Abstract
Patients with high FLT3 internal tandem duplication allelic ratios (FLT3/ITD-ARs) have a poor prognosis. Single-nucleotide polymorphism/comparative genomic hybridization, single-cell PCR and colony-forming assays were used to evaluate genotypic evolution of high FLT3/ITD-ARs in 85 acute myeloid leukemia (AML) patients. Microarrays were used to examine molecular pathways disrupted in leukemic blasts with high FLT3/ITD-ARs. Copy-neutral loss of heterozygosity (CN-LOH) was identified at the FLT3 locus in diagnostic samples with high FLT3/ITD-ARs (N=11), but not in samples with low FLT3/ITD-ARs (N=24), FLT3-activating loop mutations (N=11) or wild-type FLT3 (N=39). Single-cell assays showed that homozygous FLT3/ITD genotype was present in subsets of leukemic blasts at diagnosis but became the dominant clone at relapse. Less differentiated CD34+/CD33− progenitor colonies were heterozygous for FLT3/ITD, whereas more differentiated CD34+/CD33+ progenitor colonies were homozygous for FLT3/ITD. Expression profiling revealed that samples harboring high FLT3/ITD-ARs aberrantly expressed genes within the recombination/DNA repair pathway. Thus, the development of CN-LOH at the FLT3 locus, which results in high FLT3/ITD-ARs, likely represents a late genomic event that occurs after the acquisition of the FLT3/ITD. Although the etiology underlying the development of CN-LOH remains to be clarified, the disruption in recombination/DNA repair pathway, which is present before the development of LOH, may have a role.
Introduction
Activating mutations of the FLT3 gene occur as a result of an internal tandem duplication (FLT3/ITD) of the juxtamembrane domain-coding sequence or a point mutation within the activation loop domain (FLT3/ALM).1, 2, 3 Although both mutations lead to the constitutive activation of the receptor, only presence of FLT3/ITD has consistently been associated with clinical outcome.4, 5, 6, 7, 8, 9, 10, 11, 12, 13 We and others have demonstrated that allelic ratio (AR) of FLT3/ITD has prognostic significance, such that those patients with a high FLT3/ITD-AR have an inferior outcome compared with those with a low FLT3/ITD-AR.8, 12, 13, 14 The central mechanism underpinning the variation in AR and its prognostic significance remains poorly understood.
Analyses of microsatellite markers have demonstrated that a small subset of patients with very high ARs actually harbor a loss of heterozygosity (LOH) of the FLT3 gene at chr13q locus.9, 15, 16 Recent reports suggest that copy-neutral LOH (CN-LOH), which is also known as acquired uniparental disomy, may have a role in the development of high FLT3/ITD-AR within leukemic blasts.17, 18 However, additional studies are warranted to better characterize the frequency of CN-LOH in patients with FLT3/ITD and how this process may contribute to the development of high FLT3/ITD-AR. In this study, single-nucleotide polymorphism/comparative genomic hybridization (SNP/CGH) profiling was used to better define the underlying mechanism and temporal evolution of allelic variation in AML blasts containing FLT3 mutations.
Materials and methods
Patients and treatment
Pediatric patients with acute myeloid leukemia (AML) enrolled in the Children's Cancer Group, AML clinical protocols CCG 2941 and 2961, were candidates for this study. Details of the aforementioned protocols are described elsewhere.19, 20 Evaluated patients were limited to those with >80% leukemic blasts in the bone marrow (BM) or peripheral blood sample. All samples were further enriched for leukemic blast by ficoll preparation. DNA was extracted by Qiagen (Valencia, CA, USA) DNA extraction kit per manufacturer instructions.
Affymetrix mapping 250K SNP/CGH arrays and data processing
Samples were genotyped with Affymetrix 250K Nsp GeneChip Human Mapping arrays (Affymetrix, Inc., Santa Clara, CA, USA), which interrogated over 262 000 loci and allowed the evaluation of genomic copy number and LOH. DNA was digested with restriction enzymes, amplified by PCR, purified, labeled, fragmented and hybridized to the arrays according to the manufacturer's instructions. Briefly, 250 ng of DNA was digested with XbaI, HindIII or StyI (New England Biolabs, Boston, MA, USA). Digested DNA was adaptor-ligated and PCR-amplified using AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA) in 100 μl PCR reactions for each enzyme-digested sample. PCR products from each reaction were pooled, concentrated and fragmented using DNase I (Invitrogen, Carlsbad, CA, USA). Fragmented PCR products were then labeled, denatured and hybridized to the arrays. Arrays were washed using Affymetrix fluidics stations, and scanned using the GeneChip Scanner 3000 (Affymetrix, Inc.). CEL files were generated using Affymetrix GeneChip Genotyping Analysis Software (GTYPE) v4.0.21
SNP calls were generated using GTYPE by incorporating the Copy Number Analysis Tool (CNAT) 4.0.1 package.22 Samples (85) passed the recommended minimum dynamic model call rate of 93%. Log2 signal ratios were estimated using the unpaired-copy-number workflow with a Gaussian smoothing window of 100 000 bp. Copy-number states were estimated by a Hidden Markov Model using CNAT as per the recommended parameters. LOH was estimated using the unpaired-LOH workflow on genotype files produced by the BRLMM algorithm at default settings. The reference set was comprised of 51 normal samples from the International HapMap Project. Results were visualized with the Partek Genomics Suite, v. 6.07.0801. The algorithms utilized by CNAT 4.0 have been previously described.21
Fluorescence-activated cell sorting for colony-forming cells
Fluorescence-activated cell sorting purification for hematopoietic progenitor subpopulations for colony-forming cell assays on methylcellulose, as well as fluorescence-activated cell sorting-based single-cell sorts for PCR were performed using anti-CD34-fluorescein isothiocyanate and anti-CD33-phycoerythrin (Becton Dickinson, San Jose, CA, USA) with appropriate isotype controls as previously described.23 Propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) was used to select viable cells. Desired populations were selected on a Vantage flow cytometer (Becton Dickinson) and collected in Iscove's modified Dulbecco's medium (Invitrogen). Colonies were grown in methylcellulose culture supplemented with granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, stem cell factor, interleukin-3, interleukin-6 and erythropoietin (all from PeproTech, Rocky Hill, NJ, USA) as previously described.23
Single-cell FLT3 genotyping
Individual colonies were harvested from methylcellulose and sorted as individual cells into 20 μl of RNase-free water supplemented with 10 U RNaseOUT RNase Inhibitor and 1 mM dithiothreitol (Invitrogen) on Vantage flow cytometer. FLT3 genotyping was carried out using nested touch-down PCR. First-round amplification was performed using primers ITDA-F (5′-CCTGATTGTCTGTGGGGAGT)-3′ and ITDB-R (5′-ACCCCAATCCACTCCATTTT-3′) in a final reaction volume of 50 μl, consisting of 25 μl 2 × Failsafe PCR Premix D (Epicentre, Madison, WI, USA), 2.5 μl PCR-grade water, 2.5 U Platinum Taq (Invitrogen), 0.2 μM of each primer and 20 μl single-cell template. Cycling conditions were as follows: initial denaturation, 94 °C for 5 min, followed by 15 cycles of 94 °C for 30 s denaturation, 70–56 °C (Δ−1 °/cycle) for 45 s annealing and 72 °C for 1 min extension; this was followed by another 25 cycles with the same parameters, but with annealing temperature of 56 °C and completed with a final extension of 7 min at 72 °C. Second-round amplification was performed using primers CG13F-6FAM (5′-[6FAM]TGCCTATTCCTAACTGACTCATCA-3′) and CG14R (5′-TCTTTGTTGCTGTCCTTCCA-3′) and a 25-μl reaction mix consisting of 12.5 μl 2 × Failsafe PCR Premix B (Epicentre), 6.25 μl PCR-grade water, 2.5 U Platinum Taq (Invitrogen), 0.2 μM of each primer and 5 μl template DNA from the first-round amplification described above. Touch-down cycling conditions were as listed above, with the exception of the annealing and extension times were reduced to 30 s each.
Fluorescence in situ hybridization (FISH) evaluation of colony-forming cell colonies
FISH was performed using bacterial artificial chromosome (BAC) RP11-136G6 (13q12.2) that contains the FLT3 gene, and BAC RP11-408E5 (13q12.11, near the centromere) as a control for chromosome 13 copy number. BAC DNA was isolated from a single-colony culture using the Large-Construct Kit according to the manufacturer's protocol (Qiagen). DNA was labeled by nick translation using SpectrumOrange dUTP (RP11-136G6) or SpectrumGreen dUTP (RP11-408E5) following the manufacturer protocol (Vysis Inc., Downers Grove, IL, USA). Labeled BAC DNA was precipitated, resuspended in LSI/WCP hybridization buffer (Vysis Inc.), and hybridized to slides according to the manufacturer's protocol. Labeled BAC DNA was initially hybridized to normal human metaphase cells to verify probe localization to the correct band on chromosome 13. At least two and up to 20 independent colonies from each cell population (30–323 total interphase nuclei per population) were scored.
Microarray expression analyses
Details of subjects included in the microarray studies are provided in Supplementary Table 1. RNA was extracted from BM and peripheral blood samples, and quality of RNA was analyzed on HP 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA).24 DNA from available samples was evaluated for NPM1, DNMT3A, IDH1 and IDH2 mutations as previously described.25, 26, 27 Five micrograms of total RNA underwent biotin-labeling using the Enzo BioArray HighYield RNA Transcript Labeling Kit (Enzo Life Technologies, Farmingdale, NY, USA) as per manufacturer's recommendations. The biotin-labeled product was fragmented as per the standard Affymetrix protocol.24 Fifteen micrograms of fragmented antisense cRNA was then hybridized to the HG-U133A arrays (Affymetrix, Inc.). DAT, CEL and CHP files were generated using GCOS 1.2.1 and MAS 5.0 software (Affymetrix, Inc.) as previously described.24 All microarray data met previously determined quality controls.28 CEL files were normalized using robust multi-array average (gcRMA).29, 30
Log2 expression values were analyzed using GenePlus software (Enodar Biologic, Seattle, WA, USA).24, 31 Two separate analyses were performed. The first analysis directly compared the expression profile between eight AML samples without FLT3/ITDs (FLT3/wild type (WT)) and five AML samples with high FLT3/ITD-AR (FLT3/ITDH-AR).24, 31 Expression changes with a Z-score ⩾4.75 or ⩽−4.75 were deemed to be statistically significant.32, 33 The second analysis examined expression changes across the three different groups: normal CD34 cells from BM (BM34), FLT3/WT and FLT3/ITDH-AR. For this analysis, the three groups were assigned a group number (BM34=0, FLT3/WT=1 and FLT3/ITDH-AR=2). A linear regression analysis examined the expression changes across the three groups.24, 31 Expression changes with a Z-score ⩾4.75 or ⩽−4.75 were deemed to be statistically significant.32, 33 Detailed clinical, cytogenetic and FLT3/ITD-AR information, along with the CEL files for each of the normal and AML samples, are available at the Gene Expression Omnibus.34 All normal BM34 samples and a portion of the AML samples have previously been used for other microarray analyses.24, 31
Network generation and pathway analyses
Affymetrix IDs and Z-scores for significant genes were analyzed using Ingenuity Pathways Analysis (IPA) software (Qiagen, Redwood City, CA, USA). Using the IPA software, we determined the significance of functional and canonical pathways as previously described.24 All relationships were supported by at least one literature reference and/or information from the Ingenuity Pathways Knowledge Base.35 Functions and canonical pathways with a P-value ⩽0.05 were considered to be statistically significant.24, 36 Networks were generated using genes identified from the microarray analyses and IPA Network algorithm. A Network Score was based on hypergeometric distribution and calculated using the right-tailed Fisher Exact Test, taking into account total number of network eligible genes in the Knowledge Base, the number of genes analyzed and network size: the higher the score, the less likely that the observed fit between the analyzed genes and the network was due to chance.24, 36
Results
SNP/CGH genomic profiling identifies CN-LOH at 13q locus
Diagnostic specimens from 85 patients with and without FLT3/ITDs were analyzed by SNP/CGH arrays. To control for the potential impact of blast variability, all specimens had >80% blasts at diagnosis, and leukemic blast populations were further enriched before the study by ficoll processing. The 85 patients displayed the following genotypes: FLT3/ITD 35/85 (41%), FLT3/ALM 11/85 (13%) and FLT3/WT 39/85 (46%). Patients with FLT3/ITD had ARs (that is, ratio of ITD to WT product) ranging from 0.2 to 7.05.
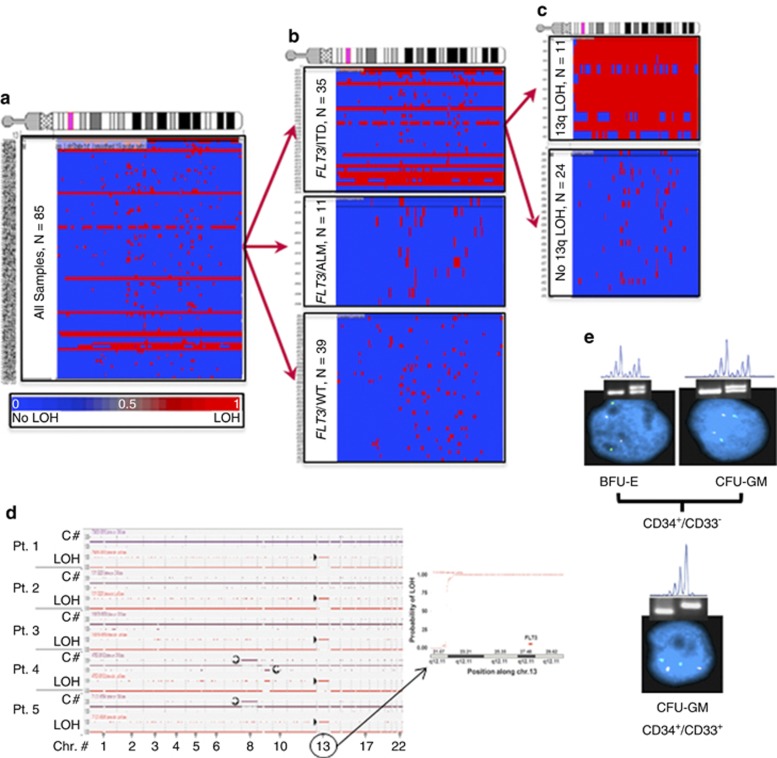
Using data from the Affymetrix Nsp arrays, chromosome 13 was evaluated for copy-number and LOH alterations, and analyses compared with samples with and without FLT3 mutations. Unclustered analysis of the entire cohort demonstrated large region of LOH in specimens from 12/85 patients (Figure 1a). Clustering analyses revealed that all samples with LOH13q contained FLT3/ITDs (Figures 1b and c), and the ITD-AR was substantially higher in those FLT3/ITD samples with LOH13q (median=2.5, range 1.21–8.88) as compared with FLT3/ITD samples without LOH13q (median=0.65, range 0.20–0.88). Those with LOH13q were further analyzed for copy-number alterations to determine whether observed LOH was due to large segmental deletion or CN-LOH. The observed LOH13q was not due to deletion, thus indicating the presence of CN-LOH at 13q (CN-LOH13q) in 12/35 specimens with FLT3/ITD. Furthermore, the region of CN-LOH conversion always involved the FLT3 gene (Figure 1d), and the LOH extended from a locus that was centromeric to FLT3 and encompassed the entire distal part of 13q. To define the transition point for the CN-LOH conversion, a detailed evaluation of the centromeric locus was performed. In 11/12 cases with CN-LOH13q, the CN-LOH transition occurred at 13q12.1, and in the remaining case, the entire 13q had undergone conversion.
Figure 1.
CN-LOH in FLT3/ITD samples with high allelic ratio. (a) Unclustered profile for chr13 for the entire cohort demonstrates segmental LOH (red color) in a subset of samples. (b) Clustering based on FLT3 genotype shows that a large segmental LOH segregates with FLT3/ITD. (c) Clustering of FLT3/ITD AML by LOH13q, segregates samples based on allelic ratio. (d) Simultaneous copy number (C#) and LOH alteration in five representative patients with LOH13q are shown (left panel). C# state represents patients with or without any copy-number changes. CN state of 1 represents no loss of copy number. Deletions are represented as a downward deflection (for example, patient 4, del9q,  ) and any duplication or trisomy are represented with a upward deflection from baseline (for example, patients 4 and 5, trisomy 8,
) and any duplication or trisomy are represented with a upward deflection from baseline (for example, patients 4 and 5, trisomy 8,  ). LOH state is represented as either 0 (no LOH, baseline) or 1 (LOH, upward deflection, patients 1–5, chr. 13,
). LOH state is represented as either 0 (no LOH, baseline) or 1 (LOH, upward deflection, patients 1–5, chr. 13,  ). Inset is a close up of Chr13 for patient number 5, demonstrating the region of transition into LOH (right panel). (e) Figure shows BFU-E or CFU-GM colonies derived from CD34+/CD33− myeloid progenitors and CFU-GM colonies derived from more differentiated CD34+/CD33+ cells. The cells were subjected to FLT3 genotyping by PCR and LOH determination by STR analysis and FISH. Colonies derived from CD34+/CD33− progenitors were heterozygous for FLT3/ITD (gel), with no LOH detected by STR and no deletions by FISH (green signals—control probe near chromosome 13 centromere, red signals—BAC probe that includes FLT3). Cultured colonies from more differentiated CD34+/CD33+ progenitors were homozygous for FLT3/ITD (gel), had LOH by STR, but showed no evidence of deletion by FISH, suggesting CN-LOH.
). Inset is a close up of Chr13 for patient number 5, demonstrating the region of transition into LOH (right panel). (e) Figure shows BFU-E or CFU-GM colonies derived from CD34+/CD33− myeloid progenitors and CFU-GM colonies derived from more differentiated CD34+/CD33+ cells. The cells were subjected to FLT3 genotyping by PCR and LOH determination by STR analysis and FISH. Colonies derived from CD34+/CD33− progenitors were heterozygous for FLT3/ITD (gel), with no LOH detected by STR and no deletions by FISH (green signals—control probe near chromosome 13 centromere, red signals—BAC probe that includes FLT3). Cultured colonies from more differentiated CD34+/CD33+ progenitors were homozygous for FLT3/ITD (gel), had LOH by STR, but showed no evidence of deletion by FISH, suggesting CN-LOH.
Single-cell analysis to detect subpopulations of leukemic blasts with potential CN-LOH13q
Given the intra-individual genomic heterogeneity of AML, we examined whether subpopulations of leukemic blasts may harbor CN-LOH13q, which would be undetected by standard SNP/CGH analyses. Individual leukemic blasts from five patients with known FLT3/ITD without previously identifiable CN-LOH13q by SNP/CGH analyses (see Figure 1d) were sorted into PCR tubes, and single-cell PCR assays were utilized to define FLT3/ITD status. FLT3 signal recovery for the single-cell assays ranged from 50 to 85% in each case. In each of the five diagnostic samples, three genotypic subpopulations were identified: (1) heterozygous FLT3/ITD, (2) homozygous FLT3/ITD and (3) FLT3/WT (Table 1). The homozygous FLT3/ITD genotype, likely owing to CN-LOH13q, was demonstrated in 13–34% cells of the five samples tested. These findings suggest that patients with low FLT3/ITD-AR at diagnosis may harbor CN-LOH13q in minor subpopulations of leukemic blasts. Single-cell analysis of paired diagnostic and relapsed samples from two patients demonstrated that the homozygous FLT3/ITD genotype became the dominant genotype at relapse (Table 1), indicating a clonal evolution to a more uniform population of leukemic blasts harboring CN-LOH13q upon relapse.
Table 1. Distribution of molecular subtypes of FLT3 gene in a single-cell assay.
|
Diagnostic |
Relapse |
|||||
|---|---|---|---|---|---|---|
| Heterozygous ITD | Homozygous ITD | FLT3/WT | Heterozygous ITD | Homozygous ITD | FLT3/WT | |
| Pt 1 | 27% | 21% | 62% | 10% | 83% | 7% |
| Pt 2 | 32% | 32% | 37% | 12% | 77% | 11% |
| Pt 3 | 3% | 13% | 83% | ND | ND | ND |
| Pt 4 | 21% | 26% | 53% | ND | ND | ND |
| Pt 5 | 16% | 34% | 50% | ND | ND | ND |
Abbreviations: ITD, internal tandem duplication; ND, not determined; WT, wild type.
CN-LOH13q is a late event in AML pathogenesis
In order to evaluate the sequence of events leading to CN-LOH13q, CD34+/CD33− and CD34+/CD33+ progenitor cells from two patient samples with CN-LOH13q (high FLT3/ITD-AR) were isolated and subjected to colony-forming cell culture. Erythroid burst-forming unit (BFU-E) and granulocyte, monocyte colony-forming unit (CFU-GM) colonies were sequentially evaluated by PCR for FLT3/ITD status, short tandem repeat analyses (STR) for LOH, and FISH for genetic loss. BFU-E and CFU-GM colonies grown from CD34+/CD33− leukemic blasts (less differentiated population) demonstrated heterozygous ITD and no LOH by STR, indicating that the less differentiated progenitors within the colony harbored FLT3/ITDs but had not yet developed LOH. As expected, the FISH results for this population uniformly demonstrated two copies of chromosome 13 and no deletion of FLT3. In contrast, CFU-GM colonies cultured from more differentiated CD34+/CD33+ leukemic blasts uniformly demonstrated homozygous ITD and LOH13q by STR evaluation but still showed no evidence of chromosome 13 loss or deletion of FLT3 by FISH (Figure 1e). This data demonstrates that FLT3/ITD evolves initially in the progenitor population as a heterozygous clone (that is, no CN-LOH13q), and subsequent events likely lead to the evolution of CN-LOH13q in the more differentiated population (Figure 1e).
Gene expression changes associated with CN-LOH13q
To identify expression changes potentially associated with the development of homozygous ITD status and subsequent CN-LOH13, microarray studies were performed using leukemic samples from AML patients without FLT3/ITD (FLT3/WT, N=8) and those with very high FLT3/ITD ARs (FLT3/ITDH-AR, N=5). To reduce the potential impact of normal hematopoietic cells and leukemic maturation stage on the expression profile, only AML samples with high blast counts (>80%) that expressed CD34 were selected for the microarray studies (Supplementary Table 1). A direct comparison of expression profiles between FLT3/WT and FLT3/ITDH-AR identified 84 genes and 2 unique probe sets (that is, sequences without an annotated gene) with significant expression differences between the two sample groups (Supplementary Table 2). Using FLT3/WT samples as the baseline, 43 genes and 1 unique probe set displayed increased expression in the FLT3/ITDH-AR samples, four of which were HOX family members, whereas 41 genes and 1 unique probe set displayed a decreased expression (Supplementary Figure 1). The uniformity in expression of top candidate genes suggests that presence of other known mutations (for example, NPM1 and IDH1) may not be a major contributor to expression differences observed between FLT3/ITDH-AR and FLT3/WT samples (Supplementary Table 1).
IPA software was used to identify significant functions, networks and canonical pathways associated with the 84 identified genes. The top 20 functions associated with the 84 genes are listed in Table 2, and the top 12 networks are provided in Supplementary Table 3. The most significant functions were1 cancer,2 cellular growth and proliferation and3 hematologic system function and development. Not surprisingly, the network with the highest score was comprised of genes associated with similar functions: cellular proliferation, hematologic development and function, and immune response. The only canonical pathway associated with the 84 genes was the cell cycle pathway that regulates G2/M checkpoint for DNA damage (that is, cell cycle: G2/M DNA damage checkpoint regulation).
Table 2. Top functions for genes with significant expression differences between FLT3/WT and FLT3/ITDH-AR samples.
| Function | P-value | Number of genes associated with function |
|---|---|---|
| aCancer | 4.50E-04–3.70E-02 | 28 |
| aCellular growth and proliferation | 8.66E-05–3.57E-02 | 23 |
| aHematological system development and function | 3.82E-05–3.67E-02 | 16 |
| aImmune response | 1.86E-04–3.67E-02 | 15 |
| Immune and lymphocyte development and function | 1.86E-04–3.57E-02 | 14 |
| Small molecule biochemistry | 9.31E-05–3.53E-02 | 12 |
| aHematologic disease | 4.61E-04–3.70E-02 | 12 |
| Cellular development | 3.82E-05–3.81E-02 | 11 |
| aImmunologic disease | 4.50E-04–3.33E-02 | 11 |
| Neurological disease | 1.09E-03–3.33E-02 | 9 |
| aCell cycle | 1.31E-03–3.56E-02 | 9 |
| Molecular transport | 9.31E-05–3.53E-02 | 8 |
| Tissue development | 1.86E-04–3.33E-02 | 7 |
| aCell morphology | 4.61E-04–3.33E-02 | 7 |
| Lipid metabolism | 9.31E-05–3.53E-02 | 6 |
| Cellular compromise | 4.61E-04–3.33E-02 | 5 |
| Genetic disorders | 1.09E-03–3.33E-02 | 5 |
| Gene expression | 3.08E-04–2.78E-02 | 4 |
| Nucleic acid metabolism | 1.09E-03–3.33E-02 | 4 |
| Hepatic system disease | 1.66E-03–1.66E-03 | 2 |
Abbreviations: ITD, internal tandem duplication; WT, wild type.
Represents an overlap of top functions between the two analyses evaluating (a) FLT3/WT and FLT3/ITDH-AR and (b) BM34, FLT3/WT and FLT3/ITDH-AR.
AML samples can harbor CN-LOH at loci other than 13q.17, 18, 37, 38, 39, 40 Thus, AML samples with CN-LOH at other loci may display the same or similar expression changes as those with CN-LOH13q. To further examine potential expression changes associated with CN-LOH, we incorporated the expression profiles of normal CD34 cells from the BM (BM34, N=8) into our analyses, which are unlikely to harbor CN-LOH.41 For these analyses, samples were divided into three groups based on the likelihood of harboring CN-LOH: normal BM34 (unlikely to harbor CN-LOH), FLT3/WT (unlikely to have CN-LOH13 but potentially harboring CN-LOH at other regions) and FLT3/ITDH-AR (CN-LOH13 likely in the majority of blasts and potential CN-LOH at other loci). The normal BM34 were designated as the ‘control' and given the covariate of 0, whereas FLT3/WT and FLT3/ITDH-AR were designated as having covariates of 1 and 2, respectively. Linear regression analysis identified 642 genes and 3 unique probe sets with significant expression changes (Supplementary Table 4). The analysis identified genes with relatively heterogeneous expression changes within the FLT3/WT group, which became more homogenous in the FLT3/ITDH-AR samples (Figures 2a and b). The presence of other mutations did not appear to explain these mutation differences. Approximately 55% of the genes/unique probe sets (N=364) displayed a linear increase in expression with increasing certainty of CN-LOH presence, while 45% (N=281) displayed decreasing expression with increasing certainty of CN-LOH presence (Figure 2c). Thirty-three of these genes were also identified in the previous analysis comparing AML patients with FLT3/WT vs FLT3/ITDH-AR.
Figure 2.
Linear expression changes associated with likelihood of CN-LOH. (a) Figure shows the log2 expression (y-axis) of stabilin 1 (STAB1, potential role in regulating lipid metabolism and angiogenesis) across normal BM34 (N=8), FLT3/WT (N=8) and FLT3/ITDH-AR (N=5) samples. As depicted in the figure, the expression significantly increases (Z=17.09, P<0.01), yet there is considerable heterogeneity in its expression within the FLT3/WT group. (b) Figure shows the log2 expression (y-axis) of serpin pepitidase inhibitor, clade B (SERPINE2, potential role in regulating apoptosis and differentiation) across normal BM34, FLT3/WT and FLT3/ITDH-AR samples. As depicted in the figure, the expression significantly increases (Z=−20.90, P<0.01), and as with STAB1, there is heterogeneity in its expression within the FLT3/WT group. (c) Heatmaps show increasing and decreasing expression (y-axis) of 364 and 281 genes from two analyses, respectively, across the three different subpopulations of cells (x-axis). Expression range is provided in the lower right corner. Expression for each gene within a group represents the average expression of the gene across all samples within the group. Overall, as the likelihood of CN-LOH increases, so does the uniformity of the expression and differences in the expression within the normal and malignant cell populations.
Similar to the first analysis, IPA software was used to identify significant functions, networks and canonical pathways associated with the 642 significant genes. The top 20 functions are provided in Table 3, and as expected, there was considerable overlap among the significant functions associated with 84 and 642 gene sets (Tables 2 and 3). The top five networks are provided in Supplementary Table 5. The network with the highest score was comprised of genes associated with cell cycle, cellular movement and cancer. When considering the top 20 networks (Supplementary Table 6), the three most frequently represented functions were (1) cancer, (2) cell cycle and (3) DNA replication, recombination and repair. As DNA replication, recombination and repair likely have a central role in the development of CN-LOH, we examined the genes associated with these functions and their relationship in more detail (Table 4). All the genes within this functional category displayed decreasing expression with increasing likelihood of CN-LOH being present (that is, BM34 to FLT3/WT to FLT3/ITDH-AR).
Table 3. Top functions for genes with significant linear expression changes in BM34, FLT3/WT and FLT3/ITDH-AR samples.
| Function | P-value | Number of genes associated with function |
|---|---|---|
| aCancer | 1.67E-08–1.36E-02 | 196 |
| aCellular growth and proliferation | 5.91E-06–1.33E-02 | 173 |
| Cell death | 1.37E-06–1.39E-02 | 158 |
| aHematologic disease | 2.40E-06–1.36E-02 | 104 |
| aCell cycle | 9.28E-07–1.41E-02 | 92 |
| aImmunologic disease | 1.61E-05–1.36E-02 | 91 |
| Cellular movement | 8.03E-07–1.36E-02 | 91 |
| aHematological system development and function | 8.03E-07–1.36E-02 | 86 |
| Inflammatory disease | 3.51E-07–1.41E-02 | 85 |
| aImmune response | 8.03E-07–1.41E-02 | 75 |
| DNA replication, recombination and repair | 2.79E-07–1.29E-02 | 73 |
| Gastrointestinal disease | 3.53E-06–1.36E-02 | 68 |
| Skeletal and muscular disorders | 4.24E-06–1.36E-02 | 60 |
| aCell morphology | 4.81E-06–1.36E-02 | 58 |
| Connective tissue disorders | 9.86E-07–3.79E-05 | 53 |
| Reproductive system diseases | 1.70E-06–1.03E-02 | 46 |
| Cell-to-cell signaling and interaction | 1.35E-05–1.36E-02 | 39 |
| Cellular assembly and organization | 2.79E-07–1.36E-02 | 29 |
| Cell function and maintenance | 1.35E-05–1.36E-02 | 23 |
| Renal and urological disease | 4.24E-05–1.36E-02 | 18 |
Abbreviations: ITD, internal tandem duplication; WT, wild type.
Represents an overlap of top functions between the two analyses evaluating (a) FLT3/WT and FLT3/ITDH-AR and (b) BM34, FLT3/WT and FLT3/ITDH-AR.
Table 4. Downregulated genes within DNA replication, recombination and repair pathways associated with FLT3/ITDH-AR.
| Process annotation | P-value | Genes/molecules | Number of molecules |
|---|---|---|---|
| Recombination of DNA | 2.50E-03 | CHEK1, DKFZP762E1312, HMGB1, KPNA2, MSH6, REC8, RUVBL2, XRCC5 | 8 |
| Checkpoint control | 3.67E-03 | BUB3, BUB1, CDC6, CDC20, CDK2, CHEK1, MCM7, NDC80 | 8 |
| Double-stranded DNA break repair | 4.96E-03 | FEN1, KPNA2, POLA1, SOD1, SOD2, XRCC5 | 6 |
Abbreviation: ITD, internal tandem duplication.
Process associated with the overall function category (first column), P-value for significance of the process (column 2), genes within the process (column 3, all with decreasing expression from BM34 to FLT3/WT to FLT3/ITDH-AR), and number of genes within the network (column 4) are provided. Overall, the data suggest that the expression of genes is downregulated in these functional pathways as the likelihood of harboring CN-LOH increases.
Discussion
In this study, we demonstrate that not only CN-LOH13q is detected in a subset of FLT3/ITD patients with very high AR (AR >1), but we show lack of CN-LOH13q in patients without FLT3/ITD, suggesting a potential causal link between FLT3/ITD and evolution of CN-LOH13q. We also demonstrate that presence of CN-LOH13q is not limited to those with very high ITD-AR, but in fact, as demonstrated by single-cell FLT3 genotyping, CN-LOH13q can be present as a minor clone in FLT3/ITD cases with low AR, bringing into question what are the additional events that may lead to clonal dominance of this homozygous clone. The results from our studies indicate that the evolution of CN-LOH13q likely represents a late event in the pathogenesis of high FLT3/ITD-ARs, such that FLT3/ITD initially develops as a heterozygous mutation with the corresponding WT FLT3 allele. Over time, the heterozygous FLT3/ITD blasts acquire CN-LOH13q, resulting in homozygous mutated alleles and a high FLT3/ITD-AR.
Previous genome-wide studies have identified CN-LOH at other loci in AML and in a variety of different hematopoietic malignancies.17, 18, 37, 38, 39, 40, 42 These other loci also frequently contain oncogenes or tumor suppressors (for example, TP53, MPL, NRAS and so on).39 As with FLT3/ITD and CN-LOH13q, the development of CN-LOH at these loci also results in homozygous mutant alleles and the loss of expression of the WT protein. The relative expression of mutant vs WT proteins (that is, ‘mutant dosage effect') likely has a critical role in malignant transformation and disease progression,43 and a mutant dosage effect has been demonstrated for RAS mutations.44, 45, 46, 47, 48, 49, 50 Like FLT3/ITDs, RAS mutations enhance proliferation and likely provide a growth advantage.44, 48, 49, 50 For example, hematopoietic progenitors with homozygous NRAS mutations develop a phenotypically different hematopoietic malignancy than similar progenitors harboring heterozygous NRAS mutations.50 In the case of FLT3/ITDs, CN-LOH13q also likely provides a growth and/or survival advantage through the establishment of a homozygous FLT3/ITD state.43 Higher FLT3/ITD-AR (that is, likely to harbor CN-LOH13q) are associated with higher relapse rates (that is, more resistant disease),14 and are found more frequently at relapse.
Few studies have examined the temporal relationship between the development of mutations and CN-LOH. Wang et al.50, 51 used a Cre–Lox murine model to introduce a single NRAS mutant allele into hematopoietic stem cells. Using this model, they showed that ∼40% of mice harboring a heterozygous NRAS mutation subsequently acquired CN-LOH at the NRAS locus, resulting in a homozygous NRAS mutant state. Recently, similar murine models have been used to examine the cooperative nature between FLT3/ITDs and NPM1 mutations.43 In these experiments, transgenic mice harboring heterozygous mutations for both FLT3/ITD and NPM1 rapidly developed an AML-like disease. Interestingly, LOH of the WT FLT3 locus was observed in a very high percentage of the mice with the most rapid disease progression.43 Thus, the available data from NRAS and FLT3/ITD murine models are in keeping with our findings, which indicate that FLT3/ITD mutations in humans likely precede the development of LOH.
Several theories have been proposed to explain the potential mechanism linking genomic mutations and CN-LOH.39, 52 Expanding upon previous mechanistic models, it can be hypothesized that some mutations, particularly those with extensive deletions or insertions, may destabilize the DNA structure in ways that directly promote CN-LOH during mitotic recombination or during failed attempts to correct the mutant allele. According to this model, CN-LOH develops in a ‘deterministic' manner, occurring initially at the mutated locus. Alternatively, CN-LOH may develop in a more ‘stochastic' manner. According to this model, CN-LOH randomly develops throughout the genome of leukemic blasts. Clones developing CN-LOH at loci containing advantageous mutations are perpetuated, while those clones developing CN-LOH in normal allelic regions will have no survival advantage and eventually perish. This stochastic model does not exclude the possibility that the mutations have a role in the initial development of CN-LOH. For example, certain oncogenic mutations may disrupt key regulatory pathways that control genomic fidelity, apoptosis and proliferation. In the case of FLT3/ITD mutations, the fact that CN-LOH13q is not observed in FLT3/WT cases is highly suggestive of causal link between FLT3/ITD and evolution of CN-LOH13q. In addition, the expression data from others suggest that many pathways are disrupted in leukemic blasts harboring ITD mutations.53, 54, 55 In the current study, we identified a number of expression differences between FLT3/WT and FLT3/ITDH-AR (Figure 2; Tables 2, 3, 4). Some of these expression changes involve the DNA repair/recombination pathway, which, if antedate the development of CN-LOH, may have a role in its development. Hence, the disruption of these critical functions may predispose cells to the development of CN-LOH throughout the genome, eventually leading to the emergence of a dominant clone with FLT3/ITDH-AR. However, the number of samples evaluated for expression studies were limited, and we cannot rule out that other potential confounders may be contributing to the observed expression changes. Additional studies are needed to better dissect out the molecular mechanisms underlying these expression differences and determine the potential etiology driving the development of CN-LOH in FLT3/ITD blasts.
Acknowledgments
This work was supported in part by National Institute of Health grant numbers CA114563 (SM) and CA160872 (DLS).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Author contributions
Conception and design was by DLS and SM. Development of methodology (FISH, SNP/CGH, PCR and expression arrays) was by KT, JJ and ELP-A. Analysis and interpretation of data was done by DLS, KT, ELP-A, JJ and SM. Writing, review and/or revision of the manuscript was done by DLS, KT, ELP-A, JJ and SM.
Supplementary Material
References
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- Matsuno N, Nanri T, Kawakita T, Mitsuya H, Asou N. A novel FLT3 activation loop mutation N841K in acute myeloblastic leukemia. Leukemia. 2005;19:480–481. doi: 10.1038/sj.leu.2403630. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- Lavagna-Sevenier C, Marchetto S, Birnbaum D, Rosnet O. FLT3 signaling in hematopoietic cells involves CBL, SHC and an unknown P115 as prominent tyrosine-phosphorylated substrates. Leukemia. 1998;12:301–310. doi: 10.1038/sj.leu.2400921. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- Abu-Duhier FM, Goodeve AC, Wilson GA, Gari MA, Peake IR, Rees DC, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111:190–195. doi: 10.1046/j.1365-2141.2000.02317.x. [DOI] [PubMed] [Google Scholar]
- Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of FLT3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Bacher U, Kern W, Alpermann T, Haferlach C, Haferlach T. Prognostic impact of FLT3-ITD load in NPM1 mutated acute myeloid leukemia. Leukemia. 2011;25:1297–1304. doi: 10.1038/leu.2011.97. [DOI] [PubMed] [Google Scholar]
- Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Mason J, Rindl M, Akiki S, McMullan D, Stinton V, et al. Acquired isodisomy for chromosome 13 is common in AML, and associated with FLT3-itd mutations. Leukemia. 2005;19:2355–2358. doi: 10.1038/sj.leu.2403988. [DOI] [PubMed] [Google Scholar]
- Raghavan M, Lillington DM, Skoulakis S, Debernardi S, Chaplin T, Foot NJ, et al. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–378. [PubMed] [Google Scholar]
- Dunbar AJ, Gondek LP, O'Keefe CL, Makishima H, Rataul MS, Szpurka H, et al. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 2008;68:10349–10357. doi: 10.1158/0008-5472.CAN-08-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- Lange BJ, Dinndorf P, Smith FO, Arndt C, Barnard D, Feig S, et al. Pilot study of idarubicin-based intensive-timing induction therapy for children with previously untreated acute myeloid leukemia: Children's Cancer Group Study 2941. J Clin Oncol. 2004;22:150–156. doi: 10.1200/JCO.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Affymetrix I.CNAT 4.0: Copy Number and Loss of Heterozygosity Estimation Algorithms for the GeneChip® Human Mapping 10/50/100/250/500 K Array Set, 2007 (cited 2013 0716/2013); revision version 1.2:(available from http://www.affymetrix.com/esearch/search.jsp?N=4000097&Ntt=cnat+4.0&st=doc .
- Konings P, Vanneste E, Jackmaert S, Ampe M, Verbeke G, Moreau Y, et al. Microarray analysis of copy number variation in single cells. Nat Protoc. 2012;7:281–310. doi: 10.1038/nprot.2011.426. [DOI] [PubMed] [Google Scholar]
- Pollard JA, Alonzo TA, Gerbing RB, Woods WG, Lange BJ, Sweetser DA, et al. FLT3 internal tandem duplication in CD34+/CD33− precursors predicts poor outcome in acute myeloid leukemia. Blood. 2006;108:2764–2769. doi: 10.1182/blood-2006-04-012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirewalt DL, Meshinchi S, Kopecky KJ, Fan W, Pogosova-Agadjanyan EL, Engel JH, et al. Identification of genes with abnormal expression changes in acute myeloid leukemia. Genes Chromosomes Cancer. 2008;47:8–20. doi: 10.1002/gcc.20500. [DOI] [PubMed] [Google Scholar]
- Ho PA, Kutny MA, Alonzo TA, Gerbing RB, Joaquin J, Raimondi SC, et al. Leukemic mutations in the methylation-associated genes DNMT3A and IDH2 are rare events in pediatric AML: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:204–209. doi: 10.1002/pbc.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PA, Kopecky KJ, Alonzo TA, Gerbing RB, Miller KL, Kuhn J, et al. Prognostic implications of the IDH1 synonymous SNP rs11554137 in pediatric and adult AML: a report from the Children's Oncology Group and SWOG. Blood. 2011;118:4561–4566. doi: 10.1182/blood-2011-04-348888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostronoff F, Othus M, Ho PA, Kutny M, Geraghty DE, Petersdorf SH, et al. Mutations in the DNMT3A exon 23 independently predict poor outcome in older patients with acute myeloid leukemia: a SWOG report. Leukemia. 2013;27:238–241. doi: 10.1038/leu.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, Abeygunawardena N, et al. ArrayExpress—a public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 2003;31:68–71. doi: 10.1093/nar/gkg091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Choi YE, Sharpless NE, Pogosova-Agadjanyan EL, Cronk MR, Yukawa M, et al. Decreased IRF8 expression found in aging hematopoietic progenitor/stem cells. Leukemia. 2009;23:391–393. doi: 10.1038/leu.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XL, Olson JM, Zhao LP. A regression-based method to identify differentially expressed genes in microarray time course studies and its application in an inducible Huntington's disease transgenic model. Hum Mol Genet. 2002;11:1977–1985. doi: 10.1093/hmg/11.17.1977. [DOI] [PubMed] [Google Scholar]
- Gene Expression Omnibus (database on the internet), 2007. Available from: http://www.ncbi.nlm.nih.gov/geo/ .
- Ingenuity S. FAQs about statistical considerations, 2006 (cited 2013 7/15/2013). Available from: http://ingenuity.force.com/ipa/IPATutorials?id=kA250000000TNACCA4 .
- Stirewalt DL, Mhyre AJ, Marcondes M, Pogosova-Agadjanyan E, Abbasi N, Radich JP, et al. Tumour necrosis factor-induced gene expression in human marrow stroma: clues to the pathophysiology of MDS. Br J Haematol. 2008;140:444–453. doi: 10.1111/j.1365-2141.2007.06923.x. [DOI] [PubMed] [Google Scholar]
- Akagi T, Ogawa S, Dugas M, Kawamata N, Yamamoto G, Nannya Y, et al. Frequent genomic abnormalities in acute myeloid leukemia/myelodysplastic syndrome with normal karyotype. Haematologica. 2009;94:213–223. doi: 10.3324/haematol.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T, Shih LY, Kato M, Kawamata N, Yamamoto G, Sanada M, et al. Hidden abnormalities and novel classification of t(15;17) acute promyelocytic leukemia (APL) based on genomic alterations. Blood. 2009;113:1741–1748. doi: 10.1182/blood-2007-12-130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe C, McDevitt MA, Maciejewski JP. Copy neutral loss of heterozygosity: a novel chromosomal lesion in myeloid malignancies. Blood. 2010;115:2731–2739. doi: 10.1182/blood-2009-10-201848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak D, Klaumuenzer M, Hanfstein B, Mossner M, Nolte F, Nowak V, et al. SNP array analysis of acute promyelocytic leukemia may be of prognostic relevance and identifies a potential high risk group with recurrent deletions on chromosomal subband 1q31.3. Genes Chromosomes Cancer. 2012;51:756–767. doi: 10.1002/gcc.21961. [DOI] [PubMed] [Google Scholar]
- Liehr T. Cytogenetic contribution to uniparental disomy (UPD) Mol Cytogenet. 2010;3:8. doi: 10.1186/1755-8166-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Huh J, Kim HJ, Kim SH, Kim HJ, Kim YK, et al. Adverse prognostic impact of abnormal lesions detected by genome-wide single nucleotide polymorphism array-based karyotyping analysis in acute myeloid leukemia with normal karyotype. J Clin Oncol. 2011;29:4702–4708. doi: 10.1200/JCO.2011.35.5719. [DOI] [PubMed] [Google Scholar]
- Mupo A, Celani L, Dovey O, Cooper JL, Grove C, Rad R, et al. A powerful molecular synergy between mutant Nucleophosmin and Flt3-ITD drives acute myeloid leukemia in mice. Leukemia. 2013;27:1917–1920. doi: 10.1038/leu.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistonen L, Keski-Oja J, Ulmanen I, Holtta E, Wikgren BJ, Alitalo K. Dose effects of transfected c-Ha-rasVal 12 oncogene in transformed cell clones. Exp Cell Res. 1987;168:518–530. doi: 10.1016/0014-4827(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Venkatachalam S, Shi YP, Jones SN, Vogel H, Bradley A, Pinkel D, et al. Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation. EMBO J. 1998;17:4657–4667. doi: 10.1093/emboj/17.16.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y, et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet. 2004;36:624–630. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu Y, Li Z, Wang Z, Tan LX, Ryu MJ, et al. Endogenous oncogenic Nras mutation initiates hematopoietic malignancies in a dose- and cell type-dependent manner. Blood. 2011;118:368–379. doi: 10.1182/blood-2010-12-326058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu Y, Li Z, Du J, Ryu MJ, Taylor PR, et al. Endogenous oncogenic Nras mutation promotes aberrant GM-CSF signaling in granulocytic/monocytic precursors in a murine model of chronic myelomonocytic leukemia. Blood. 2010;116:5991–6002. doi: 10.1182/blood-2010-04-281527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuna M, Knuutila S, Mills GB. Uniparental disomy in cancer. Trends Mol Med. 2009;15:120–128. doi: 10.1016/j.molmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Lacayo NJ, Meshinchi S, Kinnunen P, Yu R, Wang Y, Stuber CM, et al. Gene expression profiles at diagnosis in de novo childhood AML patients identify FLT3 mutations with good clinical outcomes. Blood. 2004;104:2646–2654. doi: 10.1182/blood-2003-12-4449. [DOI] [PubMed] [Google Scholar]
- Radmacher MD, Marcucci G, Ruppert AS, Mrozek K, Whitman SP, Vardiman JW, et al. Independent confirmation of a prognostic gene-expression signature in adult acute myeloid leukemia with a normal karyotype: a Cancer and Leukemia Group B study. Blood. 2006;108:1677–1683. doi: 10.1182/blood-2006-02-005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balgobind BV, Van den Heuvel-Eibrink MM, De Menezes RX, Reinhardt D, Hollink IH, Arentsen-Peters ST, et al. Evaluation of gene expression signatures predictive of cytogenetic and molecular subtypes of pediatric acute myeloid leukemia. Haematologica. 2011;96:221–230. doi: 10.3324/haematol.2010.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.