Multiple myeloma (MM) remains incurable despite widespread deployment of novel agents and high-dose melphalan/autologous stem cell transplantation (ASCT) earlier in the disease course.1 Recently, non-myeloablative (NMA) allogeneic stem cell transplantation (allo SCT) has been introduced in an attempt to exploit the well-documented graft-versus-myeloma effect (GVM), while at the same time reducing the prohibitively high transplant-related-mortality (TRM) associated with myeloablative allografting in the past.2 This approach, however, still results in significant rates of acute and chronic graft-versus-host disease (GVHD) and has limited cytoreductive capability. The sequential use of a cytoreductive and immunosuppressive ASCT followed by a NMA allo SCT (auto-allo SCT), with the aim of reducing TRM and optimizing tumur control through a GVM effect, has been explored by a number of groups and compared prospectively with tandem ASCT. Although this represents a promising approach, a recent meta-analysis of six biological assignment trials of almost 1200 patients found outcome data to be inconsistent because of the various conditioning regimens used, and any improvements in progression-free survival (PFS) and overall survival (OS) are frequently offset by the high allo SCT-associated TRM.3 We report our single-institution experience of delivering auto-allo SCT for MM in the ambulatory setting, associated with limited toxicity, a low TRM and minimal resource utilization.
From May 2008 to December 2012, 33 patients with a diagnosis of MM underwent an auto-allo SCT at the Alfred Hospital. Patients were considered for auto-allo SCT if they were <70 years old, had no significant co-morbidity at the time of transplant, had a suitably matched related or unrelated donor and had achieved at least a partial response (PR) to their latest line of therapy. All tandem auto-allo SCT procedures were performed as an elective treatment programe with the allo SCT timed to occur within 3 months of the ASCT. Patients were selected for an ‘upfront' (n=18) auto-allo SCT if they had high-risk disease defined as having at least 2 of the following criteria at presentation: high-risk cytogenetics, elevated LDH, ISS stage III or less than a PR with an induction regimen that included a novel agent. In contrast, patients transplanted as a ‘deferred' procedure (n=15) underwent a planned auto-allo SCT as salvage therapy for progressive or relapsed disease, usually following initial treatment with high-dose melphalan, ASCT and maintenance therapy. Baseline characteristics of all patients included in the cohort are outlined in Table 1.
Table 1. Baseline characteristics of all patients (n=33).
| Variable | Number (%) |
|---|---|
| Median age (range) | 52 (39–65) |
| Sex | |
| Male | 19 (58) |
| Female | 14 (42) |
| Disease stage (ISS) | |
| I | 5 (15) |
| II | 12 (36) |
| III | 13 (39) |
| Unknown | 3 (10) |
| Myelomas Subtype | |
| IgG | 12 (37) |
| IgA | 10 (30) |
| IgD | 1 (3) |
| IgM | 1 (3) |
| Light chain | 8 (24) |
| Non-secretory | 1 (3) |
| Cytogenetic risk stratification | |
| High risk | 10 (30) |
| Standard | 16 (48) |
| Unknown | 7 (22) |
| Donor type | |
| Sibling | 20 (61) |
| Unrelated | 13 (39) |
| Transplant timing | |
| Upfront | 18 (55) |
| Deferred | 15 (45) |
| Disease status post ASCT | |
| CR/sCR | 8 (24) |
| VGPR | 10 (30) |
| PR | 15 (43) |
| SD | 1 (3) |
| Median days between diagnosis and transplant (range) | |
| Upfront | 269 (149–3869) |
| Deferred | 1498 (261–5881) |
| Median days between ASCT and allo SCT (range) | 106 (50–693) |
Abbreviations: ASCT, autologous stem cell transplantation; ISS, International Staging System; PR, partial response; SCT, stem cell transplantation; SD, stable disease.
Standard risk cytogenetics=any result other than 17p-, t(4;14) or t(14;16).
The ASCT conditioning regimen was melphalan 200 mg/m2 on day −1 followed by infusion of G-CSF-mobilized peripheral blood stem cells (minimum dose 2 × 106/kg) on day 0. Deferred ASCT was performed using cells mobilized and collected prior to the initial ASCT. The NMA allo SCT transplant regimen was administered in the outpatient setting using a conditioning regimen of oral fludarabine 42 mg/m2 on days −4 to −2 followed by 2 Gy of total body irradiation (TBI) on day 0 in one fraction. A target cell dose of 2 × 106/kg CD34+ donor stem cells was infused on day 0. GVHD prophylaxis consisted of cyclosporine (ceased by day 56 for sibling donors and day 180 for unrelated donors in the absence of GVHD) and mycophenolate mofetil (ceased by days 27 and 96 for sibling and unrelated donors respectively). Standard opportunistic infection prophylaxis was routinely employed in all patients. The majority of patients had their allo SCT as an outpatient; four (12%) were electively admitted for geographical reasons alone. Patients were reviewed clinically in the day ward twice weekly initially until day 30 and then weekly until day 100. Responses were graded according to the International Myeloma Working Group uniform response criteria.4 Bone marrow aspirates and trephines were performed at 3 month intervals during the first year, 6 months during the second year and annually thereafter.
The median follow up for the entire cohort was 719 days (range 50–1733). The outpatient allo SCT was well tolerated leading to non-elective admission in 15 (45%) patients during the first 30 days, the majority for minor febrile episodes, poor oral intake or dehydration. The overall median length of stay was 1.5 days and only four patients were hospitalized for more than 7 days. All patients engrafted satisfactorily and median nadir neutrophil and platelet counts were 0.5 (range 0.1–1.1) × 109/l and 94 (range 23–143) × 109/l, respectively. Acute GVHD occurred in 14 (44%) patients, was mild in the majority of cases, with only 3 (9%) patients experiencing grade II-IV GVHD. There were no deaths directly attributable to acute GVHD. Of the 19 (61%) patients developing chronic GVHD, 4 (12%) and 15 (45%) had limited and extensive disease, respectively. The TRM was 6% one patient succumbed to an undetermined infection at day 240 post allo SCT in the setting of chronic GVHD management and a second patient died of disseminated nocardia infection while on immunosuppression 1025 days post transplant.
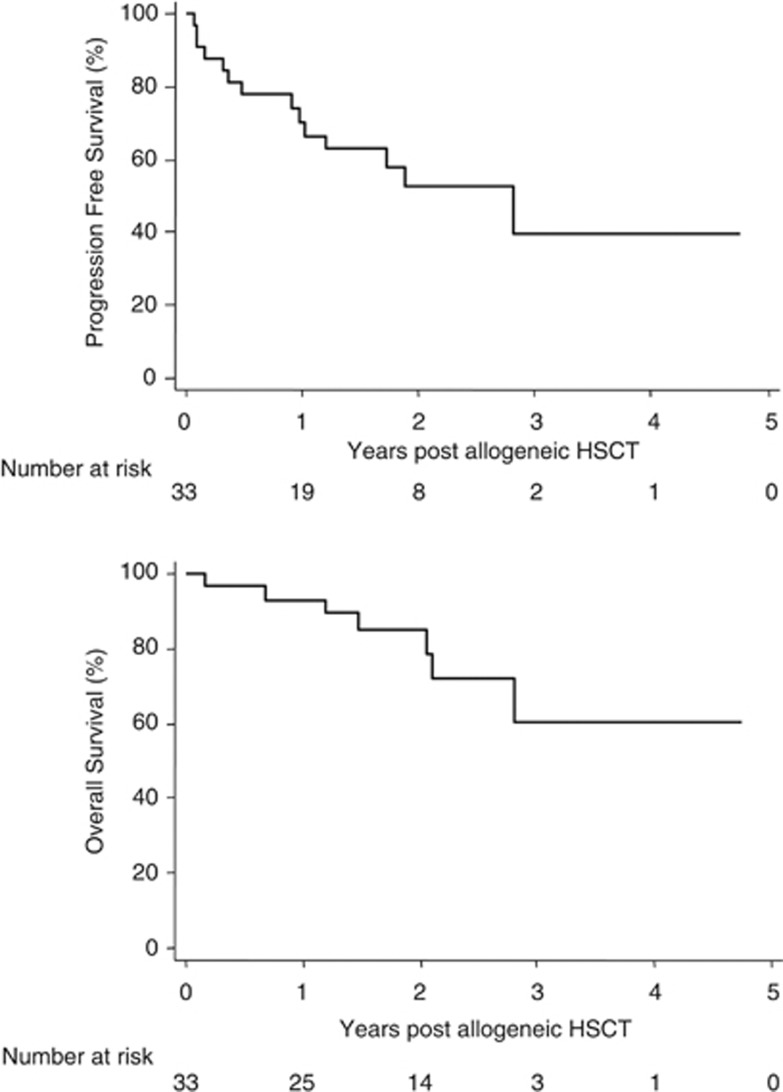
At the 3 month assessment post allo SCT, 22 (67%) of the entire cohort had achieved either a CR or VGPR, with significantly more patients in the ‘upfront' group achieving this level of response compared with recipients of a ‘deferred' auto-allo SCT (15/18 versus 7/15; P=0.03). The median time to full donor chimerism (FDC) for the cohort was 180 days (range 30–730) and there was no significant correlation between FDC status and the grade of response. In total 12 (36%) patients progressed or relapsed post allo SCT including 4 (12%) patients who progressed within 100 days of allo SCT. Relapsing patients received a range of post-allo SCT therapies including novel agents, chemotherapy and donor lymphocyte infusions, resulting in an overall response rate of 50%. At the time of the analysis, 26 (79%) patients were alive including 15 (45%) in complete remission. The median PFS for all patients was 2.8 years with the median OS for the entire cohort yet to be reached, whereas the estimated probability of OS at 4 years is 67% (95% CI: 38–85%) (Figure 1).
Figure 1.
Progression-free and overall survival for the entire cohort.
This retrospective, single-centre study of tandem auto-allo SCT for MM confirms the feasibility of delivering this potentially curative therapy in an ambulatory setting, thereby minimizing prolonged hospitalization and the extensive resource utilization historically associated with allografting myeloma patients. In addition, there were no deaths directly attributable to the conditioning regimen or acute GVHD and the observed overall TRM of 6% is considerably less than reported in the literature.When one considers the response rates, PFS and OS in this cohort of patients is comparable to that reported in the literature, we consider tandem auto-allo SCT performed as an outpatient procedure to be safe, convenient and effective antimyeloma therapy.
Although MM remains an incurable malignancy, debate continues as to the appropriate timing of tandem auto-allo SCT. A recent French registry-based study of 146 MM patients undergoing tandem auto-allo SCT across 20 centers reported superior outcomes for patients transplanted upfront, and this was confirmed by the EBMT group in their 96 month update of the EBMT—NMAM2000 study comparing tandem auto-allo SCT with ASCT in newly-diagnosed MM patients.5, 6 Similarly, in our cohort, the 18 patients undergoing tandem auto-allo SCT as part of their initial therapy had superior response rates (> VGPR 83% versus 46% P=0.03) and PFS (median not reached versus 1.2 years; P=0.03) compared with those patients undergoing the procedure at first progression or relapse. Relapse and progressive disease continue to be the principle cause of treatment failure and death in patients receiving tandem auto-allo SCT for MM. In line with previous reports, we observed significant disease responses to immunotherapy post allograft; 5 of our 12 relapsing patients responded to escalating doses of donor lymphocytes either alone or in combination with other agents. Novel agents, in particular bortezomib and lenalidomide, have well documented potent synergistic activity with alloreactive T cells post allograft and may provide a platform for enhancing tumor control post allograft, particularly in those patients with high-risk disease in CR or with minimal residual disease on flow cytometry or PCR.7, 8 The feasibility of this approach in post allo SCT relapse patients has been explored recently by two groups, where the introduction of lenalidomide following NMA allo SCT resulted in responses in over a third of patients, often however at the expense of acute GVHD.9, 10 Balancing the potential to achieve disease control with lenalidomide in this setting against the risk of inducing or exacerbating acute GVHD remains a challenge.
In conclusion, our single-centre experience with tandem auto-allo SCT, using outpatient Flu/TBI conditioning, yields durable disease control in over 60% of patients with a highly acceptable TRM when compared with a salvage ASCT.11 The low toxicity of the procedure enabled the majority of patients to be managed as outpatients and to benefit from the curative potential of allo SCT with minimal inconvenience. Given the low morbidity, mortality and ambulatory nature of auto-allo SCT in our hands, one may argue that there should be less reluctance to offer this procedure as part of initial therapy in young patients with MM or patients with high-risk disease in whom long-term disease control is unlikely to be achieved with available or emerging pharmacotherapeutic approaches.
The authors declare no conflict of interest.
References
- Michael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, et al. Management of newly diagnosed symptomatic myeloma: Updated Mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88:360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Koehne G, Giralt S. Allogeneic hematopoietic stem cell transplantation for multiple myeloma: curative but not the standard of care. Curr Opin Oncol. 2012;24:720–726. doi: 10.1097/CCO.0b013e328358f619. [DOI] [PubMed] [Google Scholar]
- Armeson KE, Hill EG, Costa LJ. Tandem autologous vs autologous plus reduced intensity allogeneic transplantation in the upfront managemet of multiple myeloma: a meta-analysis of trials with biological assignment. Bone Marrow Transplant. 2013;48:562–567. doi: 10.1038/bmt.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukaemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Gahrton G, Iacobelli S, Bjorkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of EBMT-NMAM2000 study. Blood. 2013;121:5055–5063. doi: 10.1182/blood-2012-11-469452. [DOI] [PubMed] [Google Scholar]
- Fabre C, Koscielny S, Mohty M, Fegueux N, Blaise D, Maillard N, et al. Younger donor's age and upfront tandem are two independent prognostic factors for survival in multiple myeloma patients treated by tandem autologous-allogeneic stem cell transplantation: a retrospective study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) Haematologica. 2012;97:482–490. doi: 10.3324/haematol.2011.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger N, Shimoni A, Zagrivnaja M, Ayuk F, Lioznov M, Schieder H, et al. Low-dose thalidomide and donor lymphocyte infusion as adoptive immunotherapy after allogeneic stem cell transplantation in patients with multiple myeloma. Blood. 2004;104:3361–3363. doi: 10.1182/blood-2004-05-2031. [DOI] [PubMed] [Google Scholar]
- Montefusco V, Spina F, Patriarca F, Offidani M, Bruno B, Montanari M, et al. Bortezomib plus dexamethasone followed by escalating donor lymphocyte infusions for patients with multiple myeloma relapsing or progressing after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:424–428. doi: 10.1016/j.bbmt.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Kneppers E, Van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118:2413–2419. doi: 10.1182/blood-2011-04-348292. [DOI] [PubMed] [Google Scholar]
- Coman T, Bachy E, Michallet M, Socie G, Uzunov M, Bourhis J, et al. Lenalidomide as salvage treatment for multiple myeloma relapsing after allogeneic stem cell transplantation: a report from the French Society of Bone Marrow and Cellular Therapy. Haematol. 2013;98:776–783. doi: 10.3324/haematol.2012.069328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI, et al. Salvage second haematopoietic transplantation in myeloma. Biol Blood Marrow Transplant. 2013;19:760–766. doi: 10.1016/j.bbmt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]