Abstract
The striatum of the basal ganglia demonstrates distinctive upstate and downstate membrane potential oscillations during slow-wave sleep and under anesthetic. The upstates generate calcium transients in the dendrites, and the amplitude of these calcium transients depends strongly on the timing of the action potential (AP) within the upstate. Calcium is essential for synaptic plasticity in the striatum, and these large calcium transients during the upstates may control which synapses undergo plastic changes. To investigate the mechanisms that underlie the relationship between calcium and AP timing, we have developed a realistic biophysical model of a medium spiny neuron (MSN). We have implemented sophisticated calcium dynamics including calcium diffusion, buffering, and pump extrusion, which accurately replicate published data. Using this model, we found that either the slow inactivation of dendritic sodium channels (NaSI) or the calcium inactivation of voltage-gated calcium channels (CDI) can cause high calcium corresponding to early APs and lower calcium corresponding to later APs. We found that only CDI can account for the experimental observation that sensitivity to AP timing is dependent on NMDA receptors. Additional simulations demonstrated a mechanism by which MSNs can dynamically modulate their sensitivity to AP timing and show that sensitivity to specifically timed pre- and postsynaptic pairings (as in spike timing-dependent plasticity protocols) is altered by the timing of the pairing within the upstate. These findings have implications for synaptic plasticity in vivo during sleep when the upstate-downstate pattern is prominent in the striatum.
Keywords: striatum, calcium, computational model, medium spiny neuron, upstates
medium spiny neurons (MSNs) are the sole projection neurons of the striatum, and plasticity changes in these neurons underlie motor learning (Yin et al. 2009). In vivo, the MSNs exhibit distinct membrane potential oscillations referred to as upstates and downstates. Although this upstate-downstate activity pattern was observed more than 30 years ago (Wilson and Groves 1981), its function is still unclear. This pattern of activity is displayed in anesthetized animals and occurs during slow-wave sleep but is much less prominent in the awake animal (Mahon et al. 2006). Because this activity pattern occurs most strongly when the animal is anesthetized or sleeping and consequently motionless, its main function is not likely the direct control of motor output. Rather, it may be a means to consolidate and reinforce motor skill learning by controlling which specific synapses are strengthened during sleep (Stoetzner et al. 2010) while cells replay previous wakeful sequences (Lansink et al. 2009; Ribeiro et al. 2004).
Large dendritic calcium transients coincide with the corticostriatal upstate (Kerr and Plenz 2002, 2004), and calcium elevations are necessary for striatal synaptic plasticity in vitro (Fino et al. 2010). Therefore, during the upstate the calcium elevation in the dendrite may dictate whether specific synapses are potentiated or depressed. The timing of the action potential (AP) within the upstate strongly determines the amplitude of the corresponding calcium elevation. APs early in the upstate coincide with higher calcium peaks than APs late in the upstate, resulting in a nonlinear, timing-dependent relationship (Kerr and Plenz 2004). However, the intracellular mechanisms responsible for this relationship and the consequences for synaptic plasticity during the upstate are as yet unknown.
In vitro experiments have shown that carefully timed pairings of pre- and postsynaptic activity can result in spike timing-dependent plasticity (STDP) (Fino et al. 2005; Pawlak and Kerr 2008), and computational models have demonstrated that calcium influx strongly predicts plasticity during these protocols (Evans et al. 2012). However, the noisy in vivo environment is very different from the quiet in vitro slice preparation. Specifically, a rigidly controlled single pairing of pre- and postsynaptic activity is not likely to occur in vivo. Instead, barrages of synaptic activity and an underlying upstate-downstate pattern characterize the in vivo situation. In this study, we investigate the calcium influx due to STDP pairings during these in vivo-like upstates and discuss the consequences for synaptic plasticity.
Using a multichannel, multicompartmental biophysical model and a sophisticated model of calcium diffusion, buffering, and pump extrusion, we investigate two possible mechanisms that can control the relationship between calcium and AP timing during the upstate. In the first half of this article, we determine that one mechanism is more likely and confirm its plausibility with voltage-clamp experiments. In the second half of this article, we use the model to make several experimentally testable predictions. Specifically, we show that AP timing affects calcium binding to downstream targets involved in plasticity, and we demonstrate a mechanism by which MSNs could dynamically modulate their sensitivity to AP timing in response to neuromodulation.
METHODS
General MSN Model
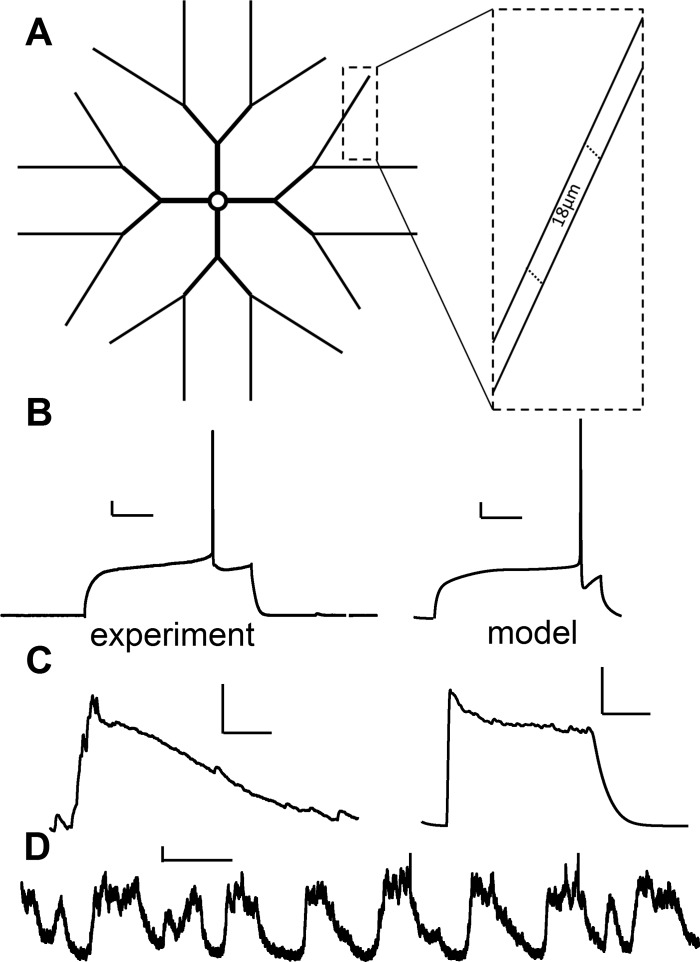
Using GENESIS simulation software, we developed a model MSN with realistic electrophysiological activity (Fig. 1) and calcium dynamics (Fig. 2) by modifying our previously published dorsal striatum MSN model (Evans et al. 2012). The morphology is similar (Fig. 1A), but the maximal dendritic length is reduced to 224 μm, and the long tertiary branches slightly taper from 0.8 to 0.7 μm in diameter (Fig. 1A). These changes are in accordance with reconstructions of MSNs from the NeuroMorpho.org neuronal reconstruction database (Halavi et al. 2008). For simplicity, this model does not contain dendritic spines except where noted for the STDP simulations.
Fig. 1.
Computational model comparison with electrophysiological data. A: morphology of model medium spiny neuron (MSN), not to scale. Soma is 16 μm in diameter, primary dendrites are 12 μm long, secondary dendrites are 14 μm long, and tertiary dendrites are divided into 11 contiguous 18-μm-long segments. B: experimental and model voltage responses to somatic current injection of 260 pA. Both demonstrate long latency to first action potential (AP). Scale bars: vertical, 10 mV; horizontal, 100 ms. C: experimental and model voltage traces showing synaptically evoked upstates. Scale bars: vertical, 10 mV; horizontal, 100 ms. Experimental upstate is a spontaneous upstate recorded from organotypic triple co-culture (Blackwell KT and Plenz D, unpublished observations). D: in vivo upstate traces show variability in upstate shape. Scale bars: vertical, 5 mV; horizontal, 1 s. [Traces used with permission from John Reynolds (unpublished observations)].
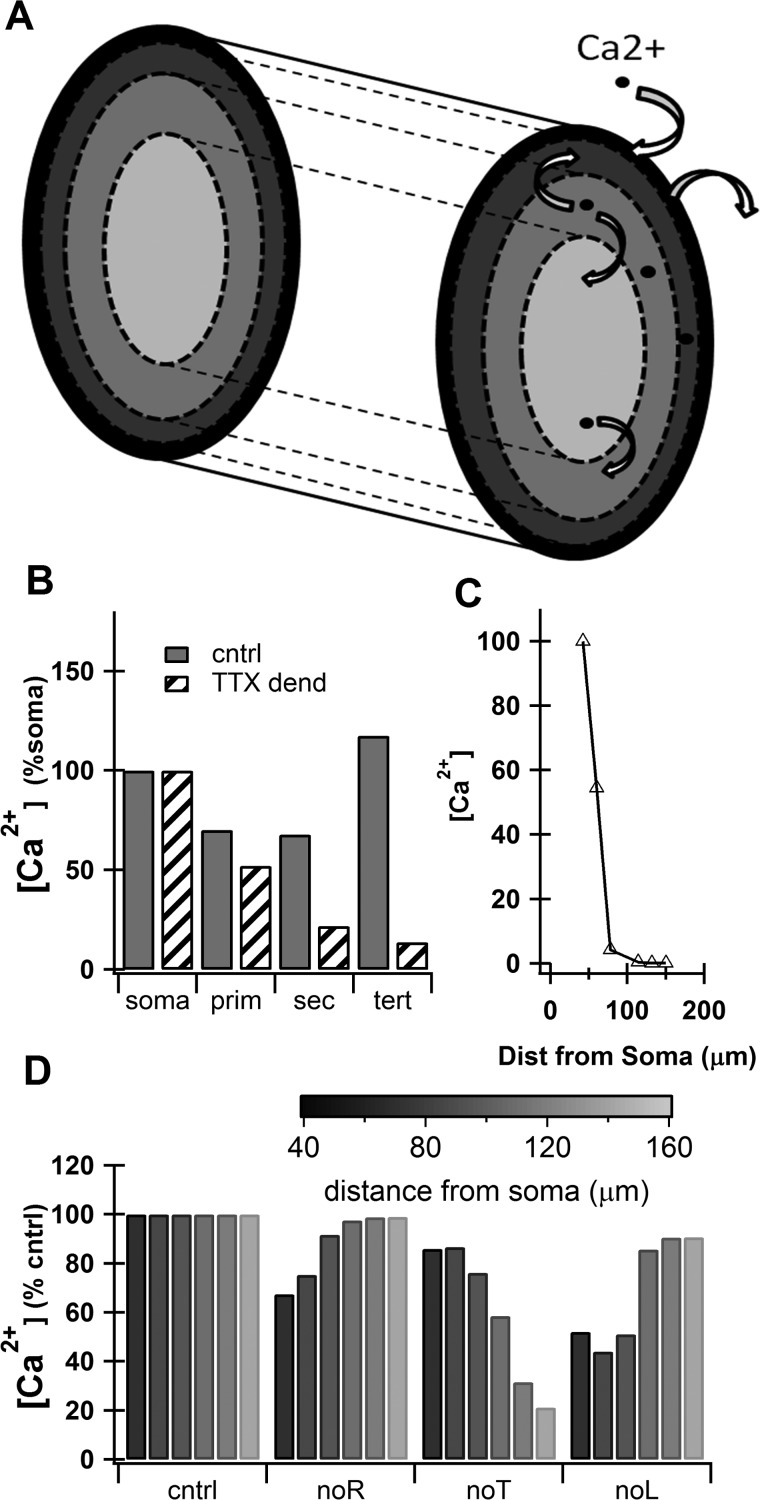
Fig. 2.
Intrinsic calcium signaling in the model matches published data. A: schematic drawing of dendritic segment showing diffusion of calcium between shells, extrusion of calcium via pumps, and influx of calcium via voltage-gated calcium channels (VGCCs). B: model calcium signal (normalized to the signal seen at the soma) strongly responds to a single backpropagating AP in proximal dendrites. When sodium channels are removed from the dendrites to represent tetrodotoxin (TTX dend), calcium decreases with distance from the soma (similar to Fig. 7G in Kerr and Plenz 2002). cntrl, Control; prim, primary; sec, secondary; tert, tertiary. C: model calcium signal (normalized to tertiary dendrite segment 1, 42 μm from soma) in distal dendrites does not strongly respond to backpropagating AP (similar to D1 neurons in Fig. 1D in Day et al. 2008). Dist, distance. D: the contribution of VGCCs changes with distance from the soma even when the conductances are the same. The relative contribution of R- and L-type calcium channels is reduced in distal dendrites, whereas the relative contribution of T-type calcium channels is increased (contributions tuned to be qualitatively similar to Fig. 2 in Carter and Sabatini 2004).
Ionic Channels
This model contains one fast sodium channel (Naf) (Ogata and Tatebayashi 1990) and four voltage-gated potassium channels: a fast potassium A current (Kaf; Kv4.2) (Tkatch et al. 2000), a slow potassium A current (Kas; Kv1.2) (Shen et al. 2004), a resistant persistent potassium current (Krp) (Nisenbaum and Wilson 1995), and an inwardly rectifying potassium current (Kir) (Steephen and Manchanda 2009). The model also contains two calcium-activated potassium channels: the big-conductance BK channel and the small-conductance SK channel. All equations governing the kinetics of these channels are given in appendix Tables A1 and A2. Intrinsic channel time constants in this model have been temperature corrected with a Q factor of 3, except those for Kaf and Naf, which are adjusted by a Q factor of 1.5 and 2.5, respectively. Equations for BK and SK are not included in appendix Tables A1 and A2 because they are the same as in our previous model (Evans et al. 2012). All channels are present in the soma and in the dendrites; however, the maximal conductances of the Naf, Kaf, and Kas channels were adjusted differentially in the soma and dendrites during model tuning (see appendix Table A3). Under the sodium slow inactivation (NaSI) conditions, an additional slow inactivation term is added to the dendritic sodium channels (Migliore 1996; Ogata and Tatebayashi 1990).
Calcium Channels
Five voltage-gated calcium channels (VGCCs) are included in this model. High-voltage-activated channels include R-type (CaR) (Brevi et al. 2001; Foehring et al. 2000), N-type (CaN; Cav2.2) (Bargas et al. 1994; Kasai and Neher 1992; McNaughton and Randall 1997), and an L-type channel (CaL1.2; Cav1.2) (Bargas et al. 1994; Kasai and Neher 1992; Tuckwell 2012; Wolf et al. 2005). Low-voltage-activated channels include T-type (CaT: Cav3.3, α1G) (McRory et al. 2001) and an L-type channel (CaL1.3; Cav1.3) (Tuckwell 2012; Wolf et al. 2005). Calcium channel kinetics and equations are given in appendix Tables A1 and A2. For each calcium channel, the Goldman-Hodgkin-Katz (GHK) formula is applied to accurately compute the driving potential for these channels. Under the calcium-dependent inactivation (CDI) conditions, an additional calcium-dependent inactivation term is added to the R-type, N-type, and both L-type calcium channels (Liang et al. 2003). The maximal permeabilities of these VGCCs were adjusted in the soma to produce calcium elevations comparable in size and shape to values from published experiments (Kerr and Plenz, 2002). Similarly, calcium permeabilities in the dendrites were adjusted to match their contributions during backpropagating APs (Carter and Sabatini 2004) (Fig. 2). Permeabilities are shown in appendix Table A3.
Calcium Diffusion, Buffers, and Pumps
Calcium dynamics were implemented using the calcium difshell object in GENESIS, which integrates changes to calcium concentration produced by calcium influx, buffers, pumps, and diffusion. A thin (0.1 μm) submembrane shell was created as the outermost shell, and concentric shells progressively doubling in thickness were added within the compartment (Fig. 2A). One-dimensional calcium diffusion between shells occurred at a rate of 200 μm2/s (Allbritton et al. 1992). Calcium extrusion was achieved by the addition of a Michaelis-Menten pump with Km = 0.3e−3 mM and Kcat = 85 pmol·(cm2/s)−1 in the soma and 12 pmol·(cm2/s)−1 in the dendrites. The endogenous calcium buffers calbindin and calmodulin (both NH2- and COOH-terminal binding site) were included with concentrations and kinetics taken from published models (Kim et al. 2010; Oliveira et al. 2012) (see appendix Table A4).
Synaptic Channels
AMPA, NMDA, and GABA receptors are distributed along the dendrites. For simplicity, there is one excitatory synapse (containing AMPA and NMDA) and one inhibitory synapse (GABA) per isopotential compartment. The maximal conductances and time constants are summarized in appendix Table A5. All synaptic channels use the facsynchan object in GENESIS, which uses Eq. 1 to calculate the conductance of the channel from the activation and inactivation time constants (τ1 and τ2, respectively), time t relative to the AP, and the maximal conductance (gmax). K is a normalization constant that is calculated from the time constants and allows Gsyn to reach a peak value of gmax.
| (1) |
where wt is synaptic weight, which depends on short-term facilitation or depression of these synapses:
| (2) |
where wt0 = 1. For NMDA and GABA channels, depr = 0. AMPA receptor desensitization, known to occur in the striatum (Akopian and Walsh 2007; Carter et al. 2007), is simulated with a time-dependent depr value. Each time an AMPA synapse is activated, depr is incremented by 1.0, and the value of depr decays with a time constant of 100 ms. NMDA receptors are modulated by the addition of a magnesium block object, where B = 99 and A = 18 (Eq. 3):
| (3) |
Extracellular magnesium concentration ([Mg2+]) is set to 1.4 mM (Kerr and Plenz 2002, 2004).These parameters closely match but slightly accentuate the magnesium sensitivity shown in Monyer et al. (1994). Excitatory inputs activate both an NMDA and an AMPA component with an NMDA-to-AMPA ratio of 2.75:1 (Ding et al. 2008), except in the “no NMDA” condition where the NMDA channel was removed. The fraction of the NMDA receptor current carried by calcium was set to 10% (Wolf et al. 2005), and the AMPA receptors were not calcium permeable. A GHK object was employed to calculate the driving potential of the calcium current through the NMDA receptor independently of the driving potential of the total NMDA current. An empirically determined multiplicative factor of 35e−9 was needed convert the conductance output of the magnesium block object to the permeability required by the GHK object.
Synaptic Inputs and Spike Timing
Upstates were generated using excitatory and inhibitory Poisson input trains of 300-ms duration as input. Inhibitory input frequencies were set to 70 Hz, and these trains were completely independent of excitatory inputs. A variety of excitatory input patterns were simulated: a flat input of 40 Hz and a series of graded inputs at different strengths (see Fig. 5 and appendix Table A6). Unless otherwise specified, gradient “G3” was used for the upstate simulations. APs were evoked at specific times within the upstate by a 5-ms, 800-pA current injection into the soma. AP timing was measured from the beginning of the upstate to the beginning of the 5-ms depolarization. The actual peak of the AP varied by <2 ms, and our previous work has shown that spike variability on this scale does not affect calcium results (Evans et al. 2012). During the STDP protocols, one segment of dendrite 44 μm from the soma was given a single input at positive or negative Δt from the AP with no other synaptic inputs.
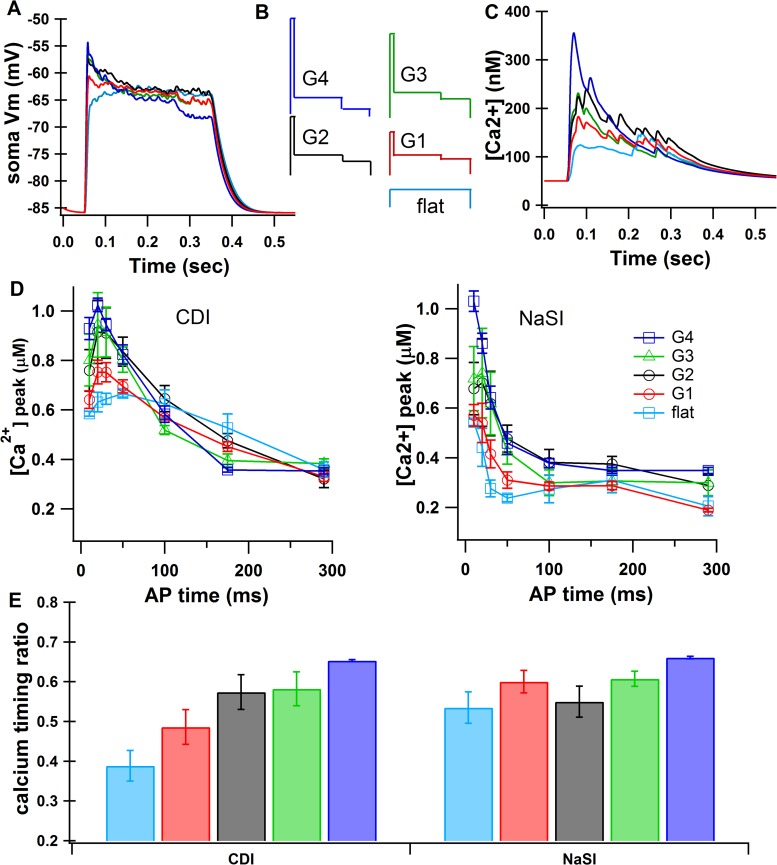
Fig. 5.
Input pattern affects calcium dependence on AP timing. A: subthreshold upstates of varying gradient patterns (see G1–G4 in B) as measured at the soma. B: schematic of input patterns (not to scale; see appendix Table A6). C: example traces of calcium in tertiary dendrites during each input pattern (no AP). D: average of 4 tertiary dendrites as a function of AP timing for each input pattern. E: calcium timing ratio for each input pattern, averaged over 4 tertiary dendrites for 1 random seed. Values are means ± SE.
Voltage Clamp of Calcium Currents
All animal handling and procedures were in accordance with the National Institutes of Health animal welfare guidelines and were approved by the George Mason University Institutional Animal Care and Use Committee. C57B6 male and female mice (Charles River; postnatal day 13–21) were anesthetized with isoflurane and euthanized. Every effort was made to minimize anxiety and pain. Brains were extracted and cut 350 μm thick with the use of a vibratome (Leica VT 1000S) in ice-cold sucrose slicing solution (in mM: 2.8 KCl, 10 dextrose, 26.2 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7 Mg2SO4, and 210 sucrose). Slices were immediately placed in an incubation chamber containing artificial cerebrospinal fluid (aCSF; in mM: 126 NaCl, 1.25 NaH2PO4, 2.8 KCl, 2 CaCl2, 1 Mg2SO4, 26.2 NaHCO3, and 11 dextrose) for 30 min at 33°C and then removed to room temperature (22–24°C) for at least 90 more minutes before use. Recording aCSF was modified by replacing Mg2SO4 with MgCl2 and including either 2 mM CaCl2 or 2 mM BaCl2. Tetrodotoxin (0.5 μM) and 4-aminopyridine (4 mM) were added to block sodium and potassium channels, respectively. Slices were individually placed in a submersion chamber (ALA Scientific), and cells were visualized using differential interference contrast imaging (Zeiss Axioskop 2 FS Plus). Pipettes (3–5 MΩ) were pulled from borosilicate glass on a laser pipette puller (Sutter P-2000), coated with candle wax (to reduce pipette capacitance), and fire-polished (Narishige MF-830). Pipettes were filled with a cesium-based internal solution (in mM: 85 Cs-gluconate, 10 Cs3-citrate, 1 KCl, 10 NaCl, 10 HEPES, 1.1 EGTA, 0.1 CaCl2, 0.25 MgCl2, 15 tetraethylammonium-Cl, 3.56 Mg-ATP, and 0.38 Na-GTP), pH 7.26 (Rankovic et al. 2011). Voltage-clamp recordings were obtained on the HEKA EPC-10 and sampled at 20 kHz. Data were acquired in PatchMaster (HEKA Elektronik). Analog three-pole (10 kHz) and four-pole (2.9 kHz) Bessel filters were applied. Series resistance (4–15 MΩ) and capacitance were compensated.
Simulation and Analysis
All simulations used a time step of 5 μs and were repeated using three random number seeds unless otherwise specified. Four dendrites from each simulation were averaged together to estimate the calcium for a single simulation. For the STDP simulation, calcium from the dendrite receiving only the single timed glutamatergic input was measured from each random number seed. The strength of the dependence of calcium on AP timing (the “calcium timing ratio”) was evaluated by averaging the two latest AP conditions (175 and 290 ms) and normalizing them by the highest calcium elevation of that condition (usually 10, 20, or 30 ms). For all these cases graphs report means and SD across the three random number seeds. Data shown in Figs. 3–5 are run for one random seed, and the data are presented as means ± SE across four measured dendritic segments. Statistical significance was evaluated using the SAS procedure GLM and considered significant for P < 0.01. In the case of multiple comparisons, a Bonferroni correction was applied. This model is available on ModelDB.
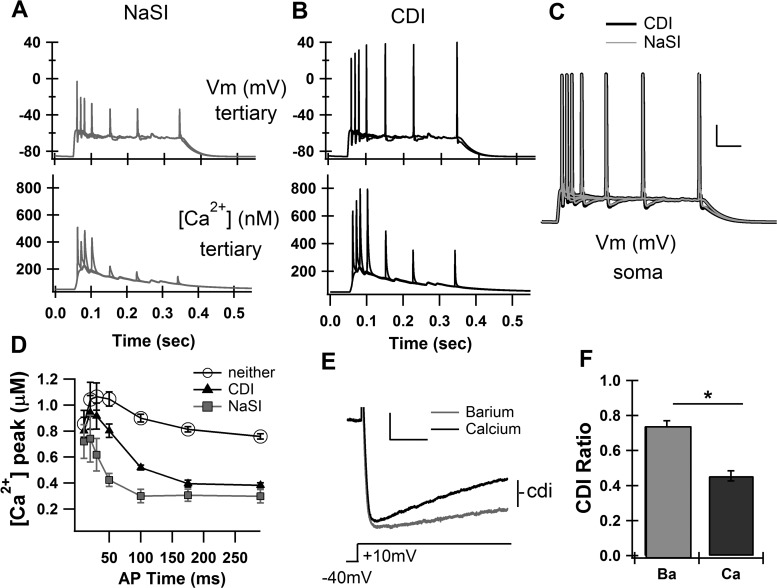
Fig. 3.
Two basic mechanisms can account for the relationship between calcium elevation and AP timing during the upstate: reduced AP backpropagatation (sodium slow inactivation, NaSI) or reduced calcium response (calcium-dependent inactivation, CDI). A: reducing the backpropagation of the APs through the implementation of NaSI causes a timing-dependent reduction in the tertiary dendrite depolarization (top) and calcium response (bottom). B: reducing the calcium response to depolarization through the implementation of CDI does not cause a reduction in the depolarization of the tertiary dendrite (top) but does cause a timing-dependent reduction of the calcium response (bottom). Vm, membrane potential. C: APs were elicited at specific times by short (5 ms) somatic depolarizations during the synaptically induced upstate. There was very little difference in the shape of the upstate between the NaSI mechanism (gray) and the CDI mechanism (black). Scale bars: vertical, 20 mV; horizontal, 50 ms. D: average over 4 tertiary dendrites as a function of AP timing. E. example traces showing CDI in high-voltage-activated calcium currents from a voltage-clamped striatal neuron. Scale bars: vertical, 100 pA; horizontal, 50 ms. F: summary averaged CDI ratio (degree of reduction by end of 200 ms) for all cells (n = 5). *P < 0.00001, paired t-test.
RESULTS
Part 1: Mechanisms Underlying the Calcium Sensitivity to AP Timing During the Upstate
Here we validate our model against published data and describe two mechanisms that may account for the relationship between calcium and AP timing during corticostriatal upstates. We confirm that both mechanisms make calcium elevation sensitive to AP timing and test how these manipulations interact with distance from the soma and the presence of NMDA receptors.
Intrinsic physiology and calcium dynamics in the MSN model match published data.
We developed a multichannel, multicompartmental biophysical model of a MSN, tuned to match electrophysiological characteristics of whole cell current-clamp recordings in a slice (Fig. 1, A and B; and see methods) and upstate characteristics (Fig. 1, C and D) such as fast depolarization into the upstate, 200- to 500-ms plateau, and slower repolarization back to the downstate. To accurately model the calcium dynamics in response to electrical activity, this model included a shell-based representation of calcium pools to simulate diffusion, calcium buffers (see appendix Table A4), and submembrane pumps. The intrinsic calcium activity of this MSN was tuned to match published data showing sodium channel-dependent backpropagation of APs into the proximal dendrites (Kerr and Plenz 2002) (Fig. 2B) and strong AP decay in distal dendrites (Day et al. 2008) (Fig. 2C). Dendritic VGCC permeabilities were adjusted to qualitatively match their contribution to dendritic calcium elevations during single backpropagating APs (Carter and Sabatini 2004) (Fig. 2D). Despite the maximal permeabilities being consistent throughout the distal dendrites (see appendix Table A3), the contribution of the T-type calcium channels increased with distance from the soma and the contributions of the L- and R-type calcium channels decreased (Fig. 2D). Though the contribution of the T-type calcium channel in our model at 44 μm from the soma is not quite as strong as shown in Carter and Sabatini (2004), we consider these results to be a reasonably good match because Carter and Sabatini were sampling a range of distances from the soma. We used this tuned model for all subsequent upstate simulations without any further adjustment to the intrinsic calcium.
Two mechanisms can account for the relationship between calcium and AP timing.
Calcium imaging studies have shown that APs evoked early in the upstate correspond to higher dendritic calcium elevations than APs evoked late in the upstate (Kerr and Plenz 2004). We hypothesize that two main mechanisms could cause this relationship between calcium and AP timing. First, if the AP backpropagated strongly when it occurred early in the upstate but weakly when it occurred late in the upstate, the calcium peaks would also be strong early and weak late. Second, even if the AP backpropagated with equal strength early and late in the upstate, the calcium response could desensitize during the upstate to show the same strong early, weak late calcium pattern. Because the dendritic voltage during upstates has not been recorded, it is not known if the AP backpropagates differently early and late in the upstate. Therefore, we tested both configurations in the computational model to see how well each matches published data.
We simulated the calcium in response to a range of AP times during an upstate for two model variations. Upstates are simulated in the model MSN using randomly generated Poisson trains of excitatory and inhibitory input. By using the same random seed to generate the pattern of input, the exact same subthreshold upstate could be repeated with several different AP timings. Each upstate was repeated with a somatic current injection evoking an AP delayed 10, 20, 30, 50, 100, 175, or 290 ms from upstate onset.
First, a slow inactivation component, which has been measured in striatal sodium channels (Ogata and Tatebayashi 1990), was added to dendritic sodium channels (Migliore 1996). This NaSI condition indeed caused the AP to backpropagate more strongly early in the upstate than late (Fig. 3A, top), and the corresponding calcium elevations varied with AP timing during the upstate (Fig. 3A, bottom). Second, CDI (see appendix TableA2 for equation) of VGCCs (Liang et al. 2003) was added to L-, N-, and R-type calcium channels in our model. When applied to the model, the CDI condition resulted in strong electrical backpropagation of the AP both early and late in the upstate (Fig. 3B, top), but the calcium elevation still varied with AP timing (Fig. 3B, bottom). These two conditions, CDI and NaSI, resulted in APs that were indistinguishable at the soma in size and shape (Fig. 3C). If neither manipulation was applied, the relationship between calcium and AP timing was very weak (Fig. 3D), and if both were applied simultaneously, the results were not different from the NaSI condition alone (data not shown).
To confirm that high-voltage-activated (HVA) channels actually undergo CDI in the striatum, we voltage-clamped HVA calcium currents (by holding at −40 mV and stepping to +10mV) and repeated measurements using barium (which causes minimal CDI) and calcium (which causes strong CDI). The ratio of the decayed current (sampled after 180 ms) to the peak current (sampled within first 50 ms) was taken as the CDI ratio. In all striatal cells recorded (n = 5), the CDI ratio was much stronger in calcium than in barium (P < 0.00001, paired t-test) (Fig. 3, E and F).
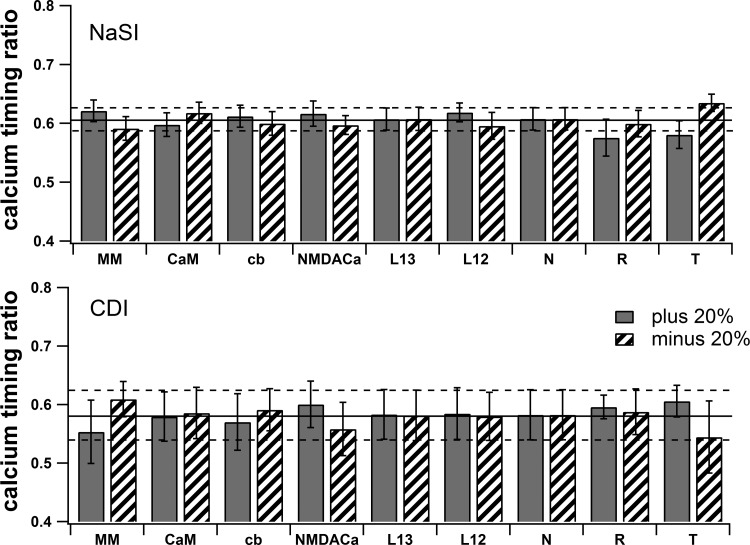
An additional mechanism could theoretically account for a decrease in calcium elevation late in the upstate. An increase in buffer capacity and/or pump capacity during the upstate could increase the rate of calcium removal and thus decrease the free calcium. Changes in buffer capacity due to protein synthesis are unlikely at the temporal scales being investigated. One report of a changes in pump capacity actually demonstrates an activity dependent decrease in the sodium calcium exchanger (Scheuss et al. 2006). Because an activity-dependent decrease in pump activity would result in more calcium late in the upstates, this mechanism is unlikely to contribute to the decrease in calcium elevation observed late in the upstate (Kerr and Plenz 2004). Furthermore, simulations that evaluate the effect of changing pump capacity reveal an insignificant effect on calcium timing ratio for both CDI and NaSI conditions (Fig. 4).
Fig. 4.
Robustness tests. Changes in calcium parameters ±20% do not significantly change the main effect of AP timing-dependent calcium concentration for either the NaSI condition (top) or the CDI condition (bottom). MM, Michaelis-Menten pump (Kcat); CaM, calmodulin [both NH2 (N) and COOH (C) site]; cb, calbindin; NMDACa, fraction of calcium through NMDA receptor; L13, L-type calcium channel Cav1.3; L12, L-type calcium channel Cav1.2; N, N-type calcium channel; R, R-type calcium channel; T, T-type calcium channel. Values are means ± SE. Solid black line represents mean for control condition, and dotted black lines indicate ±SE for control condition.
In addition to changes in pump capacity, we evaluated the robustness of our computational model to other parameter variations (Fig. 4). We systematically varied each parameter directly relating to calcium (buffers, pumps, NMDA calcium and each VGCC) by ±20% and compared the calcium relationship with AP timing for each condition to the controls. None of these manipulations significantly altered the relationship between calcium and AP timing in either CDI [F(18, 75) = 0.15, P > 0.9] or NaSI [F(18, 75) = 0.49, P > 0.9] (averaged over 4 dendrites for 1 random seed). These tests confirm that our main effect is robust to variation in calcium influx, buffering, and pump extrusion.
The calcium-AP relationship depends on input shape and distance from the soma.
The strength of the relationship between calcium elevation and AP timing is modulated by the shape of the cortical input creating the upstate. We simulated a range of input gradients (Fig. 5, A and B), and the strength of the calcium dependence on AP timing varied with the strength of the gradient (Fig. 5, D and E). Although both the NaSI and CDI conditions showed a dependence on input steepness, the effect was stronger for CDI (calcium timing ratio = 0.59 ± 0.0008 G3, 0.35 ± 0.01 flat; P < 0.0001), than for NaSI (calcium timing ratio = 0.57 ± 0.04 G3, 0.54 ± 0.07 flat; P > 0.9). This result leaves open the possibility that synaptic input pattern alone is sufficient for AP timing effects on calcium. To test this, we repeated simulations using gradient G3 in a model with neither CDI nor NaSI. Without either of these mechanisms, the calcium dependence on AP timing was essentially absent (Fig. 3D). Therefore, although the input gradient contributes to the calcium dependence on AP timing, it is not sufficient to generate it. Because the simulations fit published data more accurately when the synaptic inputs were weighted toward the beginning of the upstate, we used gradient G3 as the upstate-generating input, unless otherwise specified.
Calcium imaging in organotypic co-cultures reveals that the calcium elevation due to the upstate and the relationship between calcium and AP timing increases with distance from the soma (Kerr and Plenz 2004). To test whether both CDI and NaSI show this increase in the calcium-AP timing relationship, the calcium signal was recorded at progressively more distal dendrites during upstate simulations (Fig. 6A). Both CDI and NaSI showed an increase in the strength of the calcium dependence on AP timing (calcium timing ratio) between primary, secondary, and tertiary branches (Fig. 6, B and C, insets). Therefore, in this case, both conditions equally match the published data.
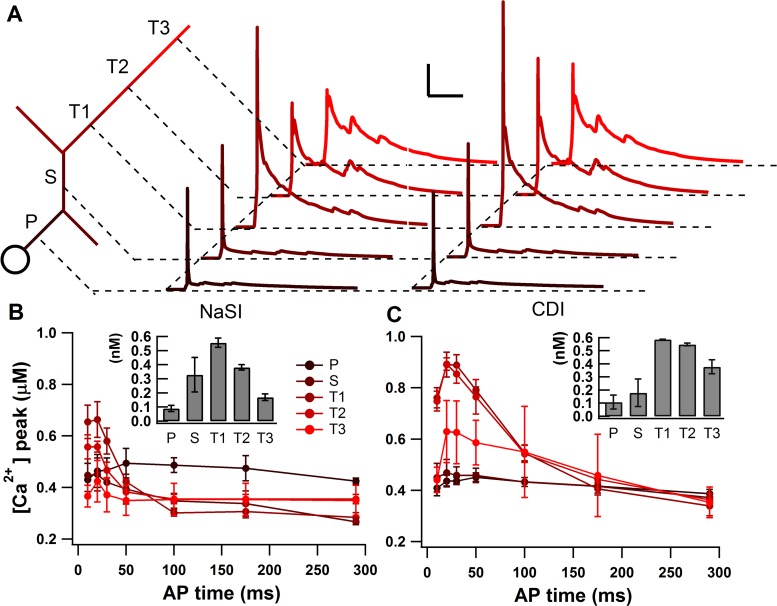
Fig. 6.
Distance from soma affects calcium dependence on AP timing. A: morphology (not to scale) of 1 dendritic branch color-coded dark to light for increasing distance from soma. Example calcium traces for each dendritic segment, primary (P), secondary (S), tertiary1 (T1), tertiary2 (T2), and tertiary3 (T3), are shown for both NaSI and CDI. Scale bars: vertical, 0.1 μM; horizontal, 100 ms. B: calcium dependence on AP timing for NaSI condition is most prominent at the proximal tertiary dendritic segment (T1). Inset: bar graph showing the calcium timing ratio between the calcium peak for early (highest point) and late (average of 2 last points) APs for each dendritic segment. Values are means ± SD. C: same as B, but for CDI condition.
Because the organotypic co-culture study did not record the calcium signals of distal dendrites (Kerr and Plenz 2004), and since we have previously shown that the effect of the backpropagating AP on calcium influx is attenuated in distal dendrites (Evans et al. 2012), we tested the relationship between calcium and AP timing distally. Figure 6 shows that distally the relationship between calcium and AP timing decreases with distance from the soma. This result implies that there is an optimal distance from the soma where the timing of the AP has the strongest effect on the corresponding calcium signal. More proximal dendrites are larger and have lower impedance, causing a reduced effect of synaptic inputs, and more distal dendrites are subject to the decay of the backpropagating AP, causing a weaker relationship between calcium and AP timing. The optimal distance in our model is the first tertiary dendritic segment, 44–62 μm from the soma. Interestingly, it is exactly this distance from the soma that has been shown to have the highest density of dendritic spines (Berlanga et al. 2011).
CDI replicates the effect of NMDA receptor blockade on the relationship between calcium and AP timing.
Previous work demonstrated that the relationship between calcium influx and AP timing is dependent on the activation of the NMDA receptors (Kerr and Plenz 2004). Therefore, we tested whether our two mechanisms (CDI and NaSI) were each dependent on the NMDA receptor by running upstate simulations with and without the NMDA receptors. Simulations revealed that removing the NMDA receptors greatly reduced the strength of the relationship between AP timing and calcium influx for the CDI condition (calcium timing ratio = 0.59 ± 0.0008 control, 0.30 ± 0.054 no NMDA; P < 0.0001) but did not reduce it for the NaSI condition (Fig. 7). In the NaSI condition, calcium elevations corresponding to both the early and the late APs were reduced with NMDA blockade, resulting in essentially the same relationship between calcium peak and AP timing (calcium timing ratio = 0.57 ± 0.04 control, 0.61 ± 0.06 no NMDA; P > 0.9; Fig. 7A, inset). Because the CDI condition matches the dependence of this calcium timing ratio on NMDA receptors and the NaSI condition does not, our model predicts that CDI is more likely to be a mechanism controlling this relationship.
Fig. 7.
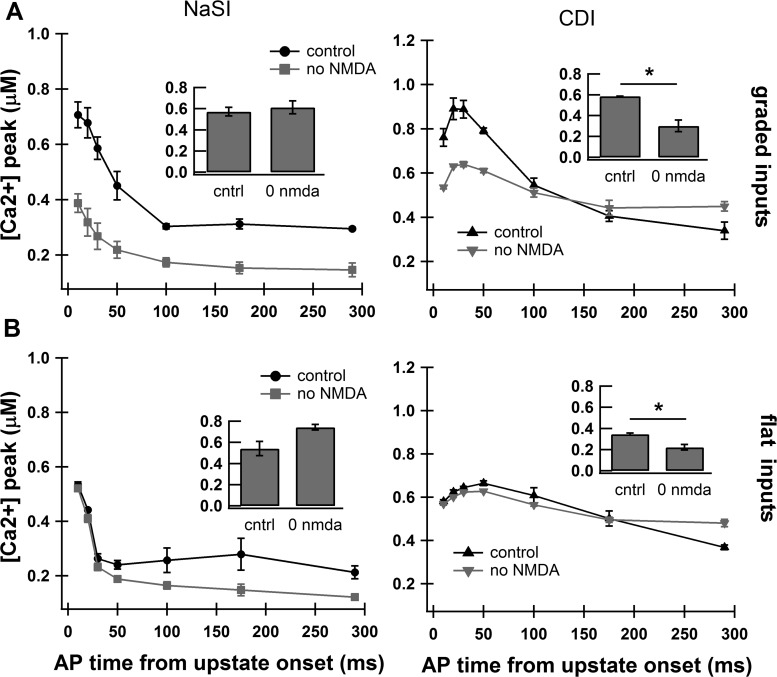
Dependence on NMDA receptors during flat and graded inputs. A: graded inputs result in a strong relationship between calcium signal and AP timing during the upstate. The calcium dependence on AP timing is reduced when the NMDA receptor is blocked only in the CDI condition. Note that the peak calcium elevations, but not the relationship between calcium and AP timing (calcium timing ratio, inset), are changed in the NaSI condition. Inset: bar graph showing the calcium timing ratio. Values are means ± SD. *P < 0.0001. B: when the upstate is elicited by flat input trains, the dependence of calcium peak on AP timing is reduced and the phenomenon is more weakly dependent on NMDA. Again, this NMDA dependence is observed for CDI but not NaSI. Inset: bar graph showing the calcium timing ratio.
Because the input gradient strongly influences the relationship between calcium and AP timing (Fig. 5), we repeated the NMDA and no-NMDA comparison for the flat input condition. In the CDI condition, removing NMDA receptors during flat inputs caused a decrease in the calcium timing ratio (0.35 ± 0.01 flat, 0.22 ± 0.03 flat no NMDA; P < 0.01; Fig. 7B), even though the effect of removing NMDA receptors was much stronger for the G3 input gradient (Fig. 7A). In the NaSI condition, neither the G3 gradient input nor the flat input resulted in NMDA dependence of the calcium timing ratio. In contrast to the flat CDI condition, the flat NaSI condition showed a surprising enhancing of the relationship between calcium and AP timing due to blocked NMDA receptors (calcium timing ratio = 0.54 ± 0.07 flat, 0.74 ± 0.03 flat no NMDA; P < 0.01; Fig. 7B, inset). This enhancement clearly does not replicate published data (Kerr and Plenz 2004), further supporting CDI as an essential mechanism underlying the relationship between calcium and AP timing.
In summary, we find that the CDI model better fits the published data. Specifically, the relationship between calcium and AP timing is dependent on NMDA receptors only in the CDI case. Our voltage-clamp experiments support this as a plausible mechanism because they show that CDI does indeed occur in the HVA calcium channels of the striatum. Therefore, all subsequent simulations are run using the CDI condition.
Part 2: Effect of Neuromodulation and Implications for In Vivo Plasticity
Here we use the model to investigate several aspects of plasticity during corticostriatal upstates. We test how intrinsic and synaptic excitability, which can balance each other in homeostatic plasticity, affect the relationship between calcium and AP timing, and we investigate the mechanisms controlling calcium binding to downstream targets, which can influence the direction of plasticity at a synapse. Finally, we test whether STDP pairings are modulated by their timing within the upstate.
Modulation of intrinsic but not synaptic excitability alters calcium relationship with AP timing.
Differences in stimulation patterns or changes in neuromodulator availability can alter MSN intrinsic excitability, which in turn can affect plasticity. The intrinsic excitability of the dendrite depends on the composition of voltage-gated potassium and sodium channels as well as the input resistance. Slice experiments have shown that dopamine depletion in the striatum increases intrinsic excitability of MSNs (Fino et al. 2007) and that this excitability increase is due to a twofold increase in the inactivation speed of A-type potassium currents (Kaf) (Azdad et al. 2009). Another potassium channel that can both modify dendritic excitability and be dynamically altered in response to neuromodulation is the inwardly rectifying potassium current (Kir). Acetylcholine M1 receptor activation causes a strong (40%) reduction of this current in one class of MSN (Shen et al. 2007).
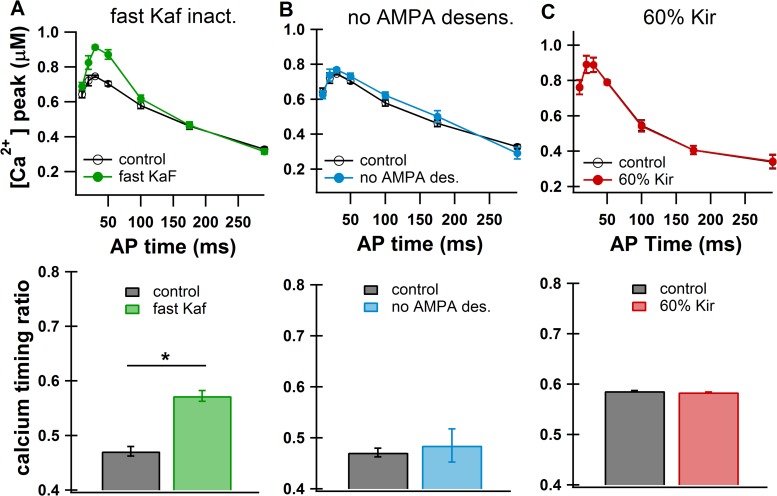
To evaluate the effect of excitability on the sensitivity of calcium to AP timing, we repeated simulations with either inactivation of Kaf twice as fast as the control condition or the conductance of the inwardly rectifying Kir channel set to 60% of its control value. Our results show that faster Kaf inactivation makes the calcium elevation corresponding to the upstate more sensitive to AP timing (Fig. 8A). Specifically, this modification causes a calcium increase in response to APs early in the upstate, but no change in calcium elevation in response to APs late in the upstate (calcium timing ratio = 0.47 ± 0.01 control, 0.57 ± 0.01 fast Kaf inact; P < 0.005). In contrast to the strong effect seen with Kaf modification, the change in Kir affects neither the absolute calcium nor the relationship between AP timing and calcium elevation (calcium timing ratio = 0.59 ± 0.0008 control, 0.58 ± 0.0005 60% Kir; P > 0.9; Fig. 8C).
Fig. 8.
A change in intrinsic excitability alters calcium relationship with AP timing. A: an increase in fast potassium A current (Kaf) inactivation speed (green) caused an increase in the strength of the calcium relationship with AP timing (*P < 0.005). Synaptic input with gradient G1 (see Fig. 5) was used to generate upstates to avoid spontaneous APs due to increased excitability. B: removing AMPA receptor desensitization (blue) did not affect the calcium relationship with AP timing. Gradient G1 was used to generate upstates. C: reducing inwardly rectifying potassium current (Kir) by 40% (red) did not affect the calcium relationship with AP timing.
The increase in intrinsic excitability (via fast Kaf inactivation) may be a homeostatic mechanism deployed by the neuron to compensate for a decrease in synaptic excitability (via reduced AMPA receptor activity) that occurs with dopamine depletion (Azdad et al. 2009). The AMPA receptor plays a strong role in the local depolarization of the dendrite and has been shown to influence the effect of backpropagating APs on NMDA calcium (Holbro et al. 2010). We therefore hypothesized that increased synaptic excitability implemented by removing AMPA receptor desensitization would reduce calcium sensitivity to AP timing. Surprisingly, when AMPA receptor desensitization was removed, the calcium dependence on AP timing was not altered (calcium timing ratio = 0.47 ± 0.009 control, 0.48 ± 0.03 no-AMPA desensitization; P > 0.9; Fig. 8B). These results show that increases in synaptic excitability and increases in intrinsic excitability do not affect calcium during the upstate in the same way. This also suggests that a change in intrinsic excitability cannot fully compensate for a change in synaptic excitability with regard to calcium sensitivity to AP timing and therefore has implications for upstates during dopamine depletion pathologies such as Parkinson's disease.
Fast and slow calcium binding partners are differentially influenced by AP timing and AP number.
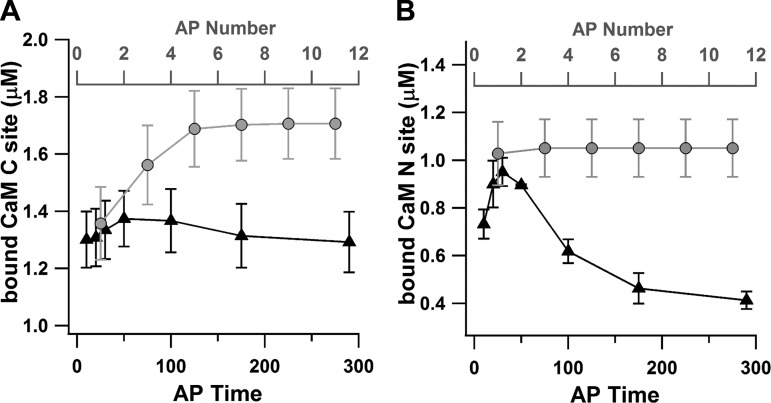
Since calcium is required for plasticity in the striatum (Fino et al. 2010), it has been suggested that the calcium elevations during upstates could cause potentiation or depression (Kerr and Plenz 2004). However, the factors that determine whether calcium causes potentiation or depression are still not clear. Recent studies suggest that the dynamics of calcium influx control preferential binding of calcium to disparate targets (Goldberg et al. 2009; Kubota and Waxham 2010). To test whether the timing of the AP within the upstate would influence not only calcium elevation but also the binding partners which calcium preferred, we made use of our calmodulin buffer, which has a fast-binding NH2 site (N site; CaMN) and a slow-binding COOH site (C site; CaMC).
During the upstate simulations, the peak concentration of bound CaMN is more strongly affected by the timing of the AP within the upstate than the peak concentration of bound CaMC (Fig. 9A). The slower binding and unbinding of calcium from the CaMC site smoothes the effect of the AP, making its timing irrelevant, whereas the fast binding and unbinding of the CaMN site makes it sensitive to AP timing. Figure 9 shows the peak bound CaMC (A) and CaMN (B) sites as a function of AP timing within the upstate (black symbols). Although the peak bound CaMC is always higher than the peak bound CaMN (due to the higher affinity of calcium for the C site than the N site), it is clear that binding to the CaMN site is sensitive to AP timing, but binding to the CaMC site is not.
Fig. 9.
Preference for calcium binding partners differs with AP timing and AP number. A: peak bound calmodulin C site (CaMC) is sensitive to AP number (gray) but not AP time (black). B: peak bound calmodulin N site (CaMN) is sensitive to AP time (black) but not AP number (gray). Values are means ± SE.
Another way that information can be conveyed during the upstate is through number of APs, rather than the specific timing of a single AP. To test whether the N and C sites of calmodulin were differentially sensitive to AP number, we ran upstate simulations with 1 to 11 APs. To prevent AP timing from influencing the bound buffer elevation, the timing of the initial AP was kept constant (20 ms from upstate onset) and each AP was added 20 ms after the most recent one (for a frequency of 50 Hz). In contrast to the AP timing condition, the peak bound CaMC site increased with the total number of APs, whereas the peak bound CaMN remained constant (Fig. 9, gray symbols). These results demonstrate that the two lobes of calmodulin are differentially sensitive to either the precise timing of the AP (CaMN) or the total number of APs (CaMC) within the upstate. Because the bound N and bound C sites differentially influence CaM's ability to bind to CaM-dependent kinase type 2 (CaMKII), a molecule implicated in synaptic strengthening (Forest et al. 2008), the specific combination of AP timing and AP number during the upstate may determine whether a given synapse will undergo potentiation or depression.
AP timing during the upstate interacts with STDP protocols.
When APs are paired with precisely timed glutamatergic inputs, a corticostriatal synapse can undergo STDP (Fino et al. 2010). However, in vivo neurons are subject to barrages of synaptic inputs during the upstate; thus a crucial question is whether spike timing influences calcium under in vivo-like conditions. We have previously shown that the NMDA-mediated calcium during STDP protocols correlates with synaptic potentiation (Evans et al. 2012). Here we use similar measurements to test how specifically timed pairings during the upstate would affect NMDA-mediated calcium.
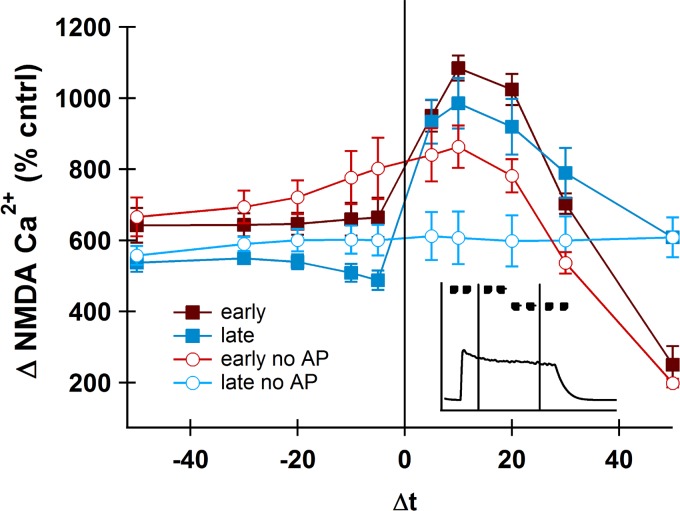
Because the spatial constraints of dendritic spines could alter the calcium dynamics, we added a single spine with an NMDA calcium pool to the first tertiary dendritic segment. To precisely control the timing of the pre and postsynaptic stimulations, we reserved a single tertiary dendritic segment, 44 μm from the soma, and stimulated its spine only one time during the entire upstate. The time of this stimulation was varied (Δt) around an early (20 ms after onset) upstate AP and a late (175 ms after onset) upstate AP (Fig. 10, inset). The NMDA-mediated calcium was recorded for each interval through a separate NMDA calcium pool (Evans et al. 2012). Pairing synaptic input with the AP showed a distinctive STDP curve both early and late in the upstate (Fig. 10). As in our previous model (Evans et al. 2012), the positive intervals showed higher NMDA-mediated calcium peaks.
Fig. 10.
Spike timing-dependent plasticity (STDP) can occur during an upstate. NMDA receptor-mediated calcium in the spine head shows an STDP curve shape early and late in the upstate (filled squares), which requires the AP. Cntrl, NMDA stimulation alone; Δt, stimulation time relative to the AP. Inset: schematic of early and late AP with stimulation times (not to scale) during the upstate. Vertical lines represent timing of AP; dots represent timing of presynaptic stimulation.
The upstate depolarizes the dendrite even without an AP, and subthreshold depolarizations have been shown to cause synaptic plasticity in MSNs (Fino et al. 2009). Thus it is possible that the timing of the synaptic input relative to the subthreshold upstate could produce an STDP-like curve even without an AP. To demonstrate that the timing of the AP is indeed contributing to the shape of the STDP curve, we ran simulations in the absence of an AP (Fig. 10, open symbols). The stimulations given late in the upstate have no temporal sensitivity without the AP, whereas the stimulations early in the upstate demonstrate moderate temporal sensitivity even without the AP. In both the early and late AP cases, however, the AP “sharpens” the curve, lowering the NMDA calcium during negative intervals and increasing NMDA calcium during positive intervals relative to the no-AP condition. This “sharpening” effect is much stronger when the stimulated synapse is on the spine than when on the dendritic shaft (data not shown), suggesting that indeed the spatial constraints of the spine head influence the relationship between calcium and the backpropagating AP.
These results suggest that specific pre- and postsynaptic pairings could undergo STDP even during an upstate. Because the NMDA-mediated calcium is higher early in the upstate than later in the upstate, our model predicts that pairings occurring early in the upstate will have a slight bias toward potentiation compared with pairings occurring late in the upstate.
DISCUSSION
Upstates in the striatum drive large dendritic calcium transients, which may control naturally occurring potentiation and depression of MSN synapses during sleep. The distinct upstate and downstate pattern is a trademark of the anesthetized or sleeping striatum and is much weaker during wakefulness (Mahon et al. 2006). We have implemented highly realistic calcium dynamics in a biophysical model of a MSN to study the factors governing these calcium elevations during upstates and to investigate the implications of a timing-dependent calcium elevation on synaptic plasticity.
Is CDI or NaSI More Likely?
We first evaluated two mechanisms that cause the strong relationship between calcium elevation and AP timing within the upstate: NaSI and CDI. Both mechanisms matched the published data regarding the dependence of calcium on AP timing and the effects of distance from the soma on calcium elevation, but they did not both match other published effects such as NMDA dependence. Because CDI matched the published NMDA dependence of the timing-dependent calcium elevations whereas NaSI did not, our model predicts that CDI is more likely the mechanism producing the effect of AP timing.
It is important to note that these are not the only possible mechanisms that could account for a calcium relationship with AP timing. Pump extrusion, for example, could be altered over the course of the upstate or dependent on number or frequency of APs (Scheuss et al. 2006). Further experiments are needed to confirm that CDI is the main mechanism for the calcium relationship with AP timing.
Three Testable Predictions
First, the simulations fit published data more accurately when the synaptic inputs are weighted toward the beginning of the upstate (Fig. 5). Thus our model predicts that the corticostriatal upstates are initiated by strong glutamatergic inputs from the cortex. Electrophysiological recordings from organotypic triple co-cultures (Kerr and Plenz 2004) and in vivo (Schulz et al. 2009) demonstrate variability in upstate shape. However, the upstates often show steep rise times at initiation, rather than slow increases (Fig. 1, C and D). In addition, recent experimental work has shown that striatal upstates can be sustained hundreds of milliseconds after the glutamatergic barrage has ended (Flores-Barrera et al. 2009; Plotkin et al. 2011; but see also Kasanetz et al. 2006), indicating that it is indeed possible that naturally occurring upstates could have strong initial inputs that decrease or even disappear over the duration of the upstate. Although one study found that distal inputs were able to induce upstates more strongly than proximal (Plotkin et al. 2011), the excitatory and inhibitory inputs in our model are distributed equally throughout the cell, and differences based on spatial distribution of inputs were not explored. Experiments using calcium hotspot imaging (Varga et al. 2011) in organotypic co-cultures could test our model prediction that glutamatergic inputs during the upstate are weighted toward upstate onset. In addition, this technique could be used to test the spatial distribution of synaptic inputs during spontaneous upstates.
Second, simulations demonstrate that there is a specific distance from the soma that shows optimal sensitivity to AP timing. In our model it is the first segment of the tertiary dendrite, 44–62 μm from the soma. However, because each neuron is slightly different, it is likely that the size and location of this optimal distance could be variable. Therefore, our simulations predict that for a given neuron there will be an optimal distance that displays a very strong relationship between calcium and AP timing, although it will not necessarily be the exact same distance for every neuron. Synapse and spine counting studies have shown that excitatory synapse density and spine density are highest between 30 and 60 μm from the soma, declining sharply in the proximal direction and declining more gradually in the distal direction (Berlanga et al. 2011). It is possible that optimal sensitivity to AP timing at this location increases calcium-based plasticity and consequently spine growth and excitatory synapse development. Although the changes in sensitivity to AP timing proximal to the soma have already been shown (Kerr and Plenz 2004), experiments imaging calcium on very distal dendrites during striatal upstates could test our model prediction that the sensitivity to AP timing decreases in the distal dendrites.
Third, simulations reveal a means through which MSNs can dynamically alter their sensitivity to AP timing during the upstate by modulating their intrinsic excitability. Increasing the speed of Kaf inactivation accentuated calcium's dependence on AP timing during the upstate. This doubling of the Kaf inactivation kinetics occurs in the striatum in response to dopamine depletion (Azdad et al. 2009). Azdad et al. (2009) suggest that this excitability change is a form of homeostatic plasticity, increasing intrinsic excitability to compensate for a loss in synaptic excitability. However, as we have shown, this specific intrinsic excitability change increases the calcium dependence on AP timing, whereas a synaptic excitability change (the removal of AMPA receptor desensitization) does not alter the relationship. Thus the balance is not necessarily restored accurately. Such a compensation would result in an increased sensitivity to AP timing that might be too strong for optimal function. Indeed, a change in calcium dependence on AP timing could result in plasticity imbalances and consequently contribute to symptoms of dopamine depletion pathologies such as Parkinson's disease. Because we did not see an effect of Kir modification, our simulations show that not all changes in potassium-based intrinsic excitability will result in altered calcium dynamics. Although we did not model all the effects of dopamine depletion, our model predicts that alterations in Kaf observed during dopamine depletion will increase the dependence of calcium on AP timing. This prediction could be experimentally tested by pharmacologically or genetically manipulating the Kaf channel in organotypic co-cultures to test how changes in kinetics affect the calcium elevation during the upstate. Similarly, application of cyclothiazide to inhibit AMPA desensitization under the same conditions could reveal whether this aspect of synaptic excitability affects the calcium elevation during the upstate.
Implications for Plasticity
Calcium elevation is necessary for both the potentiation and depression of corticostriatal synapses (Fino et al. 2007). How calcium can cause these opposing effects is still a question up for debate. Several mechanisms have been postulated, for example, that the total amount of calcium determines the direction of plasticity (Graupner and Brunel 2012), that the channel allowing calcium influx determines plasticity (Fino et al. 2010), or that the binding of calcium to downstream targets makes the difference (Lisman 1989).
It is clear from our results that if the total amount of calcium determines the direction of plasticity (high calcium = potentiation; low calcium = depression), upstates that contain early APs will be more likely to potentiate synapses than upstates containing late APs. However, it is not clear that the amount of calcium is the only important factor. Therefore, we investigated differences in calcium binding during early and late APs. We found that the fast binding of the CaMN site was sensitive to AP timing, but the slow binding of the CaMC site was not. Previous studies have shown that fast binding partners are more sensitive to the backpropagation of a single AP than slow binding partners (Markram et al. 1998). Our work supports this idea and extends it to an in vivo-like context within the upstate. Importantly, we demonstrate that there is a dichotomy in the way that each calmodulin binding site responds to different types of information carried by the upstate. Specifically, the CaMN site is sensitive to AP timing, while the CaMC site is sensitive to AP number. These simulations reveal differential functions of calmodulin lobes without the spatial scale implemented in single spine simulations (Kubota and Waxham 2010). Calcium binding to the CaMN site alone is able to partially activate CaMKII, a molecule strongly implicated in synaptic strengthening (Forest et al. 2008). The CaMN site's preferential response to APs early in the upstate implies that the APs early in the upstate will more efficiently trigger mechanisms for increasing synaptic strength.
In addition, our results can be extended to other calcium binding partners. Our model predicts that in general fast binding partners will be sensitive to AP timing, while slow binding partners will be sensitive to AP number. This result, in combination with future research revealing the kinetics of plasticity-related calcium binding partners, will shed light on which plasticity mechanisms are sensitive to specific upstate characteristics.
STDP pairings show robust synaptic plasticity in corticostriatal brain slice, and our previous work (Evans et al. 2012) demonstrates that the simulated NMDA calcium under these conditions is an excellent predictor of synaptic potentiation. Here we show that during noisy in vivo-like conditions, STDP curves can still be established for independent dendritic and spine compartments. This finding suggests that STDP can occur during sleep, when the upstate-downstate pattern is most prominent (Mahon et al. 2006). Plasticity under these conditions may play a role in memory consolidation mediated by replay of cortical activity during sleep (Lansink et al. 2009; Ribeiro et al. 2004).
Our simulations complement the experimental findings of Fino et al. (2009), which show that subthreshold depolarizations paired with presynaptic stimulation can induce synaptic plasticity in MSNs. They showed that the curves elicited by pairing presynaptic stimulation with subthreshold postsynaptic depolarization are wider and less “directional” than those elicited by pairing presynaptic stimulations with suprathershold stimulations containing APs. Similarly, our simulations show that the timing intervals that evoke elevated calcium under the subthreshold (no AP) condition early in the upstate are wider and shallower than the same stimulations paired with an AP.
It has been hypothesized that one population of synapses controls the upstate but that a separate set of inputs drives the AP (Kasanetz et al. 2006; O'Donnell and Grace 1995). If this is indeed the case, a specific STDP-like pairing could easily occur on a dendritic segment or spine during the upstate. If an input consistently drives an AP during the upstate, it would consistently show a positive Δt and be potentiated. If an input simply drives the upstate, but not the AP, it would be equally likely to fall in a positive or negative Δt relative to the AP and would not be consistently potentiated. In this way the STDP control of calcium influx could control the direction of plasticity for specific synapses despite the calcium influx due to the upstate as a whole. On the other hand, the STDP control of calcium influx is not completely independent of timing during the upstate. We found that the whole STDP curve is shifted slightly upward, toward higher NMDA calcium, when the AP is early in the upstate compared with when the AP is late. This suggests that even if the plasticity of a synapse is based solely on STDP principles, the pairings that occur early in the upstate will be slightly biased toward potentiation.
Conclusion
Our model is the most advanced biophysical MSN model to date and is a useful tool for studying the calcium dynamics in the striatum. We have used it to investigate the mechanisms that underlie the nonlinear calcium dynamics corresponding to AP timing during striatal upstates, and it could easily be extended to answer other essential questions about striatal function and plasticity.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke National Research Service Award 1F31NS066645-01A2 (to R. Evans), National Institute on Alcohol Abuse and Alcoholism Grant R01-AA-16022, and Office of Naval Research Grant MURI N00014-10-1-0198.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.C.E. and K.T.B. conception and design of research; R.C.E. performed experiments; R.C.E. and Y.M.M. analyzed data; R.C.E. and K.T.B. interpreted results of experiments; R.C.E. and Y.M.M. prepared figures; R.C.E. drafted manuscript; R.C.E. and K.T.B. edited and revised manuscript; R.C.E. and K.T.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Reynolds for providing traces of in vivo upstates and Sriram Damodaran and Wonryull Koh for feedback on earlier versions of this manuscript.
Appendix
This model contains one fast sodium channel (Naf), four voltage-gated potassium channels, two calcium-activated potassium channels, and five VGCCs. All equations governing the kinetics of these channels, other than for the calcium-activated potassium channels, are given in Tables A1 and A2. The maximal conductances of these channels are listed in Table A3. Mechanisms controlling calcium dynamics included the endogenous calcium buffers calbindin and calmodulin, whose concentrations and kinetics are listed in Table A4. The maximal conductances and time constants for AMPA, NMDA, and GABA receptors are summarized in Table A5. Upstates were generated by providing excitatory and inhibitory Poisson input trains of 300-ms duration to the synapses, using several different temporal patterns (Table A6).
Table A1.
Steady-state equations
| Channel | mh Form | Steady State | Tau, ms | α or β Equation | Vhalf, mV | Slope, mV | Rate, ms |
|---|---|---|---|---|---|---|---|
| Naf | m3 | sigmoid | See Table A2 | −25 | −10 | 1 | |
| h | sigmoid | See Table A2 | −60 | 6 | 1 | ||
| Kir | m | α/(α + β) | [1e−3/(α + β)]×2 | α (exp) | −11 | 1e−5 | |
| β (sigmoid) | 30 | −50 | 1.2 | ||||
| Kaf | m2 | α/(α + β) | 1e−3/(α + β) | α (sigmoid) | −18 | −13 | 1.8 |
| β (sigmoid) | 2 | 11 | 0.45 | ||||
| h | α/(α + β) | 1e−3/(α + β) | α (sigmoid) | −121 | 22 | 0.105 | |
| β (sigmoid) | −55 | −11 | 0.065 | ||||
| Kas | m2 | α/(α + β) | 1/(α + β) | α (sigmoid) | 54 | −22 | 0.25 |
| β (sigmoid) | −100 | 35 | 0.05 | ||||
| h | 0.8 + [α/(α + β)]×0.2 | 1/(α + β) | α (sigmoid) | −95 | 16 | 2.5 | |
| β (sigmoid) | 50 | −70 | 2 | ||||
| Krp | m2 | α/(α + β) | 1/(α + β) | α (exp) | 24 | 16 | |
| β (exp) | −45 | 2.4 | |||||
| h | 0.87 + [α/(α + β)]×0.13 | 1/(α + β) | α (exp) | −100 | 0.01 | ||
| β (exp) | 18 | 0.4 | |||||
| CaL1.2 | m | sigmoid | See Table A2 | −8.9 | −6.7 | 1 | |
| h | 0.17 + (sigmoid)×0.83 | 44.3 ms | −55 | 8 | 1 | ||
| CaL1.3 | m | sigmoid | See Table A2 | −40 | −5 | 1 | |
| h | sigmoid | 44.3 ms | −37 | 5 | 1 | ||
| CaN | m2 | sigmoid | See Table A2 | −3 | −8 | 1 | |
| h | 0.21 + (sigmoid)×0.79 | 70 ms | −74.8 | 6.5 | 1 | ||
| CaR | m3 | sigmoid | 5.1 ms | −29 | −9.6 | 1 | |
| h | sigmoid | See Table A2 | −33.3 | 17 | 1 | ||
| CaT | m3 | sigmoid | See Table A2 | −63 | −8 | 1 | |
| h | sigmoid | See Table A2 | −84 | 5 | 1 |
Tau values are not temperature corrected (see methods for temperature correction values). Equations: sigmoid = rate/{1 + exp[(V − Vhalf)/slope]}; exp = rate×[exp(V/slope)]. See text for description of channels.
Table A2.
Tau equations for inward currents
| Channel | mh Form | Tau | α or β Equation | Rate* | Slope, mV | Vhalf, mV |
|---|---|---|---|---|---|---|
| Naf | m3 | Eq. A1 | 1.45 | 8 | −62 | |
| h | Eq. A2 | 1.2 | 3 | −42 | ||
| CaL1.2† | m | 1/(α + β) | α (linoid) | 39.8 | 8.124 | 9.005 |
| β (exp) | 0.99 | 31.4 | ||||
| CaL1.3† | m | 1/(α + β) | α (linoid) | 39.8 | 67.24 | 15.005 |
| β (exp) | 3.5 | 31.4 | ||||
| CaN† | m2 | 1/(α + β) | α (linoid) | 39.8 | 17.19 | 15.22 |
| β (exp) | 0.3842 | 23.82 | ||||
| CaR† | h | [1/(α + β)] + 20 | α (linoid) | 10e3 | 94.5 | 5.12 |
| β (exp) | 0.0842 | 13 | ||||
| CaT | m3 | [1/(α + β)] + 2.2 | α (linoid) | 14.552 | 84.5 | 7.12 |
| β (exp) | 4.9842 | 13 | ||||
| h | [1/(α + β)] + 100 | α (linoid) | 2.652 | 94.5 | 5.12 | |
| β (exp) | 0.6842 | 13 |
Tau values are not temperature corrected (see methods for temperature correction values). Equations: Eq. A1 = 0.1 + (rate/{1 + exp[(V − Vhalf)/slope]})(rate/{1 + exp[(V − Vhalf)/−slope]}; Eq. A2 = 0.2754 + (rate/{1 + exp[(V − Vhalf)/slope]}); linoid = [rate×(V + Vhalf)]/{exp[(V + Vhalf)/slope] − 1}; exp = rate×[exp(V/slope)].
Rate units are ms−1 for linoid and exp, and ms for Eqs. A1 and A2.
Channels undergoing calcium-dependent inactivation (CDI) with the equation 0.00053/(0.00053 + [Ca2+]3)100 and a time constant of 47.3 ms, only when CDI is turned on.
Table A3.
Maximal conductance and permeability for ionic channels
| Channel | Soma | Prox Dend | Mid Dend | Dist Dend |
|---|---|---|---|---|
| Gbar, S/m2 | ||||
| NaF | 50,000 | 6,000 | 6,000 | 2,000 |
| Kir | 11 | 11 | 11 | 11 |
| KaF | 300 | 550 | 550 | 550 |
| KaS | 200 | 22 | 22 | 22 |
| Krp | 14 | 14 | 14 | 14 |
| SK | 1 | 1 | 1 | 1 |
| BK | 10 | 10 | 10 | 10 |
| Pbar, cm/s | ||||
| CaL1.2 | 6e-7 | 1e-7 | 1e-7 | 1e-7 |
| CaL1.3 | 3e-7 | 0.5e-8 | 0.5e-8 | 0.5e-8 |
| CaN | 12e-7 | 0 | 0 | 0 |
| CaR | 8e-7 | 10e-7 | 10e-7 | 10e-7 |
| CaT | 0 | 0 | 8e-8 | 8e-8 |
Gbar, maximal conductance; Pbar, maximal calcium permeability; Prox dend, proximal dendrites (≤42 μm from soma); Mid Dend, middle dendrites (42–60 μm from soma); Dist Dend, distal dendrites (60–224 μm from soma).
Table A4.
Calcium buffer parameters
| Concentration, μM | Kf, μM−1×s−1 | Kb, s−1 | Diff, m2/s | |
|---|---|---|---|---|
| CaMN | 15 | 100 | 1000 | 11e−12 |
| CaMC | 15 | 6 | 9.1 | 11e−12 |
| Calbindin | 80 | 28 | 19.6 | 0 |
CaMN, calmodulin NH2-terminal binding site; CaMC, calmodulin COOH-terminal binding site; Kf, forward rate constant; Kb, backward rate constant; Diff, diffusion constant; Calbindin, concentration of binding sites.
Table A5.
Conductances and time constants for synaptic channels
| Gbar, pS | τ1, ms | τ2, ms | |
|---|---|---|---|
| NMDA | 470 | 2.2312 | 25 |
| AMPA | 171 | 1.1 | 5.75 |
| GABA | 900 | 0.25 | 3.75 |
Time constants have already been temperature corrected by a Q factor of 2. τ1, activation time constant; τ2, inactivation time constant.
Table A6.
Input gradients used to create upstates
| Frequency, Hz |
|||
|---|---|---|---|
| Input | First 10 ms | Middle 200 ms | Last 90 ms |
| Flat | 40 | 40 | 40 |
| G1 | 200 | 40 | 10 |
| G2 | 400 | 50 | 20 |
| G3 | 500 | 30 | 10 |
| G4 | 600 | 20 | 0 |
Values are input frequencies for different input shapes. G1–G4, gradients 1–4. Input G3 was used for simulations unless otherwise specified (see also Fig. 5).
REFERENCES
- Akopian G, Walsh JP. Reliable long-lasting depression interacts with variable short-term facilitation to determine corticostriatal paired-pulse plasticity in young rats. J Physiol 580: 225–240, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815, 1992 [DOI] [PubMed] [Google Scholar]
- Azdad K, Chàvez M, Don Bischop P, Wetzelaer P, Marescau B, De Deyn PP, Gall D, Schiffmann SN. Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion. PLoS One 4: e6908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci 14: 6667–6686, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga ML, Price DL, Phung BS, Giuly R, Terada M, Yamada N, Cyr M, Caron MG, Laakso A, Martone ME, Ellisman MH. Multiscale imaging characterization of dopamine transporter knockout mice reveals regional alterations in spine density of medium spiny neurons. Brain Res 1390: 41–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevi S, de Curtis M, Magistretti J. Pharmacological and biophysical characterization of voltage-gated calcium currents in the endopiriform nucleus of the guinea pig. J Neurophysiol 85: 2076–2087, 2001 [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44: 483–493, 2004 [DOI] [PubMed] [Google Scholar]
- Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J Neurosci 27: 8967–8977, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci 28: 11603–11614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci 28: 6483–6492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RC, Morera-Herreras T, Cui Y, Du K, Sheehan T, Kotaleski JH, Venance L, Blackwell KT. The effects of NMDA subunit composition on calcium influx and spike timing-dependent plasticity in striatal medium spiny neurons. PLoS Comput Biol 8: e1002493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Deniau JM, Venance L. Brief subthreshold events can act as Hebbian signals for long-term plasticity. PLoS One 4: e6557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci 25: 11279–11287, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci Res 58: 305–316, 2007 [DOI] [PubMed] [Google Scholar]
- Fino E, Paille V, Cui Y, Morera-Herreras T, Deniau JM, Venance L. Distinct coincidence detectors govern the corticostriatal spike timing-dependent plasticity. J Physiol 588: 3045–3062, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Laville A, Plata V, Tapia D, Bargas J, Galarraga E. Inhibitory contribution to suprathreshold corticostriatal responses: an experimental and modeling study. Cell Mol Neurobiol 29: 719–731, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Mermelstein PG, Song WJ, Ulrich S, Surmeier DJ. Unique properties of R-type calcium currents in neocortical and neostriatal neurons. J Neurophysiol 84: 2225–2236, 2000 [DOI] [PubMed] [Google Scholar]
- Forest A, Swulius MT, Tse JKY, Bradshaw JM, Gaertner T, Waxham MN. Role of the N- and C-lobes of calmodulin in the activation of Ca2+/calmodulin-dependent protein kinase II. Biochemistry 47: 10587–10599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Teagarden MA, Foehring RC, Wilson CJ. Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J Neurosci 29: 8396–8407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner M, Brunel N. Calcium-based plasticity model explains sensitivity of synaptic changes to spike pattern, rate, and dendritic location. Proc Natl Acad Sci USA 109: 3991–3996, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halavi M, Polavaram S, Donohue DE, Hamilton G, Hoyt J, Smith KP, Ascoli GA. NeuroMorpho.Org implementation of digital neuroscience: dense coverage and integration with the NIF. Neuroinformatics 6: 241–252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Wiegert JS, Oertner TG. AMPA receptors gate spine Ca2+ transients and spike-timing-dependent potentiation. Proc Natl Acad Sci USA 107: 15975–15980, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol 448: 161–188, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme LA, O'Donnell P, Murer MG. Turning off cortical ensembles stops striatal Up states and elicits phase perturbations in cortical and striatal slow oscillations in rat in vivo. J Physiol 577: 97–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci 22: 1499–1512, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Action potential timing determines dendritic calcium during striatal up-states. J Neurosci 24: 877–885, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Huang T, Abel T, Blackwell KT. Temporal sensitivity of protein kinase a activation in late-phase long term potentiation. PLoS Comput Biol 6: e1000691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Waxham MN. Lobe specific Ca2+-calmodulin nano-domain in neuronal spines: a single molecule level analysis. PLoS Comput Biol 6: e1000987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol 7: e1000173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron 39: 951–960, 2003 [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA 86: 9574–9578, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci 26: 12587–12595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Roth A, Helmchen F. Competitive calcium binding: implications for dendritic calcium signaling. J Comput Neurosci 5: 331–348, 1998 [DOI] [PubMed] [Google Scholar]
- McNaughton NC, Randall AD. Electrophysiological properties of the human N-type Ca2+ channel: I. Channel gating in Ca2+, Ba2+ and Sr2+ containing solutions. Neuropharmacology 36: 895–915, 1997 [DOI] [PubMed] [Google Scholar]
- McRory JE, Santi CM, Hamming KS, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem 276: 3999–4011, 2001 [DOI] [PubMed] [Google Scholar]
- Migliore M. Modeling the attenuation and failure of action potentials in the dendrites of hippocampal neurons. Biophys J 71: 2394–2403, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15: 4449–4463, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15: 3622–3639, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Tatebayashi H. Sodium current kinetics in freshly isolated neostriatal neurones of the adult guinea pig. Pflügers Arch 416: 594–603, 1990 [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Kim M, Blackwell KT. Subcellular location of PKA controls striatal plasticity: stochastic simulations in spiny dendrites. PLoS Comput Biol 8: e1002383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci 28: 2435–2446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JL, Day M, Surmeier DJ. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat Neurosci 14: 881–888, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic V, Landgraf P, Kanyshkova T, Ehling P, Meuth SG, Kreutz MR, Budde T, Munsch T. Modulation of calcium-dependent inactivation of L-type Ca2+ channels via β-adrenergic signaling in thalamocortical relay neurons. PLoS One 6: e27474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, Lavine M, Nicolelis MA. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol 2: E24, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuss V, Yasuda R, Sobczyk A, Svoboda K. Nonlinear [Ca2+] signaling in dendrites and spines caused by activity-dependent depression of Ca2+ extrusion. J Neurosci 26: 8183–8194, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JM, Redgrave P, Mehring C, Aertsen A, Clements KM, Wickens JR, Reynolds JN. Short-latency activation of striatal spiny neurons via subcortical visual pathways. J Neurosci 29: 6336–6347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hernandez-Lopez S, Tkatch T, Held JE, Surmeier DJ. Kv1.2-containing K+ channels regulate subthreshold excitability of striatal medium spiny neurons. J Neurophysiol 91: 1337–1349, 2004 [DOI] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci 10: 1458–1466, 2007 [DOI] [PubMed] [Google Scholar]
- Steephen JE, Manchanda R. Differences in biophysical properties of nucleus accumbens medium spiny neurons emerging from inactivation of inward rectifying potassium currents. J Comput Neurosci 27: 453–470, 2009 [DOI] [PubMed] [Google Scholar]
- Stoetzner CR, Pettibone JR, Berke JD. State-dependent plasticity of the corticostriatal pathway. Neuroscience 165: 1013–1018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci 20: 579–588, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckwell HC. Quantitative aspects of L-type Ca2+ currents. Prog Neurobiol 96: 1–31, 2012 [DOI] [PubMed] [Google Scholar]
- Varga Z, Jia H, Sakmann B, Konnerth A. Dendritic coding of multiple sensory inputs in single cortical neurons in vivo. Proc Natl Acad Sci USA 108: 15420–15425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res 220: 67–80, 1981 [DOI] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O'Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci 25: 9080–9095, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12: 333–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]