Abstract
Olfactory bulb (OB) glomeruli, the initial sites of synaptic processing of odor information, exhibit high levels of nicotinic acetylcholine receptor (nAChR) expression and receive strong cholinergic input from the basal forebrain. The role of glomerular nAChRs in olfactory processing, however, remains to be elucidated. External tufted (ET) cells are a major source of excitation in the glomerulus and an important component of OB physiology. We have examined the role of nAChRs in modulating ET cell activity using whole-cell electrophysiology in mouse OB slices. We show here that the activation of glomerular nAChRs leads to direct ET cell excitation, as well as an increase in the frequency of spontaneous postsynaptic GABAergic currents. β2-containing nAChRs, likely the α4β2*-nAChR subtype (* represents the possible presence of other subunits), were significant contributors to these effects. The nAChR-mediated increase in spontaneous postsynaptic GABAergic current frequency on ET cells was, for the most part, dependent on glutamate receptor activation, thus implicating a role for excitation-dependent inhibition within the glomerulus. β2-containing nAChRs also regulate the frequency of miniature inhibitory postsynaptic currents on ET cells, implying nicotinic modulation of dendrodendritic signaling between ET and periglomerular cells. Our data also indicate that nAChR activation does not affect spontaneous or evoked transmission at the olfactory nerve-to-ET cell synapse. The results from this study suggest that ET cells, along with mitral cells, play an important role in the nicotinic modulation of glomerular inhibition.
Keywords: glomerulus, inhibition, nicotinic receptors, olfactory bulb
acetylcholine (ach) plays a key role in perceptual learning and sensory processing, but the cellular mechanisms by which ACh modulates brain circuits are not well understood. The main olfactory bulb (OB) provides for an attractive model to study modulation of neuronal circuits because of its laminar organization, the subsequent layer-specific expression of cholinergic receptor subtypes, and because its output neurons, the mitral cells (MCs), project directly to cortex. In the OB, MCs receive sensory information from the olfactory nerve (ON) at neuropil structures called glomeruli. Within a glomerulus, external tufted (ET) cells receive direct glutamatergic input from the ON fibers and also make dendrodendritic connections with the inhibitory periglomerular (PG) cells (Hayar et al. 2004, 2005; Wachowiak and Shipley 2006).
In recent years, ET cells have been shown to play a major role in OB function. They are a major source of excitation not only for local glomerular microcircuits (Hayar et al. 2004), but also for the MCs, via a feed-forward mechanism (De Saint et al. 2009; Gire et al. 2012), thus potentially playing a key role in shaping the output of the OB (Gire and Schoppa 2009; Shao et al. 2012). Examining the modulation of ET cell function, therefore, would help unravel the complexity of the olfactory glomerular circuit.
The OB receives cholinergic inputs from the horizontal limb of the diagonal band of Broca (Luskin and Price 1982; Zaborszky et al. 1986). Within the OB, the glomerulus exhibits the highest level of cholinergic innervation (Gomez et al. 2006; Le Jeune et al. 1995; Salcedo et al. 2011), and its distribution within the locus is sculpted by olfactory activity (Salcedo et al. 2011). Radiolabeling studies indicate that the glomerulus also exhibits a high expression of nAChRs (Le Jeune et al. 1995). However, detailed studies of cholinergic modulation at the glomerulus are lacking. Behavioral work suggests an important role of ACh in modulating odor discrimination, odor detection, and olfactory perceptual learning (Chaudhury et al. 2009; Hellier et al. 2012; Mandairon et al. 2006; Rushforth et al. 2010). Previous electrophysiological studies have demonstrated the role of muscarinic ACh receptors in modulating the reciprocal dendrodendritic synapses between MCs and the GABAergic granule cells (Castillo et al. 1999; Ghatpande et al. 2006; Pressler et al. 2007), while also providing insights into the role of nicotinic ACh receptors (nAChRs) in regulating the activity of MCs and a subpopulation of juxtaglomerular interneurons. Activation of nAChRs has been shown to excite MCs in the main, as well as in the accessory, OB (Castillo et al. 1999; D'Souza and Vijayaraghavan 2012; Smith and Araneda 2010) and control ON-to-MC transmission (D'Souza and Vijayaraghavan 2012). In agreement with the latter study, a more recent in vivo study has demonstrated that activation of basal forebrain cholinergic neurons regulate the responses of MCs to inhaled odors (Ma and Luo 2012).
Despite these recent advances in our understanding of cholinergic control of the OB circuitry, the modulation of identified ET cells has not been examined, arguably because the importance of this population of cells in olfactory information processing has only recently been demonstrated. In this study, we demonstrate that glomerular α4β2*-nAChR activation leads to two distinct effects on ET cells: direct excitation, and an increase in the frequency of postsynaptic GABAergic currents. Our results imply that ET cell nAChRs play an important role in modulating glomerular inhibition.
MATERIALS AND METHODS
Animals.
All experiments were performed using protocols approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus. Eleven- to sixteen-day-old FVB mice (Charles River) were used for the experiments described. The mice were anesthetized using isofluorane inhalation, after which they were decapitated using a guillotine.
Slice preparation.
Horizontal slices 280 μm thick were prepared from mouse OBs using a Leica VT1000S (Nussloch, Germany) vibratome, in ice-cold sucrose-artificial cerebrospinal fluid (aCSF) containing (in mM) 72 sucrose, 83 NaCl, 26 NaHCO3, 2.5 KCl, 1 NaH2PO4, 20 glucose, 3 MgCl2, and 0.5 CaCl2, adjusted to 285–290 mosM. The slices were then allowed to rest in a custom-made chamber containing aCSF at ∼32°C for ∼45 min before being incubated in the same solution at room temperature until recordings. This resting solution had the following substitutions made (in mM): 120 NaCl, no sucrose. Recordings were performed in aCSF containing (in mM) 1 MgCl2 and 2 CaCl2. All solutions were bubbled with 95% O2 and 5% CO2.
Cell identification and electrophysiology.
All recordings were performed between 32°C and 35°C, using a Dual Automatic Temperature Controller from Warner Instrument. Slices were continuously perfused with bubbled aCSF at a rate of ∼2 ml/min.
ET cells were filled with 50 μM Alexa 488 dextran and identified by their pear-shaped bodies in the glomerular layer, a distinct primary dendrite that ramifies extensively within a single glomerulus, and relatively large (>10 μm) cell bodies (Antal et al. 2006; Hayar et al. 2004). Imaging was performed using a charge-coupled device camera (Imago SensiCam, PCO Germany) and a Lambda DG-4 light source (Sutter Instruments, Novato, CA), under the control of Imaging Workbench image data acquisition software (Imaging Workbench, Santa Clara, CA). Optical excitation filters for epifluorescence were obtained from Chroma Technology. The input resistances of ET cells from which we recorded had a mean value of 280 ± 15.5 MΩ (n = 108).
The internal solution for whole-cell recordings contained (in mM) 123 potassium-gluconate, 2 KCl, 0.1 EGTA, 10 HEPES, 2 Na-ATP, and 0.5 Na-GTP (pH = 7.2). Voltage and current recordings were acquired using AxoGraph X and a MultiClamp 700B amplifier (Molecular Devices). Data were low-pass filtered at 2 kHz using a Bessel filter and acquired every 10 μs. Access resistances were monitored to ensure the stability of recordings. Recordings were made with patch pipettes with resistances ranging from 4 to 7 MΩ. For recording miniature inhibitory postsynaptic currents (mIPSCs), the internal solution was changed to a cesium chloride-based one as described previously, with an chloride equilibrium potential of 0.85 mV (Ghatpande et al. 2006). Tetrodotoxin (TTx) 1 μM, 10 μM 6,7-dinitroquinoxaline-2,3 dione (DNQX), and 50 μM D-2-amino-5-phosphonopentanoic acid (D-AP5) were present throughout these experiments in order block action potentials (APs) and ionotropic glutamate receptors (iGluRs), respectively. Under these conditions, no events were observed if 10 μM gabazine were added to the medium (data not shown).
Focal pressure application of agonists was performed using Picospritzer III (Parker Instruments, Cleveland, OH). Pressures were typically <5 psi. Electrical stimulations of the ON were performed using a Stimulus Isolator from WPI (Sarasota, FL). The stimulus intensities were adjusted to elicit submaximal responses from ET cells.
Data analyses.
Axograph X was used to measure the peak amplitudes and charge transfer of nAChR currents as well as to identify and count spontaneous inhibitory postsynaptic currents (sIPSCs) and spontaneous excitatory postsynaptic currents (sEPSCs). Mean values were compared for statistical significance using Student's t-test on Origin 6.0 software (Microcal). Unless otherwise stated, values given are as means ± SE. The Kolmogorov-Smirnov (K-S) test was used for comparing distributions for statistical significance. Analyses of mIPSCs were carried out using the Mini Analysis software (Synaptosoft).
Materials.
Alexa Fluor 488 dextran was obtained from Invitrogen (Life Technologies, Carlsbad, CA). All other chemicals were obtained from either Tocris Bioscience (Ellisville, MO) or Sigma (St. Louis, MO).
RESULTS
Modulation of ET cell activity by glomerular nAChR activation.
We first sought to observe the effects of glomerular nAChR activation on ET cells. We recorded from ET cells during focal (puff) application of ACh in the presence of the muscarinic ACh receptor antagonist atropine (At; 2 μM, in the bath solution as well as in the puffer pipette). Such applications of ACh in the presence of 2 μM At will be referred to as “ACh/At” throughout the paper. The concentration of At used was sufficient to block muscarinic effects in this preparation (D'Souza and Vijayaraghavan 2012). Puff applications of 1 mM ACh/At, on the glomerular arborization of ET cells, resulted in two distinct effects. ET cells voltage clamped at −70 mV exhibited small, and slow, inward currents (Fig. 1Aii) whose amplitudes showed large variations between cells. In general, the currents were much smaller (84 ± 4.2% smaller, P < 0.0001) than the currents we previously observed in MCs (D'Souza and Vijayaraghavan 2012). Since the currents were slow and had low peak amplitudes, we calculated both the peak amplitudes as well as the net charge transfer upon ACh/At application. The peak amplitudes of the ACh/At-induced slow currents, at −70 mV, ranged from 4.1 pA to 129 pA (mean = 39 ± 5.4 pA, n = 42). The mean integral for the slow inward currents in cells held at −70 mV was 186 ± 38 pA.s (n = 42); the currents were usually not detectable at more positive potentials (see Fig. 1Aii, top trace). In the presence of 1μM TTx, 50 μM DNQX, and 100 μM D-AP5, to block APs and iGluRs, the peak current obtained after ACh/At application was 33.3 ± 7 pA (n = 10; P = 0.5 compared with current in the absence of the blockers; range 10 pA to 134 pA). The results suggest that a large fraction of the observed currents was due to direct activation of nAChRs on ET cells.
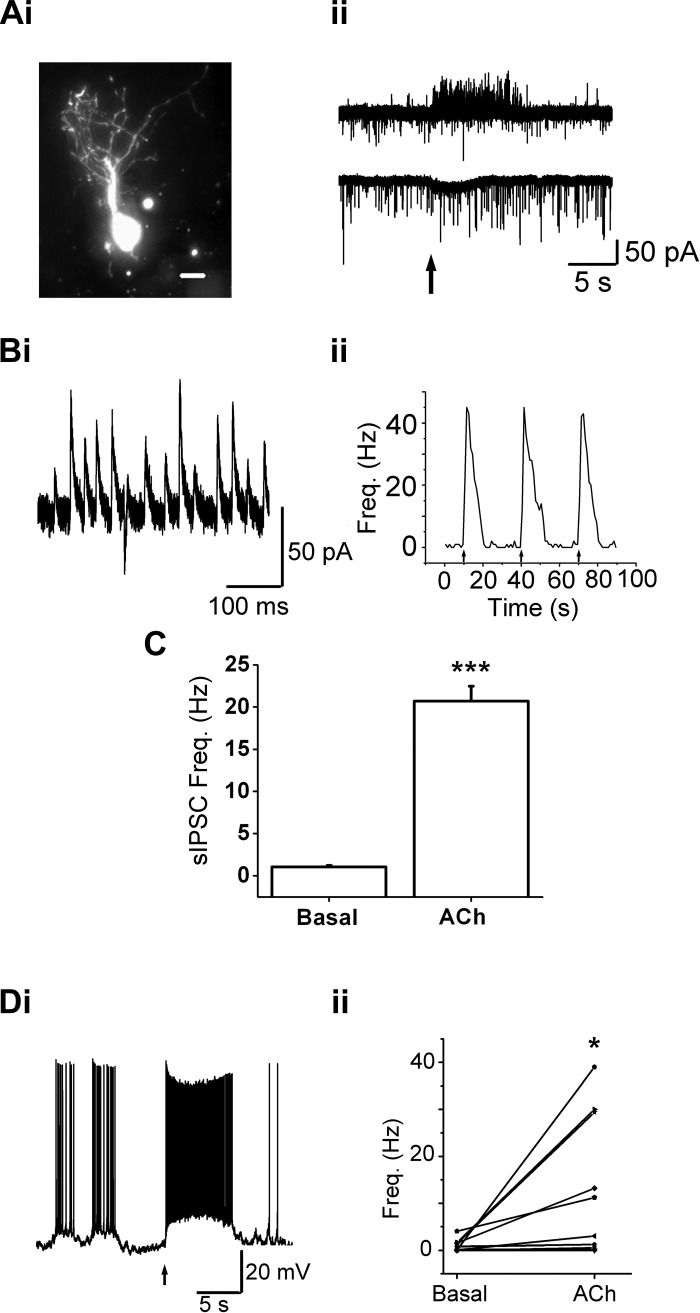
Fig. 1.
Acetylcholine (ACh)-mediated slow currents and increased spontaneous inhibitory postsynaptic current (sIPSC) frequency in external tufted (ET) cells. Ai: an ET cell loaded with Alexa 488 dextran displays the extensive dendritic arborization within a glomerulus. Scale bar = 10 μm. Aii, top trace: a 1-s, 1 mM ACh/atropine (At) application (arrow) leads to a barrage of sIPSCs (upward deflections) in the ET cell from Ai, held at −30 mV. Bottom trace: the same cell, when held at −70 mV, displays a small, slow inward current upon ACh/At application, that is superimposed on spontaneous excitatory postsynaptic currents (sEPSCs) (downward deflections). Bi: a few individual sIPSCs from the barrage in the upper trace in Aii. Bii: frequency plot of sIPSCs in the ET cell upon three consecutive ACh/At (1 s) applications (beginning at arrows). C: ACh/At application increased the average sIPSC frequency by 20.3 ± 1.7-fold (n = 52, ***P < 0.001). Di: an ET cell, under current clamp, exhibits an increase in its average spike frequency upon 1 mM ACh/At application (1 s beginning at arrow). Action potential (AP) frequencies were calculated for 5 s from the onset of agonist application. Dii: ET cells exhibit a significant increase in average firing frequency (n = 11, *P < 0.05) upon nicotinic acetylcholine receptor (nAChR) activation, recorded under whole-cell current clamp.
ET cells also exhibited an increase in the frequency of sIPSCs upon nAChR activation (Figs. 1, Aii, B, and C). To detect sIPSCs, the cells were held at more positive potentials (typically −30 or −40 mV). sIPSCs were detected as outward synaptic currents (Fig. 1Bi) and were blocked by the GABAA receptor antagonist gabazine (10 μM). Bath application of gabazine abolished all sIPSCs, both in control as well as upon ACh/At applications (n = 4). Upon focal applications of 1 mM ACh/At, the mean sIPSC frequency rose from a basal rate of 1.02 ± 0.2 Hz to an increased rate of 20.7 ± 1.77 Hz in the presence of the agonist (n = 52; P < 1E-12, paired t-test; Fig. 1C). Thus nAChR activation leads to a significant increase in the release of GABA onto ET cells.
Under current clamp, ACh/At applications led to depolarization of ET cells that, on average, resulted in increased AP firing (Fig. 1Di). In agreement with our observations from cells under voltage clamp conditions, the ACh-mediated increase in firing was variable between cells (Fig. 1Dii). 1 mM ACh/At application (1 s) increased average firing rates from 0.93 ± 0.36 Hz to 12.15 ± 4.3 Hz (n = 11; P < 0.03, paired t-test; Fig. 1Dii). These data suggest that excitatory nAChR currents can cause sufficient depolarization of the membrane to increase spiking in ET cells. The finding suggests that, even though nAChR currents are small in the ET cells, they are still sufficient to affect ET cell excitability in a significant manner.
The ACh/At-mediated inward currents on ET cells were blocked by the nAChR-antagonist mecamylamine (Mec; 5 μM; Fig. 2, Ai–Aiii), confirming the effect to be nAChR mediated. Mec reduced mean nAChR currents to 4.2 ± 1.4% of control (n = 5, P < 1E-10, paired t-test). Upon washout, the currents recovered to 27.4 ± 3.8% of the control ACh/At response (n = 4, P < 1E-5; compared with blocked levels; Fig. 2Aiii). Mec also abolished the ACh/At-induced increase in the sIPSC frequency (n = 10; P = 0.86, paired t-test; not different from basal sIPSC frequency; Fig. 2, Bi–Biii).
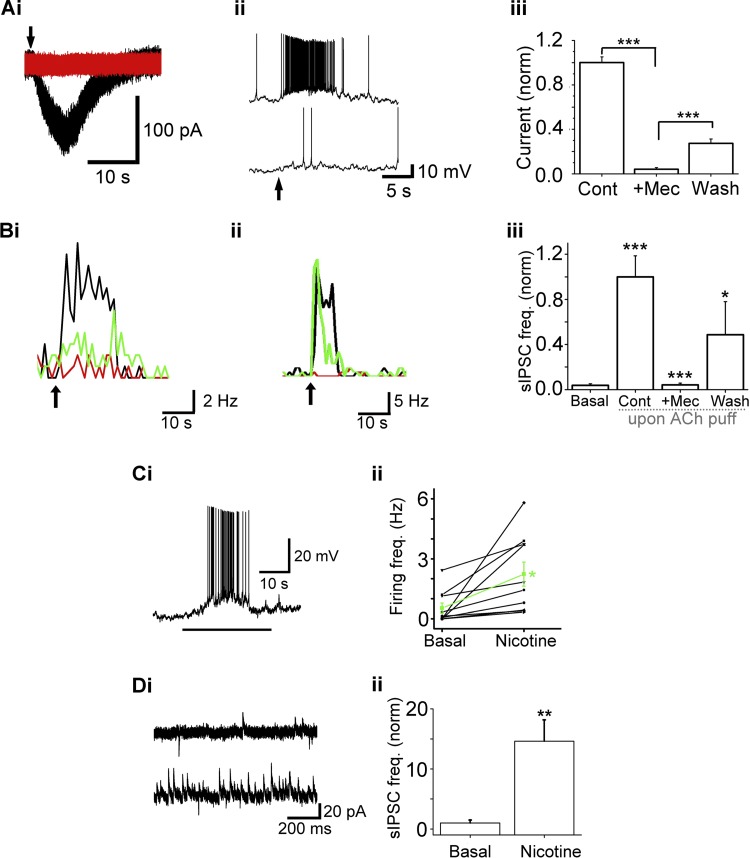
Fig. 2.
Nicotine and mecamylamine (Mec) confirm nAChR effect. Ai: ACh/At-induced inward current [black trace, control (Cont) conditions] is abolished upon bath application of 5 μM Mec (red trace). Aii: the same cell as in Ai is depolarized upon ACh/At application (upper trace). The depolarization is blocked during bath application of 5 μM Mec (bottom trace). Aiii: effect of Mec on nAChR currents (Cont, ACh/At alone; +Mec, ACh/AT-induced currents in the presence of 5 μM Mec; Wash, ACh/At response after washout of the antagonist). Mec causes a reversible block of 1 mM ACh/At-induced currents (***P < 0.001). Cont: n = 5; +Mec: n = 5; Wash: n = 4. Bi and Bii: 5 μM Mec reversibly blocks the increase in sIPSC frequency in ET cells. Frequency plots from two cells are shown. Arrows denote the start of 1-s, 1 mM ACh/At applications. Black: ACh/At; red: ACh/At responses in the presence of 5 μM Mec; green: ACh/At responses after washout of the antagonist. In Bi, Mec reduced ACh/At responses by 80% of the Cont responses, which recovered to 65% of Cont values after a 15-min wash. In Bii, Mec blocked responses by 97.3%, and the response recovered to 77% of Cont values. Biii: Mec reversibly abolishes the ACh/At-induced increase in sIPSC frequency (***P < 0.001, *P < 0.05). Basal: sIPSC frequency under basal conditions; n = 10; ACh/At: n = 10; +Mec: n = 10; Wash: n = 4. Events were calculated over 10 s from the time of agonist application. Ci: a 30-s application of 5 μM nicotine (horizontal bar) results in the depolarization and increased firing of an ET cell. Cii: 5 μM nicotine application increases the mean firing rates of individual ET cells. Mean increase in frequency is represented by the green line (n = 10, *P < 0.05). Di: 5 μM nicotine application increases the average sIPSC frequency in an ET cell. Top trace, basal; bottom trace, response to nicotine. Dii: nicotine application resulted in a 14.6 ± 3.5-fold increase in sIPSC frequency (n = 9, **P < 0.01).
The excitation of ET cells, as well as the increase in sIPSC frequency, upon nAChR activation was mimicked by application of nicotine. Glomerular applications of nicotine (5 μM, 30 s) increased the average spike frequency, measured under current clamp configuration, from 0.53 ± 0.26 Hz to 2.24 ± 0.60 Hz (n = 10; P < 0.05, paired t-test; Fig. 2, Ci and Cii). Nicotine also increased the sIPSC frequency from 1.00 ± 0.5 Hz to 14.6 ± 3.5 Hz (n = 9; P < 0.01, paired t-test; Fig. 2, Di and Dii). Data from experiments in this section confirm the nicotinic nature of the ACh/At responses on ET cells.
Excitation-dependent inhibition in the glomerulus.
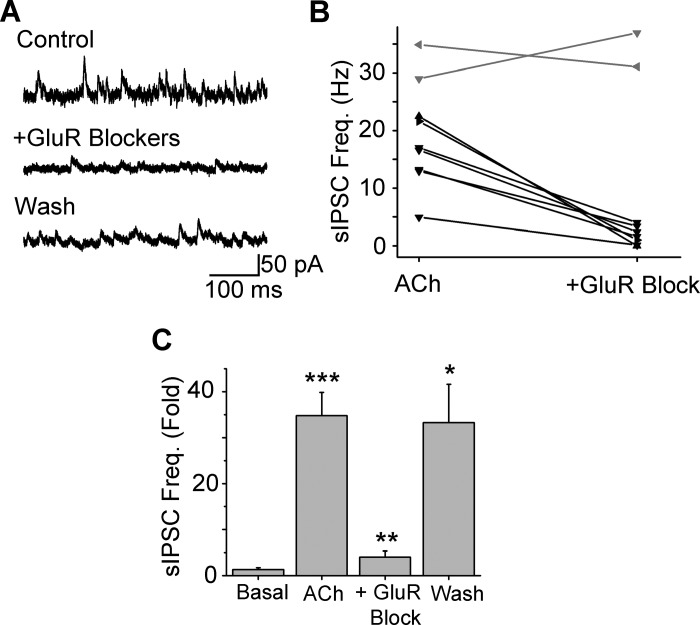
We next examined whether the increase in GABA release on ET cells was due to the direct activation of nAChRs on GABAergic interneurons, such as the local PG cells, as has been suggested (Castillo et al. 1999). nAChR-mediated sIPSC increases were examined under conditions where the excitatory glutamate receptors (GluRs) were blocked by having DNQX (50 μM) and D-AP5 (100 μM) in the aCSF. Due to previous studies implicating a role of metabotropic glutamate receptors (mGluRs) in OB processing (Dong et al. 2007, 2009), the mGluR blocker, (+)-α-methyl-4-carboxyphenylglycine (1 mM) was also included in the bath. Consistent with the idea of GluR-dependent GABA release, the cocktail of GluR blockers significantly blocked nAChR-evoked sIPSC frequency increases in 7 out of 9 ET cells tested (Fig. 3, A and B; P < 0.001; K-S test for each of the 7 cells). The mean inhibition of the sIPSC increase was 88.5 ± 3.8% (Fig. 3C; n = 7, P < 0.002).
Fig. 3.
nAChR-induced sIPSC frequency increase is excitation dependent. A: the ACh/At increase in sIPSC frequency in a representative ET cell is reversibly blocked by bath application of 50 μM 6,7-dinitroquinoxaline-2,3 dione (DNQX), 100 μM D-AP5, and 1 mM (+)-α-methyl-4-carboxyphenylglycine (MCPG). B: bath application of 50 μM DNQX, 100 μM D-AP5, and 1 mM MCPG significantly reduced the sIPSC frequency upon ACh/At application in 7 out of 9 cells [black; P < 0.05, Kolmogorov-Smirnov (K-S) test]. No significant block of sIPSC frequency by glutamate receptor (GluR) blockade was observed in the other 2 cells (gray). C: summary of effects with GluR blockade (n = 7 cells). GluR blockers reversibly block ACh/At-induced increases in sIPSC frequency. ***P < 0.001 (basal vs. ACh); **P < 0.01 (ACh/At alone vs. GluR block); *P < 0.05 (block vs. wash).
These observations, taken together, suggest that nAChR activation primarily depolarizes the ET cells, which in turn, activate GABAergic synapses (likely, the dendrodendritic synapses with PG cells), leading to GABA release back onto the ET cells. These data are consistent with excitation-dependent GABA release observed in MCs in an earlier study (D'Souza and Vijayaraghavan 2012). The excitatory drive, thus, appears to be provided by both ET cells and MCs. Whether a fraction of the GABA release occurs from direct action of ACh/At on PG cells, short axon cells, or any other GABAergic inputs to ET cells, remains to be resolved.
Pharmacology of nAChR effects on ET cells.
The sensitivity of the ACh/At-mediated effects to low concentrations of Mec, and a previous autoradiographic study suggesting a high level of α4β2-containing nAChR expression in the glomerulus (Le Jeune et al. 1995), suggested that these effects might be mediated by the α4β2* nAChR subtype (* indicates the possible presence of other subunits). We, therefore, tested the effects of the α4β2-receptor antagonist dihydro-β-erythroidine hydrobromide (DHβE) on the ACh/At-induced currents. 10 μM DHβE significantly blocked the slow, inward current (n = 9; 70 ± 6.6% decrease in charge transfer, P < 0.01; Figs. 4, Ai and Aii), indicating that the current is mediated by the α4β2* nAChR. The currents were recovered upon washout (to 97.1% of control values, n = 4, P < 0.05 compared with the blocked response). On the other hand, the α3β4*-nAChR antagonist conotoxin AuIB (CTx AuIB; 10 μM) did not significantly block the nAChR currents on ET cells (responses in the presence of the toxin was 104 ± 29% of the control ACh/At responses; n = 6; Fig. 4, Ci and Cii).
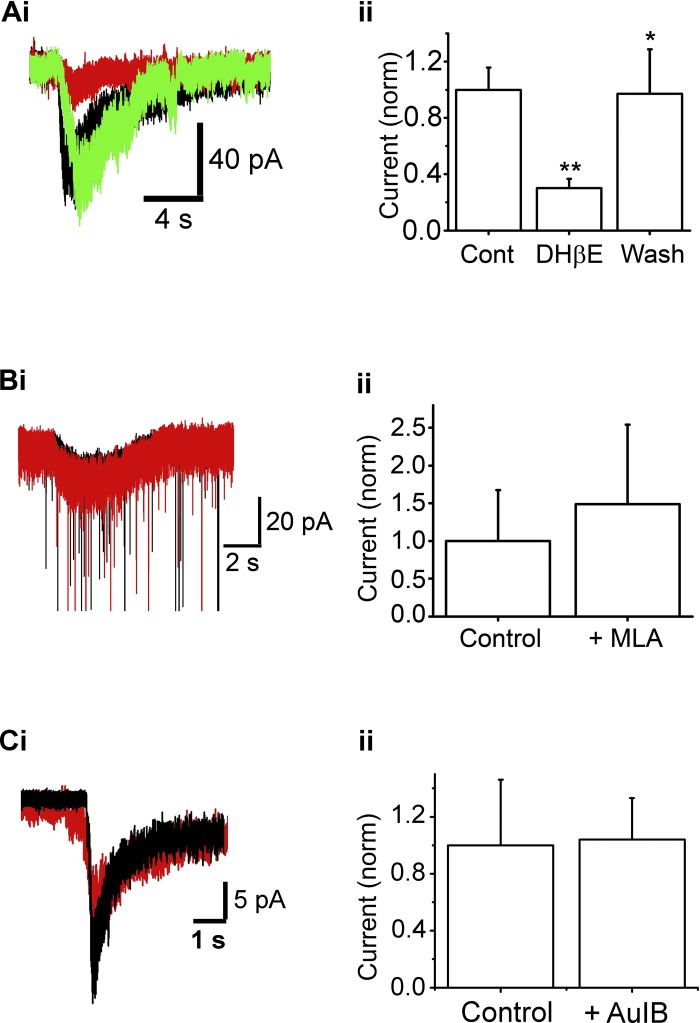
Fig. 4.
Functional α4β2-nAChRs are expressed on ET cells. Ai: raw trace of ACh/At-induced current in an ET cell under Cont conditions (black), in the presence of 10 μM dihydro-β-erythroidine hydrobromide (DHβE; red), and after washout of DHβE (green). Cell was held at −70 mV. Aii: summary of effect of 10 μM DHβE on ACh/At-induced currents normalized to currents under Cont conditions (**P < 0.01, *P < 0.05). n = 9 (Cont and DHβE); n = 4 (Wash). Bi: 1-s, 1 mM ACh/At (starts at arrow) results in a slow inward current under Cont (black trace) as well as in the presence of 10 nM methyllycaconitine (MLA; red), at −70 mV. Some sEPSCs have been truncated. Bii: MLA application does not significantly alter the ACh/At-induced current (n = 5, P > 0.2). Ci: nAChR current response in the absence (black) and presence (red) of 10 μM CTx-AuIB. The current was not significantly inhibited. Cii: mean values from 6 cells. The presence of CTx AuIB did not significantly attenuate ACh/At responses, suggesting the α3β4*-nAChR subtype is not a major contributor to the nAChR currents on ET cells.
Because α7-nAChRs are thought to be expressed in the glomerular layer (Hellier et al. 2010; Le Jeune et al. 1995), we examined whether blocking this receptor subtype altered ACh/At-induced effects in ET cells. Bath application of 10 nM methyllycaconitine (MLA), a selective blocker for the α7-nAChR, did not significantly affect the ACh-induced slow current. In the presence of the α7-nAChR antagonist, the mean current, upon ACh/At application was 41.9 ± 29 pA (control amplitude: 28.2 ± 19 pA; n = 4, P > 0.06, paired t-test; Fig. 4, Bi and Bii).
These results suggest that the major component of the ET-cell response to agonist application arises from the activation of α4β2*-nAChRs with minor, if any, contributions from the other receptor subtypes expressed in the glomerulus, viz. the α3β4*-nAChRs and α7*-nAChRs.
We next examined the pharmacology of the nAChR-mediated changes in sIPSCs on ET cells. DHβE 10 μM also resulted in a significant decrease in the frequency of ACh/At-induced sIPSCs. In the presence of the blocker, the ACh/At-mediated increase in sIPSC frequency was reduced by 33 ± 5.8% (n = 7, P < 0.05; Fig. 5A). Basal frequency of sIPSCs did not change upon application of DHβE (n = 7, P > 0.2), thus arguing against tonic control by the endogenous transmitter or sIPSC rundown. The mean sIPSC frequency upon ACh/At application was 31 ± 8.2 Hz under control conditions and 25 ± 9.5 Hz in the presence of 10 nM MLA (n = 5, P = 0.4; Fig. 5B).
Fig. 5.
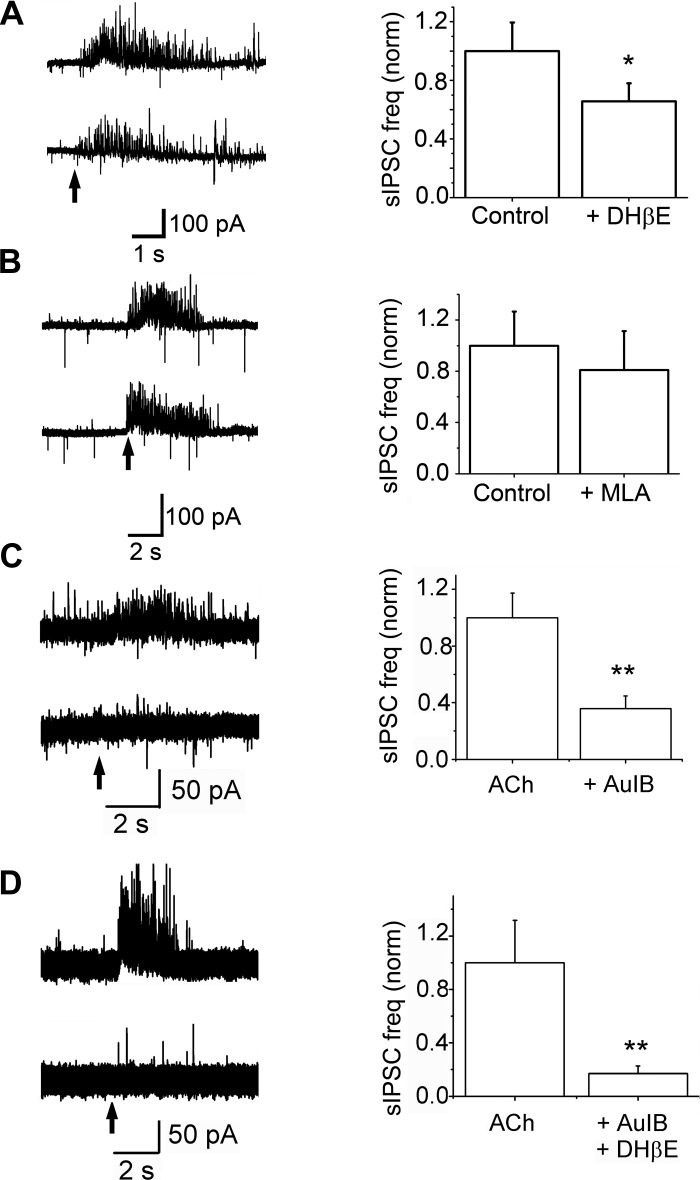
Pharmacology of nAChR-mediated increase in sIPSC frequencies. A, left: raw trace of an ET cell, held at −30 mV, under Cont conditions (top trace) and in the presence of 10 μM DHβE (bottom trace). Arrow indicates the start of a 1-s, 1 mM ACh/At application. Right: bath application of 10 μM DHβE results in a 33 ± 5% decrease in ACh/At-induced sIPSC frequency (n = 7, *P < 0.05). B, left: at −30 mV, ACh/At application (arrow) results in an increase in sIPSC frequency under Cont (top trace) as well as during bath application of MLA (bottom). Right: 10 nM MLA does not significantly change the sIPSC frequency increase caused by ACh/At application (n = 5, P > 0.5). C, left: raw trace showing ACh/At-induced increases in sIPSCs in the absence (top) and presence (bottom) of 10 μM CTx-AuIB applied for 3 min via a puffer pipette. Right: cumulative data from 5 cells. CTx-AuIB blocked the increase in sIPSC frequencies by 64 ± 9% (**P = 0.011, paired t-test). D, left: raw trace showing sIPSC increases in the absence (top trace) and in the presence (bottom trace) of 10 μM DHβE and 10 μM CTx-AuIB applied for 2 min as in C. Right: cumulative data from 7 cells. The two antagonists together blocked the sIPSC frequency increases by 83 ± 6% (**P = 0.02, paired t-test).
To determine if some of the sIPSC increases on ET cells come from the activation of α3β4*-nAChRs, we examined the effects of CTx AuIB (10 μM). After eliciting a control response, the drug was applied to the recorded cell via a puffer pipette for 3 min prior to a second agonist application. Results are shown in Fig. 5C. Application of CTx AuIB reduced nAChR-mediated increases in sIPSC frequencies by 64 ± 9% (n = 5; P < 0.01).
In the presence of both CTx AuIB and DHβE, nAChR-mediated increase in sIPSC frequencies was reduced by 87 ± 27% (n = 7; P = 0.02; Fig. 5D). In four of these seven cells, a 5-min wash resulted in recovery of the response to 103 ± 23% of control responses (P = 0.5; control vs. wash).
An interesting result obtained from the pharmacology studies is that DHβE blocks a lesser fraction of sIPSCs on ET cells (33% inhibition) than the nAChR currents (70% inhibition), while an α3β4*-nAChR antagonist had no significant effect on the ET-cell currents, but a larger inhibition of the sIPSC increases. A possible explanation for this apparent discrepancy is that, upon glomerular network activation by nAChRs, both MCs and ET cells contribute to the excitation-dependent feedback inhibition.
nAChR effects on mIPSCs.
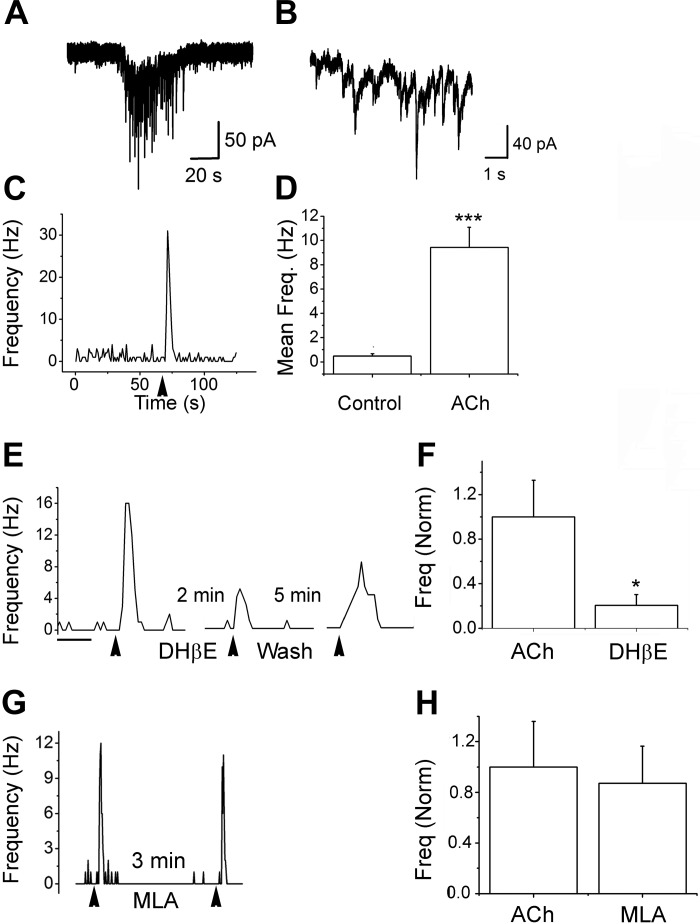
Under conditions that do not activate the glomerular network, do α4β2*-nAChRs on ET cells have local effects on ET-PG signaling? To examine this, we tested the effects of ACh/At on mIPSCs. For these experiments, the recording pipette contained CsCl-based internal solutions. iGluR blockers and TTx were included in the aCSF to isolate gabazine-sensitive events as shown before (Ghatpande et al. 2006). A brief (3 s) application of the agonist resulted in a burst of mIPSCs from the ET cells (Fig. 6, A–C). Upon ACh/At application, the mean mIPSC frequencies rose from a basal 0.48 ± 0.17 Hz to 9.44 ± 1.64 Hz (n = 17; P = 6E-5; Fig. 6D). Local application of 10 μM DHβE for 3 min reduced nAChR effects by 80 ± 9% (n = 5, P = 0.03; Fig. 6, E and F). In two cells, a 5-min wash resulted in recovery of responses to 78% of control values. On the other hand, a 3-min application of 10 nM MLA resulted in small and variable effects that did not reach statistical significance (mean mIPSC frequencies in the presence of MLA was 87 ± 21% of that obtained from ACh/At alone; n = 7; P = 0.2; Fig. 6, G and H). These results suggest that α4β2*-nAChRs might regulate local changes in synaptic strength at the ET-PG dendrodendritic synapses. Further experiments need to be done to determine the location of the receptors at these synapses. Interestingly, in three cells the increase in mIPSCs was completely blocked by bath application of the mGluR type 1 blocker (+)-α-methyl-4-carboxyphenylglycine (1 mM; 90 ± 5% inhibition, P = 0.02, recovered to 42% and 158% of control values after a 15-min wash; iGluR blockers and TTx were present as well). These results suggest that local AP-independent sIPSC increases mediated by α4β2*-nAChRs might involve feedback mechanisms as well. More information about nAChR effects on PG cells is required to confirm this finding.
Fig. 6.
α4β2*-nAChRs increase miniature inhibitory postsynaptic current (mIPSC) frequencies on ET cells. mIPSCs were examined under voltage-clamp in the presence of 1 μM tetrodotoxin, 10 μM DNQX, and 50 μM D-AP5 to block action potentials and ionotropic GluRs. The internal solution was based on CsCl to give a chloride equilibrium potential of 0.85 mV (Ghatpande et al. 2006). Thus mIPSCs are inward. All events were abolished by the addition of 10 μM gabazine. A: application of 1 mM ACh/At for 3 s resulted in an inward nAChR current with a barrage of mIPSCs superimposed on it. B: expanded segment of the trace in A. C: frequency plot for the cell shown in A. Application of ACh/At results in a burst of mIPSCs with a peak frequency ∼30-fold that of the baseline. D: cumulative data for mean frequency change from 17 cells. nAChR activation caused a 20-fold increase in mIPSC frequencies (***P < 0.0001). E: frequency plot for the nAChR effect. Three applications of ACh/At (3 s/application) were performed at the arrowheads. After the first application, 10 μM DHβE were applied via a puffer pipette for 2 min. The second nAChR response in the presence of the antagonist was attenuated. A third agonist application was carried out in this cell after a 5-min washout of the antagonist and shows a partial recovery. F: cumulative data from 5 cells. DHβE blocked the mIPSC frequency increases by 80 ± 9% (*P = 0.03). G: two agonist applications before and after a 3-min application of 10 nM MLA. The two responses were not different from each other. H: cumulative data from 7 cells. mIPSC frequencies in the presence of MLA were 87 ± 29% of the responses with ACh/At alone (P = 0.2).
ACh/At application does not modulate ON-to-ET cell transmission.
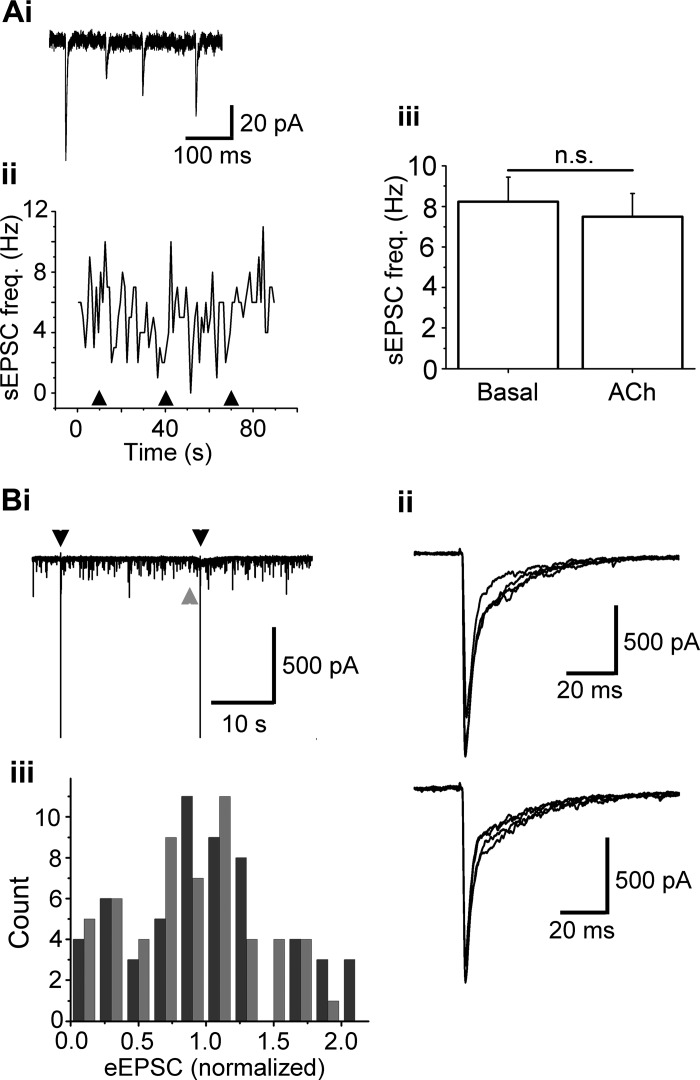
In MCs, the activation of nAChRs results in inhibition of ON-evoked EPSCs (D'Souza and Vijayaraghavan 2012), resulting from feedback GABA release in the glomerulus. As ET cells receive direct input from the ON, and as there is evidence that ET cells drive MC output (De Saint et al. 2009; Gire et al. 2012), we examined the effects of nAChR activation on ON-ET transmission. A 1-s ACh/At (1 mM) application did not significantly change the sEPSC frequency in ET cells. At −70 mV, ET cells exhibited a basal, mean sEPSC frequency of 8.2 ± 1.2 Hz; upon ACh/At application the mean frequency was 7.5 ± 1.1 Hz (n = 21; P > 0.1, paired t-test; Fig. 7, Aii and Aiii). The sEPSCs were abolished by 50 μM DNQX + 100 μM D-AP5, indicating that they were mediated by the ionotropic GluRs (n = 5). The nAChR-mediated slow, inward ET cell currents persisted even in the presence of the GluR blockers (mean peak current amplitude = 36 ± 15 pA; n = 7; also see above), suggesting a direct action of nAChRs expressed on ET cells. Since, to the best of our knowledge, these fast glutamatergic sEPSCs arise from the ON terminals, our observations suggest that glomerular nAChR activation does not affect spontaneous glutamate release from these terminals.
Fig. 7.
nAChR activation does not alter olfactory nerve (ON)-to-ET cell transmission. Ai: a few individual sEPSCs from the bottom trace in Fig. 1Aii on an expanded scale. Aii: frequency plot of sEPSCs from the same cell as in Fig. 1, A and B, voltage clamped at −70 mV. Arrowheads denote the start of a 1-s, 1 mM ACh/At application. Aiii: ACh/At does not alter the sEPSC frequency in ET cells (n = 21, P > 0.1). n.s., nonsignificant. Bi: recordings from an ET cell held at −70 mV, to observe the effect of glomerular ACh/At application (gray arrowhead) on evoked postsynaptic responses [evoked excitatory postsynaptic currents (eEPSCs)] to electrical stimulation of the ON (black arrowheads). Bii: expanded overlaid traces of eEPSCs under Cont conditions (top) and during the slow, ACh/At-induced current (1.5 s after the start of ACh/At application; bottom). Biii: nAChR activation does not alter eEPSC amplitudes. Black: Cont; gray: 1.5 s after the start of ACh/At puff; n = 8 cells, P > 0.6, K-S test. eEPSCs are normalized to the mean of the amplitudes under Cont condition.
Further, we asked if ET cell responses to ON input can be modulated by the activation of glomerular nAChRs, as has been observed for MCs (D'Souza and Vijayaraghavan 2012). We electrically stimulated the ON, and recorded evoked EPSCs (eEPSCs) from ET cells under voltage clamp, before and during focal applications of 1 mM ACh/At. Interestingly, unlike in MCs (D'Souza and Vijayaraghavan 2012), nAChR activation did not alter the amplitude distribution of eEPSCs in ET cells (n = 8; Fig. 7B, P = 0.64; K-S test). The lack of effect of ACh/At on sEPSC frequencies as well as on eEPSC amplitudes suggest that nAChRs on ON terminals, if present, do not modulate glutamate release at the ON-ET cell synapse.
DISCUSSION
Each OB glomerulus, which is considered a functional and anatomical unit for odor processing, receives converging homogenous information from olfactory sensory neurons that express the same olfactory receptor protein (Johnson et al. 1998; Rubin and Katz 1999; Wachowiak and Cohen 2001). Different combinations of activated glomeruli are therefore thought to code for different individual odors (Mori et al. 1999). Consequently, modulation of glomerular signaling should play a crucial role in the detection, perception, and discrimination of different odors. We have demonstrated that activation of glomerular nAChRs depolarizes ET cells, a major class of juxtaglomerular neurons. In addition, ET cells also exhibit an increase in sIPSC frequency upon nAChR activation. This increase in the frequency of sIPSCs was, for the most part, blocked by GluR blockers. Such an excitation-dependent inhibitory mechanism was also observed in MCs (D'Souza and Vijayaraghavan 2012) and could play a key role in shaping the output of the olfactory glomerulus during active sniffing of an odor, by potentially limiting the window of glomerular excitation, as well as bringing the glomerular circuitry to basal levels of activity before the start of a new inhalation of odorants (Shao et al. 2012).
A previous electrophysiological study of cholinergic modulation of the OB (Castillo et al. 1999) concluded that a subpopulation of juxtaglomerular neurons (which they referred to as “bipolar PG cells”) exhibits nicotinic currents. As the Castillo et al. study was carried out prior to a detailed characterization of the juxtaglomerular cell populations, the identity of nAChR-expressing neurons in this region remains unresolved.
Recent studies have provided interesting insights into the function of individual glomeruli in odor processing. ET cells, through glutamate release within the glomerulus, drive the output of the OB (De Saint et al. 2009; Gire et al. 2012) and would therefore be an important target for modulation. Indeed, the activity of ET cells has been recently shown to be modulated by the activation of serotonergic (Liu et al. 2012) as well as endocannabinoid receptors (Wang et al. 2012). Modulation of ET cell function by nAChRs is, therefore, also likely to modulate bulbar output.
nAChRs are ubiquitous in the central nervous system and are expressed in a laminar fashion in the OB (Le Jeune et al. 1995). Our experiments suggest the existence of α4β2* subtype of the receptor on ET cells. Our previous work (D'Souza and Vijayaraghavan 2012) showed the existence of α3β4*-nAChRs on MCs, and an earlier report demonstrated the presence of α-bungarotoxin binding sites (presumably the α7-nAChRs) in the glomerular neuropil (Le Jeune et al. 1995, 1996). Taken together, these results suggest the existence of at least three nAChR subtypes that potentially influence the input-output relationship in the OB. What purpose would such diversity serve? Different EC50 values for the endogenous agonist and different current kinetics could selectively activate receptors on distinct locations. Similarly, the subcellular location of the receptors, dendritic or somatic, synaptic or nonsynaptic, could make a difference in downstream signaling in response to ACh release. All of these forms of signaling are consistent with what we know of the cholinergic innervation to the OB. Studies have shown that the highest density of cholinergic fibers in the OB is seen in the glomerular layer, although the distribution in this layer is nonuniform (Macrides et al. 1981; Salcedo et al. 2011; Zheng et al. 1987). Studies on central nervous system cholinergic innervations also reveal that incoming axons exhibit multiple transmitter-containing varicosities: only a fraction of these make synaptic contacts (Contant et al. 1996; Descarries et al. 1997; Mechawar et al. 2002; Umbriaco et al. 1994). These studies indicate the possibility of diffusion-based signaling that might make receptor affinities and location more significant.
An unresolved issue is the role of the α7-nAChR subtype. Effects of MLA at 10 nM, if any, in this study and D'Souza and Vijayaraghavan (2012) have been subtle and inconclusive, in addition to being variable. Yet autoradiographic studies demonstrate dense α-bungarotoxin labeling in the glomerular neuropil (Hellier et al. 2010; Le Jeune et al. 1995). Furthermore, α7-subunit knockout mice show deficits in both odor discrimination (Hellier et al. 2010) and early odor learning preference tasks (Hellier et al. 2012), although these effects need not be mediated by bulbar nAChRs. A number of possibilities need to be examined. Are these receptors restricted to ONs? If so, they might mediate short- or long-term plasticity at the ON-PG synapses. Another possibility, based on our studies on this receptor subtype in the hippocampus (Sharma et al. 2008; Sharma and Vijayaraghavan 2003), is that these receptors control homeostatic plasticity at glomerular synapses and act as foil to the positive feedback loops inherent in hebbian processes. Our work in the hippocampus indicates that the activation of these receptors drives signaling mainly via presynaptic ER calcium stores, and that these effects are slow, requiring prolonged exposure to agonists. If true, it is likely that experimental paradigms used in this study do not reveal effects of this receptor subtype. Another possibility is the existence of heteromeric α7-nAChRs. The existence of functional α7β2*-nAChRs, with mixed pharmacology, has been demonstrated in the basal forebrain cholinergic receptors (Azam et al. 2003; Murray et al. 2012). A more detailed study is required to elucidate the role of this receptor subtype in the glomerular circuit, one not relying on the purely pharmacological approaches used in this study.
The overall idea behind this work and our previous one (D'Souza and Vijayaraghavan 2012) is that nAChR activation serves as a gain control mechanism for glomerular output in the OB. This mechanism allows for filtering out weak inputs while allowing stronger ones through. Such a mechanism could contribute to nAChR-driven increase in odor discrimination and perceptual learning. The key process is inhibition of MC output by GABA release from inhibitory interneurons in the glomerulus. The GABA release, presumably from PG cells, and perhaps short-axon cells (Whitesell et al. 2013), occurs because of the nAChR-mediated excitation of both ET cells and MC primary dendrites. Two subtypes of nAChRs, viz. the α4β2* and the α3β4*, appear to mediate this feedback inhibition. Our data suggests that the relative contributions, to nAChR responses, of the two receptor subtypes are different in ET cells and MCs. Based on our knowledge, thus far, we would predict that the two subclasses contribute synergistically to the net feedback inhibition mediated by glomerular network activation. The ET-specific action of α4β2* nAChRs might be to alter dendrodendritic synapses between these cells and PG neurons controlling, locally, the strength of these synapses, independent of network activation.
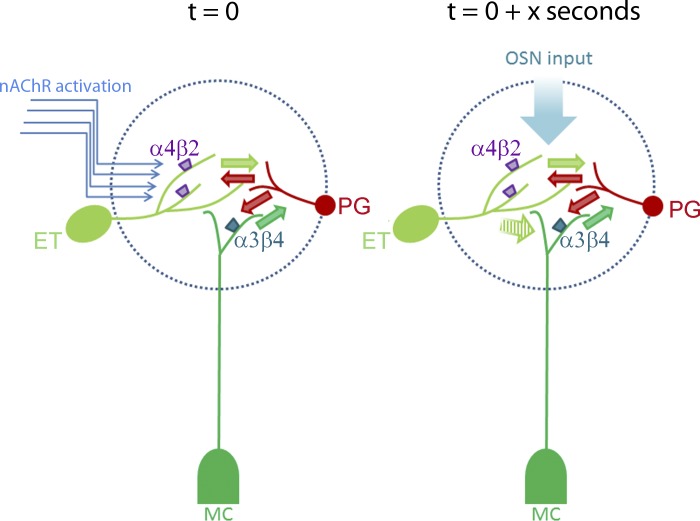
The key assumption in this model, based on our data (current study and D'Souza and Vijayaraghavan 2012) is that nAChR-mediated inhibition is due to excitation-driven feedback from PG cells (Fig. 8). As far as cholinergic regulation is concerned, it appears that feed-forward inhibition via PG cell activation might play a lesser role, if any. This assumption needs to be tested by examining nAChRs signals on PG cells, currently under examination.
Fig. 8.
A model summarizing the role of nAChRs in modulating glomerular output. The model summarizes our current state of knowledge based on this work and our previous study (D'Souza and Vijayaraghavan 2012). The arrival of cholinergic input (t = 0 s) has an excitatory effect on ET cells and mitral cells (MCs), via the activation of 4β2*-nAChRs and α3β4*-nAChRs, respectively, resulting in glutamate release onto inhibitory interneurons (green arrows). The net consequence of this excitation is a strong feedback inhibition from surrounding periglomerular (PG) cells (and perhaps, the short-axon cells). If an ON input arrives at time 0 + x seconds (the value of x to be determined), the feedback GABA release (red arrows) results in inhibition of MC output, resulting in failures upon weak stimulation. A possible locus for inhibition is ET-MC signaling (hatched green arrow). This possibility is based on our observation that slow eEPSCs on MCs are inhibited upon nAChR activation (D'Souza and Vijayaraghavan, 2012). Thus nicotinic modulation of the glomerular circuit results in an effective filtering mechanism for odor input. Modulation of direct ON-MC inputs (Najac et al. 2011) by nAChRs remains to be investigated. OSN, olfactory sensory neuron.
A tonic cholinergic tone seen during the awake state of an animal serves as a baseline tone for OB activity, maintaining a low level of MC firing. We expect that under specific behavioral situations like arousal and attention, the cholinergic input serves to enhance excitation-driven inhibition across glomerular circuits across the entire bulb, for a specific time period. ON inputs impinging upon MCs in this time window will be attenuated, enabling filtering of weak inputs. A key requirement for this model to work is that cholinergic input has to arrive within a specific time window prior to odor presentation. Such a scenario would also imply control of cholinergic modulation by basal forebrain neurons rather than the local OB circuitry, consistent with mediation of common attentional/learning mechanisms. This idea is supported by findings in the prefrontal cortex, where there is behavioral cue-evoked, phasic, spike in ACh levels, not present if the animal misses these cues (Parikh et al. 2007; Sarter et al. 2009). This transient increase in ACh levels occurs in the time course of seconds, similar to that tested here.
GRANTS
The work was funded by National Institute of Deafness and Other Communications Disorders Grant R01 DC-008855 (S. Vijayaraghavan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.D.D. and S.V. conception and design of research; R.D.D. and P.V.P. performed experiments; R.D.D., P.V.P., and S.V. analyzed data; R.D.D. and S.V. interpreted results of experiments; R.D.D. and S.V. prepared figures; R.D.D. drafted manuscript; R.D.D., P.V.P., and S.V. edited and revised manuscript; R.D.D., P.V.P., and S.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Nathan Schoppa and Diego Restrepo for helpful discussions.
Present address of R. D. D'Souza: Department of Anatomy and Neurobiology, Washington University School of Medicine, St. Louis, MO 63110.
REFERENCES
- Antal M, Eyre M, Finklea B, Nusser Z. External tufted cells in the main olfactory bulb form two distinct subpopulations. Eur J Neurosci 24: 1124–1136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119: 965–977, 2003 [DOI] [PubMed] [Google Scholar]
- Castillo PE, Carleton A, Vincent JD, Lledo PM. Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J Neurosci 19: 9180–9191, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci 29: 52–60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience 71: 937–947, 1996 [DOI] [PubMed] [Google Scholar]
- D'Souza RD, Vijayaraghavan S. Nicotinic receptor-mediated filtering of mitral cell responses to olfactory nerve inputs involves the alpha3beta4 Subtype. J Neurosci 32: 3261–3266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint Jan D, Hirnet D, Westbrook GL, Charpak S. External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29: 2043–2052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 53: 603–625, 1997 [DOI] [PubMed] [Google Scholar]
- Dong HW, Hayar A, Ennis M. Activation of group I metabotropic glutamate receptors on main olfactory bulb granule cells and periglomerular cells enhances synaptic inhibition of mitral cells. J Neurosci 27: 5654–5663, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Heinbockel T, Hamilton KA, Hayar A, Ennis M. Metabotropic glutamate receptors and dendrodendritic synapses in the main olfactory bulb. Ann NY Acad Sci 1170: 224–238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatpande AS, Sivaraaman K, Vijayaraghavan S. Store calcium mediates cholinergic effects on mIPSCs in the rat main olfactory bulb. J Neurophysiol 95: 1345–1355, 2006 [DOI] [PubMed] [Google Scholar]
- Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R, Schoppa NE. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J Neurosci 32: 2964–2975, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29: 13454–13464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Brinon JG, Colado MI, Orio L, Vidal M, Barbado MV, Alonso JR. Differential effects of unilateral olfactory deprivation on noradrenergic and cholinergic systems in the main olfactory bulb of the rat. Neuroscience 141: 2117–2128, 2006 [DOI] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24: 6676–6685, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Arevalo NL, Blatner MJ, Dang AK, Clevenger AC, Adams CE, Restrepo D. Olfactory discrimination varies in mice with different levels of alpha7-nicotinic acetylcholine receptor expression. Brain Res 1358: 140–150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Arevalo NL, Smith L, Xiong KN, Restrepo D. alpha7-Nicotinic acetylcholine receptor: role in early odor learning preference in mice. PLos One 7: e35251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol 393: 457–471, 1998 [DOI] [PubMed] [Google Scholar]
- Le Jeune H, Aubert I, Jourdan F, Quirion R. Comparative laminar distribution of various autoradiographic cholinergic markers in adult rat main olfactory bulb. J Chem Neuroanat 9: 99–112, 1995 [DOI] [PubMed] [Google Scholar]
- Le Jeune H, Aubert I, Jourdan F, Quirion R. Developmental profiles of various cholinergic markers in the rat main olfactory bulb using quantitative autoradiography. J Comp Neurol 373: 433–450, 1996 [DOI] [PubMed] [Google Scholar]
- Liu S, Aungst JL, Puche AC, Shipley MT. Serotonin modulates the population activity profile of olfactory bulb external tufted cells. J Neurophysiol 107: 473–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The distribution of axon collaterals from the olfactory bulb and the nucleus of the horizontal limb of the diagonal band to the olfactory cortex, demonstrated by double retrograde labeling techniques. J Comp Neurol 209: 249–263, 1982 [DOI] [PubMed] [Google Scholar]
- Ma M, Luo M. Optogenetic activation of Basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci 32: 10105–10116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Davis BJ, Youngs WM, Nadi NS, Margolis FL. Cholinergic and catecholaminergic afferents to the olfactory bulb in the hamster: a neuroanatomical, biochemical, and histochemical investigation. J Comp Neurol 203: 495–514, 1981 [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci 24: 3234–3244, 2006 [DOI] [PubMed] [Google Scholar]
- Mechawar N, Watkins KC, Descarries L. Ultrastructural features of the acetylcholine innervation in the developing parietal cortex of rat. J Comp Neurol 443: 250–258, 2002 [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science 286: 711–715, 1999 [DOI] [PubMed] [Google Scholar]
- Murray TA, Bertrand D, Papke RL, George AA, Pantoja R, Srinivasan R, Liu Q, Wu J, Whiteaker P, Lester HA, Lukas RJ. alpha7beta2 nicotinic acetylcholine receptors assemble, function, and are activated primarily via their alpha7-alpha7 interfaces. Mol Pharmacol 81: 175–188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P, Charpak S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31: 8722–8729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56: 141–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, Inoue T, Strowbridge BW. Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci 27: 10969–10981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron 23: 499–511, 1999 [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett 471: 114–118, 2010 [DOI] [PubMed] [Google Scholar]
- Salcedo E, Tran T, Ly X, Lopez R, Barbica C, Restrepo D, Vijayaraghavan S. Activity-dependent changes in cholinergic innervation of the mouse olfactory bulb. PLos One 6: e25441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci 10: 383–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Liu S, Shipley MT. Intraglomerular inhibition shapes the strength and temporal structure of glomerular output. J Neurophysiol 108: 782–793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Grybko M, Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J Neurosci 28: 2563–2575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron 38: 929–939, 2003 [DOI] [PubMed] [Google Scholar]
- Smith RS, Araneda RC. Cholinergic modulation of neuronal excitability in the accessory olfactory bulb. J Neurophysiol 104: 2963–2974, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol 348: 351–373, 1994 [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723–735, 2001 [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol 17: 411–423, 2006 [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Sun L, Heinbockel T. Cannabinoid receptor-mediated regulation of neuronal activity and signaling in glomeruli of the main olfactory bulb. J Neurosci 32: 8475–8479, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell JD, Sorensen KA, Jarvie BC, Hentges ST, Schoppa NE. Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci 33: 1552–1563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol 243: 488–509, 1986 [DOI] [PubMed] [Google Scholar]
- Zheng LM, Ravel N, Jourdan F. Topography of centrifugal acetylcholinesterase-positive fibres in the olfactory bulb of the rat: evidence for original projections in atypical glomeruli. Neuroscience 23: 1083–1093, 1987 [DOI] [PubMed] [Google Scholar]