Abstract
Aging alters the ability of the brain to respond to injury. One of the major differences between the adult and aged brain is that comparable injuries lead to greater blood brain barrier disruption in the aged brain. The goals of these studies were to quantify the effects of age on BBB permeability using high field strength MRI T1 mapping and to determine whether activation of matrix metalloproteases, their inhibitors, or expression of blood brain barrier structural proteins, occludin, zonnula occludins-1 (ZO-1) and claudin-5 were altered following injury to the aged C57/Bl6 mouse brain. T1 mapping studies revealed greater blood brain barrier permeability in the aged (21–15 months old) brain than in the adult (4–6 months old) following controlled cortical impact. The increased blood brain barrier permeability in the pericontusional region was confirmed with IgG immunohistochemistry. MMP-9 activity was increased following controlled cortical impact in the aged brain, and this was accompanied by increased MMP-9 gene expression. MMP-2 activity was higher in the uninjured aged brain than in the adult brain. Occludin and ZO-1 mRNA levels were unchanged following injury in either age group, but claudin-5 mRNA levels were lower in the aged than the adult brain following injury. These results demonstrate quantitative increases in blood brain barrier permeability in the aged brain following injury that are accompanied by increased MMP-9 activation and decreased blood brain barrier repair responses.
Keywords: Controlled cortical impact, Traumatic brain injury, Aging, T1 mapping, Blood brain barrier, MMP-9, MMP-2, Occludin, ZO-1, Claudin-5
Introduction
Traumatic Brain Injury (TBI) affects over 1.5 million Americans each year, claims over 50,000 American lives (Conroy and Kraus, 1988; Kraus et al., 1984; Thurman and Guerrero, 1999), and often causes permanent disability (Susman et al., 2002). TBI most often affects toddlers, adolescents, and the elderly (Thurman et al., 1999). In the elderly, TBI is most commonly caused by falls (Masson et al., 2001), and the rate of TBI-related hospitalizations is higher in people over 65 than in any other age group (Coronado et al., 2005). In addition to the increased incidence, there is strong evidence of worsened outcomes after TBI in the elderly (Hukkelhoven et al., 2003; Pennings et al., 1993), and mortality rates are high even when the patients are responsive and alert at admission (Susman et al., 2002). Elderly patients who survive TBI also have higher rates of disability than younger adults (Coronado et al., 2005; Mosenthal et al., 2004). Thus, in the elderly, injuries are worse (Luerssen et al., 1988; Pennings et al., 1993; Vollmer and Dacey, 1991) and recovery of skills needed for daily living is poorer than in younger adults (Mosenthal et al., 2004).
Worsened outcomes after TBI in the elderly suggest that aging alters the brain’s responses to injury. Our previous studies have demonstrated that several mechanistic aspects of the response to brain injury are altered in aged mice. Behavioral outcomes are worse, inflammatory responses to injury are increased and neuroprotective responses are decreased (Anderson et al., 2009; Onyszchuk et al., 2008; Sandhir et al., 2004; Sandhir et al., 2008; Sandhir and Berman, 2010). In particular, expression of several proinflammatory mediators is increased (Sandhir et al., 2004). Many of these mediators regulate the matrix metalloproteinases, MMP-2 and MMP-9, that degrade extracellular matrix and basement membranes and cleave blood brain barrier (BBB) proteins (Asahi et al., 2001; Bojarski et al., 2004; Giebel et al., 2005; Yang et al., 2007). MMP activity contributes to TBI outcomes. MMP-2 activity is involved in early opening of the blood brain barrier after ischemia (Rosenberg and Yang, 2007). MMP-9 increases vasogenic edema and disrupts the blood brain barrier following a delay (Mun-Bryce and Rosenberg, 1998; Shigemori et al., 2006; Tejima et al., 2006). MMP-9 ablation improves behavioral outcomes and reduces contusion volume following controlled cortical impact (Asahi et al., 2001; Wang et al., 2000). MMPs are regulated by transcription, activation of proMMPs, and by their specific inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). In the brain, microglia and astrocytes are major sources of many proinflammatory cytokines and other mediators that stimulate increased MMP production (del Zoppo et al., 2007; Kauppinen and Swanson, 2005). Our previous studies have shown that the responses of microglia and astrocytes to injury increase during aging (Sandhir et al., 2004; Sandhir et al., 2008), and that BBB disruption is increased following controlled cortical impact in the aged brain (Onyszchuk et al., 2008), suggesting that the increased inflammatory response may result in increased MMP activation that, in turn, increases BBB disruption.
Approximately 40% of all traumatic brain injuries in the elderly are contusions (Pennings et al., 1993). The established model of contusion injury is controlled cortical impact (CCI). This model reproduces many of the features of human brain injuries including motor deficits, memory loss, and neuron loss (Colicos et al., 1996). Responses following controlled cortical impact include glial activation, cytokine expression, and BBB disruption (Koshinaga et al., 2007; Raghavendra Rao et al., 2003) throughout the ipsilateral cortex and hippocampus (Smith et al., 1995).
Disruption of the BBB has conventionally been measured using radiolabeled substrates including 14C-labelled sucrose or histochemical methods. However, these invasive techniques are not suitable for within subject, longitudinal studies. Alternatively, MRI has been used in clinical and research settings to probe altered BBB permeability in diseases (e.g., multiple sclerosis and cancer) or injury (e.g., stroke) using gadolinium based MRI contrast agents. When the contrast agent transfers from blood to injured tissue due to BBB disruption, the altered relaxation characteristics are visible by T1-weighted MRI. However, quantification of the BBB alteration is difficult because T1-weighted MRI intensities are affected by other factors. A variant approach, quantitative T1 mapping with gadolinium contrast administration, provides comparable information of the BBB permeability alterations to other techniques such as the 14C sucrose technique (Sood et al., 2007). An important advantage of MRI approaches is that they allow longitudinal within subjects studies to determine the duration of injury responses and to determine effectiveness of treatment on BBB integrity. Moreover, contrast based MRI studies are equally effective in pre-clinical and clinical studies which is advantageous in translational research.
The major tight junction structural proteins of the BBB, occludin, zona occludens-1 (ZO-1) and claudin-5 are required for barrier function (Hirase et al., 1997; Yang and Rosenberg, 2011), and the microvascular basal lamina, comprised of collagen IV, laminin and fibronectin, also contributes to barrier function (Hawkins and Davis, 2005; Yurchenco and Schittny, 1990). Both the tight junction proteins and the basal lamina proteins are cleaved by MMPs (Yang et al., 2007). Disruption of the BBB allows serum proteins to enter the brain, many of which are either toxic to neurons or detrimental to neuron function (Herrera et al., 2007; Rao et al., 2007). Thus, increased extravasation of serum proteins following injury could contribute to worsened outcomes, but little is known about how age may alter BBB disruption following injury.
There were two goals of these studies. The first was to quantify the effects of age on BBB disruption using T1 mapping. The second was to determine whether TBI-related MMP activation and MMP, TIMP, or BBB structural proteins gene expression are altered with brain aging. All of these mechanisms have the potential to contribute to the worsened outcomes that follow brain injury in the elderly.
Materials and Methods
Animals
All animal care and procedures in the study were approved by the Institutional Animal Care and Use Committee, and were performed in compliance with the regulations and policies of the laboratory animal care and use at the University of Kansas Medical Center. Adult, 4–6 months old, and aged, 21–24 months old, C57/BL6 male mice were obtained from the National Institute on Aging colony maintained by Charles River Laboratories International, Inc., Wilmington, MA. Mice 21–24 months old are senescent, which is defined experimentally as the age at which 50% of the population dies of natural causes, which occurs at 24 months in C57/BL6 mice (Adams et al., 2001; Pugh et al., 1999). All mice were given free access to chow and water throughout the study.
Controlled cortical impact
The injury model used in this study damages sensorimotor cortex and produces unilateral behavioral deficits. This location avoids the middle meningeal artery, reducing the risk of major vascular damage. Mice were anesthetized with 2.5% isoflurane in medical grade compressed air. Core temperature was measured with a rectal thermometer and kept at 38°C using a heating pad. The skull was observed at 60X with an operating microscope, and the bone over the cortex removed using a dental drill, exposing a 3.5 mm region on one side, with AP coordinates centered at bregma, 2.5 mm lateral to the midline. A small amount of bone wax was applied to the craniotomy to prevent bone bleeding. Cortical areas under the impact tip included primary motor cortex (M1), and hindlimb and forelimb representation in primary somatosensory cortex (S1HL and S1FL). The impact device was assembled from a linear motor (P01–23×80, LinMot Inc., Zurich, Switzerland) and an electronic servo controller (E100-MT, LinMot Inc., Zurich, Switzerland), and has been used in previous studies (Bilgen, 2005; Narayana et al., 2004; Onyszchuk et al., 2007; Onyszchuk et al., 2008).
Magnetic Resonance Imaging
High field MRI was used to assess BBB disruption using gadolinium enhanced T1 parametric mapping. Two hours prior to MRI scans, Gd-DTPA (Magnevist, Berlex), an MRI contrast agent, was administered (0.2 mmol/kg) by an intraperitoneal injection to all animals on postinjury days 3, 7 and 14. For each MRI scan, the mouse was anesthetized by 1–1.5 % isoflurane in compressed air 4L/min supplemented oxygen, 1L/min, delivered through a nose cone. A warm water blanket with a rectal feedback thermosensor (Cole-Palmer, Vernon Hills, Illinois) was used to maintain the animal body temperature at 37±0.5°C. The respiratory rate of the spontaneously breathing animal was also monitored (SA Instruments, Stony Brook, NY).
All MRI measurements were performed on a 9.4 T Varian INOVA system (Varian Inc., CA) equipped with a 12 cm gradient insert (40 G/cm, 250 μs). A radiofrequency (RF) coil set with a 6 cm diameter Helmholtz volume transmit coil and a 7 mm diameter de-tunable surface receive coil was used for all MRI: quantitative T1 mapping, RF magnetic field (B1) mapping, and transverse relaxation time (T2)-weighted MRI. T1 mapping was performed using a modified Look-Locker multislice sequence (Kim et al., 2011) with multiple phase encodings per successive inversion pulse (repetition time/echo time (TR/TE) = 4/2 ms, matrix = 128×128, Field of view (FOV) = 2×2 cm2, slice thickness = 0.5 mm, flip angle = 20°, 22 inversion times, acquisition time = 8.5 min). B1 mapping was performed using a standard double excitation method (TR/TE = 200/3.7 ms, matrix = 128 × 128, slice thickness = 0.5 mm, averages = 4) to correct for flip angle variations on T1 mapping. T2-weighted MRI was performed using a multi-slice spin echo sequence (TR/TE = 1500/60 ms, matrix = 192×192, FOV = 2×2 cm2, slice thickness = 0.5 mm, averages = 2) to identify lesions following CCI and to place regions of interest. Because aged mice are often unstable under extended anesthesia exposure, especially early after CCI, we acquired MRI data in two separate, but shorter, sessions. After ~20 minutes of scanning to obtain localizer MRI, T2-weighted MRI, and the baseline T1 mapping, animals were allowed to recover before preparing them for the subsequent study. The second MRI scans (localizer MRI and T1 mapping) following contrast agent administration lasted 15 minutes. All scans for each time point were completed on the same day.
T1 calculation
The T1 and B1 values in each pixel were calculated using software developed at the Hoglund Brain Imaging Center, as described previously (Kim et al., 2011). In brief, the T1 values were calculated by a non-linear curve fit of inversion recovery MRI signals using the Levenberg-Marquardt algorithm on a pixel-by-pixel basis. The B1 maps were calculated from the ratio of the image intensity of the dual excitation MRI. The effect of flip angle variability on T1 values was corrected using the actual flip angle obtained from the B1 map variability in each pixel.
BBB permeability index calculation
First, the region of interest (ROI) was assigned in three brain regions including the ipsilateral, contralateral, and peri-injury cortices (Fig. 1A) using STIMULATE software (Strupp, 1996). Second, an average T1 value was obtained from each ROI (2×2 mm2) in T1 maps acquired pre-and post-Gd-DTPA administration. Third, the BBB permeability index defined as the difference of R1 (= 1/T1) values between post- and pre-Gd-DTPA administration was calculated for each ROI at all time points. The BBB permeability indices were compared between adult and aged groups using the pooled Student’s t-test. All data are presented as mean ± standard error (SE). A p-value of less than 0.05 was considered statistically significant.
Figure 1. Time course of T1 contrast enhancement of a mouse brain following i.p. Gd-DTPA administration.
A. T1 maps were acquired at before (pre), 1, 2, 3, and 4 hr after Gd-DTPA (0.2 mmol/kg) administration. The areas of interest where the time course of R1 (=1/T1) were obtained are marked as squares 1 (core) and 3 (peri-contusional area). The vertical gray scale bar indicates the range of T1 values. B. Time courses of R1 of the core (open circle) and peri-contusional area (filled square). Error bars indicate +/−SE.
Immunohistochemistry
Extravasation of serum proteins into the brain parenchyma was also evaluated by immunohistochemistry for IgG as described previously (Berman et al., 1999; Stephens et al., 2003). Mice were anesthetized and perfused transcardially with 4% buffered paraformaldehyde. Serial 40 μm frozen coronal sections through the contusion site were prepared and IgG staining was measured in the peri-CCI cortex region of adult and aged mice and at days 3 and 7 post CCI. Sections containing contused cortex were selected for each group, rinsed 3 times in 1X PBS, incubated for 30 minutes in 1% H2O2 in 1x PBS, rinsed 3 times in 1x PBS, placed in blocking solution (Pierce Super block + 2% donor goat serum) for 1 hour at room temperature followed by a 1:1000 dilution of biotinylated anti-mouse IgG (H+L) antibody (Vector Labs, Burlingame, CA, BA-9200) in 10% blocking solution for 1 hour at room temperature. The sections were washed 3 times for 5 minutes then incubated for 30 min with the standard Vectastain ABC kit (Vector Labs). Sections were rinsed briefly, then incubated for 5 minutes in the ImmPACT SG peroxidase substrate kit (Vector Labs) before stopping the reaction with water. Sections were analyzed by capturing images at 1x power on a Nikon 80i microscope and Image J was used to determine the intensity of staining of an equivalent 0.44 mm2 area of peri-CCI cortex in each section. The data were analyzed using Student’s t-tests.
Zymography
Mice were perfused with 0.9 % sterile saline, and samples of cortex extending 2.5 mm in each direction from the injury epicenter were dissected and snap frozen at −80°C. These samples were homogenized in 500 μl of lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5 mM CaCl, 0.05% Brij-35, 0.02% NaN3) and supernatant collected after centrifugation. The supernatant was incubated with 50 μl gelatin-Sepharose 4B (Pharmacia Biotech), centrifuged and the pellet rinsed with 500 μl of lysis buffer, and centrifuged again. The pellet was incubated for 30 min with 50 μl of elution buffer consisting of lysis buffer plus 10% dimethylsulfoxide (DMSO). Each 25 μl of eluted sample from was mixed with 25 μl of 2x nonreducing sample buffer (0.125 M Tris-HCl, 20% glycerol, 4% SDS, 0.003% bromophenol blue, pH 6.8), loaded onto 7.5% SDS-PAGE containing 0.1% gelatin, and electrophoresed at 125 V. Recombinant mouse MMP-2 and -9 (0.1–0.5 ng; EMD Bioscience) provided positive controls to identify the pro-MMP-2 (pro-) and active forms of MMP-2 and -9. The gel was incubated for 20 h at 37°C in buffer containing 21 mM Tris-HCl, 10 mM CaCl2, and 0.04% NaN3, pH 7.6, after two washes in 2.5% Triton X-100 for 20 min. After incubation, the gel was stained for 1 h with 0.1% Coomassie Blue and destained until clear bands were visible on a blue background. For quantitation, the contrast of these images was reversed using Photoshop and their intensity was measured using Image J. Data were analyzed using Student’s t-tests.
Quantitative real-time polymerase chain reaction (qRT-PCR)
At specific time points following CCI, mice were sacrificed by decapitation following anesthesia with Beuthanasia-D (Schering-Plough) at a dose of 60mg/kg IP, following recommendations of the Panel on Euthanasia, American Veterinary Medical Association. The brain was removed and placed in RNALater (Ambion, Foster City, CA) and kept overnight at 4C. The next day, a sample extending 2.5 mm in each direction from the injury epicenter though the depth of the cerebral cortex was dissected and total RNA was extracted by homogenation with Polytron 2000 using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Isolated RNA was treated with DNaseI (Ambion PCR Kit) to remove any contaminating genomic DNA according to manufacturer’s instruction, and RNA quantification was performed using a Nanodrop (Thermo Scientific, RNA quality was assessed by measuring the RNA Integrity Number (RIN) with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and samples that did not meet quality standards were discarded. Transcript levels of MMP-9, TIMP-1, MMP-2, TIMP-2, occludin, ZO-1 and claudin-5 (details provided in Table 1A) were measured using an array custom designed for the RT2 Profiler PCR array system (SA Biosciences, Qiagen, Frederick, MD). Gene expression levels were normalized against levels of GAPDH, the most stable housekeeping gene in mouse frontal neocortex (Boda et al., 2009), which was the region of the brain analyzed in the present study. RNA (500 ng) from each pool was used to prepare cDNA using the RT2 first-strand kit (SA Biosciences) and then added to the qPCR master mix, and thermal cycling was performed using a 7900HT FAST 96-well block (Applied Biosystems, Foster City, CA). Data were analyzed using software provided by the manufacturer.
Table 1A.
Primers Used in RT PCR Array (SABiosciences, Frederick, MD). Column 1, SAB Biosciences Catalog number, Column 2, Description= Gene name, Column 3, Gene= Gene abbreviation used in text, Column 4, UniGene #, Column 5, Refseq Accession 3, Column 6, Band (pb), Column 7, Reference position, primer start location.
| Catalog Number | Description | Gene Symbol | UniGene # | Refseq Accession # | Band Size (bp) | Reference Position |
|---|---|---|---|---|---|---|
| PPM02946E | Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | Mm.343110 | NM_008084 | 140 | 309 |
| PPM03693E | Tissue inhibitor of metalloproteinase 1 | Timp-1 | Mm.8245 | NM_011593 | 85 | 39 |
| PPM03614B | Tissue inhibitor of metalloproteinase 2 | Timp-2 | Mm.206505 | NM_011594 | 100 | 2892 |
| PPM03661B | Matrix metallopeptidase 9 | MMP-9 | Mm.4406 | NM_013599 | 119 | 2761 |
| PPM03642B | Matrix metallopeptidase 2 | MMP-2 | Mm.29564 | NM_008610 | 155 | 2900 |
| PPM05314A | Occludin | Ocln | Mm.4807 | NM_008756 | 181 | 718 |
| PPM26345A | Claudin -5 | Cldn-5 | Mm.22768 | NM_013805 | 179 | 180 |
| PPM25091A | Tight junction protein 1 | Tjp1 (ZO-1) | Mm.4342 | NM_009386 | 191 | 5279 |
Selected results from the custom array were replicated using quantitative real-time polymerase chain reaction as described previously (Stucky et al., 2011). Complementary DNA (cDNA) was synthesized by using 10 μg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA). A 2μg cDNA quantity of each sample was synthesized in reaction tubes using 2μg total RNA from each sample, random hexamers (10% by volume), and reverse transcriptase (1.25 U/μL) in a reaction volume 50μL. Reaction tubes were placed in a Hybaid PCR thermal cycler (Thermo Scientific) and run as follows: 10min at 25°C, 30min at 48°C, 5 min at 95°C. cDNA samples were stored for up to a month in a −80°C freezer before performing qRT-PCR. Gene-specific primers were selected based on literature review and in silico specificity screen with BLAST (Table 1) and qPCR was performed in 96 well MicroAmp Optical reaction plates (Applied Biosystems, Foster City, CA). A sample of 20–40ng cDNA was added to a 20 μL reaction volume of SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaqDNA polymerase, dNTPs mixture, dUTP, and optimal buffer components (Applied Biosystems), 1ul primer mixture (10 μg forward and 10 μg reverse primers in 150μl Sigma water) and Sigma water (SigmaAldrich, St. Louis, MO). Each plate was subjected to PCR amplification using an Applied Biosystems 7500 qPCR thermocycler. Thermocycling parameters were 40 cycles of 95°C for 15 seconds for denaturation and 60°C for 1 minute for annealing and extension. Thermocycling was followed by a melting curve stage of 60°C for 20 seconds with a 1% temperature ramp to 95°C. Parameters and reaction conditions were identical for all sets of primers. PCR reactions were conducted in duplicate with the exception of the internal control which was run in triplicate. The amplified transcripts were quantified with the comparative double delta Cq method using GAPDH and as internal control. These values are presented as relative expression in the results. To validate the use of GAPDH as a housekeeping gene, the ratio of GAPDH expression to total RNA(delta Cq/ng/μl) was calculated for each experimental group. This ratio was not significantly changed with age or injury. Cq values for non-template controls (NTC) were greater than 40 cycles. The amplicon qPCR products of each primer were visualized on agarose gels to check size, reaction specificity, and to confirm results. Applied Biosystems 7500 Software v 2.0.2 was used for outlier removal and qPCR analysis. Outliers were identified and removed if there were anomalous melting curves with multiple, wide, or blunted peaks (< 3:1 ratio) upon fluorescence derivative analysis. Cq of NTC below 40 for a given experiment was an additional criterion for disposition for the associated experimental values. Two independent sample Wilcoxon rank sum tests were used for statistical comparisons because some of the data were not normally distributed.
Results
T1 mapping studies
Determination of optimal wait time for post contrast agent MRI
The optimal wait time for detecting MRI signal changes following intraperitoneal Gd-DTPA administration was determined using two adult mice at 3 days after CCI. The T1 maps of Fig. 1 highlight the temporal changes of MRI signals in the core and surrounding areas of the CCI and as well as in non-brain tissues including skin and muscle that are in free exchange with the vascular space (Fig. 1A). Of note to this study is the CCI region where decreased signal indicates Gd-DTPA induced T1 shortening. The signal gradually recovered to pre-contrast levels over 3–4 hrs. The average time courses obtained from the core and pericontusional areas are shown in Fig. 1B. R1 (=1/T1) reached its peak at 1h post Gd-DTPA administration in the core and at 2h in the pericontusional area, indicating the maximum concentration of Gd-DTPA in the tissue. The optimal delay time at 2h was selected for use in all subsequent experiments.
Comparisons of BBB opening in adult and aged mice using MRI
Longitudinal measurements of the BBB permeability index performed on D3, D7, and D14 are shown in Fig. 2. The hyperintense regions on the T2-weighted images of an adult mouse (arrow heads) result from longer T2 values indicating edema at D3 and the post-contusional cyst at D14 (Fig. 2A, left column). T1 maps acquired before contrast agent administration showed longer T1 values at the areas of injury at both day 3 and 14 (Fig. 2A, center column). Substantial reduction of T1 values at the injury site was evident in the post-contrast T1 maps at D3 but to a lesser extent at day 14 (Fig. 2A, right column) compared to the pre-contrast T1 maps. A BBB permeability index, represented as the R1 changes following the Gd-DTPA administration, was calculated from the three regions of interest indicated on the day 14 T2-weighted images. Figure 2B compares the BBB permeability indices at the contusion core in adult and aged mice at days 3, 7, and 14 post CCI. Both groups showed initially increased BBB permeability followed by gradual recovery. Aged mice showed 65% (p = 0.05) and 120% (p = 0.02) higher BBB permeability index values than adult mice at day 3 and day 7, respectively. The peri-contusional area surrounding the core showed a similar pattern over time as the core (Fig. 2C). Aged mice showed 86% (p = 0.03) and 160% (p = 0.0004) higher BBB permeability index values than adult mice at days 3 and 7, respectively. In contrast to the ipsi-contusional area, no significant BBB opening was detected in the contra-contusional area following contrast administration (Fig. 2D).
Figure 2. T2 and T1 mapping of gadolinium enhancement in adult and aged mouse brain after controlled cortical impact.
A. T2-weighted image (left column) and corresponding T1 maps acquired before (middle column) and 2 hr after (right column) Gd-DTPA administration in an adult mouse. Images in the top row were acquired at day 3 and images at the bottom row were acquired at day 14. Three areas of interest marked by 1 (core), 2 (contra-contusional area) and 3 (peri-contusional area) are shown in the T2-weighted image at D14 (bottom left). The gray scale bars indicate the range of T1 values between 0 to 2.3 s. B. R1 changes at the core of contusional area (1) after Gd-DTPA administration in adult (black bars) and aged mice (white bars) acquired at days 3, 7 and 14. Error bars indicate +/− SE. C. R1 changes at the peri-contusional area (3) after Gd-DTPA administration in adult (black bars) and aged mice (white bars). D. R1 changes at the contra-contusional area after Gd-DTPA. Error bars indicate +/− SE. N=6 adult, 6 aged mice.
Immunohistochemistry
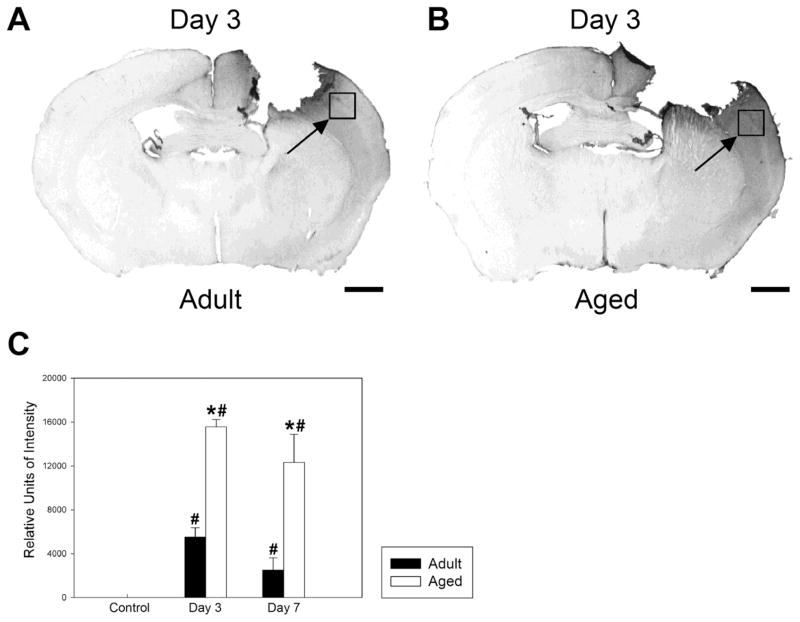
An example of IgG staining 3 days post CCI in adult is shown in Fig. 3A, and in aged mice in Fig. 3B. IgG staining confirmed the increased BBB leakage in the pericontusional region, the same region of the brain measured with T1 mapping. Fig. 3C shows results of measurements of staining intensity in control uninjured, 3 and 7 days post CCI from the region shown in the photomicrographs. There was a significant increase in staining in both groups compared with pre-injury levels. The aged mice had significantly more IgG staining at day 3 after injury (p < 0.001) and day 7 after injury (p < 0.05) than injured adult mice. Staining in the pericontusional area was 160% higher in the aged brain than in the adult brain at day 3 and 369% higher at day 7. In the adult mice, there was a significant decline in staining from day 3 to day 7 (p<0.05), while in aged mice, the decline was not significant. There were no significant differences in IgG staining in the region of cortex comparable to the pericontusional region between uninjured adult and aged mice.
Figure 3. Immunohistochemistry of extravasated IgG reveals greater blood brain barrier disruption following controlled cortical impact in aged than in adult mice.
A. IgG staining in an adult brain, 3 days post CCI. B. IgG staining in an aged mouse brain, 3 days post CCI. In both cases, the boxes indicated by arrows outline the regions in which image intensity was measured. C. Quantitation of staining intensity in the region shown in A and B. Both the adult and aged mice showed increased IgG staining in the outlined region at 3 and 7 days (p<0.05). In addition, the aged mice had significantly more IgG staining at day 3 after injury (p-value < 0.001) and day 7 after injury (p < 0.05) than adult injured mice. Differences from uninjured values are indicated by #, while differences between adult and aged values are indicated by *. There were no significant IgG staining differences in this region of the cortex between aged and adult non-injured control mice. Error bars indicate +/−SE. N= 5 adult control, 3 aged control, 6 adult injured day 3, 6 aged injured day 3, 4 adult injured day 7, 4 aged injured day 6. Scale bar = 1mm.
MMP activity
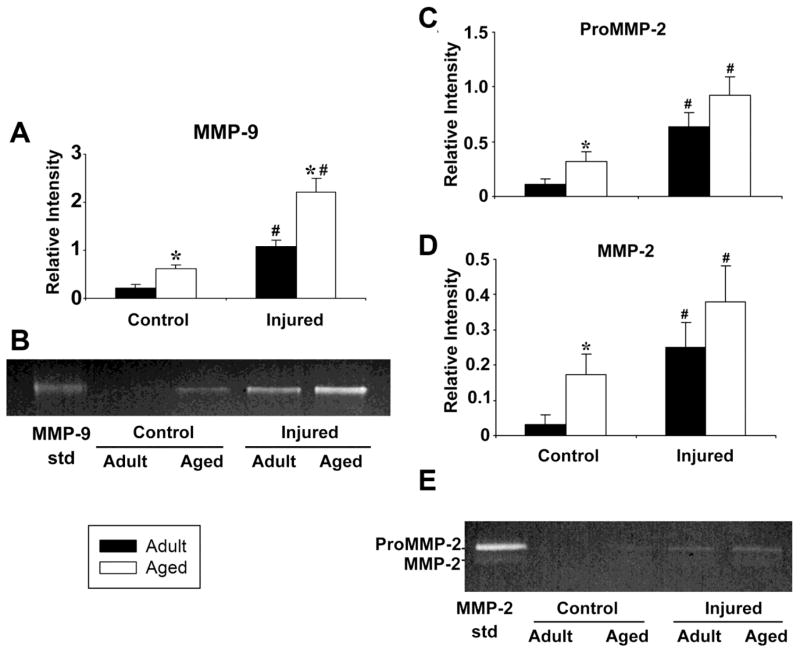
MMP activity was measured by zymography at day 3, when significant age-related BBB differences in BBB disruption were demonstrated in both the quantitative MRI and immunohistochemistry studies (Figs. 2 and 3). In addition, at this time point proinflammatory genes are maximally upregulated after CCI in both adult and aged brain (Sandhir et al., 2008). Fig. 4A and B show a significant age-related difference in the MMP-9 response. Both baseline MMP-9 activity and the MMP-9 injury response were significantly higher in the aged brain (t test, p<0.05). No pro-MMP-9 band was detected, as has been reported by others, possibly because pro-MMP-9 is processed rapidly after brain injury (Tejima et al., 2009). Fig. 4C–E show that both pro- (Fig. 4C) and active (Fig. 4D) MMP-2 were increased following injury in both adult and aged mice, but the magnitude of the injury response was not significantly different between age groups. However, MMP-2 activity was significantly higher in the uninjured aged brain than in the uninjured adult brain.
Figure 4. MMP activity after controlled cortical impact is higher in the aged than in the adult mouse brain.
Quantitation of zymographic assay of (A) MMP-9 activity in injured cortex 3 days after CCI in adult and aged mice. B. Zymogram of MMP-9 activity including standard (MMP-9 std). C. Quantitation of zymographic assay of pro-MMP-2 activity in injured cortex 3 days after CCI in adult and aged mice. D. Quantitation of zymographic assay of MMP-2 activity in injured cortex 3 days after CCI in adult and aged mice. E. Image of zymogram of proMMP-2 and active MMP-2 including standards (MMP-2 std). The increase in the MMP-9 baseline activity and the difference in injury response between adult and aged mice are both statistically significant, while the difference in baseline MMP-2 activity between adult and aged mice is significant. *= p<.05 adult vs. aged, #= p<0.05 injured vs. control. N=3 adult controls, 3 aged controls, 3 adult injured, 3 aged injured. Error bars indicate +/− SE
Gene expression
Expression of the genes most associated with disruption of BBB function, MMP-9 and MMP-2 and their corresponding inhibitors, TIMP-1 and TIMP-2, was examined in the pericontusional area in adult and aged mice 1–14 days postinjury using qRT-PCR. The aged mice showed a 4.8 fold increase in MMP-9 mRNA at day 1 (p<0.05) and a 4.2 fold increase at day 3 (p<0.01) compared with uninjured control values. In addition, at day 3, MMP-9 mRNA levels were 2.9 fold higher in the aged than in the adult mice (p<0.05). Age differences were not significant at other time points. In adult mice, increases in TIMP-1 mRNA relative to uninjured adult controls were 23.9 fold at day 1, 22.8 fold at day 2, 21.2 fold at day 3, 3.5 fold at day 7 and 2.9 fold at day 14. Aged mice showed comparable increases relative to uninjured aged control mice, 25.1 fold at day 1, 13.8 fold at day 2, 12.6 fold at day 3, 5.4 fold at day 7 and 2.9 fold at day 14 (Fig. 5B). All of these injury-related increases (i.e. differences from uninjured controls) were significant, but there were no age-related differences (p<0.05). Because MMP-9 showed an age-related difference but TIMP-1 did not, these data suggest that higher MMP-9 rather than lower TIMP-1 injury responses contribute to the increased MMP-9 activity after TBI in aged mice. MMP-2 mRNA levels (Fig. 5C) were 13.4 fold higher in the aged than in the adult mice at day 2 (p<0.05), and the difference between day 0 and day 2 was also significant in the aged mice (p<0.05). TIMP-2 mRNA levels (Fig. 5D) were 2.0 fold higher in the adult than in the aged mice (p<0.05), and significant injury responses were present in the adult brain at day 3 (1.7 fold upregulated compared to control, p<0.05), and in the aged brain at day 3 (3.1 fold upregulated compared to control, p<0.05) and day 7 (2.5 fold upregulated compared to control, p<0.01) and day 14 (2.1 fold upregulated compared to control, p<0.05). Those data suggest that the higher baseline MMP-2 activity in the aged brain is due to reduced TIMP-2 expression. The data are also consistent with the zymography data showing that changes in MMP-9 after injury are greater than changes in MMP-2 after injury in both the adult and aged brain.
Figure 5. Assessment of gene expression of MMP, TIMP, and blood brain barrier structural proteins following controlled cortical impact in the adult and aged brain.
Gene expression was measured in uninjured cortex (D0) and at 1,2,3,7 and 14 days post CCI. A. MMP-9, B. MMP-2, C. TIMP-1, D. TIMP-2, E. occludin, F. ZO-1, G. Claudin-5.. All measurements are relative to GAPDH levels in the same sample. D0=uninjured control mice. Y axes are relative expression. *= p<.05 comparing adult vs. aged, #= p<0.05 comparing injured vs. control using two independent sample Wilcoxon rank sum tests. N= Uninjured: 7 adult, 7 aged; D1: 6 adult, 6 aged; D2: 6 adult, 6 aged; D3: 7 adult, 7 aged; D7: 6 adult; 6 aged; D14: 6 adult, 6 aged. Error bars indicate +/− SE
Gene expression of the major structural BBB proteins occludin, ZO-1, and claudin-5 was also assessed at these time points. There were no significant injury or age-related differences in occludin mRNA levels (Fig. 5E) or in ZO-1 mRNA levels (Fig. 5F). In contrast, claudin-5 mRNA levels (Fig. 5G) were 1.6 fold higher in the adult uninjured brain than in the aged uninjured brain (p<0.01), indicating lower baseline claudin-5 expression in the aged brain. Claudin-5 mRNA levels were 1.9 fold higher in the adult (p<0.01) and 2.0 fold higher in the aged brain (p<0.01) compared to control at day 3, indicating that there is a repair response after injury in both age groups. However, claudin-5 mRNA levels were 1.8 fold higher at day 2 (p<0.01), 1.5 fold higher at day 3 (p<0.05) and 1.5 fold higher at day 14 (p<0.05) in the adult than in the aged injured brain, suggesting that the claudin-5 repair response is impaired in the aged brain.
Discussion
Disruption of the BBB in response to injury is exacerbated in the aged brain, likely contributing to worsened outcome after traumatic brain injury. In addition, loss of BBB function with age is thought to contribute to development and progression of neurodegenerative diseases (Furuno et al., 2002). However, the mechanism of BBB disruption following injury to the aged brain has received little attention. The present study demonstrated increased BBB disruption after controlled cortical impact in the aged brain using quantitative T1 MRI techniques that were verified by IgG immunohistochemistry. It also demonstrated that the increased BBB disruption in the aged brain is accompanied by increased MMP-9 gene expression and activity and reduced claudin-5 expression.
Magnetic Resonance Imaging
Most contrast-enhanced MRI has been performed following intravenous administration of gadolinium-containing contrast agents. However, since access to blood vessels in small animals such as mice is challenging, especially in longitudinal studies requiring repeated injections, we combined intraperitoneal Gd-DTPA administration with T1 mapping techniques. Intraperitoneal administration has been justified by a previous study showing that the route of injection did not alter the amount of the tracer Evans Blue seen in the mouse brain (Manaenko et al., 2011).
Quantitative T1 mapping provides reliable repeated estimation of Gd-DTPA extravasation as the measured T1 values are independent of experimental conditions including RF coil desigs, receiver gains, and flip angles, unlike conventional T1-weighted MR techniques. Although T1 mapping has not been used to measure BBB permeability in mouse models of traumatic brain injury, it has been employed in other rodent models of brain injury (Sood et al., 2008) and validated against 14C-labeled Gd-DTPA (Nagaraja et al., 2011). In the present study, mice were scanned using T1 mapping in multiple sessions before and after contrast agent administration with a short scan time of ~20 min per session to reduce anesthesia stress. In aged mice, lower anesthesia exposure in a session was especially important for animal survival in the acute phase of TBI (i.e., Day 3). The multiple session approach also allowed for longer duration between injection and scanning which provided time for the contrast agent to accumulate in the tissue.
T1 mapping studies revealed increased BBB permeability in the pericontusional region following TBI in the aged brain. At the acute time point, day 3, the BBB permeability was 86% higher in the aged brain. In addition, the repair process in the aged brain was slower than in the adult brain, as the difference increased to 160% at day 7. If this delay in BBB repair is a general feature of the aged brain, it could contribute to disease progression in neurodegenerative diseases such as Alzheimer’s disease, where blood brain barrier disruption is common (Farrall and Wardlaw, 2009).
Immunohistochemistry
Immunohistochemistry of extravasated IgG confirmed the results of the T1 mapping studies. The amount of staining in the pericontusional region was significantly higher in the aged mice. In addition, the proportional differences between adult and aged brains were similar when the two methods were used to measure BBB disruption in the same pericontusional region. T1 mapping data indicated 86% greater permeability at day 3 and 169% at day 7 in aged than adult mice, while the comparable differences revealed by IgG staining were 160% higher in aged brain at day 3 and 369% higher in aged brain at day 7. Although the measured magnitude of the differences was greater in the T1 mapping approach than in the immunohistochemical studies, perhaps a result of the molecular size of the tracer, both methods agree that the difference between the adult and aged brains is greater at day 7 than at day 3. Those data demonstrate that BBB disruption is both greater in magnitude and more prolonged in the aged than in the adult brain.
MMP activity
The data demonstrate significantly higher baseline MMP activity in aged animals as well as significant age-related differences between MMP-2 and MMP-9 in response to injury. Baseline pro-MMP-2, MMP-2 and MMP-9 activity were all higher in the aged brain than in the adult brain. All showed significant injury responses, but only MMP-9 activity showed a significantly higher injury response in the aged than in the adult brain. Other studies have shown that MMP-9 activity is increased after cortical contusion, and that the MMP inhibitor GM6001 reduced BBB disruption (Shigemori et al., 2006). In addition, previous studies have shown that MMP-2 and MMP-9 are both upregulated after brain injury (Sifringer et al., 2007; Wang et al., 2000). Interestingly, MMP-2 and MMP-9 are differentially regulated in young and adult brains. MMP-2 activity is elevated after TBI in infant rats, while MMP-9 is elevated after TBI in adult mice (Sifringer et al., 2007; Wang et al., 2000). The present study shows that this trend continues in the aging brain.
The effects of focal brain injuries such as contusions often expand into normal brain tissue due to BBB failure, leading to poor outcomes. In patients with expanding contusions, both MMP-2 and MMP-9 levels are high (Vajtr et al., 2008). Therefore, if MMP-9 activity is high following contusion in the aged human brain, the expansion of the lesion into normal tissue is expected to increase more than it would in a younger brain, thereby contributing to the worsened outcomes. It is likely that increased MMP activity in the aged brain contributes to blood brain barrier dysfunction (Farrall and Wardlaw, 2009), but the present study has not addressed the question of whether changes in the expression/function of MMP-9 are the cause or the effect of more severe disruption of BBB permeability in aged mice. Treatment of aged mice with pharmacological MMP inhibitors after TBI would address that question.
Gene expression of MMPs, TIMPs and BBB structural proteins
RNA studies examined expression of MMPs and their tissue inhibitors to determine how the age and injury-related differences in MMP-9 and MMP-2 activity are achieved. The results revealed upregulation of MMP-9 mRNA in aged mice 1 day after CCI, and that MMP-9 mRNA was significantly higher at 3 days post CCI in the aged mice than in the adult mice. Both adult and aged mice demonstrated increases in TIMP-1 mRNA in response to injury at all time points, but there were no age-related differences. Consequently, the increase in MMP-9 mRNA should result in increased MMP-9 protein levels and, without a concurrent increase in TIMP-1 mRNA, increased MMP-9 activity at day 3 in the aged mice, as was observed by zymography. These data suggest that increased gene expression of MMP-9, rather than decreased gene expression of TIMP-1, is a major contributor to the increased MMP-9 activity after TBI in aged mice. In contrast, although MMP-2 mRNA increased after injury and was higher in the aged than in the adult mice at day 2, TIMP-2 mRNA was lower in the aged mice at baseline. That result is consistent with the higher MMP-2 activity in aged mice seen in the zymography results, and it suggests that higher MMP-2 activity in the aged brain is due to lower TIMP-2 gene expression. Taken together, these data demonstrate that MMP-9 and MMP-2 are differentially regulated in normal aging and following traumatic brain injury.
MMP-2 and MMP-9 have different potential effects on the BBB due to differences in their cellular location. While MMP-2 is tethered to the cell surface, MMP-9 is released extracellularly where it has access to vessels and to the matrix surrounding neurons (Candelario-Jalil et al., 2008). Thus, an increased MMP-9 response is likely to have widespread effects on both BBB integrity and neuronal function. The source of the increased MMP-9 activity may be activated microglia (Cross and Woodroofe, 1999; Rosell et al., 2006) as well as invading neutrophils (Rosell et al., 2006; Rosell et al., 2008). Microglial activation after controlled cortical impact is known to be higher in the aged than in the adult brain (Sandhir et al., 2008). In addition to the other detrimental effects of activated microglia, increased MMP-9 may contribute to the increased BBB disruption, further increasing BBB disruption by allowing increased neutrophil infiltration.
Occludin, ZO-1 and claudin-5 are sensitive indicators of the functional state of the BBB (Dobrogowska and Vorbrodt, 2004). Occludin is a transmembrane protein that seals the tight junctions (Tavelin et al., 2003). Claudin-5 is a structural component of the transmembrane tight junction protein strands (Krause et al., 2008). ZO-1 is a cytoplasmic protein that links claudin-5 to the actin cytoskeletal network (Stevenson et al., 1986; Van Itallie et al., 2009). All three proteins are degraded by MMP-2 and MMP-9 (Yang et al., 2007; Yang and Rosenberg, 2011). In the present study, there were no significant changes in occludin or ZO-1, either in aged brains or in response to injury. In contrast, claudin-5 mRNA levels (Fig. 6G) were lower in the aged brain than in the adult brain, which may reflect the reduced blood brain barrier function of the aged brain (Furuno et al., 2002). In addition, upregulation of claudin-5 in response to injury was significantly lower in the aged brain than in the adult brain, suggesting that the BBB repair response is also diminished in the aged brain. Claudin-5 regulates the size of molecules that can cross the BBB, precluding peptides and smaller proteins. In claudin-5 knockout mice, molecules smaller than 800 kDa can cross the BBB (Nitta et al., 2003). That size group includes Gd-DPTA (0.94 kDa), IgG (150kDa), and other proteins such as thrombin (37kDa), which can be toxic to neurons (Rohatgi et al., 2004; Suo et al., 2004). The reduced injury response in the aged brain may result from increased TNF-α expression following injury in the aged brain (Sandhir et al., 2004), as TNF-α is known to reduce claudin-5 expression (Burek and Forster, 2009). Tight junction disruption is thought to contribute to disease progression in Alzheimer’s disease (Biron et al., 2011). The present study suggests that slower repair of disrupted tight junction proteins may be a generalized feature of the aged brain, contributing to worsened outcomes after TBI and disease progression in neurodegenerative disorders of aging.
Conclusions
In this study we used quantitative T1 mapping to demonstrate that BBB permeability following TBI is greater in aged than in adult mice. Increased BBB permeability was accompanied by increased MMP-9 activation and gene expression, and decreased claudin-5 gene expression. These age-related changes in the brain’s response to injury may contribute to worsened outcomes following brain injury in the elderly.
Table 1B.
Primers used in real time RT-PCR. Column 1 mRNA, gene abbreviation. Columns 2 and 3 Primer Forward and Reverse sequences. Column 4, Primer position in mRNA, Column 5, Gene Accession number.
| mRNA | Priimer | Sequence | Position(nt) | Accession Number |
|---|---|---|---|---|
| MMP-9 | Forward | GCCCTGGAACTCACACGACA | 2031–2050 | NM_013599.2 |
| Reverse | GGAAACTCACACGCCAGAAG | 2094–2113 | ||
| MMP-2 | Forward | CGGTTTATTTGGCGGACAGT | 1774–1792 | NM_008610.2 |
| Reverse | GCCTCATACACAGCGTCAATCTT | 1858–1880 | ||
| TIMP-1 | Forward | GGCATCCTCTTGTTGCTATCAC | 197–218 | NM_001044384.1 |
| Reverse | TATGACCAGGTCCGAGTTGC | 276–295 | ||
| TIMP-2 | Forward | CACGCTTAGCATCACCCA | 618–635 | M82858 |
| Reverse | TGACCCAGTCCATCCAGAG | 733–751 | ||
| OCLN | Forward | GCTGTGATGTGTGTTGAGCT | 2054–2073 | NM_008756.2 |
| Reverse | GACGGTCTACCTGGAGGAAC | 2105–2124 | ||
| CLDN5 | Forward | GTGGAACGCTCAGATTTCAT | 1054–1073 | NM_013805.4 |
| Reverse | TGGACATTAAGGCAGCATCT | 1131–1150 | ||
| ZO-1 | Forward | AGGACACCAAAGCATGTGAG | 6047–6066 | NM_001163574.1 |
| Reverse | GGCATTCCTGCTGGTTACA | 6115–6133 | ||
| GAPDH | Forward | ATGACATCAAGAAGGTGGTG | 811–830 | NM_008084.2 |
| Reverse | CATACCAGGAAATGAGCTTG | 968–987 |
MMP-9(M)- F: (matrix metallopeptidase)
MMP-2 (mus) R: matrix metallopeptidase 2
TIMP-1 Tissue inhibitor of metalloproteinase 1 (Timp1), transcript variant 2, Mrna
OCLN occludin
CLDN5 claudin-5
ZO-1-Tjp1 tight junction protein 1
GAPDH-glyceraldehyde-3-phosphate dehydrogenase
Highlights.
Blood brain barrier disruption after traumic brain injury is greater in aged than adult mice.
Activation of MMP-9 after traumatic brain injury is greater in aged than adult mice.
Expression of the blood brain barrier structural protein claudin-5 is impaired after traumatic brain injury in aged mice.
Increased blood brain barrier disruption and decreased repair may contribute to poor outcomes after injury to the aged brain.
Acknowledgments
Supported by NIH grant R01AG31140, P30 HD02528, P20 RR016475, and the Hoglund Family Foundation. We would like to thank Dr.Y.Y. He for assistance with surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DD, Lucas WO, Williams BG, Berkeley BB, Turner KW, Schofield JC. A mouse genetic locus with death clock and life clock features. Mech Ageing Dev. 2001;122:173–89. doi: 10.1016/s0047-6374(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009;26:1557–66. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman NE, Marcario JK, Yong C, Raghavan R, Raymond LA, Joag SV, Narayan O, Cheney PD. Microglial activation and neurological symptoms in the SIV model of NeuroAIDS: association of MHC-II and MMP-9 expression with behavioral deficits and evoked potential changes. Neurobiol Dis. 1999;6:486–98. doi: 10.1006/nbdi.1999.0261. [DOI] [PubMed] [Google Scholar]
- Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–26. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS One. 2011;6:e23789. doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda E, Pini A, Hoxha E, Parolisi R, Tempia F. Selection of reference genes for quantitative real-time RT-PCR studies in mouse brain. J Mol Neurosci. 2009;37:238–53. doi: 10.1007/s12031-008-9128-9. [DOI] [PubMed] [Google Scholar]
- Bojarski C, Weiske J, Schoneberg T, Schroder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- Burek M, Forster CY. Cloning and characterization of the murine claudin-5 promoter. Mol Cell Endocrinol. 2009;298:19–24. doi: 10.1016/j.mce.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicos MA, Dixon CE, Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–9. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- Conroy C, Kraus JF. Survival after brain injury. Cause of death, length of survival, and prognostic variables in a cohort of brain-injured people. Neuroepidemiology. 1988;7:13–22. doi: 10.1159/000110131. [DOI] [PubMed] [Google Scholar]
- Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20:215–28. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Cross AK, Woodroofe MN. Chemokine modulation of matrix metalloproteinase and TIMP production in adult rat brain microglia and a human microglial cell line in vitro. Glia. 1999;28:183–9. [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–51. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- Dobrogowska DH, Vorbrodt AW. Immunogold localization of tight junctional proteins in normal and osmotically-affected rat blood-brain barrier. J Mol Histol. 2004;35:529–39. doi: 10.1007/10.1007/s10735-004-1318-3. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–52. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Furuno T, Landi MT, Ceroni M, Caporaso N, Bernucci I, Nappi G, Martignoni E, Schaeffeler E, Eichelbaum M, Schwab M, Zanger UM. Expression polymorphism of the blood-brain barrier component P-glycoprotein (MDR1) in relation to Parkinson’s disease. Pharmacogenetics. 2002;12:529–34. doi: 10.1097/00008571-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Herrera AJ, de Pablos RM, Carreno-Muller E, Villaran RF, Venero JL, Tomas-Camardiel M, Cano J, Machado A. The intrastriatal injection of thrombin in rat induced a retrograde apoptotic degeneration of nigral dopaminergic neurons through synaptic elimination. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.05170.x. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–13. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, Murray GD, Maas AI. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–73. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–96. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- Kim J, Choi IY, Michaelis ML, Lee P. Quantitative in vivo measurement of early axonal transport deficits in a triple transgenic mouse model of Alzheimer’s disease using manganese-enhanced MRI. Neuroimage. 2011;56:1286–92. doi: 10.1016/j.neuroimage.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshinaga M, Suma T, Fukushima M, Tsuboi I, Aizawa S, Katayama Y. Rapid microglial activation induced by traumatic brain injury is independent of blood brain barrier disruption. Histol Histopathol. 2007;22:129–35. doi: 10.14670/HH-22.129. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Black MA, Hessol N, Ley P, Rokaw W, Sullivan C, Bowers S, Knowlton S, Marshall L. The incidence of acute brain injury and serious impairment in a defined population. Am J Epidemiol. 1984;119:186–201. doi: 10.1093/oxfordjournals.aje.a113737. [DOI] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–16. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195:206–10. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson F, Thicoipe M, Aye P, Mokni T, Senjean P, Schmitt V, Dessalles PH, Cazaugade M, Labadens P. Epidemiology of severe brain injuries: a prospective population-based study. J Trauma. 2001;51:481–9. doi: 10.1097/00005373-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Mosenthal AC, Livingston DH, Lavery RF, Knudson MM, Lee S, Morabito D, Manley GT, Nathens A, Jurkovich G, Hoyt DB, Coimbra R. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma. 2004;56:1042–8. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–11. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN, Ewing JR, Karki K, Jacobs PE, Divine GW, Fenstermacher JD, Patlak CS, Knight RA. MRI and quantitative autoradiographic studies following bolus injections of unlabeled and (14)C-labeled gadolinium-diethylenetriaminepentaacetic acid in a rat model of stroke yield similar distribution volumes and blood-to-brain influx rate constants. NMR Biomed. 2011;24:547–58. doi: 10.1002/nbm.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Grill RJ, Chacko T, Vang R. Endogenous recovery of injured spinal cord: longitudinal in vivo magnetic resonance imaging. J Neurosci Res. 2004;78:749–59. doi: 10.1002/jnr.20275. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–96. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental Effects of Aging on Outcome from Traumatic Brain Injury: A Behavioral, Magnetic Resonance Imaging, and Histological Study in Mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Pennings JL, Bachulis BL, Simons CT, Slazinski T. Survival after severe brain injury in the aged. Arch Surg. 1993;128:787–93. doi: 10.1001/archsurg.1993.01420190083011. discussion 793–4. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 1999;59:1642–8. [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dhodda VK, Song G, Bowen KK, Dempsey RJ. Traumatic brain injury-induced acute gene expression changes in rat cerebral cortex identified by GeneChip analysis. J Neurosci Res. 2003;71:208–19. doi: 10.1002/jnr.10486. [DOI] [PubMed] [Google Scholar]
- Rao HV, Thirumangalakudi L, Desmond P, Grammas P. Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J Neurochem. 2007;101:498–505. doi: 10.1111/j.1471-4159.2006.04389.x. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Sedehizade F, Reymann KG, Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist. 2004;10:501–12. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–6. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–80. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Berman NE. Age-dependent response of CCAAT/enhancer binding proteins following traumatic brain injury in mice. Neurochem Int. 2010;56:188–93. doi: 10.1016/j.neuint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006;96:130–3. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, Ikonomidou C. The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiol Dis. 2007;25:526–35. doi: 10.1016/j.nbd.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–78. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Sood R, Taheri S, Estrada EY, Rosenberg GA. Quantitative evaluation of the effect of propylene glycol on BBB permeability. J Magn Reson Imaging. 2007;25:39–47. doi: 10.1002/jmri.20802. [DOI] [PubMed] [Google Scholar]
- Sood RR, Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Early beneficial effect of matrix metalloproteinase inhibition on blood-brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. J Cereb Blood Flow Metab. 2008;28:431–8. doi: 10.1038/sj.jcbfm.9600534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens EB, Singh DK, Kohler ME, Jackson M, Pacyniak E, Berman NE. The primary phase of infection by pathogenic simian-human immunodeficiency virus results in disruption of the blood-brain barrier. AIDS Res Hum Retroviruses. 2003;19:837–46. doi: 10.1089/088922203322493003. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–66. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp JP. Stimulate: A GUI based fMRI Analysis Software Package. NeuroImage. 1996;3:S607. [Google Scholar]
- Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache. 2011;51:674–92. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Z, Citron BA, Festoff BW. Thrombin: a potential proinflammatory mediator in neurotrauma and neurodegenerative disorders. Curr Drug Targets Inflamm Allergy. 2004;3:105–14. doi: 10.2174/1568010043483953. [DOI] [PubMed] [Google Scholar]
- Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53:219–23. doi: 10.1097/00005373-200208000-00004. discussion 223–4. [DOI] [PubMed] [Google Scholar]
- Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol. 2003;64:1530–40. doi: 10.1124/mol.64.6.1530. [DOI] [PubMed] [Google Scholar]
- Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, Montaner J, Wang X, Lo EH. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, Rosell A, Wang X, Lo EH. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26:1935–41. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. Jama. 1999;282:954–7. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Vajtr D, Benada O, Kukacka J, Prusa R, Houstava L, Toupalik P, Kizek R. Correlation of ultrastructural changes of endothelial cells and astrocytes occuring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2008 doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–40. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer DG, Dacey RG., Jr The management of mild and moderate head injuries. Neurosurg Clin N Am. 1991;2:437–55. [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–42. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. MMP-Mediated Disruption of Claudin-5 in the Blood-Brain Barrier of Rat Brain After Cerebral Ischemia. Methods Mol Biol. 2011;762:333–45. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Schittny JC. Molecular architecture of basement membranes. Faseb J. 1990;4:1577–90. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]