Abstract
Midazolam and morphine are often used in pediatric intensive care unit (ICU) for analgesia and sedation. However, how these two drugs interact behaviorally remains unclear. Here, we examined whether 1) co-administration of midazolam with morphine would exacerbate morphine tolerance and morphine-induced hyperactive behaviors, and 2) protein kinase C (PKC) would contribute to these behavioral changes. Male rats of 3 to 4 weeks old were exposed to a hindpaw burn injury. In Experiment 1, burn-injured young rats received once daily saline or morphine (10 mg/kg, subcutaneous, s.c.), followed 30 min later by either saline or midazolam (2 mg/kg, intraperitoneal, i.p.), for 14 days beginning 3 days after burn injury. In Experiment 2, young rats with burn injury were administered with morphine (10 mg/kg, s.c.), midazolam (2 mg/kg, i.p.), and chelerythrine chloride (a non-specific PKC inhibitor 10 nmol, intrathecal) for 14 days. For both experiments, cumulative morphine anti-nociceptive dose-response (ED50) was tested and hyperactive behaviors such as jumping and scratching were recorded. Following 2 weeks of each treatment, ED50 dose was significantly increased in rats receiving morphine alone as compared with rats receiving saline or midazolam alone. The ED50 dose was further increased in rats receiving both morphine and midazolam. Co-administration of morphine and midazolam also exacerbated morphine-induced hyperactive behaviors. Expression of the NR1 subunit of the N-methyl-D-aspartate (NMDA) receptor and PKCγ in the spinal cord dorsal horn (immunohistochemistry; Western blot) was upregulated in burn-injured young rats receiving morphine alone or in combination with midazolam, and chelerythrine prevented the development of morphine tolerance. These results indicate that midazolam exacerbated morphine tolerance through a spinal NMDA/PKC-mediated mechanism.
Keywords: Midazolam, Morphine tolerance, Hyperactivity, Young rats, Burn injury, NMDA, NR1, PKC
1. Introduction
Over 30% of people who experience burn injury in the United States are children (Borse et al., 2009). Clinically, opioids are among the first-line drugs used to treat severe pain resulting from burn injury particularly in the intensive care unit (ICU) setting (Stoddard et al., 2006). However, the development of opioid analgesic tolerance, a diminished opioid anti-nociceptive effect following repeated exposure to opioid, could significantly hamper the clinical effectiveness of opioid therapy.
Many pediatric patients with burn injury have a prolonged stay in ICU and require both analgesia and sedation. Benzodiazepines are the most frequently used sedatives in ICU settings. At the cellular level, benzodiazepines potentiate γ–amino butyric acid (GABA) actions by increasing the frequency of chloride channel opening and by prolonging its open state (Matsumoto, 1989; Reynolds et al., 1992). Midazolam is a water-soluble and short-acting benzodiazepine. Acting on a benzodiazepine receptor-GABAA ionophore complex, midazolam reduces excitability of second-order neurons in the spinal cord dorsal horn and brain stem (Haefely et al., 1988; Richards et al., 1986). However, intraperitoneal or intrathecal application of midazolam alone has been shown to induce both hyperalgesia and antinociception (Clavier et al., 1992; Kontinen et al., 2000; Lim et al., 2006; Niv et al., 1988; Rattan et al., 1991; Shih et al., 2008; Tatsuo et al., 1999; Yanez et al., 1990). Clinically, midazolam and morphine are the most frequently used drugs in ICU, including pediatric ICU, to achieve sedation and analgesia (Chamorro et al., 2010; Soliman et al., 2001). To date, it remains unclear as to how morphine and midazolam might interact at the behavioral and cellular level, particularly in pediatric populations.
Our previous studies indicate that burn injury itself may have a differential effect on the development of morphine tolerance in adult versus young rats (Wang et al., 2005, 2011). In this study we used a burn injury model of young rats to examine whether 1) co-administration of midazolam with morphine would exacerbate morphine tolerance and morphine-induced hyperactive behaviors, and 2) protein kinase C (PKC) would contribute to the tolerance and behavioral hyperactivity. In this study, we found that co-administration of morphine with midazolam reduced the analgesic effect of morphine and increased hyperactive behaviors such as scratching and jumping in the absence of naloxone-precipitated withdrawal. Co-administration of morphine and midazolam upregulated the expression of the NR1 subunit of the N-methyl-D-aspartate (NMDA) receptor and PKC within the spinal cord dorsal horn.
2. Results
2.1. Burn injury induced hindpaw hyperalgesia
There were no differences in baseline nociceptive threshold to thermal stimulation between sham and burn-injured groups (Fig. 2A). Burn injury induced hyperalgesia in the ipsilateral hindpaw when compared to sham injured rats (Fig. 2A). No changes in baseline nociceptive threshold in the contralateral hindpaw in burn-injured or sham rats. Moreover, in burn injured rats treated with 1ml/kg saline (SAL-SAL), 1ml/kg saline plus 2 mg/kg midazolam (SAL-MID), 10 mg/kg morphine plus 1ml/kg saline (MOR-SAL), or10 mg/kg morphine plus 2 mg/kg midazolam (MOR-MID), there was no significant interaction between groups and days after burn injury (P = 0.145), indicating that midazolam per se did not further exacerbate burn injury-induced hyperalgesia. Figure 2B shows the time course of hyperalgesia in various groups of rats after burn injury. All groups of rats in figure 2B were burn-injured rats and all groups were tested on a designated day of behavioral testing before a drug administration.
Fig. 2. Burn injury-induced hyperalgesia.
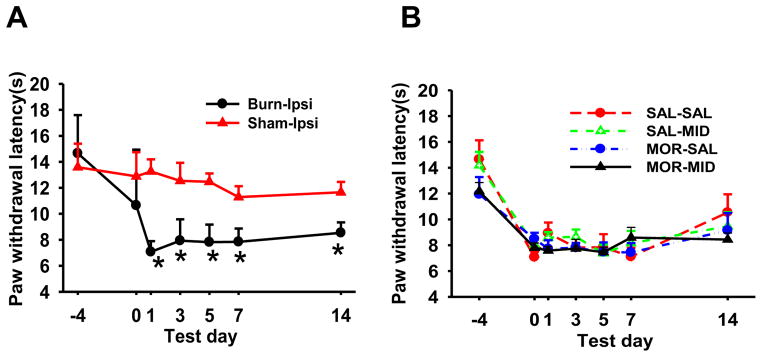
Hindpaw withdrawal latency to thermal stimulation in burn-injured young rats was determined on days -4, 0, 1, 3, 5, 7, and 14. Nociceptive threshold was decreased on the ipsilateral side of burn-injured rats. There were significant differences (P<0.0001) between pre-injury baseline (day -4) and the remaining time points (A). However, the ipsilateral withdrawal latency to thermal stimulation were not significantly different in all groups of burn-injured rats (P = 0.145) treated with morphine, midazolam, or both (B). The time point day 0 (post-injury baseline, day 0) represents 3 days after burn injury but before the first morphine or midazolam administration. These behavioral tests were made before each injection on the designated days. Data are presented as mean ± SD for 6 rats per group.
*P<0.05, as compared with the Sham-Ipsi (ipsilateral) group.
2.2. Midazolam exacerbated morphine tolerance
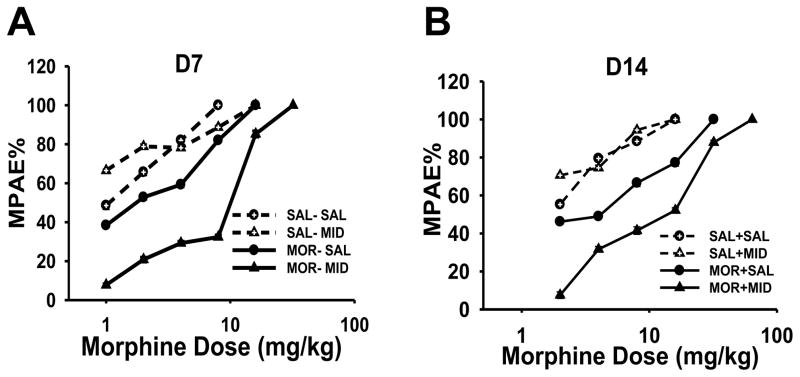
Morphine tolerance was developed in the MOR-SAL group and further exacerbated in the MOR-MID group in burn-injured young rats over a 2-week period (Fig. 3). As shown in Table 2 (day 7 and 14), when compared to the SAL-SAL group, the morphine ED50 dose (the morphine dose producing 50% of the anti-nociceptive effect) for morphine was increased in the MOR-SAL group on day 7 (P = 0.008) and 14 (P = 0.004). However, the morphine ED50 dose in the MOR-MID group was further increased as compared to that in the MOR-SAL group on day 14 (P = 0.04) (see Table 2 for ED50 and 95% CI for all groups). In contrast, the ED50 dose in the SAL-MID group was not increased as compared to that in the SAL-SAL group on day 7 (P = 0.781) and day 14 (P = 0.758) (Table 2). The ED50 dose in the SAL-SAL group on day 14 also was not increased when compared with its own ED50 dose on day 7 (P = 0.098). Neither morphine nor midazolam within the experimental dose range caused motor changes (e.g., gait abnormality).
Fig. 3. Effect of midazolam on cumulative morphine dose-response curves.
Morphine anti-nociceptive dose-responses in young rats with burn injury (n=6) were assessed on day 7 (A) and day 14 (B). A rightward shift of morphine dose-response was demonstrated in burn-injured rats of MOR-SAL and MOR-MID groups on day 7 (A) and day 14 (B). And the development of morphine tolerance was exacerbated when morphine was administered with midazolam for 14 days B. ED50 and 95% confidence intervals (CI) are presented in Table 2. MPAE%: percent of maximal possible anti-nociceptive effect.
The percent of maximal possible anti-nociceptive effect (%MPAE) was determined by comparing hindpaw withdrawal latency before (baseline, BL) and after each morphine administration (TL) using the equation: MPAE% = [(TL-BL)/(20−BL) ×100% (20s as the cut-off time).
Table 2.
ED50 and 95% CI in Burn-injured Young Rats
| ED50 [95% CI] (mg/kg)
|
|||
|---|---|---|---|
| Day 7 | Day 14 | P-value | |
|
| |||
| SAL-SAL | 3.19 [2.20 to 5.40] | 5.91 [3.90 to 10.06] | 0.098 |
| SAL-MID | 4.59 [2.55 to 8.49] | 5.14 [3.40 to 7.63] | 0.382 |
| MOR-SAL | 7.70 [5.66 to 13.93]* | 15.41 [11.32 to 27.85]* | 0.041 |
| MOR-MID | 13.68 [10.18 to 20.97]* | 30.79 [22.83 to 53.68]*# | 0.017 |
ED50: the morphine dose that produced 50% of the anti-nociception effect
95% CI: 95% confidence intervals
P < 0.01, as compared with the SAL-SAL or SAL-MID group on Day 7 or 14
P = 0.04 as compared with the MOR-SAL group on Day 14
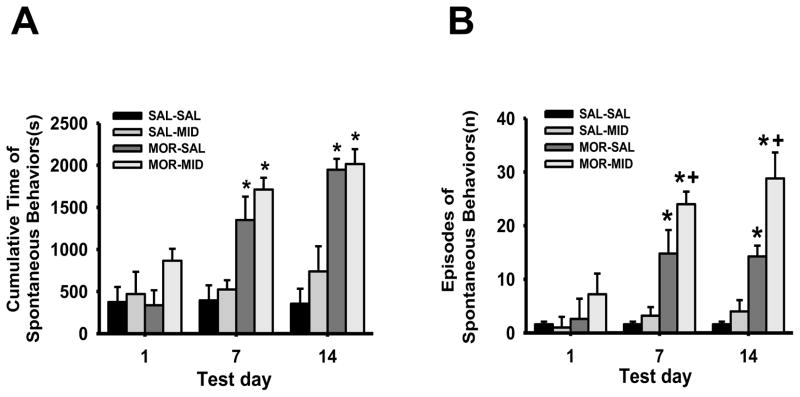
2.3. Midazolam enhanced morphine-induced hyperactive behaviors
The cumulative duration of hyperactive behaviors in burn-injured young rats, including scratching, jumping, and licking, was significantly increased in both MOR-SAL and MOR-MID groups as compared to the SAL-SAL or SAL-MID group on day 7 and day 14 (Fig. 3A, P < 0.0001). Moreover, the number of episodes of such hyperactive behaviors in burn-injured young rats was significantly increased in the MOR-MID group, as compared to the MOR-SAL group on both day 7 and day 14 (Fig. 3B, P < 0.05). These results indicate that repeated co-administration of morphine and midazolam increased hyperactive behaviors in the absence of naloxone precipitation.
2.4. PKC inhibitor prevented midazolam-exacerbated morphine tolerance
To examine whether inhibition of PKC at the spinal level would prevent the development of morphine tolerance, chelerythrine chloride (a non-specific PKC inhibitor, 10 nmol; intrathecally, once daily × 14 days) or vehicle was added to the daily treatment regimen with morphine (10 mg/kg, s.c.) and/or midazolam (2 mg/kg, i.p.) over a 2-week period. The chelerythrine dose was referenced from previous publications showing its effect on preventing morphine tolerance (Hargreaves et al., 1988; Mao et al., 1995). When examined on day 14, intrathecal administration of chelerythrine (MOR-MID-Chelerythrine group) but not vehicle (MOR-MID-Vehicle group) prevented the increase in ED50 dose in rats administrated with morphine and midazolam (P = 0.017) (Table 3 for ED50 and 95% CI for each group). Intrathecal chelerythrine or vehicle administration alone (SAL-SAL-Chelerythrine and SAL-SAL-Vehicle groups) for two weeks did not alter the morphine anti-nociceptive effect when examined on day 14 (P = 0.763). These results indicate that inhibition of PKC at the spinal level prevented the development of morphine tolerance that was exacerbated by co-administration with midazolam.
Table 3.
Effect of chelerythrine on ED50
| ED50 (mg/kg) | 95% CI (mg/kg) | |
|---|---|---|
| SAL-SAL-Vehicle | 4.27 | 3.31 to 7.24 |
| SAL-SAL-Chelerythrine | 5.25 | 3.74 to 8.81 |
| MOR-MID-Vehicle | 23.98 | 17.71 to 36.10 |
| MOR-MID-Chelerythrine | 10.47 | 7.64 to 15.07* |
ED50: the morphine dose that produced 50% of the anti-nociception effect
95% CI: 95% confidence intervals
P = 0.017, as compared with the MOR-MID-Vehicle group
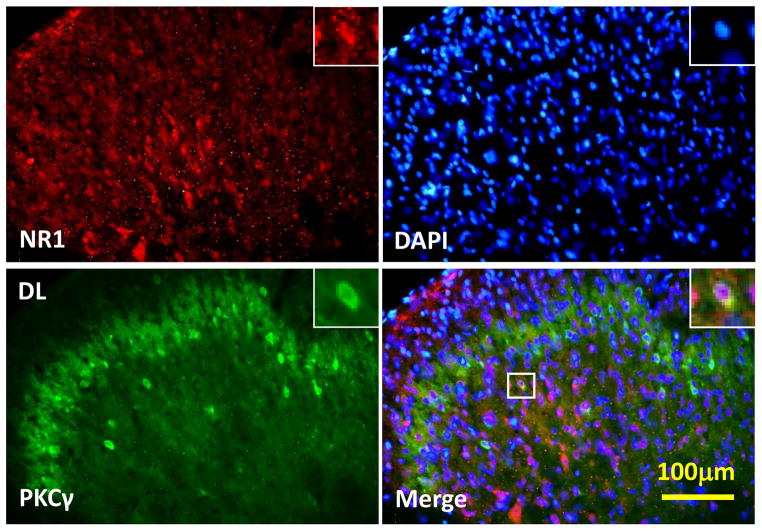
2.5. Expression of NR1 and PKCγ in the spinal cord dorsal horn
As detected by immunohistochemistry, NR1 and PKCγ were co-localized in superficial layers (I & II) of the spinal cord dorsal horn in burn-injured young rats (Fig. 4). When examined on day 14 by Western blot, expression of NR1 was increased in burn-injured young rats treated with either morphine alone or a combination of morphine and midazolam, as compared to that in the SAL-SAL or SAL-MID group (Fig. 5A, P < 0.01). The expression of PKCγ was also increased in burn-injured young rats treated with a combination of morphine and midazolam (Fig. 5B, P < 0.05). Collectively, these results indicate that co-administration with morphine and midazolam enhanced the spinal expression of NR1 and PKCγ.
Fig. 4. Effect of midazolam on morphine-induced hyperactive behaviors.
Both the duration (A) and number (B) of episodes of hyperactive behaviors were increased in burn-injured young rats treated with morphine or a morphine and midazolam combination. Data are presented as mean ±SD for 6 rats per group. * P < 0.0001 as compared with day 1 in the same group; + P < 0.05 as compared with the MOR-SAL group on the same day.
Fig. 5. Co-localization of spinal NR1 and PKCγ expression.
There was co-localization of NR1 and PKCγ immunoreactivity in the superficial layers (I & II) of the spinal cord dorsal horns at the lumbar (L4) level. Spinal cord samples were taken from burn-injured young rats receiving a combination of morphine and midazolam treatment for 14 days (n=3). Blue: DAPI for nucleus. Scale bar: 100 μm. DL: the dorsolateral part of the spinal cord dorsal horn.
3. Discussion
We have demonstrated that morphine tolerance in young rats with burn injury was exacerbated by co-administration of midazolam over a 2-week period, reflected by the increased ED50 dose for morphine anti-nociception. The 2-week morphine treatment regimen also increased the number and duration of hyperactive behaviors in burn-injured young rats, which were also exacerbated by co-administration with midazolam. These behavioral changes were associated with the upregulation of spinal NMDA and PKC expression and attenuated by inhibition of spinal PKC activity.
Methodological considerations
Our previous results showed significant changes in nociceptive behavior on the ipsilateral hindpaw in both young and adult rats following burn injury. These nociceptive behaviors lasted for at least 2 weeks after initial burn injury without causing overall distress in rats (Wang et al., 2005, 2011). Previous studies also found that nociceptive threshold to mechanical or thermal stimulation was reduced in morphine-treated sham rats (Mao et al., 1994, 1995; Mayer et al., 1995). In this study, young rats with burn injury exhibited a lower thermal nociceptive threshold (i.e., burn injury-induced hyperalgesia), making it difficult to examine opioid-induced hyperalgesia and the impact of midazolam on this behavioral change. Therefore, we did not see further exacerbation of baseline hyperalgesia in these burn-injured rats after a period of drug administration possibly due to a maximum degree of burn injury-induced hyperalgesia in these rats. Accordingly, we focused our study on examining the effect midazolam on morphine tolerance. While young rats were used in this study, we intend to examine the effect of midazolam on morphine tolerance in adult rats as well in future studies.
Behavioral hyperactivity including scratching, jumping, and licking may occur following a course of administration with opioid and/or midazolam even in the absence of naloxone-precipitated withdrawal. Accordingly, we specifically examined these hyperactive behaviors along with possible locomotor abnormality. These hyperactive behaviors, especially scratching and licking of the injured hindpaw, were increased in burn-injured young rats and lasted for at least 4 h after each morphine administration. It was previously reported that burn injury is associated with the local release of bradykinin, prostaglandins, interleukins, and/or monoamines (Arturson et al., 1996). Therefore, it is possible that the observed behaviors could be induced by a reaction at the injured site or due to changes in the central nervous system induced by systemic morphine administration. Nonetheless, our findings indicate that this burn injury model is useful to examine persistent nociception and morphine-induced behavioral changes in young rats.
Comparisons between young and adult burn-injured rats
In the present study, burn-injured young rats exposed to chronic morphine administration demonstrated an increased ED50 dose for morphine anti-nociception on day 7 and 14, indicative of the development of morphine tolerance. However, burn-injured young rats without exposure to this 2-week morphine treatment regimen (Saline-Saline group) did not show an increased ED50 dose, suggesting that burn injury itself may not change the responsiveness to morphine anti-nociception in the absence of a prolonged course of morphine exposure. It should be noted that these were between-group but not within-group (before and after burn injury) comparisons. Therefore, the exact impact of burn injury itself on morphine anti-nociception is yet to be fully assessed. While these findings are consistent with our previous finding in young rats (Wang et al., 2011), they are different from adult rats (Wang et al., 2005). In adult rats with burn injury, the ED50 dose was increased even in the absence of daily morphine exposure (Wang et al., 2005), suggesting that burn injury alone is sufficient to make a rightward shift of the morphine dose-response curve. This difference between young and adult rats suggests possible intrinsic age-related differences with regard to the influence of burn injury per se on morphine anti-nociception. Moreover, since our data were collected using only young rats, it is not clear whether the observed morphine-midazolam interaction would be present in rats at their developmental stage. These issues should be further explored in future studies.
Interaction between morphine and midazolam
We demonstrated that co-administration of morphine with midazolam resulted in exacerbation of morphine tolerance as well as morphine-induced hyperactive behaviors. Midazolam is a benzodiazepine receptor agonist and widely used as a hypnotic and anxiolytic (Richards et al., 1986). It enhances GABA function by acting on the GABAA receptor, thereby facilitating the descending inhibitory system (Matsumoto et al., 1989). However, high doses of midazolam have been shown to affect motor functions (Niv et al 1988). This non-specific inhibitory effect on motor functions by benzodiazepines might be a confounding factor in behavioral tests (Matsumoto et al., 1989). Indeed, controversial evidence exists regarding the analgesic versus hyperalgesic effect of midazolam in both human subjects and rodents (Clavier et al., 1992; Kontinen et al., 2000; Lim et al., 2006; Niv et al., 1988; Rattan et al., 1991; Shih et al., 2008; Tatsuo et al., 1999; Yanez et al., 1990). At higher doses, midazolam has a predominantly hyperalgesic effect, possibly due to its supraspinal effect (Ito et al., 2008; Niv et al 1988). In this study, we used a low midazolam dose (2 mg/kg) which by itself did not cause any abnormal motor function (e.g., gait abnormality).
Benzodiazepines have been reported to both potentiate and attenuate morphine analgesia (Ito et al., 2008; Mantegazza et al., 1982). For example, intrathecal administration of midazolam potentiated morphine anti-nociception, whereas administration of midazolam intracerebroventricularly or into the dorsal raphe-periaqueductal gray area reduced the anti-nociceptive effect of morphine (Ito et al., 2008). Systemic midazolam has been shown to inhibit the anti-nociceptive effect of opioids, which is at least partially reversed by bicuculline (a GABA antagonist) injected into the periaqueductal gray area (Mantegazza et al., 1982). Another interaction between morphine and midazolam is that co-administration of both agents resulted in a decrease in the endogenous β-endorphin level in the pituitary, hippocampus, cortex, spinal cord, and spleen (Rattan et al., 1996). Of interest to note is that, although in this study midazolam exacerbated morphine tolerance when daily co-administered with morphine for 2 weeks, the morphine anti-nociceptive effect was not affected in those rats exposed only to midazolam when a single dose of morphine was administrated only on day 7 or 14, suggesting that co-administration of morphine and midazolam is necessary to demonstrate the impact of midazolam on morphine anti-nociception. In this regard, our data appears to be consistent with the literature regarding the influence of clinical factors on the effectiveness of opioid therapy (Jadad et al., 1992; Kupers et al., 1991; Portenoy et al., 1990; Portenoy, 1994).
Cellular mechanism of the interaction between morphine and midazolam
Intracellular second messenger systems such as PKC have been shown to modulate NMDA receptor activation and play a critical role in the neural and molecular mechanisms of morphine tolerance and opioid-induced hyperalgesia (Chen et al., 1992; Lim et al., 2005; Mao et al., 1993). These effects are likely to result from the PKC-mediated removal of an Mg2+ blockade of the NMDA receptor, as supported by the data showing that PKCγ immunoreactivity was increased in postsynaptic sites within the spinal cord dorsal horn (Chen et al., 1992; Mao et al., 1995a). Indeed, previous studies have shown that membrane-bound PKC was upregulated in association with the development of morphine tolerance, and inhibition of PKC translocation and activation reduced opioid tolerance (Chen et al., 1992; Mao et al., 1993, 1995b, 2001; Mayer et al., 1995a, 1995b, 1999). In the present study, we demonstrated that NR1 and PKCγ were co-localized within superficial layers of the spinal cord dorsal horn and that expression of NR1 and PKCγ was upregulated in the spinal cord dorsal horn of burn-injured young rats exposed to morphine and midazolam. Since intrathecal chelerythrine prevented midazolam-exacerbated morphine tolerance, it suggests that morphine tolerance and its exacerbation by midazolam are likely to share a common NMDA receptor/PKC-medicated cellular mechanism. Of note is that the effect of chelerythrine by itself dose not exclude the role of other PKC isoforms in this process. Other possibilities such as the effect of GABA agonist on dorsal root reflexes and the influence of AMPA receptor (a non-NMDA receptor) expression and function on the opioid responsiveness may be considered as well (Lim et al., 2006).
4. Conclusion
The present data may have clinical implications for pain management and sedation in ICU settings although the exact relevance to pediatric patients with burn injury is yet to be determined. Since co-administration of an opioid and midazolam exacerbated morphine tolerance and morphine-induce hyperactive behaviors, the current strategy of combining midazolam and opioid analgesics in ICU settings may warrant further investigation. Future studies need to explore the mechanisms of interaction between different benzodiazepines and opioids in order to search for effective drug combinations that could provide effective sedation and analgesia but fewer side effects in ICU settings.
5. Experimental procedures
5.1. Experimental animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 60±5g, 3 to 4 weeks old at the beginning of experiments were used. Rats were housed in individual cages with free access to water and food pellets. Room temperature was maintained at 24°C with a 12-hour light/darkness cycle. The general experimental protocol was approved through our Institutional Animal Care and Use Committee. The overall experimental design is illustrated in Figure 1 and Table 1.
Fig. 1. Illustration of experimental design.
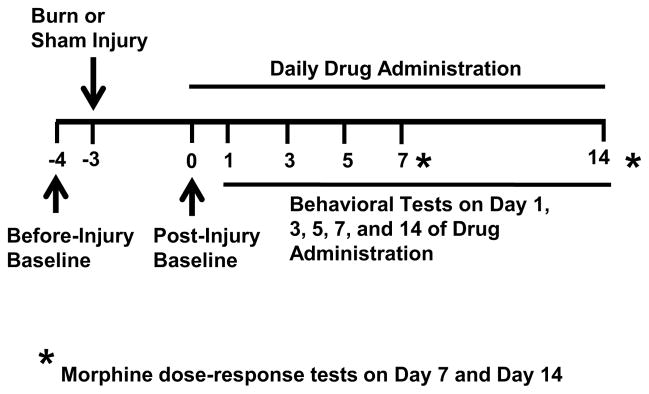
The scheme shows time points for drug injection and behavioral testing. Burn injury was induced after baseline behavior tests (day -4) and three days before (day -3) the first drug administration. Once daily drug administration regimen began on day 0 and continued over a 2-week period. The morphine anti-nociceptive effect was assessed on day 7 and 14. Additional behavioral tests were performed on day 0, 1, 3, 5, 7, and 14.
Table 1.
Drug administration and behavioral tests of burn-injured young rats
| Groups | Administration | Tests |
|---|---|---|
| SAL-SAL | Saline(1ml/kg)-Saline(1ml/kg) | Nociceptive behavioral test on days -4, 0, 1, 3, 5, 7, and 14; Morphine dose-response test on Day 7 and 14 |
| SAL-MID | Saline(1ml/kg)-midazolam (2mg/kg) | Nociceptive behavioral test on days -4, 0, 1, 3, 5, 7, and 14; Morphine dose-response test on Day 7 and 14 |
| MOR-SAL | Morphine(10mg/kg)-Saline(1ml/kg) | Nociceptive behavioral test on days -4, 0, 1, 3, 5, 7, and 14; Morphine dose-response test on Day 7 and 14 |
| MOR-MID | Morphine(10mg/kg)-midazolam (2mg/kg) | Nociceptive behavioral test on days -4, 0, 1, 3, 5, 7, and 14; Morphine dose-response test on Day 7 and 14 |
| SAL-SAL-Vehicle | Saline(1ml/kg)-Saline(1ml/k g) +3%DMSO(5μl) | Morphine dose-response test on Day 14 |
| SAL-SAL-Chelerythrine | Saline(1ml/kg)-Saline(1ml/k g)+ chelerythrine chloride (10 nmol) | Morphine dose-response test on Day 14 |
| MOR-MID-Vehicle | Morphine(10mg/kg)-midazolam (2mg/kg)+ 3%DMSO(5μl) | Morphine dose-response test on Day 14 |
| MOR-MID-Chelerythrine | Morphine(10mg/kg)-midazolam (2mg/kg)+ chelerythrine chloride (10 nmol) | Morphine dose-response test on Day 14 |
5.2. Behavioral assessments
Animals were habituated to the testing environment for 1 h × 3 days before the first behavioral test on day -4 (Fig. 1). Habituation consisted of moving rats from their home room to the testing room and keeping them in a testing apparatus for 30 min. Burn injury was then produced on day -3 (Fig. 1). Behavioral assessments were carried out by an examiner unaware of the group design as outlined in Figure 1, including a) before burn injury (before-injury baseline, day -4), b) 3 days after burn injury but before the first morphine or midazolam administration (post-injury baseline, day 0), and c) on day 1, 3, 5, 7, and 14 of drug administration. On day 7 and 14, cumulative morphine anti-nociceptive dose-response curves were generated (see below). Withdrawal threshold to thermal stimulation was examined on both ipsilateral and contralateral hindpaws before any drug administration on each designated test day. Rats were euthanized after the final behavioral test and spinal cord samples were harvested.
Thermal hyperalgesia
Nociceptive threshold to radiant heat was determined according to a previously described method using a 390 Analgesia Meter (IITC Inc., Woodland Hills, CA) (Hargreaves et al., 1988). Rats were placed individually into plexiglas cubicles placed on a transparent glass surface. The light beam from a projection bulb, located below the glass, was directed at the plantar surface of each hindpaw. Hindpaw withdrawal latency was defined as the time from the onset of radiant heat to withdrawal of the hindpaw. Radiant heat intensity was adjusted to result in a baseline latency of about 12s and a cut-off time of 20s. Two trials with an interval of 5min were made for each hindpaw and scores from both trials were averaged to yield mean withdrawal latency for each hindpaw.
Morphine Tolerance
Tolerance to the anti-nociceptive effect of morphine was assessed by the hindpaw thermal withdrawal test. Cumulative dose-response in burn-injured rats was assessed on day 7 and day 14 in each group. Details of assessing cumulative dose-response were described elsewhere (Mao et al., 1994; Mao et al., 1995; Mayer et al., 1995). In brief, increment doses (1, 2, 4, 8, 16, 32, 64 mg/kg) of morphine were given to the same rats until no further increase in the withdrawal latency was demonstrated in response to a higher dose or the cut-off time (20s) was reached. Hindpaw thermal withdrawal tests were made 15 min after each dose of morphine injection, followed by the next morphine injection (a total of 30 min between two injections).
Morphine-induced hyperactive behaviors
To examine whether morphine alone or a morphine-midazolam combination would enhance spontaneous behaviors (without naloxone precipitation) such as scratching, sniffing, jumping, biting, and licking, the total number (episodes) and duration of these behaviors were observed on day 1, 7, and 14. Rats were placed individually into viewing boxes (20×20×40cm) and spontaneous behaviors were observed via a video system over a 4-hour period. Morphine-induced hyperactive behaviors were digitally recorded and manually counted later from a computer screen.
5.3. Hindpaw burn injury
Burn injury in young rats has been previously described (Wang et al., 2011). Briefly, under isoflurane anesthesia (1.5 to 2%), the dorsal part of a rat’s right hindpaw was immersed into a hot water bath (85°C) for 12s. Burn injury was limited to an area of approximately 0.75cm2 by pressing the hindpaw firmly against a holed plastic template. Exposure to this temperature and duration has been shown to produce burn injury and nociceptive response including mechanical allodynia and thermal hyperalgesia in both adult and young rats (Wang et al., 2005, 2011). Silver sulfadiazine ointment was applied twice daily to the injured surface until scar tissue was fully formed. Similar to previous studies, young rats with burn injury showed a burn surface area of about 1% of the body surface, normal development (comparable to that of sham rats), normal movement pattern, and no signs of general distress, poor food and water intake, or self-mutilation (Wang et al., 2005, 2011).
5.4. Drugs and treatment routes
In Experiment 1, burn-injured young rats were randomly assigned to receive subcutaneous (s.c.) saline (1 ml/kg) or morphine (10 mg/kg) followed 30 min later by another saline or midazolam (2 mg/kg, i.p.) administration. Each regimen was given once daily for 14 consecutive days beginning 3 days after burn injury (Day 0 in Fig. 1).
In Experiment 2, the PKC inhibitor chelerythrine chloride (10 nmol; Sigma, St. Louis, MO) was intrathecally injected according to the method of Hylden and Wilcox (Hylden et al., 1980). A 30-gauge needle was inserted from the paraspinal side of the L5 or L6 spine process under isoflurane anesthesia. The injection volume was 5 μl per rat and given 30 min before administration of midazolam (2 mg/kg) combined with morphine (10 mg/kg). Chelerythrine was dissolved in 3% dimethyl sulfoxide (DMSO) in saline, which served as a vehicle control.
The number of rats in each group from both experiments is reported in the figure legend. Timelines for drug administration and behavioral tests in Experiment 1 and 2 are detailed in Table 1.
5.5. Immunohistochemistry
One half (n=3) of rats from each experimental group were deeply anesthetized with sodium pentobarbital (50mg/kg, i.p.) and transcardially perfused with 100 ml of 0.01M phosphate-buffered saline (PBS, pH 7.35) followed by 300ml of 4% paraformaldehyde in 0.1M phosphate buffer (PB, pH 7.35). Lumbar spinal cord (L4 and L5) segments were removed, post-fixed in the same fixative for 4 h and cryoprotected in 0.1M PB buffered 30% sucrose until the segments sank to the bottom. Transverse sections of 25μm thickness were cut using a cryostat and mounted onto slides. Sections were rinsed in 0.01M PBS for 3×10min. For fluorescence immunostaining of NR1 and PKCγ, sections were blocked for 30min in PBS containing 1% BSA, 5% donkey serum and 0.3% Triton X-100. After rinsing 3×10min, sections were incubated overnight at 4°C with a primary antibody against NR1 (1:500, rabbit anti-rat polyclonal; Novus Biologicals, Littleton, CO) or PKCγ (1:1000; Mouse anti-rat PKCγ, Zymed Laboratories Inc., South San Francisco, CA). After rinsing in PBS (3×10min), a secondary antibody (1:400; CY3 or FITC conjugated donkey anti-rabbit or anti-mouse IgG, Jackson ImmunoResearch, West Grove, PA) was added and sections were incubated for 1h at room temperature. These sections were again rinsed with PBS (3×10min) and slip-covered. Controls were made by omitting a primary antibody followed by the same incubation procedure as described above. For double staining, a second primary antibody was added after incubation with the first primary antibody following the same procedure. Four to six nonadjacent sections were randomly selected and analyzed using an Olympus fluorescence microscope, photographed with a digital camera, and processed with Adobe Photoshop.
5.6. Western blot
The other half (n=3) of rats from each experimental group were deeply anesthetized with isoflurane and decapitated for rapid tissue harvesting. Tissues from lumber (L4 and L5) spinal cord segments, divided into the ipsilateral and contralateral side as well as the dorsal and ventral horn, were dissected and rapidly frozen on dry ice and stored at −80°C for later use. Soluble proteins were prepared as follows. Frozen tissues were first homogenized in a homogenization buffer (59mM Tris–HCl, 0.1mM EDTA, 0.1mM EGTA, 1mM phenymethylsulfonyl fluoride, 1μM leupeptin, 2 μM pepstain A). The homogenate was centrifuged at 4°C for 10 min at 7,000×g. Protein concentration of the supernatants was measured using a microplate reader (Bio-TeK Instrument Inc. Winooski, VT). Supernatants (40 μg) were heated for 10min at 100°C and loaded onto 4% stacking/10% separating SDS-polyacrylamide gels for the protein separation. The protein was then electrophoretically transferred onto polyvinylidenedifluoride membrane (Millipore). The membrane was blocked with 3% non-fat dry milk solution and subsequently incubated overnight in a cold room with a primary antibody (Rabbit anti-rat NR1, 1:1000; 100kDa or Mouse anti-rat PKCγ, 1:500; 84kDa) with moderate shaking. A corresponding horseradish peroxidase -conjugated secondary antibody (Donkey anti-rabbit or mouse, 1:7,000; Amersham Biosciences, Arlington Heights, IL) and chemiluminescent solution (NEN) were used to visualize a blot, followed by exposing the blot onto hyperfilm (Amersham) for 1 to 10 min. Blots were then incubated in a stripping buffer (67.5mM Tris, pH 6.8, 2% SDS, and 0.7% b-mercaptoethanol) for 30min at room temperature and reprobed with a polyclonal rabbit anti-β-actin antibody (1:20,000; Alpha Diagnostic International, San Antonio, TX) as a loading control. Tissues from each rat were probed in triplicate. The density of each band was measured with Adobe Photoshop and normalized against each corresponding β-actin loading control.
5.7. Statistical data analysis
All data are expressed as mean and standard deviation (SD). By running software SPSS 16.0 for Windows (Chicago SPSS, Inc.), the raw data from behavioral tests of thermal hyperalgesia (hindpaw withdrawal latency in seconds) were used for repeated measures of one-way analysis of variance (ANOVA) to determine the differences between the group, day, and group×day in sham and burn-injured young rats. All multiple-comparison analyses were followed by post hoc Bonferroni’s correction if necessary. ANOVA was also used to examine differences across groups with regard to morphine-induced hyperactive behaviors as well as expression of NR1 and PKCγ. To examine the degree of morphine tolerance, the ED50 dose and 95% confidence intervals (95% CI) of morphine anti-nociception effect was obtained from the up-down reversals and by probit analysis. In each case, the statistical significance was set at P < 0.05.
Fig. 6. Upregulation of spinal NR1 and PKCγ expression in burn-injured young rats.
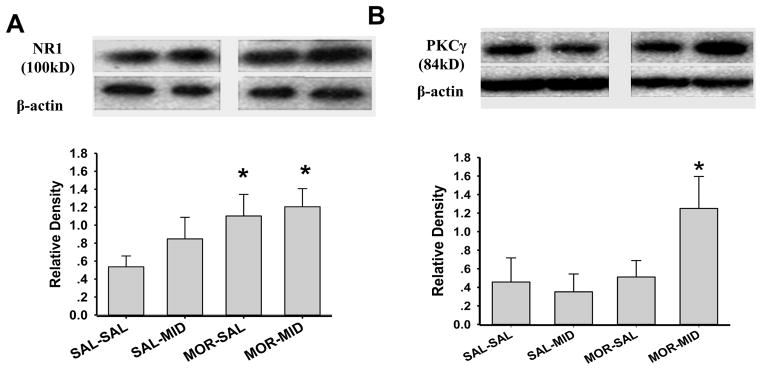
Expression (Western blot) of NR1 (A) and PKCγ (B) in the spinal cord dorsal horn was upregulated in burn-injured young rats (n=3) following 14 days of administration with morphine or a morphine and midazolam combination. Data are presented as mean ± SD. * P<0.05, as compared with the SAL-SAL group.
Highlights.
Midazolam reduces morphine analgesia;
Midazolam increases morphine-induced hyperactive behaviors;
Midazolam exacerbates morphine tolerance via spinal NMDA/PKC.
Acknowledgments
This work was supported by NIH grants DE18538, DE22901 and P20DA26002. We wish to thank the Chinese Society of Anesthesiology and the Department of Anesthesiology of West China Hospital for assistance and financial support.
Footnotes
Reprints will not be available from the authors.
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arturson G. Pathophysiology of burn wound and pharmacological treatment. The Rudi Hermans lecture. Burns. 1996;22:255–274. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Borse N, Sleet DA. CDC Childhood Injury Report: Patterns of Unintentional Injuries Among 0- to 19-Year Olds in the United States, 2000–2006. Fam Community Health. 2009;32:189. doi: 10.1097/01.FCH.0000347986.44810.59. [DOI] [PubMed] [Google Scholar]
- Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandín B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Anal. 2010;110:1328–1335. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Clavier N, Lombard MC, Besson JM. Benzodiazepines and pain: effects of midazolam on the activities of nociceptive non-specific dorsal horn neurons in the rat spinal cord. Pain. 1992;48:61–71. doi: 10.1016/0304-3959(92)90132-U. [DOI] [PubMed] [Google Scholar]
- Haefely W. Endogenous ligands of the benzodiazepine receptor. Pharmacopsychiatry. 1988;21:43–46. doi: 10.1055/s-2007-1014645. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Ito K, Yoshikawa M, Maeda M, Jin XL, Takahashi S, Matsuda M, Tamaki R, Kobayashi H, Suzuki T, Hashimoto A. Midazolam attenuates the antinociception induced by d-serine or morphine at the supraspinal level in rats. Eur J Pharmacol. 2008;586:139–144. doi: 10.1016/j.ejphar.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Carroll D, Glynn CJ, Moore RA, McQuay HJ. Morphine responsiveness of chronic pain: double-blind randomised crossover study with patient-controlled analgesia. Lancet. 1992;339:1367–1371. doi: 10.1016/0140-6736(92)91194-d. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Dickenso AH. Effects of midazolam in the spinal nerve ligation model of neuropathic pain in rats. Pain. 2000;85:425–431. doi: 10.1016/S0304-3959(99)00298-5. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Konings H, Adriaensen H, Gybels JM. Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain. Pain. 1991;47:5–12. doi: 10.1016/0304-3959(91)90004-H. [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Zeng Q, Sung B, Yang L, Mao J. Expression of spinal NMDA receptor and PKCgamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–11154. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lim G, Sung B, Wang S, Mao J. Intrathecal midazolam regulates spinal AMPA receptor expression and function after nerve injury in rats. Brain Res. 2006;1123:80–88. doi: 10.1016/j.brainres.2006.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza P, Parenti M, Tammiso R, Vita P, Zambotti F, Zonta N. Modification of the antinociceptive effect of morphine by centrally administered diazepam and midazolam. Brit J Pharmacol. 1982;75:569–572. doi: 10.1111/j.1476-5381.1982.tb09175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Mayer DJ, Hayes RL, Price DD. Spatial patterns of increased spinal cord membrane-bound protein kinase C and their relation to increases in 14C-2-deoxyglucose metabolic activity in rats with painful peripheral mononeuropathy. J Neurophysiol. 1993;70:470–481. doi: 10.1152/jn.1993.70.2.470. [DOI] [PubMed] [Google Scholar]
- Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann NY Acad Sci. 2001;933:175–184. doi: 10.1111/j.1749-6632.2001.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory aminoacid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: Implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995a;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Phillips LL, Lu J, Mayer DJ. Increases in protein kinase C gamma immunoreactivity in the spinal cord of rats associated with tolerance to the analgesic effects of morphine. Brain Res. 1995b;677:257–267. doi: 10.1016/0006-8993(95)00161-i. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. GABA receptors are cellular differences reflected in function? Brain Res. 1989;14:203–225. doi: 10.1016/0165-0173(89)90001-5. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci USA. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Price DD. The association of neuropathic pain, morphine tolerance and dependence, and the translocation of protein kinase C. NIDA Res Monogr. 1995a;147:269–298. [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Price DD. The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain. 1995b;61:365–374. doi: 10.1016/0304-3959(95)00023-L. [DOI] [PubMed] [Google Scholar]
- Niv D, Davidovich S, Geller E, Urca G. Analgesic and hyperalgesic effects of midazolam: dependence on route of administration. Anesth Anal. 1988;67:1169–1173. [PubMed] [Google Scholar]
- Portenoy RK, Foley KM. Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990;43:73–86. doi: 10.1016/0304-3959(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Portenoy RK. Tolerance to opioid analgesics: clinical aspects. Cancer Surv. 1994;1:49–65. [PubMed] [Google Scholar]
- Rattan AK, McDonald JS, Tejwani GA. A Differential effects of intrathecal midazolam on morphine-induced antinociception in the rat: role of spinal opioid receptors. Anesth Anal. 1991;73:124–131. doi: 10.1213/00000539-199108000-00004. [DOI] [PubMed] [Google Scholar]
- Rattan AK, Tejwani GA. Effect of chronic treatment with morphine, midazolam, and both together on beta-endorphin levels in the rat. Brain Res Bull. 1996;41:335–341. doi: 10.1016/s0361-9230(96)00022-6. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, MacDonald JF. Ethanol modulation of GABA receptor- activated Cl- currents in neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol. 1992;224:173–181. doi: 10.1016/0014-2999(92)90802-b. [DOI] [PubMed] [Google Scholar]
- Richards JG, Schoch P, Möhler H, Haefely W. Benzodiazepine receptors resolved. Experientia. 1986;42:121–126. doi: 10.1007/BF01952428. [DOI] [PubMed] [Google Scholar]
- Shih A, Miletic V, Miletic G, Smith LJ. Midazolam administration reverses thermal hyperalgesia and prevents gamma-aminobutyric acid transporter loss in a rodent model of neuropathic pain. Anesth Anal. 2008;106:1296–1302. doi: 10.1213/ane.0b013e318164f1e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman HM, Mélot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth. 2001;87:186–192. doi: 10.1093/bja/87.2.186. [DOI] [PubMed] [Google Scholar]
- Stoddard FJ, Saxe G, Ronfeldt H, Drake JE, Burns J, Edgren C, Sheridan R. Acute stress symptoms in young children with burns. J Am Acad Child Adolesc Psychiatry. 2006;45:87–93. doi: 10.1097/01.chi.0000184934.71917.3a. [DOI] [PubMed] [Google Scholar]
- Tatsuo MA, Salgado JV, Yokoro CM, Duarte ID, Francischi JN. Midazolam-induced hyperalgesia in rats: modulation via GABA(A) receptors at supraspinal level. Eur J Pharmacol. 1999;370:9–15. doi: 10.1016/s0014-2999(99)00096-5. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Yang L, Zen Q, Sung B, Jeevendra Martyn JA, Mao J. A rat model of unilateral hindpaw burn injury: slowly developing rightwards shift of the morphine dose-response curve. Pain. 2005;116:87–95. doi: 10.1016/j.pain.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang L, Ma Y, Chen L, Tian Y, Mao J, Martyn JJ. Nociceptive behavior following hindpaw burn injury in young rats: response to systemic morphine. Pain Med. 2011;12:87–98. doi: 10.1111/j.1526-4637.2010.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez A, Sabbe MB, Stevens CW, Yaksh TL. Interaction of midazolam and morphine in the spinal cord of the rat. Neuropharmacology. 1990;29:359–364. doi: 10.1016/0028-3908(90)90094-8. [DOI] [PubMed] [Google Scholar]