Abstract
we describe the research ethics capacity needs of the countries from the Middle East region. Against this background, we relate the experience of an international training program focused on providing long-term training in research ethics to individuals from low- and middle-income countries in the Middle East area. We describe our pedagogical approach to training, program changes to address challenges faced, and accomplishments of trainees. Many former trainees developed research ethics curricula in their home institutions, established or enhanced their institutions’ research ethics committees, provided leadership to national research ethics systems, and conducted research in research ethics. Based on our analysis, we make recommendations for how trainees can further address current regional research ethics needs in the Middle East and conduct future research. This paper is part of a collection of papers analyzing the Fogarty International Center’s International Research Ethics Education and Curriculum Development program.
Keywords: research ethics, Middle East, education
Health research has become an increasingly global endeavor, as witnessed by the increases in the number of clinical trials conducted in low- and middle-income countries (LMICs) throughout the world (Glickman et al., 2009; Normile, 2008). Many commentators have expressed concerns about the lack of regulatory oversight and research ethics capacity in LMICs (Ahmad, 2003; Bhutta, 2002; Hyder et al., 2009; Nuffield Council on Bioethics, 2002). Similar concerns apply to research ethics capacity in the Middle East region (Abdur Rab et al., 2008). Such concerns are pressing, as the number of clinical trials performed in the Middle East has substantially increased over the last decade (Kermani, 2010; Normile, 2008). For example, clinical trial activity in Egypt nearly tripled between 2008 and 2011 (U.S. National Institutes of Health, 2011). Also, despite growing political volatility, the Middle East region is poised for clinical trials growth as pharmaceutical companies continue their search for regions with large, treatment-naive populations and increasing numbers of Contract Research Organizations (CROs) are established to handle increased clinical trial activity (Next Level Pharma, 2010).
Indigenous scholarship and expertise to contribute to the complex debates surrounding the conduct of clinical research in resource-limited countries, as well as to provide appropriate oversight of health research, is therefore needed in LMICs (Bhutta, 2002; Macklin, 2004). However, varied economic and political structures, as well as extreme levels of poverty, accentuate differences in the environment in which research is conducted in high-income countries (HICs) and LMICs. Also, applying Western notions of bioethics can be challenging in countries that embrace dissimilar cultural, social, and religious values (Bhutta, 2002; Christakis, 1992; Macklin, 2004). Accordingly, to address regional needs in training, there have been regional educational efforts at enhancing training capacity in research ethics in the Middle East region, including those at the WHO Regional Office for the Eastern Mediterranean (EMRO), the United Nations Educational, Scientific and Cultural Organization (UNESCO), and the American University of Beirut (Eastern Mediterranean Region Office of the WHO, 2013; Salim El-Hoss Bioethics and Professionalism Program [SHBPP], 2013; United Nations Educational Scientific and Cultural Organization [UNESCO], 2013). Since 2000, the Fogarty International Center at the National Institutes of Health has sponsored training in research ethics for individuals from LMICs, including the Middle East, North Africa, and Iran—see Table 1 (Fogarty International Center, 2012).
TABLE 1. FIC Bioethics Programs Accepting Trainees from the Middle East, North Africa, and Iran.
| Name of Program | Years Funded |
Awardee Institutions |
Degree or Non- degree |
Length of Training Program [range or by course] |
Locations of Teaching |
Nationalities of Trainees |
|---|---|---|---|---|---|---|
| Middle East Research Ethics Training Initiative (MERETI) www.mereti.net, www.mereti-network.net |
2005– present |
University of Maryland |
|
|
University of Maryland, Baltimore, MD |
Egypt, Jordan, Sudan Yemen, Lebanon, Libya, and Syria |
| University of Toronto | 2004– present |
University of Toronto |
Master’s of Health Sciences in Bioethics, International Stream | Master’s: 2 years | University of Toronto |
One each from Iraq (2008), Iran (2005), Syria (2006), Libya (2007), Lebanon (2007), Egypt (2008),and Sudan (2008) |
We describe the past and current gaps in research ethics capacity in the Middle East region and review the experience and challenges of a Fogarty International Center/NIH–sponsored program in addressing these gaps—the Middle East Research Ethics Training Initiative (MERETI). The efforts of regional organizations in addressing these gaps are also chronicled. Based on this information and our analysis, we provide recommendations for the future activities of trainees from the MERETI Fogarty training program in enhancing research ethics capacity for the Middle East region.
ASPECTS OF RESEARCH ETHICS CAPACITY
To describe the many facets that comprise research ethics capacity, we present an ecological model adapted from a framework developed by Lavery and Hyder (Hyder et al., 2009; Lavery, 2001). Our model conceptualizes the research ethics system as five linked determinants of human subjects protection (Table 2): (1) national capacity: national laws and guidelines in research ethics, legal and regulatory mechanisms for oversight of clinical trials and RECs, and budget priorities for research funding and training programs in research ethics; (2) institutional commitments: organizational structures and procedures that support and promote a culture of ethical conduct (e.g., conflict of interest policies, appeal mechanisms, systems of awards and penalties), and institutional curriculum and degree programs in ethics and research ethics; (3) research ethics committee (REC) capacity: diversity of membership, training of members; adequate budgets, and policies that support the independence of RECs; (4) researchers’ knowledge, attitudes, and practices in research ethics: awareness of research ethics guidelines, respect for REC authority, optimal practices regarding informed consent and other human subjects protections, and responsible conduct of research; and (5) research participants’ awareness of their rights and understanding of research.
TABLE 2. Aspects of Research Ethics Capacity: Existing Gaps and Future Directions.
| Category of Capacity | Gaps in Research Ethics | Future Directions for Trainees |
|---|---|---|
| National Capacity |
|
|
| Institutional Commitments |
|
|
| Research Ethics Committee Capacity |
|
|
| Researchers’ Knowledge, Attitudes, and Practices in Research Ethics |
|
|
| Research Participants’ Awareness of Their Rights and Under- standing of Research |
|
|
Surrounding this research ethics system are enabling conditions, including strong civil society, public accountability, and trust in basic transactional processes, which are in turn influenced by developmental freedoms that include political freedoms, economic facilities, social opportunities, and transparency guarantees. The fundamental idea is that for guidelines and ethics review systems to be effective, a culture of ethical conduct is needed, which is more likely to exist when there are adequate development and enabling conditions.
The current state of development in the Middle East region has implications for the success of the range of activities supported and promoted by research ethics training programs. Countries in the Middle East region can be categorized as follows: (1) lower- and middle-income countries (e.g., Egypt, Sudan, South Sudan, Iraq, Syria, Yemen, and Morocco); (2) upper-middle-income countries (Jordan, Libya, Lebanon, Algeria, and Tunisia); and (3) high-income countries, otherwise referred to as the Gulf Countries (Saudi Arabia, Oman, Qatar, Kuwait, United Arab Emirates (UAE), and Bahrain) (World Bank, 2013). Table 3 describes the social, economic, and political characteristics of the LMICs in the Middle East from where the trainees of the Fogarty training programs have been drawn, as well as a few of the HICs for comparison purposes.
TABLE 3. Economic, Social, and Political Characteristics of the Middle East and North African Region.
| Health Expenditure |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Population | Area (Km2) |
GDP* per Capita ($US) |
Human Development Index † |
% below Poverty Line |
%GDP* | Per capita ($US) |
Education Index (mean years of schooling) |
Freedom Rating (Political rights/Civil liberties) 1.0 free and 7.0 not free |
Corruption Perceptions Index †† |
Gender Inequality Index ††† |
Registered Clinical Trials [Clinical Trials.gov] |
| Egypt | 85,294,388 | 1,002,000 | 6,600 | 112 | 20.0 | 4.7 | 137 | 6.4 | Partly Free (5.0/5.0) | 32 | .590 | 462 |
| Jordan | 6,482,081 | 89,342 | 6,000 | 100 | 14.2 | 8.0 | 392 | 8.6 | Not Free (6.0/5.0) | 48 | .482 | 60 |
| Lebanon | 4,131,583 | 10,452 | 15,900 | 72 | 28.0 | 7.0 | 622 | 7.9 | Partly Free (5.0/4.0) | 30 | .433 | 171 |
| Sudan | 34,847,910 | 1,861,484 | 2,400 | 171 | 46.5 | 6.3 | 104 | 3.1 | Not Free (7.0/7.0) | 13 | .604 | 19 |
| Syria | 22,457,336 | 185,180 | 5,100 | 116 | 11.9 | 3.4 | 101 | 5.7 | Not Free (7.0/7.0) | 26 | .551 | 13 |
| Yemen | 25,408,288 | 527,968 | 2,200 | 160 | 45.2 | 5.2 | 88 | 2.5 | Not Free (6.0/6.0) | 23 | .747 | 2 |
| Iraq | 31,858,481 | 438,317 | 4,600 | 131 | 25.0 | 8.4 | 332 | 5.6 | Not Free (6.0/6.0) | 18 | .557 | 15 |
| Morocco | 36,649,130 | 446,550 | 5,300 | 130 | 15.0 | 5.2 | 186 | 4.4 | Partly Free (5.0/4.0) | 37 | .444 | 67 |
| Qatar | 2,042,444 | 11,586 | 102,800 | 36 | NA | 1.8 | 1776 | 7.3 | Not Free (6.0/5.0) | 68 | .546 | 34 |
| Saudi Arabia | 26,939,583 | 2,149,690 | 25,700 | 57 | NA | 4.3 | 758 | 7.8 | Not Free (7.0/7.0) | 44 | .682 | 242 |
| Kuwait | 2,695,316 | 17,818 | 43,800 | 54 | NA | 2.6 | 1500 | 6.1 | Partly Free (5.0/5.0) | 44 | .274 | 38 |
| UAE | 5,473,972 | 83,600 | 49,000 | 41 | 19.5 | 3.7 | 1640 | 8.9 | Not Free (6.0/6.0) | 68 | .241 | 79 |
GDP = gross domestic product.
Range (1-186); 1 is highest.
Scale (1-100); 0 is highly corrupt.
Scale (0-1); 0 is woman and man fare equally.
Large economic disparities in the region accompanied modern development, which was largely based on the presence or lack of oil in these countries, thought to be both an asset and a hindrance for developing other economic resources and infrastructure. In comparison to other developing world regions, the Middle East region outperforms sub-Saharan Africa and South Asia in its overall Human Development Index (HDI), but has yet to reach the levels attained by East Asia and Latin America (United Nations Development Programme, 2013).
In general, the Middle East region is hampered by deficits in political freedom, women’s empowerment, and freedom of the press, and suffers from a high level of corruption, which may influence the environment in which research is conducted. All countries are characterized as "Partly Free" or "Not Free," and the region has the lowest freedom scores when compared to other world regions (Freedom House, 2013). The Middle East region ranks next to last among the other regions of the world in the Gender Inequality Index; only sub-Saharan Africa has lower scores on gender inequality. Egypt, Lebanon, Morocco, Sudan, and Yemen are thought to have a high level of public sector corruption, as measured by the Corruption Perceptions Index (CPI) (Transparency International, 2012). The Middle East region, compared with the other world regions, is last overall in CPI, somewhat behind Eastern Europe and Central Asia.
MAPPING OF RESEARCH ETHICS CAPACITY IN THE MIDDLE EAST REGION
Table 2 summarizes known gaps in research ethics capacity in the Middle East region, which are now detailed.
National Capacity
National regulations and associated guidelines can define appropriate legal and ethical conduct in research. In a study conducted among RECs in Egypt, 92% of the respondents listed the lack of national guidelines as one of several challenges to the effective functioning of RECs (Alahmad, Al-Jumah, & Dierickx, 2012; Sleem, El-Kamary, & Silverman, 2010). Table 4 lists national guidelines and regulations for research in Middle East countries compiled from several sources (Alahmad et al., 2012; Office for Human Research Protections, 2012). Alahmad and colleagues (2012) surveyed 13 countries in the Middle East and reported that national guidelines addressing research ethics exist in the United Arab Emirates, Qatar, Bahrain, Kuwait, Saudi Arabia, and Jordan. In two other countries (Egypt and Lebanon), guidance regarding research ethics is mentioned in medical ethics or medical professional guidelines. The remaining countries either refer to international guidelines or are silent on research ethics.
TABLE 4. National Guidelines and Regulations.
| Country | National Legislation | Regulatory | Guidelines |
|---|---|---|---|
| Egypt | None | Egyptian Drug Authority: http://www.eda.mohp.gov.eg/. | Professional Ethics Regulations: Conducting Medical Research on Human Beings, Articles 49-61 (2003): http://www.ems.org.eg/2_4/2_4_4/2_4_4.htm. |
| Egyptian Scientific and Research Eth- ics Committee: http://www.mohp.gov.eg/services/he/he_HealthDev/All.aspx. |
|||
| Jordan | Clinical Research Law2001: http://www.jfda.jo/custom/law/23.doc. | Jordan Food and Drug Administration: http://www.jfda.jo/en/default/. |
|
| Yemen | None | ||
| Sudan | Act on Pharmaceuticals and Poisons (2001) |
National Guidelines for Ethical Con- duct of Research Involving Human Subjects (2008): http://sites.google.com/site/healthresearchlibrary/national-guidelines. |
|
| Lebanon | Law of Medical Ethics No. 288 | ||
| Syria | Refers to Helsinki and CIOMS | ||
| Iraq | Refers to unknown documents | ||
| Saudi Arabia | Saudi Food and Drug Authority Clinical Trial Requirement Guidelines |
Regulations of the Law of Ethics of Research on Living Things. National Committee of BioEthics (NCBE): http://www.kacst.edu.sa/ar/depts/bioethics/Pages/WShp.aspx. |
|
| Qatar | Guidelines, Regulations and Policies for Research Involving Human Subjects |
||
| UAE | Guidance for Conducting Clinical Trials Based on Drugs/Medical Products & Good Clinical Practice |
||
| Iran | Ministry of Health and Medical Education, Office for the Study of Humanistic and Islamic Science in Medicine and Medical Ethics: http://www.mohme.gov.ir/. |
Protection Code for Human Subjects in Medical Research (1999) |
A careful review of the documents listed in Table 4 reveal several omissions in protections for human subjects when compared with international guidelines, such as CIOMS. For example, while Jordan’s Clinical Trial Law includes several unique aspects (e.g., a health research insurance requirement and a system of penalties for noncompliance to the law), key items are missing. For example, the law does not require a favorable assessment of benefits compared to risks, fair selection of subjects, and does not mention protection of the rights and welfare of children and other vulnerable subjects (Ramahi & Silverman, 2009). The majority of the Middle East guidelines do not mention safeguards for vulnerable populations, though two make general statements regarding vulnerable groups (Qatar and Egypt), and three mention protections for children (Saudi Arabia, Qatar, and Egypt). All guidelines state the obligation to obtain informed consent, but only seven mention the assessment of benefits and risks and confidentiality protections, and only five contain statements regarding inducements to participate.
Institutional Commitment to Ethics
A recent study of Egyptian RECs using a self-assessment tool (Sleem et al., 2012) showed suboptimal institutional commitment, as measured by whether the REC was established under a high ranking institutional official, the presence of a budget for the RECs, and the requirement for investigators to be trained in research ethics in order to submit protocols to the REC.
Several institutions in the Middle East region and Iran have established departments and centers in ethics and medical ethics (United Nations Educational Scientific and Cultural Organization [UNESCO], 2013; Zali, Shahraz, & Borzabadi). Regarding the existence of graduate programs in ethics, the UNESCO database reveals four programs (United Nations Educational Scientific and Cultural Organization [UNESCO], 2013): a diploma in ethics at the Universite Hassan II in Morocco; a graduate program in Forensic Medicine, Medical Law, Deontology and Ethics at the Universite de Sousse in Tunisia; a graduate program in bioethics at Qatar University in Qatar; and a Basic Biomedical Ethics Program for Medical Residents at the King Saud Bin Abdulaziz University for Health Sciences in Saudi Arabia. The King Abdullah International Research Center offers the only master’s degree program in bio-ethics in the region (http://www.kaimrc.med.sa/) (Ghiath Alahmad, personal communication, August 10, 2013).
Research Ethics Committee Capacity
Several studies assessed the operating characteristics and challenges of RECs in the region. Abou-Zeid and colleagues surveyed 15 national bioethics committees (NBCs) in the Eastern Mediterranean Region in 2008, many of which (14) devote a large portion of their activities to research ethics, and found that only 25% of the members and 20% of the chairs of 16 NBCs received training in research ethics (Abou-Zeid, Afzal, & Silverman, 2009). Studies involving Egyptian RECs reveal barriers to effective REC functioning, including insufficient member training, lack of diverse membership, limited human and capital resources, and lack of continuing education in research ethics (Matar & Silverman, 2013; Sleem et al., 2012; Sleem et al., 2010).
Researchers’ Knowledge, Attitudes, and Practices Regarding Research Ethics
Several studies assessed the knowledge, attitudes, and practices of investigators related to research ethics. A survey of the faculty at Cairo University showed that 48% never attended a course in research ethics (Asem & Silverman, 2009). Several faculty revealed research practices that might be considered suboptimal; for example, 48% thought it was not necessary to obtain prospective informed consent for research involving biological samples taken for clinical purposes, 38% thought that informed consent is not necessary in research involving minimal risks, and 17% thought it is proper to enroll vulnerable subjects if family members are not available to give informed consent. Another study surveying faculty at four other Egyptian universities reported similar results (Kandeel et al., 2011). Regarding attitudes towards RECs, El-Dessouky and colleagues surveyed dental faculty at two institutions in Egypt and Saudi Arabia and showed that many endorsed the existence of RECs; however, almost half thought that such committees would cause delays and make it more difficult to perform research, irrespective of their prior research experience (El-Dessouky et al., 2011). Regarding responsible conduct in research, these two survey studies from the Middle East demonstrated that approximately 6.5% to 11% of investigators agreed "it would be acceptable to fabricate data in order to improve the outcome of a study." These results assessing attitudes are similar to the prevalence of falsification and fabrication of data reported in Western universities (Eastwood et al., 1996; Fanelli, 2009; Kalichman & Freidman, 1992). Finally, the above survey studies in the Middle East showed that greater than 90% of the surveyed faculty endorsed the establishment of RECs and favored the educational programs in research ethics. However, Abdur Rab and colleagues showed that 28% of regional researchers did not obtain ethical clearance for research proposals submitted for funding to the Middle East/WHO office in Cairo in 2008, 24% failed to mention plans to obtain informed consent (Abdur Rab et al., 2008), and many basic elements of informed consent were omitted from informed consent forms (Abou-Zeid, Afzal, & Silverman, 2006).
Research Participants’ Awareness of Their Rights and Understanding of Research
Similar to findings obtained in Western countries, several studies from the Middle East demonstrated that many potential research participants lack an understanding of research concepts and are susceptible to the therapeutic misconception (Khalil et al., 2007; Waifazy, Khalil, & Silverman, 2009). Studies from Turkey and Lithuania demonstrated that 77% and 44% of patients, respectively, were not aware of their rights as patients. Research participants’ knowledge of their rights therefore might also be lacking. Lack of awareness of rights might also exist regarding patients and research subjects in the Middle East, but await further studies.
EDUCATIONAL PROGRAMS IN RESEARCH ETHICS TO ADDRESS EXISTING GAPS
In 2001, the United States National Bioethics Advisory Commission (NBAC) stated that "educational programs aimed at the responsibilities of all parties" are "the foundation of the oversight system and [are] essential to protecting research participants" (National Bioethics Advisory Commission [NBAC], 2001). Several such educational efforts exist that recruit individuals in the Middle East region. For example, the Salim El-Hoss Bioethics and Professionalism Program at the American University of Beirut Faculty of Medicine (AUB) is an interdisciplinary resource for faculty, students, and healthcare providers who are involved in bioethics education, research, and consultation in Lebanon and the region (Salim El-Hoss Bioethics and Professionalism Program [SHBPP], 2013). Launched in April 2010, this initiative seeks to educate, research, and champion issues related to professionalism, medical humanism, and bioethics. This program also organizes annual conferences focused on ethical issues confronting the region, including issues involving research ethics. The EMRO/WHO office has supported in the past several multiday in-country workshops in research ethics in Oman, Sudan, and Saudi Arabia and has also organized several regional conferences in research ethics (Eastern Mediterranean Region Office of the WHO, 2013). UNESCO initiated the Ethics Education Programme in 2004 with the overall objective of increasing the capacities of member states in the area of ethics education (United Nations Educational Scientific and Cultural Organization [UNESCO], 2013). The Programme acts as an adviser to Member States wishing to promote reflection and debate on bioethics, to set up national ethics committees, and to define national standards and/or legislation in the field. Finally, an ad hoc National Academy of Science committee, in partnership with the Bibliotheca Alexandrina in Egypt and the World Academy of Science (TWAS) in Italy, organized a onetime regional program to develop capacities for teaching responsible conduct of science in the Middle East region (National Academy of Science, 2013).
MIDDLE EAST RESEARCH ETHICS TRAINING INITIATIVE (MERETI)
Since 2005, the Middle East Research Ethics Training Initiative (MERETI) has offered training to individuals from LMICs in the Middle East region (Table 1). The ultimate goal of this program is to provide trainees with the necessary knowledge and skills to enhance institutional and country capacity in research ethics by: developing curricula in research ethics, taking leadership roles in their institutions (e.g., members and chairs of RECs) and countries (e.g., serve on national ethics committees), engaging in scholarly activities, and facilitating institutional change to improve research practices. The MERETI program also supports the continued career development of its graduates through a continuing education program, collaboration in research projects, and opportunities to present at international conferences.
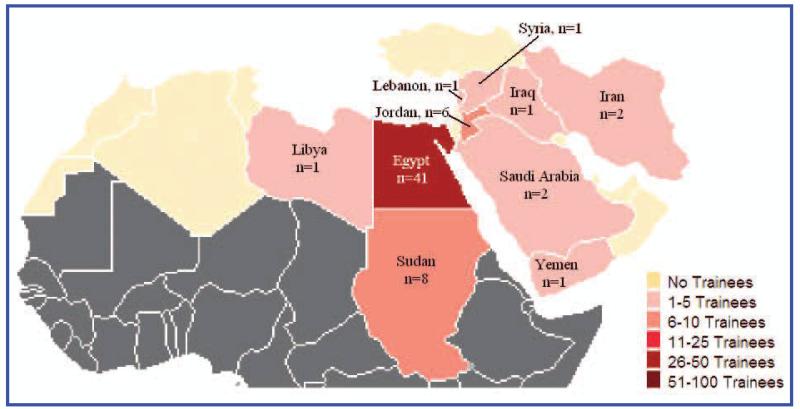
Individuals eligible for support by MERETI include those from the World Bank–designated LMIC categories. While individuals from HICs in the region are not eligible for funding from the program, they may participate as self-funded students. Since 2005, the MERETI program has provided long-term training (>1 year) to more than 60 individuals from several of the countries in the Middle East region (Figure 1). The MERETI program also collaborated with regional partners (EMRO/WHO, UNESCO, and AUB) to conduct conferences and training in research ethics in the Middle East region.
FIG. 1.
Middle East Long-Term Trainees by Country (2004-2010).
STRUCTURE OF THE PROGRAM TRAINING
MERETI offers several options for long-term training. First, it offers a two-year certificate program in research ethics targeting mid-level and senior professionals. As academia in the Middle East is highly hierarchical, the training of senior academics may support the legitimacy of research ethics, as well as promote the efforts of their junior counterparts at their institutions. Initially, the certificate program was a one-year program that consisted of two months of face-to-face training at UMB followed by a research ethics project at the trainees’ home institutions for the remainder of the year. In an effort to provide more rigor and transfer some educational activities to the Middle East region, the program evolved into a blended program (face-to-face and eLearning) consisting of the following elements: (a) a five-day workshop in research ethics located in a country in the Middle East region; (b) one-month face-to-face training at UMB during the summer; and (c) a distance-learning component for the subsequent nine months of the first year. During the face-to-face component at UMB, trainees observe institutional review boards, perform mock reviews of research protocols, and develop grant-writing, teaching, and presentation skills. During the first year, all trainees plan research ethics projects (e.g., survey studies, curriculum development, analysis of a research ethics issue) that will be completed during the second year.
Second, MERETI recently developed a completely online 12-credit graduate certificate degree offered through the UMB Graduate School (University of Maryland Graduate School, 2013). This program consists of six courses: Introduction to Research Ethics, Issues in International Research Ethics, Regulatory Aspects of Research Ethics Committees, Introduction to Ethical Theory, Responsible Conduct in International Research, and Global Ethics. An online degree program allows participation by individuals who would not be able to enroll at UMB due to professional and personal obligations. It also expands participation in the program without incurring housing and living expenses or travel prohibitions. Current plans include the development of practical experiences in research ethics, which are now part of the face-to-face UMB component via the online medium. The development of online components also makes it possible for trainees to adapt such courses for online programs at their institutions, which enhances the sustainability of research ethics education in the region.
Finally, through UMB, MERETI offers a Master’s of Science in Clinical Research with a concentration in research ethics for junior faculty who have time to devote to an intensive graduate program. The combination of training in research methodology and research ethics is designed to meet the need for additional expertise in research methodology and to enhance career prospects where a traditional master’s degree solely focused in research ethics is not thought to increase marketability.
CAREER DEVELOPMENT OF TRAINEES
To ensure continued career development of the trainees after they complete either of the MERETI training options, a Post-Training Development Program was instituted consisting of: (1) travel grants to present at regional and international conferences (e.g., AUB conference, PRIM&R conference, the World Congress of Bioethics); (2) UMB faculty collaboration on research projects; (3) teaching opportunities at regional MERETI workshops and at the summer face-to-face MERETI certificate program at UMB; and (4) mentored activities that include writing blogs and reviewing journal articles for the MERETI-NETWORK website (www.mereti-network.net).
Accomplishments of the Trainees
The accomplishments of the MERETI trainees can be categorized according to the ecological model presented previously: (1) development of national capacity through service on regulatory agencies at the Ministries of Health (e.g., Egypt and Jordan), providing consultative services and training to national ethics committees (e.g., Egypt and Jordan), establishing nongovernmental organizations in research ethics in Egypt, establishing CROs, and advocating for public awareness regarding human subjects protection; (2) strengthening institutional commitments by developing curriculum and organizing workshops in research ethics; (3) enhancing REC capacity by taking on lead roles in RECs (e.g., chairs and vice-chairs), enhancing the administrative processes of RECs (e.g., establishing Standard Operating Procedures, developing procedures for protocol submissions and reviews) and providing training to REC members; and (4) enhancing researchers’ knowledge and attitudes and influencing researcher conduct by teaching courses on research ethics and responsible conduct in research.
Of particular significance, two trainees from Egypt established an Egyptian Network of Research Ethics Committees (ENREC–www.enrec.org) in 2010, whose membership now includes more than 40 Egyptian RECs. ENREC organizes periodic meetings that serve as a forum for continued educational activities and the discussion of difficult research ethics issues. Recently, it provided consultation to the Ministry of Health National Ethics Committee and developed guidelines for stem cell research.
MERETI trainees have also been involved in various scholarly pursuits that have included the conduct of surveys that identify gaps in research ethics that can subsequently influence policy changes, published articles in peer review journals, presentations of abstracts at regional and international ethics conferences,, obtainment of grant funding (e.g., Welcome Trust), and service on advisory boards of international journals.
Several trainees were involved with developing the Virtual Collaboratory MERETI Network (www.mereti-network.net), which connects all MERETI trainees and other interested individuals as a resource community. Through this networking website, trainees can keep up to date with each other’s activities, share ideas, and communicate issues that arise in their institutions. This virtual collaboratory network includes a blogging page, discussion forum, document exchange repository, resource directory, archived audio of trainee presentations at conferences, and capacity for webinars (online meetings).
Challenges Experienced by the MERETI Training Program
Through the years, we have experienced several challenges with implementing MERETI activities. The following are examples of specific challenges the program faced and our responses to them, as we adapted the training to trainees with diverse professional backgrounds and working in varied cultural settings.
IMPLEMENTATION OF RESEARCH ETHICS TRAINING IN UNIVERSITIES
When the MERETI program began recruitment efforts in 2005, the willingness of top officials at major universities in the Middle East region to implement research ethics training in their institutions mirrored the previous unenthusiastic acceptance of curricular time and funding devoted to medical ethics programs in U.S. universities (Lehmann et al., 2004). Presidents and deans advised us that there would be minimal interest in research ethics training among their faculty and that time spent by faculty on such training would detract from efforts towards academic promotion. However, the following developments appeared to enhance interest in research ethics training among faculty: (1) former trainees, especially those at senior academic levels, served as "ambassadors" for research ethics, using their influence to promote the value of research ethics, encourage teaching research ethics, and establish RECs in their institutions; (2) the increasing presence of pharmaceutical companies and CROs in the region motivated universities to establish clinical trials centers and enhance faculty clinical research skills so that the scholarly and financial rewards of conducting clinical trials could be pursued; (3) new academic requirements for promotion required faculty to publish in international journals that require ethical review of research; and (4) national university accreditation standards in Egypt required the establishment of RECs.
TURNOVER OF TOP OFFICIALS AND POLITICAL TENSIONS IN THE MIDDLE EAST REGION
Revolutionary movements that occurred during the Arab Spring of 2011, most notably in Egypt, Tunisia, Libya, Yemen, and Syria, initially presented major challenges. For example, the region witnessed frequent turnover of top officials at universities with whom we had established relationships, and hence, continuity of collaborative efforts was disturbed. In particular, academia underwent profound changes in Egypt after the Arab Spring, whereby many top officials with ties to the Mubarak regime were asked to resign. Also, political tensions and continuous demonstrations led to interruptions in many trainee activities. However, trainees resumed activities amid much uncertainty regarding the future political landscape and reported that a new emphasis on human rights, transparency, and accountability served to strengthen the awareness of research ethics in their institutions and among potential research participants.
TRAINEE SELECTION PROCESS
Application forms, curricula vitae, reference letters, and interviews (face-to-face or via the Internet) were insufficient to detect in the applicants the presence of critical thinking skills, good study work habits, and the ability to work in groups. After several enrollment cycles in which several trainees performed below expectations or shelved their interest in research ethics, we developed a two-step selection process: (1) preliminary selection of candidates based on the previous application criteria; followed by (2) assessment of their competence and skills at an annual five-day workshop research ethics workshop held in a Middle East country. At these workshops, applicants are required to participate in discussions, work in groups, submit written assignments, and contribute to an online discussion forum. We make final selections of the applicants based on performance during this workshop.
CHALLENGES IN CURRICULUM DESIGN
Our training program attracted applicants from different specialties, at different stages in their careers, and with different learning styles, which presented challenges in developing a pedagogy that is responsive to the needs of all trainees. Furthermore, in the initial years of the training program, attempts at curriculum design lacked information regarding the appropriate curriculum elements (i.e., content and skills) that would ensure post-training success of participants. Subsequently, based on feedback from trainees, we implemented the following program changes: (1) review of epidemiologic and social behavioral research protocols, as such research is more common than clinical trials in the Middle East region; (2) addition of practical skills to the curriculum, such as writing skills, presentation skills, and more instruction in research methodology; (3) development of a performance-based assessment tool to monitor trainee progress; (4) scheduled webinars to discuss trainees’ activities at their home institutions; and (5) regional conferences where current and past trainees can present the results of their activities.
TRAINEE DIFFICULTY WITH IMPLEMENTATION OF RESEARCH ETHICS ACTIVITIES IN THEIR INSTITUTIONS
Due to the hierarchical structure of academia in the Middle East region, junior trainees reported difficulties assuming major roles on their institution’s REC and implementing research ethics into the curriculum. To alleviate these difficulties, prior to trainee selection, we discuss with top university officials how they will promote and support trainee efforts when they return home, as well as selecting senior and junior faculty from the same institution so that junior trainees could be supported in advocating for and implementing research ethics activities.
GRADUATE RECORD EXAMINATION (GRE) REQUIREMENT
Applicants to the master’s program apply to the UMB Graduate School, which requires the Graduate Record Examination (GRE). Many academics in the Middle East region consider this exam to be biased towards Western learning styles that put their students at a disadvantage. Accordingly, many potential applicants delay their application until time is available to study for the exam or suspend efforts to apply. To overcome this barrier, we encourage potential applicants who are hesitant to take the GRE to first apply and gain acceptance to our two-year certificate program, and for those who demonstrate progression, we plan to give financial support for them to take preparatory GRE courses so that they can make subsequent application to the master’s degree program. Also, we have convinced the Graduate School not to require the GRE exam for those applying to the 12-credit online certificate degree program.
Best Practices: Future Directions to Address Existing Gaps
To address the existing gaps in research ethics capacity, trainees can pursue further activities that involve educational efforts, administrative functions, and advocacy roles to convince key stakeholders to implement changes at the national and institutional levels. A discussion of these future efforts to address the existing gaps in research ethics in the Middle East region follows (summarized in Table 3).
NATIONAL CAPACITY
Graduates of Middle East region training programs may play a role in influencing public opinion, motivating governmental policy changes, and developing regulations and guidelines for research (Edwards, Hifnawy, & Silverman, 2013). As consultants to their Ministries of Health, trainees can play a role in developing national regulations and guidelines in research ethics and provide advice regarding appropriate regulatory structures for the conduct of clinical trials. Trainees from Sudan may apply for funding from the European and Developing Countries Clinical Trials Partnership (EDCTP), whose grant schemes aim to strengthen local capacity in both ethical review and the national regulatory framework in Africa (European and Developing Countries Clinical Trials Partnership [EDCTP], 2013).
INSTITUTIONAL COMMITMENT TO RESEARCH ETHICS
Trainees may guide their institutions to adopt certain organizational structures and processes that can promote ethical conduct in research, such as conflict of interest policies, appropriate appeal mechanisms, and forums for discussing ethics issues among faculty and staff. Finally, they may be able to improve educational efforts, such as through research ethics curriculum requirements for medical and science programs, and degree programs in research ethics, and advocate for budgets that provide for these improvements.
REC CAPACITY
Trainees can take on administrative roles within RECs that enhance REC functioning, such as development of standard operating procedures, submission and review policies, member selection policies, and conflict of interest policies (World Health Organization [WHO], 2011). Trainees can also develop a continuing education program in research ethics for the members and also help RECs take on quality assurance activities that include the use of a REC self-assessment tool (Sleem et al., 2012).
Finally, the establishment of an Egyptian network of RECs has been successful with enhancing collaborations among the many RECs in Egypt. Trainees can develop similar networks in other countries in the region.
RESEARCHERS’ KNOWLEDGE, ATTITUDES, AND PRACTICES
Trainees should develop training courses in research ethics and good clinical practice (face-to-face or online) (Williams et al., 2013) that target researchers and members of their staff. There should be requirements to declare existing conflicts of interest that threaten the objectivity of research conduct.
RESEARCH PARTICIPANTS’ AWARENESS OF THEIR RIGHTS AND UNDERSTANDING OF RESEARCH
Trainees may develop awareness campaigns in research ethics both within their institutions and within NGOs that target members of the public. Such campaigns may enhance the public’s understanding of the goals of research and of their rights as research participants.
Research Agenda
Further research is needed to document existing gaps in research ethics in the Middle East countries at three levels: research staff, the lay public, and national capacity. Accordingly, survey and interview studies could be conducted that further document investigators’ knowledge, attitudes, and practices regarding research ethics. Such information would help guide the development of educational programs in research ethics for those involved in research. Quantitative and qualitative studies of the public are also needed to further understand reasons for their enrollment and nonenrollment in research studies, the existence of the therapeutic misconception, and the extent to which research participants are aware of rights. Results of these studies may help trainees advocate for research policy changes to key stakeholders. Finally, surveys that include interviews of key informants (e.g., top officials of universities, governmental policy makers, members of pharmaceutical companies and contract research organizations) are needed to understand the barriers to the adoption of policies that promote ethical conduct in research at the national level.
Biographies
Authors’ Biographical Sketches
Henry Silverman is Professor of Medicine at the University of Maryland School of Medicine in the United States. He is Program Director of the Middle East Research Ethics Training Initiative (MERETI) sponsored by the Fogarty International Center at the National Institutes of Health. He helped to conceptualize the focus of this paper and was primarily responsible for the writing of this article.
Hillary Anne Edwards is currently earning her Master of Public Health degree at the University of Maryland, Baltimore, and is Director of the Wellness and Academic-Life Balance Program at the University of Maryland, Baltimore. She helped with the conception of the paper and reviewed the final drafts.
Adil Shamoo is a Professor of Biochemistry and Molecular Biology at the University of Maryland, Baltimore. Since 1988, he has been the Editor-in-Chief of the journal, Accountability in Research. He helped write several sections of this article.
Amal Matar received her master’s degree in the Department of Biotechnology at American University in Cairo, graduated from Ain Shams Medical School in Cairo, and worked for the Ministry of Health and Population in Egypt for seven years. She is currently enrolled in a doctoral bioethics program at Uppsala University, Sweden. She provided information regarding research ethics capacity in the Middle East region and reviewed the final drafts of the paper.
Contributor Information
Henry Silverman, University of Maryland, Baltimore (USA).
Hillary Edwards, University of Maryland, Baltimore (USA).
Adil Shamoo, University of Maryland, Baltimore (USA).
Amal Matar, Uppsala University (Sweden).
References
- Abdur Rab M, Abou-Zeid A, Afzal M, Silverman HJ. Ethical practices for health research in the Eastern Mediterranean Region of the World Health Organization: A retrospective data analysis. PLoS ONE. 2008;3:e2094. doi: 10.1371/journal.pone.0002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Zeid A, Afzal M, Silverman HJ. Informed consent as an ethical requirement for health research in the Eastern Mediterranean Region of the World Health Organization. Presented at PRIM&R Conference; Washington, DC. 2006. [Google Scholar]
- Abou-Zeid A, Afzal M, Silverman HJ. Capacity mapping of national ethics committees in the Eastern Mediterranean Region. BMC Medical Ethics. 2009;4:8. doi: 10.1186/1472-6939-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad K. Developing countries need effective ethics review committees. Lancet. 2003;362:627. doi: 10.1016/S0140-6736(03)14203-1. [DOI] [PubMed] [Google Scholar]
- Alahmad G, Al-Jumah M, Dierickx K. Review of national research ethics regulations and guidelines in Middle Eastern Arab countries. BMC Medical Ethics. 2012;13:34. doi: 10.1186/1472-6939-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asem N, Silverman HJ. Perspectives of faculty at Cairo Universitiy towards research ethics and informed consent [abstract]; Presented at PRIM&R Conference; Nashville, TN. 2009. [Google Scholar]
- Bhutta AZ. Ethics in international health research: A perspective from the developing world. Bulletin of the World Health Organization. 2002;80:114–120. [PMC free article] [PubMed] [Google Scholar]
- Christakis NA. Ethics are local: Engaging cross-cultural variation in the ethics for clinical research. Social Science and Medicine. 1992;35:1079–1091. doi: 10.1016/0277-9536(92)90220-k. [DOI] [PubMed] [Google Scholar]
- Eastern Mediterranean Region Office Of The Who Research policy and cooperation. 2013 Retrieved from www.emro.who.int/entity/research/
- Eastwood S, Derish P, Leash E, Ordway S. Ethical issues in biomedical research: Perceptions and practices of postdoctoral research fellow responding to a survey. Science and Engineering Ethics. 1996;2:89–114. doi: 10.1007/BF02639320. [DOI] [PubMed] [Google Scholar]
- Edwards H, Hifnawy T, Silverman HJ. Enhancing research ethics review systems in Egypt: The focus of an international training program informed by an developmental systems approach to enhancing research ethics capacity; Accepted for Presentation: Advancing Ethical Research Conference. PRIM&R; Boston. Nov 7-9, 2013. Retrieved from www.primr.org/aer2013/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dessouky HF, Abdel-Aziz AM, Ibrahim C, Moni M, Abul Fadl R, Silverman H. Knowledge, awareness, and attitudes about research ethics among dental faculty in the Middle East: A pilot study. International Journal of Dentistry. 20112011:694–759. doi: 10.1155/2011/694759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European And Developing Countries Clinical Trials Partnership (EDCTP) Types of grants. 2013 Retrieved from www.edctp.org/Types_of_Grants.2489.2010.html.
- Fanelli D. How many scientists fabricate and falsify research? A systematic review and meta-analysis of survey data. PLoS One. 2009:4. doi: 10.1371/journal.pone.0005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty International Center International Bioethics Education and Career Development Award. 2012 Retrieved from www.fic.nih.gov/programs/training_grants/bioethics/%5D.
- Freedom House Freedom in the World. 2013 Retrieved from www.freedomhouse.org/report-types/freedom-world.
- Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, et al. Ethical and scientific implications of the globalization of clinical research. New England Journal of Medicine. 2009;360(8):816–823. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Dawson L, Bachani AM, Lavery JV. Moving from research ethics review to research ethics systems in low-income and middle-income countries. Lancet. 2009;373:862–865. doi: 10.1016/S0140-6736(09)60488-8. [DOI] [PubMed] [Google Scholar]
- Kalichman M, Freidman P. A pilot study of biomedical trainees’ perceptions concerning research ethics. Academic Medicine. 1992;67:769–775. doi: 10.1097/00001888-199211000-00015. [DOI] [PubMed] [Google Scholar]
- Kandeel N, El-Nemer A, Ali NM, Kassem H, El-Setouhy M, Elgharieb ME, et al. A multicenter study of the awareness and attitudes of Egyptian faculty towards research ethics: A pilot study. Journal of Empirical Research on Human Research Ethics. 2011;6(4):99–108. doi: 10.1525/jer.2011.6.4.99. [DOI] [PubMed] [Google Scholar]
- Kermani F. How to run clinical trials in the Middle East. SCRIP. 2010 Retrieved from www.scripnews.com. [Google Scholar]
- Khalil SS, Silverman HJ, Raafat M, El-Kamary S, El-Setouhy M. Attitudes, understanding, and concerns regarding medical research amongst Egyptians: A qualitative pilot study. BMC Medical Ethics. 2007;8:9. doi: 10.1186/1472-6939-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery JV. CMH Working Paper Series (Working Paper No. WG2:5) Commission on Macroeconomics and Health; WHO: 2001. A culture of ethical conduct in research: The proper goal of capacity-building in international research ethics. [Google Scholar]
- Lehmann LS, Kasoff WS, Koch P, Federman DD. A survey of medical ethics education at U.S. and Canadian medical schools. Academic Medicine: Journal of the Association of American Medical Colleges. 2004;79(7):682–689. doi: 10.1097/00001888-200407000-00015. [DOI] [PubMed] [Google Scholar]
- Macklin R. Double standards in medical research in developing countries. Cambridge University Press; Cambridge: 2004. [Google Scholar]
- Matar A, Silverman H. Perspectives of Egyptian research ethics committees regarding their effective functioning. Journal of Empirical Research on Human Research Ethics. 2013;8(1):32–44. doi: 10.1525/jer.2013.8.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academcy of Science Developing capacities for teaching responsible science in the MENA region: Refashioning scientific dialogue. 2013 Retrieved from http://dels.nas.edu/Report/Developing-Capacities-Teaching-Responsible-Science/18356.
- National Bioethics Advisory Commission (NBAC) Ethical and policy issues in research involving human participants. U.S. Government Printing Office; Rockville, MD: 2001. [Google Scholar]
- Next Level Pharma Advancing clinical research in Turkey, Middle East, and North Africa. 2010 Retrieved from www.nextlevelpharma.com.
- Normile D. The promise and pitfalls of clinical trials overseas. Science. 2008;322(5899):214–216. doi: 10.1126/science.322.5899.214. [DOI] [PubMed] [Google Scholar]
- Nuffield Council on Bioethics . The ethics of research related to healthcare in developing countries. Nuffield Council on Bioethics; London: 2002. [Google Scholar]
- Office for Human Research Protections International compilation of human subject research protections. 2012 Retrieved from www.dhhs.gov/ohrp/international/
- Ramahi I, Silverman H. Clinical research law in Jordan: An ethical analysis. Developing World Bioethics. 2009;9(1):26–33. doi: 10.1111/j.1471-8847.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- Salim El-Hoss. Bioethics and Professionalism Program (SHBPP) 2013 Retrieved from www.aub.edu.lb/fm/shbpp/Pages/index.aspx.
- Sleem H, Moodley K, Kumar N, Moni M, Naidoo S, Silverman H. Self-assessment of the operations and functions of research ethics committees in developing countries. Paper presented at the International Association of Bioethics; Rotterdam, Neth: 2012. [Google Scholar]
- Sleem H, El-Kamary SS, Silverman HJ. Identifying structures, processes, resources and needs of research ethics committees in Egypt. BMC Medical Ethics. 2010;11:12. doi: 10.1186/1472-6939-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transparency International Corruption Perception Index. 2012 Retrieved from www.transparency.org/cpi2012/results.
- U.S. National Institutes of Health 2011 Retrieved from www.clinicaltrials.gov. [PubMed]
- United Nations Development Programme Arab human development reports. 2013 Retrieved from www.arab-hdr.org.
- United Nations Educational Scientific and Cultural Organization (UNESCO) 2013 Ethics education programme. Retrieved from www.unesco.org/new/en/social-and-human-sciences/themes/bioethics/ethics-education-programme/
- University of Maryland Graduate School Online certificate program in research ethics. 2013 Retrieved from www.graduate.umaryland.edu/research_ethics/index.html.
- Wazaify M, Khalil SS, Silverman HJ. Expression of therapeutic misconception amongst Egyptians: A qualitative pilot study. [Research Support, NIH, Extramural] BMC Medical Ethics. 2009;10:7. doi: 10.1186/1472-6939-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Sprumont D, Hirtle M, Adebamowo C, Braunschweiger P, Bull S, et al. Consensus standards for introductory e-learning courses in human participants research ethics. Journal of Medical Ethics. 2013 doi: 10.1136/medethics-2013-101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank Egypt, Arab Republic. 2013 Retrieved from http://data.worldbank.org/country/egypt-arab-republic.
- World Health Organization (WHO) Standards and operational guidance for ethics review of health-related research with human participants. WHO; Geneva: 2011. Retrieved from www.who.int/ethics/publications/research_standards_9789241502948/en/ [PubMed] [Google Scholar]
- Zali MR, Shahraz S, Borzabadi S. Bioethics in Iran: Legislation as the main problem. Archives of Iranian Medicine. 2002;5(3):136–140. [Google Scholar]