Abstract
Cell cycle regulators, such as cyclin-dependent kinases (CDKs), are appealing targets for multiple myeloma (MM) therapy given the increased proliferative rates of tumor cells in advanced vs. early stages of MM. We hypothesized that a multi-targeted CDK inhibitor with a different spectrum of activity compared to existing CDK inhibitors could trigger distinct molecular sequelae with therapeutic implications for MM. We therefore studied the small molecule heterocyclic compound NVP-LCQ195/AT9311 (LCQ195), which inhibits CDK1, CDK2 and CDK5, as well as CDK3 and CDK9. LCQ195 induced cell cycle arrest and eventual apoptotic cell death of MM cells, even at sub-μM concentrations; spared non-malignant cells; and overcome the protection conferred to MM cells by stroma or cytokines of the bone marrow milieu. In MM cells, LCQ195 triggered decreased amplitude of transcriptional signatures associated with oncogenesis, drug resistance, and stem cell renewal, including signatures of activation of key transcription factors for MM cells e.g. myc, HIF-1α, IRF4. Bortezomib-treated MM patients whose tumors had high baseline expression of genes suppressed by LCQ195 had significantly shorter progression-free and overall survival than those with low levels of these transcripts in their MM cells. These observations provide insight into the biological relevance of multi-targeted CDK inhibition in MM.
Keywords: myeloma, cancer, CDK, microenvironment, kinase inhibitor, cyclins
Introduction
Despite the clinical development of bortezomib (Richardson, et al 2003, Richardson, et al 2005), thalidomide (Thal) (Attal, et al 2006, Singhal, et al 1999) and lenalidomide (Len) (Dimopoulos, et al 2005, Hideshima, et al 2000, Richardson, et al 2006, Richardson, et al 2002, Weber, et al 2006), multiple myeloma (MM) remains incurable and identification of additional therapeutic agents is necessary. Cyclin-dependent kinases (CDKs) have been proposed as therapeutic targets for this disease because MM cells express high levels of at least one of the D-type cyclins (cyclin-D1, D2, or D3), which are key components of the CDK signaling pathway regulating cell proliferation (Bergsagel and Kuehl 2005, Bergsagel, et al 2005, Hideshima, et al 2004). Furthermore, while proliferative rates of MM cells are low in early stage of the disease, they are increased when the disease becomes resistant to various conventional or novel anti-MM agents, as evidenced both by high plasma cell labeling indices (PCLIs), which reflect cells in S-phase, and gene expression-based proliferative indices (Barlogie, et al 2001, Garcia-Sanz, et al 2004, Rajkumar, et al 2001, Rajkumar, et al 2000).
Small molecular weight CDK inhibitors, including flavopiridol (Gojo, et al 2002, Semenov, et al 2002), seliciclib (MacCallum, et al 2005, Raje, et al 2005) and SNS-032, exhibit preclinical anti-tumor activity in diverse neoplasias, including MM (as reviewed in (Shapiro 2006)), different patterns of inhibition of CDKs (e.g. seleciclib inhibits CDK1 and 2, while SNS-032 inhibits CDK2, 7 and 9). This led us to hypothesize that a multi-targeted CDK inhibitor with a different profile of kinase inhibitory activity compared to existing CDK inhibitors could trigger a distinct pattern of molecular sequelae in MM cells, which could in turn provide insight into the biology of MM with distinct therapeutic applications.
To address this hypothesis, we studied the small molecule achiral heterocyclic kinase inhibitor NVP-LCQ195/AT9311 (LCQ195), which inhibits CDK1, CDK2, CDK5, as well as CDK9 and CDK3, but is much less active against CDK7 or CDK6. LCQ195 triggered cell cycle arrest and eventual apoptotic cell death of MM cells, even at sub-μM concentrations, while sparing non-malignant cells and overcoming the protective effects conferred to MM cells by stromal cells or major cytokines of the bone marrow milieu. Importantly, LCQ195 triggered a distinct pattern of molecular sequelae hallmarked by decrease in amplitude of various transcriptional signatures associated with activation of key transcription factors for MM cells (e.g. myc, HIF-1α, IRF4). We also observed subsequent decreases in signatures of other molecular pathways associated with oncogenesis, drug resistance, and biological aggressiveness of tumor cells. Bortezomib-treated MM patients whose tumors had high baseline expression of genes suppressed by LCQ195 had significantly shorter progression-free and overall survival than those patients whose tumors had low levels of these transcripts. Taken together, our observations indicate that compounds with different patterns of inhibition of individual CDKs can induce distinct networks of transcriptional changes in MM cells. The correlation of these changes with clinical outcome provides insight into the biological relevance of multi-targeted CDK inhibition. Moreover, analysis of treatment-induced changes in expression profiles of tumor cells may represent a valuable strategy to address the currently intractable problem of dissecting the significance of effects triggered by a multi-targeted inhibitor.
Material and Methods
Cell lines
We evaluated a panel of human MM cell lines (Delta 47, Dox6, Dox40, EJM, JJN3, Karpas 620, KMS-12-BM, KMS-12-PE, KMS-28-BM, L363, LP-1, LR5, MM144, MM.1R, MM.1S, MR20, NCI-H929, NOP2, OCI-MY1, OCI-MY5, OPM-1, OPM-2, RPMI-8226/S, S6B45, SKMM1, U266, UTMC2, XG1), as well as the human immortalized bone marrow stromal cell (BMSC) line HS-5 and the immortalized hepatocyte cell line THLE-3. Cells were grown in Roswell Park Memorial Institute medium (RPMI)-1640 (BioWhittaker) with 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal calf serum (FCS) (GIBCO/BRL, Gaithersburg, MD), unless stated otherwise.
Compound
LCQ195 was discovered by Astex Therapeutics and emerged from collaboration between Astex and Novartis. Compound was provided by Novartis (Basel, Switzerland) and referenced in Patent # WO/2005/012256 and resuspended in DMSO at 10 mM and used at the concentrations indicated.
Cell viability assessment
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT; Chemicon International, Temecula, CA) colorimetric survival assay, as previously published (Mitsiades, et al 2001) or CellTiterGlo (CTG; drug combination studies). In brief, MM cell lines were plated in 48-well plates at a density of 20,000 cells/well. LCQ195 was added at the concentrations indicated and compared to vehicle treated controls. Cultures were then incubated for 48 hrs in a 37°C incubator with 5% CO2. MTT was added to each well for the final 4 hours of treatment, followed by addition of isopropanol containing 0.04 N HCl to dissolve the crystals (at volume 1.5-3-fold of the total culture volume). Optical absorbance of the culture medium was then measured at 570/630 nm using a spectrophotometer (Molecular Devices Corp., Sunnyvale CA). Recombinant human IL-6 and IGF (R&D Systems; Minneapolis, MN) were used at 10 ng/mL and 50 ng/mL, respectively, in cultures of MM.1S cells as indicated.
Peripheral blood samples from healthy donors were collected using heparinized tubes and processed by Ficoll (Amersham Biosciences, Piscataway, NJ)-density gradient centrifugation to isolate peripheral blood mononuclear cells (PBMCs), which were plated at 150,000 cells per well and pre-stimulated with 5 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich, St Louis, MO) for 24 hrs. LCQ195 was administered at the doses indicated in the respective experiments for 48 hrs, followed by the addition of MTT for the final 4 hrs of treatment and measurement as described above. Following consent, myeloma patient samples were collected, Ficoll gradient separated, and selected using CD138 micro-beads (Miltenyi Biotec; Bergisch Gladbach, Germany). Cells were cultured in Roswell Park Memorial Institute medium (RPMI)-1640 (BioWhittaker) with 100 U/ml penicillin, 100 μg/ml streptomycin and 20% fetal calf serum (FCS) (GIBCO/BRL) and treated as indicated in the figures. Viability was measured by CTG assay, as described above.
Cell death commitment assay
The minimum exposure of MM cells to LCQ195 that is required to commit them to death was evaluated by incubating cells in 24-well plates with LCQ195 (2 μM) for 1-24 hrs. Following incubation, the cells were washed with drug-free medium to remove any residual drug, and then incubated in drug-free medium for an additional 3 days, resulting in equal length of incubation for all experimental conditions. MM cell survival was quantified by MTT and expressed as percentage of the value obtained from respective controls.
Stromal cell co-culture
The luciferase (luc)-expressing cell line MM.1S-GFP/luc was generated by retroviral transduction with the pGC-gfp/luc vector (kind gift of C.G. Fathman, Stanford University) and used for co-culture experiments with the luc-negative human stromal line HS-5, as described previously (McMillin, et al 2010). Briefly, HS-5 stromal cells were plated in 96-well plates and allowed to attach overnight. MM cells were then plated and treated with LCQ195 for 48hrs at the doses indicated. Following incubation, luciferin substrate (Xenogen Corp, Alameda, CA) was added to the culture and the resulting bioluminescence signal was measured using a Luminoskan luminometer (Labsystems, Franklin, MA).
Flow cytometric analysis of cell cycle and apoptosis
The MM cell line MM.1S was treated with 2 μM LCQ195 for 0-48 hrs. For cell cycle analyses, cells were stained using a solution of propidium iodide (Sigma) and RNase A (Sigma) following 70% EtOH fixation. For evaluation of apoptosis, cells were processed using an Annexin V / PI kit (Becton Dickinson Biosciences, San Jose, CA). For both assays, cells were passed through a flow cytometer (Beckman Coulter) and analyzed using FlowJo analysis software (Treestar; Ashland, OR).
Cytochrome C release Assay
According to the InnoCyte Cytochrome C release assay instructions (Calbiochem; San Diego, CA), MM.1S cell were plated and exposed to 2 μM LCQ195 for up to 16 hrs. Cells were collected, washed in PBS and resuspended in Permeabilization Buffer. Cells were then fixed with 2% Paraformaldehyde, washed and then stained with cytochrome C specific antibody that remains in the mitochondria. Data was acquired using a Canto flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Tree Star Inc.; Ashland, OR).
In vitro kinase activity assay
Kinases involved in cell cycle were screened using the Invitrogen Z’-LYTE™ technology. Selected kinases were in the presence of 500 nM of LCQ195 (in 1% DMSO) and 100 μM ATP. The Z’-LYTE system first transfers the gamma-phosphate of ATP to a single tyrosine, serine or threonine residue in a synthetic Fluorescence Resonance Energy Transfer (FRET)-peptide. In the secondary reaction, a site-specific protease recognizes and cleaves non-phosphorylated FRET-peptides, whereas phosphorylated FRET-peptides are not cleaved. The uncleaved phosporylated FRET-peptides maintain the interaction between the donor (coumarin) and acceptor (fluorescein) fluorophores on the FRET-peptide and the ratio of donor emission to acceptor emission after excitation of the donor fluorophore at 400 nm is used to quantitate the reaction.
Immunoblotting analysis
For immunoblotting analyses, MM.1S cells (10×106 cells per condition) were plated in RPMI-1640 medium with 10%FBS, penicillin, and streptomycin as previously described. LCQ195 was added at a concentration of 2 μM for 0-24 hrs. Cell pellets were collected and treated with Triton X-100 lysis buffer containing 1 × PBS, Triton X-100 (1% v/v), sodium deoxycholate (0.5% w/v), SDS (0.1% w/v), EDTA (1 mmol/L), 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 Ag/mL aprotinin, 5 Ag/mL leupeptin, and 5 Ag/mL pepstatin A. The samples were cleared by centrifugation (14,000 rpm, 30 min, 4°C) and assessed for protein concentration by Bradford assay (Sigma). SDS-polyacrylamide gel electrophoresis (12%) was performed (30-50 μg of protein per lane), and proteins were electroblotted onto PVDF membranes. After 1 hr incubation in blocking solution (5% milk in TBS-T buffer), membranes were exposed to primary antibody overnight at 4°C. Following washing in TBS-T, the respective secondary horseradish peroxidase (HRP)-labeled antibody was added at 1:20,000 dilution for 1 hr at room temperature. The membrane was then washed with TBS-T for 45-60 min with multiple changes of the wash buffer, and the protein expression was visualized using the ECL technique. The primary antibodies used for immunoblotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), Upstate Biotechnologies (Lake Placid, NY) or Cell Signaling (Beverly, MA). Secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA).
Gene expression profiling of cells treated with LCQ195
Total RNA extraction and purification, cDNA synthesis and cRNA labeling, Affymetrix chip (human HT-U133A and HT-U133B) hybridization and data analysis were performed as previously described (Mitsiades, et al 2004a, Mitsiades, et al 2004b, Mitsiades, et al 2002). Briefly, MM.1S cells were treated with 2 μM LCQ195 or with DMSO as a control for 2, 4, 8, 16, and 24 hrs; total RNA was then extracted and purified, cDNA synthesized and cRNA labeled prior to hybridization to the HT-U133 A and B Arrays. Pathway activation status analysis is detailed in the supplement.
Results
In vitro kinase activity assays of LCQ195
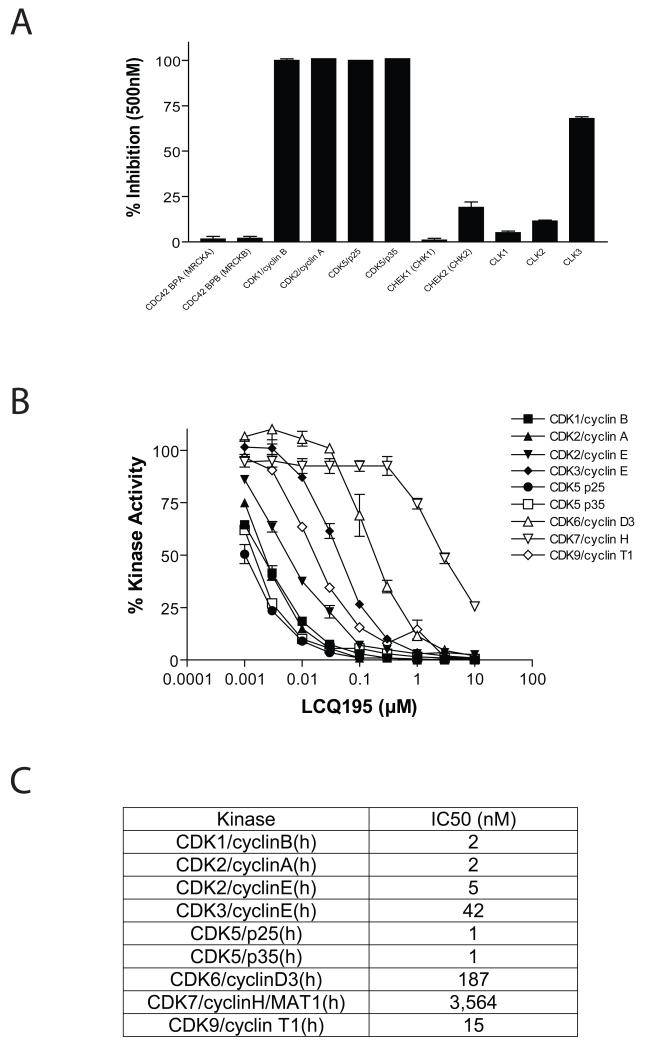
In vitro kinase activity assays were performed for cell cycle relevant kinases to evaluate the pattern of CDKs inhibited by LCQ195. Screening was performed using 500 nM of LCQ195 and 100uM ATP in the Invitrogen Z’-LYTE™ kinase assay platform. LCQ195 blocks the activity of CDK1/cyclin B and CDK2/cyclin A, as well as CDK5 (both CDK5 p25 and CDK5 p35) and modestly inhibits CLK3 and CHEK2 (CHK2) (Fig. 1A). In a second kinase screening platform we validated the inhibition of CDK1/cyclin B, CDK2/cyclin A, CDK2/cyclin E, CDK5 p25 and CDK5 p35. In addition, we show that LCQ195 is active, although with higher IC50 values, against CDK3/cyclin E, CDK9/cyclin T1, CDK7/cyclin H and CDK6/cyclin D3 (Fig. 1B, C). These results confirm the multi-targeted nature of LCQ195, which is distinct from other reported CDK inhibitors (Conroy, et al 2009, Gojo, et al 2002, MacCallum, et al 2005, Raje, et al 2005, Semenov, et al 2002)
Figure 1. In vitro kinase activity in the presence of LCQ195.
Cell cycle-related kinases were screened for their activity in the presence of 500 nM of LCQ195 (in 1% DMSO) and 100 μM ATP using the Invitrogen Z’-LYTE™ kinase assay platform. LCQ195 causes potent inhibition of CDK1/cyclin B, CDK2/cyclin A, CDK5/p25, CDK5/p35 and partial inhibition of CLK3 and CHEK2. Experiments were performed in duplicate, with averages shown (A). In addition, using Millipore’s KinaseProfilerXL platform, the in vitro kinase activities of various CDKs were profiled in the presence of a 5-log dose titration of LCQ195 (0.001-10μM). Drug was active at sub-μM concentrations against CDK1/cyclin B, CDK2/cyclin A, CDK2/cyclin E, CDK3/cyclin E, CDK5/p25, CDK5/p35, CDK6/cyclin D3, CDK9/cyclin T1 (B) and IC50 values were calculated for each kinase (C).
Selective in vitro activity of LCQ195 against MM cells vs. non-malignant cells
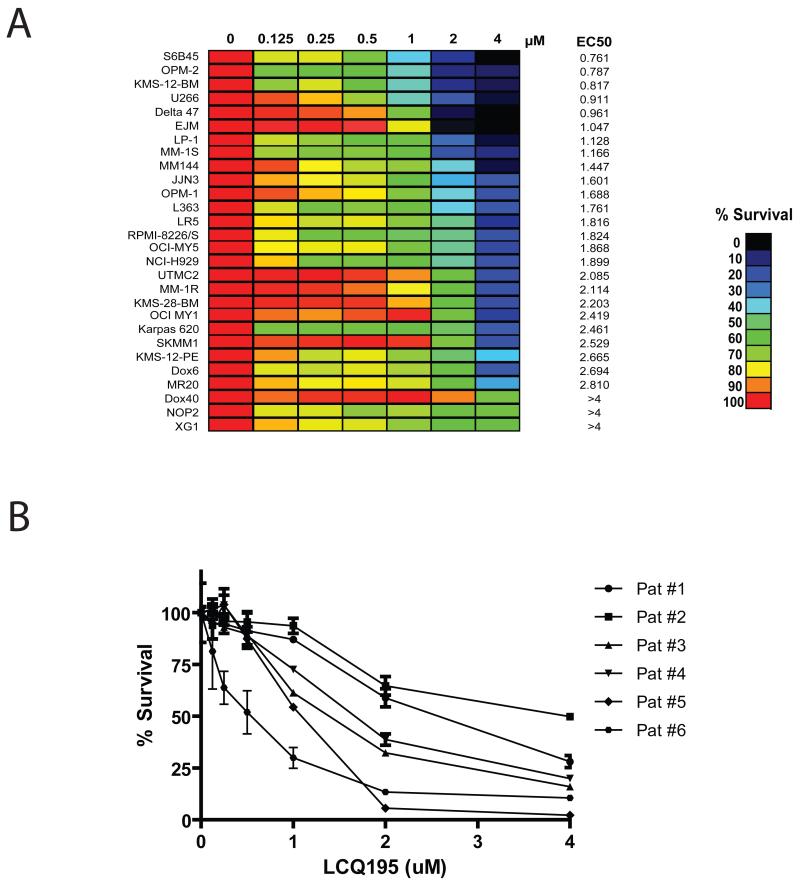
We treated a panel of MM cell lines with LCQ195 (0-4 μM) for 48 hrs and evaluated viability using MTT colorimetric survival assays. The majority of cell lines had EC50 values ~1 μM (Fig. 2A). The most sensitive cells lines, such as S6B45, OPM2, KMS-12-BM, and U266, exhibited EC50 values <1 μM. In addition, purified primary tumor cells from MM patients, including patients with advanced MM, responded to LCQ195 treatment in vitro (Fig. 2B).
Figure 2. Activity of LCQ195 on MM cells.
MM cell lines were treated with LCQ195 (0-4 μM, for 48 hrs) and viability was assessed by MTT assay. The most sensitive cell lines (S6B45, OPM2, KMS-12-BM and Delta 47) have EC50 values < 1 μM, whereas resistant MM cell lines (Dox40, NOP2, XG-1) have EC50 values > 4 μM (A). Bone marrow aspirates from MM patients were processed using Miltenyi anti-CD138 microbeads for purification of MM cells, which were then cultured in RPMI1640 media containing 20% serum. CD138+ selected cells were treated with increasing doses of LCQ195 for 48 hrs and viability was assessed by CellTiterGlo (B).
In addition, cell death commitment assays were performed on OPM-2, MM.1S, LR5 and Dox40 cells by exposing them to LCQ195 (0.5 μM) for 2-24 hrs, followed by drug washout and incubation in drug-free medium for an additional 72 hrs. LCQ195 treatment for <24 hrs resulted in commitment to death in the LCQ195-sensitive lines OPM-2 and MM.1S (Suppl. Fig. 1), suggesting that molecular events initiating cell death can occur as early as 8-16 hrs of LCQ195 treatment.
We next evaluated the impact of LCQ195 on PBMCs, stromal cells and the immortalized hepatocyte cell line THLE-3. Non-malignant HS-5 BMSCs and immortalized hepatocytes were less sensitive to LCQ195 than the majority of MM cell lines (Suppl. Fig 2A), with calculated EC50 values >4 μM. PBMCs from healthy donors were PHA-stimulated to induce their cycling and proliferation, which resulted in a 1.8-2.2 fold increase in the number of viable cells following 48 hrs of culture (Suppl. Fig 2B). Moreover, the percentage of viable PBMCs was ≥ 50% following LCQ195 treatment (up to 8 μM for 48 hrs, Suppl. Fig 2C). Of note, although sensitivity of PBMCs to LCQ195 was increased as a result of PHA-stimulation it remained less than the majority of MM cell lines.
LCQ195 overcomes cytokine- and stromal-derived protection of MM cells
MM.1S cells were cultured in the presence of IL-6 (10 ng/mL), IGF-1 (50ng/mL) and HS-5 stromal cells for 72hrs, which triggered increased cell proliferation (Suppl. Fig 3A). Under these same conditions, MM.1S cells were treated with LCQ195 and cell viability measured at 72hrs. Cell viability was normalized to each respective drug-free control and was similar at all drug concentrations irrespective of IL-6 (Suppl. Fig 3B), IGF-1 (Suppl. Fig 3C) or HS-5 (Suppl. Fig 3D) stimulation, indicating that LCQ195 can overcome the protective effects of bone microenvironmental factors.
Cell cycle and apoptotic features of LCQ195-treated MM cells
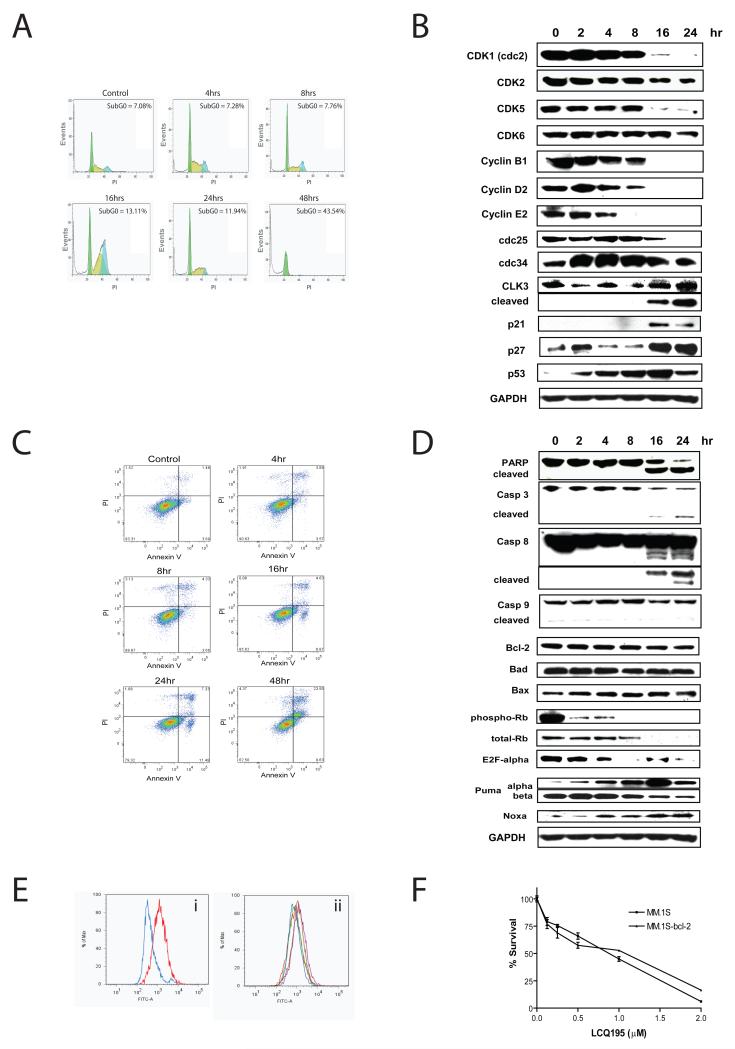
The cell cycle profile of LCQ195-treated MM cell lines was next evaluated by flow cytometric analysis. Treatment of MM.1S cells with LCQ195 (2 μM for up to 48 hours) increased the percentage of cells in S and G2/M phases early, followed by an increase in sub-G1 population (Fig. 3A). This dimorphic pattern may be due to early CDK1 and CDK2 regulating cyclic progression through S phase, with longer treatment inducing apoptosis. In addition, immunoblotting revealed decreasing CDK2, CDK5, cyclin B1, cyclin D2, cyclin E2 and cdc25 with cleavage of CLK3 as early as 8 hrs, indicating rapid changes in cell cycle regulators (Fig. 3B).
Figure 3. LCQ195 cause cell cycle arrest and cell death.
MM.1S cells were exposed to 2 μM LCQ195 for 4-48 hrs and their cycle profiles were compared, using propidium iodide staining, to the control cells cultured for 48 hrs in the absence of drug. LCQ195 blocked cell cycle progression in S-phase (light green) and G2/M (blue) before inducing cell death, evidenced by increased sub-G0 (white) events at later time points (A). In addition, immunoblotting analysis revealed decreases in proteins involved in cell cycle checkpoints including CDK1, CDK5, cdc25 and cyclin D2. In addition, CLK3 was cleaved, while p53, p21, and p27 levels increased with longer duration of treatment (B). MM.1S cells were exposed to LCQ195 (2 μM, for 4-48 hrs), collected, washed, stained for Annexin V and propidium iodide (PI), and analyzed using flow cytometry. Exposure to LCQ195 resulted in a time-dependent increase in the fraction of Annexin V+ events, with an increase in the number AnnexinV+PI− events at 16-24hrs indicating apoptotic cell death of MM.1S cells. The increase in AnnexinV+PI+ dead cells at 48hrs is compatible with the late stages of apoptotic cell death (C). Immunoblotting analysis of MM.1S cells treated with 2 μM LCQ195 revealed cleavage of caspase 3, caspase 8 and PARP, but not of caspase 9. Rb phosphorylation was decreased at early time points, followed by decreases in total Rb and E2F-α at later time points. In addition, p53 downstream targets, Puma (alpha) and Noxa, are increased in response to LCQ195 treatment but no changes in Bcl-2, Bad and Bax protein levels were observed up to 24hrs (D). LCQ195-treated MM.1S cells were evaluated for cytochrome c release by flow cytometric analysis. The positive control, MM.1S cells treated with PS-341 (50 nM for 12 hrs), showed significant release of cytochrome C in the cytoplasm (E; i), whereas no changes were observed in MM.1S cells treated with 2 μM of LCQ195 for 4-24hrs (E; ii). LCQ195 sensitivity (0–2 μM, 72hrs) was similar in the Bcl-2 over-expressing MM.1S and their parental cell line (F).
Annexin V / PI staining and flow cytometric analysis revealed that LCQ195 induces apoptosis, evidenced by increased % of Annexin V+/ PI− cells, followed by increased % of secondary necrosis evidenced by increased % of Annexin V+ / PI+ cells (Fig. 3C). In addition, immunoblotting revealed caspase 8, caspase 3 and PARP cleavage as early as 16 hrs, but no cleavage of caspase 9. A pronounced decrease in Rb phosphorylation is noted as early as 2 hours (Fig. 3D) and is associated with a significant decrease in levels of other cell cycle regulators. For example, we observed decreased levels of the E2F1, a key downstream target of Rb, D-type cyclins (e.g. cyclin D1); and Cyclin E2 (Fig. 3D). More detailed analysis of the p53 pathway and its downstream targets revealed increased levels of Puma (alpha) and Noxa (Fig. 3D). No changes in Bcl-2, Bad and Bax were observed in response to LCQ195 treatment (Fig. 3D). Consistent with these data, release of cytochrome C was not observed in MM.1S cells exposed to LCQ195 for up to 16 hrs (Fig. 3E). In addition, overexpression of bcl-2 in MM.1S cells had minimal effect on cell survival after LCQ195 treatment for 48 hrs (Fig. 3F). These data suggest that the anti-MM activity of LCQ195 is independent of mitochondrial mechanisms of apoptosis.
Importantly, these molecular events triggered by LCQ195 do not appear to be mere consequences of cell cycle arrest, For example, MM.1S cells were exposed to equipotent doses of LCQ195 (1 μM) and 2 other agents which cause cell cycle arrest through inhibition of CDKs (flavopiridol at 50 nM), or non-CDK dependent mechanisms (the microtubule stabilizing agent Taxol at 100 nM) for 4-24 hrs (Suppl. Fig. 4). Several molecular targets analyzed by immunoblotting exhibited a distinct pattern of changes with LCQ195 compared to flavopiridol and Taxol. For instance, Mcl-1 remained stable in LCQ195 and Taxol treated cells but decreased in flavopiridol treated cells. In contrast, p21 levels increased in LCQ195 treated cells, was stable in Taxol treated cells and decreased in flavopiridol treated cells. Phosphorylation of Rb and p107 levels decreased in response to LCQ195 treatment was stable in response to flavopiridol treatment but phosphorylation of Rb increased in Taxol treated cells.
LCQ195 combinations with other anti-MM agents
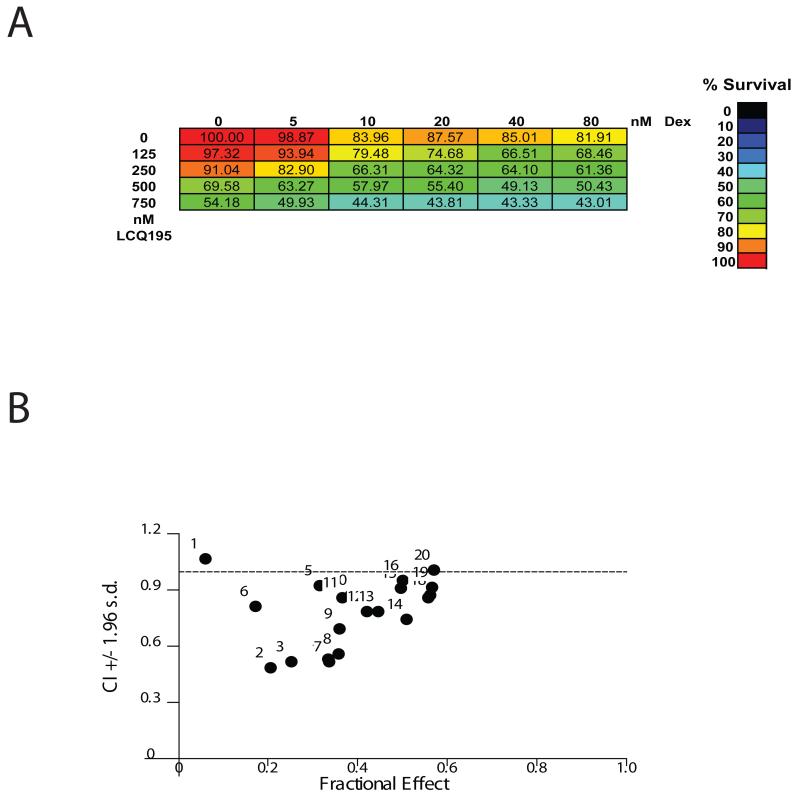
We next evaluated combinations of LCQ195 with agents used for clinical management of MM (Dex, bortezomib, doxorubicin). Although no synergy was observed between LCQ195 and bortezomib and doxorubicin, there also was no antagonism observed (data not shown), suggesting that LCQ195 does not adversely impact the anti-MM activity of these approved agents. We did observe enhanced killing with dexamethasone and LCQ195 at various drug doses (Fig. 4A), with combination indices (CI) <1 for the majority of conditions (Fig. 4B).
Figure 4. LCQ195 enhances the anti-MM activity of dexamethasone in vitro.
MM.1S cells were plated in 384-well format and treated with increasing doses of LCQ195 for 72 hrs, with increasing doses of Dex added during the final 48 hrs. Cell viability was then assessed using the CellTiterGlo (CTG) assay (A). Cell viability was normalized to non-treated controls, visualized in a color coded format and compared to treatment with each drug alone (A). Isobologram analysis (Calcusyn software) was performed to calculate the combination index (CI) for each LCQ195/Dex combination (B). The x-axis corresponds to the fractional effect at various combination doses and the y-axis represents the CI for each data point in which CI values <1.0 indicate drug synergy. The majority of combinations tested had CI values <1.0, indicating that LCQ195 synergizes with Dex at multiple doses of either compound.
Cell cycle-related and oncogenic transcripts down-regulated in response to LCQ195
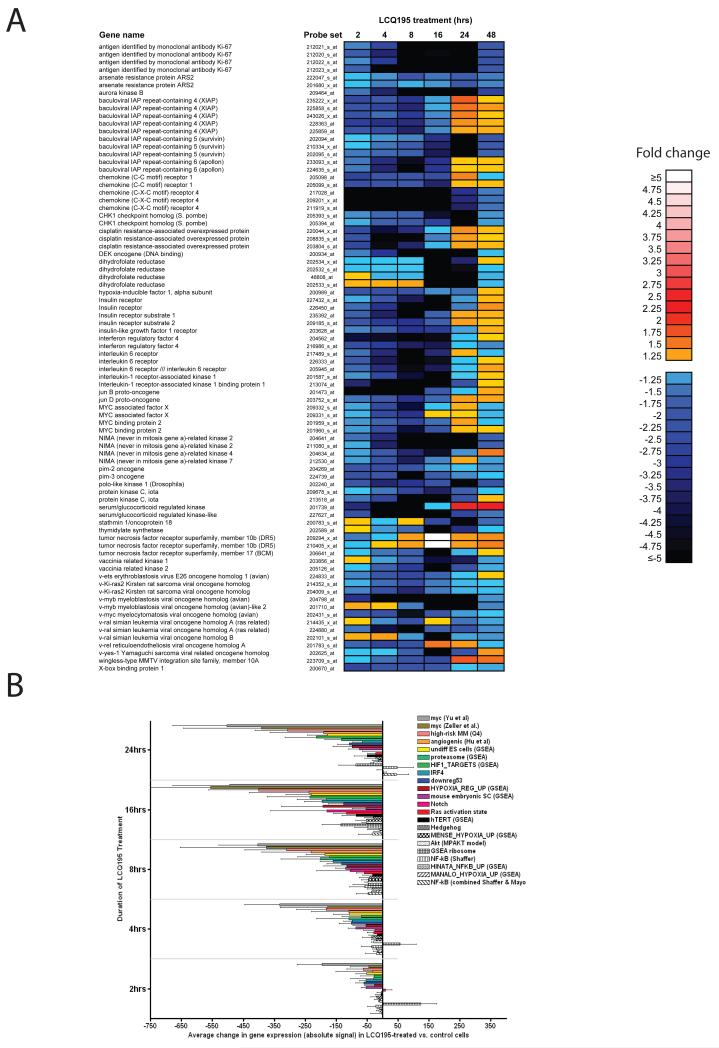
We evaluated the transcriptional profiles of LCQ195-treated MM1.S cells. As anticipated by its effects on CDK function, it modulates the expression of a broad range of cell cycle-related transcripts (Suppl. Fig. 5). We also observed that LCQ195 significantly suppressed the expression of transcripts known to promote oncogenesis, drug resistance and tumor cell protection from apoptosis in MM and/or other neoplasias. For example, LCQ195 treatment suppressed the expression of transcription factors with key regulatory roles on MM cell behavior, including myc, IRF4 and XBP-1 (Carrasco, et al 2005, Lee, et al 2003, Shaffer, et al 2008, Yu, et al 2005, Zeller, et al 2003) (Fig. 5A). Furthermore, LCQ915 suppressed the transcript levels of genes with roles in drug resistance, hypoxia response (e.g. HIF-1a); or genes involved in growth factor/cytokine signaling (Fig. 5A). LCQ195 also suppressed the expression of anti-apoptotic transcripts (e.g. XIAP, survivin, apollon) and induced pro-apoptotic transcripts (e.g. TRAIL receptor DR5) (Fig. 5A).
Figure 5. Treatment of MM.1S cells with LCQ195 leads to distinct changes in transcriptional signatures of activation of key molecular pathways in tumor cell pathophysiology.
MM.1S cells were cultured in the absence or presence of LCQ195 (2-24hrs, 2 μM) and gene expression profiling analysis was performed using Affymetrix HT-U133 A&B oligonucleotide microarrays. The fold change in transcripts with previous known functions in oncogenesis, growth factor/cytokine signaling, drug resistance and regulation of apoptosis in MM and/or other neoplasias is visually represented according to a linear color scale (as indicated in panel A). Transcripts previously shown to reflect the activation state of the indicated pathways were evaluated for differences in expression in the presence vs. absence of LCQ195 treatment (2 μM) at each of the indicated time-points. The graph presents, for each transcriptional signature and each time-point, the average value (+/− 95% confidence limit) of the difference in absolute expression signal in MM.1S cells cultured in the presence vs. absence of LCQ195 (B).
Pathway activation status analysis of MM cells treated with LCQ195
We evaluated LCQ195-induced changes in transcripts previously shown to reflect the activation state of specific pathways pertinent to tumor cell biology, including cell proliferation, survival and drug resistance. We observed that LCQ195 treatment causes decreased amplitude in various transcriptional signatures indicative of the activity of transcription factors (e.g. myc, HIF-1α, IRF4, and NF-κB), signaling cascades (e.q. Notch, Ras, Akt, and Hedgehog), the ubiquitin/proteasome and ribosomal pathways (Fig. 5B). Importantly, we observed decreases in many signatures previously reported in the literature which could be important in MM tumor cell function, including cancer stem cell self-renewal, hTERT overexpression, p53 function. angiogenic state, response to hypoxia (Hu, et al 2005, Leonard, et al 2003, Manalo, et al 2005, Mense, et al 2006, Semenza 2001, Shaughnessy, et al 2007). These molecular events were more pronounced at later time points (16 and 24 hrs), but prominent suppression of transcriptional signatures for myc activity, high-risk MM, angiogenic state, undifferentiated embryonic stem cells, and proteasome pathway were observed at earlier time points (Fig. 5B).
Molecular signature of LCQ195 treatment correlates with clinical outcome in MM patients
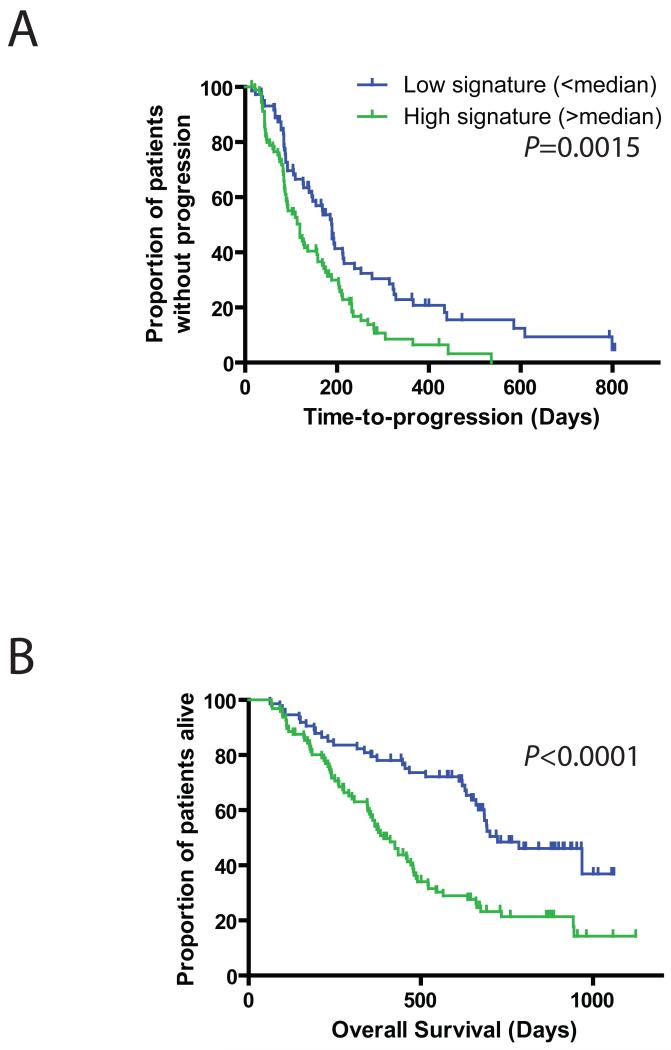
The observation that LCQ195 suppresses the transcriptional signatures for activation of pathways with prominent roles in the biological aggressiveness of MM, such as myc, IRF4, or the transcriptional signature for high-risk MM, suggested that LCQ195 treatment induces a transcriptional response that includes genes which may correlate with clinical outcome. To evaluate this hypothesis, we identified a transcriptional index of early (2hrs of drug exposure) MM.1S cell response to LCQ195. As described in Supplemental Information, we applied this index to gene expression data of bortezomib-treated MM patients with advanced disease who had enrolled in the SUMMIT and APEX clinical trials (Richardson, et al 2005). Bortezomib-treated patients with high expression of genes suppressed by LCQ195 had significantly shorter progression-free and overall survival than those with low levels of these transcripts (p=0.0015 and p<0.0001, respectively log-rank test, Fig. 6).
Figure 6. LCQ195 treatment induces a transcriptional response of genes which correlate with clinical outcome.
We identified a transcriptional index of early (2hrs of drug exposure) MM.1S gene responses to LCQ195 (described in Supplemental Information). This index was applied to gene expression data of bortezomib-treated MM patients with advanced disease in the SUMMIT and APEX clinical trial trials of bortezomib. We observed that the bortezomib-treated patients with high expression of genes suppressed by LCQ195 had significantly shorter progression-free (A; p=0.0015; log-rank test) and overall survival (B; p<0.0001; log-rank test) than those with low levels of these transcripts.
Discussion
CDKs are legitimate targets for therapeutic intervention against different cancers, including MM, and several CDKs inhibitors are currently being evaluated pre-clinically and clinically. In this study we demonstrate that LCQ195 potently inhibits CDK 1, 2 and 5 activity and also modestly targets CDK3 and 9. This profile of activity is distinct from other CDK inhibitors studied in MM, including the broad-spectrum CDK inhibitor flavopiridol (Gojo, et al 2002, Semenov, et al 2002), the potent CDK7 and 9 inhibitor seliciclib (MacCallum, et al 2005, Raje, et al 2005) and the multi-targeted CDK inhibitor AT7519 (Wyatt, et al 2008). These distinct patterns of inhibition of CDKs correlate with different patterns of activity against MM cells. For example, EC50 values with seliciclib for most MM cell lines are >10 μM (MacCallum, et al 2005, Raje, et al 2005) compared to ~1 μM or lower for similar cell lines treated with LCQ195. In addition, flavopiridol has molecular effects distinct from these other CDK inhibitors, indicating that not all CDK inhibitors are causing identical molecular and cellular effects, even in cases where their patterns of activity overlap.
Different patterns of CDK inhibition may lead to distinct mechanisms of cell cycle checkpoint control. Targeting of CDK1 has been associated with G2/M blockade in MM cells (Stromberg, et al 2006), inhibition of CDK7 and 9 has been shown to block cell cycle in S phase (Dai, et al 2006), CDK2 and 3 have been reported to have a role in G1/S transition (van den Heuvel and Harlow 1993), inhibition of CDK4/6 blocks cells in G1 (Menu, et al 2008), and the effect cell cycle by CDK2/7/9 inhibition is dependent on the cell line tested (Lacrima, et al 2007), therefore, it is conceivable that multi-targeted CDK inhibition can have pleotropic effects cell cycle and cell survival. In addition, molecular effects are distinct when inhibiting specific sets of CDKs. For example, signaling through the Rb pathway is reduced by LCQ195, but remains largely unaffected by flavopiridol. In addition, the anti-MM activity of LCQ195 does not involve the release of cytochrome c and is not suppressed by the over-expression of bcl-2. LCQ195, therefore, appears to operate through mitochondrial-independent mechanisms of apoptosis. This is distinct from other CDK inhibitors (Duffin, et al 2009). In terms of therapeutic applications, these differences could be useful, because they imply that different multi-targeted CDK inhibitors with different spectra of targets may reduce cross resistance and provide opportunity for design of effective drug combination strategies in MM.
An intriguing feature of LCQ1995 is that it decreases several gene expression signatures important for tumor cell biology. Importantly the transcriptional signature of LCQ195 treatment correlates with clinical outcome of bortezomib-treated MM patients in the SUMMIT and APEX trials. This correlation specifically highlights that LCQ195 may target patients with molecularly distinct features which may predispose them for sub-optimal clinical benefit with bort treatment.
Our data highlight the notion that not all CDK inhibitors have the same biological effects, and their distinct molecular properties may be therapeutically useful in specific settings. The development of multiple agents of the same class has been particularly useful in the case for Bcr-Abl inhibitors. Similar to the development of Bcr-Abl inhibitors, inhibitors of CDKs which have overlapping targets do not always have the same pattern of activity. Since it is difficult to characterize the mechanism of action of a multi-targeted agent by attempting to dissect the individual role of each of its different targets, we specifically studied how LCQ195 affects transcriptional signatures of key pathways for MM cells. We specifically show that the cluster of genes suppressed by LCQ195 defines a population of MM patients with adverse clinical outcome in the context of bortezomib treatment. This approach may be useful, not only for the study of CDK inhibitors, but also for other classes of multi-targeted agents, because it allows the identification of agents within a given class which trigger a clinically relevant set of molecular changes.
Supplementary Material
Acknowledgments
Supported in part by the “Dunkin Donuts Rising Stars” Program at the Dana-Farber Cancer Institute (to C.S.M), the Chambers Medical Foundation (P.G.R and C.S.M.), the Cobb Family Fellowship (to D.W.M), and National Institutes of Health Grants RO1-50947 and PO-1-78378.
We would like to thank Dr. Towia Libermann, Marie Joseph-Bruno and Xuesong Gu of the BIDMC Genomics Center for their help with gene expression profiling
Footnotes
Disclosures
D.W.M., J.D., J.N., M.O, L.B., J.J., M.O., P.H., R.S. have nothing to disclose. C.L. was formerly an employee of Novartis. N.C.M was in the Speakers’ bureau (until 7/1/09) and Scientific Advisory Boards of and received honoraria from Celgene, Millennium, Novartis. P.G.R. was in the Speakers’ bureau (until 7/1/09) of and received honoraria from Millennium and Celgene. K.C.A. has received research grants and honoraria from Millennium and Celgene and was is in the Speakers’ bureau (until 7/1/09) of Millennium and Celgene. CSM has received in the past Consultant honoraria from Millennium Pharmaceuticals, Novartis Pharmaceuticals, Bristol-Myers Squibb, Merck &Co., Kosan Pharmaceuticals and Pharmion, licensing royalties from PharmaMar, and research funding from Amgen Pharmaceuticals, AVEO Pharma, EMD Serono, Sunesis, Gloucester Pharmaceuticals and Genzyme.
References
- Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, Yakoub Agha I, Bourhis JH, Garderet L, Pegourie B, Dumontet C, Renaud M, Voillat L, Berthou C, Marit G, Monconduit M, Caillot D, Grobois B, Avet-Loiseau H, Moreau P, Facon T. Maintenance therapy with thalidomide improves survival in multiple myeloma patients. Blood. 2006 doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, Badros A, Zangari M, Anaissie E, Epstein J, Shaughnessy J, Ayers D, Spoon D, Tricot G. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–494. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333–6338. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J., Jr. Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco RD, Sukhdeo K, Protopopova M, German M, Henderson J, Anderson KC, DePinho RA. Targeted overexpression of the transcription factor XBP-1 in B cells promotes plasma cell and lymphoplasmacytic neoplasms in transgenic mice. Blood. 2005;106:109A–109A. [Google Scholar]
- Conroy A, Stockett DE, Walker D, Arkin MR, Hoch U, Fox JA, Hawtin RE. SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother Pharmacol. 2009;64:723–732. doi: 10.1007/s00280-008-0921-5. [DOI] [PubMed] [Google Scholar]
- Dai Y, Hamm TE, Dent P, Grant S. Cyclin D1 overexpression increases the susceptibility of human U266 myeloma cells to CDK inhibitors through a process involving p130-, p107- and E2F-dependent S phase entry. Cell Cycle. 2006;5:437–446. doi: 10.4161/cc.5.4.2441. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Spencer A, Attal M, Prince M, Harousseau JL, Dmoszynska A, Yu ZU, Olesnyckyj M, Zeldis J, Knight R. Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): Results of a phase 3 study (MM-010) Blood. 2005;106:6A–7A. [Google Scholar]
- Duffin R, Leitch AE, Sheldrake TA, Hallett JM, Meyer C, Fox S, Alessandri AL, Martin MC, Brady HJ, Teixeira MM, Dransfield I, Haslett C, Rossi AG. The CDK inhibitor, R-roscovitine, promotes eosinophil apoptosis by down-regulation of Mcl-1. FEBS Lett. 2009;583:2540–2546. doi: 10.1016/j.febslet.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Garcia-Sanz R, Gonzalez-Fraile MI, Mateo G, Hernandez JM, Lopez-Berges MC, de las Heras N, Fernandez-Calvo J, Ortega F, Portero JA, Barez A, Galende J, Orfao A, San Miguel JF. Proliferative activity of plasma cells is the most relevant prognostic factor in elderly multiple myeloma patients. Int J Cancer. 2004;112:884–889. doi: 10.1002/ijc.20491. [DOI] [PubMed] [Google Scholar]
- Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- Hu J, Bianchi F, Ferguson M, Cesario A, Margaritora S, Granone P, Goldstraw P, Tetlow M, Ratcliffe C, Nicholson AG, Harris A, Gatter K, Pezzella F. Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene. 2005;24:1212–1219. doi: 10.1038/sj.onc.1208242. [DOI] [PubMed] [Google Scholar]
- Lacrima K, Rinaldi A, Vignati S, Martin V, Tibiletti MG, Gaidano G, Catapano CV, Bertoni F. Cyclin-dependent kinase inhibitor seliciclib shows in vitro activity in diffuse large B-cell lymphomas. Leuk Lymphoma. 2007;48:158–167. doi: 10.1080/10428190601026562. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1 alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem. 2003;278:40296–40304. doi: 10.1074/jbc.M302560200. [DOI] [PubMed] [Google Scholar]
- MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, Lane DP, Green SR. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65:5399–5407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, Griffin JD, Richardson PG, Anderson KC, Mitsiades CS. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Zhou M, Lan C, Bentsman G, Volsky DJ, Zhang L. Gene expression profiling reveals the profound upregulation of hypoxia-responsive genes in primary human astrocytes. Physiol Genomics. 2006;25:435–449. doi: 10.1152/physiolgenomics.00315.2005. [DOI] [PubMed] [Google Scholar]
- Menu E, Garcia J, Huang X, Di Liberto M, Toogood PL, Chen I, Vanderkerken K, Chen-Kiang S. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res. 2008;68:5519–5523. doi: 10.1158/0008-5472.CAN-07-6404. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D, Joseph M, Libermann TA, Garcia-Echeverria C, Pearson MA, Hofmann F, Anderson KC, Kung AL. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004a;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann TA, Richon VM, Marks PA, Anderson KC. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci U S A. 2004b;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R, Hideshima T, Anderson KC. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98:795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H, Shiraishi N, Chauhan D, Munshi NC, Green SR, Anderson KC. Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma. Blood. 2005;106:1042–1047. doi: 10.1182/blood-2005-01-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Therneau TM, Kyle RA, Greipp PR, Gertz MA. Methods for estimation of bone marrow plasma cell involvement in myeloma: predictive value for response and survival in patients undergoing autologous stem cell transplantation. Am J Hematol. 2001;68:269–275. doi: 10.1002/ajh.10003. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Witzig TE, Lust JA, Larson D, Therneau TM, Kyle RA, Litzow MR, Greipp PR, Gertz MA. Effect of complete response on outcome following autologous stem cell transplantation for myeloma. Bone Marrow Transplant. 2000;26:979–983. doi: 10.1038/sj.bmt.1702640. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, Schlossman RL, Rajkumar SV, Desikan KR, Hideshima T, Munshi NC, Kelly-Colson K, Doss D, McKenney ML, Gorelik S, Warren D, Freeman A, Rich R, Wu A, Olesnyckyj M, Wride K, Dalton WS, Zeldis J, Knight R, Weller E, Anderson KC. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, LeBlanc R, Catley LP, Doss D, Kelly K, McKenney M, Mechlowicz J, Freeman A, Deocampo R, Rich R, Ryoo JJ, Chauhan D, Balinski K, Zeldis J, Anderson KC. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Semenov I, Akyuz C, Roginskaya V, Chauhan D, Corey SJ. Growth inhibition and apoptosis of myeloma cells by the CDK inhibitor flavopiridol. Leuk Res. 2002;26:271–280. doi: 10.1016/s0145-2126(01)00103-5. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, Zeng Y, Chen B, Epstein J, Staudt LM. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- Shaughnessy JD, Jr., Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR, Xiao Y, Xu H, Epstein J, Anaissie E, Krishna SG, Cottler-Fox M, Hollmig K, Mohiuddin A, Pineda-Roman M, Tricot G, van Rhee F, Sawyer J, Alsayed Y, Walker R, Zangari M, Crowley J, Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- Stromberg T, Ekman S, Girnita L, Dimberg LY, Larsson O, Axelson M, Lennartsson J, Hellman U, Carlson K, Osterborg A, Vanderkerken K, Nilsson K, Jernberg-Wiklund H. IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M-phase accumulation and apoptosis in multiple myeloma cells. Blood. 2006;107:669–678. doi: 10.1182/blood-2005-01-0306. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- Weber D, Wang M, Chen C, Belch A, Stadtmauer EA, Niesvisky R, Yu Z, Olesnyckyj M, Zeldis J, Knight RD, Dimopoulos M. Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): Results of 2 phase III studies (MM-009, MM-010) and subgroup analysis of patients with impaired renal function. Blood. 2006;108:1012A–1013A. [Google Scholar]
- Wyatt PG, Woodhead AJ, Berdini V, Boulstridge JA, Carr MG, Cross DM, Davis DJ, Devine LA, Early TR, Feltell RE, Lewis EJ, McMenamin RL, Navarro EF, O’Brien MA, O’Reilly M, Reule M, Saxty G, Seavers LC, Smith DM, Squires MS, Trewartha G, Walker MT, Woolford AJ. Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J Med Chem. 2008;51:4986–4999. doi: 10.1021/jm800382h. [DOI] [PubMed] [Google Scholar]
- Yu D, Cozma D, Park A, Thomas-Tikhonenko A. Functional validation of genes implicated in lymphomagenesis: an in vivo selection assay using a Myc-induced B-cell tumor. Ann N Y Acad Sci. 2005;1059:145–159. doi: 10.1196/annals.1339.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller KI, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4:R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.