Abstract
Immunosuppressive therapy (IST), consisting of antithymocyte globulin and cyclosporine A, is effective in refractory cytopenia of childhood (RCC), suggesting that, similar to low-grade myelodysplastic syndromes in adult patients, T lymphocytes are involved in suppressing hematopoiesis in a subset of RCC patients. However, the potential role of a T-cell-mediated pathophysiology in RCC remains poorly explored. In a cohort of 92 RCC patients, we prospectively assessed the frequency of T-cell receptor (TCR) β-chain variable (Vβ) domain skewing in bone marrow and peripheral blood by heteroduplex PCR, and analyzed T-cell subsets in peripheral blood by flow cytometry. TCRVβ skewing was present in 40% of RCC patients. TCRVβ skewing did not correlate with bone marrow cellularity, karyotype, transfusion history, HLA-DR15 or the presence of a PNH clone. In 28 patients treated with IST, TCRVβ skewing was not clearly related with treatment response. However, TCRVβ skewing did correlate with a disturbed CD4+/CD8+ T-cell ratio, a reduction in naive CD8+ T cells, an expansion of effector CD8+ T cells and an increase in activated CD8+ T cells (defined as HLA-DR+, CD57+ or CD56+). These data suggest that T lymphocytes contribute to RCC pathogenesis in a proportion of patients, and provide a rationale for treatment with IST in selected patients with RCC.
Introduction
Myelodysplastic syndromes (MDS), which are characterized by clonal hematopoiesis, impaired differentiation and maturation of myeloid cells, peripheral blood cytopenias and a risk of progression to acute myeloid leukemia, are rare in childhood, with an estimated annual incidence of 0.8–1.8 per million children aged 0–14 years.1, 2, 3 The most common variant of pediatric MDS is refractory cytopenia of childhood (RCC), defined as myelodysplasia without an increased blast count. About 80% of children with RCC have a hypocellular bone marrow, and karyotype is normal in the majority of patients.4, 5
Intrinsic hematopoietic stem cell defects, caused by acquired cytogenetic and molecular aberrations or by epigenetic changes, result in hallmark features of MDS.6, 7 However, evidence obtained in adult MDS patients also suggests that a T-cell-mediated immune response directed against hematopoietic progenitor cells contributes to MDS pathophysiology. Clinically, immunosuppressive therapy (IST) consisting of antithymocyte globulin (ATG), which specifically targets T cells, with or without cyclosporine A, is effective in selected patients.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Furthermore, in vitro experiments demonstrated that autologous peripheral blood lymphocytes of MDS patients inhibit granulocyte colony formation in a major histocompatibility complex class I-dependent manner;19, 20, 21, 22 this inhibitory effect was abrogated by ATG in the few patients studied.19 Subsequently, analysis of the T-cell receptor (TCR) β-chain variable (Vβ) domain usage by flow cytometry and PCR-based methods showed oligoclonal expansions of mainly CD8+ T cells in MDS patients.19, 21, 23, 24, 25, 26 These clonally expanded T cells were revealed to have an activated and effector phenotype.21, 27, 28, 29
We recently reported that cyclosporine A and ATG are effective in RCC,30 and that over half of RCC patients display a skewed TCRVβ complementarity-determining region 3 (CDR3) usage,31 which is representative of clonal T-cell expansion. These findings indicate that an immune-mediated pathophysiology might also be present in a proportion of RCC patients. However, apart from the latter studies, the potential role of a T-cell-mediated pathophysiology in RCC remains unexplored. In a prospective study conducted by the European Working Group of MDS in Childhood (EWOG-MDS), we therefore assessed the frequency of TCRVβ skewing in bone marrow and peripheral blood obtained from a cohort of 92 RCC patients, correlated TCRVβ skewing with clinical and laboratory characteristics, and analyzed the T-cell subset composition of peripheral blood. We here describe that T-cell oligoclonality is frequently present in RCC, correlates with a disturbed CD4+/CD8+ T-cell ratio, an expansion of effector CD4+ and CD8+ T cells, and an activated phenotype of CD8+ T cells. Altogether, our data suggest that T cells are actively involved in RCC pathogenesis in a substantial proportion of patients.
Materials and methods
Patients and controls
Peripheral blood samples for PNH analysis were obtained from 92 consecutive, treatment-naive primary RCC patients, ⩽18 years of age (Table 1). Patients were diagnosed according to World Health Organization criteria5 between June 2005 and December 2011, enrolled in the prospective, multicenter studies EWOG-MDS 2006 and EWOG-MDS RC06 (ClinicalTrial.gov identifiers: NCT00662090 and NCT00499070). Peripheral blood and bone marrow samples obtained from 29 pediatric patients (median age: 13.2 years; range: 2–18) with (very) severe aplastic anemia ((v)SAA) served as controls for TCRVβ analysis. Peripheral blood samples obtained from 152 healthy subjects (age <2 years, n=53; 2–4 years, n=27; 5–9 years, n=30; 10–15 years, n=20; >15 years, n=22) served as controls for T-cell subset analysis. Further details are described in Supplementary Methods.
Table 1. Clinical and laboratory characteristics of included RCC patients.
| Characteristic | |
|---|---|
| Number of patients | 92 |
| Median age at diagnosis, years (range) | 10.3 (1–18) |
| Male sex, no. (%) | 46 (50) |
| Hypocellular bone marrow, no. (%) | 74 of 90 (82) |
| Cytogenetics, no. (%)a | |
| Normal | 67 of 78 (86) |
| Monosomy 7b | 5 of 78 (6) |
| Otherc | 6 of 78 (8) |
Abbreviation: RCC, refractory cytopenia of childhood.
In 12 of 90 patients (13%) no karyotype was obtained due to insufficient metaphases; in 2 patients no data were available.
Includes one patient with monosomy 7 and other aberrations.
Includes one patient with del(7q), two patients with trisomy 8 and three patients with rare aberrations.
Immunosuppressive therapy
A subset of RCC patients included for TCRVβ analysis was treated with IST consisting of horse or rabbit-ATG, prednisolone and cyclosporine A. Response to IST was evaluated on day 180. Treatment details and response criteria are described in Supplementary Methods.
TCRVβ analysis by heteroduplex PCR
TCRVβ CDR3 repertoire analysis by heteroduplex PCR in mononuclear cells was performed as previously described.31, 32 Skewing was defined when two or more TCRVβ families showed an oligoclonal pattern on heteroduplex gel. This cutoff was based on healthy controls, in whom skewing in more than one TCRVβ family was present in only 2 of 18 cases (11%).31 Skewing was further subdivided into weak skewing, when an oligoclonal pattern was found in 2–5 TCRVβ families, and strong skewing, when an oligoclonal pattern was found in >5 TCRVβ families.31 Detailed methods may be found in Supplementary Methods.
T-cell subset analysis by flow cytometry
Fresh peripheral blood samples were processed, stained and acquired on a FACSCanto II flow cytometer (BD Biosciences, Erembodegem, Belgium). Data were analyzed using FlowJo software version 7.6.5 (Tree Star, Ashland, OR, USA). Lymphocyte subsets were gated and defined as described in Supplementary Methods, and compared between RCC patients and healthy controls after categorizing patients and controls into age groups as previously published (<2, 2–4, 5–9, 10–15, >15 years),33 whereas lymphocyte and T-cell subset distributions in skewed and non-skewed RCC patients were compared after normalization according to age group using Z-scores as described below.
Statistical analyses
Statistical analyses were performed with SPSS 20 (IBM, Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Categorical variables were compared using the chi-square test or Fisher's exact test. Continuous variables were compared using the Mann–Whitney U-test or the Kruskal–Wallis test when more than two groups were compared. To compare relative lymphocyte and T-cell subset distributions between RCC patients with and without a skewed T-cell repertoire, taking age-dependent changes in into account, Z-scores were calculated as Z=(X–μ)/σ, with X=raw score, μ=mean of age-matched control group, σ=s.d. of the age-matched control group. All reported P-values are two-sided and were considered statistically significant when <0.05; P-values >0.1 were reported as nonsignificant (NS), whereas those between 0.05 and 0.1 were reported in detail.
Results
Patient characteristics
A total of 92 treatment-naive RCC patients (46 male, 46 female), with a median age at diagnosis of 10 years (range: 1–18 years), were analyzed by heteroduplex PCR for TCRVβ CDR3 skewing in bone marrow and/or peripheral blood. Median time from diagnosis to TCRVβ analysis was 43 days. Clinical characteristics of the included patients are summarized in Table 1. Hypocellular bone marrow was reported in 82% of patients, which is comparable to the previously reported frequency of 81% of primary RCC patients in an interim analysis of studies EWOG-MDS 1998 and 2006.4 Conventional cytogenetics displayed a normal karyotype in 67 of 78 patients (86%), which is slightly higher than the previously reported frequency of 77%4 monosomy 7 in 5 of 78 patients (including 1 patient with monosomy 7 and additional aberrations) (6%) and other cytogenetic aberrations in 6 of 78 patients (trisomy 8 in 2 patients, rare aberrations in 3 patients and del(7q) in 1 patient) (8%). In 12 patients karyotype was not obtained owing to insufficient metaphases and in 2 patients no information was available.
TCRVβ skewing frequently occurs in bone marrow and peripheral blood of RCC patients
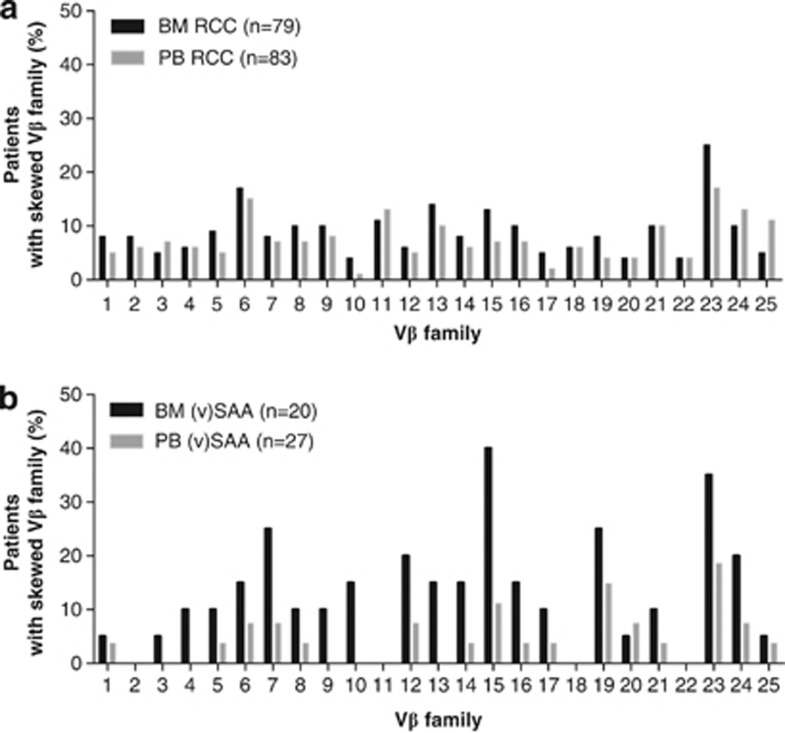
TCRVβ CDR3 skewing in the bone marrow occurred in 31 of 79 RCC patients (39%) who were successfully analyzed. Of the 31 patients with a skewed TCRVβ CDR3 usage, 20 (25%) displayed weak skewing and 11 (14%) strong skewing. In the peripheral blood, 33 of 83 analyzed RCC patients (40%) showed a skewed TCRVβ usage, 23 (28%) of whom had a weakly skewed and 10 (12%) a strongly skewed repertoire (Table 2). In the 70 RCC patients successfully analyzed in both bone marrow and peripheral blood, skewing was similar in bone marrow and peripheral blood: 29 of 70 patients (41%) displayed weak or strong skewing in the bone marrow compared with 26 of 70 patients (37%) in the peripheral blood. The frequency of skewing of individual TCRVβ families in peripheral blood and bone marrow within all RCC patients is depicted in Figure 1a: there appeared to be no preferential skewing of specific TCRVβ families and TCRVβ usage was comparable in bone marrow and peripheral blood.
Table 2. Frequency of TCRVβ CDR3 skewing in RCC and (v)SAA patients.
| RCC | (v)SAA | P | |
|---|---|---|---|
| Bone marrow skewing, no. (%) | |||
| No skewing (0–1 families skewed) | 48 of 79 (61) | 7 of 20 (35) | 0.038a |
| Weak skewing (2–5 families skewed) | 20 of 79 (25) | 10 of 20 (50) | |
| Strong skewing (>5 families skewed) | 11 of 79 (14) | 3 of 20 (15) | |
| Peripheral blood, no. (%) | |||
| No skewing (0–1 families skewed) | 50 of 83 (60) | 17 of 28 (61) | NSa |
| Weak skewing (2–5 families skewed) | 23 of 83 (28) | 8 of 28 (29) | |
| Strong skewing (>5 families skewed) | 10 of 83 (12) | 3 of 28 (11) | |
Abbreviations: CDR3, complementarity-determining region 3; NS, nonsignificant; RCC, refractory cytopenia of childhood; TCRVβ, T-cell receptor β-chain variable; (v)SAA, (very) severe aplastic anemia.
No skewing versus weak or strong skewing; chi-square test. Bold indicates P-value <0.05.
Figure 1.
Frequency of skewing of individual TCRVβ families in bone marrow and peripheral blood of RCC and (v)SAA patients. (a) Frequency of skewing in RCC patients. (b) Frequency of skewing in (v)SAA patients.
TCRVβ skewing occurs less frequently in bone marrow of RCC than of (v)SAA patients
In a previous pilot study, RCC and (v)SAA patients displayed a similar frequency of TCRVβ skewing in bone marrow and peripheral blood combined.31 In the present study, we compared the frequency of TCRVβ skewing in (v)SAA with a larger cohort of RCC patients. TCRVβ skewing in the bone marrow, but not in the peripheral blood, occurred significantly less frequently in RCC patients than in (v)SAA patients: 48 of 79 RCC patients (61%) did not display skewing in the bone marrow compared with only 7 of 20 (v)SAA patients (35%, P=0.038); 20 of 79 RCC patients (25%) versus 10 of 20 (v)SAA patients (50%) displayed weak skewing in the bone marrow; and 11 of 79 RCC patients (14%) versus 3 of 20 (15%) of (v)SAA patients had a strongly skewed TCRVβ repertoire in the bone marrow (Table 2). The frequency of skewing of individual TCRVβ families in (v)SAA is depicted in Figure 1b.
Clinical characteristics of RCC patients with a skewed TCRVβ repertoire
We next compared clinical characteristics of RCC patients with and without a skewed TCRVβ repertoire. Because characteristics of patients with weak and strong skewing were similar, these patients were grouped for further analyses (Table 3). Patients with a skewed usage of the TCRVβ in the peripheral blood were older than patients with a polyclonal T-cell repertoire (median age: 13 versus 10 years, P=0.013). A comparable relationship between age and skewing of the T-cell repertoire in the bone marrow was observed, but this difference was not statistically significant. Skewing of the TCRVβ repertoire did not correlate with gender, bone marrow cellularity or transfusion dependency for platelets or erythrocytes. As the occurrence of trisomy 8 has been linked with response to IST and the presence of trisomy 8-specific clonal T-cell expansions,17, 34, 35 we compared cytogenetic results among patients with and without T-cell skewing. The distribution of patients with a normal karyotype or with monosomy 7 did not differ among the groups with or without a skewed T-cell repertoire, neither in bone marrow nor in peripheral blood. However, all five patients with other abnormalities in the karyotype, including both patients with trisomy 8 that were included in the study, had a skewed T-cell repertoire in the bone marrow (P=0.014). In the peripheral blood the same trend was observed, but differences were not statistically significant. Finally, although HLA-DR15 and the presence of minor PNH clones are predictors of IST response in some studies and thought to be indicators of a T-cell-mediated pathophysiology of bone marrow failure,17, 36, 37, 38, 39 we observed no differences in the frequency of HLA-DR15 and PNH clone positivity between the groups with and without skewing.
Table 3. Clinical characteristics of RCC patients without or with a skewed TCRVβ repertoire in BM or PB.
|
TCRVβ skewing BM |
TCRVβ skewing PB |
|||||
|---|---|---|---|---|---|---|
| No skewing | Skewing | P | No skewing | Skewing | P | |
| Number of patients | 48 | 31 | 50 | 33 | ||
| Median age at diagnosis, years (range) | 10 (1–18) | 13 (1–18) | NS | 10 (1–18) | 13 (4–18) | 0.013 |
| Male sex, no. (%) | 22 of 48 (46) | 16 of 31 (52) | NS | 27 of 50 (46) | 15 of 33 (46) | NS |
| Hypocellular bone marrow, no. (%) | 35 of 47 (75) | 26 of 30 (87) | NS | 40 of 49 (82) | 29 of 33 (88) | NS |
| Transfusion dependency at TCRVβ analysis, no. (%) | ||||||
| Platelets | 15 of 32 (47) | 9 of 18 (50) | NS | 16 of 32 (50) | 9 of 18 (50) | NS |
| Erythrocytes | 12 of 32 (38) | 6 of 18 (33) | NS | 12 of 32 (38) | 7 of 18 (39) | NS |
| Cytogenetics, no. (%) | ||||||
| Normal | 35 of 38 (92) | 23 of 30 (77) | 0.094 | 34 of 38 (90) | 26 of 32 (81) | NS |
| Monosomy 7 | 3 of 38 (8) | 2 of 30 (7) | NS | 3 of 38 (8) | 1 of 32 (3) | NS |
| Other | 0 of 38 (0) | 5 of 30 (17) | 0.014 | 1 of 38 (3) | 5 of 32 (16) | 0.086 |
| HLA-DR15, no. (%) | 13 of 37 (35) | 6 of 23 (26) | NS | 10 of 39 (26) | 10 of 28 (36) | NS |
| PNH clone at diagnosis, no. (%) | ||||||
| >0.01% | 17 of 43 (40) | 14 of 30 (47) | NS | 20 of 48 (42) | 13 of 33 (40) | NS |
| >0.1% | 10 of 43 (23) | 7 of 30 (23) | NS | 13 of 48 (27) | 8 of 33 (24) | NS |
| Response to IST, no. (%) | 4 of 13 (31) | 5 of 8 (63) | NS | 8 of 16 (50) | 6 of 10 (60) | NS |
Abbreviations: BM, bone marrow; IST, immunosuppressive therapy; NS, nonsignificant; PB, peripheral blood; RCC, refractory cytopenia of childhood; TCRVβ, T-cell receptor β-chain variable. Bold indicates P-value <0.05.
Laboratory characteristics of RCC patients with a skewed TCRVβ repertoire
RCC patients with or without T-cell skewing in the peripheral blood had similar peripheral blood leukocyte, neutrophil, lymphocyte, T-cell and platelet counts, and comparable hemoglobin and mean corpuscular volume levels (determined at the time of peripheral blood and bone marrow collection for TCRVβ repertoire analysis) (data not shown, all P-values >0.01). However, patients with strong, but not those with weak, skewing in the bone marrow had significantly lower leukocyte, absolute lymphocyte and T-cell counts in the peripheral blood than patients without skewing (median leukocyte count, 3.3 × 109/l (range, 0.8–7.5) versus 2.1 × 109/l (range, 0.8–2.9); median lymphocyte count, 1.9 × 109/l (range, 0.6–6.1) versus 1.1 × 109/l (0.1–2.2); median T-cell count, 1.2 × 109/l (range, 0.5–3.6) versus 0.6 × 109/l (range, 0.4–0.7); P=0.020, P=0.002 and P=0.020, respectively).
Association of TCRVβ repertoire skewing with response to IST
Twenty-eight RCC patients were treated with IST and evaluated for response at day 180 after the start of therapy. Response to IST was not significantly different between patients with or without T-cell skewing (Table 3). In particular, 5 of 8 patients (63%) with skewing in the bone marrow responded to IST, whereas 4 of 13 patients (31%) without skewing in the bone marrow responded to IST; 6 of 10 patients (60%) with skewing in the peripheral blood responded to IST, whereas 8 of 16 patients (50%) without skewing in the peripheral blood responded to IST. Furthermore, there were no significant differences in the frequency of skewing of specific TCRVβ families (data not shown) and in the median total number of skewed TCRVβ families among IST responding and nonresponding RCC patients (medians of 2 and 0.5 expanded families in the bone marrow, respectively, and 1 and 0.5 in the peripheral blood, respectively). In responding and nonresponding patients in whom follow-up bone marrow or peripheral blood samples were available (11 responders, median follow-up time after start of IST: 367 days (range: 234–1233 days); and 8 non-responders, median follow-up time after start of IST: 160 days (range: 62–1018 days)), no consistent increases or decreases in the number of skewed TCRVβ families were observed (data not shown).
TCRVβ skewing is associated with a reduction of naive CD8+ T cells and an expansion of effector CD4+ and CD8+ T cells in peripheral blood
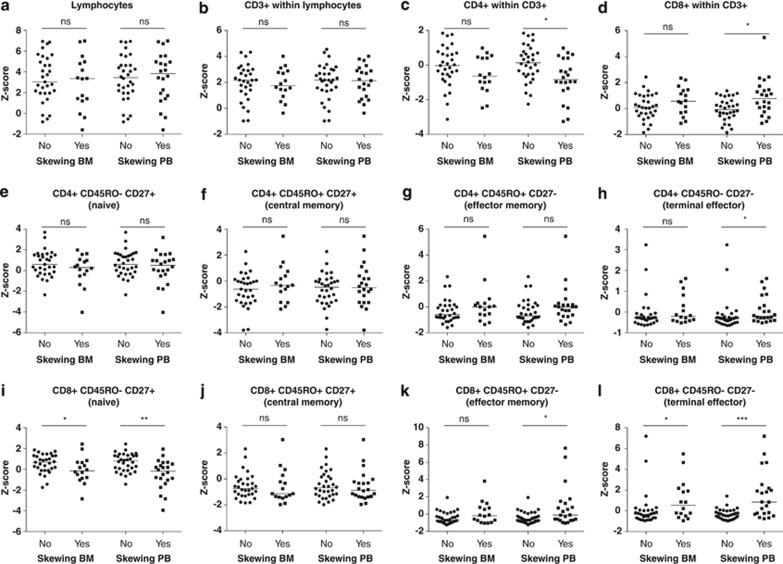
Previous studies have shown that adult MDS patients, in comparison with healthy controls, display a disturbed CD4+/CD8+ T-cell ratio29 and a reduction in naive CD8+ T cells with an increase in CD8+ effector cells.21, 25, 29 In the present RCC cohort, we therefore compared the relative distribution of these parameters with healthy children. RCC patients showed a strong relative expansion of lymphocytes (Supplementary Figure S1), mainly due to an absolute reduction in granulocytes and monocytes (data not shown). Within the lymphocytes, T cells were relatively increased (Supplementary Figure S1), mainly due to an absolute reduction in B cells and natural killer cells (Supplementary Figure S2). Within the T-cell compartment, no consistent differences were observed in the frequency of CD4+ and CD8+ T cells and in the distribution of naive and effector CD4+ and CD8+ T cells (Supplementary Figure S1) between RCC patients and healthy children. However, RCC patients with a skewed TCRVβ repertoire in the bone marrow had significantly less naive CD8+CD45RO−CD27+ T cells and more terminally differentiated effector CD8+CD45RO−CD27− T cells in the peripheral blood than RCC patients without a skewed T-cell repertoire (Figure 2 and Supplementary Table S1). Moreover, when compared with RCC patients without skewing, patients with a skewed T-cell repertoire in the peripheral blood showed significantly different relative peripheral blood T-cell subset numbers, with less CD4+ T cells, more CD8+ T cells and consequently a decreased CD4+/CD8+ T-cell ratio, more terminally differentiated effector CD4+CD45RO−CD27− T cells, less naive CD8+CD45RO−CD27+ T cells, more effector memory CD8+CD45RO+CD27− T cells and more terminally differentiated effector CD8+CD45RO−CD27− T cells (Figure 2; medians, ranges and P-values are shown in Supplementary Table S1). The relative number of terminally differentiated effector CD8+CD45RO−CD27− T cells showed an inverse correlation with the relative number of naive CD8+CD45RO−CD27+ T cells, irrespective of TCRVβ skewing (Pearson's r=−0.557, P=0.000).
Figure 2.
Naive and effector CD4+ and CD8+ T cells in RCC patients with or without a skewed bone marrow or peripheral blood TCRVβ repertoire (Z-scores of relative distribution). (a) Lymphocytes within leukocytes. (b) CD3+/T cells within lymphocytes. (c) CD4+ within CD3+/T cells. (d) CD8+ within CD3+/T cells. (e) Naive CD4+ T cells. (f) Central memory CD4+ T cells. (g) Effector memory CD4+ T cells. (h) Terminally differentiated effector CD4+ T cells. (i) Naive CD8+ T cells. (j) Central memory CD8+ T cells. (k) Effector memory CD8+ T cells. (l) Terminally differentiated effector CD8+ T cells. Lines indicate medians. Z-scores <0 indicate a decrease and Z-scores >0 indicate an increase compared with age-matched controls. ns, nonsignificant; *P<0.05; **P<0.01; ***P<0.001.
TCRVβ skewing is associated with an expansion of activated CD8+ T cells in peripheral blood
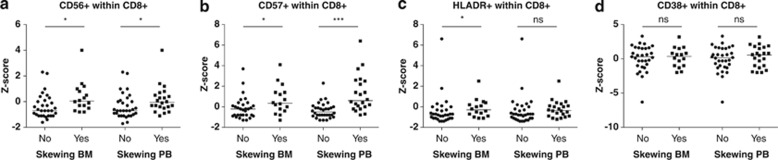
When compared with healthy pediatric controls, no consistent differences were observed in the frequency of activated CD8+ T cells in RCC patients (Supplementary Figure S3). However, the frequency of CD8+ T cells positive for activation markers CD56 and CD57 in patients with a skewed TCRVβ repertoire in the bone marrow and peripheral blood, and HLA-DR in patients with a skewed TCRVβ repertoire in the bone marrow, was significantly increased when compared with patients with a polyclonal T-cell repertoire (Figures 3a–c, Supplementary Table S2). No differences were observed in the frequency of CD8+ T cells positive for the activation marker CD38 (Figure 3d; medians, ranges and P-values are shown in Supplementary Table S2).
Figure 3.
Activation markers on CD8+ T cells in RCC patients with or without a skewed TCRVβ repertoire in bone marrow or peripheral blood (Z-scores of relative distribution). (a) CD56+CD8+ T cells. (b) CD57+CD8+ T cells. (c) HLA-DR+CD8+ T cells. (d) CD38+CD8+ T cells. Lines indicate medians. ns, nonsignificant; *P<0.05; ***P<0.001.
Discussion
In this study we show that TCRVβ skewing occurs in about 40% of RCC patients, suggesting an immune-mediated suppression of hematopoiesis in a considerable proportion of these patients. The frequency of TCRVβ skewing in the bone marrow, but not in the peripheral blood, of RCC patients was lower than the frequency of 65% in pediatric (v)SAA patients, indicating that an immune-mediated pathophysiology of bone marrow failure might be more common in (v)SAA than in RCC. Distinguishing RCC from (v)SAA, however, can be challenging, mainly due to the high frequency of bone marrow hypocellularity and the low frequency of karyotypic abnormalities in RCC, and a pathophysiological overlap between both diseases seems likely.
TCRVβ skewing was most prevalent in older children, but no correlation was found between skewing and bone marrow cellularity, the presence or absence of a normal karyotype or transfusion history. Previous reports in adult MDS patients also failed to show any significant correlation between clinical characteristics and TCRVβ skewing.25, 26 With respect to laboratory characteristics, we observed no difference in hemoglobin or mean corpuscular volume levels, platelet, leukocyte, absolute neutrophil, lymphocyte and T-cell counts when patients with TCRVβ skewing were compared with those without skewing, which is in line with reports in adult MDS patients.25, 26 However, patients with skewing in >5 TCRVβ families had significantly lower absolute lymphocyte and T-cell counts than patients without skewing. This indicates that in patients with strong TCRVβ skewing, but not in patients with skewing in 2–5 TCRVβ families, skewing might reflect a contracted T-cell repertoire. This phenomenon was previously described in aplastic anemia patients after IST.40 Furthermore, although HLA-DR15 positivity and the presence of a PNH clone are thought to be indicators of an immune-mediated pathophysiology of MDS, we did not find an increased frequency of TCRVβ skewing in HLA-DR15- or PNH-positive patients.
TCRVβ skewing was not predictive of IST response in the present pediatric cohort. Although the number of patients treated with IST was small, and results should be interpreted cautiously, this is consistent with studies in adult MDS patients, in whom a predictive role of TCRVβ skewing for IST response has not been found.23, 25, 26 Nevertheless, alterations in TCRVβ profiles or loss of clonal dominance of specific TCRVβ families after response to IST were described.19, 24 Likely, the specific T-cell subsets (for example, FOXP3+ regulatory T cells, Th17 T cells)41, 42, 43, 44 involved in the clonal expansion, the type of IST the patient received and patient characteristics, such as age, HLA-type, bone marrow cellularity and underlying molecular aberrations, all modulate response to IST and TCRVβ skewing alone is insufficient to predict response to therapy.
TCRVβ skewing was associated with a reduced CD4+/CD8+ T-cell ratio in peripheral blood, a reduction in naive CD8+ T cells, an expansion of effector CD8+ T cells and an increase in activated CD8+ T cells. Owing to a limited availability of material, regulatory T cells and Th17 T cells could not be assayed. However, our data confirm that RCC patients with a skewed TCRVβ repertoire indeed have an expanded population of activated T cells that might mediate suppression of hematopoiesis in RCC. A limitation of our study is that we did not provide direct evidence that T cells indeed suppress hematopoiesis. Future studies could employ coculture experiments with clonally expanded T cells and autologous myeloid (progenitor) cells, using adequate controls, to support the hypothesis that hematopoiesis in RCC is inhibited in a T-cell-dependent manner. Yet, the limited numbers of myeloid (progenitor) cells in RCC bone marrow might hamper these experiments.
The exact meaning of TCRVβ skewing and expansion of activated effector CD8+ T cells, and presumed T-cell-mediated suppression of hematopoiesis in MDS, remains unclear. It might either be a reflection of immune surveillance against hematopoietic cells expressing a neo-antigen or might be a result of breaking of self-tolerance leading to an autoimmune-like response against normal hematopoietic progenitor cells, or a combination of both. Evidence for the latter hypothesis was provided in the specific subgroup of MDS patients with trisomy 8, who are likely to respond to IST, but in whom the proportion of trisomy 8 cells increases after IST response.17, 34 T cells of clonally expanded TCRVβ families obtained from these patients selectively inhibited trisomy 8 cell growth.34 WT1, overexpressed in trisomy 8-positive bone marrow mononuclear cells, but also expressed at a low level in normal hematopoietic stem cells, was subsequently implicated to be one of the antigens inducing T-cell-mediated myelosuppression in trisomy 8-positive MDS.35 Future efforts to gain more insight into the precise nature and antigenic target(s) of the skewed and activated T cells in trisomy 8-positive and other subtypes of MDS could benefit from TCR repertoire deep sequencing approaches, which have now become readily available. These approaches should preferably be performed in sorted T-cell subsets, taking into account differences between human leukocyte antigen types.
In summary, we show that TCRVβ skewing is frequently detected in RCC. TCRVβ skewing is associated with a disturbed CD4+/CD8+ T-cell ratio, an expansion of effector CD4+ and CD8+ T cells, and an activated phenotype of CD8+ T cells. These data, in conjunction with the previously described response to IST in RCC patients,30 suggest that T cells are involved in the pathogenesis of RCC in a proportion of patients, and provide a rationale for treatment with IST in selected patients with RCC according to the existing treatment recommendations by EWOG-MDS.30
Acknowledgments
AMA and this research were supported by the KiKa Foundation, Amstelveen, the Netherlands. In the Czech Republic, RCC diagnosis and treatment were supported by a grant of the Ministry of Health for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic). The authors acknowledge excellent technical support by research technicians from the Departments of Immunology and Pediatric Oncology/Hematology, Erasmus MC.
Author contributions
MHE, CMN, VHJV and AWL conceived and designed the study; AMA analyzed the data, AMA, MHE, JJMD, RP, VHJV and AWL interpreted the data; MHE, GJD, MD, HH, FL, BDM, MS, JS, MZ, CMZ and CMN treated patients and contributed patient or control samples; AF and AY collected clinical data; PN provided statistical advice; IB performed central review of bone marrow morphology; GG and HBB performed central review of cytogenetics; AMA, VHJV, MHE and AWL wrote the paper; all authors approved the final version of the paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Hasle H, Kerndrup G, Jacobsen BB. Childhood myelodysplastic syndrome in Denmark: incidence and predisposing conditions. Leukemia. 1995;9:1569–1572. [PubMed] [Google Scholar]
- Hasle H, Wadsworth LD, Massing BG, McBride M, Schultz KR. A population-based study of childhood myelodysplastic syndrome in British Columbia, Canada. Br J Haematol. 1999;106:1027–1032. doi: 10.1046/j.1365-2141.1999.01645.x. [DOI] [PubMed] [Google Scholar]
- Passmore SJ, Chessells JM, Kempski H, Hann IM, Brownbill PA, Stiller CA. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia in the UK: a population-based study of incidence and survival. Br J Haematol. 2003;121:758–767. doi: 10.1046/j.1365-2141.2003.04361.x. [DOI] [PubMed] [Google Scholar]
- Niemeyer CM, Baumann I. Classification of childhood aplastic anemia and myelodysplastic syndrome. Hematology Am Soc Hematol Educ Program. 2011;2011:84–89. doi: 10.1182/asheducation-2011.1.84. [DOI] [PubMed] [Google Scholar]
- Baumann I, Niemeyer CM, Bennett JM, Shannon K.Childhood myelodysplastic syndromesIn: Swerdlow SH, Campo E, Harris NL, et al(eds).WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues IARC: Lyon, France; 2008104–107. [Google Scholar]
- Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012;12:849–859. doi: 10.1038/nrc3321. [DOI] [PubMed] [Google Scholar]
- Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997;99:699–705. doi: 10.1046/j.1365-2141.1997.4423249.x. [DOI] [PubMed] [Google Scholar]
- Jonasova A, Neuwirtova R, Cermak J, Vozobulova V, Mocikova K, Siskova M, et al. Cyclosporin A therapy in hypoplastic MDS patients and certain refractory anaemias without hypoplastic bone marrow. Br J Haematol. 1998;100:304–309. doi: 10.1046/j.1365-2141.1998.00551.x. [DOI] [PubMed] [Google Scholar]
- Asano Y, Maeda M, Uchida N, Yokoyama T, Osaki K, Shimoda K, et al. Immunosuppressive therapy for patients with refractory anemia. Ann Hematol. 2001;80:634–638. doi: 10.1007/s002770100360. [DOI] [PubMed] [Google Scholar]
- Aivado M, Rong A, Stadler M, Germing U, Giagounidis A, Strupp C, et al. Favourable response to antithymocyte or antilymphocyte globulin in low-risk myelodysplastic syndrome patients with a 'non-clonal' pattern of X-chromosome inactivation in bone marrow cells. Eur J Haematol. 2002;68:210–216. doi: 10.1034/j.1600-0609.2002.01625.x. [DOI] [PubMed] [Google Scholar]
- Molldrem JJ, Leifer E, Bahceci E, Saunthararajah Y, Rivera M, Dunbar C, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med. 2002;137:156–163. doi: 10.7326/0003-4819-137-3-200208060-00007. [DOI] [PubMed] [Google Scholar]
- Killick SB, Mufti G, Cavenagh JD, Mijovic A, Peacock JL, Gordon-Smith EC, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with 'low-risk' myelodysplasia. Br J Haematol. 2003;120:679–684. doi: 10.1046/j.1365-2141.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- Yazji S, Giles FJ, Tsimberidou AM, Estey EH, Kantarjian HM, O'Brien SA, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17:2101–2106. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]
- Stadler M, Germing U, Kliche KO, Josten KM, Kuse R, Hofmann WK, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18:460–465. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- Broliden PA, Dahl IM, Hast R, Johansson B, Juvonen E, Kjeldsen L, et al. Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica. 2006;91:667–670. [PubMed] [Google Scholar]
- Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Giagounidis AA, Simcock M, Aul C, Dobbelstein C, Stadler M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care—SAKK 33/99. J Clin Oncol. 2011;29:303–309. doi: 10.1200/JCO.2010.31.2686. [DOI] [PubMed] [Google Scholar]
- Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, Barrett AJ. Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol. 1998;102:1314–1322. doi: 10.1046/j.1365-2141.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- Baumann I, Scheid C, Koref MS, Swindell R, Stern P, Testa NG. Autologous lymphocytes inhibit hemopoiesis in long-term culture in patients with myelodysplastic syndrome. Exp Hematol. 2002;30:1405–1411. doi: 10.1016/s0301-472x(02)00968-2. [DOI] [PubMed] [Google Scholar]
- Chamuleau ME, Westers TM, van Dreunen L, Groenland J, Zevenbergen A, Eeltink CM, et al. Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica. 2009;94:496–506. doi: 10.3324/haematol.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Qianqiao Z, Qi H, Feng X, Chunkang C, Xiao L. In vitro deprivation of CD8(+)CD57(+)T cells promotes the malignant growth of bone marrow colony cells in patients with lower-risk myelodysplastic syndrome. Exp Hematol. 2010;38:677–684. doi: 10.1016/j.exphem.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Epperson DE, Nakamura R, Saunthararajah Y, Melenhorst J, Barrett AJ. Oligoclonal T cell expansion in myelodysplastic syndrome: evidence for an autoimmune process. Leuk Res. 2001;25:1075–1083. doi: 10.1016/s0145-2126(01)00083-2. [DOI] [PubMed] [Google Scholar]
- Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100:3639–3645. doi: 10.1182/blood-2002-01-0155. [DOI] [PubMed] [Google Scholar]
- Epling-Burnette PK, Painter JS, Rollison DE, Ku E, Vendron D, Widen R, et al. Prevalence and clinical association of clonal T-cell expansions in myelodysplastic syndrome. Leukemia. 2007;21:659–667. doi: 10.1038/sj.leu.2404590. [DOI] [PubMed] [Google Scholar]
- Fozza C, Contini S, Galleu A, Simula MP, Virdis P, Bonfigli S, et al. Patients with myelodysplastic syndromes display several T-cell expansions, which are mostly polyclonal in the CD4(+) subset and oligoclonal in the CD8(+) subset. Exp Hematol. 2009;37:947–955. doi: 10.1016/j.exphem.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol. 2001;29:1270–1277. doi: 10.1016/s0301-472x(01)00736-6. [DOI] [PubMed] [Google Scholar]
- Meers S, Vandenberghe P, Boogaerts M, Verhoef G, Delforge M. The clinical significance of activated lymphocytes in patients with myelodysplastic syndromes: a single centre study of 131 patients. Leuk Res. 2008;32:1026–1035. doi: 10.1016/j.leukres.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zou JX, Rollison DE, Boulware D, Chen DT, Sloand EM, Pfannes LV, et al. Altered naive and memory CD4+ T-cell homeostasis and immunosenescence characterize younger patients with myelodysplastic syndrome. Leukemia. 2009;23:1288–1296. doi: 10.1038/leu.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi A, van den Heuvel-Eibrink MM, Baumann I, Schwarz S, Simonitsch-Klupp I, de Paepe P, et al. Comparison of horse and rabbit anti-thymocyte globulin in immunosuppressive therapy for refractory cytopenia of childhood. Haematologica. 2013;99:656–663. doi: 10.3324/haematol.2013.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries AC, Langerak AW, Verhaaf B, Niemeyer CM, Stary J, Schmiegelow K, et al. T-cell receptor Vbeta CDR3 oligoclonality frequently occurs in childhood refractory cytopenia (MDS-RC) and severe aplastic anemia. Leukemia. 2008;22:1170–1174. doi: 10.1038/leu.2008.23. [DOI] [PubMed] [Google Scholar]
- Langerak AW, van Den Beemd R, Wolvers-Tettero IL, Boor PP, van Lochem EG, Hooijkaas H, et al. Molecular and flow cytometric analysis of the Vbeta repertoire for clonality assessment in mature TCRalphabeta T-cell proliferations. Blood. 2001;98:165–173. doi: 10.1182/blood.v98.1.165. [DOI] [PubMed] [Google Scholar]
- Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- Sloand EM, Mainwaring L, Fuhrer M, Ramkissoon S, Risitano AM, Keyvanafar K, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106:841–851. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloand EM, Melenhorst JJ, Tucker ZC, Pfannes L, Brenchley JM, Yong A, et al. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011;117:2691–2699. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn DE, Tanawattanacharoen P, Boccuni P, Nagakura S, Green SW, Kirby MR, et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131:401–408. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Follmann D, Nakamura R, Saunthararajah Y, Rivera CE, Simonis T, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98:3513–3519. doi: 10.1182/blood.v98.13.3513. [DOI] [PubMed] [Google Scholar]
- Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100:3897–3902. doi: 10.1182/blood-2002-03-0799. [DOI] [PubMed] [Google Scholar]
- Aalbers AM, van der Velden VH, Yoshimi A, Fischer A, Noellke P, Zwaan CM, et al. The clinical relevance of minor paroxysmal nocturnal hemoglobinuria clones in refractory cytopenia of childhood: a prospective study by EWOG-MDS. Leukemia. 2013;28:189–192. doi: 10.1038/leu.2013.195. [DOI] [PubMed] [Google Scholar]
- Kook H, Risitano AM, Zeng W, Wlodarski M, Lottemann C, Nakamura R, et al. Changes in T-cell receptor VB repertoire in aplastic anemia: effects of different immunosuppressive regimens. Blood. 2002;99:3668–3675. doi: 10.1182/blood.v99.10.3668. [DOI] [PubMed] [Google Scholar]
- Kotsianidis I, Bouchliou I, Nakou E, Spanoudakis E, Margaritis D, Christophoridou AV, et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS) Leukemia. 2009;23:510–518. doi: 10.1038/leu.2008.333. [DOI] [PubMed] [Google Scholar]
- Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145:64–72. doi: 10.1111/j.1365-2141.2009.07593.x. [DOI] [PubMed] [Google Scholar]
- Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847–850. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- Mailloux AW, Sugimori C, Komrokji RS, Yang L, Maciejewski JP, Sekeres MA, et al. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol. 2012;189:3198–3208. doi: 10.4049/jimmunol.1200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.