Abstract
The reticulospinal tract was recently shown to have synaptic connections to the intrinsic muscles of the fingers in nonhuman primates, indicating it may contribute to hand function long thought to be controlled exclusively through corticospinal pathways. Our objective was to obtain evidence supporting the hypothesis that these same anatomical connections exist in humans. startReact, an involuntary release of a planned movement via the startle reflex, provides a noninvasive means to examine the reticulospinal tract in humans. We found that startReact was triggered during coordinated grasp but not individuated finger movements. This result suggests that the reticulospinal tract does have connections to the intrinsic muscles of the fingers in humans but its functional role is limited to coordinated movement of the whole hand. These results do not diminish the well-established role of corticospinal pathways in the control of hand movement. Indeed, they cement the significance of corticospinal pathways in individuated finger movement control. Still, these results point to an updated and expanded view of distal hand control where reticulospinal and corticospinal pathways work in parallel to generate a large repertoire of diverse, coordinated movement in the hand. Finally, the presence of reticulospinal pathways to the muscles of the hand makes this pathway an attractive therapeutic target for clinical populations where the corticospinal tract is absent or injured.
Keywords: startle, reticulospinal, corticospinal, hand
the nervous system controls voluntary movement through a system of seemingly redundant (Sherrington 1906) neural tracts emanating from the cortex (corticospinal) and brainstem (lateral: rubrospinal and medial: reticulospinal, tectospinal, and vestibulospinal). The seminal work of Lawrence and Kuypers (1968a,b) in nonhuman primates demonstrated that these tracts are not as redundant as initially thought. Instead, they showed that each tract has a unique contribution to voluntary movement control. Through selective lesioning, it was demonstrated that the corticospinal tract controls individuated movement of the fingers, the lateral brainstem tracts control larger independent movement of the extremities (particularly the hands), and finally the medial brainstem tracts control the proximal limb and global movements like posture and locomotion. It was concluded that the medial brainstem, lateral brainstem, and corticospinal tracts existed within a proximal-distal gradient that defined their influence.
These studies from nonhuman primates have shaped our understanding of how the nervous system controls movement in humans. Various reports confirm the analogous function of the corticospinal tract in nonhuman primates and humans (Lemon 2008; Schieber 2011, 2004), but the brainstem tracts have important anatomical distinctions. The most dramatic is that the lateral brainstem tract is largely absent in humans (Nathan and Smith 1955, 1982). This leaves the medial brainstem tracts as the major descending systems to compensate for losses of the corticospinal tract following stroke and certain incomplete spinal cord injuries, which can cause devastating loss of hand function. Of the medial brainstem tracts, the reticulospinal tract receives the most prominent projections from the cortex (Kuypers 1981; Lemon 2008), suggesting it could provide an appropriate alternative to the corticospinal tract.
The reticulospinal tract was recently shown to have connections to the intrinsic muscles of the hand in nonhuman primates (Riddle and Baker 2010), indicating that it may contribute to hand function. Compared with projections from the corticospinal tract, those from the reticulospinal tract in nonhuman primates were more distributed, weaker, and fewer in number. Still, if this same anatomical distribution exists in humans, it would suggest that reticulospinal pathways could play a role in control of hand movement.
Our objective was to obtain evidence supporting the hypothesis that these same anatomical connections exist in humans. The startReact response, an involuntary release of a planned movement via a startling stimulus, provides a noninvasive means to study the role of the reticulospinal tract in humans (Valls-Solé et al. 1999). This phenomenon is easily triggered through unexpected exposure to a loud starting acoustic stimulus, delivered after the subject has prepared a movement. The startReact response is associated with activity in the same neural circuits that mediate the startle reflex. Animal studies demonstrate that the startle reflex is generated in the reticular formation and expressed through the reticulospinal tract (Davis et al. 1982; Davis and Gendelman 1977). Movements mediated without the reticulospinal tract in nonhuman primates (Kuypers 1981; Lawrence and Kuypers 1968a,b) and humans (Lemon 2008; Schieber 2011, 2004) are not susceptible to startReact in humans (Carlsen et al. 2009). Alternatively, proximal movements, known to involve brainstem pathways (Davidson et al. 2007) in nonhuman primates, exhibit startReact in humans (Carlsen et al. 2010). Based on the recent anatomical findings in nonhuman primates (Baker 2011), we hypothesized that coordinated movements of the hand (grasp) would be susceptible to startReact indicating that reticulospinal pathways can exert influence on finger muscles in humans.
METHODS
Subjects.
Seventeen participants (8 males, 9 females; age: 25.9 ± 2.8 yr) with no apparent physical abnormalities or sensory or motor dysfunctions volunteered to participate in the study. Before experimentation, a detailed explanation of procedures and risks was provided to all subjects and express written consent for participation was obtained in accordance with the provisions set forth by the Northwestern University Institutional Review Board Institutional Review Board (STU9204). All subjects were interviewed and screened for recent upper body injury and hearing sensitivity before participating in the experiment.
Equipment and setup.
Electromyography (EMG) was recorded from the right sternocleidomastoid muscle (RSCM) and the right first dorsal interosseous muscles (FDI). Bipolar EMG electrodes (solid gel, Ag-AgCl surface electrode; MVAP Medical Supplies, Newbury Park, CA) were placed on the belly of the RSCM and FDI muscles. A unipolar ground electrode (solid gel, Ag-AgCl surface electrode; MVAP Medical Supplies) was placed over the right ulnar styloid process. EMG data were passed through preamplifiers (model no. AMT-8; base system, model no. APE-500; 500-gain Bortec, Calgary, Alberta, Canada) with a band-pass filter of 10–1,000 Hz. All electrodes and preamplifier wires were secured to minimize motion artifact. The resulting signals were antialias filtered using fifth order Bessel filters with a 500-Hz cut-off frequency and sampled at 2,500 Hz (PCI-DAS1602/16; Measurement Computing).
Subjects were comfortably seated in a height-adjustable chair with arm rests. The hand and arm were supported against gravity by the arm rest. They were restrained across the chest with padded straps to minimize motion during the experiment. The elbow joint was oriented in line with the shoulder and flexed at 90°. In each trial, subjects performed either Finger or a Grasp task. The Finger task consisted of index finger abduction from a rested position towards the ceiling (Fig. 1A). A switch device was used to ensure task completion (D2VW-5L1B-3HS; Omron). The switch was positioned such that it was depressed when the subject was at rest. The switch height and angle of the device were made adjustable to fit each subject's unique hand and finger size/shape. This allowed each subject to keep digits 3, 4, and 5 bent 90° at the proximal interphalangeal joint, leaving digit 2 (index finger) pointing straight along the axis of the forearm and free to move.
Fig. 1.
Task depiction. Hand configuration during the Finger task (A) and Grasp task (B).
The Grasp task consisted of flexion of the fingers about the metacarpophalangeal joint (Fig. 1B). Subjects were given a stress ball with an embedded switch that was positioned in the subject's palm against the metacarpophalangeal joints. We positioned the switch within the stress ball such that application of a force along the latitudinal axis of the ball, which occurred when the subject grasped the object, resulted in depression of the switch. Subjects were asked to keep their wrist in a neutral position during experimental trials with the thumb resting on the top of the stress ball. The hand position and body posture for this task were almost identical to the Finger task; the only major difference is that the index finger is now bent around the stress ball.
We used switch data to confirm completion of the task by determining whether or not the switch state was modulated during a trial. The initial states of the switch were different (compressed: Finger task; open: Grasp task) for each task by convention to allow differentiation during postprocessing that the appropriate task was completed.
Protocol.
Two nonstartling, low-intensity (80 dB) acoustic sounds were delivered to subjects. Subjects were instructed to treat the first sound as a WARNING and prepare to move. The second sound was to be treated as a GO after which subjects were asked to perform the movement as quickly as possible. The time between the WARNING and GO signals was randomized between 1.5 and 3.5 s to prevent anticipation of the GO cue.
Before experiment trials, subjects were trained on the task until they generated consistent reaction latencies. Following training, participants experienced blocks of 15 experimental trials. Each block consisted either of the Grasp or Finger task, the order of which was randomly assigned for each subject. During each block five trials, the GO cue was randomly replaced with a high-intensity startling acoustic stimulus of 128 dB delivered through a loudspeaker fixed to the chair directly behind the head of the subject.
Data analysis.
The onset latency and amplitude of FDI muscle activity were calculated for each trial. The DC offset was removed from the EMGs, which were then rectified and smoothed using a 10-point moving average filter. The average background activity and standard deviation were calculated for a 500-ms window before the GO. Next, an automated program identified the time at which the processed EMG increased above three times the standard deviation of the background activity for a period of at least 5 ms. Following the automatic detection of EMG onset, each trial was evaluated visually to ensure accuracy. The onset latency was then used to calculate the average amplitude of the first 70 ms of FDI muscle activity.
Next, all trials were evaluated for SCM activity, an indicator of the startle reflex (Brown et al. 1991). We used a conservative automated program that captured any SCM activity that exceeded the maximum background activity for at least 0.8 ms. This approach ensured that all possible SCM+ trials were tagged for visual inspection. Each SCM trace was visually inspected to determine if activity was large enough to be classified as SCM+. Any trials where SCM+ could not be definitely assessed or background activation was abnormal were not included in further analysis. Task and trial type were blinded to the reviewer. To be consistent with previous literature (Brown et al. 1991; Carlsen et al. 2004a,b; Carlsen and Mackinnon 2010; Valls-Solé et al. 1995, 1999, 2008) activity within 120 ms after the GO cue was used to identify trials in which a startle occurred, designated as SCM+. Trials where activity in the SCM occurred after 120 ms or was not present were designated as SCM−.
To determine if each task (Finger and Grasp) was susceptible to startReact, the intensity-dependent and startle effects must be evaluated separately. When a task is susceptible to startReact, the presence of the startle reflex decreases the onset latency and increases the amplitude of muscle activity. However, onset latencies also decrease in response to increasing auditory stimulus intensities (Kohfeld 1969, 1971), i.e., subjects react more quickly when the GO stimulus is more intense (louder sound or brighter light). To differentiate between these two factors, it is necessary to compare low-intensity SCM− trials and high-intensity SCM+ and SCM− trials. Low-intensity SCM− trials are compared with high-intensity SCM− trials to quantify the intensity-dependent effect. High-intensity SCM+ and SCM− trials are then compared with determine if the response is susceptible to startReact. There are only rare low-intensity SCM+ trials, so those are not considered in our analyses.
Onset latency and amplitude differences between the high-intensity SCM+ and SCM− trials indicate that the task is susceptible to startReact, i.e., that the presence of startle influences the behavior of the task. The startReact response is associated with activity in the same neural circuits that mediate the startle reflex. Animal studies demonstrate that the startle reflex is generated in the reticular formation and expressed through the reticulospinal tract (Davis et al. 1982; Davis and Gendelman 1977). Movements that have been shown to not utilize these pathways for expression, such as individuated finger movements that rely on corticospinal pathways, are not susceptible to startReact. In contrast, if the movement is susceptible to startReact, performance of the voluntary movement and the startle reflex utilize common (reticulospinal) pathways.
All trials were visually inspected to ensure the task was completed, i.e., the switch condition was altered. Voluntary trials were further inspected to ensure that 1) subjects did not move before the GO or 2) moved too late (motion onset >300 ms after the GO cue). All EMG data were processed in Matlab (R2011b; The MathWorks). Subjects were eliminated on the basis that they did not provide a minimum of three of each trial type (voluntary, SCM+, and SCM−). This was necessary to ensure enough variance for accurate statistical comparisons. Application of this criterion reduced our data set from 17 subjects to 10. Two subjects responded with no SCM− trials and one subject with no SCM+ trials; the remaining four subjects provided fewer than three data points in either SCM+ or SCM− condition for the task. Without sufficient replication of the SCM+ and SCM− trials within each subject, it is not possible to obtain reliable within-subject estimates (Pinheiro and Bates 2000).
Statistical analyses.
Based on the behavioral data from Lawrence and Kuypers (1968a,b) and the anatomical data from Riddle and Baker (2010), we hypothesized that the Grasp task would be susceptible to startReact but not the Finger task. This hypothesis was tested using a linear mixed-effect model with condition (voluntary, SCM+, and SCM−) and task (Finger and Grasp) as the independent factors while latency was the dependent factor. Subjects were treated as a random factor, and the model did not assume equal variances. In accordance with recent standardization of statistical practices, all individual trials were included in our analysis of the linear mixed-effects models (Hedeker and Gibbons 2006). This method has been shown to be more rigorous and powerful than using a single mean for each subject. The use of all trials allows more independent information than a single measurement decreasing the probability of statistical error by capturing all the variability within a data set. Additionally, the mixed-effects models take into account the number of trials into the ANOVA analysis ensuring that data are not misrepresented or inflated due to differences in trial number across subjects in unbalanced data sets.
Tukey's honestly significant difference, which corrects for multiple comparisons, was used for all post hoc comparisons. All statistical analyses were performed using R (R Development Core Team, 2006). All statistical tests were made at a significance level of P < 0.05. P values are noted in main text when they are not otherwise depicted in Figs. 1–5. All error bars correspond to SD. All statistical measures were completed and verified with an independent statistician.
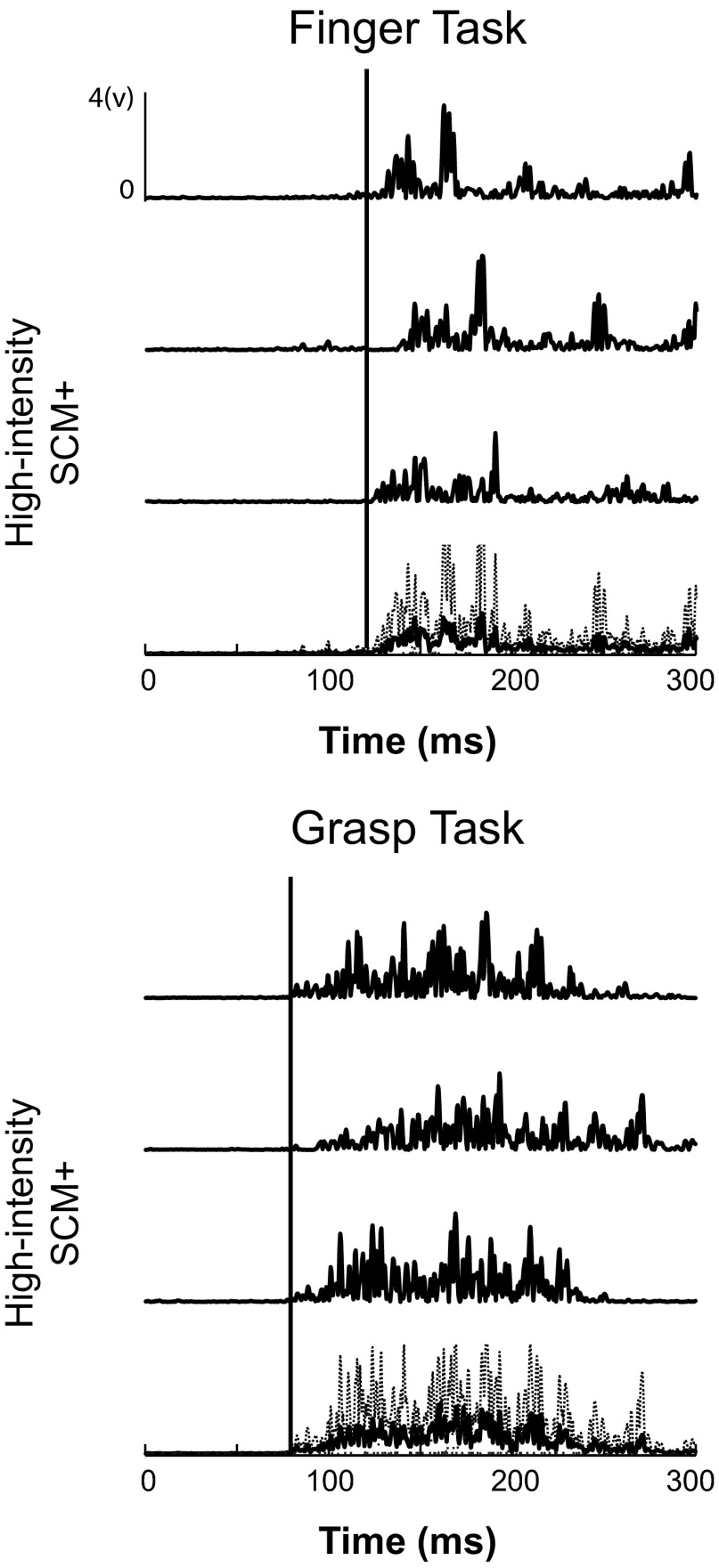
Fig. 5.
Individual and average HI SCM+ trials during Finger and Grasp tasks. Three raw HI SCM+ trials during the Finger task (top) are shown above the average (solid) and standard deviation (dashed) of all traces. Same responses during the Grasp task (bottom) are shown. Solid black lines represent the start of the average response.
RESULTS
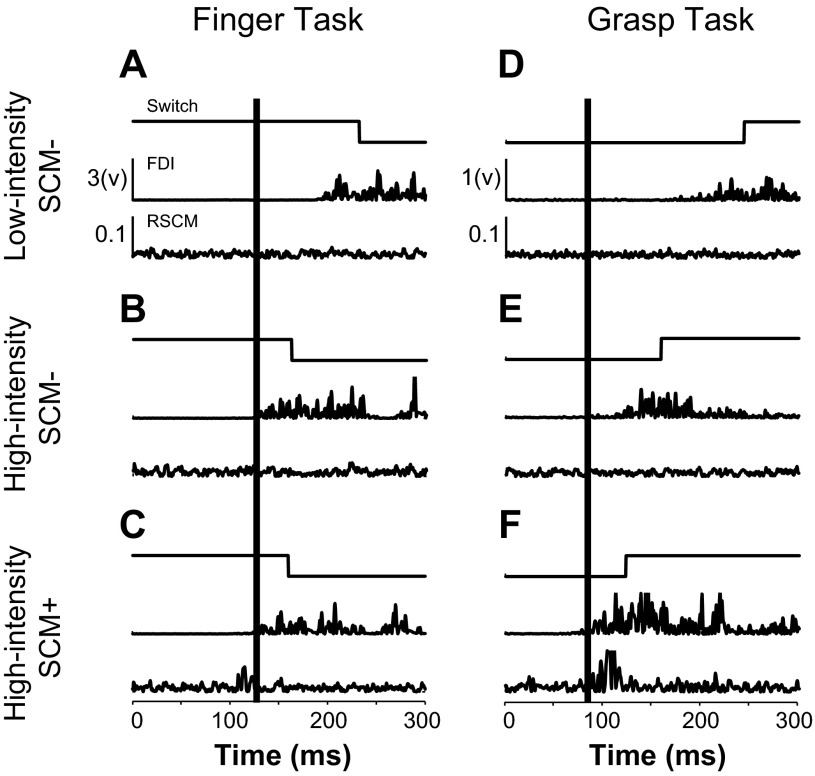
Individuated finger movements (Finger task) were not susceptible to startReact. The presence of startle (SCM+) did not influence onset latency or amplitude of the FDI muscle (Fig. 2C). While both SCM+ and SCM− high-intensity trials were faster than low-intensity trials, there was no difference in onset latency and amplitude between these conditions (Fig. 2, A–C). The average FDI latency during low-intensity SCM− movements was 176 ± 32.5 (n = 224) ms compared with 98 ± 14.8 (n = 102) and 96 ± 15.3 (n = 68) for high-intensity SCM+ and SCM− conditions, respectively.
Fig. 2.
Sample data from Finger and Grasp tasks. A–C: switch and EMG data from the first dorsal interosseous (FDI) and right sternocleidomastoid muscle (SCM) muscles during the Finger task. D–F: switch and EMG data during the Grasp task. A and D: low-intensity (LI) SCM−. B and E: high-intensity (HI) SCM−. C and F: HI SCM+. GO cue or startling acoustic stimulus occurred at zero. Vertical black line is placed to show the onset of FDI activity during HI SCM+ trials.
Coordinated hand movements (Grasp task) were susceptible to startReact. The presence of startle (SCM+) during the high-intensity trials decreased the onset latency and increased the amplitude of muscle activity relative to high-intensity SCM− trials (Fig. 2F). Both high-intensity SCM+ and SCM− trials were faster than voluntary trials (Fig. 2, D–F). The average FDI latency during low-intensity SCM− grasp movement was 171 ± 31.2 (n = 199) ms compared with 87 ± 10 (n = 94) and 96 ± 16.7 (n = 80) for high-intensity SCM+ and SCM− conditions, respectively.
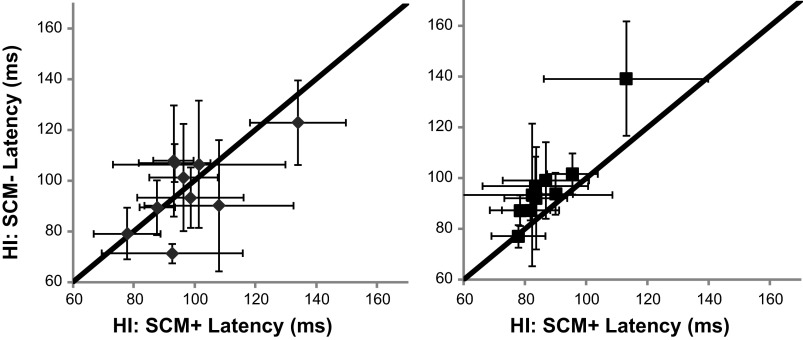
Group results demonstrated these comparisons were consistent across subjects. Main effects condition (low-intensity SCM−, high-intensity SCM+, and high-intensity SCM−) and task (Finger and Grasp) showed significant effects F(2,843) = 407.0: P < 0.0001 and F(1,843) = 15.3: P = 0.0001, respectively. Post hoc comparisons of the Finger task showed that the latency of FDI EMG activity between the high-intensity SCM+ and SCM− conditions were not different (Fig. 3A, left; P = 0.68). Conversely during the Grasp task, high-intensity SCM+ and SCM− conditions were different (Fig. 3A, right; P = 0.0002). Graphically represented, no relationship was found between FDI latency during high-intensity SCM+ and high-intensity SCM− trials during the Finger task (Fig. 4, left); however, FDI latency was faster during high-intensity SCM+ trials in the Grasp task with all relationships falling to the left of the unity line (Fig. 4, right). FDI muscle latency during the low-intensity SCM− was different from both high-intensity SCM+ (P ≈ 0) and high-intensity SCM− (P ≈ 0) conditions during both Finger and Grasp tasks (Fig. 3A).
Fig. 3.
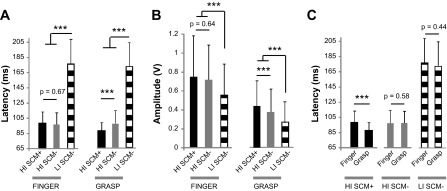
Group results. A: comparison of FDI muscle onset latencies during LI SCM−, HI SCM−, and HI SCM+ conditions during the Finger (left) and Grasp (right) tasks. B: comparison of FDI muscle amplitude. C: comparison of FDI latency between tasks. ***P < 0.001.
Fig. 4.
Relationship between SCM+ and SCM− latencies. HI SCM+ FDI latency as a function of HI SCM− FDI latency for the Finger (left: gray) and Grasp (right: black) tasks. A unity line is presented in black.
Finger and grasp FDI latency was different during high-intensity SCM+ trials but was not during both low- and high-intensity SCM− conditions indicating that the tasks were the same in cases where a startle was not reported. Individual and average high-intensity SCM+ trials during the Grasp task were faster than those during the Finger task in each subject (Fig. 5). Group results confirm there was no difference between low-intensity and high-intensity SCM− trials between Finger and Grasp tasks (Fig. 3C; P = 0.44 and 0.58, respectively) However, the latency of the FDI muscle was faster during high-intensity SCM+ Grasp tasks compared with the Finger task (P ≈ 0).
Group amplitude comparisons were similar to latency results demonstrating that the Grasp task was susceptible to startReact while the Finger task was not. Both the Finger and Grasp tasks showed intensity dependent increase in FDI amplitude between the low-intensity SCM− and high-intensity SCM+ conditions (Fig. 3B). However, the Grasp task showed an additional increase in amplitude during high-intensity trials when startle was present (SCM+) while the amplitude of the Finger task remained the same. Group results confirmed these results. Condition and task showed significant effects F(2,843 = 76.9): P < 0.0001 and F(1,843) = 301.6: P < 0.0001. During the Finger task, there was no significant difference between high-intensity SCM+ and SCM− conditions (P = 0.64) while a difference was found during the Grasp task (P = 0). The FDI muscle amplitude during the low-intensity SCM− was different from both high-intensity SCM+ (p ≈ 0) and high-intensity SCM− (P ≈ 0) conditions during both Finger and Grasp tasks.
The differences present between tasks were not related to unique properties of the startle reflex between tasks. Specifically, the probability of startle (SCM+) during Finger (54% ± 15) and Grasp (61% ± 13) tasks was not different F(1,9) = 1.58: P = 0.24. Further, the latency of SCM activity during Finger (81.07 ± 19.34) and Grasp tasks (82.64 ± 18.47) tasks was not different F(1,183) = 0.24: P = 0.62.
DISCUSSION
Our main finding was that coordinated hand movements (Grasp task) were susceptible to startReact but that individuated hand movements (Finger task) were not. The latency and amplitude of FDI muscle activation was influenced by the presence of startle (high-intensity SCM+) in the Grasp but not the Finger task. This effect was not influenced by the difficulty of the task as the latency of FDI muscle activation was not different between tasks when startle was not present (high-intensity SCM− and low-intensity SCM−). This task-dependent behavior was also not related to differences in expression of the startle reflex between the two tasks. Specifically, the probability of eliciting a startle was not different between the tasks and the latency of startle, as measured by activity in the SCM muscle, was the same. While it is not possible to definitively test the anatomical connections in humans, our results suggest that the reticulospinal projections found in nonhuman primates (Riddle et al. 2009) also exist in humans. The functional role of these projections appears to be important for coordinating whole hand movements, more than to assist with the individuated movements that appear to rely more on corticospinal projections. This is consistent with the distributed nature of reticulospinal projections, many of which have divergent connections to multiple muscles (Baker 2011; Matsuyama et al. 1999, 1997; Peterson et al. 1975; Riddle et al. 2009). Still, recent evidence demonstrates that reticular neurons are strongly modulated even during fine finger movements (Soteropoulos et al. 2012), suggesting that differences in the task-dependent involvement of corticospinal and reticulospinal pathways are likely to lie along a continuum rather than be absolute.
Use of startReact to evaluate reticulospinal connections in humans.
The startReact response has previously been used as a noninvasive tool to investigate the role of the reticulospinal tract in humans (Valls-Solé et al. 1999). This response is the involuntary release of a planned motor action associated with activity in the same circuits that mediate startle. Numerous animal studies have demonstrated that the startle reflex is generated by the reticular formation and modulated by cortical inputs. In rats, the startle reflex is completed blocked by lesions either of the caudal pontine reticular formation or medullary reticular formation (Davis et al. 1982; Groves et al. 1974; Hammond 1973) but remains intact following lesion (Davis et al. 1982) or removal (Davis and Gendelman 1977) of the cerebral cortexes. Still, cortical lesion causes a hypermetric startle reflex (Davis and Gendelman 1977) highlighting the important modulatory role of the cortex over startle.
The use of startReact to investigate the reticular formation in humans is predicated on animal work; however, recent evidence demonstrates that the role of the reticular formation and cortex during startReact is likely maintained in humans. Inhibition of the cortex through transcranial magnetic stimulation (TMS) suppresses the expression of startReact and enhances the expression of startle (SCM activity) (Alibiglou and MacKinnon 2012) demonstrating that the cortex modulates these responses in humans. Further, human patient populations with cortical lesions often have hypermetric startle reflexes (Jankelowitz and Colebatch 2004), a further indication of cortical modulation of startle in humans. These results suggest that the cortex modulates startle and startReact in humans similarly to animals.
Still, there is evidence that startReact does not require the cortex for expression in humans. Specifically, startReact remains intact following cortical lesion in stroke survivors. While voluntary movements in stroke survivors are substantially impaired, startReact elbow flexion movements are not different in latency and muscle activation patterns from unimpaired individuals of the same age (Honeycutt and Perreault 2012). Further, individuated movements of the hand are not susceptible to startReact (Carlsen et al. 2009; and Finger task presented here). These movements have been shown in human (Schieber 2011, 2004) and animal (Kuypers 1981; Lawrence and Kuypers 1968a,b; Lemon et al. 2012) experiments to be expressed predominantly through the corticospinal tract and not reliant on the reticulospinal tract. Together, these results suggest that only movements that rely predominantly on pathways used by the startle reflex (reticulospinal tract) are easily susceptible to startReact. Thus, while current technology and ethical considerations restrict our ability to concretely state that the reticulospinal tract influences finger movement in humans, this report and the cited literature provide evidence in support of that role.
There are some recent results that question the use of startReact to evaluate the reticulospinal projections in humans, since they suggest that the startReact and startle reflexes can involve separate pathways. In brief, an auditory prepulse or “warning” delivered to subjects before startReact diminishes the activity in the SCM muscle (startle indicator) but does not alter the early release of movement (startReact) (Maslovat et al. 2012). This result indicates that these two phenomena can be modulated differently, even though expression of startReact remained associated with the expression of startle. Further, TMS can be used to delay the early release of movement (startReact), which has been interpreted as involvement from the primary motor cortex (Alibiglou and MacKinnon 2012). However, it has since been shown that TMS also has powerful effects on the reticular formation (Fisher et al. 2012), making it difficult to discount the role of these pathways using cortical TMS alone. While there is no doubt that the cortex is involved in motor planning (Rushworth 2003; Stinear et al. 2009) and that the startle and startReact rely on some distinct neural circuits, the available data still suggest that these phenomena are fundamentally linked, as the startReact is always expressed with startle. Hence, while startle and startReact have distinctive elements, the available literature suggests that they are mediated by overlapping neural circuitry.
Latency difference between SCM+ and SCM− grasp trials.
The difference between high-intensity SCM− and high-intensity SCM+ latencies during the Grasp task was found to be 9 ms. This latency is reasonable when one considers that the conduction time between the reticular formation and the cortex is only 2.88 ms (Fisher et al. 2012). During a startle-triggered movement, the auditory stimulus travels from the cochlea through the auditory nerve to the reticular formation (located between the pons and midbrain) where startle triggers movement. Alternatively, during a voluntary movement, the auditory signal must travel additionally from the pons and midbrain to the primary auditory cortex to the primary motor cortex to initiate movement. Thus the signal must travel the additional distance ascending and descending between the midbrain and cortex. If we assume corticospinal and reticulospinal conduction times are similar (Peterson et al. 1975), the conduction time from cortex to reticular formation is 2.88 ms (Fisher et al. 2012) in the monkey. Factoring in one to two additional synapses for the connection between the auditory and motor cortexes and the additional length in humans, a 9- to 10-ms difference between these conditions is a reasonable estimate. Other reports have reported a larger differences between high-intensity SCM− and high-intensity SCM+ trials (Carlsen et al. 2009); however, this study was completed the proximal elbow joint, which is distinctive in its neural control and biomechanics.
Role of the reticulospinal and corticospinal tracts in movement.
Our results along with those in nonhuman primates demonstrating reticulospinal connections to the intrinsic muscles of the hand (Riddle et al. 2009; Soteropoulos et al. 2012) do not diminish the well-established role of the corticospinal tract in movement control of the hand. The corticospinal tract is uniquely able to activate muscle independently allowing fine, fractionated control (Lemon 1993; Schieber 2011, 2004). This is of particular advantage in muscles of the hand where proper dexterity requires detailed and specific movement. It is known that severing the corticospinal tract in nonhuman primates causes a considerable deficit in hand function (Lawrence and Kuypers 1968a,b) that is paralleled in humans by the devastating loss of hand function following injury to the corticospinal tract after stroke (Handley et al. 2009; Krakauer 2005; Langhorne et al. 2009).
Rather than contradicting the traditional view of the corticospinal pathways, our results point to an updated and expanded view of movement control at the distal limb. The cortex communicates with peripheral muscles through two major descending projections: corticospinal and corticobular. While the corticospinal tract offers the most direct access to the spinal cord, the corticobular pathway connecting through the reticular formation provides an alternative pathway (Lemon 2008). While the reticulospinal tract has well-established role in posture and locomotion (Deliagina et al. 2008; Honeycutt et al. 2009; Honeycutt and Nichols 2010; Mori 1987; Mori et al. 1989; Musienko et al. 2008; Schepens et al. 2008; Stapley and Drew 2009), new evidence expands this traditional view highlighting its role during voluntary movements like reaching (Buford and Davidson 2004; Davidson et al. 2007; Sakai et al. 2009). Although this type of task is known to also be mediated by corticospinal pathways, recordings from the reticular formation demonstrate that these cells are strongly modulated during reaching. Thus during reaching, corticospinal and reticulospinal pathways work in concert to deliver appropriately coordinated movement of the arm. This is supported by anatomical data demonstrating that 48% of interneurons in the intermediate zone, associated with both proximal and distal joint, receive inputs from both corticospinal and reticulospinal inputs (Alstermark et al. 1984; Baker 2011; Drew et al. 2004; Riddle and Baker 2010). These previous findings, in conjunction with the results presented in this report, suggest that this same type of collaboration between pathways occurs at the level of the distal hand.
The notion of a proximal-distal gradient defining the influence of corticospinal/medial brainstem tracts, while based on empirical evidence, may not fully express the versatility of these pathways. The proximal-distal gradient view of the nervous system is based on the data from Lawrence and Kuypers showcasing the gradation of control of these different tracts (Lawrence and Kuypers 1968a; b; Lemon et al. 2012). It is supplemented from data that corticospinal connections increase in number in a proximal-distal fashion while reticulospinal synapses follow the opposite trend (Baker 2011). We suggest that this organization may result from the different muscular requirements of tasks completed at proximal and distal joints. Proximal joint movements (shoulder and hip) require coordinated activity across multiple muscle groups to generate the desired movement. Movement at these joints also requires postural adjustments of the whole body to maintain stability. The reticulospinal tract, with its distributed, pervasive connections, is ideal to coordinate such movements. Alternatively, the highly specified tasks completed by the distal hand (typing and writing) require fractionated control of small groups of muscles or individual muscle activation. The selective corticospinal tract is anatomically best suited to these types of tasks. The biomechanical challenges of the proximal and distal joint likely dictate these distinctive roles but importantly do not preclude corticospinal influences at proximal joint or reticulospinal influences at the distal joint. We, therefore, propose that these two systems work in parallel within a neural organization based on functional control with the reticulospinal and corticospinal tracts mediating coordinated and individuated movements, respectively, throughout the entire arm.
Clinical significance.
The presence of distal reticulospinal projections makes it an attractive therapeutic target. We recently demonstrated that following stroke elbow movements elicited via startReact were improved from voluntarily activated movement (Honeycutt and Perreault 2012). Therefore, it may be possible to utilize startReact as a training tool during therapy to elicit more appropriate movements in stroke patients. It is known that reticulospinal projections are strengthened following injury to the corticospinal tracts (Zaaimi et al. 2012) and are utilized more strongly following stroke (Mazevet 2003). Still, although the reticulospinal tract has the capacity to serve as an alternative pathway to access the muscles of the hand, these projections are significantly fewer in number than those of the corticospinal tract (Baker 2011). Further research is necessary to evaluate if there are enough of these projections to strengthen following stroke to generate functionally significant results.
GRANTS
This work was supported by the National Institutes of Health Grants K12 GM-088020, T32 HD-07418-18, and R01 NS-053813.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.F.H., M.K., and E.J.P. conception and design of research; C.F.H. and M.K. performed experiments; C.F.H. and M.K. analyzed data; C.F.H., M.K., and E.J.P. interpreted results of experiments; C.F.H., M.K., and E.J.P. prepared figures; C.F.H., M.K., and E.J.P. drafted manuscript; C.F.H., M.K., and E.J.P. edited and revised manuscript; C.F.H., M.K., and E.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tim Goetz-Haswell technical and scientific expertise. In addition, we thank Dr. Jungwha “Julia” Lee for statistical expertise.
REFERENCES
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol 590: 919–936, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 12. Interneurones which may mediate descending feed-forward inhibition and feed-back inhibition from the forelimb to C3–C4 propriospinal neurones. Exp Brain Res 56: 308–322, 1984 [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 589: 5603–5612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain 114: 1891–1902, 1991 [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004 [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res 159: 301–309, 2004a [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Motor Behav 36: 253–264, 2004b [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Differential effects of startle on reaction time for finger and arm movements. J Neurophysiol 101: 306–314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Mackinnon CD. Motor preparation is modulated by the resolution of the response timing information. Brain Res 1322: 38–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35: 366–376, 2010 [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 2: 791–805, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Gendelman PM. Plasticity of the acoustic startle response in the acutely decerebrate rat. J Comp Physiol Psychol 91: 549–563, 1977 [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev 57: 212–221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 143: 251–261, 2004 [DOI] [PubMed] [Google Scholar]
- Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol 590: 4045–4060, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Boyle RD. Brain stem pathways, cortical modulation, and habituation of the acoustic startle response. Behav Biol 10: 391–418, 1974 [DOI] [PubMed] [Google Scholar]
- Hammond GR. Lesions of pontine and medullary reticular formation and prestimulus inhibition of the acoustic startle reaction in rats. Physiol Behav 10: 239–243, 1973 [DOI] [PubMed] [Google Scholar]
- Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing 38: 260–266, 2009 [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal Data Analysis. New York: John Wiley & Sons, chapt. 4, 2006 [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol 101: 2751–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Nichols TR. The decerebrate cat generates the essential features of the force constraint strategy. J Neurophysiol 103: 3266–3273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Planning of ballistic movement following stroke: insights from the startle reflex. PLoS ONE 7: e43097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG. The acoustic startle reflex in ischemic stroke. Neurology 62: 114–116, 2004 [DOI] [PubMed] [Google Scholar]
- Kohfeld DL. Effects of the intensity of auditory and visual ready signals on simple reaction time. J Exp Psychol 82: 88–95, 1969 [DOI] [PubMed] [Google Scholar]
- Kohfeld DL. Simple reaction time as a function of stimulus intensity in decibels of light and sound. J Exp Psychol 88: 251–257, 1971 [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Arm function after stroke: from physiology to recovery. Semin Neurol 25: 384–395, 2005 [DOI] [PubMed] [Google Scholar]
- Kuypers H. Anatomy of descending pathways. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am Physiol Soc, 1981, sect I, vol II, p. 597–666 [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 8: 741–754, 2009 [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. Functional organization of motor system in monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968a [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. Functional organization of motor system in monkey. 2. The effects of lesions of descending brain-stem pathways. Brain 91: 15–26, 1968b [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008 [DOI] [PubMed] [Google Scholar]
- Lemon RN. The G. L. Brown Prize Lecture. Cortical control of the primate hand. Exp Physiol 78: 263–301, 1993 [DOI] [PubMed] [Google Scholar]
- Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers (1968a,b) revisited: copies of the original filmed material from their classic papers in Brain. Brain 135: 2290–2295, 2012 [DOI] [PubMed] [Google Scholar]
- Maslovat D, Kennedy PM, Forgaard CJ, Chua R, Franks IM. The effects of prepulse inhibition timing on the startle reflex and reaction time. Neurosci Lett 513: 243–247, 2012 [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol 410: 413–430, 1999 [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol 377: 234–250, 1997 [PubMed] [Google Scholar]
- Mazevet D. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain 126: 988–1000, 2003 [DOI] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol 28: 161–195, 1987 [DOI] [PubMed] [Google Scholar]
- Mori S, Sakamoto T, Ohta Y, Takakusaki K, Matsuyama K. Site-specific postural and locomotor changes evoked in awake, freely moving intact cats by stimulating the brainstem. Brain Res 505: 66–74, 1989 [DOI] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res 190: 124–134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. Long descending tracts in man. 1. Review of present knowledge. Brain 78: 248–303, 1955 [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain 105: 223–269, 1982 [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and braching of reticulospinal neurons. Exp Brain Res 23: 333–351, 1975 [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Statistics and Computing: Mixed-Effects Models in S and S-Plus. New York: Springer, 2000 [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol 103: 2821–2832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M. The left parietal and premotor cortices: motor attention and selection. Neuroimage 20: S89–S100, 2003 [DOI] [PubMed] [Google Scholar]
- Sakai ST, Davidson G, Buford J. Reticulospinal neurons in the pontomedullary reticular formation of the monkey (Macaca fascicularis). Neuroscience 163: 1158–1170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol 100: 2235–2253, 2008 [DOI] [PubMed] [Google Scholar]
- Schieber MH. Dissociating motor cortex from the motor. J Physiol 589: 5613–5624, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Motor control: basic units of cortical output? Curr Biol 14: R353–354, 2004 [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale Univ. Press, 1906 [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol 590: 4011–4027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101: 1334–1350, 2009 [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Verryt TS, Acharya PP, Byblow WD. Repetitive stimulation of premotor cortex affects primary motor cortex excitability and movement preparation. Brain Stimul 2: 152–162, 2009 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res 187: 497–507, 2008 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516: 931–938, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett 195: 97–100, 1995 [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135: 2277–2289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]