.1).
Women’s views
There was no information reported on women’s views.
Other outcomes
There was no information reported on other pre-specified secondary outcomes.
13. Rectal misoprostol versus oral misoprostol (no studies)
There were no studies that addressed this comparison.
14. Oral misoprostol 600 ug versus oral misoprostol 1200 ug (two studies, 469 women, Analyses 14.1 to 14.26)
For women less than 13 weeks’ gestation
Two studies involving 469 women addressed this comparison for women with incomplete miscarriage (Blanchard 2004; Ngoc 2005).
One study was of reasonably good quality (Blanchard 2004) with adequate sequence generation, concealment of allocation, low loss to follow up and other sources of bias were not apparent. There was no blinding of participants, clinicians and assessors, and it was unclear whether there was selective reporting bias. The other study (Ngoc 2005) was similar but it was unclear whether there was adequate allocation concealment.
Primary outcomes
Complete miscarriage
There was no difference identified in complete miscarriage (RR 1.00, 95% CI 0.93 to 1.07; two studies, 464 women, Analysis 14.1.1).
Surgical evacuation
There was no difference identified in surgical evacuation (RR 0.76, 95% CI 0.29 to 1.99; one study, 295 women, Analysis 14.2.1). The success rate with the single 600 ug dose ranged from 66% to 95% and the success rate with the repeat 600 ug dose (total 1200 ug) from 67% to 94%.
Death or serious complication
One study provided data (Ngoc 2005) but it was not possible to produce a risk ratio (Analysis 14.3.1).
Secondary outcomes
Unplanned surgical intervention
There was no difference identified in unplanned surgical intervention (RR 0.76, 95% CI 0.29 to 1.99; one study, 295 women, Analysis 14.4.1).
Women’s views/satisfaction
There was no difference identified in women’s satisfaction (RR 1.02, 95% CI 0.96 to 1.09; two studies, 460 women, Analysis 14.18.1).
Digestive disorders
There was also no difference identified between the two doses of oral misoprostol for nausea (average RR 1.19, 95% CI 0.57 to 2.46, two studies, 463 women, random-effects [T2 = 0.19; Chi2 P = 0.07; I2 = 70%], Analysis 14.24.1) or vomiting (RR 1.01, 95% CI 0.60 to 1.72; two studies, 463 women, Analysis 14.25.1). There was a reduction in diarrhoea for women allocated to one dose of misoprostol (RR 0.73, 95% CI 0.55 to 0.97; one study, 294 women, Analysis 14.26.1). The confidence interval and the data being from one small study, makes the clinical significance unclear.
Other outcomes
The other pre-specified secondary outcomes were not assessed in this study.
15. Oral mifepristone + vaginal misoprostol versus surgery (one study, 19 women, Analyses 15.1 to 15.26)
This study included women with many kinds of miscarriage (missed abortion, anembryonic pregnancies, incomplete miscarriage) but the authors were able to send us the data split by the types of miscarriage (Niinimaki 2006). The study also covered women less than 24 weeks’ gestation, some of whom were less than 13 weeks and some not.
For women less than 13 weeks’ gestation
For the 16 women who were less than 13 weeks’ gestation, treatments were equally successful with 10/10 (100%) women in the medical group and 6/6 (100%) women in the surgical group achieving complete miscarriage. There were no additional surgical evacuations required and none of the women had a pelvic infections.
For women 13 to 23 weeks’ gestation
For the three women who were between 13 and 24 weeks’ gestation, treatments again were equally successful with 1/1 (100%) women in th medical group and 2/2 (100%) women in the surgical group achieving complete miscarriage. There were no additional surgical evacuations required and none of the women had a pelvic infections.
16. Vaginal prostaglandin E1 (gemeprost) versus surgery (one study, 34 women, Analyses 16.1 to 16.4)
For women less than 13 weeks’ gestation
One study involving 34 women compared vaginal prostaglandin E1 (gemeprost) with surgery (Clevin 2001). The study was of uncertain quality. It had adequate sequence generation and low risk of other potential sources of bias. However, the allocation concealment was unclear, as was the completeness of the outcome data and potential for selective reporting bias. It was not possible to blind participants and clinicians.
Primary outcomes
None of the pre-specified primary outcomes were reported.
Secondary outcomes
Unplanned surgical intervention
Although data were reported on this outcome it was not possible to report a risk ratio (Analysis 18.4.1).
DISCUSSION
The studies we identified virtually all covered women less than 13 weeks; there was one study which included three women greater than 13 weeks (Niinimaki 2006). Misoprostol was the drug studied most frequently and it was assessed against expectant care and surgery, and the possible routes of administration were vaginal, oral, vaginal plus oral, sublingual and rectal.
Summary of main results
The limited data available for all these comparisons can be summarised as follows.
Misoprostol compared with expectant care (Comparison 1)
No statistically significant differences were identified between misoprostol and expectant care although the review was underpowered to assess this comparison with only three studies involving 335 women. Vaginal misoprostol was the only route of administration used in these comparisons and further studies would be needed to be sure of the findings.
Misoprostol compared with surgery (Comparison 2)
Misoprostol was slightly less effective than surgery but the difference was probably not clinically relevant with the success rate for both treatments being high. There was a large and significant reduction in surgery required when misoprostol was used. There was more blood loss with misoprostol, though cervical damage seemed less; however, this was just assessed in one study with possible risk of bias in loss to follow up. In addition, the findings were not statistically significant and thus compatible with both benefit and harm. There was more nausea and vomiting with misoprostol (particularly oral misoprostol) but no difference in women’s satisfaction was identified.
Vaginal misoprostol compared with expectant care (Comparison 3)
No statistically significant differences were identified between vaginal misoprostol compared with expectant care in terms of women achieving a complete miscarriage. However, in one study vaginal misoprostol was significantly more effective than expectant care (Blohm 2005) and in the other study was equally effective (Shelley 2005). This difference seems to lie in the differing success in the expectant care group between the two studies. Complete miscarriage was 52% (32/64) in the study assessing this at one week (Blohm 2005) and 85% (12/14) in the study assessing it at two weeks (Shelley 2005). This is in contrast to the success rates with vaginal misoprostol which were 81% (52/64) and 80% (8/10) respectively. It may be, therefore, that if women are prepared to wait longer then more might achieve spontaneous miscarriage without the use of vaginal misoprostol. However, the numbers of participants in both these studies was small. There were no differences identified in the other outcomes assessed (surgical evacuation; death or serious complications; blood transfusions; pain relief; pelvic infection). There was no information about women’s views of these two forms of care.
Vaginal misoprostol compared with surgery (Comparison 7)
There was a small but significant reduction in women achieving a complete miscarriage with vaginal misoprostol compared with surgery. However, vaginal misoprostol still showed a success rate of between 80% to 91%. There was a large and significant reduction in the use of surgery and no difference in death or serious complications. The mean number of days of bleeding was higher with misoprostol and there was more need for pain relief. There was no significant difference in the other outcomes assessed (blood transfusion; anaemia; pelvic infection; nausea; vomiting; diarrhoea).
Oral misoprostol compared with surgery (Comparison 8)
No statistically significant difference was identified between oral misoprostol compared with surgery in terms of women achieving a complete miscarriage. There was a large and significant reduction in the use of surgery, and deaths or serious complications were not reported. There was less pain relief needed with oral misoprostol, but increased nausea and vomiting. There were no difference in other outcomes assessed (pelvic infection; cervical damage; diarrhoea).
Vaginal plus oral misoprostol compared with surgery (Comparison 9)
Based on one study of 80 women, no statistically significant differences were identified for complete miscarriage (success rates from 95% to 100%), days of bleeding and pelvic infection. There was a significant reduction in the use of surgery with the medical management.
Vaginal misoprostol compared with oral misoprostol (Comparison 12)
No significant difference was identified between vaginal misoprostol compared with oral misoprostol in terms of women achieving a complete miscarriage or in the need for additional surgical intervention. There was significantly less diarrhoea with vaginal misoprostol compared with the oral route, but no difference in other outcomes assessed (pain relief; nausea; vomiting).
600 ug oral misoprostol compared with 1200 ug oral misoprostol (Comparison 14)
The only significant difference identified in this comparison was significantly more diarrhoea with the higher dose.
Other comparisons
For other comparisons there were either no studies or the studies provided insufficient data.
Women’s views
the only study that assessed women views in any detail was a publication by Harwood in 2008 (Harwood 2008) as part of the study on vaginal misoprostol versus surgery (Zhang 2005). The 652 women in this multicentre randomised controlled trial were asked prospectively to complete a daily diary of any symptoms experienced for the two weeks after treatment. The women also completed questionnaires assessing quality of life, depression, stress and treatment acceptability at two weeks after treatment. Although a few differences were observed in some of the individual measures, overall there was no significant different in the mean scores for quality of life, though vaginal misoprostol was associated with higher levels of pain than surgery. Overall treatment acceptability was similar, and these findings can help to inform the focus of counselling for women choosing a treatment option.
Overall completeness and applicability of evidence
The review is probably underpowered to assess the effectiveness of medical treatments for incomplete miscarriage.
One study published by Smith 2009 but part of the MIST trial, undertook long-term follow up to assess any potential impact on subsequent fertility (Trinder 2006). They concluded that the method of miscarriage management did not affect subsequent pregnancy rates with around four in five women giving birth within five years of the index miscarriage. Women can be reassured that long-term fertility concerns need not affect their choice of miscarriage management.
Quality of the evidence
The quality of the evidence was generally fairly good, although it is hard to assess if there has been selective reporting bias.
Potential biases in the review process
We attempted to minimise bias by the following; two review authors assessed eligibility for inclusion and two authors carried out data extraction and assessed risk of bias. Data entry into RevMan (RevMan 2008) was undertaken by one author and checked by another. However, many of these steps involve subjective assessments and thus may carry some risk of bias.
Agreements and disagreements with other studies or reviews
We are unaware of other reviews on this topic. Our conclusions seem to agree with most of those of the included studies that women can be offered a choice of treatments because differences are small and not of major consequences. Women may have particular preferences as to the adverse effects they wish to try to avoid and this likely to influence their choice of treatment.
AUTHORS’ CONCLUSIONS
Implications for practice
Although it would be critical to have more data, the current evidence suggests there appears to be no major differences other than avoiding surgery, between misoprostol, expectant care and surgery in the treatment of incomplete miscarriage for women of less than 13 weeks’ gestation. Avoiding surgery has considerable benefits in terms of reducing adverse effects (although these were not fully assessed systematically in the included studies) and is particularly beneficial in income-poor countries. There were some differences identified in adverse effects such as pain, digestive disorders, etc, and this information should be conveyed to women to help them make an informed choice on what is important to them.
Implications for research
There is an urgent need for studies to assess medical interventions for incomplete miscarriage for women between 13 to 24 weeks’ gestation, as currently there are no trials to guide practice. Multi-centre trials would seem appropriate to give sufficient size to provide sound evidence.
There is a need for more trials comparing the use of medical treatments, by the various routes, with expectant care and surgery to confirm or refute these findings for women less than 13 weeks. This should provide more evidence on the effectiveness and adverse effects, so women can be provided with better information in order to support their choices. Future trials should separate women with non-viable pregnancies prior to miscarriage, from those with incomplete miscarriages.
Women’s views and quality of life measures should be assessed alongside the clinical outcome in any future trials. These trials should be large enough to provide definitive findings and should assess the important outcomes identified in this review.
[Note: the 34 citations in Studies awaiting classification may alter the conclusions of the review once assessed.]
PLAIN LANGUAGE SUMMARY.
Comparing medical treatments for miscarriage with waiting for nature to take its course or using surgery to empty the womb
Miscarriage is when a pregnant woman loses her baby before the baby would be considered able to survive outside the womb, i.e. before 24 weeks’ gestation. Miscarriage occurs in about 10% to 15% of pregnancies and the signs are bleeding usually with some abdominal pain and cramping. The cause of miscarriage is often unknown, but most are likely to be due to abnormalities in the baby’s chromosomes. Women experiencing miscarriage may be quite distressed, and there can be feelings of emptiness, guilt and failure. Fathers can also be affected emotionally. Traditionally, surgery (curettage or vacuum aspiration) has been the treatment used to remove any retained tissue and it is quick to perform. It has now been suggested that medical treatments (usually misoprostol) may be as effective and may carry less risk of infection. This review was undertaken to compare medical treatments with surgery or with no treatment. The review identified 15 studies involving 2750 women and all these studies were of women less than 13 weeks’ gestation. There were a number of different ways of giving the drugs and so there are limited data for each comparison. Overall, the review found no difference in the success between misoprostol and waiting for spontaneous miscarriage, nor between misoprostol and surgery. The overall success rate was over 80% and sometimes as high as 99%, and one study identified no difference in subsequent fertility between treatments. Vaginal misoprostol was compared with oral misoprostol in one study which found no difference in success but there was more diarrhoea with oral misoprostol. However, women on the whole seemed happy with their care whichever treatment they were given. The review suggests that misoprostol or waiting for spontaneous expulsion of fragments are important alternatives to surgery, but women should be offered an informed choice. Further studies are clearly needed to confirm these findings. There is an urgent need for studies on women who miscarry when more than 13 weeks’ gestation.
ACKNOWLEDGEMENTS
Jorgen Boettiger for the translation of the Clevin 2001 paper and Maria Paz de Andres for the translation of Rivero-Lopez 1998.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
• The University of Liverpool, UK.
External sources
• National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT with randomisation of individual women. Using computer-generated random numbers in sequentially numbered opaque envelopes | |
| Participants | Inclusion criteria • Women with confirmed incomplete spontaneous or induced miscarriage, less than 12 weeks’ gestation. • Diagnosis was based on past or present history of vaginal bleeding during pregnancy and an open cervical os. • > 18 years; no known allergy to misoprostol; no signs of severe infection; no haemodynamic disturbance; lived or worked within the hospitals geographic area of coverage. • N = 270 women. Exclusion criteria • Nothing specified other than inclusion criteria. |
|
| Interventions | Intervention: oral misoprostol - 600 ug single dose. N = 123. Comparison: surgery - MVA. N = 124. |
|
| Outcomes | Complete miscarriage without recourse to additional surgical intervention, experience of side effects and acceptability of treatment • Appears to be clinical assessment of complete miscarriage. • Women were assessed 1 week. • If miscarriage was still incomplete, women were given the option to wait another week or have surgery then. Women who chose to wait were reassessed 1 week later and if still no complete miscarriage then surgery. |
|
| Notes | 1. Setting: tertiary hospital in Mozambique. 2. If miscarriage incomplete at 7 days, women were given the option to wait another week or have surgery then. Women who chose to wait and were still incomplete at 2 weeks were then given surgery. 3. Additional outcomes assessed but not pre-specified in the review: bleeding; pain/cramps; fever; chills; tolerability; would choose method again; would recommend method to a friend; best and worst features. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation scheme was generated by computer…” |
| Allocation concealment (selection bias) | Low risk | “…treatment allocations printed on cards inserted into sequentially numbered opaque envelopes…a member of the study staff opened the next envelope in the sequence and assigned to women to the indicated treatment group.” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It was not possible to blind people and this is discussed by authors |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | • 23 women were excluded after randomisation because of a problem identified with the randomisation process as discussed by authors. • 35 women did not return at 1 week: 12 from misoprostol group and 23 from MVA group. • 10 women in misoprostol group had MVA prior to the 1 week follow-up time. These were included in the misoprostol group. • Not strictly speaking ITT analysis, but outcomes on the 23 women excluded were reported and similar to those included. Analysis was done on 212 women on whom data were available. |
| Selective reporting (reporting bias) | Unclear risk | As far as can tell, outcomes reported were those pre-specified, however the trial protocol was not assessed |
| Other bias | Unclear risk | The 2 groups were comparable on background characteristics. But the paper mentioned that ‘in the process of monitoring the first 20 cases, it was noted that the randomisation scheme was not being appropriately followed - the study was re-started’. More women were lost to follow up in the MVA group than the misoprostol group |
| Methods | RCT with randomisation of individual women. Sequentially numbered opaque envelopes, using pseudo-random number generator | |
| Participants | Inclusion criteria • Women with signs of incomplete miscarriage. • Diagnosis confirmed by ultrasound. • 1st trimester; good general health; no allergy to misoprostol; good access to emergency facilities. • N = 169 women. Exclusion criteria • None specified. |
|
| Interventions | Intervention: oral misoprostol - 600 ug, single dose (N = 86). Comparison: oral misoprostol - 1200 ug, 2 doses, 4 hours apart (N = 83) |
|
| Outcomes | Complete miscarriage at 48 hours; surgical evacuation; side effects and acceptability • Assessed by ultrasound at 48 hours. • If miscarriage not complete at 48 hours, women were given the option to wait additional 5 days (1 week from misoprostol administration) to see if miscarriage would be complete without further intervention. If miscarriage not complete after 1 week or if woman refused extension, then she underwent surgical evacuation according to standard practice. |
|
| Notes | 1. Setting: 2 teaching hospitals in Bangkok, Thailand. 2. Additional outcomes assessed but not pre-specified in the review: bleeding (heavy, normal, spotting); pain; fever; medically necessary interventions; satisfied or very satisfied with treatment. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Pseudo-random number generator in SSPS 9.0.” |
| Allocation concealment (selection bias) | Low risk | Women given the next “…sequentially numbered opaque envelope; the number in the envelope became her study identification number” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | “Neither the provider nor the woman was blinded to the treatment regimes.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 2 women in single-dose group and 1 woman in double-dose group were lost to follow up. 1.8% of total, so no real impact. |
| Selective reporting (reporting bias) | Unclear risk | Appears to be free of selective reporting bias but we did not assess the trial protocol |
| Other bias | Low risk | Appears to be free of other reporting bias. |
| Methods | RCT with randomisation of individual women. | |
| Participants | Inclusion criteria • Women seeking medical attention due to signs of miscarriage in 1st trimester. • To be included women had to be circulatory stable (stable blood pressure and haemoglobin > 90 g/L) and without any signs of genital infection. Only women with a gestational residue (A-P diameter) between 15 and 50 mm were included. The non-viability of the concepts had to be confirmed and accepted by both the physician and woman. Only women above the age of 18 were included. • Vaginal ultrasound confirmed the miscarriage diagnosis. • N = 126 women. Exclusion criteria • Women who were not able to understand the information provided regarding the study and women with a possible allergy or medical contraindication for analgesics or misoprostol were not included. |
|
| Interventions | Intervention: vaginal misoprostol - 400 ug. • 2 tablets of 200 ug, each self administered at home. • N = 64. Comparison: placebo. • tablets identical with the misoprostol tablets. • N = 62. |
|
| Outcomes | Complete miscarriage assessed at 6-7 days; infection; bleeding; gastrointestinal side effects; subjective pain; use of analgesics and length of sick leave • Assessed at 7 days. • Successful miscarriage was defined as A-P diameter for the gestational residue was < 15 mm. |
|
| Notes | 1. Setting: University Hospital, Goteborg, Sweden. 2. Confirmed with the author that the women had incomplete miscarriages diagnosed by ultrasound and there were no IUF. 3. Additional outcomes assessed but not pre-specified in the review: serum haemoglobin; reduction in serum haemoglobin and days of sick leave. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…random table system…” |
| Allocation concealment (selection bias) | Low risk | “Patients were randomised…by drawing a sealed envelope from a box…tablets were delivered to the independent pharmacy where they were inserted by the pharmacy staff into numbered envelopes in blocks of 10…the randomisation list was retained by the hospital pharmacy and was not broken until after completion of the study when statistical analyses were performed”. However, no mention of the envelopes being opaque - so concealment allocation unclear but because tablets are identical, it seems unlikely there is a problem here |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The placebo tablets “…were identical in appearance to the active misoprostol tablets” and clinicians “…unaware of the randomisation sequence” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | There was no loss to follow up and women received their appropriate allocation. the analysis appears to be ITT |
| Selective reporting (reporting bias) | Unclear risk | Seems to be free of bias here, though the secondary outcome of ‘total number of days of bleeding’ was not reported. However, we did not assess the trial protocol |
| Other bias | Unclear risk | 1. There was an imbalance in baseline data for gestational age: misoprostol: 72.8 (SD 12.2) and placebo 77.8 (SD 12.9). This might favour better outcomes for the placebo group, but probably no important bias here. 2. Women chose whether they wanted a D&C if miscarriage not complete after 1 week or whether to wait longer. So we used the outcome of complete miscarriage at 1 week which excludes problems with choice after that time, but the problem is present for the outcome of surgical evacuation. |
| Methods | RCT with randomisation of individual women. | |
| Participants | Inclusion criteria • Women with miscarriage up to 12 weeks’ gestation. • Transvaginal ultrasound used. • Only those women (N = 34) with endometrial thickness > 10 cm were randomised, the remaining women (N = 27) had endometrial thickness < 10 cm an were managed by expectancy. • N = 61 women. Exclusion criteria • Women with intrauterine device in situ, missed abortion flow/blighted ovum, extrauterine pregnancy or mola. |
|
| Interventions | Intervention: vaginal prostaglandin. • Prostaglandin E1 analogue (gemeprost). • N = 17. Comparison: surgical management. • Curettage. • N = 17. |
|
| Outcomes | Duration of vaginal bleeding; pain; discomfort experienced; sick days and days of absence • Assessed at 5-8 days using transvaginal ultrasound. |
|
| Notes | 1. Setting: district hospital in Glostrup, Copenhagen, Denmark. 2. Paper written in Danish, with English abstract. Paper was translated. 3. Additional outcomes assessed but not pre-specified in the review: bleeding; pain; days of sick leave; women’s dissatisfaction. The participants were divided into 2 groups: • Group 1 (27) with an endometrial thickness of less than 10mm and • Group 2 (34) with an endometrial thickness greater than 10mm. Group 1 was managed by expectancy and Group 2 was further divided into 2 groups again at random: • Group 2 A (17) which was given Prostaglandin E1 analogue gemeprost (1 mg). • Group 2 B (17) which underwent curettage. This review looked only at group 2A versus 2B. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The participating women were chosen at random by the drawing of lots into 2 parallel groups.” |
| Allocation concealment (selection bias) | Unclear risk | “The participating women were chosen at random by the drawing of lots into 2 parallel groups.” No further information |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Not possible to blind women nor clinicians. No mention of whether assessors were blinded or not |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 4 women did not complete the trial period. 2 from Gemeprost group and 0 from curettage group (2 from expectant group). 6% loss but both from the medical management group |
| Selective reporting (reporting bias) | Unclear risk | There is no mention of the outcomes to be measured although there was a questionnaire sent to women and this may well have been designed before the study began. Also, we did not assess the trial protocol |
| Other bias | Low risk | “The patients in all groups were comparable regarding age, previous births and previous spontaneous or instigated abortions.” There was no other information which would suggest other biases |
| Methods | RCT with randomisation of individual women, in blocks of 10 and stratified by site (2 sites involved) | |
| Participants | Inclusion criteria • Women with incomplete spontaneous or induced miscarriage, less than 12 weeks’ gestation, diagnosed using ultrasound. • Uterine size equivalent to a gestation of less than 12 weeks LMP, open cervical os, past or present history of vaginal bleeding during pregnancy and ultrasound evidence of substantial uterine debris with evidence of fetal demise. • Women living or working within the hospital’s geographical area of coverage, no known contraindications to misoprostol, no signs of severe infection, temperature < 38 °C and general good health. • N = 460 women. Exclusion criteria • Women with very high fever; signs of severe infection. |
|
| Interventions | Intervention: oral misoprostol. • 600 ug, single dose. • N = 233. Comparison: surgery. • MVA. • N = 227. |
|
| Outcomes | Complete miscarriage following initial treatment; adverse effects, bleeding; pain (7-point Likert scale), acceptability (5-point Likert scale) • Assessed at 1 week using clinical assessments and US. Women could wait a further week before surgery (MVA) if they wished. |
|
| Notes | 1. Setting: 2 large university teaching hospitals in Burkina Faso, Sub-Saharan Africa. 2. Additional outcomes assessed but not pre-specified in the review: pain/cramps; fever; chills; bleeding; overall experience; overall satisfaction; would choose again; would recommend to a friend; hospitalisation; managed pain with paracetamol; would have liked stronger pain killers; sought contact with providers; made phone calls to providers; best and worst features. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…computer-generated random sequence provided by Genunity Health Projects…” |
| Allocation concealment (selection bias) | Low risk | “The assignment was concealed from providers and participants until after informed consent was given when the next sequential opaque sealed study envelope was opened to reveal allocation…” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | “Neither women nor providers were blinded to treatment assignment…” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | • Lost after randomisation and before Rx: 10 in misoprostol group and 3 in the MVA group. • Exclusion after randomisation: 5 in the misoprostol group and 1 in the MVA group. • Overall, there were uneven loses to follow and some exclusions, but as numbers are small and we think this is unlikely to cause bias. |
| Selective reporting (reporting bias) | Unclear risk | Pre-specified outcomes reported on, but the trial protocol not assessed |
| Other bias | Low risk | No apparent biases from other sources. Baseline data showed no statistically significant differences between the groups |
| Methods | RCT with randomisation of individual women. Sequentially numbered sealed envelopes, using a computer-generated number allocation | |
| Participants | Womenwith spontaneous incomplete miscarriage after up to 13 weeks’ gestation assessed by ultrasound N = 94 women. |
|
| Interventions | Intervention: vaginal misoprostol - 600 ug (plus a second dose 24 hours later if miscarriage still not complete). N = 47 Comparison: surgical ERPC by sharp curettage following 20 U of oxytocin per litre of normal saline under GA with no prophylactic antibiotics but oral analgaesics were prescribed. N = 47 |
|
| Outcomes | Women requiring ERPC after failed medical management; number of doses of misoprostol required; duration of bleeding; adverse effect profile (nausea, vomiting and/or diarrhoea); time spent away from work; use of analgesia | |
| Notes | 1. Setting: Gynaecology Outpatient Dept, Durban, South Africa. 2. Additional outcomes assessed but not pre-specified in the review: Hb at 4 days; pain (VAS); duration of analgaesia; days of sick leave; satisfaction (VAS); would use same treatment again; would recommend treatment to friend. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer-generated patient number allocation.” |
| Allocation concealment (selection bias) | Low risk | “The number was sealed consecutively numbered envelopes by staff not involved in the study. Sealed envelopes were opened and consecutively enrolled women had their allocated treatment. It is not clear, however, whether the enveloped were opaque or not.” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | It was not possible to blind women nor clinicians, and it was unclear whether assessors were blinded for some of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss of participants nor exclusions reported. |
| Selective reporting (reporting bias) | Unclear risk | It would appear so though the pre-specified outcomes do not match the reported outcomes fully. Also we did not assess the trial protocol |
| Other bias | Unclear risk | No figures given on baseline data, only reported as “those who were randomised were well matched for demographic and clinical data”. Study not stopped early for benefit and no other apparent biases |
| Methods | RCT with randomisation of individual women. | |
| Participants | Inclusion criteria • Women with incomplete miscarriage. • Women of 18 years or older, living or working within 1 h of the study hospital, no known contraindication to misoprostol and general good health. • N = 300 women. Exclusion criteria • None specified. |
|
| Interventions | Intervention: oral misoprostol - 600 ug. N = 150. Comparison: oral misoprostol - 1200 ug (2 × 600 ug, 4 hours apart). N = 150 |
|
| Outcomes | Complete evacuation without recourse to surgery; women’s satisfaction and acceptability | |
| Notes | 1. Setting: large tertiary facility in Ho Chi Minh City in Southern Vietnam. 2. Mean gestational age was 8.1 weeks, so we consider all to be less than 13 weeks’ gestation, this was confirmed by personal communication with co-author J Blum but we have emailed the first author to confirm as suggested. 3. Additional outcomes assessed but not pre-specified in the review: bleeding; pain/cramps; fever/chills; tolerability; would choose again; would recommend to a friend. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…computer generated random sequence…” |
| Allocation concealment (selection bias) | Unclear risk | “…opening the next study envelope…” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Although comparing drug doses, because the comparator group were given second dose 4 hours later, this was not blinded from participants not caregivers |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 5 women lost to follow up: 1/150 for single dose and 4/150 for repeat dose. Authors made every effort to contact women, both by phone and visits but unsuccessfully for these 5 women. There were no exclusion reported although some outcomes were only available on 145 of the women in the double dose group rather than 146. The analysis was not by intention to treat because of the lost data, but we considered the loss was small enough for there to be no important bias |
| Selective reporting (reporting bias) | Unclear risk | Seem to have reported all pre-specified outcomes, but we did not access the trial protocol |
| Other bias | Low risk | There was nothing to suggest any other risk of bias. |
| Methods | RCT with randomisation of individual women. | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention: oral mifepristone + vaginal misoprostol.
|
|
| Outcomes | Complete abortion rate; bleeding; pain; satisfaction; complications including infection (clinical signs or elevated infection parameters in lab tests) treated with oral or IV antibiotics; continuous and heavy bleeding; blood transfusions; curettage for any reason; intense pain requiring admission | |
| Notes | Setting: Oulu University Hospital, Finland. The 19 women with incomplete spontaneous miscarriage were part of a larger study of 98 women who had had various forms of miscarriage (incomplete spontaneous miscarriage; anembryonic pregnancy; missed miscarriage). Separate data were available from the authors for the women with incomplete spontaneous miscarriage. Of the 19 women, 16 were < 13 weeks’ gestation and 3 were between 13 and 23 weeks’ gestation. This information is held at the Cochrane Pregnancy and Childbirth Group Office |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…computer randomised program with the block length of 6.” |
| Allocation concealment (selection bias) | Low risk | “An independent consult performed the randomisation and assigned the randomisation list to a secretary, who made the numbered opaque envelopes for the study. …Allocation concealment was used to confirm that neither the clinician nor the patient knew the type of treatment in advance… After informed consent the next numbered envelope was opened to define the type of treatment of each patient.” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Cannot blind women nor clinicians to the treatment because this study compared medical versus surgical treatment |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Authors randomised 98 women (19 with ICM and 79 with IUFD) with 49 in each group. Of these, they reported on 48 in medical and 47 in surgical groups because 1 woman in the medical management group had an ERPC and in the surgical group one woman had an emergency ERPC and one had a spontaneous complete miscarriage. Of these women only 19 had incomplete miscarriage (the remainder had intrauterine deaths) and of these all appear to be accounted for in the analysis |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the protocol, and additionally the authors did not report on bleeding; blood transfusions’ |
| Other bias | Low risk | No apparent additional biases apparent. |
| Methods | RCT with randomisation of individual women. | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention: vaginal misoprostol.
|
|
| Outcomes | Efficacy; side effects; short-term complications.
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated set of random numbers in blocks of 5. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes labelled serially. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Of the 201 women randomised, 198 got the treatment allocated, but only 186 were analysed because 12 were lost to follow up - 7.5%. It is unclear whether ITT analysis was undertaken |
| Selective reporting (reporting bias) | Unclear risk | No obvious outcome reporting bias but authors do not list their outcomes and although only report significant differences in abstract, in paper they report several adverse outcomes with data. We did not assess the trial protocol. |
| Other bias | Low risk | Significantly more women in oral group had a past history of termination, P < 0. 001, but this was thought to probably not to create important bias |
| Methods | RCT with randomisation of individual women. | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention: vaginal misoprostol.
|
|
| Outcomes | Number of days of vaginal bleeding; rate of complications (fall in Hb, infection, perforation) and women’s satisfaction
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided except to say women were randomised. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Participants nor clinicians cannot be blinded. There is nomention as to whether the outcome assessor was blinded. For outcomes where participants assessed for themselves, these were not blinded |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No losses and no exclusions were reported, but nothing is described. As there is no deviation from protocol it is assumed that analysis was by ITT |
| Selective reporting (reporting bias) | Unclear risk | Seem to report on all outcomes specified in ‘Materials and methods’ but we did not assess the trial protocol |
| Other bias | Unclear risk | No imbalances in baseline data identified (assessed: age, gravity, parity, gestational age, anterior-posterior diameter). Study not stopped early and no apparent differential diagnosis |
| Methods | RCT with randomisation of individual women. Used a centralised computer-based enrolment and randomisation service. The Coordinating Centre used the biased coin method of maintaining balance between study arms, and was stratified by hospital and gestation (< 7 weeks; 8-10 weeks; 11-13 weeks) | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention 1: vaginal misoprostol.
|
|
| Outcomes | Successful evacuation; infection; haemorrhage; pain; bleeding; physical and emotional recovery; anxiety and depression
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…a centralised computer-based enrolment and randomisation service…using the biased coin method of maintaining balance between study arms, and was stratified by hospital and gestation (7 weeks or less, 8 - 10 weeks, 11 - 13 weeks).” |
| Allocation concealment (selection bias) | Low risk | “…a centralised computer-based enrolment and randomisation service, available by telephone 24 hours a day.” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | “Participants and clinicians could not be blind. Unclear of outcome assessor blind or not, though not for outcomes assessed by women. Reports that “The data analyst had access to unblinded data but no contact with any study participant” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1 woman randomised to medical Rx (misoprostol) withdrew following randomisation and was not included in the analyses Medical group: 1 woman was lost to follow up at 10 to 14 days; 1 woman was lost to follow up at 8 weeks Surgical group: 1 woman was lost to follow up at 8 weeks. Expectant group: 1 woman was lost to follow up at 8 weeks. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures are listed in the methods section and are those reported in the results section. We did not assess the trial protocol |
| Other bias | Low risk | Study was planned to recruit 831 women from power calculation 80% power to detect of 5% (99% to 91%) at 0.05 level, but staff were recruiting < 50% eligible women and of these only 22% agreed. So, in effect stopped early but not because of benefit, so probably no bias, just underpowered. No data provided on base line balance, but reported that: “there were no marked or systematic differences between the groups at trial entry with regards to gestation, women’s age, reproductive history, methods of diagnosis, days of bleeding, pain, haemoglobin or white cell count”. There seemed to be no differential diagnosis. |
| Methods | RCT with randomisation of individual women in blocks of 10. | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention: oral misoprostol.
|
|
| Outcomes | Successful miscarriage; adverse effects; women’s satisfaction
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “….computer-generated random code, created in bocks of 10 at Genunity Health Projects’ Office in New York City.” |
| Allocation concealment (selection bias) | Low risk | “The code was used by a Genunity employee who was not part of the research team as a basis for sealing cards in consecutively numbered envelopes… staff would open the next envelope in the numbered series…”. Although not opaque enveloped, we think the numbered series should be alright |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Not able to blind participants or clinicians. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss to followup and no deviations from protocol allocation reported. ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Report on pre-specified outcomes, though we have not assessed the trial protocol |
| Other bias | Low risk | On most characteristics women did not differ significantly. But significantly more women in the misoprostol group had spontaneous miscarriage and were married. However, we considered that this probably will not have any impact on differences in outcome |
| Methods | RCT with randomisation of individual women. Randomisation was by a central telephone system at the Clinical Trials Unit using minimisation to ensure comparability | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention 1: vaginal misoprostol.
|
|
| Outcomes | Primary outcome: gynaecological infection within 14 days of trial entry Secondary outcomes: antibiotics for presumed gynaecological infection within 14 days and within 8 weeks; duration of clinical symptoms (pain, additional analgesia, vaginal bleeding; days off work, days before return to usual daily activities); complications (fall in haemoglobin at 10-14 days, blood transfusion, unplanned consultations or admission within 14 days and within eight weeks); efficacy; psychological outcomes (depression and anxiety); and return to normal activity
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was by a central telephone system at the Clinical Trials Unit in Oxford. We used minimisation to ensure comparability between women with respect to participating centres, parity, type of miscarriage and gestation” |
| Allocation concealment (selection bias) | Low risk | Randomisation was by a central telephone system at the Clinical Trials Unit in Oxford, and although no specific information given on randomisation, clinical trials units generally use computer-generated random numbers list |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Not possible to blind women nor clinicians, because women given medical or surgical intervention were treatment in hospital and women in expectant arm were able to go home. Cannot blind surgery vs medical treatment or expectant care. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss of participants to follow up:
Exclusions after randomisation: In each of the surgical group and expectant care group, one woman with a viable pregnancy was excluded. Analysis was by ITT |
| Selective reporting (reporting bias) | Unclear risk | All important pre-specified outcomes were reported but we have not assessed the trial protocol |
| Other bias | Unclear risk | Study stopped early because struggling to recruit and not because of benefit, so bias unlikely. There were no important baseline differences between the 3 groups However, the randomisation was not reported as stratified by women with ICM and women with IUFD and so these may not have similar groups for comparison |
| Methods | RCT with randomisation of individual women. Consecutively numbered sealed opaque envelopes, using a computer-generated random number | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention: oral misoprostol.
|
|
| Outcomes | Completeness of evacuation; adverse effects, maximum pain and blood loss
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer-generated random numbers…” |
| Allocation concealment (selection bias) | Low risk | “The allocation was written on cards and placed in consecutively numbered opaque sealed envelopes.” |
| Blinding (performance bias and detection bias) All outcomes |
High risk | “‘Neither the patients, assessors, nor the data analysers were blinded to the allocation.” It was not possible to blind women or clinicians because a drug was compared with surgery. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Loss of participants to follow up from 317 women randomised was considerable and was discussed by authors. Many women in the rural communities of Uganda did not come for follow up after discharge.
|
| Selective reporting (reporting bias) | Unclear risk | Seem to report findings fully, there is just the problem of large losses to follow up - due to the low-income country setting probably. We have not assessed the trial protocol. |
| Other bias | Unclear risk | Study was stopped for pragmatic reasons and not for benefit (principle investigator moved) and os probably no bias Clinical characteristics at presentation were similar between the 2 groups, though no P values reported Women in the MVA group were given routine analgesia where women in the medical management group had analgesia on re-quest. However, women in MVA still had more pain, so pain with MVA likely to be under-estimated |
| Methods | RCT with randomisation of individual women in ratio of 3:1. | |
| Participants | Inclusion criteria
|
|
| Interventions | Intervention: vaginal misoprostol.
Comparison: surgery.
|
|
| Outcomes | Success, Hb, fever, nausea, vomiting, diarrhoea and acceptability
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “…centralised, computer-automated telephone response system…” used to ran-domly assign women to groups in a 3:1 ratio |
| Allocation concealment (selection bias) | Low risk | A centralised, computer-automated telephone response system. It was considered that because it was an automated computer response, then allocation concealment would be good |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Not possible to blind either women or clinicians. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1 woman lost to follow up - surgical group. |
| Selective reporting (reporting bias) | Unclear risk | Assessed all the pre-specified outcomes from the paper, but the trial protocol was not assessed |
| Other bias | Unclear risk | No significant difference in baseline data reported on the criteria assessed, but difficult to say anything about all other types of bias |
BP: blood pressure
ERPC: evacuation of retained products of conception
GA: general anaesthetic
Hb: haemoglobin
ICM: incomplete miscarriage
ITT: intention to treat
IUFD: intrauterine fetal death
MVA: manual vacuum aspiration
RCT: randomised controlled trial
SD: standard deviation
VAS: visual analogue scale
vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Abdel 1997 | Participants were women with an intrauterine fetal death. |
| Al Inizi 2003 | Participants were women with an intrauterine fetal death. |
| Al-Bdour 2007 | Participants were women with an intrauterine fetal death. |
| Almog 2005> | Participants were women having a termination of pregnancy. |
| Amjad 1999 | Participants were women with an intrauterine fetal death. |
| Anderman 2000 | Participants were women with an intrauterine fetal death. |
| Autry 1999 | Participants were women with an intrauterine fetal death. |
| Avila-Vergara 1997 | Participants were women with an intrauterine fetal death. |
| Ayudhaya 2006 | Participants were women with an intrauterine fetal death. |
| Bagratee 2004 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICM and women with IUFD. To date we have not had a response |
| Bani-Irshaid 2006 | Participants were women having a termination of pregnancy. |
| Bebbington 2002 | Participants were women having a termination of pregnancy. |
| Behrashi 2008 | Participants were women having a second trimester termination of pregnancy, some of whom had an intrauterine fetal death |
| Betstadt 2008 | Participants were women with an intrauterine fetal death. |
| Biswas 2007 | Participants were women having a termination of pregnancy. |
| Cabrol 1990 | Participants were women with an intrauterine fetal death. |
| Caliskan 2005 | Participants were women having a termination of pregnancy. |
| Caliskan 2009 | Participants were women having a second trimester termination of pregnancy, some of whom had an intrauterine fetal death |
| Chittacharoen 2003 | Participants were women having a termination of pregnancy. |
| Chung 1999 | Study included women with ICM and women with IUFD. We contacted the authors who were extremely helpful and did provide additional data which is held at the OPCG editorial office. However, unfortunately they could not provide their data split by women with ICM and women with IUFD so we were unable to include their data in this review |
| Creinin 1997 | Participants were women with an intrauterine fetal death. |
| David 2003 | Participants were women with an intrauterine fetal death. |
| David 2005 | Participants were women with an intrauterine fetal death. |
| de Jonge 1995 | Quasi RCT. |
| Demetroulis 2001 | Study included women with ICM and women with IUFD. We have contacted the authors who have tried to help us but are unable to separate their data by women with ICM and women with IUFD |
| Dickinson 1998 | Participants were women having a termination of pregnancy, including some with intrauterine fetal death |
| Dickinson 2002 | Participants were women having a termination of pregnancy, including some with intrauterine fetal death |
| Dickinson 2003 | Participants were women having a termination of pregnancy for fetal abnormality |
| Egarter 1995 | Participants were women with an intrauterine fetal death. |
| Elhassan 2008 | Participants were women having a termination of pregnancy, including some with intrauterine fetal death |
| Eng 1997 | Participants were women with an intrauterine fetal death in the 2nd trimester |
| Eppel 2005 | Participants were women having a termination of pregnancy in the 2nd trimester, including some with intrauterine fetal death |
| Fadalla 2004 | Participants were women having a termination of pregnancy in the 2nd trimester, including some with intrauterine fetal death |
| Feldman 2003 | Participants were women having a termination of pregnancy in the 2nd trimester, including some with intrauterine fetal death |
| Fiala 2005 | Participants were women having a termination of pregnancy for fetalmalformations and socio-economic reasons |
| Gazvani 2000 | Study was of women with incomplete miscarriage, but it assessed surgery versus expectant care, rather than medical management |
| Ghorab 1998 | Participants were women having a termination of pregnancy for fetalmalformations or intrauterine fetal death |
| Gilles 2004 | Participants were women with an intrauterine fetal death. |
| Gonzalez 2001 | Participants were women having a termination of pregnancy for intrauterine fetal death or medical or genetic reasons |
| Graziosi 2004 | Participants were women with an intrauterine fetal death. |
| Grimes 2005 | Participants were women having a termination of pregnancy, including some with intrauterine fetal death |
| Gronlund 2002 | Not an RCT, but a prospective cross-over study by alternate regimes every 4 months |
| Guix 2005 | Participants were women having a termination of pregnancy. Also allocated to different treatments at the discretion of the clinician, so not an RCT |
| Hassan 2007 | Quasi RCT, women allocated to groups based on alternate sequence |
| Hausler 1997 | Participants were women with complete spontaneous miscarriage and endometrial width up to 8 mm |
| Heard 2002 | Participants were women with an intrauterine fetal death. |
| Herabutya 1997a | Participants were women with an intrauterine fetal death. |
| Herabutya 1997b | Participants were women with an intrauterine fetal death. |
| Herabutya 2005 | Participants were women having a termination of pregnancy. |
| Hernandez-Valencia 2003 | Participants were women with an intrauterine fetal death. |
| Hidar 2001 | Participants were women having a termination of pregnancy. |
| Hidar 2005 | Participants were women having a termination of pregnancy for intrauterine fetal death |
| Hill 1991 | Participants were women with an intrauterine fetal death. |
| Hinshaw 1995 | Participants were women with an intrauterine fetal death. |
| Hinshaw 1997 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICM and women with IUFD. To date we have not had a response |
| Hogg 2000 | Participants were women having a termination of pregnancy, with some for intrauterine fetal death but mostly for fetal anomalies |
| Islam 2006 | Participants were women with an intrauterine fetal death. |
| Jain 1994 | Participants were women having a termination of pregnancy for intrauterine fetal death |
| Jain 1996 | Participants were women having a termination of pregnancy for intrauterine fetal death or fetal anomalies |
| Jain 1999 | Participants were women having a termination of pregnancy. |
| Johnson 1997 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICM and women with IUFD. To date we have not had a response |
| Kanhai 1989 | Participants were women with an intrauterine fetal death. |
| Kapp 2007 | Participants were women having a termination of pregnancy, including women with an intrauterine fetal death |
| Kara 1999 | Participants were women with an intrauterine fetal death. |
| Kovavisarach 2002 | Participants were women with an intrauterine fetal death. |
| Kovavisarach 2005 | Participants were women with an intrauterine fetal death. |
| Kushwah 2009 | Participants were women with an intrauterine fetal death. |
| Lelaidier 1993 | Participants were women with an intrauterine fetal death. |
| Lippert 1978 | Not an RCT. Women were divided into two groups. |
| Lister 2005 | Participants were women with an intrauterine fetal death. |
| Louey 2000 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICM and women with IUFD. To date we have not had a response |
| Lughmani 2007 | Participants were women with an intrauterine fetal death. |
| Lughmani 2008 | Participants were women with an intrauterine fetal death. |
| Machtinger 2004 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICM and women with IUFD. To date we have not had a response |
| Makhlouf 2003 | Participants were women having a termination of pregnancy. |
| Martin 1955 | Not an RCT, alternate allocation. |
| Muffley 2002 | Participants were women with an intrauterine fetal death. |
| Mulayim 2009 | Participants were women with an intrauterine fetal death and some having a termination of pregnancy |
| Nakintu 2001 | Participants were women with an intrauterine fetal death. |
| Nassar 2006 | Participants were women with an intrauterine fetal death. |
| Ngai 2001 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICM and women with IUFD. To date we have not had a response |
| Ngoc 2004 | Participants were women with an intrauterine fetal death, classified as ‘missed abortion’ |
| Nielsen 1999 | Study included women with ICM and women with IUFD. We have tried to contact the authors to ask if they could provide their data spilt by women with ICMand women with IUFD. To date we have had no response but we are still trying to contact the authors using a different email address |
| Niromanesh 2005 | Participants were women with an intrauterine fetal death. |
| Nor Azlin 2006 | Participants were women having a termination of pregnancy, including women with an intrauterine fetal death |
| Nuthalapaty 2005 | Participants were women having a termination of pregnancy, including women with an intrauterine fetal death |
| Nuutila 1997 | Participants were women having a termination of pregnancy, including women with an intrauterine fetal death |
| Owen 1999 | Participants were women with indication for termination of pregnancy |
| Paraskevaides 1992 | Included both women with incomplete miscarriage and women with intrauterine fetal death. We were unable to contact the authors to request split data |
| Perry 1999 | Participants were women with indication for termination of pregnancy |
| Piotrowski 1979 | Participants were women with an intrauterine fetal death. |
| Pongsatha 2004 | Participants were women with indication for termination of pregnancy |
| Ramsey 2004 | Participants were women with indication for termination of pregnancy |
| Rita 2006 | Participants were women with an intrauterine fetal death. |
| Rivero-Lopez 1998 | Participants were women with an intrauterine fetal death. |
| Rosenberg 2006 | Participants were women with an intrauterine fetal death. |
| Roy 2003 | Participants were women with indication for termination of pregnancy |
| Ruangchainikhom 2006 | Participants were women with indication for termination of pregnancy |
| Saichua 2009 | Participants were women with an intrauterine fetal death. |
| Salamalekis 1990 | Not an RCT, no mention of randomisation. |
| Sathapanachai 2000 | Participants were women with an intrauterine fetal death. |
| Shobeira 2007 | Participants were women with an intrauterine fetal death. |
| Stockheim 2006 | Participants were women with an intrauterine fetal death. |
| Su 2005 | Participants were women with indication for termination of pregnancy |
| Suchonwanit 1999 | Participants were women with an intrauterine fetal death. |
| Surita 1997 | Participants were women with an intrauterine fetal death. |
| Tang 2003 | Participants were women with ‘silentmis carriage’ and women with complete and incomplete miscarriages were excluded |
| Tang 2006a | Participants were women with ‘silent miscarriage’ and women with incomplete miscarriages were excluded |
| Thavarasah 1986 | Participants were women with an intrauterine fetal death. |
| Toppozada 1994 | Participants were women with indication for termination of pregnancy for intrauterine fetal death |
| Wood 2002 | Participants were women with an intrauterine fetal death. |
| Yapar 1996 | Participants were women with indication for termination of pregnancy including women with intrauterine fetal death |
| Yilmaz 2005 | Participants were women with indication for termination of pregnancy including women with intrauterine fetal death |
| Yilmaz 2007 | Participants were women having termination of pregnancy, some of whom had an intrauterine fetal death |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods | RCT with randomisation of individual women. |
| Participants | Women with incomplete miscarriage with uterine size < 12 weeks’ gestation N = 300. |
| Interventions | Intervention: oral misoprostol (600 mcg). Comparison: sublingual misoprostol (400 mcg). |
| Outcomes | Complete miscarriage, satisfaction, side effects and pain. |
| Notes | We have still to assess this study for inclusion. |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We are trying to get a copy of this paper for assessment. |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods | RCT with randomisation of individual women. |
| Participants | Women with missed or incomplete miscarriage before 10 weeks’ gestation N = 200 women. |
| Interventions | Intervention: 1200 ug oral misoprostol (400 ug doses every 4 hours for 3 doses) Comparison: 400 ug oral misoprostol 2 hours before MVA under cervical block |
| Outcomes | Success; adverse effects (abdominal cramps}; acceptability to women |
| Notes | We aim to contact the authors for information on the methodology and outcome data |
| Methods |
| Participants |
| Intervention |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We are trying to get a copy of this paper for assessment. |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
MVA: manual vacuum aspiration
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Is misoprostol a safe alternative to manual vacuum aspiration in women with incomplete abortions in developing countries? |
| Methods | Evaluator-blinded, single centre, randomised controlled non-inferiority trial |
| Participants | Women with first trimester pregnancy loss. |
| Interventions | Intervention: sublingual misoprostol - 600 ug (3 doses of 200 ug each every 4 hours) Comparison: surgery (MVA). |
| Outcomes | Ultrasonagraphic thickness; change in Hb; pain; adverse effects; women’s satisfaction and acceptability |
| Starting date | 11 February 2008. |
| Contact information | Dr Regina Unkels, PO Box 97, Lindi, Tanzania. |
| Notes | Study No: ISRCTN65305620 Website: www.tgpsh.or.tz |
MVA: manual vacuum aspiration
DATA AND ANALYSES
Comparison 1.
Misoprostol versus expectant care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 2 | 150 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.72, 2.10] |
| 1.1 Vaginal misoprostol | 2 | 150 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.72, 2.10] |
| 1.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 2.1 Vaginal misoprostol | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 2.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 1 | 126 | Risk Ratio (M-H, Random, 95% CI) | 2.91 [0.12, 70.05] |
| 3.1 Vaginal misoprostol | 1 | 126 | Risk Ratio (M-H, Random, 95% CI) | 2.91 [0.12, 70.05] |
| 3.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 4.1 Vaginal misoprostol | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 4.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 3 | 332 | Risk Ratio (M-H, Random, 95% CI) | 3.07 [0.13, 74.28] |
| 5.1 Vaginal misoprostol | 3 | 332 | Risk Ratio (M-H, Random, 95% CI) | 3.07 [0.13, 74.28] |
| 5.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.67, 1.88] |
| 10.1 Vaginal misoprostol | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.67, 1.88] |
| 10.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection < 14 days | 3 | 333 | Risk Ratio (M-H, Random, 95% CI) | 2.42 [0.59, 9.98] |
| 11.1 Vaginal misoprostol | 3 | 333 | Risk Ratio (M-H, Random, 95% CI) | 2.42 [0.59, 9.98] |
| 11.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of placental tissue | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Misoprostol versus surgery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 8 | 1377 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.92, 1.00] |
| 1.1 Vaginal misoprostol | 3 | 154 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.83, 0.99] |
| 1.2 Oral misoprostol | 4 | 1143 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.93, 1.02] |
| 1.3 Vaginal + oral misoprostol | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 1.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 8 | 1538 | Risk Ratio (M-H, Random, 95% CI) | 0.07 [0.03, 0.18] |
| 2.1 Vaginal misoprostol | 3 | 315 | Risk Ratio (M-H, Random, 95% CI) | 0.18 [0.08, 0.44] |
| 2.2 Oral misoprostol | 4 | 1143 | Risk Ratio (M-H, Random, 95% CI) | 0.05 [0.02, 0.10] |
| 2.3 Vaginal + oral misoprostol | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 0.04 [0.01, 0.18] |
| 2.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 2 | 132 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.04, 22.64] |
| 3.1 Vaginal misoprostol | 2 | 132 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.04, 22.64] |
| 3.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 6 | 1158 | Risk Ratio (M-H, Random, 95% CI) | 6.32 [2.90, 13.77] |
| 4.1 Vaginal misoprostol | 3 | 315 | Risk Ratio (M-H, Random, 95% CI) | 5.56 [1.11, 27.90] |
| 4.2 Oral misoprostol | 3 | 843 | Risk Ratio (M-H, Random, 95% CI) | 6.12 [1.99, 18.84] |
| 4.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 4 | 430 | Risk Ratio (M-H, Random, 95% CI) | 1.73 [0.19, 16.08] |
| 5.1 Vaginal misoprostol | 3 | 241 | Risk Ratio (M-H, Random, 95% CI) | 1.73 [0.19, 16.08] |
| 5.2 Oral misoprostol | 1 | 189 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 1 | 36 | Risk Ratio (M-H, Random, 95% CI) | 1.71 [0.24, 12.24] |
| 8.1 Vaginal misoprostol | 1 | 36 | Risk Ratio (M-H, Random, 95% CI) | 1.71 [0.24, 12.24] |
| 8.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 3 | 211 | Mean Difference (IV, Random, 95% CI) | 2.12 [1.18, 3.07] |
| 9.1 Vaginal misoprostol | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 2.76 [1.55, 3.97] |
| 9.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Vaginal + oral misoprostol | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 1.55 [0.58, 2.52] |
| 9.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 4 | 525 | Risk Ratio (M-H, Random, 95% CI) | 1.48 [0.67, 3.25] |
| 10.1 Vaginal misoprostol | 3 | 313 | Risk Ratio (M-H, Random, 95% CI) | 1.64 [1.05, 2.55] |
| 10.2 Oral misoprostol | 1 | 212 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.77, 0.92] |
| 10.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection < 14 days | 7 | 907 | Risk Ratio (M-H, Random, 95% CI) | 0.70 [0.25, 1.99] |
| 11.1 Vaginal misoprostol | 4 | 338 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.29, 4.44] |
| 11.2 Oral misoprostol | 2 | 489 | Risk Ratio (M-H, Random, 95% CI) | 0.26 [0.03, 2.41] |
| 11.3 Vaginal + oral misoprostol | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 0.5 [0.05, 5.30] |
| 11.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 1 | 189 | Risk Ratio (M-H, Random, 95% CI) | 0.07 [0.00, 1.25] |
| 12.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Oral misoprostol | 1 | 189 | Risk Ratio (M-H, Random, 95% CI) | 0.07 [0.00, 1.25] |
| 12.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 4 | 1134 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.98, 1.01] |
| 18.1 Vaginal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Oral misoprostol | 4 | 1134 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.98, 1.01] |
| 18.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.01, 2.00] |
| 19.1 Vaginal misoprostol | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.01, 2.00] |
| 19.2 Oral misoprostol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Vaginal + oral misoprostol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Rectal misoprostol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.5 Sublingual misoprostol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 1 | 38 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Vaginal misoprostol | 1 | 38 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Vaginal misoprostol | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of placental tissue | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Vaginal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Vaginal + oral misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 Rectal misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.5 Sublingual misoprostol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 6 | 1115 | Risk Ratio (M-H, Random, 95% CI) | 3.18 [1.78, 5.70] |
| 24.1 Vaginal misoprostol | 3 | 156 | Risk Ratio (M-H, Random, 95% CI) | 1.46 [0.61, 3.48] |
| 24.2 Oral misoprostol | 3 | 959 | Risk Ratio (M-H, Random, 95% CI) | 4.69 [2.64, 8.34] |
| 24.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 5 | 1090 | Risk Ratio (M-H, Random, 95% CI) | 2.25 [1.14, 4.43] |
| 25.1 Vaginal misoprostol | 2 | 131 | Risk Ratio (M-H, Random, 95% CI) | 1.26 [0.12, 13.73] |
| 25.2 Oral misoprostol | 3 | 959 | Risk Ratio (M-H, Random, 95% CI) | 2.39 [1.13, 5.02] |
| 25.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 3 | 437 | Risk Ratio (M-H, Random, 95% CI) | 4.25 [0.76, 23.73] |
| 26.1 Vaginal misoprostol | 2 | 131 | Risk Ratio (M-H, Random, 95% CI) | 4.09 [0.51, 32.97] |
| 26.2 Oral misoprostol | 1 | 306 | Risk Ratio (M-H, Random, 95% CI) | 4.63 [0.22, 95.55] |
| 26.3 Vaginal + oral misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.4 Rectal misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.5 Sublingual misoprostol | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
Vaginal misoprostol versus expectant care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 2 | 150 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.72, 2.10] |
| 1.1 Gestation < 13 weeks | 2 | 150 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.72, 2.10] |
| 1.2 Geststion 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 2.1 Gestation < 13 weeks | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 1 | 126 | Risk Ratio (M-H, Fixed, 95% CI) | 2.91 [0.12, 70.05] |
| 3.1 Gestation < 13 weeks | 1 | 126 | Risk Ratio (M-H, Fixed, 95% CI) | 2.91 [0.12, 70.05] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 4.1 Gestation < 13 weeks | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.17, 2.26] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 3 | 332 | Risk Ratio (M-H, Fixed, 95% CI) | 3.07 [0.13, 74.28] |
| 5.1 Gestation < 13 weeks | 3 | 332 | Risk Ratio (M-H, Fixed, 95% CI) | 3.07 [0.13, 74.28] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.67, 1.88] |
| 10.1 Gestation < 13 weeks | 2 | 308 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.67, 1.88] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection < 14 days | 3 | 333 | Risk Ratio (M-H, Fixed, 95% CI) | 2.81 [0.77, 10.33] |
| 11.1 Gestation < 13 weeks | 3 | 333 | Risk Ratio (M-H, Fixed, 95% CI) | 2.81 [0.77, 10.33] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4.
Oral misoprostol versus expectant care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Geststion 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7.
Vaginal misoprostol versus surgery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 3 | 154 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 1.1 Gestation < 13 weeks | 3 | 154 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [00.0, 0.0] |
| 2 Surgical evacuation | 3 | 315 | Risk Ratio (M-H, Random, 95% CI) | 0.18 [0.08, 0.44] |
| 2.1 Gestation < 13 weeks | 3 | 315 | Risk Ratio (M-H, Random, 95% CI) | 0.18 [0.08, 0.44] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [00.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 2 | 132 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.04, 22.64] |
| 3.1 Gestation < 13 weeks | 2 | 132 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.04, 22.64] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [00.0, 0.0] |
| 4 Unplanned surgical intervention | 3 | 315 | Risk Ratio (M-H, Random, 95% CI) | 5.56 [1.11, 27.90] |
| 4.1 Gestation < 13 weeks | 3 | 315 | Risk Ratio (M-H, Random, 95% CI) | 5.56 [1.11, 27.90] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [00.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 3 | 241 | Risk Ratio (M-H, Fixed, 95% CI) | 1.82 [0.21, 15.70] |
| 5.1 Gestation < 13 weeks | 3 | 241 | Risk Ratio (M-H, Fixed, 95% CI) | 1.82 [0.21, 15.70] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [00.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.71 [0.24, 12.24] |
| 8.1 Gestation < 13 weeks | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.71 [0.24, 12.24] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | 2.76 [1.55, 3.97] |
| 9.1 Gestation < 13 weeks | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | 2.76 [1.55, 3.97] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 3 | 313 | Risk Ratio (M-H, Fixed, 95% CI) | 1.75 [1.21, 2.54] |
| 10.1 Gestation < 13 weeks | 3 | 313 | Risk Ratio (M-H, Fixed, 95% CI) | 1.75 [1.21, 2.54] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection < 14 days | 4 | 338 | Risk Ratio (M-H, Fixed, 95% CI) | 1.27 [0.37, 4.42] |
| 11.1 Gestation < 13 weeks | 4 | 338 | Risk Ratio (M-H, Fixed, 95% CI) | 1.27 [0.37, 4.42] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.01, 2.00] |
| 19.1 Gestation < 13 weeks | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.01, 2.00] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 1 | 38 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 1 | 38 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 3 | 156 | Risk Ratio (M-H, Fixed, 95% CI) | 1.37 [0.58, 3.22] |
| 24.1 Gestation < 13 weeks | 3 | 156 | Risk Ratio (M-H, Fixed, 95% CI) | 1.37 [0.58, 3.22] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 2 | 131 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [0.25, 8.93] |
| 25.1 Gestation < 13 weeks | 2 | 131 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [0.25, 8.93] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 2 | 131 | Risk Ratio (M-H, Fixed, 95% CI) | 4.30 [0.52, 35.36] |
| 26.1 Gestation < 13 weeks | 2 | 131 | Risk Ratio (M-H, Fixed, 95% CI) | 4.30 [0.52, 35.36] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8.
Oral misoprostol versus surgery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 4 | 1143 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.93, 1.02] |
| 1.1 Gestation < 13 weeks | 4 | 1143 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.93, 1.02] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 4 | 1143 | Risk Ratio (M-H, Random, 95% CI) | 0.05 [0.02, 0.10] |
| 2.1 Gestation < 13 weeks | 4 | 1143 | Risk Ratio (M-H, Random, 95% CI) | 0.05 [0.02, 0.10] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 3 | 843 | Risk Ratio (M-H, Fixed, 95% CI) | 7.07 [2.34, 21.30] |
| 4.1 Gestation < 13 weeks | 3 | 843 | Risk Ratio (M-H, Fixed, 95% CI) | 7.07 [2.34, 21.30] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 1 | 189 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 1 | 189 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 1 | 212 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.77, 0.92] |
| 10.1 Gestation < 13 weeks | 1 | 212 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.77, 0.92] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection | 2 | 489 | Risk Ratio (M-H, Fixed, 95% CI) | 0.26 [0.03, 2.41] |
| 11.1 Gestation < 13 weeks | 2 | 489 | Risk Ratio (M-H, Fixed, 95% CI) | 0.26 [0.03, 2.41] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 1 | 189 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.00, 1.25] |
| 12.1 Gestation < 13 weeks | 1 | 189 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.00, 1.25] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 4 | 1134 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.97, 1.01] |
| 18.1 Gestation < 13 weeks | 4 | 1134 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.97, 1.01] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 3 | 959 | Risk Ratio (M-H, Fixed, 95% CI) | 4.77 [2.68, 8.49] |
| 24.1 Gestation < 13 weeks | 3 | 959 | Risk Ratio (M-H, Fixed, 95% CI) | 4.77 [2.68, 8.49] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 3 | 959 | Risk Ratio (M-H, Fixed, 95% CI) | 2.59 [1.29, 5.21] |
| 25.1 Gestation < 13 weeks | 3 | 959 | Risk Ratio (M-H, Fixed, 95% CI) | 2.59 [1.29, 5.21] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 1 | 306 | Risk Ratio (M-H, Fixed, 95% CI) | 4.63 [0.22, 95.55] |
| 26.1 Gestation < 13 weeks | 1 | 306 | Risk Ratio (M-H, Fixed, 95% CI) | 4.63 [0.22, 95.55] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 New Outcome | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9.
Vaginal + oral misoprostol versus surgery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.87, 1.04] |
| 1.1 Gestation < 13 weeks | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.87, 1.04] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.04 [0.01, 0.18] |
| 2.1 Gestation < 13 weeks | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.04 [0.01, 0.18] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [0.58, 2.52] |
| 9.1 Gestation < 13 weeks | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [0.58, 2.52] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 11.1 Gestation < 13 weeks | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0 0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Cost | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12.
Vaginal misoprostol versus oral misoprostol
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 198 | Risk Ratio (M-H,Fixed, 95% CI) | 0.94 [0.76, 1.16] |
| 1.1 Gestation < 13 weeks | 1 | 198 | Risk Ratio (M-H,Fixed, 95% CI) | 0.94 [0.76, 1.16] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 1 | 198 | Risk Ratio (M-H,Fixed, 95% CI) | 1.11 [0.77, 1.60] |
| 2.1 Gestation < 13 weeks | 1 | 198 | Risk Ratio (M-H,Fixed, 95% CI) | 1.11 [0.77, 1.60] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 1 | 186 | Risk Ratio (M-H,Fixed, 95% CI) | 0.36 [0.01, 8.80] |
| 4.1 Gestation < 13 weeks | 1 | 186 | Risk Ratio (M-H,Fixed, 95% CI) | 0.36 [0.01, 8.80] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H,Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 1 | 186 | Risk Ratio (M-H Fixed, 95% CI) | 1.43 [0.93, 2.17] |
| 10.1 Gestation < 13 weeks | 1 | 186 | Risk Ratio (M-H, Fixed, 95% CI) | 1.43 [0.93, 2.17] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 063 [0.26, 1.54] |
| 24.1 Gestation < 13 weeks | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.26, 1.54] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.07, 1.75] |
| 25.1 Gestation < 13 weeks | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.07, 1.75] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.12, 0.36] |
| 26.1 Gestation < 13 weeks | 1 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.12, 0.36] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13.
Rectal misoprostol versus oral misoprostol
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss - severe | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H’ Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14.
Oral misoprostol: 600 ug versus 1200 ug
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 2 | 464 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 1.1 Gestation < 13 weeks | 2 | 464 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.29, 1.99] |
| 2.1 Gestation < 13 weeks | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.29, 1.99] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation <13 weeks | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.29, 1.99] |
| 4.1 Gestation < 13 weeks | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.29, 1.99] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation <13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 2 | 460 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 18.1 Gestation <13 weeks | 2 | 460 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation <13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation <13 weeks | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 2 | 463 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.57, 2.46] |
| 24.1 Gestation <13 weeks | 2 | 463 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.57, 2.46] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 2 | 463 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.60, 1.72] |
| 25.1 Gestation <13 weeks | 2 | 463 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.60, 1.72] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 1 | 294 | Risk Ratio (M-H, Fixed, 95% CI) | 0.73 [0.55, 0.97] |
| 26.1 Gestation < 13 weeks | 1 | 294 | Risk Ratio (M-H, Fixed, 95% CI) | 0.73 [0.55, 0.97] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15.
Oral mifepristone + vaginal misoprostol versus surgery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 19 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.77, 1.31] |
| 1.1 Gestation < 13 weeks | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.78, 1.27] |
| 1.2 Gestation 13-23 weeks | 1 | 3 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.39, 2.58] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 1 | 19 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Gestation < 13 weeks | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gestation 13-23 weeks | 1 | 3 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Blood transfusion | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Haemorrrhage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Blood loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Days of bleeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Pain relief | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pelvic infection < 14 days | 1 | 19 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Gestation < 13 weeks | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Gestation 13-23 weeks | 1 | 3 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cervical damage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Digestive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Hypertensive disorders | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Duration of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Gestation <13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Psychological effects | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Subsequent fertility | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Gestation <13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Women’s views/satisfaction - continuous data | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Pathology of fetal/placental tissue | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Gestation < 13 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Gestation 13-23 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Gestation not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Gestation <13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Diarrhoea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16.
Vaginal prostaglandin E1 (gemeprost) versus surgery
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Surgical evacuation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or serious complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Gestation < 13 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Unplanned surgical intervention | 1 | 34 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Gestation < 13 weeks | 1 | 34 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Gestation 13-23 weeks | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gestation not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
WHAT’S NEW
Last assessed as up-to-date: 21 September 2009.
| Date | Event | Description |
|---|---|---|
| 23 July 2012 | Amended | Search updated. Thirty reports added to Studies awaiting classification |
HISTORY
Protocol first published: Issue 3, 2008
Review first published: Issue 1, 2010
Footnotes
DECLARATIONS OF INTEREST: None known.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW: We have modified the wording in the methods sections for Assessment of heterogeneity, Assessment of reporting biases and Data synthesis to update them with the new methods being used by the group, developed in conjunction with the group’s statistician, Simon Gates, and Richard Riley. We have used these new methods in the review.
References to studies included in this review
- Bique 2007 [published data only] .Bique C, Usta M, Debora B, Chong E, Westheimer E, Winikoff B. Comparison of misoprostol and manual vacuum aspiration for the treatment of incomplete abortion. International Journal of Gynecology & Obstetrics. 2007;98(3):222–6. doi: 10.1016/j.ijgo.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Blanchard 2004 [published data only] .* Blanchard K, Taneepanichskul S, Kiriwat O, Sirimai K, Svirirojana N, Mavimbela N, et al. Two regimens of misoprostol for treatment of incomplete abortion. Obstetrics & Gynecology. 2004;103(5 Pt 1):860–5. doi: 10.1097/01.AOG.0000124274.47717.a7. [DOI] [PubMed] [Google Scholar]; Phupong V, Taneepanichskul S, Kriengsinyot R, Sriyirojana N, Blanchard K, Winikoff B. Comparative study between single dose 600 mg and repeated dose oral misoprostol for treatment of incomplete abortion. Contraception. 2004;70:307–11. doi: 10.1016/j.contraception.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Blohm 2005 [published data only] .Blohm F, Friden BE, Milsom I, Platz-Christensen JJ, Nielsen S. A randomised double blind trial comparing misoprostol or placebo in the management of early miscarriage. BJOG: an international journal of obstetrics and gynaecology. 2005;Vol. 112(issue 8):1090–5. doi: 10.1111/j.1471-0528.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- Clevin 2001 [published data only] .Clevin L, Munk T, Hansen TR. Spontaneous abortion. Drug treatment versus surgery [Spontan abort. Medicinsk versus kirurgisk behandling]. Ugeskrift for Laeger. 2001;163(15):2136–9. [PubMed] [Google Scholar]
- Dao 2007 [published data only] .Dao B, Blum J, Thieba B, Raghavan S, Ouedraego M, Lankoande J, et al. Is misoprostol a safe, effective and acceptable alternative to manual vacuum aspiration for postabortion care? Results from a randomised trial in Burkina Faso, West Africa. BJOG: an international journal of obstetrics and gynaecology. 2007;114(11):1368–75. doi: 10.1111/j.1471-0528.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- Moodliar 2005 [published data only] .Moodliar S, Bagratee JS, Moodley J. Medical vs. surgical evacuation of first-trimester spontaneous abortion. International Journal of Gynecology & Obstetrics. 2005;91:21–6. doi: 10.1016/j.ijgo.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ngoc 2005 [published data only] .Nguyen TNN, Blum J, Durocher J, Quan TTV, Winikoff B. A randomized controlled study comparing 600 versus 1, 200 micrograms oral misoprostol for medical management of incomplete abortion. Contraception. 2005;Vol. 72(issue 6):438–42. doi: 10.1016/j.contraception.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Niinimaki 2006 [published data only] .* Niinimaki M, Jouppila P, Martikainen H, Talvensaari-Mattila A. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertility and Sterility. 2006;86(2):367–72. doi: 10.1016/j.fertnstert.2005.12.072. [DOI] [PubMed] [Google Scholar]; Niinimaki M, Karinen P, Hartikainen AL, Pouta A. Treating miscarriages: a randomised study of cost-effectiveness in medical or surgical choice. BJOG: an international journal of obstetrics and gynaecology. 2009;116(7):984–90. doi: 10.1111/j.1471-0528.2009.02161.x. [DOI] [PubMed] [Google Scholar]
- Pang 2001 [published data only] .* Pang MW, Chung T. Incomplete miscarriage: a randomized controlled trial comparing oral with vaginal misoprostol for medical evacuation. Human Reproduction. 2001;16(11):2283–7. doi: 10.1093/humrep/16.11.2283. [DOI] [PubMed] [Google Scholar]; Pang SMW, Chung TKH. A randomized clinical trial comparing oral and vaginal misoprostol for medical evacuation of spontaneous abortion [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 3); Washington DC, USA. 2000 Sept 3-8.2000. p. 69. [Google Scholar]
- Sahin 2001 [published data only] .Sahin HG, Sahin HA, Kocer M. Randomized outpatient clinical trial of medical evacuation and surgical curettage in incomplete miscarriage. European Journal of Contraception & Reproductive Health Care. 2001;6(3):141–4. [PubMed] [Google Scholar]
- Shelley 2005 [published data only] .Shelley JM, Healy D, Grover S. A randomised trial of surgical, medical and expectant management of first trimester spontaneous miscarriage. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2005;Vol. 45:122–7. doi: 10.1111/j.1479-828X.2005.00357.x. [DOI] [PubMed] [Google Scholar]
- Shwekerela 2007 [published data only] .Shwekerela B, Kalumuna R, Kipingili R, Mashaka N, Westheimer E, Clark W, et al. Misoprostol for treatment of incomplete abortion at the regional hospital level: results from Tanzania. BJOG: an international journal of obstetrics and gynaecology. 2007;114(11):1363–7. doi: 10.1111/j.1471-0528.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- Trinder 2006 [published data only] .Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of firsttrimester miscarriage based on results from the MIST trial. BJOG: an international journal of obstetrics and gynaecology. 2006;113(8):879–89. doi: 10.1111/j.1471-0528.2006.00998.x. [DOI] [PubMed] [Google Scholar]; Smith L. [accessed 7 March 2006];Extension to: randomised controlled trial of expectant, medical and surgical management of early miscarriage. Research Findings Register. www.refer.nhs.uk.; Smith LF, Frost J, Levitas R, Bradley H, Garcia J. Women’s experiences of three early miscarriage management options: a qualitative study. British Journal of General Practice. 2006;56(524):198–205. [PMC free article] [PubMed] [Google Scholar]; Smith LFP, Ewings PD, Quinlan C. Incidence of pregnancy after expectant, medical, or surgical management of spontaneous first trimester miscarriage: long term follow-up of miscarriage treatment (MIST) randomised controlled trial. BMJ. 2009;339(1766):b3827. doi: 10.1136/bmj.b3827. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Trinder J, Brocklehurst P, Porter R, Read M, Vyas S, Smith L. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial) BMJ. 2006;332(7552):1235–40. doi: 10.1136/bmj.38828.593125.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks 2005 [published data only] .Weeks A, Alia G, Blum J, Blanchard K, Winikoff B. A randomised trial of oral misoprostol (600mcg) versus manual vacuum aspiration in the treatment of incomplete miscarriage in Kampala, Uganda. 30th British Congress of Obstetrics and Gynaecology; Glasgow, UK. 2004 July 7-9.2004. p. 27. [Google Scholar]; * Weeks A, Alia G, Blum J, Winikoff B, Ekwaru P, Durocher J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstetrics & Gynecology. 2005;106(3):540–7. doi: 10.1097/01.AOG.0000173799.82687.dc. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Zhang 2005 [published data only] .Chen BA, Reeves MF, Creinin MD, Gilles JM, Barnhart K, Westhoff C, et al. Misoprostol for treatment of early pregnancy failure in women with previous uterine surgery. American Journal of Obstetrics and Gynecology. 2008;198(6):626.e1–626.e5. doi: 10.1016/j.ajog.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]; Creinin MD, Huang X, Westhoff C, Barnhart K, Gilles JM, Zhang J, et al. Factors related to successful misoprostol treatment for early pregnancy failure. Obstetrics & Gynecology. 2006;107(4):901–7. doi: 10.1097/01.AOG.0000206737.68709.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]; Harwood B, Nansel T, for the National Institute of Child Health and Human Development Management of Early Pregnancy Failure Trial Quality of life and acceptability of medical versus surgical management of early pregnancy failure. BJOG: an international journal of obstetrics and gynaecology. 2008;115(4):501–8. doi: 10.1111/j.1471-0528.2007.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reeves MF, Lohr PA, Harwood B, Creinin MD. Sonographic findings after misoprostol or vacuum aspiration for early pregnancy failure. Contraception. 2006;74(2):182. [Google Scholar]; Reeves MF, Lohr PA, Harwood BJ, Creinin MD. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstetrics & Gynecology. 2008;111(1):106–12. doi: 10.1097/01.AOG.0000296655.26362.6d. [DOI] [PubMed] [Google Scholar]; Robledo C, Zhang J, Troendle J, Barnhart K, Creinin MD, Westhoff C, et al. Clinical indicators for success of misoprostol treatment after early pregnancy failure. International Journal of Gynecology & Obstetrics. 2007;99(1):46–51. doi: 10.1016/j.ijgo.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang J, Gilles J, Barnhart K, Creinin M, Westhoff C, Frederick MM. Medical management with misoprostol for early pregnancy failure: a multicenter, randomized equivalence trial [abstract] Fertility and Sterility. 2004;82(Suppl 2):S53–4. [Google Scholar]; * Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. New England Journal of Medicine. 2005;353(8):761–9. doi: 10.1056/NEJMoa044064. [DOI] [PubMed] [Google Scholar]
- Abdel 1997 [published data only] .Abdel Fattah IH. PGE1 analogue for the induction of midtrimester abortion in cases of intrauterine fetal death. Acta Obstetricia et Gynecologica Scandinavica Supplement. 1997;76(167:2):26. [Google Scholar]
- Al-Bdour 2007 [published data only] .Al-Bdour AN, Akasheh H, Al-Jayousi T. Missed abortion: termination using single-dose versus two doses of vaginal misoprostol tablets. Pakistan Journal of Medical Sciences. 2007;23(6):920–3. [Google Scholar]
- Al Inizi 2003 [published data only] .Al Inizi SA, Ezimokhai M. Vaginal misoprostol versus dinoprostone for the management of missed abortion. International Journal of Gynecology & Obstetrics. 2003;83:73–4. doi: 10.1016/s0020-7292(03)00199-1. [DOI] [PubMed] [Google Scholar]
- Almog 2005 [published data only] .Almog B, Levin I, Winkler N, Fainaru O, Pauzner D, Lessing JB, et al. The contribution of laminaria placement for cervical ripening in second trimester termination of pregnancy induced by intra-amniotic injection of prostaglandin f(2)alpha followed by concentrated oxytocin infusion. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005;118:32–5. doi: 10.1016/j.ejogrb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Amjad 1999 [published data only] .Amjad T, Akhtar S. Termination of pregnancy with foetal death in second trimester: Foley’s catheter versus extra amniotic prostaglandins. Journal of the College of Physicians & Surgeons Pakistan. 1999;9:403–5. [Google Scholar]
- Anderman 2000 [published data only] .Anderman S, Jaschevatzky OE, Ballas S. Comparison between a double balloon device and the foley catheter in extraamniotic prostaglandin F2a infusion for termination of midtrimester missed abortion [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 2); Washington DC, USA. 2000 Sept 3-8.2000. p. 161. [Google Scholar]
- Autry 1999 [published data only] .Autry A, Jacobson G, Sandhu R, Isbill K. Medical management of non-viable early first trimester pregnancy. International Journal of Gynecology & Obstetrics. 1999;Vol. 67(issue 1):9–13. doi: 10.1016/s0020-7292(99)00115-0. [DOI] [PubMed] [Google Scholar]
- Avila-Vergara 1997 [published data only] .Avila-Vergara MA, Morgan-Ortiz F, Fragoza-Sosa O, Haro-Garcia L. Cervical labor induction with prostaglandin E2 in patients with fetal death [Maduracion cervical con prostaglandina E2 en pacientes con feto muerto] Ginecologia y Obstetricia de Mexico. 1997;65:155–8. [PubMed] [Google Scholar]
- Ayudhaya 2006 [published data only] .Ayudhaya OP, Herabutya Y, Chanrachakul B, Ayuthaya NI, O-Prasertsawat P. A comparison of the efficacy of sublingual and oral misoprostol 400 microgram in the management of early pregnancy failure: a randomized controlled trial. Journal of the Medical Association of Thailand. 2006;89(Suppl 4):S5–S10. [PubMed] [Google Scholar]
- Bagratee 2004 [published data only] .Bagratee JS, Khullar V, Regan L, Moodley J, Kagoro H. A randomized controlled trial comparing medical and expectant management of first trimester miscarriage. Human Reproduction. 2004;19(2):266–71. doi: 10.1093/humrep/deh049. [DOI] [PubMed] [Google Scholar]
- Bani-Irshaid 2006 [published data only] .Bani-Irshaid I, Athamneh TZ, Bani-Khaled D, Al-Momani M, Dahamsheh H. Termination of second and early third trimester pregnancy: comparison of 3 methods. Eastern Mediterranean Health Journal. 2006;12(5):605–9. [PubMed] [Google Scholar]
- Bebbington 2002 [published data only] .Bebbington MW, Kent N, Lim K, Gagnon A, Delisle MF, Tessier F, et al. A randomized controlled trial comparing two protocols for the use of misoprostol in midtrimester pregnancy termination. American Journal of Obstetrics and Gynecology. 2002;187(4):853–7. doi: 10.1067/mob.2002.127461. [DOI] [PubMed] [Google Scholar]
- Behrashi 2008 [published data only] .Behrashi M, Mahdian M. Vaginal versus oral misoprostol for second-trimester pregnancy termination: a randomized trial. Pakistan Journal of Biological Sciences. 2008;11(21):2505–8. doi: 10.3923/pjbs.2008.2505.2508. [DOI] [PubMed] [Google Scholar]
- Betstadt 2008 [published data only] .Betstadt S. [accessed 20 February 2008];MiMi: a randomized trial of mifepristone and misoprostol for treatment of early pregnancy failure. ClinicalTrials.gov. clinicaltrials.gov.
- Biswas 2007 [published data only] .Biswas SC, Dey R, Jana R, Chattopadhyay N. Comparative study of intravaginal misoprostol and extra amniotic ethacridine lactate instillation for mid trimester pregnancy termination. Journal of Obstetrics and Gynaecology of India. 2007;57(3):211–3. [Google Scholar]
- Cabrol 1990 [published data only] .Cabrol D, Dubois C, Cronje H, Gonnet JM, Guillot M, Maria B, et al. Induction of labor with mifepristone (RU 486) in intrauterine fetal death. American Journal of Obstetrics and Gynecology. 1990;163:540–2. doi: 10.1016/0002-9378(90)91193-g. [DOI] [PubMed] [Google Scholar]
- Caliskan 2005 [published data only] .Caliskan E, Dilbaz S, Doger E, Ozeren S, Dilbaz B. Randomized comparison of 3 misoprostol protocols for abortion induction at 13-20 weeks of gestation. Journal of Reproductive Medicine. 2005;50(3):173–80. [PubMed] [Google Scholar]
- Caliskan 2009 [published data only] .Caliskan E, Doger E, Cakiroglu Y, Corakci A, Yucesoy I. Sublingual misoprostol 100 microgram versus 200 microgram for second trimester abortion: a randomised trial. European Journal of Contraception & Reproductive Health Care. 2009;14(1):55–60. doi: 10.1080/13625180802360865. [DOI] [PubMed] [Google Scholar]
- Chittacharoen 2003 [published data only] .Chittacharoen A, Herabutya Y, Punyavachira P. A randomized trial of oral and vaginal misoprostol to manage delivery in cases of fetal death. Obstetrics & Gynecology. 2003;101:70–3. doi: 10.1016/s0029-7844(02)02506-1. [DOI] [PubMed] [Google Scholar]
- Chung 1999 [published data only] .Chung TKH, Cheung LP, Haines CJ. Spontaneous abortion: A randomized controlled trial of misoprostol versus routine surgical evacuation. Acta Obstetricia et Gynecologica Scandinavica. 1997;76(167):72. [PubMed] [Google Scholar]; Chung TKH, Cheung LP, Lee DTS, Haines CJ. Spontaneous abortion: a randomized controlled trial of misoprostol versus routine uterine curettage. British Journal of Obstetrics and Gynaecology. 1998;105(Suppl 17):127. [Google Scholar]; Chung TKH, Lee DTC. A randomised controlled clinical trial involving women who have aborted spontaneously: the health, social and operational cost outcomes of conservative and routine management protocols. Personal communication. 1998. ; * Chung TKH, Lee DTS, Cheung LP, Haines CJ, Chang AMZ. Spontaneous abortion: a randomised controlled trial comparing surgical evacuation with conservative management using misoprostol. Fertility and Sterility. 1999;71(6):1054–9. doi: 10.1016/s0015-0282(99)00128-4. [DOI] [PubMed] [Google Scholar]; Lee DTS, Cheung LP, Haines CJ, Chan KPM, Chung TKH. A comparison of the psychologic impact and client satisfaction of surgical treatment with medical treatment of spontaneous abortion: a randomized controlled trial. American Journal of Obstetrics and Gynecology. 2001;185:953–8. doi: 10.1067/mob.2001.117661. [DOI] [PubMed] [Google Scholar]; Tam WH, Lau WC, Cheung LP, Yuen PM, Chung TK. Intrauterine adhesions after conservative and surgical management of spontaneous abortion. Journal of the American Association of Gynecologic Laparoscopists. 2002;9(2):182–5. doi: 10.1016/s1074-3804(05)60129-6. [DOI] [PubMed] [Google Scholar]; Tam WH, Tsui MH, Lok IH, Yip SK, Yuen PM, Chung TK. Long-term reproductive outcome subsequent to medical versus surgical treatment for miscarriage. Human Reproduction. 2005;20(12):3355–9. doi: 10.1093/humrep/dei257. [DOI] [PubMed] [Google Scholar]
- Creinin 1997 [published data only] .Creinin MD, Moyer R, Guido R. Misoprostol for medical evacuation of early pregnancy failure. Obstetrics & Gynecology. 1997;89:768–72. doi: 10.1016/s0029-7844(97)81438-x. [DOI] [PubMed] [Google Scholar]
- David 2003 [published data only] .David M, Chen FCK, Lichtenegger W. NO-donor nitroglycerin versus the prostaglandin gemeprost for cervical ripening in first trimester missed abortion. International Journal of Gynecology & Obstetrics. 2003;83:71–2. doi: 10.1016/s0020-7292(03)00195-4. [DOI] [PubMed] [Google Scholar]
- David 2005 [published data only] .David M, Chen FCK. Comparison of isosorbide mononitrate (Mono Mack) and misoprostol (Cytotec) for cervical ripening in the first trimester missed abortion. Archives of Gynecology and Obstetrics. 2005;273(3):144–5. doi: 10.1007/s00404-005-0014-1. [DOI] [PubMed] [Google Scholar]
- de Jonge 1995 [published data only] .de Jonge ET, Makin JD, Manefeldt E, De Wet GH, Pattinson RC. Randomised clinical trial of medical evacuation and surgical curettage for incomplete miscarriage. BMJ. 1995;311(7006):662. doi: 10.1136/bmj.311.7006.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetroulis 2001 [published data only] .* Demetroulis C, Saridogan E, Kunde D, Naftalin AA. A prospective randomized control trial comparing medical and surgical treatment for early pregnancy failure. Human Reproduction. 2001;16(2):365–9. doi: 10.1093/humrep/16.2.365. [DOI] [PubMed] [Google Scholar]; Demetroulis C, Saridogan E, Kunde D, Naftalin AA. A prospective randomized control trial comparing medical and surgical treatment for early pregnancy failure [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 3); Washington DC, USA. 2000 Sept 3-8; 2000. p. 69. [DOI] [PubMed] [Google Scholar]
- Dickinson 1998 [published data only] .Dickinson JE, Godfrey M, Evans SF. Efficacy of intravaginal misoprostol in second-trimester pregnancy termination: a randomized controlled trial. Journal of Maternal-Fetal Medicine. 1998;7:115–9. doi: 10.1002/(SICI)1520-6661(199805/06)7:3<115::AID-MFM3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dickinson 2002 [published data only] .Dickinson JE, Evans SF. The optimization of intravaginal misoprostol dosing schedules in second-trimester pregnancy termination. American Journal of Obstetrics and Gynecology. 2002;Vol. 186(issue 3):470–4. doi: 10.1067/mob.2002.121085. [DOI] [PubMed] [Google Scholar]
- Dickinson 2003 [published data only] .Dickinson JE, Evans SF. A comparison of oral misoprostol with vaginal misoprostol administration in second-trimester pregnancy termination for fetal abnormality. Obstetrics & Gynecology. 2003;101:1294–9. doi: 10.1016/s0029-7844(03)00357-0. [DOI] [PubMed] [Google Scholar]
- Egarter 1995 [published data only] .Egarter C, Lederhilger J, Kurz C, Karas H, Reisenberger K. Gemeprost for first trimester missed abortion. Archives of Gynecology & Obstetrics. 1995;256(1):29–32. doi: 10.1007/BF00634345. [DOI] [PubMed] [Google Scholar]
- Elhassan 2008 [published data only] .Elhassan EM, Abubaker MS, Adam I. Sublingual compared with oral and vaginal misoprostol for termination of pregnancy with second-trimester fetal demise. International Journal of Gynecology & Obstetrics. 2008;100(1):82–3. doi: 10.1016/j.ijgo.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Eng 1997 [published data only] .Eng NS, Guan AC. Comparative study of intravaginal misoprostol with gemeprost as an abortifacient in second trimester missed abortion. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1997;37(3):331–4. doi: 10.1111/j.1479-828x.1997.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Eppel 2005 [published data only] .Eppel W, Facchinetti F, Schleussner E, Piccinini F, Pizzi C, Gruber DM, et al. Second trimester abortion using isosorbide mononitrate in addition to gemeprost compared with gemeprost alone: a double-blind randomized, placebo-controlled multicenter trial. American Journal of Obstetrics and Gynecology. 2005;192:856–61. doi: 10.1016/j.ajog.2004.10.597. [DOI] [PubMed] [Google Scholar]
- Fadalla 2004 [published data only] .Fadalla F, Mirghani OA, Adam I. Oral misoprostol vs. vaginal misoprostol for termination of pregnancy with intrauterine fetal demise in the second-trimester. International Journal of Gynecology & Obstetrics. 2004;86:52–3. doi: 10.1016/j.ijgo.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Feldman 2003 [published data only] .Feldman DM, Borgida AF, Rodis JF, Leo MV, Campbell WA. A randomized comparison of two regimens of misoprostol for second-trimester pregnancy termination. American Journal of Obstetrics and Gynecology. 2003;Vol. 189:710–3. doi: 10.1067/s0002-9378(03)00659-8. [DOI] [PubMed] [Google Scholar]
- Fiala 2005 [published data only] .Fiala C, Swahn ML, Stephansson O, Gemzell-Danielsson K. The effect of non-steroidal anti-inflammatory drugs on medical abortion with mifepristone and misoprostol at 13-22 weeks gestation. Human Reproduction. 2005;Vol. 20(issue 11):3072–7. doi: 10.1093/humrep/dei216. [DOI] [PubMed] [Google Scholar]
- Gazvani 2000 [published data only] .Gazvani R, Templeton A. A pilot study for the use of manual vacuum aspiration in early pregnancy loss. Journal of Obstetrics and Gynaecology. 2000;20(1):105. [Google Scholar]
- Ghorab 1998 [published data only] .Ghorab MN, El Helw BA. Second-trimester termination of pregnancy by extra-amniotic prostaglandin F2alpha or endocervical misoprostol A comparative study. Acta Obstetricia et Gynecologica Scandinavica. 1998;77:429–32. [PubMed] [Google Scholar]
- Gilles 2004 [published data only] .Barnhart KT, Bader T, Huang X, Frederick MM, Timbers KA, Zhang JJ. Hormone pattern after misoprostol administration for a nonviable first-trimester gestation. Fertility and Sterility. 2004;81(4):1099–105. doi: 10.1016/j.fertnstert.2003.08.041. [DOI] [PubMed] [Google Scholar]; Creinin MD, Harwood B, Guido RS, Fox MC, Zhang J, NICHD Management of Early Pregnancy Failure Trial Endometrial thickness after misoprostol use for early pregnancy failure. International Journal of Gynecology & Obstetrics. 2004;86(1):22–6. doi: 10.1016/j.ijgo.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis AR, Robilotto CM, Westhoff CL, Forman S, Zhang J, NICHD Management of Early Pregnancy Failure Trial Bleeding patterns after vaginal misoprostol for treatment of early pregnancy failure. Human Reproduction. 2004;19(7):1655–8. doi: 10.1093/humrep/deh291. [DOI] [PubMed] [Google Scholar]; Gilles J, Creinin MM, Barnhart KT, Westhoff C, Frederick MM, Zang J. Wet versus dry intravaginal misoprostol application for treatment of early pregnancy failure [abstract] Fertility and Sterility. 2002;78(3 Suppl 1):S64–5. [Google Scholar]; * Gilles JM, Creinin MD, Barnhart K, Westhoff C, Frederick MM, Zhang J. A randomized trial of saline solution-moistened misoprostol versus dry misoprostol for first-trimester pregnancy failure. American Journal of Obstetrics and Gynecology. 2004;Vol. 190:389–94. doi: 10.1016/j.ajog.2003.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez 2001 [published data only] .Gonzalez JA, Carlan SJ, Alverson MW. Outpatient second trimester pregnancy termination. Contraception. 2001;63(2):89–93. doi: 10.1016/s0010-7824(01)00171-8. [DOI] [PubMed] [Google Scholar]
- Graziosi 2004 [published data only] .Graziosi G, Bruinse HW, Reuwer PJH, Teteringen O, Mol BJW. Fertility outcome after a randomized trial comparing curettage with misoprostol for treatment of early pregnancy failure. Human Reproduction. 2005;20:1749–50. doi: 10.1093/humrep/deh754. [DOI] [PubMed] [Google Scholar]; Graziosi GC, van der Steeg JW, Reuwer PH, Drogtrop AP, Bruinse HW, Mol BW. Economic evaluation of misoprostol in the treatment of early pregnancy failure compared to curettage after an expectant management. Human Reproduction. 2005;20(4):1067–71. doi: 10.1093/humrep/deh709. [DOI] [PubMed] [Google Scholar]; Graziosi GCM, Bruinse HW, Reuwer PJH, van Kessel PH, Westerweel PE, Mol BW. Misoprostol versus curettage in women with early pregnancy failure: impact on women’s health-related quality life. A randomized controlled trial. Human Reproduction. 2005;20(8):2340–7. doi: 10.1093/humrep/dei019. [DOI] [PubMed] [Google Scholar]; * Graziosi GCM, Mol BWJ, Reuwer PJH, Drogtrop A, Bruinse HW. Misoprostol versus curettage in women with early pregnancy failure after initial expectant management: a randomized trial. Human Reproduction. 2004;19(8):1894–9. doi: 10.1093/humrep/deh344. [DOI] [PubMed] [Google Scholar]
- Grimes 2005 [published data only] .Grimes DA, Smith MS, Witham AD. Mifepristone and misoprostol versus dilation and evacuation for midtrimester abortion: a pilot randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology. 2005;111:148–53. doi: 10.1046/j.1471-0528.2003.00044.x-i1. [DOI] [PubMed] [Google Scholar]
- Gronlund 2002 [published data only] .Gronlund A, Gronlund L, Clevin L, Andersen B, Palmgren N, Lidegaard O. Management of missed abortion: comparison of medical treatment with either mifepristone + misoprostol or misoprostol alone with surgical evacuation. A multi-center trial in Copenhagen county, Denmark. Acta Obstetricia et Gynecologica Scandinavica. 2002;81:1060–5. [PubMed] [Google Scholar]
- Guix 2005 [published data only] .Guix C, Palacio M, Figueras F, Bennasar M, Zamora L, Coll O, et al. Efficacy of two regimens of misoprostol for early second-trimester pregnancy termination. Fetal Diagnosis and Therapy. 2005;20(6):544–8. doi: 10.1159/000088048. [DOI] [PubMed] [Google Scholar]
- Hassan 2007 [published data only] .Hassan FI, Mostapha MK, Sattar MA, Marouf E, Azim SA. Oral versus rectal route of misoprostol administration: a randomized controlled trial. Middle East Fertility Society Journal. 2007;12(1):53–6. [Google Scholar]
- Hausler 1997 [published data only] .Hausler MCH, Koroschetz F, Tamussino K, Walcher W. Is a curettage after spontaneous abortion still relevant? [Ist eine currettage nach abortus completus noch zeitgemals?] Geburtshilfe und Frauenheilkunde. 1997;57:396–9. [Google Scholar]
- Heard 2002 [published data only] .Heard MJ, Stewart GM, Buster JE, Carson SA, Miller HJ. Outpatient management of missed abortion with vaginal misoprostol [abstract] Obstetrics & Gynecology. 2002;99(4 Suppl):20S. [Google Scholar]
- Herabutya 1997a [published data only] .Herabutya Y, O-Prasertsawat P. Misoprostol in the management of missed abortion. International Journal of Gynecology & Obstetrics. 1997;56(3):263–6. doi: 10.1016/s0020-7292(96)02815-9. [DOI] [PubMed] [Google Scholar]
- Herabutya 1997b [published data only] .Herabutya Y, O-Prasertsawat P. A comparison of intravaginal misoprostol with intracervical prostaglandin E2 gel for the management of dead fetus in utero. Thai Journal of Obstetrics and Gynaecology. 1997;Vol. 9(issue 2):95–8. [Google Scholar]
- Herabutya 2005 [published data only] .Herabutya Y, Chanrachakul B, Punyavachira P. A randomised controlled trial of 6 and 12 hourly administration of vaginal misoprostol for second trimester pregnancy termination. BJOG: an international journal of obstetrics and gynaecology. 2005;112(9):1297–301. doi: 10.1111/j.1471-0528.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Valencia 2003 [published data only] .Hernandez-Valencia M. Cervical ripening with prostaglandin E1: how an ambulatory method decreases the hospital stay in abortus with intrauterine fetal demise. Fetal Diagnosis & Therapy. 2003;18:54–8. doi: 10.1159/000066386. [DOI] [PubMed] [Google Scholar]
- Hidar 2001 [published data only] .Hidar S, Fekih M, Chaieb A, Bibi M, Mellouli R, Khairi H. Oxytocin and misoprostol administered intravaginally for termination of pregnancy at 13 to 29 weeks of amenorrhea A prospective randomized trial [Apport de l’association d’ocytocine au misoprostol administre en intravaginal au cours des interruptions de grossesses entre 13 et 29 semaines d’amenorrhee. Essai clinique prospectif randomise] Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 2001;30(5):439–43. [PubMed] [Google Scholar]
- Hidar 2005 [published data only] .Hidar S, Bouddebous M, Chaieb A, Jerbi M, Bibi M, Khairi H. Randomized controlled trial of vaginal misoprostol versus vaginal misoprostol and isosorbide dinitrate for termination of pregnancy at 13-29 weeks. Archives of Gynecology and Obstetrics. 2005;273(3):157–60. doi: 10.1007/s00404-005-0053-7. [DOI] [PubMed] [Google Scholar]
- Hill 1991 [published data only] .Hill NCW, Selinger M, Ferguson J, MacKenzie IZ. Management of intra-uterine fetal death with vaginal administration of gemeprost or prostaglandin E2: a random allocation controlled trial. Journal of Obstetrics and Gynaecology. 1991;11:422–6. [Google Scholar]
- Hinshaw 1995 [published data only] .Hinshaw K, Rispin R, Henshaw R, Smith N, Templeton A. Medical versus surgical uterine evacuation in first trimester miscarriage: a prospective, pragmatic randomised trial. 27th British Congress of Obstetrics and Gynaecology; Dublin, Ireland. 1995 July 4-7.1995. p. 4. [Google Scholar]
- Hinshaw 1997 [published data only] .Davis AR, Hendlish SK, Westhoff C, Frederick MM, Zhang J, Gilles JM, et al. Bleeding patterns after misoprostol vs surgical treatment of early pregnancy failure: results from a randomized trial. American Journal of Obstetrics and Gynecology. 2007;196(1):31. doi: 10.1016/j.ajog.2006.07.053. [DOI] [PubMed] [Google Scholar]; Henshaw RC, Hinshaw K, Smith NC, Templeton AA. Fertility Society of Australia/Australasian Gynaecological Endoscopy Society; Melbourne, Australia: Nov 19-25, 1995. The medical management of miscarriage. [Google Scholar]; * Hinshaw HKS. Medical management of miscarriage. In: Grundzinkas JG, editor. Problems in early pregnancy -advances in diagnosis and management. RCOG; London: 1997. [Google Scholar]; Hinshaw K, Rispin R, Smith N, Templeton A. Medical vs surgical management in first trimester miscarriage: a prospective, pragmatic random allocation trial. Journal of Obstetrics and Gynaecology. 1993;13(5):404–5. [Google Scholar]; Rispin R, Hinshaw K, Henshaw R, Smith N, Templeton A. New aspects of care in the management of miscarriage. Proceedings of Research in Midwifery Conference; Birmingham, UK. 1993 Sept 14.1993. [Google Scholar]
- Hogg 2000 [published data only] .Hogg B, Owen J. Laminaria versus extraamniotic saline infusion (EASI) for cervical ripening and mid-trimester labor induction [abstract] American Journal of Obstetrics and Gynecology. 2000;182(1 Pt 2):S135. doi: 10.1067/mob.2001.112903. [DOI] [PubMed] [Google Scholar]
- Islam 2006 [published data only] .Islam A, Abbasi AN, Sarwar I. Use of Foley’s catheter and prostaglandin F-2 alpha in second trimester termination of pregnancy. Journal of Ayub Medical College, Abbottabad. 2006;18(3):35–9. [PubMed] [Google Scholar]
- Jain 1994 [published data only] .Jain JK, Mishell DR. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of second-trimester pregnancy. New England Journal of Medicine. 1994;331:290–3. doi: 10.1056/NEJM199408043310502. [DOI] [PubMed] [Google Scholar]
- Jain 1996 [published data only] .Jain JK, Mishell DR. A comparison of misoprostol with and without laminaria tents for induction of second-trimester abortion. American Journal of Obstetrics and Gynecology. 1996;175:173–7. doi: 10.1016/s0002-9378(96)70270-3. [DOI] [PubMed] [Google Scholar]
- Jain 1999 [published data only] .Jain JK, Mishell DR. A comparison of two dosing regimens of intravaginal misoprostol for second-trimester pregnancy termination. Obstetrics & Gynecology. 1999;93:571–5. doi: 10.1016/s0029-7844(98)00485-2. [DOI] [PubMed] [Google Scholar]
- Johnson 1997 [published data only] .Johnson N, Priestnall M, Marsay T, Ballard P, Watters J. A randomised trial evaluating pain and bleeding after a first trimester miscarriage treated surgically or medically. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1997;72(2):213–5. doi: 10.1016/s0301-2115(96)02668-1. [DOI] [PubMed] [Google Scholar]
- Kanhai 1989 [published data only] .* Kanhai HHH, Keirse MJNC. Induction of labour after fetal death: a randomized controlled trial of two prostaglandin regimens. British Journal of Obstetrics and Gynaecology. 1989;96:1400–4. doi: 10.1111/j.1471-0528.1989.tb06302.x. [DOI] [PubMed] [Google Scholar]; Kanhai HHH, Keirse MJNC. Intravenous administration of sulfprostone for the induction of labour after fetal death: a randomized comparison of two dose schedules. 12th FIGO World Congress of Gynecology and Obstetrics; Brazil. 1988 October 23-28.1988. pp. 201–2. [Google Scholar]; Kanhai HHH, Keirse MJNC. Intravenous administration of sulprostone for the induction of labour after fetal death: a randomized comparison of two dose schedules. Proceedings of 1st European Congress on Prostaglandins in Reproduction; Vienna, Austria. 1988 July 6-9.1988. p. 45. [Google Scholar]
- Kapp 2007 [published data only] .Kapp N, Todd CS, Yadgarova KT, Alibayeva G, Nazarova D, Loza O, et al. A randomized comparison of misoprostol to intrauterine instillation of hypertonic saline plus a prostaglandin F2alpha analogue for second-trimester induction termination in Uzbekistan. Contraception. 2007;76(6):461–6. doi: 10.1016/j.contraception.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kara 1999 [published data only] .Kara M, Ozden S, Eroglu M, Cetin A, Arioglu P. Comparison of misoprostol and dinoproston administration for the induction of labour in second trimester pregnancies in cases of intrauterine fetal loss. Italian Journal of Gynaecology & Obstetrics. 1999;11:13–6. [Google Scholar]
- Kovavisarach 2002 [published data only] .Kovavisarach E, Sathapanachai U. Intravaginal 400 micrograms misoprostol for pregnancy termination in cases of blighted ovum: a randomised controlled trial. Australian & New Zealand Journal of Obstetrics and Gynaecology. 2002;42(2):161–3. doi: 10.1111/j.0004-8666.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- Kovavisarach 2005 [published data only] .Kovavisarach E, Jamnansiri C. Intravaginal misoprostol 600ug and 800ug for the treatment of early pregnancy failure. International Journal of Gynecology & Obstetrics. 2005;90(3):208–12. doi: 10.1016/j.ijgo.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Kushwah 2009 [published data only] .Kushwah B, Singh A. Sublingual versus oral misoprostol for uterine evacuation following early pregnancy failure. International Journal of Gynecology & Obstetrics. 2009;106(1):43–5. doi: 10.1016/j.ijgo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Lelaidier 1993 [published data only] .Lelaidier C, Baton-Saint-Mleux C, Fernandez H, Bourget P, Frydman R. Mifepristone (RU486) induces embryo expulsion in first trimester non-developing pregnancies: a prospective randomized trial. Human Reproduction. 1993;8(3):492–5. doi: 10.1093/oxfordjournals.humrep.a138078. [DOI] [PubMed] [Google Scholar]
- Lippert 1978 [published data only] .Lippert TH, Luthi A. Induction of labour with prostaglandin E2 gel in cases of intrauterine fetal death. Prostaglandins. 1978;15:533–42. doi: 10.1016/0090-6980(78)90137-5. [DOI] [PubMed] [Google Scholar]
- Lister 2005 [published data only] .Lister MS, Shaffer LET, Bell JG, Lutter KQ, Moorma KH. Randomized, double-blind, placebo-controlled trial of vaginal misoprostol for management of early pregnancy failures. American Journal of Obstetrics and Gynecology. 2005;193:1338–43. doi: 10.1016/j.ajog.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Louey 2000 [published data only] .Louey K. Misoprostol for the medical management of miscarriage (ongoing trial) Personal communication. 2000.
- Lughmani 2007 [published data only] .Lughmani ST. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of 1st trimester pregnancy. Double blind randomized trial [abstract]. 31st British International Congress of Obstetrics and Gynaecology; London, UK. 2007 July 4-6.2007. p. 209. [Google Scholar]
- Lughmani 2008 [published data only] .Lughmani ST. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of 1st trimester pregnancy. BJOG: an international journal of obstetrics and gynaecology. 2008;115(s1):179. [Google Scholar]
- Machtinger 2004 [published data only] .Machtinger R, Stockheim D, Goldenberg M, Soriano D, Atlas M, Seidman DS. A randomized prospective study of misoprostol alone or combined with mifepristone for treatment of first trimester spontaneous abortion [abstract] Fertility and Sterility. 2002;Vol. 78(issue 3 Suppl 1):S64. [Google Scholar]; Machtinger R, Stockheim D, Shulman A, Dulitzki M, Schiff E, Seidman DS. A randomized prospective study comparing the effectiveness of four protocols for treatment of first trimester spontaneous abortion [abstract] Fertility and Sterility. 2004;82(Suppl 2):S80. [Google Scholar]
- Makhlouf 2003 [published data only] .Makhlouf AM, Al-Hussaini TK, Habib DM, Makarem MH. Second-trimester pregnancy termination: comparison of three different methods. Journal of Obstetrics and Gynaecology. 2003;23:407–11. doi: 10.1080/0144361031000120923. [DOI] [PubMed] [Google Scholar]
- Martin 1955 [published data only] .Martin RH, Menzies DN. Oestrogen therapy in missed abortion and labour. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1955;62:256–8. doi: 10.1111/j.1471-0528.1955.tb14129.x. [DOI] [PubMed] [Google Scholar]
- Muffley 2002 [published data only] .Muffley PE, Stitely ML, Gherman RB. Early intrauterine pregnancy failure: a randomized trial of medical versus surgical treatment. American Journal of Obstetrics and Gynecology. 2002;187:321–6. doi: 10.1067/mob.2002.126205. [DOI] [PubMed] [Google Scholar]
- Mulayim 2009 [published data only] .Mulayim B, Celik NY, Onalan G, Zeyneloglu HB, Kuscu E. Sublingual misoprostol after surgical management of early termination of pregnancy. Fertility & Sterility. 2009;92(2):678–81. doi: 10.1016/j.fertnstert.2008.07.1706. [DOI] [PubMed] [Google Scholar]
- Nakintu 2001 [published data only] .Nakintu N. A comparative study of vaginal misoprostol and intravenous oxytocin for induction of labour in women with intra uterine fetal death in Mulago Hospital, Uganda. African Health Sciences. 2001;1(2):55–9. [PMC free article] [PubMed] [Google Scholar]
- Nassar 2006 [published data only] .Nassar AH. [accessed 21 March 2006];A randomized trial of two regimens of misoprostol for second trimester termination for intrauterine fetal death (ongoing trial) ClinicalTrials.gov. www.clinicaltrials.gov.
- Ngai 2001 [published data only] .Ngai SW, Chan YM, Tang OS, Ho PC. Vaginal misoprostol as medical treatment for first trimester spontaneous miscarriage. Human Reproduction. 2001;16(7):1493–6. doi: 10.1093/humrep/16.7.1493. [DOI] [PubMed] [Google Scholar]
- Ngoc 2004 [published data only] .Ngoc NTN, Blum J, Westheimer E, Quan TV, Winikoff B. Medical treatment of missed abortion using misoprostol. International Journal of Gynecology & Obstetrics. 2004;87:138–42. doi: 10.1016/j.ijgo.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Nielsen 1999 [published data only] .Nielsen S, Hahlin M, Platz-Christensen J. Expectant management or pharmacological treatment for first trimester spontaneous abortion: a randomised trial. Acta Obstetricia et Gynecologica Scandinavica. 1997;Vol. 76(issue 167):77. [Google Scholar]; * Nielsen S, Hahlin M, Platz-Christensen J. Randomised trial comparing expectant with medical management for first trimester miscarriages. British Journal of Obstetrics and Gynaecology. 1999;106(8):804–7. doi: 10.1111/j.1471-0528.1999.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Niromanesh 2005 [published data only] .Niromanesh S, Hashemi-Fesharaki M, Mosavi-Jarrahi A. Second trimester abortion using intravaginal misoprostol. International Journal of Gynecology & Obstetrics. 2005;89:276–7. doi: 10.1016/j.ijgo.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Nor Azlin 2006 [published data only] .Nor Azlin MI, Abdullah HS, Zainul Rashid MR, Jamil MA. Misoprostol (alone) in second trimester terminations of pregnancy: as effective as Gemeprost? Journal of Obstetrics and Gynaecology. 2006;26(6):546–9. doi: 10.1080/01443610600811383. [DOI] [PubMed] [Google Scholar]
- Nuthalapaty 2005 [published data only] .Nuthalapaty F, Ramsey P, Biggio J, Owen J. Comparative efficacy of high dose vaginal misoprostol for mid-trimester labor induction [abstract] American Journal of Obstetrics and Gynecology. 2004;191(6 Suppl 1):S73. [Google Scholar]; * Nuthalapaty FS, Ramsey PS, Biggio JR, Owen JO. High-dose vaginal misoprostol versus concentrated oxytocin plus low-dose vaginal misoprostol for midtrimester labor induction: a randomized trial. American Journal of Obstetrics and Gynecology. 2005;193:1065–70. doi: 10.1016/j.ajog.2005.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutila 1997 [published data only] .Nuutila M, Toivonen J, Ylikorkala O, Halmesmaki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second-trimester abortion. Obstetrics & Gynecology. 1997;90(6):896–900. doi: 10.1016/s0029-7844(97)00491-2. [DOI] [PubMed] [Google Scholar]
- Owen 1999 [published data only] .Owen J, Hauth JC. Vaginal misoprostol vs. concentrated oxytocin plus low-dose prostaglandin E2 for second trimester pregnancy termination. Journal of Maternal-Fetal Medicine. 1999;8(2):48–50. doi: 10.1002/(SICI)1520-6661(199903/04)8:2<48::AID-MFM3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Paraskevaides 1992 [published data only] .Paraskevaides E, Prendiville W, Stuart B, Scanaill SN, Walsh D, McGuinness N, et al. Medical evacuation of first trimester (twelve weeks gestation) incomplete abortion and missed abortion. Journal of Gynecologic Surgery. 1992;8(3):159–63. [Google Scholar]
- Perry 1999 [published data only] .Perry KG, Rinehart BK, Terrone DA, Martin RW, May WL, Roberts WE. Second-trimester uterine evacuation: a comparison of intra-amniotic (15S)-15-methylprostaglandin F2alpha and intravaginal misoprostol. American Journal of Obstetrics and Gynecology. 1999;181:1057–61. doi: 10.1016/s0002-9378(99)70081-5. [DOI] [PubMed] [Google Scholar]
- Piotrowski 1979 [published data only] .Piotrowski J, Basta A, Klimczyk K, Malolepazy A, Dluzniewska M, Splawinski JA. Indomethacin increases abortifacient effect of PGE2 in man. Prostaglandins. 1979;17:451–9. doi: 10.1016/s0090-6980(79)80013-1. [DOI] [PubMed] [Google Scholar]
- Pongsatha 2004 [published data only] .Pongsatha S, Tongsong T. Intravaginal misoprostol for pregnancy termination. International Journal of Gynecology & Obstetrics. 2004;87:176–7. doi: 10.1016/j.ijgo.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Ramsey 2004 [published data only] .Ramsey PS, Savage K, Lincoln T, Owen J. Vaginal misoprostol versus concentrated oxytocin and vaginal PGE2 for second-trimester labor induction. Obstetrics & Gynecology. 2004;104(1):138–45. doi: 10.1097/01.AOG.0000128947.31887.94. [DOI] [PubMed] [Google Scholar]
- Rita 2006 [published data only] .Rita Gupta S, Kumar S. A randomised comparison of oral and vaginal misoprostol for medical management of first trimester missed abortion. JK Science. 2006;8(1):35–8. [Google Scholar]
- Rivero-Lopez 1998 [published data only] .Rivero-Lopez E, Marquez-Maraver F, Duenas-Diez JL, Cabezas-Sanchez B. Deferred miscarriage: effectiveness of intravaginal misoprostol versus laminaria stems [Aborto diferido: eficacia del misoprostol intravaginal versus la aplicación de tallos de laminaria] Progresos de Obstetricia y Ginecologia. 1998;41:579–81. [Google Scholar]
- Rosenberg 2006 [published data only] .Rosenberg P. [accessed 21 March 2006];Expectant versus immediate medical management for the evacuation of the nonevolutives pregnancies before 13 GW (ongoing trial) ClinicalTrials.gov. www.clinicaltrials.gov.
- Roy 2003 [published data only] .Roy G, Ferreira E, Hudon L, Marquette G. The efficacy of oral versus vaginal misoprostol for second-trimester termination of pregnancy: a double-blind, randomized, placebo controlled trial [abstract] American Journal of Obstetrics and Gynecology. 2003;189(6 Suppl 1):S70. [Google Scholar]
- Ruangchainikhom 2006 [published data only] .Ruangchainikhom W, Phongphissanou E, Bhekasuta J, Sarapak S. Effectiveness of 400 or 600 micrograms of vaginal misoprostol for terminations of early pregnancies. Journal of the Medical Association of Thailand. 2006;89(7):928–33. [PubMed] [Google Scholar]
- Saichua 2009 [published data only] .Saichua C, Phupong V. A randomized controlled trial comparing powdery sublingual misoprostol and sublingual misoprostol tablet for management of embryonic death or anembryonic pregnancy. Archives of Gynecology and Obstetrics. 2009;280(3):431–5. doi: 10.1007/s00404-009-0947-x. [DOI] [PubMed] [Google Scholar]
- Salamalekis 1990 [published data only] .Salamalekis E, Loghis C, Kassanos D, Traka A, Zourlas PA. Comparison of extra-amniotic prostaglandin F2alpha and dinoprostone use for labor induction after second trimester intrauterine fetal death. Proceedings of 12th European Congress of Perinatal Medicine; Lyon, France. 1990 Sept 11-14.1990. p. 228. [Google Scholar]
- Sathapanachai 2000 [published data only] .Sathapanachai U. Intravaginal 400 micrograms misoprostol for pregnancy termination in cases of blighted ovum. Thai Journal of Obstetrics and Gynaecology. 2000;12(4):363. doi: 10.1111/j.0004-8666.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- Shobeira 2007 [published data only] .Shobeira JM, Atashkhoii S. Second trimester pregnancy termination by intravaginal and parenteral form of prostaglandin E2 [abstract]. 31st British International Congress of Obstetrics and Gynaecology; London, UK. 2007 July 4-6.2007. p. 210. [Google Scholar]
- Stockheim 2006 [published data only] .Stockheim D, Machtinger R, Wiser A, Dulitzky M, Soriano D, Goldenberg M, et al. A randomized prospective study of misoprostol or mifepristone followed by misoprostol when needed for the treatment of women with early pregnancy failure. Fertility and Sterility. 2006;Vol. 86(issue 4):956–60. doi: 10.1016/j.fertnstert.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Su 2005 [published data only] .Su L-L, Biswas A, Choolani M, Kalaichelvan V, Singh K. A prospective, randomized comparison of vaginal misoprostol versus intra-amniotic prostaglandins for midtrimester termination of pregnancy. American Journal of Obstetrics and Gynecology. 2005;193:1410–4. doi: 10.1016/j.ajog.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Suchonwanit 1999 [published data only] .Suchonwanit P. Comparative study between vaginal misoprostol 200 mg and 400 mg in first trimester intrauterine fetal death and anembryonic gestation. Thai Journal of Obstetrics and Gynaecology. 1999;11(4):263. [Google Scholar]
- Surita 1997 [published data only] .Surita FGC. Misoprostol versus laminaria for cervical ripening in intrauterine fetal death. Acta Obstetricia et Gynecologica Scandinavica Supplement. 1997;76(167:2):32. [Google Scholar]
- Tang 2003 [published data only] .Tang OS, Lau WNT, Ng EHY, Lee SWH, Ho PCH. A prospective randomized study to compare the use of repeated doses of vaginal with sublingual misoprostol in the management of first trimester silent miscarriage. Human Reproduction. 2003;18(1):176–81. doi: 10.1093/humrep/deg013. [DOI] [PubMed] [Google Scholar]
- Tang 2006a [published data only] .Tang OS, Ong CY, Tse KY, Ng EH, Lee SW, Ho PC. A randomized trial to compare the use of sublingual misoprostol with or without an additional 1 week course for the management of first trimester silent miscarriage. Human Reproduction. 2006;21(1):189–92. doi: 10.1093/humrep/dei303. [DOI] [PubMed] [Google Scholar]
- Thavarasah 1986 [published data only] .Thavarasah AS, Almohdzar SA. Prostaglandin (F2 alpha) in missed abortion Intravenous, extra-amniotic and intramuscular administration--a randomized study. Biological Research in Pregnancy and Perinatology. 1986;7:106–10. [PubMed] [Google Scholar]
- Toppozada 1994 [published data only] .Toppozada MK, Shaala SA, Anwar MY, Haiba NA, Abdrabbo S, El-Absy HM. Termination of pregnancy with fetal death in the second and third trimesters - the double balloon versus extra-amniotic prostaglandin. International Journal of Gynecology & Obstetrics. 1994;45:269–73. doi: 10.1016/0020-7292(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Wood 2002 [published data only] .Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstetrics & Gynecology. 2002;99(4):563–6. doi: 10.1016/s0029-7844(01)01765-3. [DOI] [PubMed] [Google Scholar]
- Yapar 1996 [published data only] .Yapar EG, Senoz S, Urkutur M, Batioglu S, Gokmen O. Second trimester pregnancy termination including fetal death: comparison of five different methods. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1996;69:97–102. doi: 10.1016/0301-2115(95)02548-0. [DOI] [PubMed] [Google Scholar]
- Yilmaz 2005 [published data only] .Yilmaz B, Kelekci S, Ertas IE, Kahyaoglu S, Ozel M, Sut N, et al. Misoprostol moistened with acetic acid or saline for second trimester pregnancy termination: a randomized prospective double-blind trial. Human Reproduction. 2005;20(11):3067–71. doi: 10.1093/humrep/dei204. [DOI] [PubMed] [Google Scholar]
- Yilmaz 2007 [published data only] .Yilmaz B, Kelekci S, Ertas IE, Ozel M, Sut N, Mollamahmutoglu L, et al. Randomized comparison of second trimester pregnancy termination utilizing saline moistened or dry misoprostol. Archives of Gynecology and Obstetrics. 2007;276(5):511–6. doi: 10.1007/s00404-007-0374-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Altaf 2006 [published data only] .Altaf F, Sultana N, Iqbal N. Therapeutic abortions; efficacy of intra-vaginal misoprostol in comparison to extra amniotically administered prostaglandin f2a. Professional Medical Journal. 2006;13(3):417–22. [Google Scholar]
- Anderson 2009 [published data only] .Anderson J, Gouk E, Young L, Turnbull L, Sayeed G, Elattar A, et al. A randomised controlled trial of oral versus vaginal misoprostol for medical management of early fetal demise. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S533. [Google Scholar]
- Ara 2009 [published data only] .Ara G, Nargis S, Khatun R, Saha A. Vaginal misoprostol as a medical management in early pregnancy loss. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S533–4. [Google Scholar]
- Arellano 2009 [published data only] .Arellano M, Durocher J, Leon W, Montesinos R, Pena M, Winikoff B. Introduction of misoprostol for incomplete abortion care in Latin Amerca: evidence from Ecuador. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S49. [Google Scholar]
- Azra 2007 [published data only] .Azra B, Shakeel S, Nilofer M. A comparison of two protocols of intra vaginal misoprostol for second trimester medical termination of pregnancy. Pakistan Armed Forces Medical Journal. 2007;57(1):61–5. [Google Scholar]
- Bagratee 2009 [published data only] .Bagratee J, Regan L, Khullar V, Moodley J, Connolly C. Does the volume of retained products of conception and hormonal parameters influence the success of conservative methods of management of first trimester miscarriage? International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S116. [Google Scholar]
- Behrashi 2010 [published data only] .Behrashi M. [accessed 6 December 2010];Comparison between the oral and vaginal misoprostol effects on pregnancy termination in second. IRCT Iranian Registry of Clinical Trials. 2010 www.irct.ir.
- Ben-Meir 2009 [published data only] .Ben-Meir A, Erez Y, Feigenberg T, Hamani Y, Laufer N, Rojansky N. Mifepristone followed by high-dose oxytocin drip for second-trimester abortion: a randomized, double-blind, placebo-controlled, pilot study. Journal of Reproductive Medicine. 2009;54(8):511–6. [PubMed] [Google Scholar]
- Dabash 2009 [published data only] .Dabash R, Cherine M, Darwish E, Blum J, Hassanein N, Abdel Daiem T, et al. Bleeding following surgical (MVA) and medical (400 ug sublingual misoprostol) treatment of incomplete abortion. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S150–1. [Google Scholar]
- Dabash 2010 [published data only] .Dabash R, Ramadan MC, Darwish E, Hassanein N, Blum J, Winikoff B. A randomized controlled trial of 400-mug sublingual misoprostol versus manual vacuum aspiration for the treatment of incomplete abortion in two Egyptian hospitals. International Journal of Gynecology & Obstetrics. 2010;111(2):131–5. doi: 10.1016/j.ijgo.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Diop 2009 [published data only] .Diop A, Raghavan S, Rakotovao JP, Comendant R, Blumenthal PD, Winikoff B. Two routes of administration for misoprostol in the treatment of incomplete abortion: a randomized clinical trial. Contraception. 2009;79(6):456–62. doi: 10.1016/j.contraception.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Fang 2009 [published data only] .Fang AH, Chen QF, Zheng W, Li YH, Chen RY. Termination of missed abortion in a combined procedure: a randomized controlled trial. Journal of Reproduction and Contraception. 2009;20(1):45–9. [Google Scholar]
- Hughes 1996 [published data only] .Hughes J, Ryan M, Hinshaw K, Henshaw R, Rispin R, Templeton A. The costs of treating miscarriage: a comparison of medical and surgical management. British Journal of Obstetrics and Gynaecology. 1996;103(12):1217–21. doi: 10.1111/j.1471-0528.1996.tb09632.x. [DOI] [PubMed] [Google Scholar]
- Jabir 2009 [published data only] .Jabir M, Smeet RI. Comparison of oral and vaginal misoprostol for cervical ripening before evacuation of first trimester missed miscarriage. Saudi Medical Journal. 2009;30(1):82–7. [PubMed] [Google Scholar]
- Jabir 2009a [published data only] .Jabir M, Smeet R. Comparison of oral and vaginal misoprostol for cervical ripening before evacuation of first trimester missed miscarriage. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S209. [PubMed] [Google Scholar]
- Kushwah 2011 [published data only] .Kushwah DS, Kushwah B, Salman MT, Verma VK. Acceptability and safety profile of oral and sublingual misoprostol for uterine evacuation following early fetal demise. Indian Journal of Pharmacology. 2011;43(3):306–10. doi: 10.4103/0253-7613.81513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos 2011 [published data only] .Montesinos R, Durocher J, Leon W, Arellano M, Pena M, Pinto E, et al. Oral misoprostol for the management of incomplete abortion in Ecuador. International Journal of Gynecology & Obstetrics. 2011;115(2):135–9. doi: 10.1016/j.ijgo.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Moran 2005 [published data only] .Moran T, Deutsch R. Methotrexate/misoprostol vs. a more standard approach for termination of pregnancies of undetermined location: a randomized, controlled trial. Journal of Reproductive Medicine. 2005;50(10):784–92. [PubMed] [Google Scholar]
- Mostafa-Gharebaghi 2010 [published data only] .Mostafa-Gharebaghi P, Mansourfar M, Sadeghi-Bazargani H. Low dose vaginal misoprostol versus prostaglandin E2 suppository for early uterine evacuation: a randomized clinical trial. Pakistan Journal of Biological Sciences. 2010;13(19):946–50. doi: 10.3923/pjbs.2010.946.950. [DOI] [PubMed] [Google Scholar]
- Nasreen 2009 [published data only] .Nasreen Z. What for early pregnancy failure manual vacuum aspiration (MVA) with small dose misoprostol or misoprostol alone? International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S539–40. [Google Scholar]
- Paritakul 2010 [published data only] .Paritakul P, Phupong V. Comparative study between oral and sublingual 600 microg misoprostol for the treatment of incomplete abortion. Journal of Obstetrics and Gynaecology Research. 2010;36(5):978–83. doi: 10.1111/j.1447-0756.2010.01264.x. [DOI] [PubMed] [Google Scholar]
- Ramadan 2009 [published data only] .Ramadan MC. Misoprostol versus MVA for incomplete abortion: results from a randomized controlled trial in Egypt. International Journal of Gynecology & Obstetrics. 2009;107(Suppl 2):S68–9. [Google Scholar]
- Rausch 2012 [published data only] .Rausch M, Lorch S, Chung K, Frederick M, Zhang J, Barnhart K. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertility and Sterility. 2012;97(2):355–60. doi: 10.1016/j.fertnstert.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah 2010 [published data only] .Shah N, Azam SI, Khan NH. Sublingual versus vaginal misoprostol in the management of missed miscarriage. JPMA - Journal of the Pakistan Medical Association. 2010;60(2):113–6. [PubMed] [Google Scholar]
- Shaikh 2008 [published data only] .Shaikh ZAN. Comparison between misoprostol alone and misoprostol with manual vacuum aspiration for the treatment of missed and incomplete miscarriage. BJOG: an international journal of obstetrics and gynaecology. 2008;115(s1):83. [Google Scholar]
- Shokry 2009 [published data only] .Shokry M, Shahin AY, Fathalla MM, Shaaban OM. Oral misoprostol reduces vaginal bleeding following surgical evacuation for first trimester spontaneous abortion. International Journal of Gynecology & Obstetrics. 2009;107(2):117–20. doi: 10.1016/j.ijgo.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sripramote 2000 [published data only] .Sripramote M, Chatsuphang W. A randomized comparison of oral and vaginal misoprostol for cervical priming before uterine curettage in the first trimester of pregnancy. Vajira Medical Journal. 2000;44(3):207–15. [Google Scholar]
- Tanha 2010 [published data only] .Tanha FD. [accessed 6 December 2010];Comparison of the efficacy of two routes of misoprostol administration (sublingual and vaginal) for termination of second trimester pregnancy. IRCT Iranian Registry of Clinical Trials. www.irct.ir.
- Tanha 2010a [published data only] .Tanha FD, Feizi M, Shariat M. Sublingual versus vaginal misoprostol for the management of missed abortion. Journal of Obstetrics and Gynaecology Research. 2010;36(3):525–32. doi: 10.1111/j.1447-0756.2010.01229.x. [DOI] [PubMed] [Google Scholar]
- Taylor 2011 [published data only] .Taylor J, Diop A, Blum J, Dolo O, Winikoff B. Oral misoprostol as an alternative to surgical management for incomplete abortion in Ghana. International Journal of Gynecology & Obstetrics. 2011;112(1):40–4. doi: 10.1016/j.ijgo.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Torre 2012 [published data only] .Torre A, Huchon C, Bussieres L, Machevin E, Camus E, Fauconnier A. Immediate versus delayed medical treatment for first-trimester miscarriage: a randomized trial. American Journal of Obstetrics & Gynecology. 2012;206(3):215.e1–6. doi: 10.1016/j.ajog.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Yu 2000 [published data only] .Yu XH. Clinical observation of 42 cases of mifepristone and Misoprostol in missed abortion. Journal of Chinese Medical Writing. 2000;7(5):552–3. [Google Scholar]
- Zanganeh 2010 [published data only] .Zanganeh M. [accessed 6 December 2010];Comparing the effects of multiple doses of misoprostol with single dose of misoprostol plus oxitocin in induction of second trimester abortion. IRCT Iranian Registry of Clinical Trials. www.irct.ir.
- Zhang 2000 [published data only] .Zhang C, Cheng W. A contrastive analysis of the efficacy of misoprostol and li fan nuo in intermediate term of pregnancy. Journal of Wuhan University of Science and Technology (Natural Science Edition) 2000;23(4):409–11. [Google Scholar]
References to ongoing studies
- Unkels 2008 [published data only] .Unkels R. [accessed 9 April 2008];Is misoprostol a safe alternative to manual vacuum aspiration in women with early pregnancy failure in a low resource setting?: a randomized controlled trial. http://www.controlled-trials.com/ISRCTN65305620/
Additional references
- Aleman 2005 .Aleman A, Althabe F, Belizán J, Bergel E. Bed rest during pregnancy for preventing miscarriage. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD003576.pub2. [DOI: 10.1002/14651858.CD003576.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfirevic 2006 .Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD001338.pub2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
- Ankum 2001 .Ankum WM, Wieringa-de Waard M, Bindels PJE. Management of spontaneous miscarriage in the first trimester: an example of putting informed shared decision making into practice. BMJ. 2001;322:1343–6. doi: 10.1136/bmj.322.7298.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur 2007 .Arthur P, Taggart MJ, Mitchell BF. Oxytocin and parturition: a role for increased myometrial calcium and calcium sensitization? Frontiers in Bioscience. 2007;12:619–33. doi: 10.2741/2087. [DOI] [PubMed] [Google Scholar]
- Bamigboye 2003 .Bamigboye AA, Morris J. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database of Systematic Reviews. 2003;(Issue 3) doi: 10.1002/14651858.CD004353. [DOI: 10.1002/14651858.CD004353] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung 1995 .Chung TKH, Cheung LP, Leung TY, Haines CJ, Chang AMZ. Misoprostol in the management of spontaneous abortion. British Journal of Obstetrics and Gynaecology. 1995;102:832–5. doi: 10.1111/j.1471-0528.1995.tb10852.x. [DOI] [PubMed] [Google Scholar]
- Costa 1993 .Costa SH, Vessey MP. Misoprostol and illegal abortion in Rio de Janeiro, Brazil. Lancet. 1993;341:1258–61. doi: 10.1016/0140-6736(93)91156-g. [DOI] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books; London: 2001. [Google Scholar]
- Dodd 2004 .Dodd JM, Crowther CA. Misoprostol for induction of labour to terminate pregnancy in the second or third trimester for women with a fetal anomaly or after intrauterine fetal death. Cochrane Database of Systematic Reviews. 2004;(Issue 3) doi: 10.1002/14651858.CD004901.pub2. [DOI: 10.1002/14651858.CD004901] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggar 1997 .Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson 2005 .Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD002859.pub2. [DOI: 10.1002/14651858.CD002859.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca 1991 .Fonseca W, Alencar AJC, Mota FSB, Coelho HLL. Misoprostol and congenital malformations. Lancet. 1991;338:56. doi: 10.1016/0140-6736(91)90046-r. [DOI] [PubMed] [Google Scholar]
- Forna 2001 .Forna F, Gülmezoglu AM. Surgical procedures to evacuate incomplete miscarriage. Cochrane Database of Systematic Reviews. 2001;(Issue 1) doi: 10.1002/14651858.CD001993. [DOI: 10.1002/14651858.CD001993] [DOI] [PubMed] [Google Scholar]
- Gates 2005 .Gates S. Methodological Guidelines. Issue 2. the Editorial Team. Pregnancy and Childbirth Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)); 2005. [Google Scholar]
- Gülmezoglu 2007 .Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews. 2007;(Issue 3) [DOI: 10.1002/14651858.CD000494.pub3] [Google Scholar]
- Haas 2008 .Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews. 2008;(Issue 2) doi: 10.1002/14651858.CD003511.pub2. [DOI: 10.1002/14651858.CD003511.pub2] [DOI] [PubMed] [Google Scholar]
- Harbord 2006 .Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Harris 2007 .Harris LH, Dalton VK, Johnson TRB. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. American Journal of Obstetrics and Gynecology. 2007;196(5):445.e1–445.e5. doi: 10.1016/j.ajog.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Harwood 2008 .Harwood B, Nansel T, for the National Institute of Child Health and Human Development Management of Early Pregnancy Failure Trial Quality of life and acceptability of medical versus surgical management of early pregnancy failure. BJOG: an international journal of obstetrics and gynaecology. 2008;115(4):501–8. doi: 10.1111/j.1471-0528.2007.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk 1985 .Hawk HW, Conley HH. Effect of prostaglandin F2 alpha, phenylephrine and ergonovine on uterine contractions in the ewe. Journal of Animal Science. 1985;60(2):537–43. doi: 10.2527/jas1985.602537x. [DOI] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; [updated September 2008]. 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Hofmeyr 2003 .Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews. 2003;(Issue 1) doi: 10.1002/14651858.CD000941. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
- Jain 2002 .Jain JK, Dutton C, Harwood B, Meckstroth KR, Mishell DR., Jr A prospective randomized, double-blinded, placebo-controlled trial comparing mifepristone and vaginal misoprostol to vaginal misoprostol alone for elective termination of early pregnancy. Human Reproduction. 2002;17(6):1477–82. doi: 10.1093/humrep/17.6.1477. [DOI] [PubMed] [Google Scholar]
- Kaandorp 2009 .Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin or anticoagulants for the treatment of recurrent miscarriage in women without antiphospholipid syndrome. Cochrane Database of Systematic Reviews. 2009;(Issue 1) doi: 10.1002/14651858.CD004734.pub3. [DOI: 10.1002/14651858.CD004734.pub3] [DOI] [PubMed] [Google Scholar]
- Kawarabayashi 1990 .Kawarabayashi T, Kishikawa T, Sugimori H. Effect of methylergometrine maleate (methergin) on electrical and mechanical activities of pregnant human myometrium. Gynecologic and Obstetric Investigation. 1990;24(4):246–9. doi: 10.1159/000293327. [DOI] [PubMed] [Google Scholar]
- Klier 2002 .Klier CM, Geller PA, Ritsher JB. Affective disorders in the aftermath of miscarriage: a comprehensive review. Archives of Women’s Mental Health. 2002;5:129–49. doi: 10.1007/s00737-002-0146-2. [DOI] [PubMed] [Google Scholar]
- Kulier 2004 .Kulier R, Gülmezoglu AM, Hofmeyr GJ, Cheng LN, Campana A. Medical methods for first trimester abortion. Cochrane Database of Systematic Reviews. 2004;(Issue 1) doi: 10.1002/14651858.CD002855.pub2. [DOI: 10.1002/14651858.CD002855.pub3] [DOI] [PubMed] [Google Scholar]
- Lede 2005 .Lede R, Duley L. Uterine muscle relaxant drugs for threatened miscarriage. Cochrane Database of Systematic Reviews. 2005;(Issue 3) doi: 10.1002/14651858.CD002857.pub2. [DOI: 10.1002/14651858.CD002857.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis 2007 .Lewis G, editor. The seventh report on confidential enquiries into maternal deaths in the United Kingdom. CEMACH; London: 2007. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer - 2003-2005. [Google Scholar]
- Lohr 2008 .Lohr PA, Hayes JL, Gemzell-Danielsson K. Surgical versus medical methods for second trimester induced abortion. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD006714.pub2. [DOI: 10.1002/14651858.CD006714.pub2] [DOI] [PubMed] [Google Scholar]
- Lok 2007 .Lok IH, Neugebauer R. Psychological morbidity following miscarriage. Best Practice & Research Clinical Obstetrics & Gynaecology. 2007;21(2):229–47. doi: 10.1016/j.bpobgyn.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Luise 2002 .Luise C, Jermy K, May C, Costello G, Collins WP, Bourne TH. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873–5. doi: 10.1136/bmj.324.7342.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema 2005 .Medema S, Wildschut HIJ, Van Gemund N, Scherjon SA. Medical methods for mid-trimester termination of pregnancy. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD005216.pub2. [DOI: 10.1002/14651858.CD005216] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Rojas 2007 .Mota-Rojas D, Villanueva-García D, Velazquez-Armenta EY, Nava-Ocampo AA, Ramírez-Necoechea R, Alonso-Spilsbury M, et al. Influence of time at which oxytocin is administered during labor on uterine activity and perinatal death in pigs. Biological Research. 2007;40(1):55–63. doi: 10.4067/s0716-97602007000100006. [DOI] [PubMed] [Google Scholar]
- Nanda 2006 .Nanda K, Peloggia A, Grimes D, Lopez L, Nanda G. Expectant care versus surgical treatment for miscarriage. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD003518.pub2. [DOI: 10.1002/14651858.CD003518.pub2] [DOI] [PubMed] [Google Scholar]
- Neilson 2006 .Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks) Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD002253.pub3. [DOI: 10.1002/14651858.CD002253.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman 1991 .Norman JE, Thong KJ, Baird DT. Uterine contractility and induction of abortion in early pregnancy by misoprostol and mifepristone. Lancet. 1991;338:1233–6. doi: 10.1016/0140-6736(91)92102-8. [DOI] [PubMed] [Google Scholar]
- Porter 2006 .Porter TF, LaCoursiere Y, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD000112.pub2. [DOI: 10.1002/14651858.CD000112.pub2] [DOI] [PubMed] [Google Scholar]
- Ramphal 2006 .Ramphal SR, Moodley J. Emergency gynaecology. Best Practice & Research Clinical Obstetrics & Gynaecology. 2006;20(5):729–50. doi: 10.1016/j.bpobgyn.2006.04.001. [DOI] [PubMed] [Google Scholar]
- RCOG 2006 .RCOG . The Management of Early Pregnancy Loss. Royal College of Obstetricians and Gynaecologists; London: 2006. (RCOG Guideline No. 25). [Google Scholar]
- RevMan 2008 .The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre: The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Rumbold 2005 .Rumbold A, Middleton P, Crowther CA. Vitamin supplementation for preventing miscarriage. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD004073.pub2. [DOI: 10.1002/14651858.CD004073.pub2] [DOI] [PubMed] [Google Scholar]
- Sawyer 2007 .Sawyer E, Ofuasia E, Ofili-Yebovi D, Helmy S, Gonzalez J, Jurkovic D. The value of measuring endometrial thickness and volume on transvaginal ultrasound scan for the diagnosis of incomplete miscarriage. Ultrasound in Obstetrics & Gynecology. 2007;29(2):205–9. doi: 10.1002/uog.3914. [DOI] [PubMed] [Google Scholar]
- Say 2002 .Say L, Kulier R, Campana A, Gülmezoglu M. Medical versus surgical methods for first trimester termination of pregnancy. Cochrane Database of Systematic Reviews. 2002;(Issue 4) doi: 10.1002/14651858.CD003037. [DOI: 10.1002/14651858.CD003037.pub2] [DOI] [PubMed] [Google Scholar]
- Shiers 2003 .Shiers C. Abnormalities of early pregnancy. In: Fraser DM, Cooper MA, editors. Myles Textbook for Midwives. 14th Edition Churchill Livingstone; Edinburgh: 2003. [Google Scholar]
- Smith 2009 .Smith LFP, Ewings PD, Quinlan C. Incidence of pregnancy after expectant, medical, or surgical management of spontaneous first trimester miscarriage: long term follow-up of miscarriage treatment (MIST) randomised controlled trial. BMJ. 2009;339(1766):b3827. doi: 10.1136/bmj.b3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson 1999 .Swanson KM. Effects of caring, measurement, and time on miscarriage impact and women’s well-being. Nursing Research. 1999;48(6):288–98. doi: 10.1097/00006199-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Tang 2006b .Tang OS, Ho PC. The use of misoprostol for early pregnancy failure. Current Opinion in Obstetrics and Gynecology. 2006;18(6):581–6. doi: 10.1097/GCO.0b013e32800feedb. [DOI] [PubMed] [Google Scholar]
- Thapar 1992 .Thapar AK, Thapar A. Psychological sequelae of miscarriage: a controlled study using the general health questionnaire and the hospital anxiety and depression scale. British Journal of General Practice. 1992;42(356):94–6. [PMC free article] [PubMed] [Google Scholar]
- Wahabi 2007 .Wahabi HA, Abed Althagafi NF, Elawad M. Progestogen for treating threatened miscarriage. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD005943.pub2. [DOI: 10.1002/14651858.CD005943.pub2] [DOI] [PubMed] [Google Scholar]
- Weeks 2001 .Weeks A, Alia G. Ultrasonography may have role in assessing spontaneous miscarriage. BMJ. 2001;323:694. [PMC free article] [PubMed] [Google Scholar]
- Zieman 1997 .Zieman M, Fong SK, Benowitz NL, Bankster D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology. 1997;90:88–92. doi: 10.1016/S0029-7844(97)00111-7. [DOI] [PubMed] [Google Scholar]
- *. Indicates the major publication for the study