Abstract
Cerebral involvement in Myotonic Dystrophy Type 1 (DM1) is well-established but not well characterized. This study applied new Diffusion Tensor Imaging (DTI) tractography to characterize white matter disturbance in adults with DM1. Forty-five participants with DM1 and 44 control participants had MRIs on a Siemens 3T TIM Trio scanner. Data were processed with TRActs Constrained by UnderLying Anatomy (TRACULA) and 7 tracts were evaluated. Bilateral disturbances in white matter integrity were seen in all tracts in participants with DM1 compared to controls. There were no right-left hemisphere differences. The resulting DTI metrics were correlated with cognitive functioning, particularly working memory and processing speed. Motor speed was not significantly correlated with white matter microstructural integrity and, thus, was not the core explanation for the working memory and processing speed findings. White matter integrity was correlated with important clinical variables including the muscular impairment rating scale (MIRS). CTG repeat length was moderately associated with white matter status in corticospinal tract and cingulum. Sleepiness (Epworth Sleepiness Scale) was moderately associated with white matter status in the superior longitudinal fasciculus and cingulum. Overall, the results add to an emerging literature showing widespread white matter disturbances in both early-onset and adult-onset DM1. Results suggest that further investigation of white matter pathology is warranted in DM1 and that non-invasive measures such as DTI have potentially important clinical value in characterizing the status of individuals with DM1.
Keywords: Myotonic Dystrophy, Brain, MRI, Diffusion Tensor Imaging, White Matter, Neuropsychology
1. Introduction
Myotonic Dystrophy Type 1 (DM1), the most common form of muscular dystrophy in adults, is a multi-faceted genetic disease caused by CTG repeat expansion in the dystrophia myotonica protein kinase (DMPK) gene located on chromosome 19q13.3 (1). In addition to profound effects in muscular, ocular, gonadal, cardiac and endocrine systems (2–5), there are widespread effects in brain (6). Although the congenital-onset form of the disease is often associated with significant intellectual impairment (7, 8), adult-onset DM1 is characterized by average or low-average IQ, often accompanied by impairments in attention, memory, and executive functioning (8–14).
The neuropathology underlying these cognitive differences in DM1 is not well understood. Neurofibrillary tangles and senile plaques have been observed in DM1, especially in older patients (15). Pathological tau proteins have been observed in hippocampus and inferior temporal cortex in DM1(16). A case report indicated severe loss and disordered arrangement of myelin in temporal white matter in a patient with DM1(17) and another study reported mutant RNA foci in various locations throughout the brain including sub-cortical white matter and corpus callosum (18).
Neuroimaging studies examining white matter in DM1 have frequently revealed non-specific hyperintensities in subcortical, periventricular, and temporal white matter (19–22). Only a handful of studies have examined the microstructural integrity of white matter using newer imaging techniques such as Diffusion Tensor Imaging (DTI). One DTI study found abnormal white matter integrity in several specific tracts including the corpus callosum (23). Ota et al. suggested that the DTI abnormalities seen might be the result of Wallerian degeneration of axons following atrophy in the cortical grey matter. Others have argued against Wallerian degeneration, citing the predominance of white matter disease in comparison to gray matter effects in DM1 (24). Minnerop et al. reported abnormalities in white matter tracts throughout the brain including the callosum, projection fibers, association fibers, and motor pathways. One other study by Fukuda et al. (25) reported lower fractional anisotropy (FA), a measure of white matter integrity, in those with DM1 compared to controls. Naka et al. (26) provided converging evidence of white matter abnormalities in normal-appearing white matter using a different imaging technique, magnetization transfer imaging (MTI). Similarly, DiCostanzo et al. (27) showed widespread white matter disruption in DM1 with T2-relaxometry.
The current study utilized newly available DTI tractography methods to extend the investigation into possible regional patterns of white matter abnormalities in DM1and to increase our understanding of relationships between white matter disturbance and important clinical variables including cognitive functioning in this population. This study represents the largest sample of patients with DM1 examined with DTI to date.
2. Methods
2.1 Informed Consent
All participants underwent a comprehensive informed consent procedure that included a discussion of the study and a signed consent form. All procedures were reviewed and approved by the University of Minnesota institutional review board.
2.2 Participants
Forty-five participants (21 male, 24 female) with DM1 and 46 control participants (18 male, 28 female) were studied. Participant characteristics are listed in Table 1. Patients were recruited from a University-based myotonic dystrophy clinic. Diagnoses were established by polymerase chain reaction (PCR) and southern blot. The mean age of the groups was equivalent for patients (38.4 years) and controls (38.5 years). CTG repeats ranged from 75 to 800, with an average of 387. Congenital/infantile-onset DM1 patients were not included in the study. Attempts to retrospectively define “age of onset” of DM1 for these patients yielded imprecise estimates, with a range of symptoms appearing across wide developmental spans in many individuals and, so, these data are not reported here. The sample included cases that were diagnosed during adolescence or adulthood. Scores on the Muscular Impairment Rating Scale (MIRS) (28), completed by participants with DM1, ranged from 1 to 4 with a mean of 3.15. Scores on the Epworth Sleepiness Scale (29), completed by those with DM1, ranged from 1 to 13 with a mean of 7.44. Control participants, who were age-matched and gender-matched, were recruited from the community. Other neurological disorders, including traumatic brain injury, were exclusionary for all participants. Control participants were excluded for psychiatric disorder, learning disability, or below-average IQ (more than 1 standard deviation below normal).
Table 1.
Demographic characteristics of included participants.
| N(%) or mean ± SD | Myotonic Dystrophy (n =45) |
Control (n =46) |
Statistical Test |
|---|---|---|---|
| Age at MRI scan | 38.4 ± 6.6 yrs. | 38.5 ± 7.2 yrs. | t(1,89) =.024, p=.981 |
| Gender | |||
| Male | 21 (47%) | 18 (39%) | |
| Female | 24 (53%) | 28 (61%) | χ2=.528, p=.468 |
| Handedness | |||
| Right | 41(91%) | 45(98%) | χ2=1.98, p=.174 |
| CTG Repeats | 387 ± 208 | -- | -- |
| Muscular Impairment Rating Scale (MIRS) | 3.15 ± .96 | -- | -- |
| Epworth Sleepiness Scale | 7.44 ± 3.3 | -- | -- |
| Years of Education | 14.0 ± 2.0 yrs. | 16.1 ± 1.5 yrs. | t(1,65)=4.77, p<.001 |
| Intellectual Functioning | |||
| WAIS-III Vocabulary Score | 10.8 ± 2.8 | 11.9 ± 2.5 | t(1,88)=1.99, p=.050 |
| WAIS-III Matrix Reasoning Score | 11.6 ± 3.3 | 12.2 ± 2.6 | t(1,88)=0.87, p=.387 |
| Estimated IQ (WTAR) | 101 ± 14.3 | 110 ± 11.1 | t(1,88)=3.31, p=.001 |
| Estimated IQ (OPIE-3) | 108 ± 12.1 | 114 ± 8.2 | t(1,88)=2.41, p=.018 |
NOTE: WTAR = Wechsler Test of Adult Reading (a proxy measure of IQ); OPIE-3 Oklahoma Premorbid Intelligence Estimate - 3
2.3 Neuropsychological Assessment
Subjects completed the following neuropsychological measures: the Wechsler Test of Adult Reading (30) (as a proxy measure of premorbid IQ), Wechsler Adult Intelligence Scale (3rd ed.) (31) [Vocabulary, Block Design, Matrix Reasoning, Digit Dan, Digit-Symbol, Letter-Number Sequencing, and Symbol Search subtests],the Grooved Pegboard test (32), the Delis-Kaplan Executive Functioning System (D-KEFS) [Trailmaking, Tower Test, and Verbal Fluency], the Wisconsin Card Sorting Test (WCST) (33), and the California Verbal Learning Test (CVLT-II) (34). All neuropsychological instruments were administered by a trained research assistant under the supervision of a neuropsychologist (J.R.W. or L.S.H.). Intelligence Quotient (IQ) was estimated with the Oklahoma Premorbid Intelligence Estimate – 3 (OPIE-3) (35).
2.4 MRI acquisition and processing
Subjects were scanned using a Siemens 3T TIM Trio MRI scanner with 12-channel receive-only head coil. The imaging sequence and parameters for each scan are listed in Table 2. Participants were not sedated for the MRI scan.
Table 2.
MRI sequence and parameters.
| Sequence | Imaging Parameters | Purpose | Time |
|---|---|---|---|
| Scout | 3 plane localizer | Positioning | 1 min |
| T1-weighted MPRAGE | TR=2350ms, TE=3.65ms, TI=1100ms, 240 slices, voxel size=1×1×1mm, FOV=256mm, flip angle=7 degrees. | Segmentation & cortical parcellation | 11 min |
| Diffusion weighted (DTI) | TR=8500ms, TE=90ms, 64 slices, voxel size=2×2×2mm, FOV=256mm, GRAPPA 2, 30 volumes with b=1000 s/mm2 & 6 with b=0 s/mm2, 2 averages (72 volumes). | Computation of the diffusion tensor | 11 min |
| DTI Field-map | Positioned to match DTI, 64 slices, voxel size=2×2×2mm, FOV=256mm TR=700ms, TE=4.62ms / 7.08ms, flip angle=90 deg. | Correction of geometric distortions for DTI | 3 min |
2.5 MRI processing
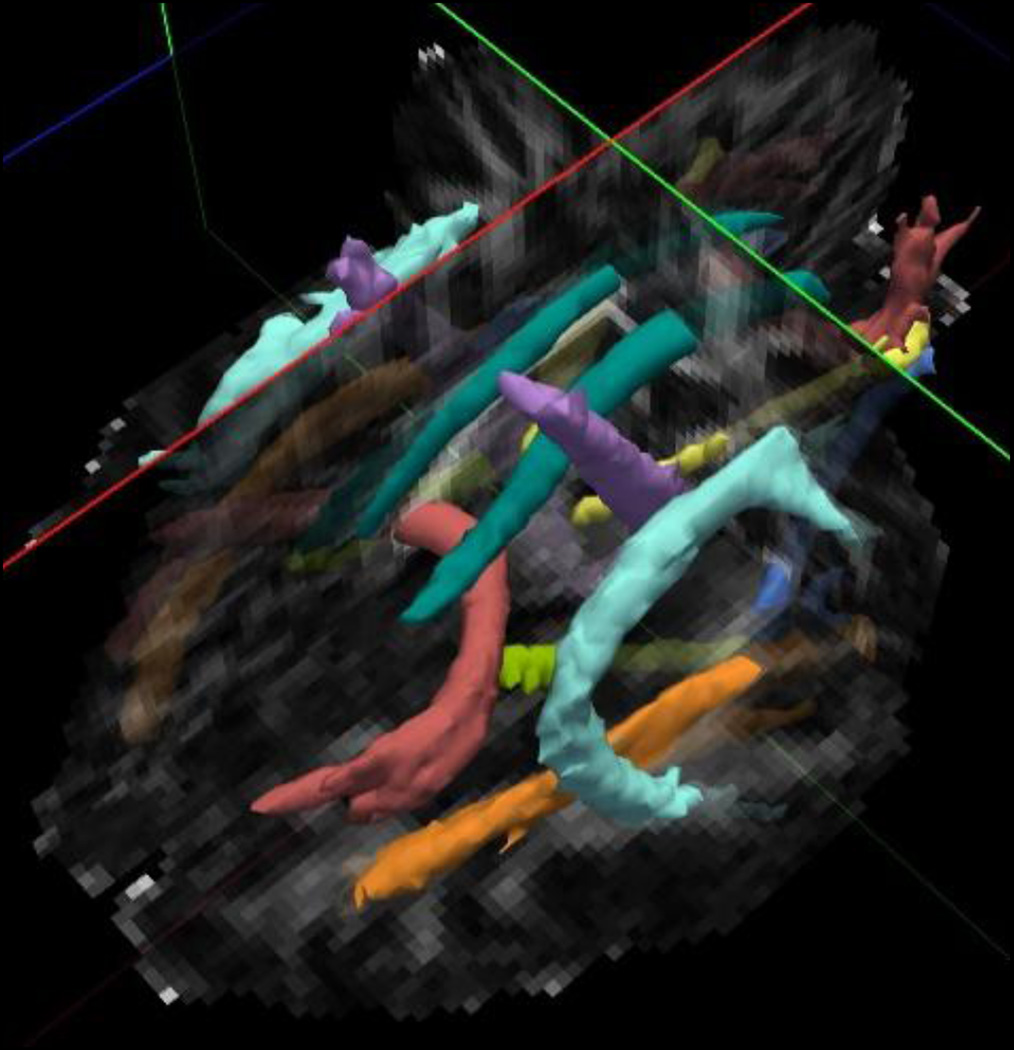
Image data were processed using the TRACULA (Tracts Constrained by Underlying Anatomy) processing stream (36), a component of the Freesurfer 5.3.0 Software Library (http://surfer.nmr.mgh.harvard.edu/). This processing stream consists of four parts: 1) Freesurfer segmentation and parcellation of the structural T1 data to define specific anatomic regions; 2) preprocessing of the DTI data including affine registration to correct for motion and eddy current effects, B0 distortion correction using the field map data, computation of the diffusion tensor and registration to the T1 data; 3) ball and stick modeling of the diffusion data; 4) probabilistic tracking and track determination. TRACULA utilizes known anatomy of the major white matter tracts to constrain a probabilistic mapping algorithm, allowing for reliable tract reconstruction with minimal user input. We examined the following tracts that were mapped and auto-labeled by TRACULA: corticospinal tracts (CST), inferior longitudinal fasciculus (ILF), uncinate fasciculus, cingulum bundle (supra-callosal and infra-callosal components combined), superior longitudinal fasciculus (SLF) (parietal and temporal components combined), forceps major, and forceps minor. Figure 1 illustrates these tracts. Note that TRACULA does not generate a whole corpus callosum tract, but instead identifies the large tracts passing through the genu (forceps minor) and the splenium (forceps major). Two scalar measures were derived from the tensor: Fractional Anisotropy or FA (the fraction of the magnitude of the tensor that is due to anisotropic water diffusion (37)) and Mean Diffusivity or MDiff (the mean of the three eigenvalues). For each tract, the mean FA and MDiff were computed from all of the voxels in the tract.
Figure 1.
Illustration of white matter tracts from TRACULA (purple = corticospinal tract; red = forceps major & forceps minor; teal= cingulum bundle {cingulum gyrus component}; light green = cingulum bundle {angular gyrus component}; light blue = superior longitudinal fasciculus {temporal component}; orange = inferior longitudinal fasciculus; medium blue = uncinated fasciculus; yellow = thalamic radiations {not examined}.
3. Results
As indicated in Table 1, the groups did not differ in age, gender, or handedness. There was a modest difference in education, with control participants having approximately two more years than those with DM1. There was also a small but significant group difference in estimated IQ, with the control group approximately 6–10 points higher than those with DM1. Comparison of the Wechsler Vocabulary and Matrix Reasoning subtests suggests the controls had higher scores that those with DM1 particularly in verbal IQ rather than non-verbal IQ.
Table 3 contains results from t-tests comparing the groups on measures of neurocognitive performance. As expected, patients with DM1 performed significantly below controls on measures of working memory (short-term memory / concentration), processing speed, motor speed, and some aspects of executive functioning. Those with DM1 performed below controls on the Trailmaking subtest, which required working memory and flexible shifting of attention. In addition, the DM1 group performed below controls on the category-switching component of the Verbal Fluency subtest (there were no differences in basic phonemic-fluency or category fluency). In contrast, there were no group differences for the Wisconsin Card Sorting test (a measure of systematic problem-solving and mental flexibility), nor were there differences in verbal memory. Grooved Pegboard performance indicated significant slowing in basic motor speed for those with DM1.
Table 3.
Comparison of patients with myotonic dystrophy type 1 and control participants on neuropsychological tests.
| Measure |
Control Mean (SD) |
Myotonic Dystrophy Mean (SD) |
Significance (p) |
|---|---|---|---|
| Wechsler Working Memory | 105.7 (11.1) | 97.3 (12.1) | <.001 |
| Wechsler Processing Speed | 109.7 (11.2) | 95.1 (12.1) | <.001 |
| California Verbal Learning Test (total) | 52.9 (10.3) | 49.8 (8.7) | .126 |
| Wisconsin Card Sorting Test (total errors) | 91.6 (23.0) | 95.7 (19.2) | .464 |
| D-KEFS Trailmaking (letter-number switching) | 11.4 (2.2) | 8.9 (3.3) | <.001 |
| D-KEFS Tower Test | 10.9 (2.4) | 10.7 (2.8) | .648 |
| D-KEFS Verbal Fluency (category switching) | 9.7 (3.4) | 11.5 (3.4) | .010 |
| Grooved Pegboard (dominant hand) | −2.9 (3.9) | −0.1 (1.1) | <.001 |
| Grooved Pegboard (non-dominant hand) | −2.3 (2.9) | −0.1 (0.9) | <.001 |
Note: SD = Standard Deviation. D-KEFS = Delis-Kaplan Executive Functioning System
An ANOVA tested for differences in FA by group (control vs. DM1), hemisphere (right vs. left), and white matter tract (see Table 4 for list of tracts). The overall ANOVA was significant, F(31,1455)=163.38, p<.001. The group by hemisphere interaction was non-significant, F(1, 1455)=.580, p=.446 and, therefore, the right and left hemisphere tracts were averaged together for remaining analyses. The group by tract interaction was significant, F(1,1455)=17.98, p<.001. Therefore, group differences (control vs. DM1) in FA were examined for all tracts. To reduce the number of analyses, the two components of the cingulum (supra-callosal and infra-callosal) were averaged together and the two components of the SLF (parietal and temporal) were averaged together. Group differences are illustrated in Table 4.
Table 4.
Comparison of patients with myotonic dystrophy type 1 and control participants on measures of fractional anisotropy (FA) and mean diffusivity (MDiff) in white matter tracts.
| Control (n =46) |
Myotonic Dystrophy (n =45) |
Statistical tests | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | Effect size (Cohen’s d) |
|
| Fractional Anisotropy (FA) | |||||||
| Corticospinal tract | .497 | .024 | .486 | .025 | 2.15 | .034 | .45 |
| Inferior Longitudinal Fasciculus (ILF) | .501 | .024 | .429 | .041 | 10.27 | <.001 | 2.14 |
| Superior Longitudinal Fasciculus (SLF) | .446 | .022 | .394 | .034 | 12.32 | <.001 | 1.82 |
| Cingulum | .454 | .028 | .386 | .040 | 13.44 | <.001 | 1.97 |
| Uncinate Fasciculus | .421 | .023 | .360 | .034 | 10.12 | <.001 | 2.10 |
| Forceps Major | .613 | .024 | .558 | .041 | 7.72 | <.001 | 1.63 |
| Forceps Minor | .524 | .030 | .453 | .052 | 8.11 | <.001 | 1.67 |
| Mean Diffusivity (MDiff) | |||||||
| Corticospinal tract | .747 | .017 | .795 | .028 | 10.04 | <.001 | 2.07 |
| Inferior Longitudinal Fasciculus (ILF) | .812 | .022 | .928 | .060 | 12.33 | <.001 | 2.57 |
| Superior Longitudinal Fasciculus (SLF) | .740 | .020 | .841 | .053 | 16.94 | <.001 | 2.52 |
| Cingulum | .784 | .025 | .874 | .051 | 15.29 | <.001 | 2.24 |
| Uncinate Fasciculus | .809 | .022 | .924 | .059 | 12.56 | <.001 | 2.58 |
| Forceps Major | .830 | .027 | .896 | .051 | 7.74 | <.001 | 1.61 |
| Forceps Minor | .796 | .026 | .905 | .063 | 10.93 | <.001 | 2.26 |
Note: Mean Diffusivity values are ×10−6 mm2 / second; Mean values represent the average of the right and left tracts; Superior Longitudinal Fasciculus values represent the average of parietal and temporal components; Cingulum values represent the average of supracallosal and infracallosal components.
indicates significant difference after Bonferroni correction (p<.0035).
A second ANOVA tested for differences in mean diffusivity (MDiff) by group, hemisphere, and tract. The overall ANOVA was significant, F(31,1455)=104.48, p<.001. The group by hemisphere interaction was non-significant, F(1,1455)=0.0, p=1.0 and, therefore, right and left hemisphere tracts were averaged together for remaining analyses. The group by tract interaction was significant, F(1,1455)=13.0, p<.001. Therefore, group differences in MDiff were examined for all tracts. As above, the averaged cingulum and SLF components were examined. Group differences are illustrated in Table 4. Effect sizes were large for MDiff in all tracts and FA in all but one tract, indicating that there are substantial, meaningful abnormalities in white matter integrity in DM1 compared to controls.
Pearson correlations were used to test the relationships between cognitive functioning and white matter status. These correlations were done for the DM1 group only in order to avoid the confounding effects of group differences in cognitive functioning, white matter integrity, and other related factors such as education level and IQ. In order to reduce the number of correlations, only MDiff was examined from the 7 tracts (right and left tracts were averaged together). MDiff was chosen because it appeared to be the more sensitive of the two DTI metrics to the group differences (DM1 vs. control) in white matter status. Only cognitive measures that showed significant group differences (DM1 vs. control) were analyzed. Because Grooved Pegboard dominant-hand and non-dominant hand performance were highly correlated with each other (r=.90, p<.001), these scores were averaged together for subsequent analysis. As shown in Table 5, the results demonstrate strong relationships between white matter integrity in nearly all tracts and working memory, with the exception of the forceps major. Similarly, strong relationships were also seen with processing speed in the corticospinal tracts, SLF, cingulate, and uncinate. The correlations between white matter status and measures of executive functioning and motor functioning were much lower and were all non-significant.
Table 5.
Correlations between white matter tract mean diffusivity (MDiff) and measures of cognitive performance in participants with DM1.
| Wechsler WMI |
Wechsler PSI |
D-KEFS Fluency Category Switching |
Grooved Pegboard |
|
|---|---|---|---|---|
| Corticospinal tract | r=.−585, p<.001* | r=−.596, p<.001* | r=−.045, p=.771 | r=−.274, p=.072 |
| Inferior Longitudinal Fasciculus (ILF) | r=−.484, p<.001* | r=−.435, p=.003 | r=−.170, p=.269 | r=−.413, p=.005 |
| Superior Longitudinal Fasciculus (SLF) | r=−.509, p<.001* | r=−.474, p=.001* | r=−.132, p=.392 | r=−.297, p=.050 |
| Cingulum | r=−.545, p<.001* | r=−.528, p<.001* | r=−.188, p=.222 | r=−.366, p=.015 |
| Uncinate Fasciculus | r=−.571, p<.001* | r=−.480, p<.001* | r=−.147, p=.342 | r=−.350, p=.020 |
| Forceps Major | r=−.431, p<.001 | r=−.385, p=.009 | r=−.064, p=.679 | r=−.179, p=.245 |
| Forceps Minor | r=−.586, p<.001* | r=−.438, p=.003 | r=−.038, p=.806 | r=−.339, p=.025 |
NOTE:
significant correlation after Bonferroni correction (p<.0018);
WMI = Working Memory index; PSI = Processing Speed Index; D-KEFS = Delis-Kaplan Executive Functioning System.
Of the two cognitive domains that were associated with white matter integrity (working memory and processing speed), Pearson correlations show that neither was significantly correlated with education level for those in the DM1 group (r=.302, p=.126 for Working Memory Index (WMI) and r=.363, p=.063 for Processing Speed Index (PSI)). In contrast, estimated IQ was significantly correlated with WMI (r=.542, p=<.001) and PSI (r=.580, p<.001). Nonetheless, follow-up partial correlation analyses revealed that the correlations between both WMI and PSI and white matter status in each of the tracts remained significant at the p<.01 level after controlling for IQ.
Lastly, relationships between white matter status and three important clinical variables were examined. As shown in Table 6, CTG repeat length was correlated with MDiff in the corticospinal tracts and cingulum. MIRS scores were strongly associated with white matter integrity in all tracts with the exception of the forceps major. Sleepiness, as defined by the Epworth Sleepiness Scale, was moderately associated with MDiff in the SLF and cingulum.
Table 6.
Correlations between white matter tract mean diffusivity (MDiff) and clinical measures in participants with DM1
| CTG Repeats | MIRS | Sleepiness Scale | |
|---|---|---|---|
| Corticospinal tract | r=.417, p<.003* | r=.420, p<.001* | r=.221, p=.069 |
| Inferior Longitudinal Fasciculus (ILF) | r=.154 p=.284 | r=.556, p<.001* | r=.250, p=.040 |
| Superior Longitudinal Fasciculus (SLF) | r=.088, p=.381 | r=.504, p<.001* | r=.242, p=.005* |
| Cingulum | r=.407, p<.001* | r=.473, p<.001* | r=.266, p=.002* |
| Uncinate Fasciculus | r=.291, p=.040 | r=.555, p<.001* | r=.241, p=.047 |
| Forceps Major | r=−.084, p=.689 | r=.345, p=.046 | r=.204, p=.246 |
| Forceps Minor | r=.126, p=.549 | r=.573, p<.001* | r=.325, p=.060 |
NOTE:
significant correlation after Bonferroni correction (p<.007);
MIRS = Muscular Impairment Rating Scale
4. Discussion
Consistent with our previous findings in adults with DM1 (38) and our findings in child and adolescent patients with early-onset DM1 (39), the data presented here clearly show significant white matter abnormalities throughout the brain in DM1. Seven specific white matter tracts were examined and all showed highly significant abnormalities in the DM1 group compared to control participants. Nearly all DTI studies of DM1 thus far have shown diffuse white matter abnormalities of this type as opposed to focal disruption. Takaba et al. (40), found widespread abnormalities (low FA and high MDiff) in DM1. Fukuda et al. (25) demonstrated that these measureable microstructural abnormalities are not only evident in abnormal-appearing white matter (i.e. hyperintensities), but also in normal-appearing white matter. Minnerop et al. (24) showed significant white matter disruption in DM1 in all regions examined. We reported very large (16–27%) differences in DTI metrics in a previous study of adults with DM1 (38) and similarly large (8–22%) differences in two studies of children / adolescents with DM1 (39, 41).
Participants in this study demonstrated several cognitive deficits that are frequently seen in DM1 and thought to be clinically relevant (42) including working memory, processing speed, attentional switching, and motor speed. Although significant executive deficits, such as those reflected by the Wisconsin Card Sorting Test, have been reported in DM1 (11, 13, 43), these may be less severe in more mildly-affected individuals (12) and were absent in our sample of patients with relatively intact IQ. Overall there was ample evidence that the observed white matter abnormalities are clinically important in DM1. Strong associations between white matter status and cognition were observed for working memory ability and processing speed. These two neurocognitive domains were correlated with MDiff in nearly all of the tracts examined. We observed similar relationships between working memory task performance and MDiff in multiple brain regions in patients with early-onset DM1 (41). Furthermore, these data also show significant relationships between white matter status and processing speed in multiple tracts including the corticospinal tracts, SLF, and cingulum. In contrast, correlations between white matter tract integrity and a measure of basic motor speed (grooved pegboard) were lower and non-significant. Thus, if white matter abnormalities do contribute to the cognitive impairment seen in MDiff, it does not appear to be strictly a function of simple motor delays.
We also observed a strong association between the level of white matter abnormality and muscular impairment, as measured by the MIRS. Minnerop et al. (24) observed similar associations with MIRS scores. This suggests that DTI metrics may be providing a useful index of overall disease burden in the CNS. CTG repeat-length, as measured in blood, is an imperfect index of disease severity because of the variable expression across tissue types. A number of studies have found significant correlations between CTG-repeats and macro-level measures like IQ (13, 44, 45) or the extent of white matter disease (19), but some have not found an association (23, 46, 47). In the current study, we observed a relationship with MDiff in two tracts, the corticospinal tract and cingulum. Minnerop et al. (24) also reported an association between FA and CTG repeat length. Lastly, we report modest correlations between MDiff in two tracts (SLF and cingulum) and sleepiness. Both sleepiness and fatigue are common clinical features of DM1, occurring in a large percentage of patients (48, 49), and future studies may help to further characterize the role of sleepiness/fatigue in the cognitive impairment seen in DM1.
At this point, there remains much to be learned about the underlying neuropathology that is reflected in DTI studies of DM1. DTI metrics are non-specific and could potentially reflect changes in myelin, axonal membrane integrity, microtubule/neurofilament structure, fast axonal transport within axons, differences in extra-axonal water between axons (50–52), or other underlying pathology. Hernandez-Hernandez (53) have speculated that abnormalities in synaptic proteins in cell membranes seen in both a mouse model of DM1 (DMSXL) and human DM1 brain tissue could represent an underlying pathophysiology that might be reflected in DTI changes like those seen here. Although limited in specificity, DTI metrics do provide unique, non-invasive measures of white matter status that have important clinical correlation. Ultimately, DTI measures may prove to be clinically useful, likely in conjunction with a broad set of neurocognitive measures, in fully characterizing the disease status in individuals with DM1.
5. Conclusion
In conclusion, the current study adds to the small existing body of studies that show diffuse white matter abnormalities in DM1. Consistent with previous studies, we observed significant relationships between white matter integrity, as measured by DTI, and cognitive functioning. This was especially true for working memory and processing speed. Future studies examining the role of sleepiness and fatigue as potential moderating / mediating factors in cognitive dysfunction in DM1 will be worthwhile.
Highlights.
DTI tractography reveals white matter disturbance in all tracts examined in DM1
These brain disturbances are associated with the level of muscle impairment
The abnormalities are also associated with working memory and cognitive speed deficits
Sleepiness was associated with white matter status in two white matter tracts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 2.Sovari AA, Bodine CK, Farokhi F. Cardiovascular manifestations of myotonic dystrophy-1. Cardiol Rev. 2007;15(4):191–194. doi: 10.1097/CRD.0b013e318070d1a7. [DOI] [PubMed] [Google Scholar]
- 3.Schara U, Schoser BG. Myotonic dystrophies type 1 and 2: a summary on current aspects. Semin Pediatr Neurol. 2006;13(2):71–79. doi: 10.1016/j.spen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Anastasopoulos D, Kimmig H, Mergner T, Psilas K. Abnormalities of ocular motility in myotonic dystrophy. Brain : a journal of neurology. 1996;119(Pt 6):1923–1932. doi: 10.1093/brain/119.6.1923. [DOI] [PubMed] [Google Scholar]
- 5.Jozefowicz RF, Griggs RC. Myotonic dystrophy. Neurol Clin. 1988;6(3):455–472. [PubMed] [Google Scholar]
- 6.Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle & nerve. 2007;36(3):294–306. doi: 10.1002/mus.20800. [DOI] [PubMed] [Google Scholar]
- 7.Censori B, Danni M, Del Pesce M, Provinciali L. Neuropsychological profile in myotonic dystrophy. Journal of neurology. 1990;237(4):251–256. doi: 10.1007/BF00314629. [DOI] [PubMed] [Google Scholar]
- 8.Modoni A, Silvestri G, Pomponi MG, Mangiola F, Tonali PA, Marra C. Characterization of the pattern of cognitive impairment in myotonic dystrophy type 1. Arch Neurol. 2004;61(12):1943–1947. doi: 10.1001/archneur.61.12.1943. [DOI] [PubMed] [Google Scholar]
- 9.Gaul C, Schmidt T, Windisch G, Wieser T, Muller T, Vielhaber S, et al. Subtle cognitive dysfunction in adult onset myotonic dystrophy type 1 (DM1) and type 2 (DM2) Neurology. 2006;67(2):350–352. doi: 10.1212/01.wnl.0000225180.27833.c1. [DOI] [PubMed] [Google Scholar]
- 10.Malloy P, Mishra SK, Adler SH. Neuropsychological deficits in myotonic muscular dystrophy. Journal of Neurology, Neurosurgery and Psychiatry. 1990;53(11):1011–1013. doi: 10.1136/jnnp.53.11.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, et al. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2) Neuromuscular Disorders. 2003;13(10):813–821. doi: 10.1016/s0960-8966(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 12.Rubinsztein JS, Rubinsztein DC, McKenna PJ, Goodburn S, Holland AJ. Mild myotonic dystrophy is associated with memory impairment in the context of normal general intelligence. Journal of Medical Genetics. 1997;34(3):229–233. doi: 10.1136/jmg.34.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winblad S, Lindberg C, Hansen S. Cognitive deficits and CTG repeat expansion size in classical myotonic dystrophy type 1 (DM1) Behav Brain Funct. 2006;2:16. doi: 10.1186/1744-9081-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber YG, Roebling R, Kassubek J, Hoffmann S, Rosenbohm A, Wolf M, et al. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology. 2010;74(14):1108–1117. doi: 10.1212/WNL.0b013e3181d8c35f. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi A, Otsuka N, Namba Y, Nakano I, Tomonaga M. Presenile appearance of abundant Alzheimer's neurofibrillary tangles without senile plaques in the brain in myotonic dystrophy. Acta neuropathologica. 1991;82(1):1–5. doi: 10.1007/BF00310916. [DOI] [PubMed] [Google Scholar]
- 16.Vermersch P, Sergeant N, Ruchoux MM, Hofmann-Radvanyi H, Wattez A, Petit H, et al. Specific tau variants in the brains of patients with myotonic dystrophy. Neurology. 1996;47(3):711–717. doi: 10.1212/wnl.47.3.711. [DOI] [PubMed] [Google Scholar]
- 17.Ogata A, Terae S, Fujita M, Tashiro K. Anterior temporal white matter lesions in myotonic dystrophy with intellectual impairment: an MRI and neuropathological study. Neuroradiology. 1998;40(7):411–415. doi: 10.1007/s002340050613. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, Mankodi A, Swanson MS, Moxley RT, Thornton CA. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Human Molecular Genetics. 2004;13(24):3079–3088. doi: 10.1093/hmg/ddh327. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann G, Damian MS, Koch M, Schilling G, Fach B, Stoppler S. The clinical and genetic correlates of MRI findings in myotonic dystrophy. Neuroradiology. 1996;38(7):629–635. doi: 10.1007/s002340050322. [DOI] [PubMed] [Google Scholar]
- 20.Glantz RH, Wright RB, Huckman MS, Garron DC, Siegel IM. Central nervous system magnetic resonance imaging findings in myotonic dystrophy. Arch Neurol. 1988;45(1):36–37. doi: 10.1001/archneur.1988.00520250042017. [DOI] [PubMed] [Google Scholar]
- 21.Huber SJ, Kissel JT, Shuttleworth EC, Chakeres DW, Clapp LE, Brogan MA. Magnetic resonance imaging and clinical correlates of intellectual impairment in myotonic dystrophy. Arch Neurol. 1989;46(5):536–540. doi: 10.1001/archneur.1989.00520410070026. [DOI] [PubMed] [Google Scholar]
- 22.Kornblum C, Reul J, Kress W, Grothe C, Amanatidis N, Klockgether T, et al. Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. Journal of neurology. 2004;251(6):710–714. doi: 10.1007/s00415-004-0408-1. [DOI] [PubMed] [Google Scholar]
- 23.Ota M, Sato N, Ohya Y, Aoki Y, Mizukami K, Mori T, et al. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci Lett. 2006;407(3):234–239. doi: 10.1016/j.neulet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 24.Minnerop M, Weber B, Schoene-Bake JC, Roeske S, Mirbach S, Anspach C, et al. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain : a journal of neurology. 2011;134(Pt 12):3530–3546. doi: 10.1093/brain/awr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda H, Horiguchi J, Ono C, Ohshita T, Takaba J, Ito K. Diffusion tensor imaging of cerebral white matter in patients with myotonic dystrophy. Acta Radiol. 2005;46(1):104–109. doi: 10.1080/02841850510015974. [DOI] [PubMed] [Google Scholar]
- 26.Naka H, Imon Y, Ohshita T, Honjo K, Kitamura T, Mimori Y, et al. Magnetization transfer measurements of cerebral white matter in patients with myotonic dystrophy. J Neurol Sci. 2002;193(2):111–116. doi: 10.1016/s0022-510x(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 27.Di Costanzo A, Di Salle F, Santoro L, Bonavita V, Tedeschi G. T2 relaxometry of brain in myotonic dystrophy. Neuroradiology. 2001;43(3):198–204. doi: 10.1007/s002340000459. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu J, Boivin H, Meunier D, Gaudreault M, Begin P. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology. 2001;56(3):336–340. doi: 10.1212/wnl.56.3.336. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Holdnack HA. Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 31.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 32.Grooved Pegboard Test - Instruction/Owner's Manual. Lafayette, IN: Lafayette Instrument; 1989. Lafayette Instrument. [Google Scholar]
- 33.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 34.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - 2nd Edition (CVLT-II) San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 35.Schoenberg MR, Scott JG, Duff K, Adams RL. Estimation of WAIS-III intelligence from combined performance and demographic variables: development of the OPIE-3. Clin Neuropsychol. 2002;16(4):426–437. doi: 10.1076/clin.16.4.426.13913. [DOI] [PubMed] [Google Scholar]
- 36.Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franc DT, Muetzel RL, Robinson PR, Rodriguez CP, Dalton JC, Naughton CE, et al. Cerebral and muscle MRI abnormalities in myotonic dystrophy. Neuromuscular Disorders. 2012 doi: 10.1016/j.nmd.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wozniak JR, Mueller BA, Ward EE, Lim KO, Day JW. White matter abnormalities and neurocognitive correlates in children and adolescents with myotonic dystrophy type 1: A Diffusion Tensor Imaging study. Neuromuscular Disorders. 2011;21(2):133–147. doi: 10.1016/j.nmd.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaba J, Abe N, Fukuda H. Evaluation of brain in myotonic dystrophy using diffusion tensor MR imaging. Nippon Hoshasen Gijutsu Gakkai Zasshi. 2003;59(7):831–838. doi: 10.6009/jjrt.kj00003174212. [DOI] [PubMed] [Google Scholar]
- 41.Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Lim KO, Day JW. Diffusion tensor imaging reveals widespread white matter abnormalities in children and adolescents with myotonic dystrophy type 1. Journal of neurology. 2013;260(4):1122–1131. doi: 10.1007/s00415-012-6771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagnon C, Meola G, Hebert LJ, Puymirat J, Laberge L, Leone M. Report of the first Outcome Measures in Myotonic Dystrophy type 1 (OMMYD-1) international workshop: Clearwater, Florida, November 30, 2011. Neuromuscular disorders : NMD. 2013;23(12):1056–1068. doi: 10.1016/j.nmd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Woodward JB, 3rd, Heaton RK, Simon DB, Ringel SP. Neuropsychological findings in myotonic dystrophy. Journal of clinical neuropsychology. 1982;4(4):335–342. doi: 10.1080/01688638208401141. [DOI] [PubMed] [Google Scholar]
- 44.Ekstrom AB, Hakenas-Plate L, Tulinius M, Wentz E. Cognition and adaptive skills in myotonic dystrophy type 1: a study of 55 individuals with congenital and childhood forms. Developmental Medicine and Child Neurology. 2009;51(12):982–990. doi: 10.1111/j.1469-8749.2009.03300.x. [DOI] [PubMed] [Google Scholar]
- 45.Angeard N, Gargiulo M, Jacquette A, Radvanyi H, Eymard B, Heron D. Cognitive profile in childhood myotonic dystrophy type 1: is there a global impairment? Neuromuscular disorders : NMD. 2007;17(6):451–458. doi: 10.1016/j.nmd.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Di Costanzo A, Santoro L, de Cristofaro M, Manganelli F, Di Salle F, Tedeschi G. Familial aggregation of white matter lesions in myotonic dystrophy type 1. Neuromuscular disorders : NMD. 2008;18(4):299–305. doi: 10.1016/j.nmd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Kuo HC, Hsieh YC, Wang HM, Chuang WL, Huang CC. Correlation among subcortical white matter lesions, intelligence and CTG repeat expansion in classic myotonic dystrophy type 1. Acta neurologica Scandinavica. 2008;117(2):101–107. doi: 10.1111/j.1600-0404.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 48.Hermans MC, Merkies IS, Laberge L, Blom EW, Tennant A, Faber CG. Fatigue and daytime sleepiness scale in myotonic dystrophy type 1. Muscle & nerve. 2013;47(1):89–95. doi: 10.1002/mus.23478. [DOI] [PubMed] [Google Scholar]
- 49.Laberge L, Begin P, Montplaisir J, Mathieu J. Sleep complaints in patients with myotonic dystrophy. Journal of sleep research. 2004;13(1):95–100. doi: 10.1111/j.1365-2869.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 51.Neil JJ, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15(7–8):543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 52.Fox RJ, Cronin T, Lin J, Wang X, Sakaie K, Ontaneda D, et al. Measuring Myelin Repair and Axonal Loss with Diffusion Tensor Imaging. AJNR American Journal of Neuroradiology. 2010 doi: 10.3174/ajnr.A2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez-Hernandez O, Guiraud-Dogan C, Sicot G, Huguet A, Luilier S, Steidl E, et al. Myotonic dystrophy CTG expansion affects synaptic vesicle proteins, neurotransmission and mouse behaviour. Brain : a journal of neurology. 2013;136(Pt 3):957–970. doi: 10.1093/brain/aws367. [DOI] [PMC free article] [PubMed] [Google Scholar]