Abstract
N-methyl-d-aspartate receptors (NMDARs) have been linked to schizophrenia because agents that bind the receptor, like ketamine and phencyclidine, are capable of inducing schizophrenia-like symptoms. Here we show that the amino acid homocysteine (HCY), which is increased in the blood of schizophrenia patients, reduces desensitization of NMDARs in cultured mouse neurons, human embryonic kidney cells transfected with GluN1 + GluN2A, GluN2B, or GluN2D subunits, and hippocampal slices. HCY also alters the peak amplitude of NMDAR currents, depending on the GluN2 subunit the receptor contains; GluN1 + GluN2A-containing NMDARs show an increase in peak amplitude when exposed to HCY, while GluN1 + GluN2B-containing NMDARs show a decrease in peak amplitude. Both peak amplitude and desensitization effects of HCY can be occluded by saturating the NMDAR with glycine. Since glycine concentrations are not saturating in the brain, HCY could play an NMDAR-modulating role in the nervous system. We also show that HCY shares characteristics with glutamate and suggest that HCY affects both the agonist and co-agonist site of the NMDAR.
Keywords: NMDAR, schizophrenia, homocysteine, GluN2, desensitization
the role of n-methyl-d-aspartate (NMDA) receptors (NMDARs) in coincidence detection is critical for neural plasticity (Bi and Poo 1998), activity-dependent development (Constantine-Paton et al. 1990; Phillips et al. 2011), and persistent neural activity related to working memory (Lisman et al. 1998; Seung et al. 2000; Wang 2001). NMDARs are also strongly linked to neurological disease. For example, the NMDAR hypothesis of schizophrenia has arisen because NMDAR antagonists like ketamine induce schizophrenia symptoms in healthy individuals (Newcomer and Krystal 2001). It is unclear, however, how dysregulation of endogenous neurotransmitters could give rise to a ketamine-like environment in the schizophrenic brain. One hint comes from a meta-analysis showing that homocysteine (HCY), an amino acid that acts on NMDARs (Lipton et al. 1997; Poddar et al. 2009), is abnormally high in the blood of schizophrenia patients (Muntjewerff et al. 2006).
In addition to its presence in the blood, HCY also arises in many brain areas after catechol-O-methyltransferase (COMT) methylates synaptically released dopamine (DA) and norepinephrine (NE) (Bigl et al. 1974; Broch Jr. and Fonnum 1972; Huang et al. 2005; Tunbridge et al. 2008). HCY is present in normal human cerebrospinal fluid (CSF), and schizophrenia patients show increased HCY in the CSF (Regland et al. 2004), but studies that have addressed the synaptic actions of extracellular HCY have produced complex results. Previous work has demonstrated that HCY induces calcium flux into cultured neurons through NMDARs, causing excitotoxicity that is blocked by NMDAR glutamate site antagonists (Lipton et al. 1997). Acutely applied HCY also mimics NMDA in reducing long-term potentiation (LTP) in hippocampal slices (Christie et al. 2009). However, while these and other studies suggest that HCY is a weak agonist at the NMDAR glutamate site (Poddar et al. 2009), HCY also shows characteristics that are not typical of glutamate site agonists (Christie et al. 2009; Lipton et al. 1997). For instance, when HCY concentrations ([HCY]) are raised above 100 μM, the effect of HCY on LTP reverses: now HCY significantly enhances NMDAR-dependent LTP, which does not occur when NMDA is applied (Christie et al. 2009). Moreover, HCY inhibits peak NMDA-induced calcium flux when glycine (GLY) concentrations ([GLY]) are subsaturating in young cultured neurons (Lipton et al. 1997). This is significant because the NMDAR GLY site may not be saturated in vivo by its co-agonists d-serine and GLY, the latter of which is shuttled away from synapses by GLY transporters (GlyTs) (Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2003). Considering the complexity of previous HCY results, we sought to directly test how the schizophrenia-related molecule HCY influences the dynamics of NMDAR currents in cultured neurons, human embryonic kidney (HEK) cells transfected with NMDAR subunits, and acute brain slices.
One feature of NMDAR currents is their rapid and extensive desensitization in response to prolonged agonist exposure (Mayer et al. 2004). Channel desensitization is thought to play a role in shaping synaptic events and protecting the neuron from calcium toxicity during repeated receptor stimulation (Lukasiewicz et al. 1995; Tong et al. 1995). Three forms of NMDAR desensitization are believed to be working in concert. The GLY-dependent component of desensitization is occluded upon raising extracellular levels of GLY or d-serine (i.e., GLY and d-serine relieve, rather than cause, this form of desensitization; Lerma et al. 1990; Mayer et al. 1989; Vyklicky et al. 1990). Calcium-dependent desensitization is caused by calcium flux through the NMDAR, involves calmodulin and calcineurin, and is prevented by fast calcium chelation and low extracellular calcium (Ehlers et al. 1996; Krupp et al. 1996; Legendre et al. 1993; Tong et al. 1995; Zilberter et al. 1991). GLY-independent desensitization is the component of desensitization that remains after GLY levels are saturated and calcium flux is prevented; the molecular motifs governing GLY-independent desensitization have been elegantly localized to the GluN2 NH2-terminal domain (Sather et al. 1990, 1992; Villarroel et al. 1998).
In the present study, we examined how NMDARs responded to HCY during prolonged agonist application. Neurons and HEK cells transfected with NMDAR subunit cDNA (GluN1 + GluN2A, GluN1 + GluN2B, or GluN1 + GluN2D) showed strongly reduced NMDAR desensitization in the presence of HCY. We demonstrate that HCY specifically reduces the GLY-dependent component of NMDAR desensitization, with pronounced effects at doses as low as 50 μM. HCY maintained its desensitization-reducing capabilities in hippocampal slices, where native GLY and d-serine levels do not appear to saturate the NMDAR. HCY also affects peak amplitude of the NMDAR response, depending on ambient GLY levels and GluN2 subunit composition. Our results are consistent with, and add to, previous literature describing HCY's effects on NMDAR currents and LTP.
It is known that GluN2A-to-GluN2B ratios vary with age and between excitatory and inhibitory neural populations (Flint et al. 1997; Kinney et al. 2006; Townsend et al. 2003; van Zundert et al. 2004). This suggests that HCY's effects will change with development and differ according to cell type within neural circuits. A change in HCY's effect with development is shown here and may be critical given the late adolescent/early adulthood onset of schizophrenia (Macdonald and Chaffee 2006).
MATERIALS AND METHODS
All experiments were carried out with the approval of the Committee on Animal Care at the Massachusetts Institute of Technology.
Primary neuron culture.
Cortical cultures were prepared from postnatal day 0 (P0) Thy-1 green fluorescent protein mice (Feng et al. 2000), while hippocampal cultures were from C57B6. Cells were plated on poly-d-lysine-coated coverslips and grown glia-free in B27 supplemented Neurobasal A media (Invitrogen, Grand Island, NY). Older culture recordings (DIV30) were obtained from cultures expressing tdTomato in parvalbumin positive neurons; however, parvalbumin positive neurons were not targeted for patching.
NMDA receptor expression in HEK293T cells.
cDNAs for mouse GluN1–1a (P35438), GluN2A (P35436), and GluN2B (Q01097) (M. Mishina, University of Tokyo, Japan) were previously cloned into DsRed2N1 (Clontech, Mountain View, CA), where the DsRed2 gene was replaced with an NMDAR subunit. All subunits were selected by full-length sequencing. The GluN2D (Q03391) construct was a gift from Dr. John Woodward (Medical University of South Carolina; Jin et al. 2008). HEK293T (HEK) cells (293tsA1609neo; ATCC, Rockville, MD) were plated onto uncoated glass coverslips in 1 ml DMEM+/+ [DMEM + GlutaMAX (Invitrogen Gibco 10569) + 10% FBS + 1% Pen/Strep]. Cells were transfected 1 h after plating with GluN1 and GluN2 subunits plus a pEGFP reporter to visualize transfected cells (1:1:1 ratio). For each reaction, cDNAs were added to 200 μl Optimem (Invitrogen), incubated for 5 min after addition of 2 μl Plus reagent (Invitrogen), then incubated for 25 min after adding 6 μl lipofectamine LTX (Invitrogen) for a total volume of 208 μl. This solution was added to the 1 ml of DMEM+/+ on plated cells for 30–45 min of transfection. Transfection media was removed, and cells were incubated in a medium containing DMEM+/+, 3 mM kynurenic acid (Sigma, St. Louis, MO), and 1 mM d,l-AP5 (Tocris, Minneapolis, MN) at 37°C, 5% CO2.
Electrophysiology drugs used/prepared.
NMDA, glutamate (l-glutamic acid), d,l-HCY, d,l-HCY thiolactone, l-HCY thiolactone, d,l-homocystine, strychnine, and l-cysteine (free base) were obtained from Sigma. Dichlorokynurenic acid (DCKA), d,l-AP5, 4-methoxy-7-nitroindolinyl (MNI)-caged glutamate, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX), gabazine, N-{3-([1,1-biphenyl]-4-yloxy)-3-(4–fluorophenyl)propyl}-N-methylglycine (NFPS), and tetrodotoxin (TTX) were obtained from Tocris. GLY was obtained from J. T. Baker (Pittsburgh, NJ). l-HCY, the form of HCY that is present in the brain, was synthesized from l-HCY thiolactone by opening the thiolactone ring. This method was generously communicated to us by Dr. Donald Jacobsen (Lerner Research Institute, Cleveland, OH). l-HCY thiolactone (154 mg) was dissolved in 5 M NaOH (0.15 g/ml concentration) and incubated for 5 min at 37°C. A solution of 2 M HCl, 0.1 M TES (pH 7.4) and dH2O (1.9:1:1.1) was then mixed with the HCY/NaOH solution at a ratio of 4:1 and vortexed. Argon gas was bubbled through the solution. Maximal possible yield of l-HCY was 200 mM; this stock solution was diluted 1:100 for experiments, leaving a possible 2 mM in experimental solutions for Fig. 7.
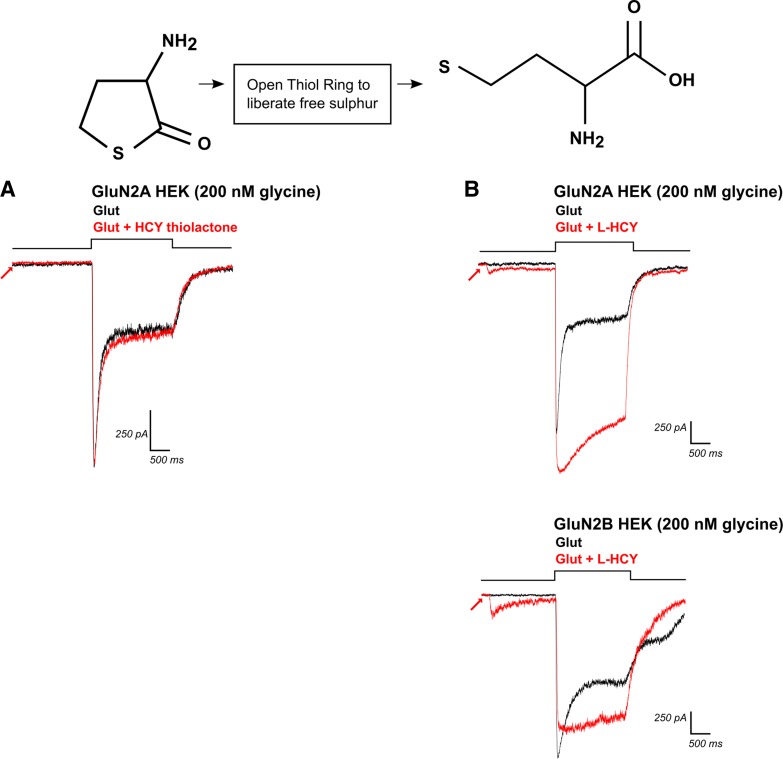
Fig. 7.
Opening the thiol ring of HCY-thiolactone creates l-HCY, which reproduces d,l-HCY effects. Top left: structure of HCY-thiolactone, with its sulfur group bound in a thiol ring. A: application of HCY-thiolactone (1 mM) did not significantly enhance charge transfer through NMDARs by glutamate (N = 5). B: opening the thiol ring of HCY-thiolactone creates l-HCY (see top right for structure). l-HCY showed effects that were qualitatively identical to d,l-HCY. l-HCY activated a small current on its own (see arrows), reduced desensitization, and enhanced peak amplitude of currents in GluN2A-transfected HEK cells (N = 3), while reducing peak amplitude and reducing desensitization of currents in GluN2B-transfected HEK cells (N = 3).
We prepared l-HCY, d,l-HCY, and all other drug solutions fresh on the day of recording. l-HCY effects were qualitatively identical to d,l-HCY (Fig. 7). Commercially available d,l-HCY consists of 50% l-HCY and 50% d-HCY (personal communication with Sigma representative), and HCY quickly degrades in solution (Hogg 1999), so it is likely that effective doses of HCY are at most one-half of what is reported here.
Neuron and HEK cell electrophysiology.
DIV7–17 cultured cortical neurons (unless otherwise noted) or transfected HEK cells (14–24 h after transfection) were transferred directly from culture media to extracellular solution before recording. Cells were patched and voltage clamped to −60 mV (unless otherwise indicated) using pulled glass pipettes (3–8 MΩ). Junction potentials were adjusted prior to break-in. Data were acquired using an Axopatch 1D patch clamp amplifier filtered at 5 kHz and sampled at 1 kHz using a Digidata 1320A and pClamp 8 software (Axon Instruments). Neuron experiments used an intracellular solution consisting of the following (in mM): 114.5 Cs-gluconate, 17.5 CsCl, 10 HEPES, 0.2 EGTA, 4 Mg-ATP, 0.4 Na-GTP, 7 phosphocreatine-Na (pH to 7.23 with CsOH). The intracellular solution used in HEK experiments contained the following (in mM): 140 CsCl, 5 BAPTA, 15 HEPES, and 4 Mg-ATP (pH to 7.3 with CsOH). To achieve fast solution exchange (10–90% rise time = ∼10 ms) for both neuron and HEK cell desensitization experiments, a gravity perfusion system equipped with computer-controlled Lee OEM solenoid valves (ALA Scientific Instruments VM-4, Farmingdale, NY) was used to apply drugs through an ALA MLF Millimanifold placed next to the patched cell. For low external calcium experiments on both neurons and HEK cells, cells were bathed and drugs were diluted in an extracellular solution (pH to 7.3 with NaOH, ∼300 mosmol) containing (in mM) 135 NaCl, 5.4 KCl, 0.2 CaCl2, 15 HEPES, 15 glucose. TTX (300 nM) was added only when neurons were used. For normal external calcium experiments in neurons, the extracellular solution used contained the following (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 10 HEPES, 10 glucose, and 300 nM TTX. Access resistance was continually monitored for all experiments throughout the recordings, and cells with access resistance over 50 MΩ were rejected for analysis. To test deactivation kinetics, GluN1 + GluN2A expressing HEK cells were patched and lifted to a 200-μm outer diameter theta perfusion tip for fast agonist application using a Burleigh PZ150M piezoelectric driver. Solutions were delivered through the theta tip using two Harvard Apparatus PHD2000 perfusion pumps.
For all culture experiments on desensitization, we accepted only cells that were tested for both conditions (drug on, drug off) and were flanked by equal amplitude recordings as a control. In other words, if an NMDA recording was followed by an NMDA + HCY recording, we required a second NMDA recording to follow with equal amplitude to the first NMDA trace. Because solution application was extremely fast, this ensured that any changes noted were due to drug application and not degradation of seal quality due to the pressure of the stream. This control also ensured that order of drug application (i.e., HCY first or control first) was not a contributing factor. For most cells, between 2 and 4 traces from each condition were averaged together to yield final traces for analysis.
Experiments on GluN2A, GluN2B, and GluN2D type NMDARs were always carried out on the same day with the same set of solutions. Recordings on cells expressing different subtypes were interleaved, so duration after transfection and time-dependent effects on drug potency were ruled out. In figures, horizontal lines above current traces mean “NMDA On” or “Glutamate On” (except Fig. 1D, where HCY is used as an agonist). Conditions are listed as agonist + HCY or HCY analog (red) or as agonist alone (black). The onset of HCY (or HCY analog) in each figure is noted with an arrow. For example, “NMDA + HCY” means that HCY was applied for 2 s before an NMDA + HCY stream was released at the step of the horizontal line above the current trace. In Fig. 9, we show the NMDA + low NMDA trace as blue to emphasize that we are not testing HCY nor an HCY analog.
Fig. 1.
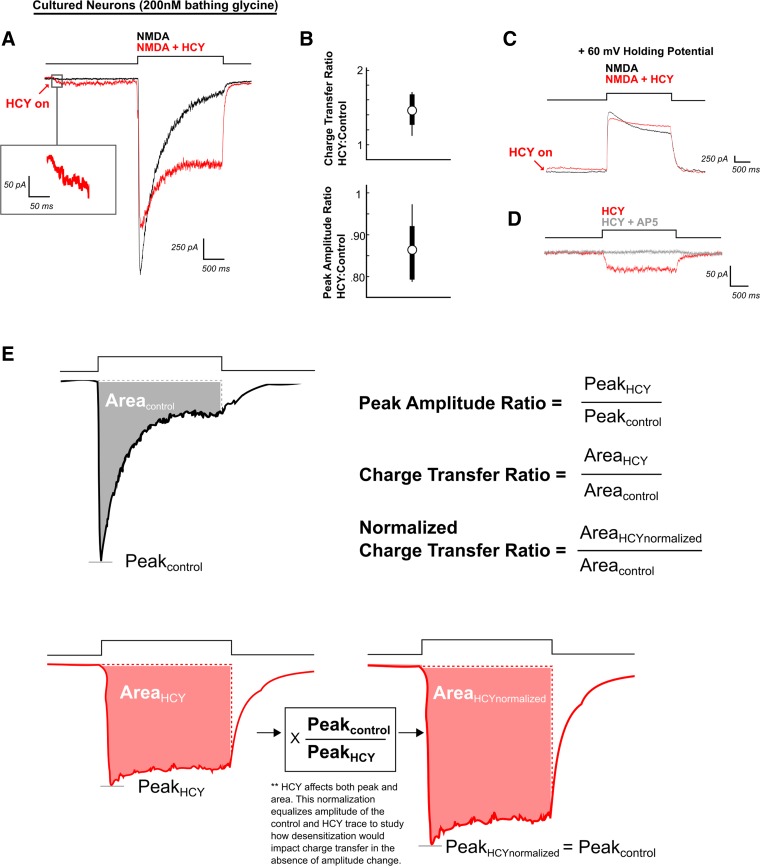
Homocysteine (HCY) induces a small depolarization in neurons and reduces desensitization of N-methyl-d-aspartate (NMDA) receptor (NMDAR) currents. A: neurons voltage clamped at −60 mV were maintained in a 2 mM Ca2+ solution containing 200 nM glycine (GLY). NMDA (100 μM) applied for 2 s produced a strongly desensitizing NMDAR response. If HCY (1 mM = 500 μM l-HCY isomer) was applied 2 s before NMDA (red arrow), it induced a low-amplitude current on its own. When NMDA + HCY was subsequently perfused onto the cell, desensitization was reduced, enhancing total charge transfer. The peak amplitude of the NMDAR response was also reduced. B: box plot showing the distribution of charge transfer ratios and peak amplitude ratios for all neurons tested (N = 6) in 200 nM GLY, 2 mM Ca2+ bathing solution. C: when held at +60 mV, neurons still showed HCY-dependent desensitization reduction and peak amplitude reduction (N = 4). D: the small initial HCY-induced current was blockable with the NMDAR glutamate site antagonist AP5 (50 μM) (N = 4). E: calculations of terms used throughout the text. Note: In this and subsequent figures, arrows indicate HCY onset, and red traces the presence of HCY (or HCY analog) before and during agonist application. “N” is the number of cells tested.
Fig. 9.
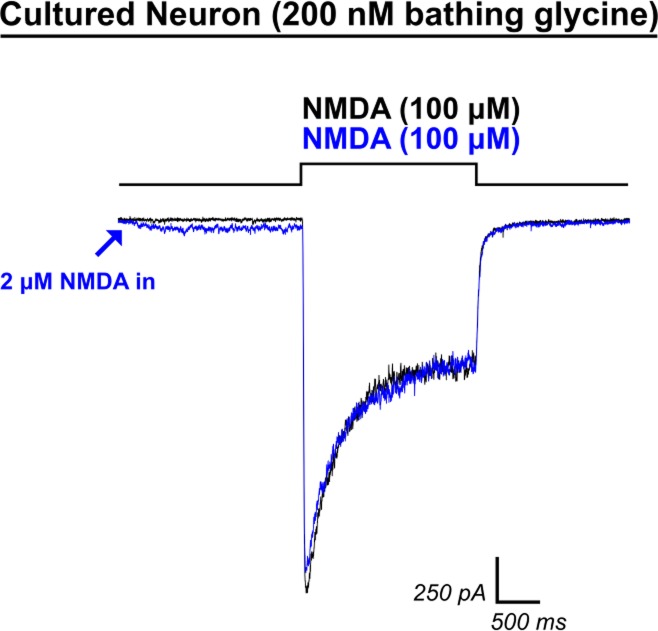
Low-dose NMDA application does not recapitulate HCY-induced reduction of desensitization. Because HCY was capable of inducing small AP5-blockable currents, we tested the hypothesis that doses of NMDA that induce current amplitudes similar to those produced by HCY could recapitulate HCY's effects on desensitization. Initial application of 2 μM NMDA to neurons (blue trace, 200 nM GLY) induced an NMDAR current similar in amplitude to HCY application (compare with Fig. 1). However, when 100 μM NMDA were then applied, desensitization was not reduced (N = 4), and charge transfer was never enhanced.
Acute hippocampal slice preparation.
Acute hippocampal slices were prepared from young (P10–P17) or older (P43–P58) mice. Mice used were from reporter lines driving the expression of tdTomato under the Drd1a or Drd2 promoter that are phenotypically wild type. These mice were used to ensure patching in a region receiving DA/NE input which could produce HCY.
Young mice were anesthetized with isofluorane and decapitated. The brain was quickly removed and placed into ice-cold carbogenated artificial CSF (ACSF) containing the following (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 24 NaHCO3, 5 HEPES, 12.5 glucose, 2 MgSO4, 2 CaCl2. Coronal or transverse hippocampal slices (200 μm) were cut in ice-cold ACSF using a Leica vibratome and placed in a chamber containing ACSF at 32°C. Fifteen minutes after slicing, the chamber was removed from the 32° incubator and allowed to recover at room temperature for 1 h. For sectioning of older slices, animals were perfused through the heart with a modified ACSF containing the following (in mM): 93 N-methyl-d-glucamine, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 Na-ascorbate, 2 thiourea, 3 Na-pyruvate, 10 MgSO4, 0.5 CaCl2 adjusted to pH 7.3 with concentrated HCl. The same procedure used for young slice preparation was repeated for older slices, except that the modified ACSF was used during cutting and 32° recovery. After 15 min of 32° incubation, slices were washed once in ACSF and then incubated in ACSF at room temperature for 1 h.
Slice electrophysiology and glutamate uncaging.
After recovery, slices were placed in a recording chamber and continuously perfused with warmed carbogenated ACSF (∼32°C, 2 ml/min). Glass pipettes (5–8 MΩ) were filled with an internal solution containing the following (in mM): 105 Cs-gluconate, 10 Na-phosphocreatine, 0.07 CaCl2, 4 EGTA, 10 HEPES, 4 Na-ATP, 1 Na-GTP, 3 MgCl2, brought to ∼290 mosmol with sucrose and pH 7.3 with CsOH. Neurons in the CA1 region of the hippocampus were patched and held at −70 mV in voltage clamp. The patched neuron was next continuously perfused with Mg-free ACSF for 10 min. After Mg2+ washout, MNI-caged glutamate (1 mM) was perfused onto the cell using an ALA millimanifold application pipette placed next to the cell and uncaged in the presence of 300 nM TTX, 10 μM NBQX, and 20 μM gabazine to isolate NMDAR currents. Uncaging was accomplished using a Zeiss HBO 100 Arclamp filtered with a DAPI filter and temporally controlled using a UniBlitz shutter system. Ultraviolet (UV) light (∼16 mW) was flashed over the entire field of a ×60 water immersion objective centered on the patched cell for 2 s to uncage glutamate both in the presence and absence of applied HCY.
Current analysis and statistics.
Using custom MATLAB software written by ADB, axon.abf files were imported and analyzed. Peak amplitude of each NMDAR current was recorded, and the area under each trace was measured by simple integration after zeroing the baseline. Areas under the NMDAR current curves during agonist application are equal to total charge transfer during agonist application in micro-Coulombs (μC).
We use “drug” in this text to mean HCY or HCY analog. “Agonist” means glutamate or NMDA. Peak amplitude and charge transfer were tested for significant differences using paired two-tailed t-tests because both agonist and agonist + drug treatments were always obtained from the same cell. Significance in these tests is indicated using a P value, which we report for both significant and insignificant results. P values ≤ 0.05 were accepted as significant differences. One-tailed t-tests were used for studies on NMDAR antagonists AP5 and DCKA (5,7-DCKA) because the actions of these drugs are known. One-tailed tests were also used in other instances when appropriate and are noted in the text. “N” in text and figure legends indicates number of cells recorded for the specified experiment, where each cell was exposed to multiple agonist and agonist + drug conditions.
The following statistics are illustrated in Fig. 1E. Charge transfer ratios (CTR) and percentages reported reflect (charge transfer induced by agonist + drug)/(charge transfer induced by the same agonist with no drug). Peak amplitude ratios (PAR) and percentages reported reflect (peak amplitude of current induced by agonist + drug)/(peak amplitude with agonist alone). For example, if on a given cell the charge transfer after glutamate application was 2 μC with a peak amplitude of 500 pA, and the charge transfer induced by glutamate with HCY preincubation was 4 μC with a peak of 400 pA, the CTR = 4 μC/2 μC = 2 (a 100% increase) and the PAR = 400 pA/500 pA = 0.8 (a 20% decrease). However, HCY effects were complex. Increasing [HCY] altered the peak amplitude of the NMDAR response while simultaneously decreasing desensitization. Therefore, we also report a normalized CTR that compares charge transfer after peak amplitude of agonist, and agonist + drug conditions have been scaled to the same size (see Fig. 1E); this measures effects on desensitization only. Distributions of these statistics from each cell tested were compiled. CTR, PAR, and normalized CTRs are not normally distributed, so averages reported for these statistics are median values. These ratios were compared across experiments (i.e., when comparing GluN2A vs. GluN2B CTR) using Wilcoxon rank sum tests (a.k.a., Mann-Whitney-Wilcoxon tests), and P values are reported. Plots display the median as a circle and the range between the 25th and 75th quartiles as a line behind the circle. If no line is present, the interquartile range is tightly contained within the bounds of the circle, indicating the median. In addition, Figs. 1, 8, and 12 show upper and lower thin lines representing the maximum and minimum values found. The thicker bars in these graphs represent the 25th to 75th quartile range.
Fig. 8.
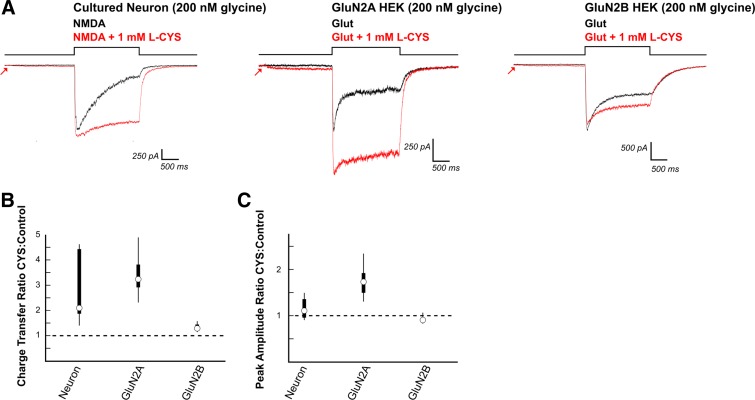
Cysteine (CYS) enhances NMDAR charge transfer through neurons and GluN2A and GluN2B expressing HEK cells. A: 2-s preincubation of neurons with l-CYS (1 mM) mimicked HCY in reducing NMDAR desensitization, which significantly enhanced charge transfer (N = 5, DIV9, 2 mM Ca2+, 200 nM [GLY] in bath). However, there was no significant effect of l-CYS on peak amplitude in these neurons. l-CYS (1 mM) enhanced charge transfer in HEK cells transfected with GluN2A (N = 7) and GluN2B (N = 8) containing NMDARs. Peak amplitudes of GluN2A responses were significantly enhanced while peak amplitudes of GluN2B NMDAR currents were only slightly reduced. B and C: box plots show that the neuron response is a hybrid between GluN2A and GluN2B expressing HEK cells.
Fig. 12.
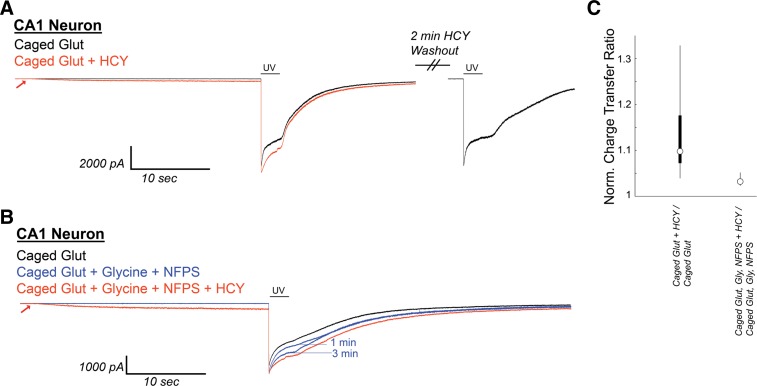
HCY induces a small initial depolarization and reduces GLY-dependent NMDAR desensitization in neurons from acute CA1 slices during uncaging of 1 mM MNI-caged glutamate. A: CA1 neurons in acute hippocampal slices [N = 6 from postnatal day 10 (P10) to P17 mice, N = 6 from P43–P58 mice] were voltage clamped in the presence of 300 nM tetrodotoxin, 10 μM NBQX, and 20 μM gabazine. Under these conditions, Schaffer collateral axon stimulation (200 μs) consistently evoked currents at +40 mV, but not at −70 mV holding, indicating the isolation of NMDAR currents. After Mg-washout, MNI-caged glutamate (1 mM) was perfused onto the neuron, and a 2-s flash of UV light was used to uncage glutamate. This induced an NMDAR current with pronounced desensitization. If HCY (1 mM d,l-HCY = 500 μM l-HCY) was washed onto the slice 30s before UV uncaging, HCY induced a small NMDAR current on its own and reduced desensitization of the NMDAR response to glutamate. When HCY was washed out (washout peak normalized to initial control), sharp desensitization returned. B: when GLY (10 μM) and the GLY transporter antagonist NFPS (100 nM) were washed onto CA1, desensitization was progressively reduced in 3 of 4 cells tested. HCY had a strongly reduced effect in the presence of GLY and NFPS. (Note 1 μM strychnine is present to avoid GLY receptor activation by exogenously applied GLY.) C: box plots showing distributions of normalized charge transfer ratios in the presence and absence of GLY and NFPS.
RESULTS
HCY reduces NMDAR desensitization and peak amplitude in cultured neurons.
We first determined the effects of HCY on NMDAR current dynamics at high temporal resolution using whole-cell voltage clamp and fast agonist application with the ALA-VM4 perfusion system. Two-second application of NMDA (100 μM) to cultured cortical neurons in 200 nM GLY induced macroscopic NMDAR currents that strongly desensitized (Fig. 1A). If HCY (1 mM) was applied to the same neuron 2 s prior to NMDA, desensitization was reduced, significantly enhancing total charge transfer by an average of 46% (N = 6, P ≤ 0.01). HCY application also attenuated peak NMDAR current amplitude by 14% (N = 6, P ≤ 0.01) (Fig. 1, A and B).
Importantly, HCY also induced a previously observed small current on its own (see arrow and box, Fig. 1A, Lipton et al. 1997). Like NMDA currents, this HCY-induced current reversed at positive holding potentials (Fig. 1C, arrow) and was blocked with AP5 (50 μM) a competitive antagonist at the NMDAR glutamate site (average 133 pA HCY, 3 pA HCY + AP5, P ≤ 0.05, N = 4, Fig. 1D). This indicates that HCY is a weak agonist at the NMDAR glutamate site.
HCY effects are dose dependent and are occluded by saturating GLY.
As seen in Fig. 1A, residual NMDAR desensitization remained even in the presence of HCY. There are at least three forms of NMDAR desensitization, one of which is caused by calcium flux into the cell (Ehlers et al. 1996; Krupp et al. 1996; Legendre et al. 1993; Tong et al. 1995; Zilberter et al. 1991). HCY reduced desensitization when neurons were held at +60 mV (Fig. 1C) and in minimal calcium (0.2 mM, Fig. 2) or zero extracellular calcium solutions. This suggests that HCY does not affect calcium-dependent desensitization resulting from calcium flux into the neuron.
Fig. 2.
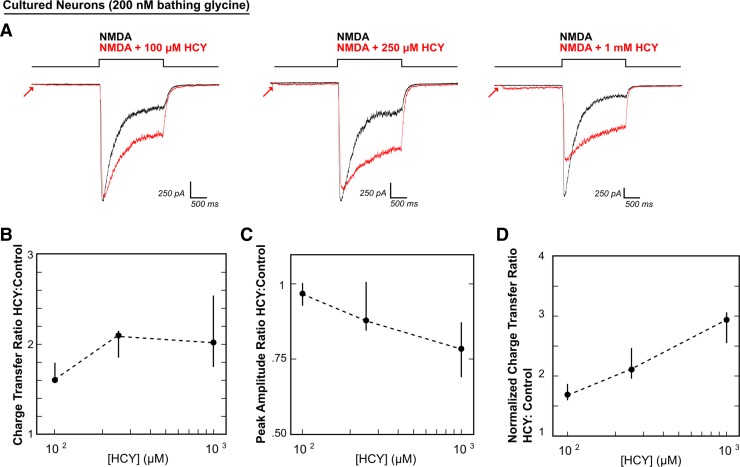
HCY effects on NMDAR current amplitude and desensitization are dose dependent. A: voltage-clamped neurons were maintained in 200 nM GLY with low extracellular calcium (0.2 mM) to prevent masking of HCY effects by calcium-dependent NMDAR desensitization. NMDA (100 μM) was applied for 2 s either with HCY or without HCY. Increasing HCY concentrations ([HCY]) decreased peak NMDAR current amplitude (A and C) and reduced desensitization (A and D) dose dependently (i.e., HCY increased normalized charge transfer: see materials and methods and Fig. 1E). Notice that HCY still strongly reduced desensitization at 1/10th of its maximal concentration (100 μM = 50 μM l-HCY isomer). There was no significant effect on amplitude at 100 μM, but a significant amplitude reduction at 1 mM HCY. This resulted in a nonmonotonic increase in charge transfer ratio (B) because, as desensitization reduction enhanced charge transfer, peak amplitude reduction decreased charge transfer (for [HCY] = 100 μM, 250 μM, 1 mM, N = 7, 5, 10).
Calcium inactivation of NMDARs can mask desensitization effects when recording whole-cell currents (Mayer et al. 1989). We therefore performed an [HCY] curve in minimal calcium (0.2 mM Ca2+) to study HCY desensitization effects in detail. NMDAR desensitization during 2 s of NMDA application in 200 nM GLY was reduced by 100 μM (N = 7), 250 μM (N = 5), and 1 mM HCY (N = 10) (Fig. 2A), resulting in significant charge transfer enhancement at all concentrations tested (P ≤ 0.001 for all [HCY], Fig. 2B). However, charge transfer enhancement through NMDARs was nonlinear with [HCY] because as HCY enhances charge transfer due to dose-dependent reduction of NMDAR desensitization, it simultaneously reduces charge transfer due to dose-dependent reduction of peak amplitude. Peak amplitude (Fig. 2C) was not significantly reduced at low 100 μM HCY (P = 0.11), but showed a strong 22% reduction at 1 mM HCY (P ≤ 0.001).
Normalized CTRs isolate desensitization effects from changes in amplitude by scaling peak amplitudes of agonist and agonist + drug conditions to the same size (Fig. 1E). This statistic therefore captures changes in charge transfer due only to altered desensitization. Increasing concentrations of HCY dose-dependently enhanced normalized charge transfer (i.e., decreased desensitization) by 69% (100 μM), 111% (250 μM), and 193% (1 mM) (P ≤ 0.005 for all [HCY], Fig. 2D).
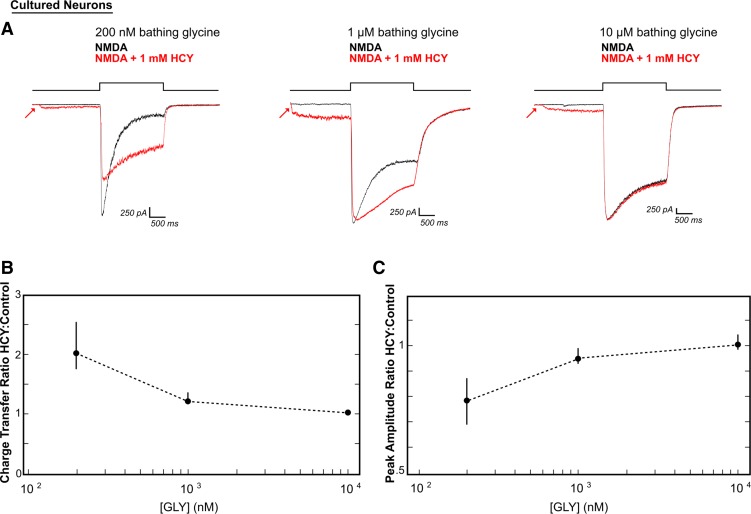
To determine if these dose-dependent HCY effects reflected a reduction in GLY-dependent desensitization, we increased extracellular GLY in the bathing solution from 200 nM to 1 μM or 10 μM. At 1 μM [GLY], 1 mM HCY showed a milder but significant enhancement of charge transfer (average 21% increase, N = 5, P ≤ 0.05, Fig. 3, A and B). Increasing [GLY] to saturating levels (10 μM, Priestley et al. 1995) eliminated the HCY effect on charge transfer (2% average enhancement, N = 4, P = 0.16, Fig. 3, A and B). These data indicate that HCY specifically reduces GLY-dependent NMDAR desensitization (Fig. 3). Furthermore, at both moderate (1 μM) and saturating (10 μM) GLY levels, the HCY reduction of peak NMDAR current amplitude also disappeared (Fig. 3C; P = 0.13 for 1 μM, P = 0.55 for 10 μM), suggesting that both of HCY's effects on NMDAR currents are fully occluded by saturating GLY. This is consistent with the result from Lipton et al. (1997), where HCY decreased calcium flux induced by brief NMDA application in low GLY, but not in saturating GLY. In addition, even when the GLY site is fully occupied, HCY continues to activate NMDARs on its own (Fig. 3A, arrows), indicating that the GLY-dependent effects of HCY are separate from a weak agonist role of HCY at the glutamate site.
Fig. 3.
Increasing bathing GLY concentrations ([GLY]) occlude HCY's desensitization effects. A: neurons were stimulated with 100 μM NMDA with or without HCY (1 mM = 500 μM l-HCY isomer). As extracellular GLY was raised, desensitization in the black control traces was reduced. HCY caused a modest enhancement of charge transfer at moderate (1 μM) GLY levels and a nonsignificant reduction of peak amplitude. HCY no longer affected the amplitude of NMDAR responses (0% reduction) and did not provide additional enhancement of charge transfer when GLY was at a saturating 10 μM (N = 5 for 1 μM GLY, N = 4 for 10 μM GLY). B and C: charge transfer ratios and peak amplitude ratios, respectively, plotted against extracellular [GLY] show that HCY lost its ability to modulate NMDA currents as GLY was raised (i.e., peak amplitude ratios and charge transfer ratios approach 1 at high [GLY]). These data suggest a common mode of action for GLY and HCY (for [GLY] = 200 nM, 1 μM, 10 μM, N = 10, 5, 4).
HCY reduces desensitization of all NMDAR subtypes, with effects on peak amplitude that are GluN2 subunit dependent.
Most NMDARs in the brain are a tetramer of two GluN1 subunits and two GluN2 subunits (GluN2A-2D). GluN1 subunits bind GLY, and GluN2 subunits bind glutamate or NMDA. However, each GluN2 subunit confers different desensitization kinetics (Vicini et al. 1998) and a different GLY affinity to the receptor (Ikeda et al. 1992; Priestley et al. 1995). NMDARs with two GluN2A subunits have the lowest reported GLY affinity (saturated at 10 μM [GLY]), NMDARs with two GluN2B subunits show an intermediate affinity (saturated at 3 μM [GLY]), and NMDARs with two GluN2D subunits have the highest GLY affinity (saturated at 1 μM [GLY]) (Ikeda et al. 1992; Priestley et al. 1995). Because cultured neuron results indicated that HCY effects were occluded by saturating extracellular GLY, we hypothesized that HCY could differentially impact the various NMDAR subtypes. We therefore transfected HEK cells with constructs expressing GluN1 and one of the three GluN2 subtypes found in cortical neurons (GluN2A, GluN2B, GluN2D; Baron et al. 2010).
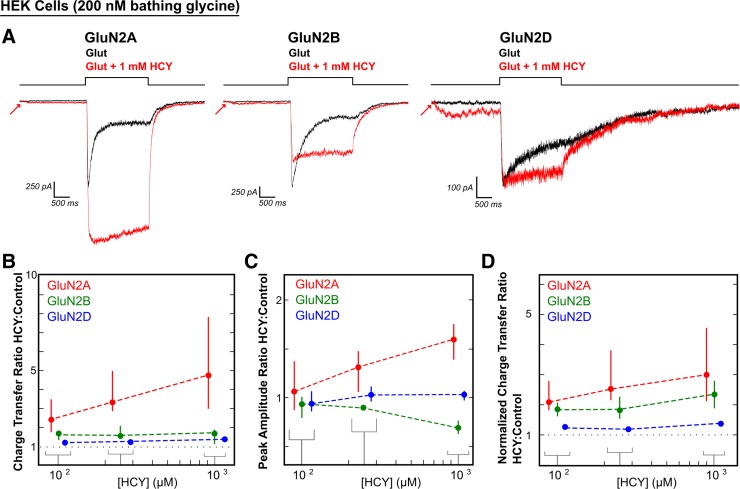
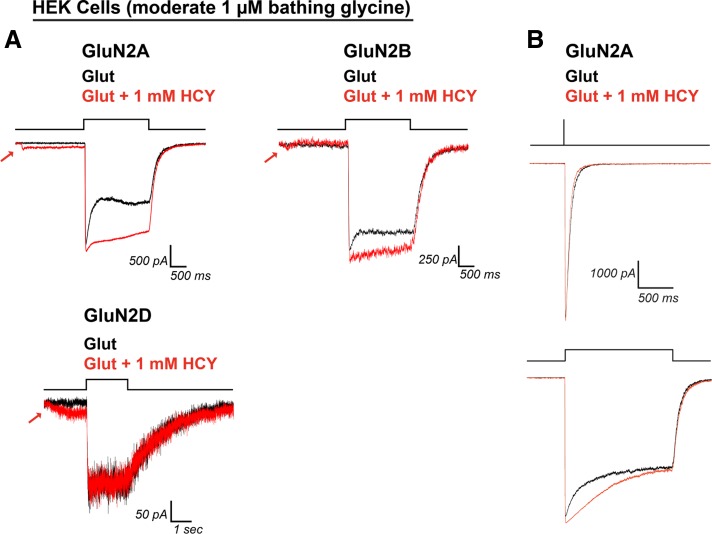
In 200 nM bathing GLY, 1 mM HCY significantly enhanced charge transfer by glutamate (100 μM) for each NMDAR subtype tested (375% enhancement for GluN2A, N = 17, P ≤ 0.00001; 68% enhancement for GluN2B, N = 17, P ≤ 0.001; 38% enhancement for GluN2D, N = 8, P ≤ 0.01; Fig. 4, A and B). CTR were significantly more enhanced through GluN2A type receptors than GluN2B and GluN2D (P ≤ 0.0001 for GluN2A vs. GluN2B and GluN2A vs. GluN2D, Wilcoxon rank sum test on CTR). This was due to the large increase in peak amplitude caused by HCY on GluN2A containing receptors (Fig. 4, A and C). The average GluN2A peak amplitude increase was 59% (N = 17, P ≤ 0.0001), while GluN2B showed the same peak amplitude decrease by HCY that we observed in neurons (31% reduction in peak amplitude, N = 17, P ≤ 0.000001). GluN2D amplitude remained unchanged (PAR = 1.03, N = 8, P = 0.31). We also tested lower [HCY] (100 μM and 250 μM). Both the charge transfer enhancement and peak amplitude changes were dose dependent, with GluN2A peak amplitude increasing as [HCY] was raised and GluN2B peak amplitude decreasing as [HCY] was raised (Fig. 4, B and C). Critically, charge transfer and normalized charge transfer were enhanced for every cell tested for all three GluN2 subtypes at 100 μM (P ≤ 0.001, N = 15) and 250 μM HCY (P ≤ 0.001, N = 13) (Fig. 4, B and D), indicating that relatively low doses of HCY could reduce the desensitization of diheteromeric GluN2A, GluN2B, and GluN2D NMDARs in the brain.
Fig. 4.
HCY effect on NMDAR currents depends on GluN2 subunit composition. A and D: HCY reduced desensitization of all three NMDAR subtypes tested in human embryonic kidney (HEK) cells in a dose-dependent manner. B: GluN2A showed the greatest enhancement in charge transfer ratio with HCY present, followed by GluN2B and GluN2D. A and C: the amplitude of GluN2A responses to glutamate was enhanced by the presence of HCY, again in a dose-dependent manner. A and C: GluN2B, similar to NMDARs in young cultured neurons, showed dose-dependent amplitude reduction by HCY. GluN2D showed no change in peak amplitude at any [HCY]. N for each [HCY] was as follows: for 100 μM = 50 μM l-HCY isomer, 250 μM = 125 μM l-HCY isomer, and 1 mM = 500 μM l-HCY isomer, GluN2A N = 4, 5, 17; GluN2B N = 5, 5, 17; and GluN2D N = 6, 3, 8.
When bathing [GLY] were raised from 200 nM to 1 μM (Fig. 5), HEK cell recordings resembled neurons in that HCY lost its ability to reduce GluN2B peak amplitude (1 mM HCY; average peak amplitude ratio = 1.02, P = 0.42, N = 5 for GluN2B) and retained statistically insignificant amplitude effects on GluN2A (average peak amplitude ratio = 1.28, P = 0.11, N = 4). Moreover, although all GluN2A and GluN2B expressing HEK cells tested showed charge transfer enhancement by HCY at this higher [GLY] (GluN2A average 142% charge transfer ratio, N = 4, P ≤ 0.05; GluN2B average 21% CTR, N = 5, P ≤ 0.05; Fig. 5), GluN2D was fully saturated at 1 μM [GLY] and did not desensitize in control conditions. Therefore, reduction of desensitization by HCY was fully occluded by 1 μM GLY for only GluN2D (average CTR = 1.00, N = 5, P = 0.51), although as expected the small HCY-dependent depolarization via the glutamate site remained. The HEK cell system also allowed us to lift patched cells to a theta perfusion pipette to study HCY's effect on deactivation kinetics independently of desensitized receptor states. Synaptic-like 1-ms pulses of 1 mM glutamate in 1 μM bathing GLY were delivered to lifted GluN1 + GluN2A HEK cells using a piezo-electric driver to briefly bump the glutamate stream onto the cell. Using this system, desensitization and deactivation of NMDAR currents could be studied on the same cell. HCY (1 mM) did not significantly alter weighted decay time NMDAR currents after a 1-ms pulse of glutamate (average 76 ms control, average 59 ms HCY, P = 0.19, Fig. 5B, top). However, on these same cells, bathing 1 mM HCY significantly reduced desensitization during 1.5-s exposure to the glutamate stream (P ≤ 0.05, N = 4, Fig. 5B, bottom). This indicates that HCY reduces desensitization by affecting transitions of the NMDAR to or from desensitized states without prolonging deactivation of the channel.
Fig. 5.
HCY reduces desensitization of GluN2A and GluN2B, but not GluN2D NMDAR currents, at 1 μM GLY, without affecting NMDAR decay kinetics. A: raising the ambient [GLY] from 200 nM to 1 μM prevented HCY from modulating peak amplitude of GluN2A- and GluN2B-mediated currents in HEK cells (N = 4, 5). However, HCY continued to enhance charge transfer through GluN2A- and GluN2B-containing receptors in 1 μM GLY. GluN2D (N = 5) is completely saturated at 1 μM GLY. B: although HCY was effective in reducing desensitization in GluN2A HEK cells, it did not alter deactivation kinetics after 1-ms pulses of glutamate, indicating an effect on desensitized states only.
HCY effects in DIV30 cultured neurons.
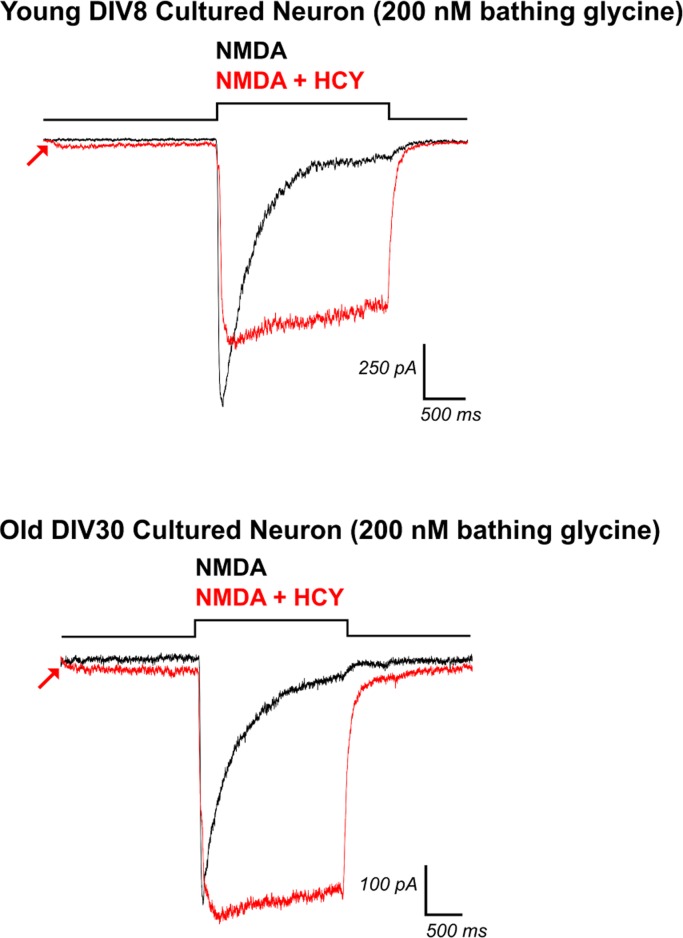
That the charge transfer enhancement and peak amplitude suppression by HCY were similar in GluN2B type NMDARs and young cultured neurons was not unexpected. Cortical neuron cultures are highly enriched for GluN2B at DIV7 (∼20:1 GluN2B:GluN2A), while GluN2A begins to express strongly at DIV21 (∼3:1 GluN2B:GluN2A, Zhong et al. 1994). GluN2A is expressed at an ∼10:1 ratio with respect to GluN2D in young cultured neurons, indicating that GluN2A and GluN2B are the main subunits at this stage and should dictate whole cell NMDAR kinetics (GluN2C is not expressed at any age tested in cortical neurons; Baron et al. 2010). We therefore predicted that, as GluN2A expression increases and balances GluN2B with age, the effects of HCY on neuron NMDAR currents should look less like GluN2B-expressing HEK cells and begin to resemble a hybrid between GluN2A and GluN2B responses. Specifically, because GluN2B peak amplitude is decreased by HCY while GluN2A is enhanced by HCY in HEK cells (Fig. 4C), a whole cell response in older neurons where GluN2B to GluN2A ratios are ∼1:1 should show little or no amplitude change. Our recordings in old (DIV30) cortical neuron cultures supported this prediction. HCY (1 mM) did not significantly reduce peak amplitude in DIV30 cultures (average 2% peak enhancement, N = 4, P = 0.96, Fig. 6). Meanwhile, every cell previously tested in our DIV7–8 culture experiments showed a peak amplitude reduction by 1 mM HCY (N = 10, average reduction of 22%, P ≤ 0.001, Fig. 2, A and C). Consequently, the average CTR per cell was 3.33 in old cultures (N = 4, P ≤ 0.05) vs. 2.02 in young cultures, consistent with the addition of the GluN2A subunit, which showed strongly enhanced charge transfer in response to HCY in HEK cells (Fig. 4, A and B).
Fig. 6.
HCY does not reduce peak amplitude in old neuronal cultures. Neurons recorded at DIV7 and DIV8 are highly enriched for GluN2B and showed strong peak amplitude reduction by HCY (upper trace, DIV8 1 mM HCY = 500 μM l-HCY, N = 10). This mirrored the HCY effect on GluN2B expressing HEK cells. However, HCY produced no significant change in peak amplitude in older cultured neurons (lower trace, DIV30, 1 mM HCY = 500 μM l-HCY, N = 4), reflecting the addition of GluN2A subunits as the cells mature.
A free sulfur group is critical for HCY's effects on NMDARs.
We next tested whether other HCY-resembling chemicals could mimic HCY effects on NMDAR current amplitude and desensitization. We used both 2- and 8-s preincubation of these chemicals in case their binding dynamics differed from HCY. All experiments were performed in 200 nM [GLY]. Homocystine, a dimer of HCY linked by disulfide bonds, did not enhance charge transfer by glutamate through NMDARs in any transfected HEK cells tested [N = 4 (2 GluN2A, 2 GluN2B), P = 0.97, one-tailed paired t-test for enhancement, data not shown]. HCY thiolactone, a cyclized form of HCY with its sulfur bound in a thiol ring, also showed insignificant charge transfer enhancement [N = 5 (3 GluN2A, 2 GluN2B), P = 0.07, Fig. 7A] without affecting current amplitude (average peak amplitude ratio = 0.99 for GluN2A, 0.93 for GluN2B). We subsequently used the procedure of Poddar et al. (2009; communicated by Dr. Donald Jacobsen, Lerner Research Institute, Cleveland, OH) to open the thiol ring of l-HCY thiolactone. This method liberates the free sulfur-group containing molecule l-HCY (final concentration dependent on efficacy of ring removal reaction, maximum possible = 2 mM), the isomer of HCY that is present in the brain. Both the amplitude and desensitization effects we observed with d,l-HCY (Fig. 4) were qualitatively identical to effects found with the l-HCY isomer (Fig. 7B). GluN2A containing receptors showed a significant increase in charge transfer (average 178% increase, N = 3, P ≤ 0.05) and peak amplitude (24% increase, N = 3, P ≤ 0.05) when l-HCY was present. l-HCY significantly reduced peak amplitude of GluN2B receptors (average 23% reduction, N = 3, P ≤ 0.05), mirroring the amplitude reduction in neurons and GluN2B expressing HEK cells by d,l-HCY, while reducing desensitization in all cells tested (average normalized charge transfer enhancement of 40%).
As mentioned earlier, the in vivo isomer l-HCY is present at a 1:1 ratio with the d-HCY isomer, which is not present in the brain, in commercially available d,l-HCY (see materials and methods). Considering that l-HCY replicated d,l-HCY's effects, it is likely that l-HCY is the only active isomer in our studies using the racemic d,l-HCY. Therefore, effective doses of HCY are likely one-half of what is reported in this paper (i.e., 100 μM d,l-HCY = 50 μM l-HCY). d-HCY thiolactone was not available for purchase.
l-Cysteine (1 mM) was the last HCY analog tested. The name “homocysteine” is derived from “homolog of cysteine”. Unlike homocystine dimers, which are disulfide bonded, and HCY thiolactone, which has its sulfur bound in a thiol ring, cysteine possesses a free sulfur group. l-Cysteine mimicked the desensitization related effects of HCY. In cultured neurons, it significantly enhanced NMDAR charge transfer (N = 5, P ≤ 0.01, average 109% increase, Fig. 8, A and B). However, l-cysteine did not cause significant changes in peak amplitude (average peak amplitude ratio = 1.11, N = 5, P = 0.30) at the young age where GluN2B:GluN2A ratios are ∼8:1 (DIV9). These results paralleled l-cysteine's effects on GluN2A and GluN2B expressing HEK cells (Fig. 8). l-Cysteine applied with glutamate to GluN2A-expressing HEK cells greatly enhanced peak amplitude by an average of 73% (P ≤ 0.001, N = 7) and strongly enhanced charge transfer by an average of 223% (N = 7, P ≤ 0.005). l-Cysteine also increased charge transfer through GluN2B type receptors (N = 8, P ≤ 0.005, 29% increase). As with HCY, l-cysteine's charge transfer enhancing effects on GluN2B expressing HEK cells were significantly less than on GluN2A expressing HEK cells (P ≤ 0.0005, Wilcoxon rank sum test on GluN2B vs. GluN2A CTR). Peak amplitude reduction of GluN2B NMDAR currents with l-cysteine was more subtle than HCY's effects. Although l-cysteine did consistently reduce peak amplitude of GluN2B currents (average 9% reduction, N = 8, P ≤ 0.05), the average reduction of only 9% was significantly less than the peak reduction of 31% produced by HCY (P ≤ 0.0005, Wilcoxon rank sum test, average 31 vs. 9% reduction). This likely explains why DIV9 neurons, which have high GluN2B:GluN2A ratios, did not show significant amplitude reduction in response to l-cysteine. The reduction of NMDAR desensitization by cysteine that spares GluN2B NMDARs from strong peak depression may explain why all concentrations of cysteine, unlike HCY, enhance LTP in hippocampal slices (Christie et al. 2009).
Finally, because HCY is a weak NMDAR glutamate-site agonist (Fig. 1D and see Lipton et al. 1997), we tested the hypothesis that low-dose NMDA itself might recapitulate the effects of HCY on charge transfer and peak amplitude. We found that 2 μM NMDA induced small currents that were similar in amplitude to currents evoked by HCY alone (Fig. 9). However, 2 μM NMDA application 2 s before high-dose (100 μM) NMDA application never enhanced charge transfer (average CTR = 0.98, N = 4, P = 0.99 for enhancement by 2 μM NMDA). This experiment provided an additional indication that the glutamate-site related and GLY-site related effects of HCY are independent of each other.
Is HCY acting at the NMDAR GLY site?
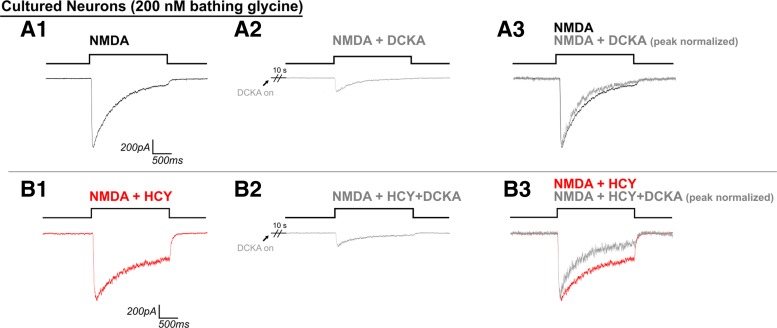
NMDA (2 μM) was not effective at reducing desensitization (Fig. 9); instead, desensitization reduction by HCY resembled and was occluded by saturating GLY (Fig. 3). This lead us to ask if the NMDAR GLY site antagonist DCKA could prevent HCY from reducing NMDAR desensitization. In control experiments in nonsaturating (200 nM) bathing GLY with no HCY, NMDA application was preceded by 10 s of 1 μM DCKA. As expected, DCKA reduced NMDAR current amplitude by an average of 82% (Fig. 10A2 vs. A1, N = 5 DIV7 neurons, P ≤ 0.001) and promoted further GLY-dependent desensitization of the NMDAR response (16% less normalized charge transfer with DCKA present, P ≤ 0.05, Fig. 10A3). We repeated this experiment with 1 mM HCY present in the bath, preincubation (1 μM DCKA + 1 mM HCY), and stimulating solutions (1 μM DCKA + 1 mM HCY + 100 μM NMDA). We hypothesized that, if HCY were blocking desensitization independently of the GLY site, then DCKA would have no effect on desensitization in the presence of HCY. However, if HCY was acting at the GLY site, we expected DCKA to have a stronger desensitization enhancing effect in the presence of HCY, as DCKA would be blocking both the effects of HCY and GLY on desensitization vs. that of GLY alone. The latter was the case, as can be seen by comparing the normalized traces in Fig. 10A3 to B3. DCKA promoted even further desensitization of the NMDAR response to NMDA application during HCY exposure (46% reduction of normalized charge transfer, N = 4, P ≤ 0.05; Wilcoxon rank sum HCY + DCKA vs. DCKA only, P ≤ 0.01, 46 vs. 16%). Thus antagonism of the GLY site appears to prevent HCY's desensitization effects. Peak amplitude was also strongly reduced by DCKA in the presence of HCY at an average of 79% per cell. This was not significantly different than trials without HCY [P = 0.90, Wilcoxon rank sum test on peak amplitude ratios with (0.21) and without (0.18) HCY].
Fig. 10.
HCY does not rescue effects of partially blocking the GLY site with dichlorokynurenic acid (DCKA). DCKA (1 μM, 10-s preincubation) inhibited peak NMDAR current amplitude by ∼80% in the presence (B2 vs. B1) and absence (A2 vs. A1) of bathing 1 mM HCY. DCKA largely prevented the ability of HCY to reduce GLY-dependent desensitization. It is as if HCY was not even present when DCKA was preincubated, because HCY + NMDA + DCKA currents desensitize similarly to NMDA + DCKA (see normalized superimposed traces A3 and B3), indicating that DCKA blocks the effects of both HCY and GLY on desensitization (N = 5 for DCKA alone, N = 4 for DCKA + HCY).
Are NMDARs in the brain susceptible to HCY?
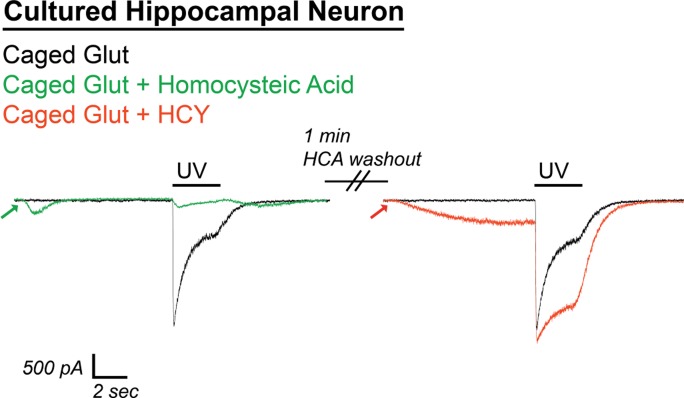
Because HCY's desensitization effects are occluded at high [GLY] and are blocked by the GLY site antagonist DCKA, NMDAR susceptibility to HCY in real neural circuits relies on whether the NMDAR GLY site is saturated at synapses in the brain. Data-based modeling experiments have presumed that the synaptic [GLY] in GlyT expressing brain areas is below 150 nM (Attwell et al. 1993; Roux and Supplisson 2000), which, by design, resembles the concentration used in our culture experiments. d-Serine, a second in vivo coagonist of the NMDAR GLY site, is strongly expressed in the forebrain (Schell et al. 1997; Wolosker 2006 for review) and may be the primary NMDAR coagonist at some synapses (Papouin et al. 2012). Nonetheless, adding extracellular GLY or d-serine to forebrain slice preparations, or blocking GlyT to allow accumulation of synaptic GLY, enhances evoked NMDAR currents in brain slices (Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2003), indicating that the combination of d-serine and GLY does not fully activate the NMDAR coagonist site. In fact, in the CA1 region of the hippocampus, GlyT block plus GLY supplementation (10 μM) enhances postsynaptic currents by up to 100% (Bergeron et al. 1998). GLY-dependent NMDAR desensitization has not been shown in a slice preparation, but the subsaturation of the NMDAR GLY site should allow this phenomenon to occur. We therefore sought to induce NMDAR desensitization with caged glutamate in CA1 to see if it could be reduced by HCY. As proof of principle, uncaging of 1 mM MNI-caged glutamate with a 2-s UV light flash was extremely effective at inducing GLY-dependent desensitization in cultured hippocampal neurons. This desensitization was strongly reduced by HCY (Fig. 11). Because GluN2A subunits express significantly earlier in the hippocampus than in other brain regions (Monyer et al. 1994), we did not expect, and did not observe, amplitude effects in hippocampal neurons. Homocysteic acid (10 μM), an NMDAR-active derivative of HCY that is produced by an unknown metabolic pathway in the brain (Benz et al. 2004, Kruger 2001), strongly damped responses to glutamate uncaging and did not resemble HCY in its actions (Fig. 11); therefore the results below are unlikely to be due to conversion of HCY to homocysteic acid in the intact circuit of the hippocampal slice.
Fig. 11.
Caged glutamate induces HCY-reducible desensitization in hippocampal cultures. UV uncaging of 1 mM 4-methoxy-7-nitroindolinyl (MNI)-caged glutamate strongly desensitized NMDAR currents in DIV11 cultured hippocampal neurons (200 nM bathing GLY). Homocysteic acid (HCA; 10 μM), an active agent at the NMDAR glutamate site, induced NMDAR currents of similar amplitude to 1 mM HCY. However, instead of enhancing charge transfer, HCA greatly damped the response to glutamate uncaging (91% reduction of charge transfer, P ≤ 0.05, N = 3). Washout of HCA lead to restoration of a desensitizing response to glutamate uncaging. Desensitization was, in turn, strongly reduced by 1 mM HCY on the same cell (49% enhancement of normal charge transfer, P ≤ 0.05). Therefore, the effects of HCY and HCA are separate, and it is unlikely that metabolic conversion of HCY to HCA that could occur in hippocampal slices is a source of HCY's charge transfer enhancement.
We next applied 1 mM MNI-caged glutamate (+300 nM TTX, 10 μM NBQX, 20 μM gabazine to isolate NMDAR currents) to voltage-clamped CA1 neurons in acute hippocampal slices (−70 mV, 2 mM Ca2+, Mg-free ACSF) and uncaged using 2-s UV flashes. Most cells tested (6/6 cells from P10–P17 mice, 6/8 from old P43–P58 mice) showed immediate NMDAR desensitization upon glutamate uncaging (Fig. 12). To each desensitizing neuron, we subsequently applied caged glutamate + HCY (1 mM d,l-HCY) for 30 s, then uncaged again for 2 s. As can be seen in Fig. 12A, the initial application of HCY induced a small NMDAR current, as seen in our culture studies, that did not appear if the cell was held at −70 mV in Mg-containing ACSF. Also, as in our culture experiments, HCY reduced glutamate-induced NMDAR desensitization on every cell tested by an average of 10% (N = 12, P ≤ 0.0005, Fig. 12C; also P ≤ 0.005, average 10% increase in CTR). If CA1 neurons in P14 or P15 slices were preincubated with GLY (10 μM) and the GlyT antagonist NFPS (100 nM), HCY only reduced desensitization by an average of 3%, which was significantly less than with HCY alone (N = 3, Fig. 12, B and C; Wilcoxon rank sum test on HCY vs. HCY + GLY + NFPS, P ≤ 0.01, 10 vs. 3% average normalized charge transfer enhancement). This indicates that neurons in CA1 of the hippocampus do show GLY-dependent desensitization that is reducible by HCY.
DISCUSSION
In this report, we use recordings from cultured neurons (Fig. 1), transfected HEK cells (Fig. 4), and acute hippocampal slices (Fig. 12) to show that HCY, a by-product of catecholamine breakdown, modulates peak amplitude and reduces desensitization of NMDAR currents. These effects are dose dependent (Figs. 2 and 4), and peak amplitude changes depend on GluN2 subunit composition of the receptor (Fig. 4). Desensitization reductions are present in all NMDAR subtypes tested in HEK cells (GluN2A, GluN2B, GluN2D; Fig. 4) and are occluded by raising ambient GLY (Figs. 3 and 5), but not by reducing or eliminating extracellular calcium. This is consistent with a specific HCY reduction of the GLY-dependent component of NMDAR desensitization (Lerma et al. 1990; Mayer et al. 1989; Vyklicky et al. 1990). The relevance of this report to NMDAR currents in vivo is therefore contingent upon the NMDAR GLY site being unsaturated in brain tissue. Nonsaturation of the NMDAR GLY site in vivo has been suggested previously (Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2003, 2004; Wilcox et al. 1996), despite the fact that GLY and the other NMDAR coagonist d-serine are both present in some brain regions (Papouin et al. 2012; Rosenberg et al. 2013; Schell et al. 1997). Using glutamate uncaging in acute hippocampal slices, we find that the NMDAR responses from CA1 neurons show desensitization upon glutamate uncaging that is significantly reduced by exposure to HCY. Thus native concentrations of GLY and d-serine are not high enough to abolish HCY desensitization reductions in the relatively intact environment of the slice (Fig. 12).
Site of HCY's action.
Previous reports concerning HCY effects on NMDARs are complex (Christie et al. 2009; Lipton et al. 1997). Our finding that HCY reduces the peak amplitude of GluN2B currents at low (200 nM) GLY is consistent with previous work showing that, in young GluN2B-enriched cortical neurons, HCY reduces calcium flux during brief applications of NMDA in low but not saturating GLY levels (Lipton et al. 1997). We also show that HCY shares some characteristics with the NMDAR coagonist GLY. First, HCY, like GLY, reduces GLY-dependent desensitization (Fig. 3). Also like GLY, HCY dose-dependently enhances the peak amplitude of GluN2A-type NMDAR currents in response to glutamate (Fig. 4; Priestley et al. 1995). Lastly, HCY loses its ability to reduce desensitization if the NMDAR GLY site is blocked by DCKA (Fig. 10). Future work may reveal why HCY decreases peak amplitude of GluN2B NMDAR responses while continuing to reduce desensitization. It may be that HCY resembles partial GLY site agonists such as l-alanine, which reduce GLY-dependent NMDAR desensitization but decrease receptor opening probability relative to full agonists (Benveniste et al. 1990; Kussius and Popescu 2009).
Unlike GLY application, the initial response of neurons and transfected HEK cells to HCY alone is a small depolarization (Figs. 1, 4, and 12; and Lipton et al. 1997). This current is blocked with AP5, a competitive antagonist at the NMDAR glutamate site (Fig. 1D), and resembles currents induced by 2 μM NMDA in amplitude (Fig. 9), suggesting that, in addition to its GLY-like properties, HCY is a low-affinity agonist at the glutamate site (Lipton et al. 1997). This may be critical if HCY is elevated during pregnancy or childhood, considering that low chronic NMDAR activation that is not correlated with presynaptic input causes functional depression and synapse elimination in the developing brain (Colonnese et al. 2006; Debski et al. 1990). We also show in Fig. 9 that preceding exposure of neurons to low levels of NMDA (2 μM) does not enhance 100 μM NMDA-induced charge transfer, indicating that HCY activation of the glutamate site is not likely to be responsible for its desensitization reducing effects. Therefore, HCY has two separate and unique effects on NMDARs that are likely to work in concert: HCY can resemble both GLY and glutamate. This may provide a basis for understanding why low HCY depresses hippocampal LTP, while higher HCY enhances LTP, characteristics of glutamate and GLY, respectively (Christie et al. 2009).
HCY and NMDAR gating.
When bound by glutamate and glycine during synaptic transmission, NMDARs show a slow rising (∼10 ms) and biphasically decaying excitatory postsynaptic current (EPSC). Patch-clamping electrophysiologists have used single-channel recordings to describe the complicated nature of NMDAR activation states governing channel opening and closing (Banke and Traynelis 2003; Lester and Jahr 1992; Popescu and Auerbach 2003). These studies reveal complex gating that entails multiple closed, open, and long-lived desensitized states. Indeed, there appears to be a “modal” gating of NMDARs where, in the continual presence of agonists, the receptor will enter three or more activity “modes” defined by the mean time between closed and open states (Popescu and Auerbach 2003; reviewed in Magleby 2004). The comprehensive models in these reports precisely predict the kinetics of the NMDAR EPSC and provide a basis for understanding the different molecular arrangements that the receptor can adopt. It will be interesting to determine the states or modes HCY modifies and how these could affect glutamatergic transmission in the brain. However, a recent single-channel study has shown that NMDAR desensitization is unlikely to affect the decay time of the NMDAR EPSC, which is accurately predicted using modal gating models that do not incorporate desensitized states (Zhang et al. 2008). This result is consistent with our finding that NMDAR decay time is not affected by HCY (Fig. 5B), which is therefore likely to only impact transitions to or from desensitized states. These data suggest that HCY may not affect single EPSCs but instead enhance NMDAR currents by reducing NMDAR transitions to desensitized states during high-frequency presynaptic firing. Transitions to desensitized states are likely also important during LTP, which is induced by a high-frequency tetanus expected to repeatedly activate the same set of NMDARs; this may explain why HCY shows strong enhancement of LTP at concentrations that reduce GLY-dependent NMDAR desensitization (Christie et al. 2009). This idea is supported by the finding that synaptic rises in GLY induced by blocking the GlyT, which would reduce GLY-dependent desensitization (Fig. 3), also enhance LTP (Martina et al. 2004).
HCY effects are age dependent.
A developmental turnover of GluN2B- to GluN2A-containing NMDARs is a common feature of cortical neuron cultures and many areas in the intact brain (Flint et al. 1997; Townsend et al. 2003; van Zundert et al. 2004; Zhong et al. 1994). NMDAR currents in young GluN2B-enriched neurons and in GluN2B-expressing HEK cells showed significant peak amplitude reductions when exposed to HCY (1 mM d,l-HCY = 500 μM l-HCY, Figs. 1, 2, and 4). Conversely, NMDAR currents in GluN2A-expressing HEK cells showed strongly enhanced peak amplitudes in response to the same HCY application (Fig. 4). Older cultured neurons did not show a change in peak amplitude, reflecting a hybrid GluN2B/GluN2A response as GluN2A is added to NMDARs during development (Baron et al. 2009; Zhong et al. 1994). Thus HCY effects on NMDARs are likely to change with maturity as synapses in the brain incorporate GluN2A subunits and receptors composed of two GluN2B subunits relocate to extrasynaptic sites (van Zundert et al. 2004).
Relevance to disease.
This report shows that low micromolar doses of HCY (50 μM l-HCY) strikingly enhance NMDAR charge transfer by 69% in neurons (P ≤ 0.001) and in every transfected HEK cell tested (P ≤ 0.001) (68% in GluN2B-transfected HEK cells, and 141% enhancement in GluN2A-transfected HEK cells; Figs. 2 and 4). Higher doses of HCY (500 μM l-HCY) caused even stronger charge transfer enhancement in all tested systems (Figs. 2, 4, 11, and 12). We will argue that these HCY levels are likely to be involved in disease states where HCY is upregulated, first outlining current clinical data.
There has been debate concerning the relevance of high HCY in the blood of schizophrenia patients to the disease itself. These observed levels nearly double the ∼10 μM level observed in controls (∼15–20 μM, Applebaum et al. 2004; Levine et al. 2002), and a 5 μM increase of HCY in the blood is known to increase schizophrenia susceptibility by 70% (Muntjewerff et al. 2006). Consistent with the fact that CSF HCY levels are also increased in schizophrenia (Regland et al. 2004), studies on HCY-injected rats have shown that increasing HCY levels in the blood causes heightened HCY in the brain that alters NMDAR-dependent plasticity (Algaidi et al. 2006). In addition, the same schizophrenia-linked molecules that produce and metabolize HCY in the blood (COMT, methyl-tetrahydrofolate reductase) also do so in brain regions where COMT is the primary clearance mechanism for synaptic DA and NE (i.e., cortex, hippocampus, superior colliculus; not the striatum; Bigl et al. 1974; Muntjewerff et al. 2006; Roffman et al. 2008; Tunbridge et al. 2006, 2008). This suggests that the heightened levels of blood HCY could reflect dysregulated HCY production and metabolism at many DA and NE synapses in the central nervous system (Tunbridge et al. 2006, 2008).
HCY doses used in this study could thus affect NMDARs in brain disorders like schizophrenia, where HCY levels average 20 μM HCY in blood, and ∼1 μM HCY is observed in some patients' CSF (Levine et al. 2002; Regland et al. 2004). Hyperhomocysteinemia and fibromyalgia, both associated with cognitive dysfunction, also show increased HCY (hyperhomocysteinemia: ∼150 μM plasma HCY levels with low micromolar CSF levels; Blom et al. 1993; Surtees et al. 1997; fibromyalgia: ∼1 μM CSF; Regland 2005). It is also possible that HCY has a role in brains of normal humans, as controls show ∼8 μM HCY in blood and ∼ 0.2 μM in CSF. These control levels of CSF HCY are higher than normal CSF levels of DA (Gjerris et al. 1987; Levine et al. 2002; Regland et al. 2004).
Although HCY CSF levels are somewhat lower than we use in this study, synaptic concentrations of neurotransmitters are not often reflected in total CSF or blood preparations. This occurs for two reasons. 1) Transporters surrounding synapses (DAT for DA or EAATs for glutamate) clear neurotransmitters from the extracellular space within milliseconds (Clements et al. 1992; Garris et al. 1994). 2) Synaptically released neurotransmitters reach high concentrations due to the miniscule volume of the synaptic cleft, but, after escaping the synapse, they are no longer confined to a small volume, lowering their absolute concentration. For example, the documented concentration of DA in the synaptic cleft after single vesicle release is 1.6 mM (Garris et al. 1994). However, even with DA transporters blocked, the local extrasynaptic DA rise due to a single vesicle is only 0.25 μM (Garris et al. 1994), and the CSF concentration of DA is only 40 nM (Gjerris et al. 1987). Glutamate also reaches millimolar (1.1 mM) concentrations at synapses, but is only found at 1.1 μM in the CSF (Clements et al. 1992; Yamamoto et al. 1999).
Like these neurotransmitters, HCY is released to the extracellular space. This occurs after DA breakdown by COMT in astrocytes (Huang et al. 2005). HCY is also cleared from the extracellular space by an as yet uncharacterized neuronal transporter (Huang et al. 2005). If HCY is released from astrocytes into the synaptic cleft, it could reach glutamate or DA-like concentrations (∼1 mM). This idea is substantiated by the growing list of “gliotransmitters” that participate in synaptic signaling (Halassa et al. 2007). Clearly, the hypothesis that HCY is a gliotransmitter should be tested experimentally; however, if HCY reaches even 5% (50 μM) of the synaptic glutamate or DA concentration at synapses, it is likely to produce the striking effects on NMDAR desensitization found in this study (Figs. 2 and 4).
An additional factor in the interpretation of the present experiments is that HCY molecules quickly dimerize in solution via their free sulfur groups, which we consistently noticed (Hogg 1999). We report in this text that dimerized HCY (homocystine) fails to reduce NMDAR desensitization; this is a significant finding because, after only 4 h in solution, 50% of HCY has dimerized to homocystine (Hogg 1999). It is therefore possible that much of our data underestimate the potency of HCY on NMDARs: a substantial amount of data were obtained +4 h after the beginning of recording sessions, and we only compensated our solutions for the slight decay that occurred between making solutions and the start of recording sessions.
An important question that arises from these HCY findings is whether the association of HCY with schizophrenia is due to its induction of a brain environment similar to that caused by drugs like ketamine and phencyclidine (PCP). These NMDAR interacting drugs produce temporary schizophrenia-like symptoms in normal subjects. Ketamine and PCP, however, are NMDAR open channel blockers. Our work does not support a ketamine or PCP-like antagonist role for HCY at the level of the NMDAR itself. Nonetheless, despite initial expectations that ketamine would depress activity in neural circuits due to antagonism of NMDARs, it is now generally accepted that ketamine produces psychotic symptoms by enhancing circuit activity through preferential inhibition of NMDARs on inhibitory interneurons (Homayoun and Moghaddam 2007; Seamans 2008). HCY may also have emergent effects on neural circuits in vivo. Indeed, the dynamic effects of HCY on desensitization and amplitude that vary according to GluN2 composition and [GLY] suggest an extremely complex role for HCY in the nervous system. Considering the growing list of brain molecules that can reduce NMDAR desensitization (e.g., spermine, GLY, d-serine, PSD-95), it may even be the case that NMDAR desensitization itself is a critical underexplored feature in vivo (Mayer et al. 1992; Sornarajah et al. 2008).
GRANTS
This work was supported by National Eye Institute Grant 5R01EY-014074-18 (M. Constantine-Paton), a National Defense Science and Engineering Graduate Fellowship (A. D. Bolton), and a National Science Foundation Graduate Research Fellowship (A. D. Bolton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.D.B., M.A.P., and M.C.-P. conception and design of research; A.D.B. and M.A.P. performed experiments; A.D.B. analyzed data; A.D.B., M.A.P., and M.C.-P. interpreted results of experiments; A.D.B. prepared figures; A.D.B. drafted manuscript; A.D.B., M.A.P., and M.C.-P. edited and revised manuscript; A.D.B. and M.C.-P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Donald Jacobsen for advice and for providing our l-HCY producing protocol. We also thank John Woodward for providing the GluN2D construct; Dr. Woodward and Gabriela Popescu both provided helpful and constructive comments on the data. Rory Kirchner and Yasunobu Murata provided helpful discussion, editing of the manuscript, experimental advice, and encouragement. Sam Cooke provided valuable advice and manuscript editing. Greg Hale, Yarden Katz, Kartik Ramamoorthi, Andrew Young, and Stuart Layton provided technical assistance. Zhe Chen wrote code used for decay fitting. Initial motivation for this project was born from discussions with Dr. Cassandra Smith, who deserves special thanks.
REFERENCES
- Algaidi SA, Christie LA, Jenkinson AM, Whalley L, Riedel G, Platt B. Long-term homocysteine exposure induces alterations in spatial learning, hippocampal signalling and synaptic plasticity. Exp Neurol 197: 8–21, 2006 [DOI] [PubMed] [Google Scholar]
- Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res 38: 413–416, 2004 [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron 11: 401–407, 1993 [DOI] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci 6: 144–152, 2003 [DOI] [PubMed] [Google Scholar]
- Baron A, Montagne A, Cassé F, Launay S, Maubert E, Ali C, Vivien D. NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity. Cell Death Differ 17: 860–871, 2009 [DOI] [PubMed] [Google Scholar]
- Benveniste M, Clements J, Vyklicky L, Mayer ML. A kinetic analysis of the modulation of N-methyl-d-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol 428: 333–357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implication in glia-neuron signaling. Neuroscience 124: 377–386, 2004 [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-d-aspartate receptor function by glycine transport. Proc Natl Acad Sci USA 95: 15730–15734, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18: 10464–10472, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigl V, Biesold D, Weisz K. The influence of functional alteration on monoamine oxidase and catechol-O-methyl transferase in the visual pathway of rats. J Neurochem 22: 505–509, 1974 [DOI] [PubMed] [Google Scholar]
- Blom HJ, Wevers RA, Verrips A, TePoele-Pothoff MT, Trijbels JM. Cerebrospinal fluid homocysteine and the cobalamin status of the brain. J Inherit Metab Dis 16: 517–519, 1993 [DOI] [PubMed] [Google Scholar]
- Broch OJ, Jr, Fonnum F. The regional and subcellular distribution of catechol-O-methyl transferase in the rat brain. J Neurochem 19: 2049–2055, 1972 [DOI] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol 89: 691–703, 2003 [DOI] [PubMed] [Google Scholar]
- Christie LA, Riedel G, Platt B. Bi-directional alterations of LTP after acute homocysteine exposure. Behav Brain Res 205: 559–563, 2009 [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science 258: 1498–1501, 1992 [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Constantine-Paton M. Developmental period for N-methyl-d-aspartate (NMDA) receptor-dependent synapse elimination correlated with visuotopic map refinement. J Comp Neurol 494: 738–751, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci 13: 129–154, 1990 [DOI] [PubMed] [Google Scholar]
- Debski EA, Cline HT, Constantine-Paton M. Activity-dependent tuning and the NMDA receptor. J Neurobiol 21: 18–32, 1990 [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84: 745–755, 1996 [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51, 2000 [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci 17: 2469–2476, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14: 6084–6093, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerris A, Werdelin L, Rafaelsen OJ, Alling C, Christensen NJ. CSF dopamine increased in depression: CSF dopamine, noradrenaline and their metabolites in depressed patients and in controls. J Affect Disord 13: 279–286, 1987 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13: 54–63, 2007 [DOI] [PubMed] [Google Scholar]
- Hogg N. The effect of cyst(e)ine on the auto-oxidation of homocysteine. Free Radic Biol Med 27: 28–33, 1999 [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Dragan M, Freeman D, Wilson JX. Activation of catechol-O-methyltransferase in astrocytes stimulates homocysteine synthesis and export to neurons. Glia 51: 47–55, 2005 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. Cloning and expression of the epsilon 4 subunit of the NMDA receptor channel. FEBS Lett 313: 34–38, 1992 [DOI] [PubMed] [Google Scholar]
- Jin C, Thetford Smothers C, Woodward JJ. Enhanced ethanol inhibition of recombinant N-methyl-d-aspartate receptors by magnesium: role of NR3A subunits. Alcohol Clin Exp Res 32: 1059–1066, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci 26: 1604–1615, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger WD. The transsulfuration pathway. In: Homocysteine in Health and Disease, edited by Carmel R, Jacobsen DW. Cambridge, UK: Cambridge University Press, 2001 [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. Calcium-dependent inactivation of recombinant N-methyl-d-aspartate receptors is NR2 subunit specific. Mol Pharmacol 50: 1680–1688, 1996 [PubMed] [Google Scholar]
- Kussius CL, Popescu GK. Kinetic basis of partial agonism at NMDA receptors. Nat Neurosci 12: 1114–1120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Rosenmund C, Westbrook GL. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci 13: 674–684, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-d-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A 87: 2354–2358, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci 12: 635–643, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Stahl Z, Sela BA, Gavendo S, Ruderman V, Belmaker RH. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry 159: 1790–1792, 2002 [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D'Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-d-aspartate receptor. Proc Natl Acad Sci U S A 94: 5923–5928, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci 1: 273–275, 1998 [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Lawrence JE, Valentino TL. Desensitizing glutamate receptors shape excitatory synaptic inputs to tiger salamander retinal ganglion cells. J Neurosci 15: 6189–6199, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Chafee MV. Translational and developmental perspective on N-methyl-d-aspartate synaptic deficits in schizophrenia. Dev Psychopathol 18: 853–876, 2006 [PubMed] [Google Scholar]
- Magleby KL. Modal gating of NMDA receptors. Trends Neurosci 27: 231–233, 2004 [DOI] [PubMed] [Google Scholar]
- Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol 557: 489–500, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Krasteniakov NV, Bergeron R. d-Serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol 548: 411–423, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol 66: 161–181, 2004 [DOI] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature 338: 425–427, 1989 [DOI] [PubMed] [Google Scholar]
- Mayer ML, Benveniste M, Patneau DK, Vyklicky L. Pharmacologic properties of NMDA receptors. Ann NY Acad Sci 648: 194–204, 1992 [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Muntjewerff JW, Kahn RS, Blom HJ, Heijer Den M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry 11: 143–149, 2006 [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus 11: 529–542, 2001 [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet J-P, Oliet SHR. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150: 633–646, 2012 [DOI] [PubMed] [Google Scholar]
- Phillips MA, Colonnese MT, Goldberg J, Lewis LD, Brown EN, Constantine-Paton M. A synaptic strategy for consolidation of convergent visuotopic maps. Neuron 71: 710–724, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R, Paul S. Homocysteine-NMDA receptor-mediated activation of extracellular signal-regulated kinase leads to neuronal cell death. J Neurochem 110: 1095–1106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci 6: 476–483, 2003 [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdellés B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-d-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol 48: 841–848, 1995 [PubMed] [Google Scholar]
- Regland B. Schizophrenia and single-carbon metabolism. Prog Neuro Psychopha 29: 1124–1132, 2005 [DOI] [PubMed] [Google Scholar]
- Regland B, Abrahamsson L, Blennow K, Grenfeldt B, Gottfries C-G. CSF-methionine is elevated in psychotic patients. J Neural Transm 111: 631–640, 2004 [DOI] [PubMed] [Google Scholar]
- Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, White T, Clark VP, Fries J, Andreasen NC, Goff DC, Manoach DS. MTHFR 677C → T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val → Met. Proc Natl Acad Sci U S A 105: 17573–17578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard J-M, Wolosker H. Neuronal d-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci 33: 3533–3544, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron 25: 373–383, 2000 [DOI] [PubMed] [Google Scholar]
- Sather W, Dieudonné S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-d-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol 450: 643–672, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W, Johnson JW, Henderson G, Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron 4: 725–731, 1990 [DOI] [PubMed] [Google Scholar]
- Schell MJ, Brady RO, Molliver ME, Snyder SH. d-Serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 17: 1604–1615, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J. Losing inhibition with ketamine. Nat Chem Biol 4: 91–93, 2008 [DOI] [PubMed] [Google Scholar]
- Seung HS, Lee DD, Reis BY, Tank DW. Stability of the memory of eye position in a recurrent network of conductance-based model neurons. Neuron 26: 259–271, 2000 [DOI] [PubMed] [Google Scholar]
- Sornarajah L, Vasuta OC, Zhang L, Sutton C, Li B, El-Husseini A, Raymond LA. NMDA receptor desensitization regulated by direct binding to PDZ1–2 domains of PSD-95. J Neurophysiol 99: 3052–3062, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees R, Leung DYM, Bowron A, Leonard J. Cerebrospinal fluid and plasma total homocysteine and related metabolites in children with cystathionine β-synthase deficiency: the effect of treatment. Pediatr Res 42: 577–582, 1997 [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science 267: 1510–1512, 1995 [DOI] [PubMed] [Google Scholar]
- Townsend M, Yoshii A, Mishina M, Constantine-Paton M. Developmental loss of miniature N-methyl-d-aspartate receptor currents in NR2A knockout mice. Proc Natl Acad Sci U S A 100: 1340–1345, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge E, Harrison P, Weinberger D. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 60: 141–151, 2006 [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Warden DR, Johnston C, Refsum H, Smith AD. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am J Med Genet B Neuropsychiatr Genet 147B: 996–999, 2008 [DOI] [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci 27: 428–437, 2004 [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J Neurophysiol 79: 555–566, 1998 [DOI] [PubMed] [Google Scholar]
- Villarroel A, Regalado MP, Lerma J. Glycine-independent NMDA receptor desensitization: localization of structural determinants. Neuron 20: 329–339, 1998 [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Benveniste M, Mayer ML. Modulation of N-methyl-d-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol 428: 313–331, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 24: 455–463, 2001 [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol 76: 3415–3424, 1996 [DOI] [PubMed] [Google Scholar]
- Wolosker H. d-Serine regulation of NMDA receptor activity. Science Signaling pe41–pe41, 2006 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Rossi S, Stiefel M, Doppenberg E, Zauner A, Bullock R, Marmarou A. CSF and ECF glutamate concentrations in head injured patients. Acta Neurochir Suppl (Wien) 75: 17–19, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang W, Howe JR, Popescu GK. Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat Neurosci 11: 1373–1375, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Russell SL, Pritchett DB, Molinoff PB, Williams K. Expression of mRNAs encoding subunits of the N-methyl-d-aspartate receptor in cultured cortical neurons. Mol Pharmacol 45: 846–853, 1994 [PubMed] [Google Scholar]
- Zilberter Y, Uteshev V, Sokolova S, Khodorov B. Desensitization of N-methyl-d-aspartate receptors in neurons dissociated from adult rat hippocampus. Mol Pharmacol 40: 337–341, 1991 [PubMed] [Google Scholar]