Abstract
Purpose
Of men with very low risk prostate cancer at biopsy recent evidence shows that black American men are at greater risk for adverse oncologic outcomes after radical prostatectomy. We studied radical prostatectomy specimens from black and white men at very low risk to determine whether there are systematic pathological differences.
Materials and Methods
Radical prostatectomy specimens were evaluated in men with National Comprehensive Cancer Network® (NCCN) very low risk prostate cancer. At diagnosis all men underwent extended biopsy sampling (10 or more cores) and were treated in the modern Gleason grade era. We analyzed tumor volume, grade and location in 87 black and 89 white men. For each specimen the dominant nodule was defined as the largest tumor with the highest grade.
Results
Compared to white men, black men were more likely to have significant prostate cancer (61% vs 29%), Gleason 7 or greater (37% vs 11%, each p <0.001) and a volume of greater than 0.5 cm3 (45% vs 21%, p = 0.001). Dominant nodules in black men were larger (median 0.28 vs 0.13 cm3, p = 0.002) and more often anterior (51% vs 29%, p = 0.003). In men who underwent pathological upgrading the dominant nodule was also more frequently anterior in black than in white men (59% vs 0%, p = 0.001).
Conclusions
Black men with very low risk prostate cancer at diagnosis have a significantly higher prevalence of anterior cancer foci that are of higher grade and larger volume. Enhanced imaging or anterior zone sampling may detect these significant anterior tumors, improving the outcome in black men considering active surveillance.
Keywords: prostate, prostatic neoplasms, African Americans, risk, neoplasm grading
Active surveillance is a treatment option recommended by the NCCN for men with very low risk PCa.1 As described by Epstein et al, very low risk criteria select men predicted to have insignificant tumors based on small pathological volume and low GS.2 These criteria, which were adopted by the NCCN, include clinical stage T1c, GS 6 or less, PSA less than 10 ng/ml, PSAD 0.15 ng/ml/gm or less, 2 or fewer positive cores and 50% or less cancer involvement per core.2, 3
In men who meet the criteria of Epstein et al2 and are enrolled in our AS program outcomes are generally excellent with 0.08% PCa specific mortality and a 12.6% rate of upgrading on followup biopsies (median followup 2.7 years).4, 5 However, evidence indicates that AS is not equally safe for all race groups.6 In particular, AA men with favorable risk cancers may have surprisingly adverse outcomes. In separate AS cohorts of 24 to 32 men at the University of Miami7 and Duke Prostate Center8 those who were AA were at higher risk for progression at biopsy and treatment, respectively. In the AS program at our institution AA men are at especially higher risk for progression by grade (unpublished data). Furthermore, analysis of a large, NCCN very low risk cohort in which RP was done showed that AA men have markedly adverse pathological outcomes compared to white men.9
In light of emerging evidence revealing significant racial disparities in PCa outcomes even among men with very low risk disease we determined whether any underlying pathological differences could explain these findings. Therefore, we identified men with NCCN very low risk PCa who were candidates for AS but underwent RP. We performed detailed pathological examination of surgical specimens, noting the volume, location, stage and grade of all tumor nodules.
MATERIALS AND METHODS
We analyzed the institutional review board approved RP database at our institution, which includes 19,142 men from the PSA era. After excluding 833 men who received neoadjuvant hormonal therapy we identified 1,801 who met all NCCN very low risk criteria, including PSA less than 10 ng/ml, PSAD 0.15 ng/ml/gm or less, 2 or fewer positive cores, 50% or less cancer involvement per core and GS less than 6.1 Also, to reflect standard clinical practice and minimize sampling heterogeneity among study subjects all men had to have been diagnosed by extended core biopsy, defined as 10 or more cores. Only men who underwent treatment since 2004 were included in analysis since that is when the modern Gleason grading system was adopted at our institution. The International Society of Urological Pathology consensus scheme assigns poorly formed glands with poorly defined lumina, glomeruloid patterns and cribriform structures to pattern 4 instead of pattern 3.10
Of NCCN men at very low risk who were diagnosed by extended biopsy sampling and underwent treatment in the modern Gleason era (2004 to 2012) 221 were white and 100 were AA. To establish symmetrical comparison groups for detailed prospective pathological examination we randomly selected 100 white men. In these 2 select cohorts of 100 white and 100 AA men, respectively, 24 RP specimens were not available for review. The final analysis cohort consisted of 89 white and 87 AA men with corresponding prostatectomy specimens.
Each surgical specimen was surface coated with india ink and fixed in 10% buffered formalin for 18 to 24 hours. The gland was step sectioned at 3 mm intervals along the coronal plane and the resulting sections were halved or quartered to fit the tissue cassette. Tissue sections were embedded in paraffin blocks, from which 4.0 µm sections were prepared and stained with hematoxylin and eosin for routine histological analysis.
Two genitourinary pathologists (ONK and JIE) examined each prostatectomy specimen and prospectively recorded the number of tumor nodules, Gleason patterns in each nodule, and stage, volume and location/extent of each nodule in the prostate. All tumor nodules were mapped on slides to calculate tumor volume and distinguish separate lesions. Tumor volume was calculated as previously described.11 Tumor nodules were considered spatially separate if they were 3 mm or more apart in a plane of a section or 4 mm or more on adjacent sections.12
We used adverse pathological findings, as defined by specific findings at prostatectomy, including 1) pT2 and GS 4 + 3 or greater, 2) pT3a and GS 3 + 3 with positive surgical margins, 3) pT3a and GS 3 + 4 or greater, or 4) pT3b or greater, to designate men predicted to have a 25% or greater 10-year probability of biochemical recurrence.13 Unfavorable pathological findings at RP were also quantified by the Cancer of the Prostate Risk Assessment (CAPRA) Post-Surgical (CAPRA-S) score, a validated risk assessment with a range of 0 to 12 points that is derived from margin status, extraprostatic disease site, Gleason pattern and PSA. CAPRA-S 3 or greater is associated with a 27% or greater 5-year probability of biochemical recurrence.14 Significant tumors were defined as those with a pathological Gleason sum of 7 or greater and those with a dominant nodule volume of 0.5 cm3 or greater.
Means were compared by the Student t-test. Medians of nonnormally distributed variables were compared by the Wilcoxon rank sum test. Proportions were compared by the chi-square test. Statistical significance was predefined at 2-tailed 0.05. Analysis was done using Stata®, version 11.0.
RESULTS
In all men NCCN very low risk disease was diagnosed by extended biopsy sampling in the modern Gleason grading era. AA and white men were similar in age, PSA, PSAD, BMI, number of cores, percent core involvement and CAPRA score at diagnosis (supplementary table 1, http://jurology.com/). There was no difference in the mean or median interval between biopsy and RP. Notably, AA men had higher Charlson comorbidity scores (supplementary table 1, http://jurology.com/). Preoperative characteristics were also similar in men whose RP specimens were not available for review, although excluded AA men were younger (53.1 vs 59.5 years, p = 0.015, table 1). At prostatectomy AA men had significantly higher rates of nonorgan confined disease (14.9% vs 3.4%, p = 0.008), positive surgical margins (19.8% vs 5.6%, p = 0.002) and upgrading to GS 7 or greater (36.8% vs 11.2%, p <0.001, supplementary table 1 and supplementary figure, http://jurology.com/). Accordingly, AA men had higher rates of adverse pathological findings (18.4% vs 5.6%, p = 0.009) and a CAPRA-S score of 3 or greater (20.7% vs 2.2%, p <0.001, supplementary table 1 and supplementary figure, http://jurology.com/).
Table 1.
Characteristics of men with missing or unevaluable surgical specimens and pathological characteristics of men at NCCN very low risk with extended biopsy sampling treated in modern Gleason grading era of 2004 or later
| White | AA | p Value | |||
|---|---|---|---|---|---|
| Missing or unevaluable surgical specimens | |||||
| No. pts | 11 | 13 | |||
| Mean age | 59.5 | 53.1 | 0.015 | ||
| Median ng/ml PSA (IQR) | 3.5 | (2.8, 5.2) | 4.2 | (2.6, 5.4) | 0.734 (Wilcoxon-Mann-Whitney test) |
| Median gm prostate size (IQR) | 48.9 | (42.0, 62.0) | 43.5 | (35.9, 51.3) | 0.885 (Wilcoxon-Mann-Whitney test) |
| Median ng/ml/gm PSAD (IQR) | 0.08 | (0.06, 0.11) | 0.08 | (0.07, 0.11) | 0.707 (Wilcoxon-Mann-Whitney test) |
| No. pos cores (%): | 0.916 | ||||
| 1 | 7 | (63.6) | 8 | (61.5) | |
| 2 | 4 | (36.4) | 5 | (38.5) | |
| Median % Ca/core (IQR) | 10.0 | (5.0, 25.0) | 10.0 | (5.0, 20.0) | 0.589 (Wilcoxon-Mann-Whitney test) |
| No. CAPRA (%): | 0.902 | ||||
| 0–1 | 10 | (90.9) | 12 | (92.3) | |
| 2–3 | 1 | (9.1) | 1 | (7.7) | |
| No. family history (%) | 4 | (36.4) | 7 | (53.8) | 0.392 |
| Median kg/m2 BMI (IQR) | 27.2 | (23.4, 31.4) | 28.1 | (27.3, 29.7) | 0.621 (Wilcoxon-Mann-Whitney test) |
| No. Charlson index (%):* | 0.168 | ||||
| 0 | 2 | (18.2) | 5 | (38.5) | |
| 1 | 1 | (9.1) | 0 | ||
| NCCN very low risk | |||||
| No. pts | 88 | 87 | |||
| Median cm3 tumor vol (IQR) | 0.185 | (0.050, 0.407) | 0.423 | (0.175, 0.837) | <0.001 (Wilcoxon-Mann-Whitney test) |
| No. multiple tumor nodules (%) | 54 | (60.7) | 73 | (83.9) | 0.001 |
| No. reclassified as significant (%): | 26 | (29.5) | 53 | (60.9) | <0.001 |
| Grade 7 or greater | 10 | (11.2) | 32 | (36.8) | <0.001 |
| Dominant nodule vol 0.5 cm3 or greater | 19 | (21.3) | 39 | (44.8) | 0.001 |
No patient had a Charlson index of 2 or 3.
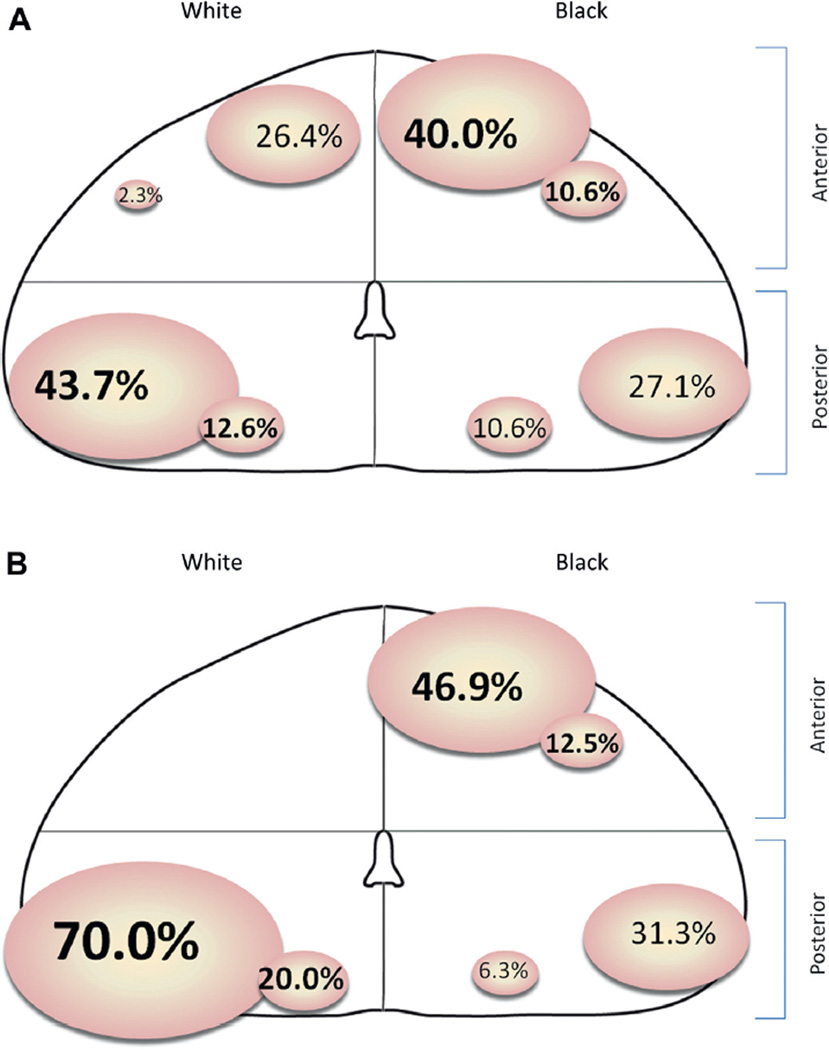
Total tumor volume in AA men was higher than in white men (median 0.423 vs 0.185 cm3, p <0.001, table 1). AA men were also more likely to have multiple tumor nodules (83.9% vs 60.7%, p = 0.001). We subsequently analyzed the dominant nodule or index lesion of each patient, defined as the largest tumor nodule with the highest Gleason grade. The dominant nodule was located in the anterior aspect of the prostate gland in 50.6% of AA men but in 28.7% of white men (p = 0.003). White men were more likely to harbor posteriorly located dominant nodules (supplementary table 2, http://jurology.com/ and part A of figure). AA men were also more likely to have a dominant nodule that exceeded the threshold size of 0.5 cm3 for insignificant tumors according to the criterion of Epstein (31.0% vs 13.5%, p = 0.005, supplementary table 2, http://jurology.com/).3
Site of anterior and posterior dominant nodules. A, in men at NCCN very low risk. B, in men at upgraded NCCN very low risk.
Since significant tumors according to the criteria of Epstein are of higher grade and/or greater volume, we analyzed dominant nodules only in cases that were upgraded at RP. Of the 42 men with upgrading those who were AA were much more likely to have anteriorly located dominant tumor nodules (59.4% vs 0.0%, p = 0.001, supplementary table 2, http://jurology.com/ and part B of figure). Of men with significant dominant nodules, as determined by volume (index lesion 0.5 cm3 or greater), the distribution and volume of tumors that were Gleason grade 6 were similar in AA and white men (supplementary table 2, http://jurology.com/). Interestingly, of the men with dominant nodules 0.5 cm3 or greater as well as GS 7 or greater those who were AA were more likely to have anterior tumors (76.9% vs 0.0%, p = 0.032, supplementary table 2, http://jurology.com/).
Because AA men had such a higher frequency of aggressive pathological findings compared to white men despite meeting all NCCN very low risk criteria preoperatively, we examined whether preoperative variables were associated with pathologically significant tumors, defined as Gleason 7 or greater, or an index lesion size of 0.5 cm3 or greater. Of white men at very low risk those who were reclassified with significant tumors at prostatectomy were more likely to present with 2 positive cores on biopsy rather than 1 (61.5% vs 31.7%, p = 0.009). Of AA men those with pathologically significant tumors had slightly higher PSAD (0.09 vs 0.07 ng/ml/gm, p = 0.003) and were also more likely to present with 2 positive biopsy cores (54.7% vs 29.4%, p = 0.021). However, PSAD in AA men with significant tumors was similar to that in white men with insignificant tumors, defined as pathological Gleason 6 or less and a dominant nodule volume of less than 0.5 cm3 (median 0.09 vs 0.08 ng/ml/gm, p = 0.269, table 2). On multivariate logistic analysis adjusting for age, PSAD, cancer volume on biopsy, BMI and family history the factor most strongly associated with pathologically significant cancer was AA race (OR 4.27, p <0.001, table 3).
Table 2.
Preoperative characteristics of men at NCCN very low risk with insignificant tumors and those reclassified with significant tumors
| White |
AA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Insignificant | Significant | p Value | Insignificant | Significant | p Value | |||||
| No. pts (%) | 63 | (70.8) | 26 | (29.2) | <0.001* | 34 | (39.1) | 53 | (60.9) | <0.001* |
| Mean age | 56.6 | 58.8 | 0.949 | 57.1 | 59.0 | 0.338 | ||||
| Median ng/ml PSA (IQR) | 4.4 | (3.4, 5.7) | 4.4 | (3.0, 5.0) | 0.459 (Wilcoxon-Mann-Whitney test)* | 4.4 | (3.0, 6.0) | 4.7 | (3.7, 5.7) | 0.617 (Wilcoxon-Mann-Whitney test) |
| Median gm prostate size (IQR) | 49.9 | (35.2, 57.0) | 44.4 | (30.6, 53.4) | 0.194 (Wilcoxon-Mann-Whitney test) | 46.0 | (36.2, 63.0) | 48.0 | (27.8, 56.0) | 0.070 (Wilcoxon-Mann-Whitney test) |
| Median ng/ml/gm PSAD (IQR) | 0.09 | (0.06, 0.12) | 0.08 | (0.07, 0.10) | 0.773 (Wilcoxon-Mann-Whitney test) | 0.07 | (0.06, 0.10) | 0.09 | (0.08, 0.12) | 0.003 (Wilcoxon-Mann-Whitney test) |
| No. pos cores (%): | 0.009 | 0.021 | ||||||||
| 1 | 43 | (68.3) | 10 | (38.5) | 24 | (70.6) | 24 | (45.3) | ||
| 2 | 20 | (31.7) | 16 | (61.5) | 10 | (29.4) | 29 | (54.7) | ||
| Median % Ca/core (IQR) | 10.0 | (5.0, 25.0) | 10.0 | (5.0, 30.0) | 0.685 (Wilcoxon-Mann-Whitney test) | 10.0 | (5.0, 30.0) | 20.0 | (5.0, 30.0) | 0.137 (Wilcoxon-Mann-Whitney test) |
| No. CAPRA (%): | 0.065 | 0.760 | ||||||||
| 0–1 | 51 | (81.0) | 25 | (96.2) | 26 | (76.5) | 42 | (79.2) | ||
| 2–3 | 12 | (19.0) | 1 | (3.8) | 8 | (23.5) | 11 | (20.8) | ||
| No. family history (%) | 28 | (44.4) | 13 | (50.0) | 0.633 | 16 | (47.1) | 20 | (37.7) | 0.389 |
| Median kg/m2 BMI (IQR) | 27.2 | (24.8, 29.7) | 27.3 | (25.1, 29.0) | 0.948 (Wilcoxon-Mann-Whitney test) | 28.2 | (26.1, 30.3) | 27.3 | (25.8, 30.1) | 0.314 (Wilcoxon-Mann-Whitney test) |
White men with significant tumors vs AA men with significant tumors.
Table 3.
Univariate and multivariate logistic regression analysis of AA race association with reclassification to pathologically significant disease
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| AA race | 3.66 (1.94, 6.89) | <0.001 | 4.27 (2.06, 8.61) | <0.001 |
| Age | 1.07 (1.01, 1.12) | 0.013 | 1.08 (1.03, 1.16) | 0.014 |
| PSAD (0.01 ng/ml/gm increments) | 1.10 (1.00, 1.22) | 0.044 | 1.18 (1.04, 1.34) | 0.011 |
| Two pos cores (referent 1) | 3.03 (1.63, 5.66) | <0.001 | 1.84 (1.08, 4.52) | 0.116 |
| Maximum % Ca involvement/core | 1.03 (1.00, 1.05) | 0.015 | 1.02 (1.00, 1.04) | 0.117 |
| CAPRA 2 or greater | 0.79 (0.36, 1.75) | 0.565 | 0.44 (0.23, 1.79) | 0.148 |
| Pos family history | 0.97 (0.53, 1.76) | 0.908 | 1.46 (0.66, 2.76) | 0.320 |
| BMI 30 kg/m2 or greater | 1.20 (0.57, 2.53) | 0.633 | 1.24 (0.55, 2.88) | 0.642 |
DISCUSSION
Evidence suggests that there are racial disparities in oncological outcomes between white and AA men who have NCCN very low risk PCa.9 The safety of monitoring AA men with favorable risk disease by AS was questioned because AA men are at significantly higher risk for clinical progression.7, 8 We determined whether there are systematic pathological differences in the tumors of very low risk, case-control matched white and AA men by studying their surgical specimens with particular attention to tumor nodule quantity, volume, location and grade.
AA men had substantially higher rates of pathological upgrading (37% vs 11%) and multifocal disease (84% vs 61%) than white men. AA men also had a median dominant lesion volume that was more than twofold larger and located more frequently in the anterior prostate (51% vs 29%). When analyzing dominant nodules in men with upgrading, the largest high grade lesions were located in the anterior prostate significantly more frequently in AA men (59% vs 0%). Such a disparity in the location of dominant tumor foci, particularly those of higher grade, may result in undersampling index lesions since a study of extended biopsy showed that the anterior location is the most difficult site to sample by 12-core biopsy techniques.15
Several groups suggested that AA men overall present with more aggressive PCa phenotypes.16–20 Investigators explored potential underlying factors, including single nucleotide polymorphisms,21 patterns of TMPRSS2-ERG gene fusion22 and inflammatory cytokines.23 The extent to which outcomes are influenced by socioeconomic status is uncertain.8, 16
We report a relatively homogenous cohort of men with NCCN very low risk PCa diagnosed by extended biopsy sampling and treated in the modern Gleason era at a single tertiary center. However, between AA and white men there were significant pathological discrepancies, most notably upgrading to Gleason sum 7 or greater. The finding that AA men have larger tumors in general may be explained by the possibility that PCa grows faster or begins at an earlier age depending on race.24 The increased tumor multifocality in AA men may be due to differences in the prostate microenvironment influenced by androgen exposure. We also postulate that the different zonal distribution of PCa between white and AA men suggests the possibility of distinct underlying biological factors. Ultimately, greater tumor multifocality and a greater extent of anteriorly located cancer volume may be associated with a greater chance of harboring high grade disease that is unrecognized by standard transrectal biopsy. This study does not reveal why cancer volume and nodules are increased in AA men, although it highlights a potential disparity in the pathogenesis of PCa in AA men that is possibly relating to an altered microenvironment or growth kinetics.
Other reports confirm this notion. In heterogeneous RP cohorts Moul25 and Bigler26 et al noted that AA men had greater pathological tumor volume than white men. Powell et al evaluated an autopsy series of men who died of unrelated causes and found that at ages 20 to 30 years AA and white men harbored incidental PCa foci that were equivalent in volume and grade.24 However, AA men presented with metastatic PCa approximately 4 times more frequently than white men starting in the fifth decade of life, leading to the inference that PCa transforms from latent to aggressive faster and more frequently in AA men.
Alternatively, the finding that very low risk AA had a much higher prevalence of anteriorly located dominant nodules, that is 51% vs 29% (p = 0.003) and in upgraded cases 59% vs 0% (p = 0.001), suggests that upgrading at RP may be a consequence of undersampling at biopsy. All men in this study underwent extended sampling with 10 or more transrectal cores but standard biopsies are posterior in approach and may miss anterior lesions. In a study of 40 AA men Pettaway et al found that they had a higher prevalence of transition zone tumors (35% vs 21%, p <0.001).27 In a report of 348 AA men Tiguert et al found that they were also more likely to have anterior tumors (16% vs 11%, p = 0.045).28 While these prior studies echo our findings, they included patients with widely ranging risk characteristics, such as clinical stage T1-T3 and PSA 0.2 to 139 ng/ml.
A key implication of the increased prevalence of anterior lesions in AA men is that significant lesions that are missed by digital rectal examination and transrectal ultrasound guided biopsy may require identification by alternate imaging or biopsy strategies. Lawrentschuk et al suggested that a subset of men with T1c PCa have what they called evasive anterior tumors, which can be characterized by prostate MRI and targeted anterior biopsies.29 Alternatively, more extensive transrectal biopsies may also be useful in AA men. In men at low risk Motamedinia et al noted that near saturation biopsies with a mean of 17 cores before initiating AS revealed previously undetected high grade cancers in 74%.30 Thus, men thought to have low risk PCa who are considering AS, especially AA men, may be well served by MRI, saturation biopsies and/or targeted anterior biopsies as part of the initial assessment.
In this study AA men with significant tumors had PSAD similar to that of white men with insignificant disease. Although increasing PSAD was independently associated with significant tumors, the association was modest (adjusted OR 1.18). Therefore, we believe that AA men with PSAD approaching the upper limit of NCCN very low risk criteria (0.15 ng/ml/gm) should be counseled that they may be at increased risk for adverse pathological findings and be less suitable candidates for surveillance.
There are several limitations to this study. Cohorts were identified retrospectively, introducing a potential selection bias. The total number of preoperative biopsies per patient was unknown, as were the reasons for electing prostatectomy over surveillance. Moreover, preoperative PSA kinetic data were not recorded, although average PSA values were low and similar in the groups at RP. Patients were treated at a single tertiary referral center, limiting generalizability, and validation in multi-institutional cohorts is warranted.
The main strength of this study is that we analyzed homogeneous cohorts of men with almost identical preoperative characteristics. All men met NCCN very low risk criteria, underwent extended biopsy sampling and were treated in the modern Gleason grading era. Therefore, the study reflects contemporary clinical practice and race based pathological disparities.
CONCLUSIONS
We performed a detailed clinicopathological study of homogeneous cohorts of white and AA men. The latter men had a markedly higher rate of adverse pathological findings at prostatectomy, including upgrading. Analysis of tumor nodules revealed higher volume cancer that was more frequently multifocal with a strong tendency of the dominant nodule to be located in the anterior aspect of the prostate gland in AA men. This suggests that AA men who are considering AS should undergo targeted tissue sampling of the anterior zone, potentially in conjunction with prostate MRI or alternative biopsy patterns to rule out significant foci of anterior disease that may be missed by standard extended transrectal biopsy. Molecular differences may underlie the disparities among men at very low risk who are highlighted in the current study. To our knowledge these distinctions remain unknown but they merit future investigation.
Supplementary Material
Acknowledgments
Supported by NIH-NIDDK Training Grant T32DK007552 (DS), the Howard Hughes Clinician-Scientist Early Careers Award, AUA/ Astellas Rising Star Award (EMS) and the Johns Hopkins Clinician Scientist Award (AER).
Abbreviations and Acronyms
- AA
black
- AS
active surveillance
- BMI
body mass index
- GS
Gleason score
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate specific antigen
- PSAD
PSA density
- RP
radical prostatectomy
REFERENCES
- 1.NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Fort Washington, Pennsylvania: National Comprehensive Cancer Network; 2012. version 3. [Google Scholar]
- 2.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368. [PubMed] [Google Scholar]
- 3.Bastian PJ, Mangold LA, Epstein J, et al. Characteristics of insignificant clinical T1c prostate tumors. A contemporary analysis. Cancer. 2004;101:2001. doi: 10.1002/cncr.20586. [DOI] [PubMed] [Google Scholar]
- 4.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Trock B, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 6.Ha Y, Salmasi A, Karelas M, et al. Increased incidence of pathologically nonorgan confined prostate cancer in African-American men eligible for active surveillance. Urology. 2013;81:831. doi: 10.1016/j.urology.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iremashvili V, Soloway MS, Rosenberg DL, et al. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187:1594. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 8.Abern MR, Bassett M, Tsivian M, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2013;16:85. doi: 10.1038/pcan.2012.38. [DOI] [PubMed] [Google Scholar]
- 9.Sundi D, Ross AE, Humphreys EB, et al. African American men with very low risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy. Should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Allsbrook WC, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hussain TO, Epstein JI. Initial high-grade prostatic intraepithelial neoplasia with carcinoma on subsequent prostate needle biopsy: findings at radical prostatectomy. Am J Surg Pathol. 2011;35:1165. doi: 10.1097/PAS.0b013e3182206da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise AM, Stamey TA, McNeal JE, et al. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 13.Warlick C, Trock BJ, Landis P, et al. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98:355. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komai Y, Numao N, Yoshida S, et al. High diagnostic ability of multi-parametric magnetic resonance imaging in detecting anterior prostate cancer missed by transrectal 12-core biopsy. J Urol. 2013;190:867. doi: 10.1016/j.juro.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 16.Chornokur G, Dalton K, Borysova ME, et al. Disparities at presentation, diagnosis, treatment, and survival in African American men affected by prostate cancer. Prostate. 2011;71:985. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamoah K, Stone N, Stock R. Impact of race on biochemical disease recurrence after prostate brachytherapy. Cancer. 2011;117:5589. doi: 10.1002/cncr.26183. [DOI] [PubMed] [Google Scholar]
- 18.Ritch CR, Morrison B, Hruby G, et al. Pathological outcome and biochemical recurrence-free survival after radical prostatectomy in African- American, Afro-Caribbean (Jamaican) and Caucasian-American men: an international comparison. BJU Int. 2013;111:E186. doi: 10.1111/j.1464-410X.2012.11540.x. [DOI] [PubMed] [Google Scholar]
- 19.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 20.Moul JW, Douglas TH, McCarthy WF, et al. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol. 1996;155:1667. [PubMed] [Google Scholar]
- 21.Reams RR, Kalari K, Wang H, et al. Detecting gene-gene interactions in prostate disease in African American men. Infect Agent Cancer, suppl. 2011;6:S1. doi: 10.1186/1750-9378-6-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magi-Galluzzi C, Tsusuki T, Elson P, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71:489. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 23.Powell IJ, Dyson G, Land S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prevent. 2013;22:891. doi: 10.1158/1055-9965.EPI-12-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moul JW, Sesterhnenn R, Connelly RR, et al. Prostate-specific antigen values at the time of prostate cancer diagnosis in African-American men. JAMA. 1995;274:1277. [PubMed] [Google Scholar]
- 26.Bigler S, Pound CR, Zhou X. A retrospective study on pathologic features and racial disparities in prostate cancer. Prostate Cancer. 2011;2011:239460. doi: 10.1155/2011/239460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettaway CA, Tronosco P, Ramirez E, et al. Prostate specific antigen and pathological features of prostate cancer in black and white patients: a comparative study based on radical prostatectomy specimens. J Urol. 1998;160:437. [PubMed] [Google Scholar]
- 28.Tiguert R, Gheiler M, Tefili M, et al. Racial differences and prognostic significance of tumor location in radical prostatectomy specimens. Prostate. 1998;37:230. doi: 10.1002/(sici)1097-0045(19981201)37:4<230::aid-pros4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Lawrentschuk N, Haider M, Daljeet N, et al. “Prostatic evasive anterior tumours”: the role of magnetic resonance imaging. BJU Int. 2010;105:1231. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 30.Motamedinia P, Richard JL, McKiernan JM, et al. Role of immediate confirmatory prostate biopsy to ensure accurate eligibility for active surveillance. Urology. 2012;80:1070. doi: 10.1016/j.urology.2012.07.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.