Abstract
Background:
Weight gain in perimenopausal women results in increased visceral adipose tissue, leading to metabolic syndrome and associated comorbidities. Despite a high prevalence of weight gain at this life stage, interventions to prevent menopausal obesity are lacking.
Aim:
To test the effectiveness of an intervention delivered by health professionals using a motivational interviewing (MI) counselling style in preventing weight gain in non-obese (body mass index (BMI) 18.5 and 29.9 kg m−2) women in late premenopause.
Methods:
In a randomised controlled trial, 54 women (mean (s.d.) age 47.3 (1.8) years; BMI 25.1 (2.4) kg m−2) who had menstruated within the preceding 3 months were randomly assigned to an MI intervention (n=28) (five health professional MI counselling sessions) or a self-directed intervention (SDI) (print materials only) (n=26). The primary outcome, body weight (kg) and secondary outcomes (blood lipids, glucose, body fat %, lean mass % and waist circumference) were measured at baseline and postintervention (12 months), and intention-to-treat analysis was conducted.
Results:
Forty women completed all measures and adhered to all protocols. The weight at 12 months for the MI group of 65.6 kg (95% CI: 64.5; 66.8) was significantly different (P=0.034) from the SDI group of 67.4 kg (95% CI: 66.2; 68.6). When stratified by baseline BMI category, the MI group lost significantly more weight (−2.6 kg; 95% CI: −3.9; −1.2) than the SDI group (−0.1 kg; 95% CI: −1.2; 1.0, P=0.002) for the healthy weight women. The overweight women lost weight regardless of the intervention group, with no between-group difference (−3.5 kg; 95% CI: −6.1, −1.0 and −2.3; 95% CI: −4.1, −0.5, P=0.467).
Conclusion:
This relatively low-intensity intervention, incorporating MI into health professional counselling, not only effectively prevented weight gain but also achieved significant weight loss and decreased diastolic blood pressure. Further refinements are required to optimise outcomes for overweight women.
Introduction
Obesity is a key public health challenge of the postindustrialised world. Physiological and psychological factors make obesity resistant to treatment, highlighting the distinct need for prevention.1 The high incidence of obesity means preventive efforts need to be directed to whole populations, or population subgroups at high risk.2 Prevention of weight gain is particularly important during the menopause transition, which is associated with oestrogen deficiency-induced visceral fat deposition,3, 4 and decreased total energy requirements secondary to loss of lean body mass,5 creating a metabolic environment in mid-age women conducive to metabolic syndrome and weight gain.3 Metabolic syndrome, characterised by insulin resistance,6, 7 accounts for an estimated 48% of coronary events in postmenopausal women.4 Observational studies provide evidence for increasing body fatness8, 9, 10 and deterioration of the lipid profile with the menopause;11 however, evidence of effective interventions designed to prevent menopausal weight gain is lacking.
To the authors' knowledge, only one intervention, the Women's Healthy Lifestyle Project (WHLP),12, 13 has been published. The WHLP randomised 535 premenopausal women into a lifestyle intervention group (15-session diet and exercise education program followed by a maintenance program for 5 years) or an assessment-only control group. The lifestyle group lost a mean (s.d.) of 0.1 (5.2) kg and only raised low-density lipoprotein (LDL)-cholesterol by 0.09 mmol l−1 after 54 months compared with the control group, which gained 2.4 (4.9) kg and had a rise in LDL-cholesterol of 0.23 mmol l−1.13 These results, while impressive, required intensive resources for the intervention, making population-wide application problematic. Lower intensity interventions that are feasible and sustainable are needed.
The Australian primary health-care setting provides a model to test an intervention for women about to enter menopause. The National Health Service, Medicare Australia, funds up to five consultations per year with an allied health practitioner (including Dietitians and Exercise Physiologists) for patients with chronic and complex medical problems referred by their general practitioners.14 Extension of this service-delivery model to prevent obesity in women in late premenopause would provide a relatively low-intensity, sustainable, national intervention aimed at preventing obesity in a high-risk group. The aim of the current study was to test the feasibility and effectiveness of a 12-month health professional intervention (the 40-Something study) in preventing obesity in non-obese premenopausal women, in comparison with women self-directing their intervention after receiving written information.
Materials and methods
Study design and participants
A parallel-group randomised controlled trial (RCT) design was used to compare the health professional-led intervention (motivational interviewing (MI) intervention), with a written information self-directed intervention (SDI). The study consisted of a 12-month intervention phase followed by 12 months of monitoring only, and conformed to CONSORT guidelines.15 The study received ethical approval from the University of Newcastle Human Research Ethics Committee (H2010-0030), and was registered with the Australian New Zealand Clinical Trials Registry (reference number ACTRN12611000064909). The detailed methods of the study have been published.16
Participants were recruited from the Newcastle region, Australia, through television, radio and print media. They were screened against inclusion criteria (women aged 44–50 years at baseline, with a body mass index (BMI) between 18.5 and 29.99 kg m−2, menstruated within preceding 3 months, free of major diseases including cardiovascular disease, diabetes and cancer) by telephone interview between May and July of 2010 and provided written informed consent. Women were categorised by BMI as healthy weight (HW) (18.5−<25 kg m−2) or overweight (OW) (25−<30 kg m−2), according to their baseline measures, and then randomly assigned to the MI or SDI group using computer-generated number sequences (one list for each BMI group) compiled by a statistician using the ProcPlan procedure in SAS Version 9.2 (SAS Institute Inc, Cary, NC, USA). As each woman presented for baseline measures, her name was recorded against the next vacant number. Later that same day, the researcher who held the allocation list phoned each woman to inform them of their intervention assignment. Reasons for withdrawal were sought from those leaving the study. The target sample size was 55, to obtain a significant (P<0.05) difference of 3.5 kg between the mean weight of the two groups at 24 months (based on an expected mean gain of 1.0 kg in 24 months in the SDI group,17 and a mean loss of 2.5 kg in the MI group based on the achievement of intervention goals).
Intervention goals and description
Goals and behaviour change strategies
All participants were given a weight management booklet (16 pages, A5 size) appropriate to their baseline BMI. The HW women were given a booklet that provided advice to maintain their weight within 1 kg by consuming 8300 kJ and walking 10 000 steps per day and performing 150 min per week of moderate to vigorous activity. The version for the OW women provided advice to lose weight to achieve a weight in the healthy BMI range (mean recommended weight loss of 5 kg (7% of a baseline of 73 kg)) by consuming 6300 kJ and walking 10 000 steps per day and performing 250 min per week of moderate to vigorous activity. Participants were encouraged to achieve these goals through individually tailored behaviour change strategies based on results of baseline and 3-month dietary intake and physical activity measures, and these were documented on a sheet headed ‘my goals'.
Health professional intervention using MI
Women assigned to the MI group received five consultations with health professionals across the intervention.18 Within 1 month of baseline measures, participants received an individual counselling session with a Dietitian (1 × 60 min) and an Exercise Physiologist (1 × 60 min). They were provided with written copies of their baseline anthropometric, dietary and physical activity assessments, which guided client-centred discussion where the women developed personal strategies for weight control. The remaining 3 × 60 min individual counselling sessions were conducted by the same Dietitian 3, 6 and 9 months after the first appointment. The health professionals (one Dietitian and two Exercise Physiologists) were registered Medicare providers who reported undertaking additional training in MI (including online courses, self-directed reading and workshops). The health professionals followed a written protocol for each session consistent with the approach of MI by aiming to develop a collaborative partnership, using a guiding method of communication to create an environment where the client felt comfortable to explore and resolve ambivalence to behaviour change,18 thereby enhancing intrinsic motivation (see Supplementary Information for the way in which MI was applied). The integrity of MI was measured by an independent psychologist using the MI Integrity Tool (MITI) version 3.19
SDI (control)
The SDI group was mailed the same weight management booklets as the MI group together with a print copy of their baseline anthropometric, biochemistry, dietary and physical activity results and materials to assist self-monitoring of weight, dietary intake and physical activity, together with documented goals and strategies tailored to their results by the health professionals. This occurred within a month of attending baseline, and 3- and 12-month measures. An important distinction is that these goals and strategies were set by the practitioners and mailed to participants, whereas the MI women developed their goals and strategies in face-to-face consultations.
Outcome measures
The primary outcome measure, weight, was collected by a trained assistant at baseline and 3 and 12 months after the intervention commenced. Participants were weighed at each visit using BIA Omron HBR-500 Body Composition Monitor with Scales (Omron Healthcare Co. Ltd, Kyoto, Japan) corrected to 0.1 kg in light indoor clothing after an overnight fast and voiding of urine. Secondary outcome measures (fat mass, lean mass, waist circumference and blood pressure) were collected at baseline and 3 and 12 months, with total cholesterol, LDL-cholesterol, high-density lipoprotein-cholesterol, triglycerides and fasting glucose collected at baseline and 12 months. Blood was collected from fasted participants by a trained phlebotomist and analysed at a nationally accredited pathology laboratory. Visceral adipose tissue (VAT) area was collected at 12 months as an addition to the original protocol when new equipment became available.
Weight control behaviours, including dietary intake (4-day weighed food record), physical activity (pedometer steps, minutes spent weekly in light, moderate and vigorous activity and International Physical Activity Questionnaire (IPAQ)20, 21) and sedentary behaviours (sitting time22, 23), were measured at baseline and 12 months to assess intervention impact, and at 3 months to assess protocol compliance. Reported health behaviours and attitudes (Three Factor Eating Questionnaire24) and health-related quality of life measures (Short Form-36; Ware et al.25) were collected, along with demographic data using a written survey at baseline and 12 months. A menstruation diary was kept throughout the study to assess change in menopause status.
Statistical management and analysis
All analyses were conducted in SPSS Version 19.0 (IBM, Armonk, NY, USA). Unpaired t-tests and χ2-tests on continuous and categorical variables, respectively, were used to compare the baseline characteristics of the two intervention groups. Continuous variables were checked for plausibility of outliers, and then for normality of distribution using the Shapiro–Wilk test. Non-normally distributed variables were transformed, and non-parametric analyses conducted any variables that did not normalise with transformation. Intention-to-treat (ITT) principles were applied to statistical analyses of the outcome variables, using the last observation carried forward method to impute values for missing data. Between-group differences for the outcomes were tested using analysis of covariance, with baseline values as covariates, and intervention group as the fixed factor, with the exception of VAT for which there was no baseline data (unpaired t-tests at 12 months were conducted in this case). To explore differences in outcomes according to whether the women were HW or OW at baseline, the tests applied to the whole group were repeated for each BMI category by intervention type (HW-MI, HW-SDI, OW-MI and OW-SDI).
Success in achieving intervention weight goals was assessed for each participant and scored as a dichotomous variable according to whether they achieved their goal (assigned a score of one) or not (assigned a score of zero). Healthy weight women scored one if they gained <1 kg from baseline to 12 months. Overweight women scored one if their 12-month weight was 7% less than baseline and/or gave a BMI within the HW range. The proportion of those achieving their goal in each intervention group was compared using χ2-tests. Those who dropped out before 12 months were included as a third category in the analysis.
Results
Participants
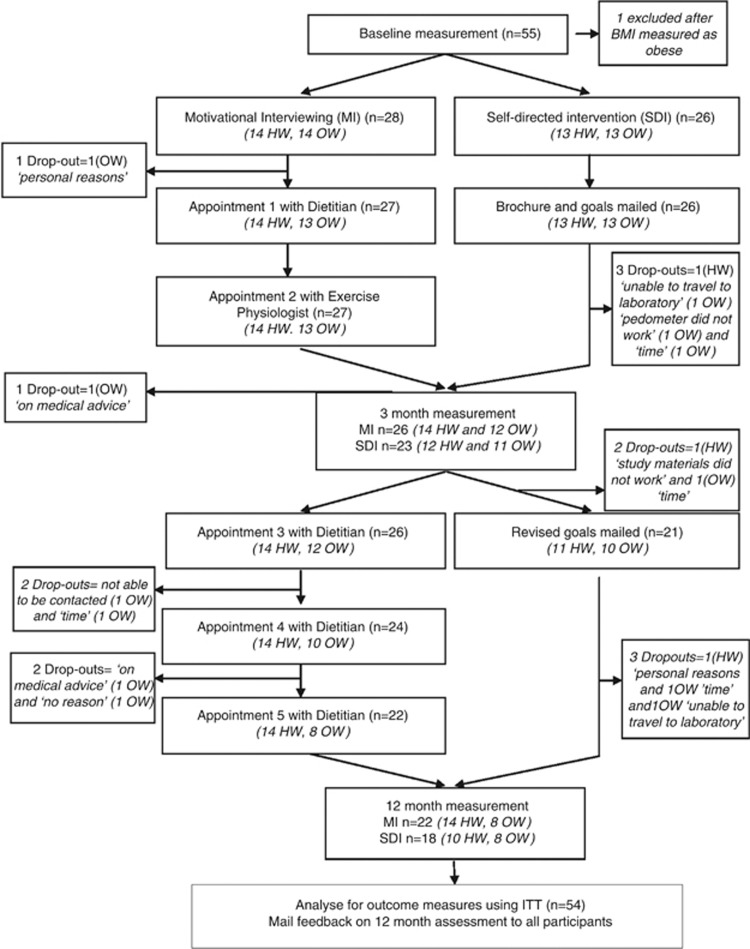
Figure 1 shows the flow of participants through the study and reasons for withdrawal (14/54, 25.9%). Baseline demographic and weight-related characteristics are shown in Table 1. Most participants were born in Australia (84.9%), were in a relationship (77.4%), had postschool education (77.4%) and were in full or part-time employment (90.6%). BMI at baseline ranged from 18.9 to 29.3 kg m−2. Four women (all MI) had become perimenopausal and none were postmenopausal by 12 months. Forty women participated in all three data collection points, with the only difference in demographic and health characteristics between them and those who did not stay in the study to 12 months, being a higher mean body fat mass in the attriters (38.4% vs 35.0%, P=0.046).
Figure 1.
Participation and dropout rates for both groups for the intervention stage of the study.
Table 1. Baseline characteristics of participants according to the intervention group.
| Characteristics | Total (n=54) | MI (n=28) | SDI (n=26) |
|---|---|---|---|
| Age (years), mean (s.d.) | 47.3 (1.8) | 47.6 (1.9) | 46.9 (1.6) |
| Weight (kg), mean (s.d.) | 68.7 (7.9) | 68.7 (8.9) | 68.6 (6.7) |
| Height (m), mean (s.d.) | 1.65 (0.06) | 1.66 (0.06) | 1.65 (0.05) |
| Body mass index, mean (s.d.) | 25.1 (2.4) | 24.9 (2.5) | 25.2 (2.4) |
| Healthy weight (N=27; MI=14; SDI=13)) | 23.0 (1.5) | 22.8 (1.4) | 23.3 (1.6) |
| Overweight (n=27; MI=14; SDI=13) | 27.1 (1.2) | 27.0 (1.0) | 27.1 (1.3) |
| Country of birtha, n (%) | |||
| Australia | 45 (84.9) | 22 (81.5) | 23 (88.5) |
| Other | 8 (15.1) | 5 (18.5) | 3 (11.5) |
| Marital statusa, n (%) | |||
| Married/defacto | 41 (77.4) | 20 (74.1) | 21 (80.8) |
| Divorced/separated/widowed/never married | 12 (22.6) | 7 (25.9) | 5 (19.2) |
| Highest qualificationa, n (%) | |||
| School only | 12 (22.6) | 6 (22.2) | 6 (23.1) |
| Postschool qualifications | 41 (77.4) | 21 (77.8) | 20 (76.9) |
| Work statusa, n (%) | |||
| Full/part time employment | 48 (90.6) | 24 (88.9) | 24 (92.3) |
| Home duties/care | 3 (5.7) | 2 (7.4) | 1 (3.8) |
| Full time student | 2 (3.8) | 1 (3.7) | 1 (3.8) |
| Difficulty managing on incomea, n (%) | |||
| Not too bad/easy | 39 (73.6) | 22 (81.5) | 17 (65.4) |
| Difficult sometimes/all times/impossible | 14 (26.4) | 5 (18.5) | 9 (34.6) |
| Self-rated healtha, n (%) | |||
| Excellent/very good/good | 52 (98.1) | 26 (96.3) | 26 (100) |
| Fair/poor | 1 (1.9) | 1 (3.7) | 0 (0) |
| Physical activity (IPAQb), n (%) | |||
| Low | 10 (22.2) | 5 (23.8) | 5 (20.8) |
| Moderate | 16 (35.6) | 9 (42.9) | 7 (29.2) |
| High | 19 (42.2) | 7 (33.3) | 12 (50.0) |
| Menopause status, n (%) | |||
| Premenopausal | 54 (100) | 28 (100) | 26 (100) |
| Perimenopausal | 0 | 0 | 0 |
| Postmenopausal | 0 | 0 | 0 |
Abbreviations: IPAQ, International Physical Activity Questionnaire; MI, motivational interviewing; s.d., standard deviation; SDI, self-directed intervention.
n=27 (MI), n=26 (SDI), N=53 (Total).
n=21 (MI), n=24 (SDI), N=45 (Total). Nine participants answered ‘don't know' in response to at least one of the questions in the IPAQ survey at baseline and according to the scoring protocol their results were excluded from the analysis.
Intervention delivery
All 40 women received all components of the relevant intervention according to the protocol. The assessment of randomly selected audiorecordings of consultations showed the clinical counselling style to be collaborative and supportive, and met proficiency requirements for open-ended questions, but fell below beginning MI proficiency level for the summary score of global spirit ratings (score=3.11, beginning proficiency=3.5) and reflections to questions ratio (score=0.75, beginning proficiency=1). Behaviour counts for percent complex reflections were below proficiency (score=7.33%, beginning proficiency=40%) and percent MI-adherent behaviours (score=45.33%, beginning proficiency=90%).
Effects on 12-month body weight of intervention and baseline BMI
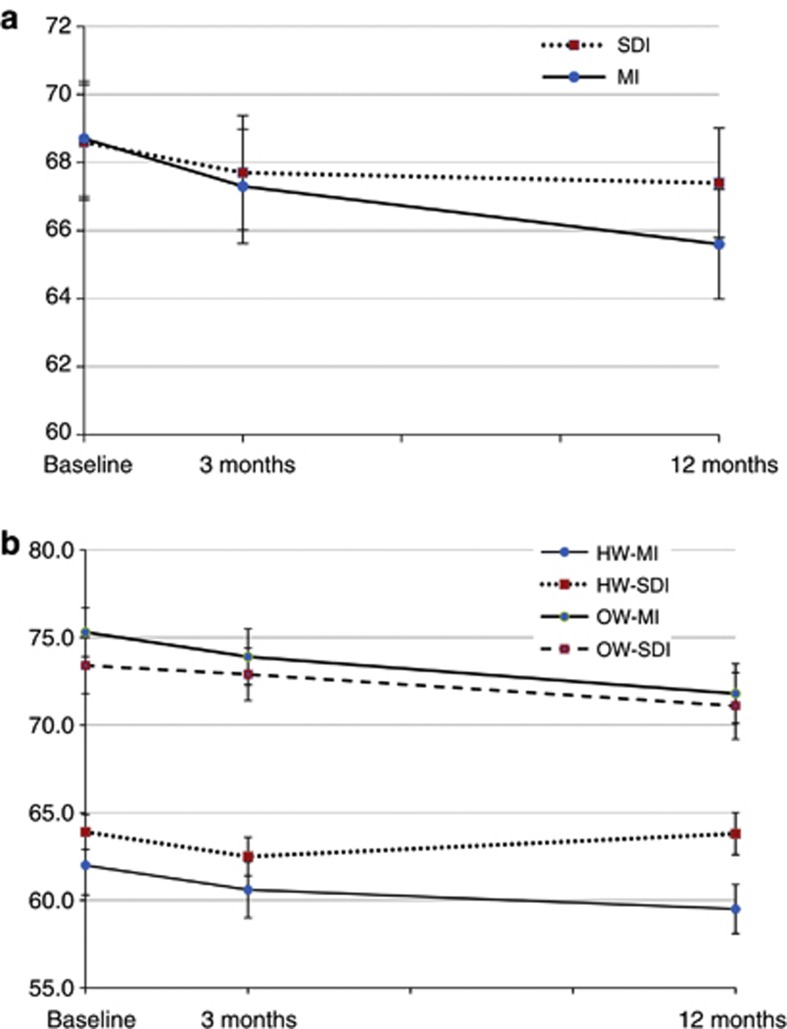
There was a significant intervention effect (P=0.034) on weight at 12 months after adjusting for baseline weight as shown by the data in Table 2. Subanalyses according to baseline BMI showed the HW-MI women lost a mean of 2.5 kg over the 12 months, a significant decrease from baseline (P=0.002), whereas the HW-SDI women showed no change over time (P=0.862). There was a significant intervention effect for the HW women at 12 months (P= 0.002) as shown in Figure 2a. The women who were OW at baseline lost a significant amount of weight over 12 months (2.9 kg; 3.9% body weight, P= 0.001). Subanalyses showed that the OW-MI and the OW-SDI groups both lost similar amounts from baseline to 12 months (3.5 and 2.3 kg, respectively), with no between-group difference at 12 months (P=0.467) (see Figure 2b).
Table 2. Mean (s.d.) weight (kg) showing weight change according to intervention type and baseline BMI (HW and) using ITT analysis.
| Data collection point | Group | MI | SDI | Unadjusted difference P-value (95% CI) | Difference at 12 months (adjusted) P-value (95% CI) |
|---|---|---|---|---|---|
| Baseline group | 68.7 (7.9) | 68.7 (8.9) | 68.6 (6.7) | 0.982 (−4.30; 4.39) | __ |
| HW only | 62.9 (5.3) | 62.0 (6.4) | 63.9 (3.7) | 0.373 (−6.01; 2.36) | __ |
| OW only | 74.4 (5.5) | 75.3 (5.4) | 73.4 (5.6) | 0.365 (−2.41; 6.32) | __ |
| 3 Months group | 67.5 (8.0) | 67.3 (8.9) | 67.7 (7.1) | 0.863 (−4.80; 4.03) | __ |
| HW only | 61.5 (5.1) | 60.6 (5.9) | 62.5 (4.0) | 0.356 (−5.86; 2.19) | __ |
| OW only | 73.4 (5.6) | 73.9 (5.9) | 72.9 (5.5) | 0.629 (−3.44; 5.58) | __ |
| 12 Months group | 66.5 (7.7) | 65.6 (8.5) | 67.4 (6.7) | 0.402 (−5.99; 2.44) | 0.034 (−3.490; −0.145) |
| HW only | 61.6 (5.3) | 59.5 (5.4) | 63.8 (4.4) | 0.032 (−8.23; −0.40) | 0.002 (−4.383; −1.085) |
| OW only | 71.5 (6.4) | 71.8 (6.3) | 71.1 (6.8) | 0.764 (−4.42; 5.95) | 0.467 (−4.258; 2.011) |
Abbreviations: BMI, body mass index; CI, confidence interval; HW, healthy weight; OW, overweight; MI, motivational interviewing; s.d., standard deviation; SDI, self-directed intervention; ITT, intention to treat.
Baseline: group—N=54, MI=28, SDI=26; HW—N=27, MI=14, SDI=13; OW—N=27, MI=14, SDI=13.
3 Months: group—N=49, MI=26, SDI=23; HW—N=25, MI=14 SDI=11; OW—N=22, MI=12, SDI=10.
12 Months: group—N=40, MI=22, SDI=18; HW—N=24, MI=14, SDI=10; OW—N=16, MI=8, SDI=8.
Figure 2.
(a) Mean (s.e.) 12-month weight change for the MI group and the SDI group (N=54) and subanalyses according to BMI at baseline. (b) Mean (s.e.) 12-month weight change for the subgroups of HW or OW and intervention group MI or SDI.
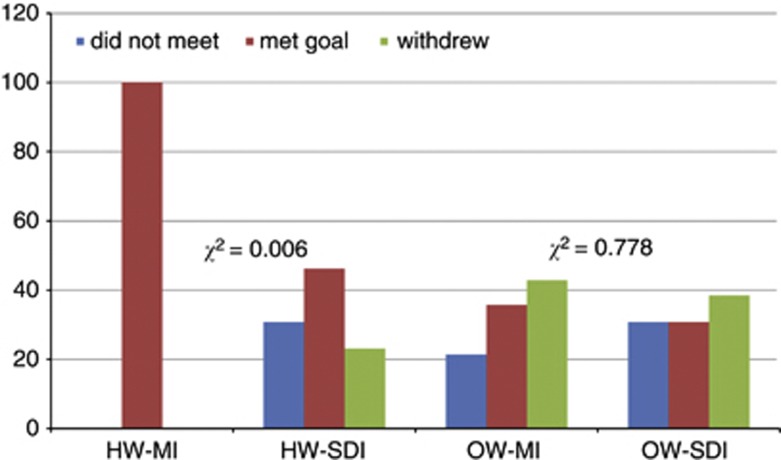
Achievement of intervention goals for weight change
Although there was no significant difference in goal achievement according to group (P=0.071), there was a difference for the HW women but not OW women (P=0.006 and 0.778, respectively) (Figure 3). All 14 HW women who received the MI intervention achieved the goal of not gaining weight, and 100% completed the intervention. This was significantly different from the HW-SDI women, six of whom did not gain weight, four of whom gained 1 kg or more and three of whom withdrew. Conversely, the type of intervention had no effect on the rate of success or withdrawal for the women who commenced the intervention in the OW BMI category, with five of 14 OW-MI women and four of the 13 OW-SDI women attaining the goal of losing 7% of baseline weight or being categorised within the HW range. The women who were HW at baseline all maintained that BMI category to 12 months, and six of the 27 women who were OW at baseline attained a BMI in the HW range. While 10 of the 16 OW women remaining in the study to 12 months failed to attain the HW range (five in each intervention condition), none gained weight.
Figure 3.
Weight goal attainment and drop-out according to the intervention group and baseline BMI.
Intervention effects on secondary outcome measures: other anthropometry and biomarkers of metabolic syndrome
Changes in the secondary outcome variables according to intervention group are reported in Table 3. After 12 months, there were statistically significant between-group differences for waist circumference and diastolic blood pressure. The mean baseline results for biomarkers of metabolic syndrome were within recommended levels, and remained so at 12 months.
Table 3. Anthropometry and biomarkers of metabolic syndrome according to the intervention type (ITT analysis).
| Variable | Group, N=54, mean (s.d.) | MI, N=28, mean (s.d.) | SDI, N=26, mean (s.d.) | Difference at baseline and 12 months unadjusted P-value (95% CI) | Difference at 12 months (adjusted model) |
|---|---|---|---|---|---|
| Body fat % | |||||
| Baseline | 35.9 (5.6) | 35.6 (5.8) | 36.2 (5.4) | 0.703 (−3.680; 2.500) | − |
| 12 Months | 34.3 (5.5) | 33.5 (6.0) | 35.2 (4.9) | 0.253 (−4.709; 1.264) | 0.235 (−2.586; 0.650) |
| Lean muscle (%) | |||||
| Baseline | 27.4 (2.7) | 27.5 (2.8) | 27.2 (2.5) | 0.649 (−1.154; 1.834) | − |
| 12 Months | 27.8 (2.5) | 28.1 (2.9) | 27.6 (2.2) | 0.432 (−0.844; 0.943) | 0.592 (−0.607; 1.053) |
| Waist circumference (cm) | |||||
| Baseline | 83.1 (7.6) | 83.3 (8.2) | 83.0 (7.0) | 0.905 (−3.937; 4.440) | − |
| 12 Months | 81.5 (8.1) | 80.4 (8.6) | 82.6 (7.5) | 0.0307 (−6.705; 2.149) | 0.045a (−4.941; −0.061) |
| Total cholesterol (mmol l−1) | |||||
| Baseline | 4.97 (0.98) | 5.05 (1.2) | 4.89 (0.74) | 0.549 (−0.376; 0.699) | − |
| 12 Months | 5.05 (0.97) | 5.07 (1.11) | 5.03 (0.82) | 0.890 (−0.498; 0.572) | 0.508 (−0.392; 0.197) |
| Triglycerides | |||||
| Baselineb (n=54) | 1.06 (0.73) | 1.07 (0.70) | 1.05 (0.40) | 0.219 | − |
| 12 Monthsb (n=39) | 0.94 (0.41) | 0.95 (0.42) | 0.93 (0.41) | 0.863 | − |
| LDL-Cc (mmol l−1) | |||||
| Baseline | 2.86 (0.85) | 2.90 (0.99) | 2.81 (0.67) | 0.705 (−0.384; 0.563) | − |
| 12 Months | 2.85 (0.87) | 2.85 (0.95) | 2.84 (0.79) | 0.944 (−0.469; 0.503) | 0.600 (−0.303; 0.177) |
| HDL-Cb,c (mmol l−1) | |||||
| Baseline | 1.62 (0.37) | 1.65 (0.34) | 1.58 (0.40) | 0.461 (−0.127; 0.277) | − |
| 12 Months | 1.73 (0.39) | 1.78 (0.36) | 1.68 (0.42) | 0.383 (−0.120; 0.308) | 0.643 (−0.084; 0.135) |
| Glucosec (mmol l−1) | |||||
| Baseline | 4.47 (0.64) | 4.45 (0.53) | 4.48 (0.75) | 0.895 (−0.377; 0.330) | − |
| 12 Months | 4.55 (0.43) | 4.53 (0.41) | 4.56 (0.45) | 0.805 (−0.266; 0.207) | 0.834 (−0.234; 0.189) |
| Visceral adipose tissued (cm3) | |||||
| Baseline | − | − | − | − | − |
| 12 Months | 79.5 (26.3) | 75.6 (26.3) | 84.4 (26.2) | 0.296 (−25.737; 8.050) | − |
| Systolic BP (mm Hg) | |||||
| Baseline | 120.3 (11.8) | 120.7 (11.6) | 119.9 (12.2) | 0.807 (−5.755; 7.356) | − |
| 12 Months | 119.1 (11.2) | 117.6 (7.8) | 120.7 (13.8) | 0.316 (−9.249; 3.048) | 0.137 (−8.341; 1.174) |
| Diastolic BP (mmol l−1) | |||||
| Baseline | 75.7 (8.3) | 76.1 (7.0) | 75.4 (9.6) | 0.741 (−3.852; 5.381) | − |
| 12 Months | 75.6 (7.3) | 74.4 (5.5) | 76.8 (8.7) | 0.237 (−6.375; 1.613) | 0.037a (−5.572; −0.175) |
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VAT, visceral adipose tissue.
Statistically significant result.
Non−normally distributed—Mann−Whitney U-test used.
Ten women (3 MI and 7 SDI) who had blood collected at 12 months are missing some results owing to a laboratory reporting error (6 missing LDL-cholesterol, 3 missing HDL-cholesterol and therefore total cholesterol to HDL ratio, and 2 missing fasting blood glucose). One woman declined blood collection at all timepoints.
VAT measures were only collected at 12 months on 40 women—differences were calculated using unpaired t–tests; n varied as follows: baseline—MI=28, SDI=26, group =54; 3 months—MI=26 (−2), SDI=23 (−3), group=49 (−5); 12 months—MI=22 (−6), SDI=18 (−8), group=40 (−14).
Discussion
The 40-Something RCT showed that a relatively low-intensity intervention (five health professional consultations) was effective in preventing menopausal obesity. Subgroup analyses revealed that, for the women who were HW at baseline, the MI intervention was more effective than the SDI. However, both interventions worked to some extent for the women OW at baseline, with both groups losing an amount of weight previously shown to improve the well-being in this target age group,26 but no between-group difference in weight at 12 months. No OW women gained weight or became obese during the 12 months, regardless of the intervention group.
While a multitude of intervention studies aimed at weight loss have been published,27 there are few obesity prevention intervention studies with which to compare the 40-Something RCT results.28, 29 An Australian RCT, the HeLP study, aimed to prevent weight gain in a group of slightly younger, premenopausal women (mean (s.d.) age of 40.39 (4.77) years) of similar BMI (27.8 (5.4) kg m−2).29 The HeLP intervention was similarly of relatively low intensity (four interactive group sessions conducted by a health professional plus monthly text messaging support) plus a 30-min education session.29 At 12 months the intervention group maintained weight (0.20 kg, 95% CI: −0.90–0.49), whereas the control group gained weight (0.83 kg, 95% CI: 0.12–0.54), with a significant between-group difference in weight change at 12 months (P<0.05).30
In the few weight gain prevention interventions in women, there is a lack of consistency in setting weight maintenance goals. The HeLP study defined within 1 kg as weight maintenance,29 which was similar to the two pounds set by Levine and co-workers31 in a US study of weight gain prevention in women 25–45 years.31 The WHLP advised women with BMI ⩽24 kg m−2 to first lose weight (between 2.3 and 6.8 kg depending on baseline BMI), to ‘prevent any weight gain above baseline'.32 So, rather than preventing weight gain because of a net weight change of zero, they actually observed an initial sharp decrease in weight and a gradual regain, which was then counted as success. The 40-Something RCT considered a gain of <1 kg as prevention of weight gain for the HW women, which was similar to the HeLP study.29 The OW women in the 40-Something RCT were encouraged to lose 5 kg or to attain an HW BMI by losing up to 5 kg.
The 40-Something RCT goals may have been too difficult to attain, especially for those commencing the study in the OW range who needed to lose up to 7% of their body weight to be deemed successful. The WHLP, using the less stringent measure of success as being ‘at or below baseline weight',32 found 55% of lifestyle intervention participants achieved this goal, compared with 26% of controls after 54 months. Applying the WHLP criterion to the 40-Something RCT results, 68% women in the MI group and 39% in the SDI group were at or below their baseline weight after 12 months. One major difference between the two studies, the final data collection point for WHLP of 54 months, should be emphasised.
One of the challenges of a weight gain prevention study, and a possible factor explaining why so few are conducted, is that the study aim is to achieve weight stability, which is zero change. While successful interventions for weight loss can demonstrate significant weight decreases, by definition, weight gain prevention studies consider zero change as success. National health goals advise populations to maintain weight, yet there is no agreed definition of weight maintenance, highlighting a need for a standardised definition to ensure consistency between studies.26, 33, 34
Given the fidelity assessment of the MI counselling sessions, we know that there was no difference in the way the intervention was delivered to the HW and OW women, but there was a difference in outcomes. One potential explanation for the differential success of the intervention in HW and OW women is that the task for the OW women was more difficult owing to problematic behaviours, an obesogenic environment and physiological challenges. Physiologically, the overweight women had significantly higher mean VAT levels at 12 months (92.0 vs 71.2 cm2, P=0.013) increasing the likelihood of insulin resistance.35 In a behavioural intervention study of a cohort of 158 healthy, mid-age (mean (s.d.) age of 48 (4.5) years), women in the United States, baseline psychosocial and behavioural characteristics of participants, including the number of previous attempts at weight loss by overweight women, were associated with increased attrition and decreased weight loss success rates,36 which may make them more resistant to treatment upon entering the study.36 This may have applied to the women in the 40-Something RCT given a similar recruitment environment.
Given these biopsychosocial barriers to weight loss, it is notable that the overweight women achieved moderate weight loss, and all those remaining in the study (16/27) managed to prevent weight gain. Interestingly, the health professional-led MI intervention did not add any benefit beyond the SDI for these women. It is possible that the MI counselling intervention was not intensive enough to make a difference above the SDI, or that the overweight women did not respond well to an approach that relies on intrinsic motivation. The extrinsic motivation received from the process of returning for measurement may have been the main driver for their weight loss, resulting in the observed equal effect for the MI and the SDI groups. In the SHED-IT weight loss RCT for men, also a low-intensity program, a positive change in the minimal intervention group resulting in a lack of between-group difference was also observed.37
While diastolic blood pressure decreased owing to the intervention, there was no statistically significant impact on the biomarkers of metabolic syndrome. An RCT conducted in the United Kingdom designed to improve cardiovascular risk factors in a male and female population (mean (s.d.) age 50.22 (50.58) years), using a similar intervention (five face-to-face MI counselling sessions by a dietitian and physical activity specialist), observed an intervention effect for cholesterol.38 However, unlike the 40-Something study, the mean baseline cholesterol was in the hypercholestrolaemic range.38 The HeLP study intervention group did not improve their metabolic profile, but the profile of the control group deteriorated.30 Similarly, the intervention group in the WHLP study had a significantly better metabolic profile (LDL-cholesterol, triglycerides and glucose) than the assessment-only group,13 because the intervention group stayed the same while the control group profile deteriorated by the end of the 54-month period.13 The 40-Something study managed to maintain the metabolic profile for both groups over 12 months. The study duration would need to be extended to assess the impact of the intervention on metabolic measures into peri- and postmenopause.11
The 40-Something study had several strengths. First, it addresses a current gap in the literature of RCT's in weight gain prevention, and secondly it targets a group at high risk of weight gain with comorbid consequences. Other strengths include the RCT design, with all but one of the researchers remaining blind to the group assignment for the study duration, and ITT analysis for all outcome measures. Having one Dietitian conduct appointments 1, 3, 4 and 5 for all participants ensured intervention consistency. Another strength of the research is its high translational potential, given that the model was based on a relatively low-intensity intervention that could be delivered through an existing primary health-care system. The limitations include not being sufficiently powered to assess the secondary outcomes, the lack of a no-treatment or wait-list control group, and the lack of an attention control group, which meant it was not possible to evaluate the effects of MI above the health professional contact. The proficiency in MI of the health professionals was not assessed before the study, although it was measured during the study. While the MI did not reach proficiency on all measures, these results were reported, which is rarely the case in MI studies.39
The 40-Something study provides learnings for future interventions with menopausal women. This includes the importance of monitoring of menstrual status to determine stage of the menopause transitions, a simple measure unnecessarily lacking in most studies that include women of menopausal age. Measurement of compliance to the intervention protocol is recommended practice, but it is especially important to determine mediators of success in complex behaviour change projects such as changing dietary intake and physical activity. In MI interventions, the 40-Something results highlight the importance of audio-recording consultations to enable MI fidelity assessment—to determine how closely intervention delivery follows the intended protocol.
The 40-Something RCT has demonstrated that a relatively low-intensity intervention is effective in preventing menopausal weight gain over 12 months. The client-centred intervention using MI counselling was effective for the HW women; however, it was no more effective than an SDI for overweight women. Future research should establish the intensity of the health professional appointments needed to optimise outcomes for overweight women.
Acknowledgments
We thank the wonderful women who participated in the study, and all the others who made the study possible: research assistants (particularly Hannah Lucas and Emma McNamara) who helped with data collection; Louana Moller, Lynn Clarke and Narelle Eddington from Hunter Area Pathology Service; and Patrick McElduff for statistical advice. We alsothank the Exercise Physiologists who contributed their expertise to the intervention. We are indebted to Kristy Parsons and Jo Ubels for assistance with preparing this manuscript. The research was funded by the University of Newcastle grants from the Faculty of Health, The School of Health Sciences and the Priority Research Centre for Physical Activity and Nutrition. CEC is supported by a National Health and Medical Research Council Career Development Fellowship. JLH is supported by an Australian Postgraduate Award Scholarship and a Barker Top-up Scholarship from the Barker Family, University of Newcastle and University Foundation Services.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Lawlor DA, Chaturvedi N. Treatment and prevention of obesity—are there critical periods for intervention. Int J Epidemiol. 2006;35:3–9. doi: 10.1093/ije/dyi309. [DOI] [PubMed] [Google Scholar]
- Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378:838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3:696–704. doi: 10.1038/ncpendmet0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaja RJ. Metabolic syndrome and the menopause. Menopause Int. 2008;14:21–25. doi: 10.1258/mi.2007.007032. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Stuenkel CA, Barrett-Connor E, Stuenkel CA. Lifestyle intervention and postmenopausal bone density. J Clin Endocrinol Metab. 2007;92:3777–3779. doi: 10.1210/jc.2007-1827. [DOI] [PubMed] [Google Scholar]
- Bittner V. Menopause and cardiovascular risk—Cause or consequence. J Am Coll Cardiol. 2006;47:1984–1986. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Macdonald HM, New SA, Campbell MK, Reid DM. Longitudinal changes in weight in perimenopausal and early postmenopausal women: effects of dietary energy intake, energy expenditure, dietary calcium intake and hormone replacement therapy. Int J Obes. 2003;27:669–676. doi: 10.1038/sj.ijo.0802283. [DOI] [PubMed] [Google Scholar]
- Samat A, Rahim A, Barnett A, Samat A, Rahim A, Barnett A. Pharmacotherapy for obesity in menopausal women. Menopause Int. 2008;14:57–62. doi: 10.1258/mi.2008.008005. [DOI] [PubMed] [Google Scholar]
- Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition. J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin-Silverman LR, Wing RR, Hansen DH, Klem ML, Pasagian-Macauley A, Meilahn EN, et al. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med. 1995;24:509–517. doi: 10.1006/pmed.1995.1081. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women's Healthy Lifestyle Project: a randomized clinical trial: results at 54 months. Circulation. 2001;103:32–37. doi: 10.1161/01.cir.103.1.32. [DOI] [PubMed] [Google Scholar]
- Health Do Medicare Benefits Schedule MBS Primary Care Items, 2010. Available at: http://www.health.gov.au/mbsprimarycareitems (last accessed 24 October 2012).
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LT, Hollis JL, Collins CE, Morgan PJ. The 40-Something randomized controlled trial to prevent weight gain in mid-age women. BMC Public Health. 2013;13:1007. doi: 10.1186/1471-2458-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Germov J, Young A. Preventing weight gain: a population cohort study of the nature and effectiveness of mid-age women's weight control practices. Int J Obes. 2007;31:978–986. doi: 10.1038/sj.ijo.0803550. [DOI] [PubMed] [Google Scholar]
- Miller W, Rollnick S.eds). Motivational Interviewing: Preparing People for Change2nd edn.The Guilford Press: New York, NY, USA; 2002 [Google Scholar]
- Moyers TB, Martin JK, Miller WR, Ernst D.Revised Global Scales: Motivational Interviewing Treatment Integrity 3.0 (MITI 3.0), 2007. Available at: : http://casaa.unm.edu/download/miti3.pdf ( last accessed 24 October 2012).
- Mader U, Martin BW, Schutz Y, Marti B. Validity of four short physical activity questionnaires in middle-aged persons. Med Sci Sports Exerc. 2006;38:1255–1266. doi: 10.1249/01.mss.0000227310.18902.28. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström L, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Marshall AL, Miller Y, Burton N, WJ B. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42:1094–1102. doi: 10.1249/MSS.0b013e3181c5ec18. [DOI] [PubMed] [Google Scholar]
- Collins CE, Morgan PJ, Warren JM, Lubans DR, Callister R. Men participating in a weight-loss intervention are able to implement key dietary messages, but not those relating to vegetables or alcohol: the Self-Help, Exercise and Diet using Internet Technology (SHED-IT) study. Public Health Nutr. 2011;14:168–175. doi: 10.1017/S1368980010001916. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Persson LO, Sjöström L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes. 2000;24:1715–1725. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A User's Manual. The Health Institute, New England Medical Centre: Boston, MA, USA; 1994. [Google Scholar]
- Williams LT, Young AF, Brown WJ. Weight gained in two years by a population of mid-aged women: how much is too much. Int J Obes. 2006;30:1229–1233. doi: 10.1038/sj.ijo.0803262. [DOI] [PubMed] [Google Scholar]
- Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes. 2005;29:1153–1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- Lemmens VEPP, Oenema A, Klepp KI, Henriksen HB, Brug J. A systematic review of the evidence regarding efficacy of obesity prevention interventions among adults. Obes Rev. 2008;9:446–455. doi: 10.1111/j.1467-789X.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- Lombard C, Deeks A, Jolley D, Teede HJ. Preventing weight gain: the baseline weight related behaviours and delivery of a randomized controlled intervention in community based women. BMC Public Health. 2009;9:2. doi: 10.1186/1471-2458-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard C, Deeks A, Jolley D, Ball K, Teede H. A low intensity, community based lifestyle programme to prevent weight gain in women with young children: cluster randomised controlled trial. BMJ. 2010;341:c3215. doi: 10.1136/bmj.c3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Klem ML, Kalarchian MA, Wing RR, Weissfeld L, Qin L, et al. Weight gain prevention among women. Obesity. 2007;15:1267–1277. doi: 10.1038/oby.2007.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH, Simkin-Silverman LR, Wing RR, et al. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26:212–220. doi: 10.1207/S15324796ABM2603_06. [DOI] [PubMed] [Google Scholar]
- Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev. 2010;11:306–321. doi: 10.1111/j.1467-789X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- Mundi MS, Karpyak MV, Koutsari C, Votruba SB, O'Brien PC, Jensen MD. Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J Clin Endocrinol Metab. 2010;95:67–73. doi: 10.1210/jc.2009-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Blew RM, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes. 2004;28:1124–1133. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12 Month outcomes and process evaluation of the SHED-IT RCT: an internet based weight loss program targeting men. Obesity. 2011;19:142–151. doi: 10.1038/oby.2010.119. [DOI] [PubMed] [Google Scholar]
- Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act. 2013;10:40. doi: 10.1186/1479-5868-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis JL, Williams LT, Collins CE, Morgan PJ. Effectiveness of interventions using Motivational Interviewing for dietary and physical activity modification in adults: a systematic review. JBI Database Syst Rev Implement Rep. 2013;11:1–27. doi: 10.11124/jbisrir-2012-171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.