Abstract
Naive CD4+ T cells require signals from the TCR and CD28 to produce IL-2, expand, and differentiate. However, these same signals are not sufficient to induce autocrine IL-2 production by naïve CD8+ T cells, which require cytokines provided by other cell types to drive their differentiation. The basis for failed autocrine IL-2 production by activated CD8+ cells is unclear. We find that Ikaros, a transcriptional repressor that silences IL-2 in anergic CD4+ T cells, also restricts autocrine IL-2 production by CD8+ T cells. We find that CD8+ T cell activation in vitro in the absence of exogenous cytokines and CD4 help leads to marked induction of Ikaros, a known repressor of the Il2 gene. Naïve murine CD8 T cells haplo-insufficient for Ikzf1 failed to upregulate Ikaros, produced autocrine IL-2, and differentiated in an IL-2-dependent manner into IFN-γ-producing CTL in response to TCR/CD28 stimulation alone. Furthermore, Ikzf1-haplo-insufficient CD8+ T cells were more effective at controlling Listeria infection and B16 melanoma growth in vivo, and could provide help to neighboring, non-IL-2-producing cells to differentiate into IFN-γ-producing effectors. Therefore, by repressing autocrine IL-2 production, Ikaros ensures that naïve CD8+ T cells remain dependent upon licensing by antigen presenting cells and CD4+ T cells, and may therefore act as a cell-intrinsic safeguard against inappropriate CTL differentiation and immunopathology.
Introduction
Naive T cell differentiation is a tightly regulated process, as aberrant activation can lead to immunopathology and disease. Naïve CD4+ and CD8+ T cells differ in their requirements for differentiation, as the latter have higher cytotoxicity potential. Naïve CD4+ T cells require TCR recognition of a cognate peptide in a class II MHC molecule and a costimulatory signal from CD28-B7 engagement. Upon receiving these two signals, CD4+ T cells can produce autocrine IL-2 and differentiate (1). In contrast, co-stimulation of naïve CD8+ T cells through the TCR and CD28 does not result in efficient autocrine IL-2 production (2), and is not sufficient for differentiation into cytolytic effectors. In addition to TCR and CD28, CD8+ T cells require a pro-inflammatory cytokines for their differentiation. For instance, IL-12, type I interferons, and IL-21 have been characterized as key inflammatory cytokines that drive naïve CD8+ T cells into full-fledged cytotoxic effectors (3). Typically, these cytokines are derived from dendritic cells or CD4+ T cells to help promote the appropriate effector immune response.
Another cytokine that strongly influences CD8+ T cell responses is IL-2. This cytokine has a pro-survival role through up-regulation of the anti-apoptotic factor Bcl-2, but also can influence CD8+ T cell differentiation through affecting the balance of effector versus memory generation. IL-2 during the priming phase is required for effective T cell memory formation, as “unhelped” CD8+ T cells fail to generate memory (4–7). However, high levels of IL-2 can promote terminal effector CD8+ T cell generation at the expense of memory formation (8–11). Thus, it is critical to regulate IL-2 production during initial phases of an immune response, as means to ensure appropriate CD8+ T cell differentiation. Naïve CD8+ T cells are highly restricted in their production of autocrine IL-2 (2), and are largely dependent upon IL-2 from CD4+ helper T cells (12–14). However, in some systems helper T cells can license CD8+ T cells to produce their own IL-2 (15), which is required during initial priming in order to generate robust memory recall responses (16).
Little is known about how autocrine IL-2 is restricted in naïve CD8+ T cells. Recently, it was demonstrated that the transcription factor Ikaros, a transcriptional repressor required for lymphocyte development (17, 18), restricts autocrine IL-2 production in mature CD4+ T cells (19, 20). We hypothesized that Ikaros may similarly regulate naïve CD8+ T cell differentiation through inhibition of autocrine IL-2 production. In this study, we demonstrate that TCR stimulation leads to strong induction of Ikaros unless exogenous cytokines are present, and that naïve CD8+ T cells with reduced Ikaros function are able to differentiate into cytolytic effectors in the absence of signal 3 cytokines and CD4 help due to a gain of autocrine IL-2 function. Thus, by restricting autocrine IL-2 production by CD8+ T cells, Ikaros ensures that induction of an inflammatory and cytotoxic program only occurs in cells that have been appropriately licensed by a third signal.
Materials and Methods
Mice, Antibodies, Cytokines
Wild-type CD45.2, CD45.1, PMEL, RAG1−/− and OT-1 mice were purchase from JAX. Mice carrying the germline Ikzf1 null allele were a gift of Dr. K. Georgopoulos, and were backcrossed on a B6 background for greater than 12 generations. RAG1−/− OT-1 Ikzf1+/− mice were generated through breeding Ikzf1+/− mice onto a RAG1−/− background to obtain RAG1−/− Ikzf1+/− mice. These mice were then crossed with the RAG1−/− OT-1 mice to generate the triple cross. Once the triple cross was generated, these mice were maintained by crossing with RAG1−/− or RAG1−/− Ikzf1+/− mice. Ikzf1+/− PMEL mice were generated by crossing Ikzf1+/− mice with PMEL mice. All procedures were approved by The Children’s Hospital of Philadelphia Research Institute animal use and care committee. Monoclonal antibodies against CD3 (2C11), CD28 (37.51), CD4 (GK1.4), MHCII (M/5114), FcR (2.4G2), and IL-2 (JES6-1A12) were purchased from BioXcell and anti-B220 (RA3-6B2), and anti-CD44 (IM7) antibodies were purchased from Biolegend. Mouse IL-2 and IL-12 were purchased from Peprotech and Roche. The SIINFEKL peptide derived from chicken ovalbumin was purchase from Biomatik.
Cell Sorting
Single cell suspensions from spleen and LN of polyclonal mice were sorted for naïve CD8+ T cells (CD62Lhi CD44-) on a MoFlo XDP (Beckman Coulter). Naïve CD8+ T cells were at >95% purity. RAG1−/− OT-1 and RAG1−/− Ikzf1+/− OT-1 single cell suspensions from spleen and LN were depleted of CD4+ T cells, monocytes, MHCII-expressing cells with Qiagen magnetic goat anti-rat IgG beads (#310107). Cells were stained with cocktail of depleting anti-CD4 (GK1.4), MHCII (M/5114), anti-FcR (2.4G2), anti-B220, and anti-CD44 (IM7). Naïve OT-1 (CD62Lhi CD44-) cells were purified to >90% purity.
Flow cytometry and applications
Fluorochrome conjugated antibodies against anti-mouse IFN-γ (XMG1), APC-Cy7 anti-mouse CD25 (PC61), anti-mouse IL-2 (JES6-1A12), anti-mouse CD8 (53-6.7), anti-mouse CD45.1 (A20), anti-mouse CD62L (MEL-14), anti-mouse CD44 (IM7), anti-mouse CD4 (GK1.5) were purchased from Biolegend. Fixable, Live/Dead Aqua stain (L34957) was purchased from Invitrogen. Fluorochrome antibody to anti-mouse Granzyme B (NGZB), and anti-mouse Eomes (Danmag11) were purchased from eBioscience. Fluorochrome anti-mouse T-Bet (4B10) was purchased from BD Biosciences. CFSE was purchased from Millipore and 7-AAD was ordered from Sigma-Aldrich. Negative gating was based on a ‘fluorescence-minus-one’ (FMO) strategy. For intracellular cytokine staining, cells were treated with Golgi Stop (BD Biosciences, 0.66 μg/ml) for 4–6 hours with PMA(30 ng/ml) and ionomycin (1 μM) or OVA peptide (1 μM) as indicated. Following harvesting, cells were fixed with 1% PFA for 30 minutes, spun down and washed once with FACS buffer. Cells were then washed with BD Perm Wash (BD Biosciences) 2 times and then stained with cytokine antibodies for 45 minutes at room temperature. Cells were washed 2 times in BD Perm Wash, then re-suspended in FACS Buffer. For transcription factor staining, cells were surfaced stained with fluorochrome-labeled primary antibodies for 20 minutes on ice. After washing in FACS buffer, cells were fixed with Fix/Perm buffer from eBioscience. Following fixation, cells were permeabilized and stained with APC anti-mouse T-bet and PE anti-mouse Eomes. For Ikaros staining, rabbit anti-mouse Ikaros (Abcam, ab26083) was used. Following staining with the Ikaros antibody, cells were washed and then stained with a PE-labeled anti-rabbit secondary antibody. Following completion of stains, cells were processed on a CyanADP (Beckman Coulter) for flow cytometric analysis.
Cell Culture
Naïve sorted CD8+ T cells were stimulated in 96-well or 24-well plates, which were coated with anti-CD3/CD28. All T cell cultures were maintained in RPMI supplemented with 10% FBS, L-glutamine, penicillin/streptomycin, and 2-β-mercaptoethanol, and maintained in 37C incubator. EL4 and EL4.ova cells lines were maintained in DMEM, supplemented with 10% FBS, L-glutamine, penicillin/streptomycin and 2-β-mercaptoethanol. EL4.ova cells were maintained in 400 μg/ml G418 (Invitrogen). All means, measures of variance, and statistical tests are based upon biological replicates, as indicated in the figure legends.
Immunoblot analysis
Immunoblotting was performed for determining the Ikaros isoform expression. For immunoblotting, 0.33×106 to 1e6 cells were lysed with Lamelli buffer and boiled for 10 minutes to denature the proteins. Total lysates were electrophoresed in Criterion precast 10% Tris-HCl gels (Bio-Rad) and transferred to nitrocellulose membrane using a Trans-Blot™ apparatus (Bio-Rad). The membrane was washed for 10 minutes (3X) with wash buffer (0.1% Tween-20 in 1X PBS). It was then incubated with HRP conjugated anti-goat antibody (1:10,000) for 1 hour at room temperature. The membrane was washed for 10 minutes 3 times with wash buffer and then developed using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). The image was developed on a Kodak X-ray film. A C-terminus reactive goat anti-mouse antibody to Ikaros (SC-9861) and a goat anti-mouse actin antibody (SC-1615) were purchased from Santa Cruz. β-actin expression level was determined to normalize the differences in loading. Prior to β-actin staining, the membrane was stripped with Restore Plus Western Stripping Buffer (Thermo Scientific) for 10 minutes and then washed for 15 minutes, 3 times. The pre-blocked membrane was stained with goat polyclonal anti-B-actin antibody followed by staining with HRP conjugated secondary antibody. Densitometry was performed using ImageJ software on background-subtracted images derived from the raw immunoblot films.
Immunohistochemistry
50–250 thousand cells were centrifuged onto glass slides and fixed for 20 minutes in 10% neutral buffered formalin. Immunohistochemical stains were performed on a Bond III system (Leica Microsystems, Bannockburn, Ill) with pH6 epitope retrieval solution (Leica), a HRP-conjugated anti-Ikaros primary antibody (ab26083, Abcam, Cambridge, MA) diluted 1:1000 in IHC diluent (Leica), and with nuclear counter stain hematoxylin, following manufacturer’s protocol (standard protocol F, Leica) but eliminating steps to de-paraffinize slides. Stained slides were analyzed on a Leica DM 2500 microscope with a 40x HCX PL Fluotar objective (∞/0.17/D). Images were captured using Leica application suite version 2.8.1 (build 1554, 2003–2007).
Cytotoxicity assays
Naïve purified OT-1 CD8+ T cells from Ikzf1+/+ or Ikzf1+/− mice were stimulated with plate bound anti-CD3/CD28 (1.0 μg/ml) for 48 hours and in presence or absence of IL-2 (10 ng/ml). Cells were then harvested, counted and re-suspended at 0.5×106 cells/ml in complete RPMI and rested overnight at 37C. After overnight rest, effectors were mixed at 10:1, 5:1, and 2.5:1 ratio with CFSE-labeled EL4 or EL4.OVA cells and incubated for 3 hours, 37C. After a 3 hour incubation period, cells were harvested, washed in FACS buffer, stained for CD8 expression and live/dead viability was assessed after addition of 7-AAD (5 μg/ml). Cells were analyzed by flow cytometry and a standard number of flow cytometric beads were collected to standardize the assay. CFSE+ tumor cells were gated on, and 7-AAD gating was measured against EL4 cells not mixed with T cells. Percent killing by CD8+ T cells was calculated by the following fashion. % Cytotoxicity of CD8+ T cells= (number of 7-AAD- cell counts/ total cell counts) *100. These numbers were then normalized to the EL4 cell fraction that had no T cells added. Specific lysis = (% cytotoxicity of CD8+ T cells − % cytotoxicity of control tumor cells without CD8+ T cells)/(% maximum cytolysis − % minimum cytolysis).
Recombinant Listeria monocytogenes infection model
Purified naive wild-type or Ikzf1+/− RAG1−/− OT-I T cells were activated for 48 hours with plate bound anti-CD3/CD28 Ab (1.0 μg/ml each). Cells were harvested and injected i.v. (5×106) into B6 mice. Two hours following transfer, mice were challenged with 1×105 live recombinant Listeria monocytogenes expressing the SIINFEKL peptide (LM-OVA). Spleen, LN, and blood were harvested at day 3 post-infection. Bacterial burden in the spleen was determined as previously described (21, 22). Blood and LN were characterized by PE H-2Kb/OVA257–264 (Kb/OVA) tetramer staining, and LN cells were assessed for cytokine production upon restimulation with OVA peptide.
B16 melanoma model
Purified naive wild-type or Ikzf1+/− PMEL CD8+ T cells were isolated and activated in vitro for 3 days with plate bound anti-CD3/CD28 (1.0 μg/ml) in the presence of IL-12 (20 ng/ml) and/or IL-2 (10 U/ml). Cells were harvested and injected i.v. into sex-matched B6 mice (1×106 cells/mouse). Twenty four hours later, mice were challenged with 1×105 cells B16 melanoma cells s.c. on each flank. Length and width of tumor was recorded and tumor volume was calculated by (L*W^2)/2.
Results
IL-2 opposes Ikaros induction and promotes CD8+ T cell differentiation
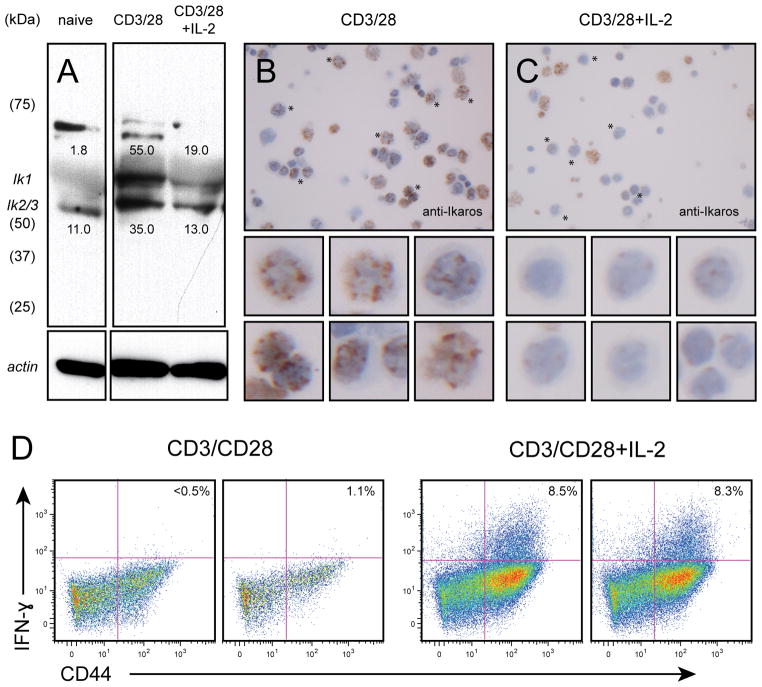
To study the differentiation of naïve CD8+ T cells into effectors in a reductionist system, we utilized agonistic anti-CD3 and anti-CD28 antibodies in an in vitro culture system devoid of other cell types or exogenous cytokines. To control for previous antigen exposure, we used polyclonal CD44loCD62Lhi CD8+ T cells enriched by flow sorting, or monoclonal CD8+ T cells developed in RAG1−/−OT-1 mice. Naïve CD8+ T cells express the full length DNA binding isoforms of Ikaros, with no evidence for expression of the smaller isoforms that lack the DNA binding domain (Fig. 1A). Ikaros exhibited a characteristic, punctate nuclear pattern by immunohistochemical staining (Fig. 1B), as observed in previous studies (23). When costimulated through the TCR and CD28 in the absence of exogenous cytokines, naïve cells showed marked upregulation of Ik1 and Ik2 (Fig. 1A and B). The Ik2 band may contain the Ik3 isoform, which also binds DNA and can co-migrate with Ik2 (24). TCR/CD28-activated cells also consistently exhibited higher molecular weight species that most likely represent post-translationally modified (e.g., sumoylated, ubiquitinylated, and/or phosphorylated) forms that have been detected in thymocytes and other cell lines (51). In some experiments, a minor low molecular weight form that is smaller than the Ik6 and Ik7 splicing isoforms is detected (see Fig. 2), which may represent an N-terminal degradation product that we have detected previously in Th1 cells (19). These modified forms were only variably detected in naive cells. The increase in Ikaros protein expression upon TCR/CD28 stimulation correlated with failure to differentiate into IFN-γ producing effector cells (Fig. 1D, left panel). The addition of exogenous IL-2 opposed the accumulation of Ikaros (Fig. 1A and C), and promoted differentiation of naïve CD8+ T cells into IFN-γ producing effectors (Fig. 1D, right panel). These results suggest that Ikaros, a known repressor of CD4+ T cell differentiation (19, 25) may also be involved in cytokine-regulated CD8+ T cell differentiation.
Fig. 1. Activation- and cytokine-dependent Ikaros expression in CD8+ T cells.
Cells from RAG1−/− OT-1 mice were stimulated with soluble CD3 and CD28 antibodies (0.5 μg/ml each) for 24 hours and in the presence (lane 3) or absence (lane 2) of IL-2 (10 ng/ml). Resting (lane 1) or stimulated (lanes 2 and 3) cells were washed in PBS, immunoblotted (A, 0.33×106 cell equivalents), and probed with antisera against Ikaros (top panels) or actin (bottom panels). The predominant Ik1, Ik2 and Ik3 isoforms are indicated. Numbers above the Ik1 band and below the Ik2/3 band indicate pixel density (x1000). Alternatively, stimulated cells were centrifuged onto glass slides and subjected to immunohistochemical staining for Ikaros (B). Arrows in B indicate intact cells with characteristic positive, punctate staining for Ikaros. Asterisks indicate intact cells that are negative or dim for Ikaros. Anucleate, apoptotic cells show a low level of background reactivity. Data are representative of 3 independent experiments. C. Naïve-enriched RAG1−/− OT-I CD8+ T cells were stimulated with plate bound anti-CD3 and anti-CD28 (1.0 μg/ml each) in the presence (right panel) or absence (left panel) of IL-2 (10 ng/ml) for 48 hours, and Golgi Stop was added for last 4 hours of stimulation. Cells were harvested, stained for CD8, CD44 and IFN-γ, and subjected to flow cytometric analysis. Gates were set using a fluorescence-minus-one (FMO) approach, and plots depict IFN-γ expression by activated (CD44hi) cells from duplicate cultures, with numbers indicating % IFN-γ+ cells. Data are representative of at least 3 independent experiments.
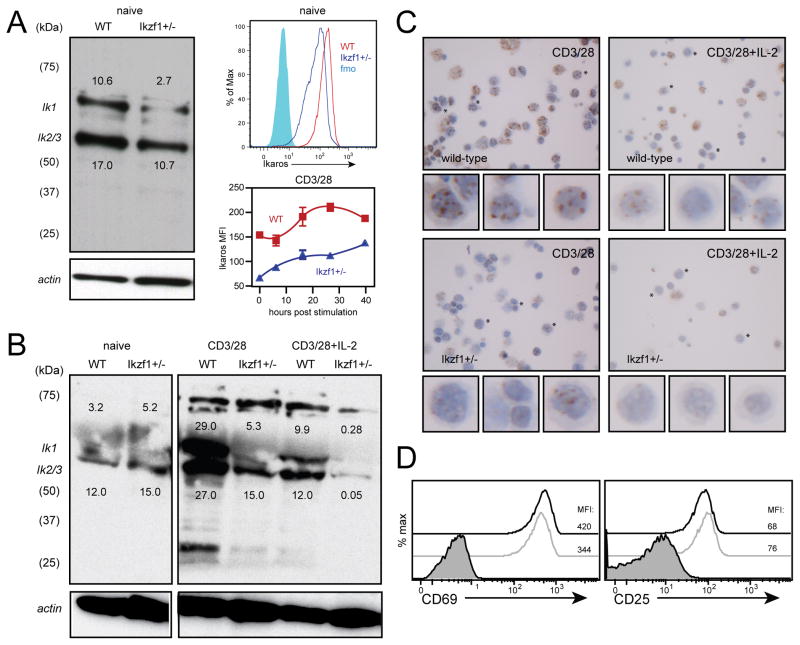
Fig. 2. CD8+ T cells hemizygous for the Ikzf1 gene exhibit reduced Ikaros protein expression.
Polyclonal naive CD8+ T cells sorted by CD62L+CD44- from wild-type or Ikzf1+/− mice (1×106 cell equivalents) were subjected to immunoblot analysis of Ikaros protein expression as in Fig. 1 (A, left panel). Ikaros expression by naive RAG1−/− OT-1 (WT, red) or RAG1−/− OT-1 Ikzf1+/− CD8+ T cells (blue) was measured by flow cytometry (A, right panels, gated on CD62L+CD44-CD8+), immunoblot analysis (B), or immunohistochemistry (C) following stimulation with plate-bound anti-CD3/CD28 in the presence or absence of IL-2 (10 ng/ml). D. CD69 and CD25 expression by 12 hour anti-CD3/CD28-stimulated RAG1−/− OT-1 (black) or RAG1−/− OT-1 Ikzf1+/− (grey) CD8+ T cells was measured by flow cytometry (gated on live CD8+ T cells). Numbers above the Ik1 band and below the Ik2/3 band indicate pixel density (x1000). Data are representative of two independent experiments.
Ikaros restricts CD8+ T cell differentiation in the absence of signal 3 cytokines
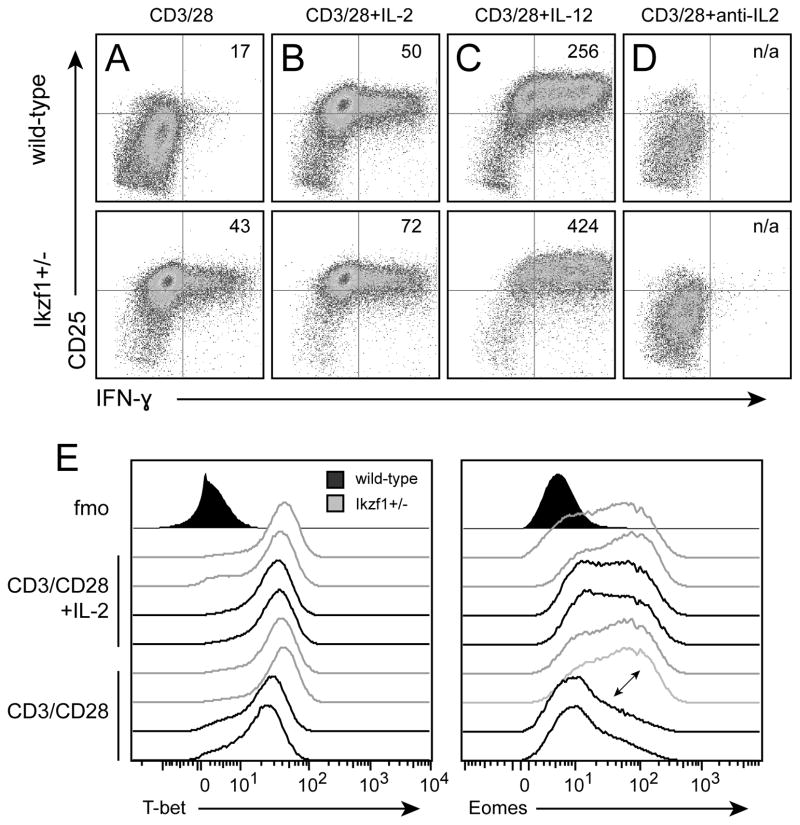
To determine whether Ikaros imposes a direct barrier to CD8+ T cell differentiation, we utilized mice that carry one null and one wild-type allele of Ikzf1, the gene encoding Ikaros. This model offers significant advantages over nullizygous mice, which do not develop an intact lymphoid immune system (26), and over mice expressing a dominant-negative mutant of Ikaros (18), which develop fatal lymphomas early in life (27), in that Ikzf1+/− mice show normal hematopoietic development and do not develop cancer (23). We find that naïve CD8+ T cells with only one functional allele of Ikzf1 exhibit a ~50% reduction in Ikaros protein, as measured by both immunoblot and flow cytometric analyses (Fig. 2A). Ikzf1+/− CD8+ T cells exhibited only modest induction of Ikaros in response to TCR/CD28 stimulation, achieving levels approximately 5-fold less than wild-type cells (Fig 2A–C). This failure was not due to under-stimulation, as wild-type and Ikzf1+/− CD8+ T cells up-regulated the activation markers CD69 and CD25 to a similar degree following TCR and CD28 stimulation (Fig. 2D). Consistent with their reduced Ikaros protein levels, Ikzf1+/− CD8+ T cells were able to upregulate CD25 and differentiate in response to TCR/CD28 co-stimulation alone (Fig. 3A), giving rise to frequencies of IFN-γ producers comparable to that observed in wild-type cultures given exogenous IL-2 (Fig. 3B). The addition of exogenous IL-2 blunted the induction of Ikaros in wild-type cells, but led to nearly complete loss of Ikaros in the Ikzf1+/− cells (Fig. 2B and C). Addition of exogenous IL-12 also led to reduced levels of Ikaros in wild-type CD8+ T cells, which was also more pronounced in Ikzf1+/− cells (data not shown), and this was associated with increased responsiveness of Ikzf1+/− cells to IL-12 at the level of IFN-γ production (Fig. 3C). Expression of the high affinity IL-2 receptor by activated T cells is amplified and stabilized by IL-2, as IL-2-induced STAT5 drives transcription of the cd25 gene in a feed-forward loop(28, 29). The high expression of CD25 therefore suggested the presence of IL-2 in TCR/CD28 costimulated Ikzf1+/− cultures. Consistent with this, neutralization of IL-2 completely blocked TCR/CD28-mediated CD25 and IFN-γ expression (Fig. 3D) and prevented down-regulation of Ikaros by Ikzf1+/− cells (data not shown), indicating that the gain of function exhibited by these cells is entirely dependent upon IL-2.
Fig. 3. CD8+ T cells hemizygous for the Ikzf1 gene exhibit enhanced effector differentiation.
CD8+ T cells from RAG1−/− OT-1 (black) or RAG1−/− OT-1 Ikzf1+/− (grey) mice were sorted by naïve (CD62LhiCD44lo) phenotype, stimulated with plate bound anti-CD3/CD28 (A) with exogenous IL-2, IL-12 or anti-IL-2 for 48 hours, and analyzed for expression of CD25 and IFNγ (MFI is depicted in upper right quadrant), or for expression of Eomes and T-bet (B) by flow cytometry. Two biological replicates are shown for each group, and data are representative of two independent experiments.
We also assessed expression of the T-box transcription factors T-bet and Eomes (30, 31), as IL-2 induces Eomes expression (8) and Ikaros has been shown to regulate T-bet during CD4+ T cell differentiation (25). TCR and CD28 signals induced T-bet expression by wild-type cells (32), which was augmented by exogenous IL-2 (Fig. 3E, left panel). In comparison, TCR/CD28-stimulated Ikzf1+/− CD8+ T cells induced more T-bet than wild-type cells, and also exhibited increased T-bet expression in response to IL-2 (Fig 3E, left panel). While wild-type CD8+ T cells required exogenous IL-2 for the induction of Eomes (Fig. 3E, right panel), Ikzf1+/− CD8+ T cells were able to induce Eomes when stimulated without additional cytokines, to levels comparable to that in wild-type cells primed in the presence of IL-2 (Fig. 3E, right panel). These data indicate that Ikaros influences the expression of T-bet and Eomes, key factors for CD8+ T cell effector differentiation (30, 31).
Ikaros influences CD8 differentiation via control of autocrine IL-2
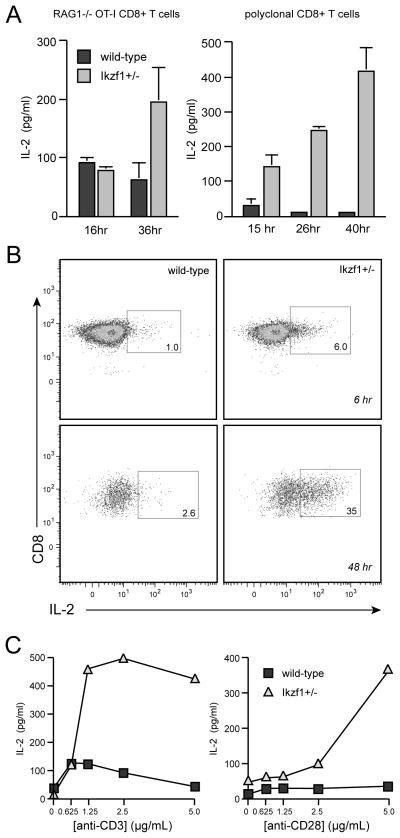
The requirement for IL-2 in the differentiation of naïve Ikzf1+/− CD8+ T cells indicated that loss of Ikaros function is accompanied by a gain of autocrine IL-2 production. To test this, we measured IL-2 levels in the supernatants of wild-type and Ikzf1+/− cultures over 48 hours of stimulation. Consistent with previous studies (2), wild-type naïve CD8 cells produced very little autocrine IL-2 in response to TCR/CD28 costimulation (Fig. 4A). However, CD8+ T cells lacking a single copy of Ikzf1 secreted significant levels of IL-2, and this was observed in both polyclonal cells and monoclonal OT-I cells (Fig. 4A). This gain of IL-2 production could also be observed at the single-cell level immediately after TCR and CD28 stimulation (Fig. 4B).
Fig. 4. Ikzf1+/− CD8+ T cells produce autocrine IL-2.
Naïve-enriched CD8+ T cells from Ikzf1+/+ or Ikzf1+/− B6 or RAG1−/− OT-I mice were stimulated as in Fig. 2, and culture supernatants were assayed for IL-2 production by ELISA at the indicated time points (A) or by intracellular staining at 6 and 48 hours (B). Plots in B are gated on CD69+ cells. In C, naïve-enriched Ikzf1+/+ or Ikzf1+/− OT-I CD8+ T cells were stimulated for 24 hours with either a fixed concentration of plate-bound anti-CD28 and a titration of anti-CD3 (left panel), or a fixed concentration of anti-CD3 and a titration of anti-CD28 (right panel). Values plotted in C represent the mean+/−SEM of duplicate cultures and are representative of 2 independent experiments.
To determine whether this enhanced IL-2 production is due to a reduced signaling threshold, or is the result of an absolute gain of autocrine function by Ikzf1+/− cells, we varied the strength of TCR or costimulatory signal received by the naïve cells in this system and measured T cell activation and IL-2 production. Naïve, wild-type CD8+ T cells produced very little IL-2, and did so only at supra-maximal concentrations of αTCR antibody (5–10 μg/mL, Fig. 4C), even though wild-type and Ikzf1+/− cells were equally able to induce the CD69 and CD25 activation markers (Fig. 2D). Similarly, increasing the strength of CD28 costimulation at a fixed, high concentration of αTCR antibody did not result in significant IL-2 production by wild-type cells, but Ikzf1+/− cells showed a strong, dose-dependent increase in IL-2 production under these conditions (Fig. 4C). Therefore, increasing TCR/CD28 signal strength could not raise the wild-type level of autocrine IL-2 production to that of the Ikzf1+/− cells, indicating that a loss of Ikaros function does not merely shift the T cell activation threshold, but results in an absolute gain of autocrine IL-2 function by naïve CD8+ T cells.
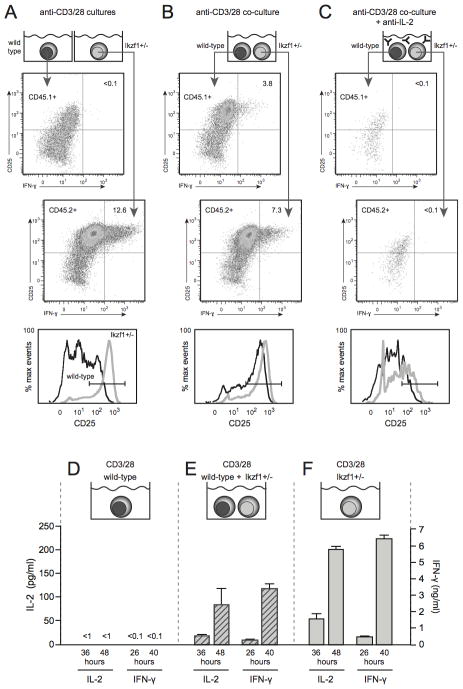
Loss of Ikaros function leads to differentiation of a relatively high frequency of CD8 T cells in this system, as measured by IFN-γ secretion at the single-cell level (Fig. 3A). While this is clearly due to a large increase in IL-2 secretion by Ikzf1+/− CD8 cultures (Fig. 4A), our ICS data indicate that this IL-2 is produced from relatively few cells at any one time (Fig 4B). To determine if Ikaros-regulated autocrine IL-2 can also drive the differentiation of neighboring, non-IL-2 producers in a paracrine manner, we utilized a mixed culture experiment. We mixed naïve-sorted, wild-type CD45.1+ CD8+ T cells with naïve CD45.2+ Ikzf1+/− CD8+ T cells and activated them in vitro in the presence or absence of IL-2. This would test if the increased autocrine IL-2 from naive Ikzf1+/− CD8 cells could act in a paracrine fashion on the wild-type cells to promote their differentiation. We also used a suboptimal dose of anti-CD3/28 to increase dependency on cytokine signals for their differentiation. As before, priming of Ikzf1+/− cells with anti-CD3/CD28 alone resulted in differentiated CD25hi, IFN-γ producing cells (Fig. 5A and F), while wild-type cells failed to differentiate under these conditions (Fig. 5A and D). However, wild-type CD8+ T cells in the presence of Ikzf1+/− CD8+ T cells became CD25hi, and a significant frequency was able to produce IFN-γ as measured by intracellular staining (Fig. 5B) and ELISA (Fig. 5E). To test if this was due to the increased autocrine IL-2 production from the CD8+ T cells with reduced Ikaros, cultures were also stimulated in the presence of a neutralizing anti-IL-2 antibody. Blockade of IL-2 resulted in a failure to differentiate into IFN-γ producing cells in these mixed cultures (Fig. 5C), indicating that the IL-2 from the Ikzf1+/− CD8+ T cells was necessary for both the autocrine and paracrine effects on differentiation. These data show that Ikaros controls not only the capacity of an activated CD8+ T cell to produce autocrine IL-2, but also its ability to ‘help’ other activated CD8+ T cells to differentiate by providing paracrine IL-2 signals.
Fig. 5. IL-2 produced by Ikzf1+/− CD8+ T cells can act in a paracrine fashion to induce differentiation of wild-type CD8+ T cells.
Naïve-enriched CD8+ T cells from Ikzf1+/+ (CD45.1+) or Ikzf1+/− (CD45.2+) mice were stimulated in individual cultures as in Fig. 1 (A), or in co-cultures at a 1:1 ratio for 48 hours in the absence (B) or presence (C) of anti-IL-2 neutralizing antibody. Expression of CD25 and IFN-γ were assessed by flow cytometric analysis, and numbers represent %IFN-γ+CD25+ cells. CD25 expression in Ikzf1+/+ (black) and Ikzf1+/− (gray) cultures is also illustrated in histogram overlays (bottom panels in A–C). Supernatants from these cultures were assessed for IL-2 and IFN-γ production by ELISA (D, E and F). Data are representative of 3 independent experiments.
Ikaros controls CD8+ T cell cytotoxicity
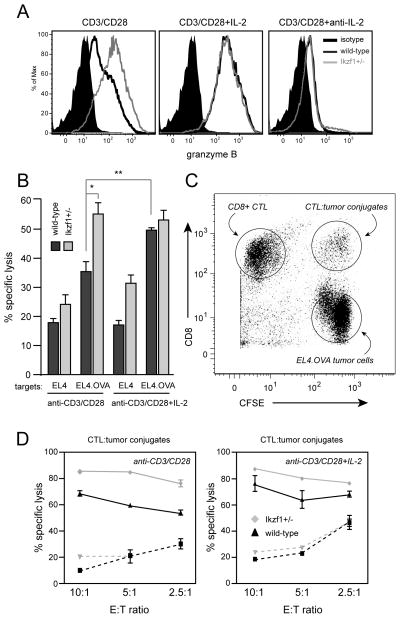
To address how a loss of Ikaros function influences the secondary effector function of CD8+ T cells, we assessed granzyme B expression and cytotoxic activity upon restimulation, functions that are driven by IL-2 (1, 8, 9, 33, 34, 35). Wild-type CD8+ T cells primed through the TCR and CD28 and re-challenged with PMA and ionomycin expressed low levels of granzyme B (Fig. 6A, left panel), and required the addition of exogenous IL-2 for high-level expression (Fig. 6A, middle panel). However, Ikzf1+/− effector cells were able to induce granzyme B to high levels in the absence of exogenous cytokines (Fig 6A), and this gain of granzyme B expression was completely dependent upon IL-2 (Fig 6A, right panel).
Fig. 6. Loss of Ikaros function leads to enhanced cytolytic capacity by CD8+ T cells.
A. Naïve-enriched RAG1−/− OT-I (dark gray) or Ikzf1+/− OT-I (light gray) CD8+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 for 48 hours in the presence or absence of IL-2 or anti-IL-2. PMA (30 ng/ml) and ionomycin (1uM) were added for the last four hours of culture. Expression of granzyme B was assessed by flow cytometry. Data are representative of two independent experiments. Filled black histograms - granzyme B FMO negative control. OT-I cells (dark gray) or Ikzf1+/− OT-I cells (light gray) were stimulated for 48 hours as in A, rested in medium overnight, then mixed at a 10:1, 5:1 and 2.5:1 ratio with CFSE-labeled EL4 or EL4.OVA targets for 3 hours. Viability of bulk CFSE+ EL4 or EL4.OVA targets at a 10:1 E:T ratio is shown in (B) or in conjugates with CD8+ T cells (CFSE+CD8+ gate in C) at all ratios in (D). Dashed lines indicate response to EL4. Data are representative of 3 independent experiments. Statistical significance was determined by Student’s T-test for triplicate values/group in B - * p<0.05, ** p<0.001, *** p<0.0001. Values plotted in D represent mean+/−SEM of duplicate cultures.
To assess cytolytic activity, OVA-specific wild-type and Ikzf1+/− OT-I cells were challenged in vitro with EL4 thymoma targets engineered to express OVA (EL4.OVA). TCR/CD28-primed OT-I cells exhibited relatively low killing of EL4.OVA cells unless exogenous IL-2 was included in the primary cultures (Fig. 6B, dark gray bars). However, consistent with their increased IL-2 and granzyme B expression, TCR/CD28 primed Ikzf1+/− OT-I cells exhibited a cytotoxic capacity similar to that observed in wild-type cells stimulated in the presence of IL-2 (Fig 6B, light gray bars). To gain more insight into the increased killing in Ikzf1+/− co-cultures, we focused on the conjugates formed between CD8+ cells and tumor cells (Fig. 6C). The frequency of CD8+ T cells engaged in conjugates with tumor cells was comparable between wild-type and Ikzf1+/− co-cultures (data not shown), suggesting that the increased tumor cell killing was not due to enhanced antigen recognition by the Ikzf1+/− CTL. However, we found that tumor cells actively engaged in a conjugate with Ikzf1+/− CTL were killed more efficiently than tumor cells engaged in a conjugate with wild-type CTL (Fig. 6D, left panel). Again, the efficiency of wild-type cell-mediated cytotoxicity was increased by exogenous IL-2, reaching levels comparable to that of the Ikzf1+/− CTL (Fig. 6D, right panel). These data indicate that Ikzf1+/− cells are more efficient at killing tumor cells in an antigen-specific manner once the cells have already engaged their targets. Together, these results demonstrate that the autocrine IL-2 that is normally repressed by Ikaros is sufficient to drive naïve CD8+ T cells to differentiate into cytotoxic, IFN-γ-producing effector cells, even in the absence of CD4+ T cell help and/or exogenous cytokines like IL-2 or IL-12.
Ikaros regulates anti-bacterial and anti-tumor CD8+ effector function in vivo
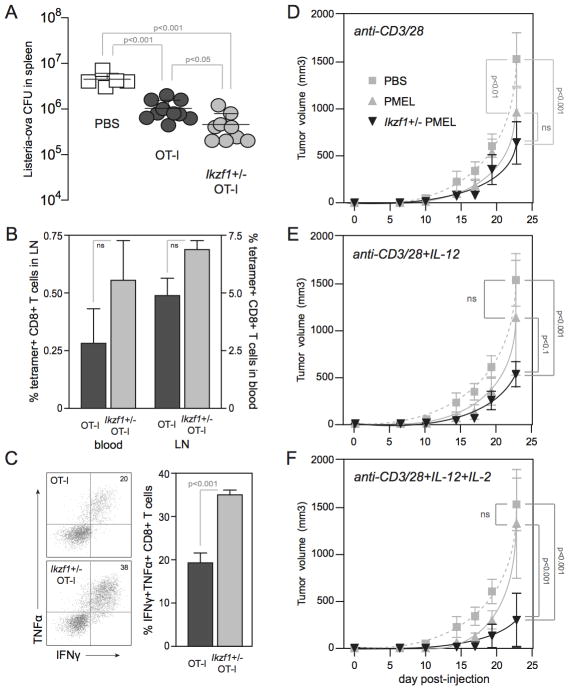
To test whether the enhanced in vitro differentiation and cytotoxicity of Ikzf1+/− CD8+ T cells translated to enhanced in vivo effector function, we utilized an IFNγ-dependent bacterial clearance model using recombinant Listeria monocytogenes expressing OVA (LM-OVA) in the context of the OVA-specific OT-I TCR, and a IFNγ-dependent tumor rejection model using the B16 melanoma in the context of a transgenic, melanocyte/melanoma antigen-specific TCR (PMEL). CD8+ T cells are crucial for the control of Listeria infection, and clear this bacterium by secreting IFN-γ, which activates macrophages to kill intracellular bacilli, and by direct killing of infected macrophages in a perforin/granzyme-dependent manner (36, 37). We primed naive OT-I or Ikzf1+/− OT-I cells through the TCR and CD28 for 48 hours and adoptively transferred these cells into B6 mice. Transfer of OT-I cells into LM-OVA-infected B6 mice resulted in a 5-fold reduction in the number of live LM-OVA in the spleen at day 3 post-infection, as compared to control animals that received no OT-I cells (Fig. 7A). However, LM-OVA replication was reduced by greater than 10-fold by day 3 in animals that received the same number of OT-I cells that lacked one copy of the Ikzf1 gene (Fig. 7A). The enhanced capacity of Ikzf1+/− CD8+ T cells to control Listeria infection was associated with a moderate (but not statistically significant) increase in the number of OVA peptide-specific CD8+ T cells in the blood and lymph nodes (Fig. 7B), and with a highly significant increase in number of antigen-specific IFNγ- and TNFα-producing cells in the LN (Fig. 7C). These cytokines have both been linked to bacterial clearance in this model (38–40). These data demonstrate that Ikaros restricts the inflammatory response of CD8+ T cells in a cell-intrinsic manner during acute bacterial infection, which has potential implications for immunity and the control of immunopathology.
Figure 7. Enhanced in vivo effector function by Ikzf1+/− CD8+ T cells.
Naive RAG1−/− OT-1 (dark grey) and Ikzf1+/− RAG1−/− OT-1 (light grey) CD8+ T cells were purified, primed with plate-bound anti-CD3/28 Ab for 48 hours, and 5×106 cells were adoptively transferred into B6 mice. Two hours later, mice were challenged with 1×105 LM-OVA, and blood, LN and spleens were harvested at day 3 post-infection. Live LM-OVA in the spleen (A) and MHC-OVA tetramer+ CD8+ T cells in blood and LN (B) were enumerated. LN cells were restimulated ex vivo with OVA peptide (1 μM) and analyzed by flow cytometry for production of IFN-γ and TNFα (C). Recipients: PBS, n=3; RAG1−/− OT-1, n=4; Ikzf1+/− RAG1−/− OT-1, n=3. Statistical significance was determined by Student’s T-test (A) and one-way ANOVA (B, C) and p values are indicated. For tumor studies, naive PMEL (grey) or Ikzf1+/− PMEL (black) CD8+ T cells were purified, stimulated in vitro with plate-bound anti-CD3/28 Ab (D), or with the addition of IL-12 (E) or IL-2 and IL-12 (F), and 1×106 cells were adoptively transferred into B6 mice. Mice were challenged subcutaneously 24 hours later with 1×105 B16 melanoma cells. Tumors: PBS, n=6; PMEL, n=6; Ikzf1+/− PMEL, n=6. Statistical significance was determined by two-way ANOVA and p values are indicated. * p<0.05, ** p<0.001, *** p<0.0001.
To further evaluate in vivo CD8+ T cell effector function, we utilized the poorly immunogenic B16 melanoma model, together with TCR-transgenic PMEL-1 T cells that recognize the melanocyte/melanoma antigen gp100 (41). Effective cellular immunotherapy of established B16 tumors in this stringent model normally requires the administration of exogenous IL-2 (42) or IL-12 (43, 44), and requires IFN-γ (45). Since our Ikzf1+/− CD8+ T cells produce more IFN-γ upon stimulation with IL-12, and IL-12 also improves IL-2 signaling (46, 47), we primed PMEL or Ikzf1+/− PMEL cells through the TCR and CD28, plus either IL-12 or the combination of IL-12 and IL-2. To test the fitness of these cells in vivo, the CD8+ T cells from these primary cultures were adoptively transferred into B6 mice and injected with highly aggressive B16 melanoma cells 24 hours later. As the endogenous T cells in the B6 host animals are ignorant of this tumor, this approach measures the in vivo efficacy of the transferred PMEL cells in absence of tumor-specific CD4+ helper T cells. Transfer of PMEL cells that were primed through the TCR and CD28 alone (Fig. 7D, dark grey line) resulted in a modest delay of tumor growth as compared to mice receiving no PMEL CD8+ T cells (Fig. 7D, dashed line). Addition of exogenous IL-12 or the combination of IL-2 and IL-12 to the in vitro priming cultures resulted in some early control of tumor growth by the adoptively transferred PMEL CD8+ T cells (e.g., days 17–19), but by day 22 tumor growth reached that observed in control mice (Fig. 7E and F, dark grey lines). Mice that received the TCR/CD28-primed Ikzf1+/− PMEL CD8+ T cells (Fig. 7A, black line) demonstrated a trend toward decreased tumor growth in comparison to the wild-type effectors, but this difference was not statistically significant. However, priming these cells in the presence of IL-12 resulted in more pronounced and significant tumor delay in comparison to Ikaros-sufficient PMEL cells (Fig. 7E, black line). This is consistent with the fact that Ikzf1+/− CD8+ T cells produce more IFN-γ in response to IL-12 than wild-type cells (Fig. 3C), and with a recent study showing that CD8+ T cells expressing a dominant-negative mutant of Ikaros also exhibit increased sensitivity to IL-12 (48). The combination of IL-2 and IL-12 resulted in the most significant tumor delay in recipients of Ikzf1+/− PMEL cells compared to tumors growing in recipients of wild-type PMEL cells (Fig. 7F, black line). These results indicate that autocrine IL-2 contributes to the enhanced responsiveness of Ikzf1+/− CD8+ T cells to IL-12, but is still limiting and can be enhanced by exogenous IL-2. Interestingly, these tumor studies show that CD8+ T cells with autoreactive TCR specificity can be driven to differentiate into functional effector CTL by inflammatory cues (i.e., IL-2 and IL-12) if activated without benefit of full Ikaros activity. This suggests that Ikaros may normally functions to protect naive CD8+ T cells from a loss of self tolerance during infection or other inflammatory situations.
Discussion
In this report we demonstrate a novel role for Ikaros in the regulation of naïve CD8+ T cell differentiation, at least in part through the control of autocrine IL-2. TCR signals in the absence of cytokines result in accumulation of Ikaros at the protein level, imposing a barrier to IL-2 production and effector differentiation. Extrinsic signals from IL-2 or IL-12 oppose the accumulation of Ikaros, and drive differentiation into IFN-γ producing CTL. Naïve CD8+ T cells with only one functional copy of the Ikzf1 gene, which could not induce Ikaros to a significant degree, were able to differentiate in the absence of CD4 help or exogenous cytokines. Neutralization of IL-2 blocked the capacity of both wild-type and Ikzf1+/− cells to differentiate. Thus, the repressive activity of Ikaros renders CD8+ T cells dependent upon environmental cues such as IL-12 from activated dendritic cells, or IL-2 from activated CD4+ T cells, to license their differentiation into effector CTL. Ikaros is also a potent repressor of IL-2 and differentiation in CD4+ T helper cells (19, 49). Importantly, we find that endogenous CD44hi memory CD8+ T cells, which have been previously licensed and can rapidly produce high levels of IFN-γ and IL-2 in response to antigenic stimulation, express lower levels of Ikaros than naïve phenotype cells (our unpublished observations), a finding predicted by our model in which Ikaros appears to integrate TCR, costimulatory and cytokine signals, and mans a global checkpoint for T cell differentiation.
While some aspects of the hematopoietic lineage-restricted control of Ikaros expression are understood, the type of dynamic ‘tuning’ of Ikaros levels by TCR vs. cytokine signals reported in this study has not been previously observed. The TCR-induced increase in Ikaros protein levels could result from increased transcription of the Ikzf1 gene and/or stability of the Ikzf1 transcript via the same mechanisms that increase expression of thousands of other genes during T cell activation. Interestingly, the larger-scale transcriptional architecture of the Ikzf1 locus includes a T cell lineage-specific enhancer region (50), which may potentially be involved in inducible expression in CD8+ T cells. Similarly, the mechanism by which IL-2 blocks induction of Ikaros in CD8+ T cells is not clear, and could either be due to transcriptional repression or post-translational regulation of Ikaros protein translation or stability. Ikaros is a known substrate of CK2 (51), Syk (52) and BTK (53), and hyper-phosphorylation of Ikaros can result in its degradation (51). IL-2 can also repress expression of Ikaros at the mRNA level (54), but the mechanism by which this occurs is unclear. It is possible that cytokine signals may induce the expression of miR-27a and miR-92, micro-RNAs known to target Ikaros expression in tumor cells (55).
IL-2 has important roles in CD8+ T cell effector differentiation and memory. IL-2 drives CD8 effector differentiation, in part by enhancing cell survival, but also by inducing the T-box transcription factor Eomes (8), which cooperates with its family member T-bet to transactivate IFN-γ, perforin, and granzyme B gene expression (30, 31). STAT5, a transcription factor activated by IL-2R signaling, has been shown to promote accessibility of the ifnγ promoter to binding by T-bet (56), and drives feed-forward expression of the high affinity IL-2 receptor chain, CD25 (29). Indeed, expression of CD25 can delineate effector from memory precursor cells. CD25 was shown to mark a terminally differentiated population of highly cytolytic KLRG1+ effector cells during acute LCMV infection (9), and high concentrations of exogenous IL-2 during CD8+ T cell activation in vitro results in CTL with limited survival and homeostatic capacity (8). In contrast, low levels of IL-2 promote the generation of effectors with reduced cytotoxicity and increased memory potential (8), resembling the CD25lo memory precursor cells that arise during LCMV infection (9). While IL-2 is crucial for tuning effector CD8 differentiation, it is also required for CD8+ T cell memory. CD8+ T cells primed in the absence of IL-2 signaling generate a blunted memory pool with poor recall responses(4, 57–59). Through the use of mixed bone marrow chimeras, it was demonstrated that CD25-deficient memory CD8+ T cells were defective in IFN-γ and IL-2 production, and exhibited poor cytotoxicity upon re-challenge with antigen. The use of IL-2/anti-IL-2 complexes to mimic strong IL-2 activity during primary immune responses also resulted in increased recall responses (60) or converted IL-2Rα−/− CD8+ T cells into competent memory CD8+ T cells (4). Adoptive transfer studies with naïve IL-2−/− cells into B6 mice also demonstrated that these cells fail to generate an effective memory recall response to Listeria challenge (16). These studies all demonstrate that failure to produce and/or respond to IL-2 during the primary response has a profound impact on CD8+ T cell memory.
The main cellular source of IL-2 is CD4+ T cells, and along with CD40-mediated licensing of dendritic cell maturation (61–63), IL-2 represents a major paracrine mechanism for CD4 help for CD8+ T cell responses (12–14). CD8+ T cells primed in the absence of CD4 help can differentiate into effectors if other signal 3 cytokines are present, but are defective in homeostasis, and exhibit markedly reduced proliferation, cytolytic capacity and cytokine production when challenged with antigen during the memory phase (4, 57, 64). Consistent with this, the Il2 and Ifnγ loci are epigenetically silenced in unhelped CD8+ T cells, at the level of both DNA methylation and chromatin structure (6, 7). The phenotype of IL-2-deprived CD8+ T cells is highly similar to that of memory CD8+ T cells generated in the absence of CD4 help, suggesting a common molecular basis for the functional defect. Interestingly, it was recently shown that CD4+ T cells can license CD8+ effector cells to produce their own IL-2 (15, 16), implicating autocrine IL-2 as an important regulatory node in the development of effective CD8+ T cell memory. Our current studies demonstrate a previously unappreciated role for the transcriptional repressor Ikaros in the control of autocrine IL-2 production by CD8+ T cells.
Ikaros interacts with the NURD, Sin3a and CtBP transcriptional co-repressor complexes (65–67), and is a potent regulator of chromatin structure and DNA methylation at its target genes (49). The repressive activity of Ikaros is required to silence gene expression programs, ensuring that only those T cells that have received the appropriate instructive signals can develop and differentiate. For example, Ikaros binds to the Il2 promoter in naïve CD4+ T cells, keeping the chromatin in this region in a ‘closed’ conformation unless signals from CD28 are received (19). Ikaros is also required to epigenetically silence the genes encoding T-bet and IFN-γ in CD4+ T cells that fail to receive Th1-promoting signals from IL-12 or IFN-γ, and cells with a loss of Ikaros function exhibit poly-lineage cytokine expression patterns upon differentiation (49), and are resistant to anergy induction (19, 20). These studies suggest that instructive signals from cytokines must oppose Ikaros-mediated repression at lineage-specific effector genes. Indeed, IL-12 inhibits the binding of Ikaros to the endogenous Tbx21 promoter in differentiating Th1 cells (49), and we show in this current study that IL-2 (Fig. 1, Fig. 2) and IL-12 (data not shown) can downregulate Ikaros in activated CD8+ T cells. These results also provide an explanation for recent finding that CD8+ T cells transduced with a dominant-negative Ikaros transgene are more responsive to IL-12 signaling (68). This suggests a model in which Ikaros integrates signals from the TCR, CD28 and the IL-2 receptor to regulate CD8+ T cell differentiation. We show that Ikaros is expressed in naïve CD8+ T cells, and is highly induced upon TCR/CD28 costimulation. Our previous studies indicate that targets such as Il2, T-box genes, Gzmb, and Ifnγ would be subject to strong Ikaros occupancy and repressive activity under these circumstances, and the chromatin at these genes would be inaccessible to transcriptional activators induced during T cell activation. However, if a naïve CD8+ T cell receives antigenic stimulation in the presence of paracrine IL-2 from a CD4+ T cell, or IL-12 from the dendritic cell, our results show that Ikaros does not accumulate, and T-bet and Eomes are induced instead. In the absence of the repressive activity of Ikaros, these factors would then be free to bind to and transactivate the accessible Ifnγ, Gzmb and other loci to drive effector differentiation.
Our studies also reinforce the importance of restricting autocrine IL-2 production by CD8+ T cells. We show that naïve CD8+ T cells that do not express the appropriate level of Ikaros are able to produce IL-2 without the need for CD4 help, driving their own differentiation into IFNγ-producing, granzyme Bhi CTL that can efficiently kill target cells in vitro and control Listeria infection in vivo. Moreover, these cells are able to help neighboring, non-IL-2 producing CD8 cells in a paracrine manner to differentiate into CD25hi IFNγ-producing effectors. From a clinical perspective, inhibition of Ikaros activity could improve vaccination, where the lack of inflammation or IL-2 derived from CD4+ T cells can limit CD8 responses, as our studies show that inhibition of Ikaros expression improves the anti-bacterial efficacy of pathogen-specific CD8+ T cells when primed in the absence of inflammatory stimuli. Similarly, barriers to anti-tumor immunity include immunologic tolerance to self/tumor antigens and the immuno-suppressive tumor microenvironment (69). Indeed, our experiments utilizing the poorly immunogenic B16 melanoma demonstrate that inhibition of Ikaros activity can cooperate with inflammatory cytokines to break tolerance to a self antigen, leading to effective control of tumor growth. On the other hand, the ability of Ikaros to control autoreactive T cell responses has important implications for autoimmunity. The IKZF1 gene has been implicated in the susceptibility to human autoimmune diseases such as systemic lupus erythematosus (SLE), type 1 diabetes (T1D) and Crohn’s/inflammatory bowel disease (IBD) in genome-wide association studies (70–72), indicating that proper Ikaros gene regulation is crucial for the maintaining self tolerance, and suggesting that interventional strategies that promote Ikaros function could lead to novel treatments for autoimmune disease or organ transplant rejection. Together, our results show that Ikaros regulates CD8+ T cell differentiation by restricting autocrine IL-2 production and enforcing dependence on paracrine signals from other cells.
Acknowledgments
The authors would like to thank Peter Morawski, Claire O’Leary, Vanessa Kurzweil, Steve Reiner, and Alex Kumar for helpful input and discussion, and Joanne Mauger for excellent technical assistance with immunohistochemistry.
Footnotes
This work was supported by grants AI070807 and AI54643 from the NIH and from the Biesecker Pediatric Liver Center at The Children’s Hospital of Philadelphia.
References
- 1.Malek TR. The Biology of Interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 2.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163:102–110. [PubMed] [Google Scholar]
- 3.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Current Opinion in Immunology. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shedlock DJ. Requirement for CD4 T Cell Help in Generating Functional CD8 T Cell Memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 6.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 7.Northrop JK, Wells AD, Shen H. Cutting edge: chromatin remodeling as a molecular basis for the enhanced functionality of memory CD8 T cells. The Journal of Immunology. 2008;181:865–868. doi: 10.4049/jimmunol.181.2.865. [DOI] [PubMed] [Google Scholar]
- 8.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and Inflammation Induce Distinct Transcriptional Programs that Promote the Differentiation of Effector Cytolytic T Cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged Interleukin-2Rα Expression on Virus-Specific CD8+ T Cells Favors Terminal-Effector Differentiation In Vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. The Journal of Immunology. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 13.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesel M, Joller N, Ehlert AK, Crouse J, Sporri R, Bachmann MF, Oxenius A. Th Cells Act Via Two Synergistic Pathways To Promote Antiviral CD8+ T Cell Responses. The Journal of Immunology. 2010;185:5188–5197. doi: 10.4049/jimmunol.1001990. [DOI] [PubMed] [Google Scholar]
- 15.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. 1AD. The CD4+ T-cell help signal is transmitted from APC to CD8+ T-cells via CD27-CD70 interactions. Nature Communications. 3:948–9. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8+ memory T cells. Nat Immunol. 2011:1–7. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 18.Georgopoulous K. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):43–56. 1–14. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 20.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2006;109(2):878–86. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 23.Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10:333–343. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- 24.Molnar A, Wu P, Largespada D, Vortkamp A, Scherer S, Copeland N, Jenkins N, Bruns G, Georgopoulos K. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. The Journal of Immunology. 1996;156:585–592. [PubMed] [Google Scholar]
- 25.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, Wells AD. Ikaros Silences T-bet Expression and Interferon- Production during T Helper 2 Differentiation. Journal of Biological Chemistry. 2010;285:2545–2553. doi: 10.1074/jbc.M109.038794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 27.Winandy SS, Wu PP, Georgopoulos KK. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):89–99. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 28.Depper JM, Leonard WJ, Drogula C, Krönke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci USA. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima H. An indirect effect of Stat5a in IL-2–induced proliferation: a critical role for Stat5a in IL-2–mediated IL-2 receptor α chain induction. Immunity. 1997;7(6):91–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- 30.Pearce EL. Control of Effector CD8+ T Cell Function by the Transcription Factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 31.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. Journal of Experimental Medicine. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imada K, Leonard WJ. The Jak-STAT pathway. Molecular Immunology. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 34.Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 35.Liu CCC, Rafii SS, Granelli-Piperno AA, Trapani JAJ, Young JDJ. Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1989;170:2105–2118. doi: 10.1084/jem.170.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes and Infection. 2007;9:1208–1215. doi: 10.10110/2/076/j.micinf.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White DW, Harty JT. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160:898–905. [PubMed] [Google Scholar]
- 39.Samsom JN, Langermans JA, Savelkoul HF, van Furth R. Tumour necrosis factor, but not interferon-gamma, is essential for acquired resistance to Listeria monocytogenes during a secondary infection in mice. Immunology. 1995;86:256–262. [PMC free article] [PubMed] [Google Scholar]
- 40.Tripp CS, Kanagawa O, Unanue ER. Secondary response to Listeria infection requires IFN-gamma but is partially independent of IL-12. J Immunol. 1995;155:3427–3432. [PubMed] [Google Scholar]
- 41.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor Regression and Autoimmunity after Reversal of a Functionally Tolerant State of Self-reactive CD8+ T Cells. Journal of Experimental Medicine. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll MW, Overwijk WW, Surman DR, Tsung K, Moss B, Restifo NP. Construction and characterization of a triple-recombinant vaccinia virus encoding B7-1, interleukin 12, and a model tumor antigen. J Natl Cancer Inst. 1998;90:1881–1887. doi: 10.1093/jnci/90.24.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, Morgan RA, Gattinoni L, Rosenberg SA, Trinchieri G, Restifo NP. Tumor-Specific CD8+ T Cells Expressing Interleukin-12 Eradicate Established Cancers in Lymphodepleted Hosts. Cancer Research. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, Powell DJ, Klebanoff CA, Finkelstein SE, Fariss RN, Yu Z, Nussenblatt RB, Rosenberg SA, Restifo NP. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen T, Wang R, Russell JH. IL-12 enhances IL-2 function by inducing CD25 expression through a p38 mitogen-activated protein kinase pathway. Eur J Immunol. 2000;30:1445–1452. doi: 10.1002/(SICI)1521-4141(200005)30:5<1445::AID-IMMU1445>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 47.Yanagida TT, Kato TT, Igarashi OO, Inoue TT, Nariuchi HH. Second signal activity of IL-12 on the proliferation and IL-2R expression of T helper cell-1 clone. J Immunol. 1994;152:4919–4928. [PubMed] [Google Scholar]
- 48.Clambey ET, Collins B, Young MH, Eberlein J, David A, Kappler JW, Marrack P. The Ikaros Transcription Factor Regulates Responsiveness to IL-12 and Expression of IL-2 Receptor Alpha in Mature, Activated CD8 T Cells. PLoS ONE. 2013;8:e57435. doi: 10.1371/journal.pone.0057435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, Wells AD. Ikaros Silences T-bet Expression and Interferon-γ Production during T Helper 2 Differentiation. Journal of Biological Chemistry. 2010;285:2545–2553. doi: 10.1074/jbc.M109.038794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida T, Landhuis E, Dose M, Hazan I, Zhang J, Naito T, Jackson AF, Wu J, Perroti EA, Kaufmann C, Gounari F, Morgan BA, Georgopoulos K. Transcriptional regulation of the Ikzf1 locus. Blood. 2013;122(3):149–59. doi: 10.1182/blood-2013-01-474916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dovat S, Song C, Payne KJ, Li Z. Ikaros, CK2 kinase, and the road to leukemia. Mol Cell Biochem. 2011;356(2):01–7. doi: 10.1007/s11010-011-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uckun FM, Ma H, Zhang J, Ozer Z, Dovat S, Mao C, Ishkhanian R, Goodman P, Qazi S. Serine phosphorylation by SYK is critical for nuclear localization and transcription factor function of Ikaros. PNAS. 2012;109:18072–18077. doi: 10.1073/pnas.1209828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H, Qazi S, Ozer Z, Zhang J, Ishkhanian R, Uckun FM. Regulatory Phosphorylation of Ikaros by Bruton’s Tyrosine Kinase. PLoS ONE. 2013;8:e71302. doi: 10.1371/journal.pone.0071302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duré M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Molecular Immunology. 2009;46:999–1006. doi: 10.1016/j.molimm.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mavrakis KJ, Van Der Meulen J, Wolfe AL, Liu X, Mets E, Taghon T, Khan AA, Setty M, Rondou P, Vandenberghe P, Delabesse E, Benoit Y, Socci NB, Leslie CS, Van Vlierberghe P, Speleman F, Wendel HG. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL) Nat Genet. 2011;43:673–678. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi M, Lin TH, Appell KC, Berg LJ. Janus-Kinase-3-Dependent Signals Induce Chromatin Remodeling at the Ifng Locus during T Helper 1 Cell Differentiation. Immunity. 2008;28:763–773. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 58.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell DM, Ravkov EV, Williams MA. Distinct Roles for IL-2 and IL-15 in the Differentiation and Survival of CD8+ Effector and Memory T Cells. The Journal of Immunology. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton SE, Schenkel JM, Akue AD, Jameson SC. IL-2 Complex Treatment Can Protect Naive Mice from Bacterial and Viral Infection. The Journal of Immunology. 2010;185:6584–6590. doi: 10.4049/jimmunol.1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 62.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 63.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 64.Shedlock DJ. Requirement for CD4 T Cell Help in Generating Functional CD8 T Cell Memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 65.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, Kingston R, Georgopoulos K. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 66.Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18:3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koipally J. Ikaros Interactions with CtBP Reveal a Repression Mechanism That Is Independent of Histone Deacetylase Activity. Journal of Biological Chemistry. 2000;275:19594–19602. doi: 10.1074/jbc.M000254200. [DOI] [PubMed] [Google Scholar]
- 68.Clambey ET, Collins B, Young MH, Eberlein J, David A, Kappler JW, Marrack P. The Ikaros transcription factor regulates responsiveness to IL-12 and expression of IL-2 receptor alpha in mature, activated CD8 T cells. PLoS ONE. 2013;8:e57435. doi: 10.1371/journal.pone.0057435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bos R, Sherman LA. CD4+ T-Cell Help in the Tumor Milieu Is Required for Recruitment and Cytolytic Function of CD8+ T Lymphocytes. Cancer Research. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvänen A-C, Rönnblom L, Behrens TW, Graham RR, Vyse TJ. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with Systemic Lupus Erythematosus. PLoS Genet. 2011;7:e1002341. doi: 10.1371/journal.pgen.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swafford ADE, Howson JMM, Davison LJ, Wallace C, Smyth DJ, Schuilenburg H, Maisuria-Armer M, Mistry T, Lenardo MJ, Todd JA. An Allele of IKZF1 (Ikaros) Conferring Susceptibility to Childhood Acute Lymphoblastic Leukemia Protects Against Type 1 Diabetes. Diabetes. 2011;60:1041–1044. doi: 10.2337/db10-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franke A, McGovern DPB, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D’Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PCF, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D’Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Nat Genet 2010 Franke. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]