Abstract
Projection neurons (PNs), located in the antennal lobe region of the insect brain, play a key role in processing olfactory information. To explore how activity is regulated at the level of single PNs within this central circuit we have recorded from these neurons in adult Drosophila melanogaster brains. Our previous study demonstrated that PNs express voltage-gated calcium currents with a transient and sustained component. We found that the sustained component is mediated by cac gene-encoded Cav2-type channels involved in regulating action potential-independent release of neurotransmitter at excitatory cholinergic synapses. The function of the transient calcium current and the gene encoding the underlying channels, however, were unknown. Here we report that the transient current blocked by prepulse inactivation is sensitive to amiloride, a vertebrate Cav3-type channel blocker. In addition PN-specific RNAi knockdown of α1T, the Drosophila Cav3-type gene, caused a dramatic reduction in the transient current without altering the sustained component. These data demonstrate that the α1T gene encodes voltage-gated calcium channels underlying the amiloride-sensitive transient current. Alterations in evoked firing and spontaneous burst firing in the α1T knockdowns demonstrate that the Cav3-type calcium channels are important in regulating excitability in adult PNs.
Keywords: transient calcium current, Drosophila brain, Cav3, excitability
a wide variety of insect behaviors are driven or modulated by olfactory input and the ensemble of neurons involved in processing olfactory information is well defined (Wilson and Mainen 2006; Vosshall and Stocker 2007). Olfactory perception begins when odorant molecules bind to receptors in olfactory receptor neurons (ORNs) located in the antennae and the maxillary palps (Hildebrand and Shepherd 1997). ORNs project to the antennal lobes, the insect equivalent of the vertebrate olfactory bulb, where they synapse onto the dendrites of projection neurons (PNs), the principal output cells that extend axons to higher order processing centers in the mushroom bodies and lateral horn (Stocker 1994; Ito et al. 1998). In Drosophila melanogaster, where genetic manipulations and behavioral assessment are routine, it is now feasible to record from identified neurons within this circuit in the adult brain (Wilson et al. 2004; Gu and O'Dowd 2006, 2007). This has made it possible to explore the mechanisms that regulate activity in a circuit important in generating specific components of an adult behavior at the single cell level. Whole cell recordings from single PNs have demonstrated that olfactory processing begins in the antennal lobe where both intra- and interglomerular interactions influence activity of these cells (Olsen et al. 2007; Olsen and Wilson 2008; Root et al. 2008).
To understand the molecular mechanisms underlying regulation of neuronal activity in individual PNs in the olfactory circuit requires identification of the ion channel subtypes that govern excitability and synaptic transmission in these cells. One important class of channels present in all neurons are voltage-gated calcium channels that mediate depolarization-induced calcium influx that influences a number of cellular processes including excitability and release of neurotransmitters at chemical synapses. The α1-subunit of these multimeric proteins forms the ion-conducting pore that defines many of the functional properties characteristic of the distinct calcium channel subtypes (Catterall 2011). There are three families of genes encoding α1 subunits and in the Drosophila genome there is one α1 subunit gene in each family: α1D (Cav1), cac (Cav2), and α1T (Cav3) (Zheng et al. 1995; Smith et al. 1996; Littleton and Ganetzky 2000; King 2007). In a recent study we found that voltage-gated calcium currents recorded from the cell bodies of PNs in the adult brain could be separated into two kinetically distinct components: a rapidly decaying transient current and a slowly decaying sustained current (Gu et al. 2009). Using a combination of pharmacological and molecular genetic strategies, we demonstrated that the Cav2-type cac gene encodes calcium channels that mediate PLTXII-sensitive sustained calcium currents. Our studies show that the CAC channels regulate action potential-independent release of neurotransmitter at excitatory cholinergic synapses in the adult brain, a novel role not predicted from previous studies at peripheral synapses (Rieckhof et al. 2003).
While a recent study suggests that Cav2-type CAC channels (aka Dmca1A) also contribute to the transient calcium currents in adult motor neurons (Ryglewski et al. 2012), our results indicated that neither PLTXII nor mutations in the cac gene reduced the transient currents in PNs. This suggests that a distinct calcium channel subtype gives rise to the transient current in adult PNs. In vertebrates, previous studies have demonstrated that Cav3 genes encode channels underlying transient calcium currents (Nowycky et al. 1985; Catterall 2011).
Here we report that amiloride, a vertebrate Cav3-type channel blocker, reduces the transient calcium current without significantly altering the sustained Cav2-type CAC channel-mediated current in adult PNs. In addition, RNAi mediated knockdown of the α1T gene, the Drosophila Cav3-type homolog, in PNs reduced the transient component significantly but the sustained component was not affected. Alterations in evoked and spontaneous firing were observed in the α1T knockdowns. These data demonstrate that α1T-encoded Cav3-type channels mediate transient calcium currents that are important in shaping the PN firing properties and, therefore, play an important role in regulating olfactory signal processing.
METHODS AND MATERIALS
Fly strains.
All recordings of wild-type PNs were from the GH146-Gal4 line. To knockdown expression of channels encoded by the Drosophila Cav3-type calcium channel gene (α1T) specifically in PNs, the GH146-Gal4 line was crossed to two independent UAS-RNAi lines from Vienna Drosophila RNAi Center targeting different sequences in the α1T gene. The knockdown flies were designated RNAi-1Ta (no.48008) and RNAi-1Tb (no. 31961), respectively. All fly lines were kept at 23°C in standard plastic vials with cotton plugs on a yeast, agar, and cornmeal diet with a 12h light/dark regimen.
Electrophysiological recordings from PNs in isolated adult brain.
Brains were obtained from adult female flies 1–2 days after eclosion. The entire brain, including optic lobes, was removed from the head, prepared for recordings as previously described, and mounted in the recording chamber with the anterior face of the brain up (Gu and O'Dowd 2006, 2007). Recordings were made from PNs in the dorsal neuron cluster using 8- to 9-mΩ resistance pipettes.
Isolated calcium currents were recorded in voltage-clamp mode using a pipette solution containing: 102 mM d-gluconic acid, 102 mM CsOH, 0.085 mM CaCl2, 1.7 mM MgCl2, 17 mM NaCl, 0.94 mM EGTA, 8.5 mM HEPES, and 4 mM Na2ATP. The osmolarity was adjusted to 235 mosM and pH to 7.2. The external solution contained: 101 mM NaCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 5.4 mM KCl, 5 mM glucose, 1.25 mM NaH2PO4, and 20.7 mM NaHCO3. Drugs were added to block synaptic transmission, sodium current, and potassium currents: 20 μM d-tubocurarine (Sigma), 10 μM picrotoxin (Sigma), 1 μM tetrodotoxin (Alomone), 2.5 mM TEA (Sigma), and 1.0 mM 4-aminopyridine (Sigma). The osmolarity was adjusted to 250 mosM, and the solution was saturated with 95% oxygen-5% CO2. For experiments evaluating the effect of amiloride (Sigma), the drug was made up fresh each day by dissolving in H2O. This was added to the extracellular solution for a final concentration of 1 mM.
Firing properties were recorded in current-clamp mode with internal solution as described above except that cesium gluconate was replaced by potassium gluconate. Intrinsic firing properties, both evoked and spontaneous, were evaluated in external solution that contained picrotoxin and d-tubocurarine to block synaptic transmission. All recordings were performed during continuous perfusion with oxygenated saline at room temperature.
Data acquisition and analysis.
A Dell computer (Dimension 8200) was used in conjunction with an EPC-7 patch clamp amplifier (List Medical Electronics, Darmstadt, Germany), Digidata 1322A analog to digital converter (Axon Instruments), and Clampex software (pClamp 9.0; Molecular Devices) filtered through a 2-kHz low-pass Bessel filter (Frequency Devices) for generation of voltage/current commands and for data acquisition. Current amplitudes were normalized to whole cell capacitance, and values are reported as current density. For evoked current-clamp experiments, cells were held at −70 mV and only cells in which three or more spikelets were evoked by current injection were included in the analysis. A spikelet was an event with a rapid rise of ≤10 ms and repolarization rate of ≤50 ms and minimum amplitude of 0.5 mV. The average spikelet amplitude and spikelet duration for each cell, measured at the inflection point on the upsweep of the spikelet, were determined from the first suprathreshold depolarization that triggered three or more spikelets.
Spontaneous activity was evaluated in the absence of holding current. A slow wave depolarization that resulted in firing of at least one spikelet was defined as a burst. Burst amplitude was measured from resting membrane potential to peak of the burst and duration was measured at half amplitude. The number of spikes/burst was determined for each burst and a mean value was calculated for each cell. Analysis was conducted in PNs in which the resting membrane potential was more hyperpolarized than −40 mV and that exhibited at least one burst of action potentials during the 2.5-min recording period.
Statistics.
Comparisons between two genotypes or treatments were made with Student's t-test. Comparisons between more than two genotypes or treatments were made using an ANOVA with Bonferroni's post hoc test for pair-wise comparisons. Data shown was corrected for the 5-mV liquid junction potential associated with the recording solutions. Statistical significance was assumed when *P < 0.05, **P < 0.01, and ***P < 0.001. Data in graphs are presented as means ± SE.
RESULTS
Amiloride inhibits transient calcium currents in adult PNs.
Antennal lobe PNs in brains of wild-type adult flies express voltage-gated calcium currents with two kinetically distinct components. In a previous study we demonstrated that Cav2-type CAC channels are major contributors to the PLTXII-sensitive sustained current based on selective reduction of this component in cac hypomorphic mutants (Gu et al. 2009). As the first step in identifying the channels underlying the transient calcium component we used a pharmacological approach focusing on the drug amiloride that blocks T-type currents in vertebrate neurons. Isolated calcium currents were recorded in physiological concentrations of extracellular calcium (1.8 mM) with drugs to block both sodium and potassium currents.
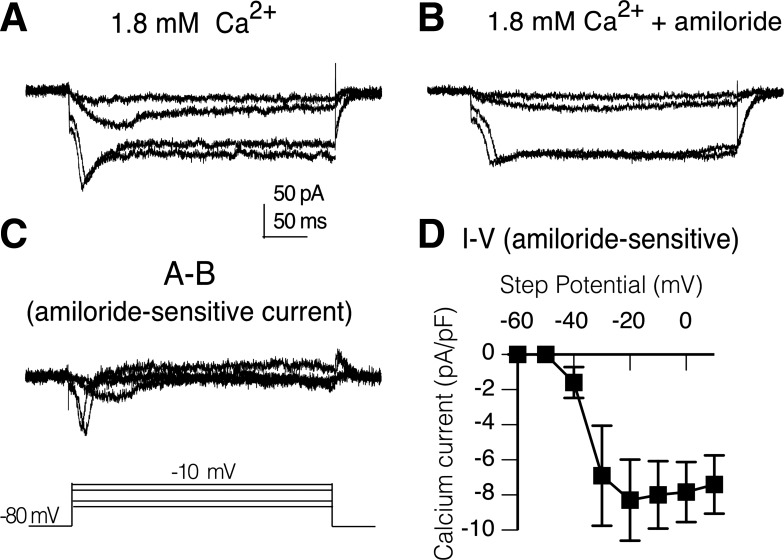
Depolarizing voltage-steps from a holding potential of −80 mV evoked calcium currents with both a transient and sustained component in a wild-type PN in the adult brain preparation (Fig. 1A). The time course of activation of the transient current is relatively slow with the current peak occurring 20–30 ms after onset of voltage-steps. Perfusion of the recording chamber with 1 mM amiloride for 10 min reduced the size of the transient current with little effect on the sustained current amplitude (Fig. 1B). Digital subtraction of traces (A-B) reveal the amiloride-sensitive component is primarily transient (Fig. 1C). The mean current density-voltage relationship of the digitally isolated amiloride-sensitive transient current in wild-type PNs shows the transient current activates between −50 and −40 mV, which does not fit the profile of classic low-voltage-activated currents (Fig. 1D).
Fig. 1.
Transient calcium currents in projections neurons (PNs) are amiloride sensitive. A: isolated calcium currents in a PN in brain of wild-type adult with both transient and sustained components. Currents elicited by a series of depolarizing voltage steps (−50, −40, −20, and −10 mV) from a holding potential of −80 mV. B: perfusion of recording chamber with 1 mM amiloride reduced the transient component with little effect on the sustained currents. C: amiloride-sensitive current isolated by digital subtraction of current records (A and B). D: current density vs. voltage curve generated from digitally generated amiloride-sensitive transient currents (I-V; n = 3). Bars indicate SE. Peak current at each voltage was normalized to cell capacitance and expressed as density (pA/pF). Isolated calcium currents were recorded in external saline containing physiological concentration of calcium (1.8 mM), tetrodotoxin (TTX), picrotoxin (PTX), curarine, TEA, and with internal cesium.
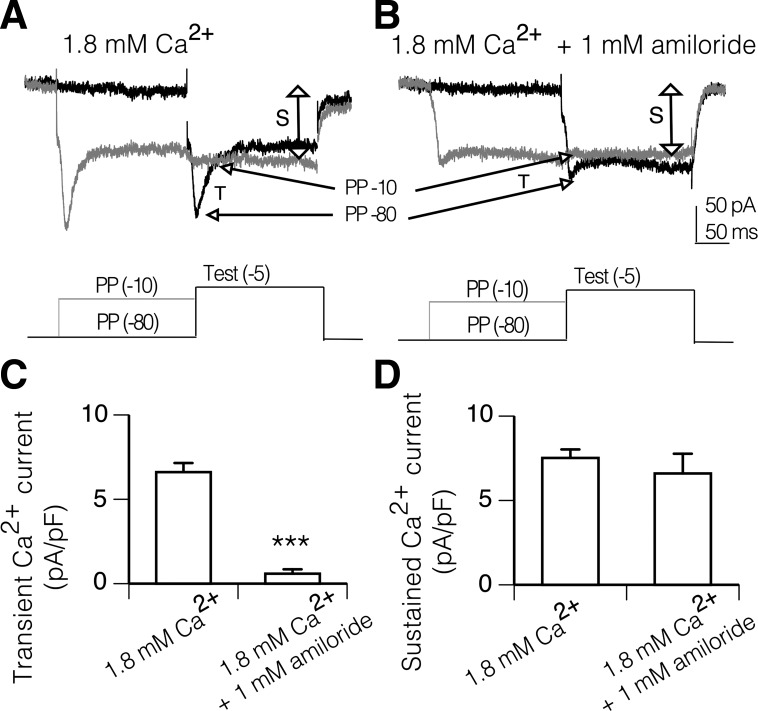
The transient and sustained currents in PNs are also differentially sensitive to prepulse inactivation. A 250-ms prepulse to −10 mV results in a dramatic reduction in the amplitude of the transient current activated during a test step to −5 mV compared with the transient current activated following a prepulse to −80 mV (Fig. 2A). In contrast, the sustained current is similar in magnitude following prepulses to −10 and −80 mV (Fig. 2A). The transient component sensitive to prepulse inactivation is reduced in magnitude following addition of 1 mM amiloride, and there is little affect on the sustained component (Fig. 2B).
Fig. 2.
Isolation of amiloride-sensitive transient calcium currents by prepulse inactivation. A: transient (T) current evoked by a test step to −5 mV from a prepulse of −80 mV (PP −80) is blocked by prepulse to −10 mV (PP −10). In contrast, the sustained current (S) is similar in size with prepulses to −10 mV and −80 mV. B: perfusion of the recording chamber with 1 mM amiloride for 10 min reduced the size of the transient current but did not alter the sustained current, confirming that the currents sensitive to prepulse inactivation are inhibited by amiloride. C: magnitude of the transient calcium current, evaluated using the prepulse protocol, is significantly reduced by 1 mM amiloride (***P < 0.001, t-test unpaired). D: magnitude of sustained calcium current was not altered by amiloride. Each bar indicates the means and SE; n = 17 for 1.8 mM Ca2+ and n = 11 for 1.8 mM Ca2+ + 1 mM amiloride. Whole cell calcium currents were isolated with TTX, PTX, d-tubocurarine, TEA, and internal cesium.
To quantify the degree of inhibition, the magnitude of the transient component was defined as the difference in magnitude of the transient current during a test step following a prepulse to −80 and a prepulse to −10 mV (Fig. 2A). The magnitude of the sustained component was measured at the end of the test step following a prepulse to −10 mV. Values were normalized to whole cell capacitance and expressed in terms of density (pA/pF) to account for variation in PN size.
Amiloride caused a significant reduction in the magnitude of the transient current in the population of PNs examined (Fig. 2C; P < 0.001, unpaired t-test). The magnitude of the sustained current was not significantly reduced by amiloride (Fig. 2D). Together these findings indicate that amiloride-sensitive calcium channels mediate transient calcium currents in PNs in adult brain.
RNAi-1T knockdown in PNs reduces transient calcium currents.
Based on kinetics and pharmacological profile, the Drosophila α1T gene, which is homologous to the vertebrate Cav3-type calcium channels, was a good candidate for encoding the channels underlying the transient current in PNs. To test this hypothesis, flies carrying UAS-RNAi targeted to the α1T gene were crossed to the GH146-Gal4 PN driver line with the goal of specifically knocking down α1T calcium channel expression in PNs. For these studies we used two different UAS-RNAi lines, targeting different sequences in the α1T gene and representing independent insertion events on the second and third chromosome. These were designated RNAi-1T-a and RNAi-1T-b.
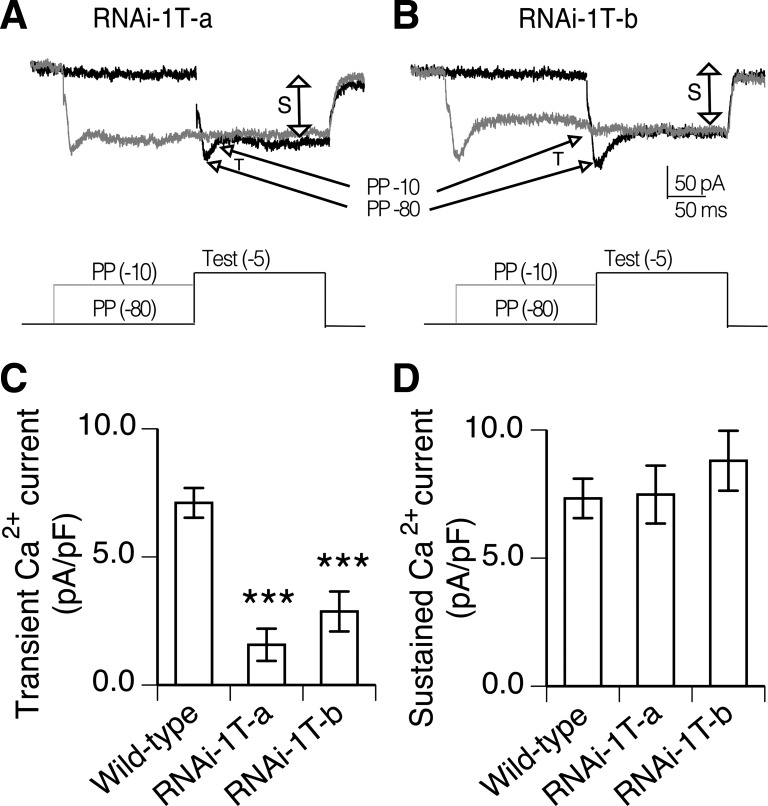
To determine if the RNAi knockdown lines exhibited altered calcium currents in PNs, the prepulse inactivation protocol was used to evaluate the magnitude of the transient and sustained current components. Representative records of calcium currents from both RNAi-1T knockdown lines illustrate a relatively small transient component while the sustained component remains prominent (Fig. 3, A and B). The magnitude of the transient current in RNAi-1T-a knockdown PNs was significantly reduced compared with wild-type PNs (Fig. 3C; P < 0.001, ANOVA, Bonferroni's post hoc test). In the RNAi-1T-b knockdown line, the reduction in transient current magnitude was not as large, but the currents were still significantly smaller than wild type (Fig. 3C, P < 0.001, ANOVA, Bonferroni's post hoc test). There was no significant difference in the sustained calcium current magnitude in either of the RNAi-1T knockdown compared with wild type (Fig. 3D). These data indicate that the α1T gene encodes calcium channels contributing to the transient calcium current in PNs.
Fig. 3.
RNAi-1T knockdown specifically reduces transient calcium currents in PNs. A and B: prepulse inactivation protocol reveals PN calcium currents with only small amplitude transient component in RNAi-1T-a and RNAi-1T-b knockdowns. The sustained component is still prominent. C: magnitude of the transient calcium current determine from prepulse protocol is significantly reduced in RNAi-1T-a (n = 8) and RNAi-1T-b (n = 9) compared with wild type (n = 14). ***P < 0.001, ANOVA, Bonferroni's post hoc test. D: sustained calcium current magnitude evaluated from same PNs were not significantly different than wild type. Whole cell calcium currents were isolated with TTX, PTX, d-tubocurarine, TEA, and internal cesium. Each bar indicates the means and SE.
RNAi-1T knockdown alters evoked firing properties of PNs.
In vertebrates, T-type calcium channels are important in regulating neuronal excitability. To investigate whether α1T-encoded calcium channels also influence excitability in Drosophila neurons, evoked firing properties were examined in wild-type and RNAi-1T-a knockdown PNs. Picrotoxin and d-tubocurarine were added to the standard physiological saline to block GABAergic and cholinergic synaptic transmission. Cells were also held at −75 mV to reduce spontaneous membrane potential oscillations characteristic of these neurons.
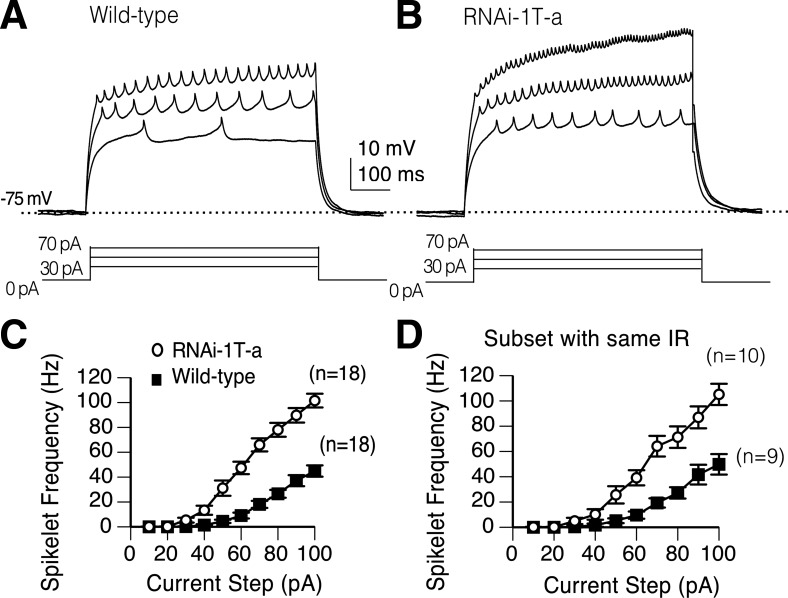
In both wild-type and RNAi-1T-a knockdown PNs, suprathreshold current injections resulted in large sustained membrane depolarizations capped by small fast spikelets (Fig. 4, A and B). Spikelets represent action potentials initiated in the axonal compartment located in the antennal lobe neuropil, electrically distant from the pipette located on the cell soma (Gouwens and Wilson 2009). In both genotypes, firing frequency increased steadily with increasing current injection but the spikelet frequency was higher in the RNAi-1T-a compared with wild type (Fig. 4, A and B). Comparison of current input vs. spikelet frequency output relationship revealed a significant increase in the firing frequency in RNAi-1T-a compared with wild-type PNs (Fig. 4C; P < 0.01, 2-way ANOVA).
Fig. 4.
Knockdown of α1T channels increases evoked firing frequency. A and B: representative current clamp recordings of a wild-type and RNAi-1T-a knockdown PN. C: spikelet frequency is plotted as a function of current step. Means determined from the whole population demonstrate that RNAi-1T-a knockdowns (n = 15) have a significantly higher mean firing frequency than wild type (n = 16). P < 0.01, two-way ANOVA. D: Significant difference in firing frequency (P < 0.05, two-way ANOVA) is still observed when analysis is confined to PNs with input resistance (IR) between 400 and 600 mΩ: wild type (n = 9, 486 ± 29 mΩ) and RNAi-1T-a (n = 10, 485 ± 24 mΩ). Each point on the line graph indicates the means and SE.
In addition to the change in firing frequency, the input resistance in the RNAi-1T-a PNs (562 ± 35 mΩ; means ± SE; n = 18) was significantly higher than wild type (459 ± 30 mΩ; means ± SE; n = 18; P < 0.05, t-test). However, this did not account for the difference in the firing frequency since it was still significantly increased in the subset of RNAi-1T-a PNs that were matched by input resistance to wild-type PNs (Fig. 4D; P < 0.05, two-way ANOVA). All wild-type (n = 9) and mutant PNs (n = 10) with input resistances between 400 and 600 mΩ were included in the analysis.
There was variability in the spikelet waveform from cell to cell, even within a genotype, but there was no significant difference in the amplitude or duration of the spikelets evoked by the first suprathreshold current step. These data demonstrate that α1T-encoded calcium channels underlying transient calcium currents are important in regulating firing frequency in PNs.
RNAi-1T knockdown alters spontaneous burst firing in PNs.
1T channels in other systems are important in regulation of spontaneous, pacemaker activity in excitable cells (Catterall 2011). To determine if 1T channels are also involved in regulating spontaneous activity in PNs, d-tubocurarine and picrotoxin were added to the bath to block cholingeric and GABAergic synaptic transmission. In addition whole cell current clamp recordings were made in the absence of applied holding current. There was no significant difference in the mean resting potential between the two genotypes (Table 1).
Table 1.
Analysis of spontaneous activity indicates no significant difference in the resting potential, burst frequency, or burst amplitude in wild-type and RNAi-1T-a knockdown PNs
| Resting Potential, mV | Burst Frequency, Hz | Burst Amplitude | n | |
|---|---|---|---|---|
| Wild type | −59.5 ± 1.47 | 0.22 + 0.1 ± | 12.2 ± 1.7 | 11 |
| RNAi-1T-a | −56.75 ± 1.63 | 0.21 + 0.05 ± | 10.1 ± 0.9 | 12 |
| t-test | P = 0.22 | P = 0.94 | P = 0.31 |
Values are means ± SE. PNs, projection neurons.
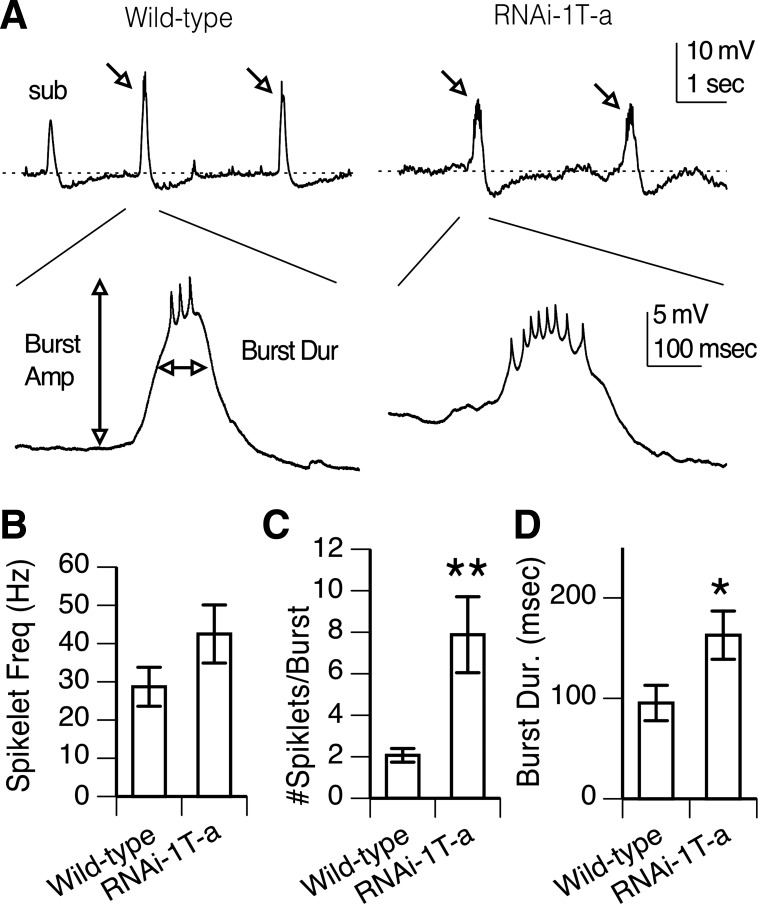
In the presence of synaptic current blockers, PNs in both wild-type and RNAi-1T-a knockdown brains exhibit spontaneous membrane potential depolarizations (Fig. 5A). These vary in frequency, amplitude, and duration during continuous recording in a single cell so evaluation of spontaneous activity was based on assessment during the first 2.5 min of each recording. Bursts were defined as suprathreshold depolarization capped by one or more spikelets (Fig. 5A). Burst properties, including mean frequency, amplitude, duration, and number of spikelets/burst were evaluated for each cell. Subthreshold depolarizations were not analyzed (Fig. 5A).
Fig. 5.
1T knockdown affects spontaneous firing in PNs. A: spontaneous activity in a wild-type and a RNAi-1T-a knockdown PN. Low-frequency, slow-wave depolarizations, classified as bursts, are marked by arrows. Subthreshold depolarizations (sub) were not analyzed. Dashed line indicates the resting membrane potential. One burst from each PN is expanded illustrating slow wave depolarizations capped by rapid spikelets. The amplitude, duration, and number of spikelets were evaluated as indicated for each burst in the first 2.5 min of recording. B: mean spikelet frequency is not significantly different in wild-type and the RNAi-1T-a knockdown. C: however, mean number of spikelets/burst is significantly higher in RNAi-1T-a knockdown vs. wild-type PNs (**P < 0.01, t-test). D. Mean burst duration is significantly longer in RNAi-1T-a knockdown PNs compared with wild-type (*P < 0.05, t-test). Whole cell current clamp recordings done in PTX and curare to block synaptic activity. Bars indicate means ± SE; n = 11 for wild-type and n = 12 for RNAi-1T-a knockdowns.
While the mean spikelet frequency/burst was larger in RNAi-1T-a than wild type, the difference was not significant (Fig. 5B). However, there was a fourfold increase in the number of spikelets/burst in the RNAi-1T-a knockdown compared with wild type (Fig. 5C, P < 0.01, t-test). Therefore, we also examined the burst duration and found this was significantly longer in RNAi-1T-a than wild type (Fig. 5, A and C, P < 0.05, t-test). The increase in number of spikelets/burst is consistent with the increase in evoked firing frequency and suggests the 1T channel is important in regulating action potential frequency in spontaneously firing PNs. The increase in burst duration suggests 1T channels also contribute to the waveform of the low-frequency depolarizations that give rise to spontaneous burst firing characteristic of PNs. In contrast, while the frequency and amplitude of the bursts were quite variable between PNs, there was no significant difference between the two genotypes (Table 1).
DISCUSSION
Projection neurons in the adult Drosophila antennal lobe process olfactory input from olfactory receptor neurons and relay this to neurons in the mushroom body and lateral horn. This well-defined neural pathway and access to these neurons in the whole brain preparation provide an excellent model system to explore how voltage-gated calcium channels regulate signaling in an identified neuronal subtype within the adult olfactory circuit. The results of this study demonstrate that the transient calcium current in the adult Drosophila antennal lobe PNs is mediated by amiloride-sensitive α1T-encoded channels. Furthermore, the α1T channels were found to modulate cell excitability, a novel role in the Drosophila central nervous system.
A recent analysis of adult Drosophila motor neurons reported three distinct calcium current phenotypes in an α1T null mutant: complete loss of all calcium currents (56%), reduction of both sustained and transient currents (28%), and no effect on calcium currents (16%) (Ryglewski et al. 2012). In contrast, when we used a PN-specific Gal4 driver in conjunction with two independently generated UAS-RNAi lines targeted to the α1T gene, this resulted in specific reductions in the transient calcium current. No significant change in the sustained calcium current was observed. Since the two RNAi lines were targeted to distinct regions of the α1T gene, this is compelling evidence that the α1T gene encodes channels underlying the transient current in PNs. The varied effects of an α1T null mutant of calcium currents previously reported in motor neurons may be associated with activation of homeostatic regulatory mechanisms caused by elimination of the channel in all cell types.
Similar to currents mediated by T-type channels in vertebrates, the transient calcium currents in adult PNs are inhibited by amiloride, a vertebrate Cav3 T-type calcium channel blocker (Tang et al. 1988; Hirano et al. 1989). Amiloride has also been reported to block a portion of the calcium current that could be inactivated by depolarization in Drosophila larva body wall fibers and embryo motor neurons (Gielow et al. 1995; Baines and Bate 1998). While the underlying gene encoding the current in muscle and motor neurons was not identified, our data suggest the α1T gene may also encode the channels in these cells. In a recent study it appears that α1T channels also underlie an amiloride-sensitive transient current that represents a relatively small component of the total calcium current in adult motor neurons (Ryglewski et al. 2012). The majority of transient current in the motor neurons, however, appears to be mediated by Cav2-type CAC channels (Ryglewski et al. 2012). This suggests that, similar to finding in the mammalian central nervous system, stage and/or cell-specific splicing events give rise to calcium channels with distinct functional properties (Bell et al. 2004; Lipscombe et al. 2012).
In contrast to T-type currents in vertebrates that typically activate at low voltages, the PN transient calcium currents are first activated at a membrane potential of between −50 and −40 mV. This activation profile is similar to that reported for transient calcium currents in neurons cultured from brains of late stage wild-type pupae (Gu et al. 2009), Drosophila embryonic motor neurons (Byerly and Leung 1988), Drosophila muscle fibers (Gielow et al. 1995), and Drosophila larval motor neurons (Worrell and Levine 2008). In contrast, studies in cytokinesis-arrested neuroblasts (Peng and Wu 2007), embryonic motor neurons (Baines and Bate 1998), and adult motor neurons (Ryglewski et al. 2012) reported both low- and high-voltage-activated calcium currents. The discrepancies in the studies could arise from the differences in cell types and developmental stages. It is also possible that contributing to the differences noted is the use of barium vs. calcium as the charge carrier for studying calcium influx. Replacing calcium with barium substantially increases the current amplitude (Byerly and Leung 1988). Barium is also known to suppress Ca2+-dependent inactivation of calcium channels. In larval motor neurons when barium was used to replace calcium as the charge carrier, this caused a substantial increase in current amplitude and altered the kinetics of calcium current decay (Worrell and Levine 2008). All of the recordings in PNs in the present study were conducted in physiological concentrations of Ca2+, and therefore, calcium-dependent inactivation of the current could contribute to the transient nature of the current.
The increase in evoked firing frequency in PNs we report in the RNAi-1T knockdowns demonstrates that the α1T channels are important in regulating excitability in adult Drosophila neurons. The increased excitability caused by reducing expression of Cav3-type channels in the adult PNs is similar to the upregulation of firing frequency documented in larval motor neurons following genetic reduction of Cav1 or Cav2 type calcium channels (Worrell and Levine 2008). In the wild-type motor neurons, the increase in firing frequency was mimicked by acute removal of calcium from the recording solution suggesting the change in excitability reflects reduced activation of Ca2+-activated K+ channels. However, Peng and Wu (2007) were not able to rule out the possibility that there was compensatory downregulation of other K+ channel genes in the Cav1 and Cav2 mutants that could have the same effect of increasing motor neuron-evoked firing frequency.
To avoid the possible contribution of developmental regulation of other channel types, it would be helpful to explore the role of α1T channels in regulating excitability by acutely blocking these channels in wild-type PNs. Unfortunately Drosophila express nonvoltage-gated sodium channels that are also sensitive to amiloride (Adams et al. 1998; Liu et al. 2003; Zelle et al. 2013), and our preliminary studies indicate these can affect membrane potential depolarizations directly (data not shown). Therefore, additional experiments with double mutant combinations will be necessary to determine if reduced activation of Ca-activated K channels and/or reduced expression of other K channels contribute to the increase in firing frequency seen in adult PNs in α1T knockdowns.
Low-voltage-activated T-type Ca2+ channels in mammals have been shown to be important in pacemaking activity in sino-artrial node of the heart (Mangoni et al. 2006), and they are crucial for generation of rhythmic bursts of action potentials in thalamic relay neurons of the thalamus (Perez-Reyes 2003). In PNs there was no significant change in the burst firing frequency, but the burst duration in α1T knockdowns was significantly increased. This suggests that while T-type calcium channels do have a role in regulating spontaneous burst firing in Drosophila PNs, their relatively high activation voltage may limit their contribution to pacemaking type activities.
Is the third calcium channel subtype encoded by the DMCA1D gene also expressed in adult PNs? Our preliminary studies revealed that calcium currents examined in PNs from hypomorphic α1D mutant AR66 and α1D-knockdowns (VDRC no.51491) had both transient and sustained currents that were not significantly different than wild-type (data not shown). However, alteration in the firing properties of DMCA1D knockdown and mutant indicates that these channels are expressed in PNs. This suggests that the currents mediated by these channels are located in the axonal compartment, electrically distant from the soma. If calcium influx is initiated at some distance from the electrode, this current change will be substantially attenuated when it reaches the soma. Unfortunately, the soma of PNs is the only location in these cells that is accessible to the patch-clamp electrode (Gouwens and Wilson 2009).
In conclusion, our results demonstrate for the first time that α1T-encoded voltage-gated calcium channels are expressed in adult PNs where they are important in regulating excitability. Further studies on how these channels regulate olfactory related behavior will be an important contribution to our understanding of how activity within specific neurons in a well-defined circuit guides behavior.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS27501 and a Howard Hughes Medical Institute Professor Grant (to D. K. O'Dowd); President's Dissertation Year Fellowship, Graduate Division, University of California, Irvine (to J. Iniguez); and America Epilepsy Society and Sunovion Pharmaceuticals Postdoctoral Fellowship (to S. S. Schutte).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.I. and D.K.O. conception and design of research; J.I. performed experiments; J.I., S.S.S., and D.K.O. analyzed data; J.I., S.S.S., and D.K.O. interpreted results of experiments; J.I. and D.K.O. prepared figures; J.I. drafted manuscript; J.I., S.S.S., and D.K.O. edited and revised manuscript; J.I., S.S.S., and D.K.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Smith and Ryan Schutte for helpful discussions.
REFERENCES
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol 140: 143–152, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci 18: 4673–4683, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron 41: 127–138, 2004 [DOI] [PubMed] [Google Scholar]
- Byerly L, Leung HT. Ionic currents of Drosophila neurons in embryonic cultures. J Neurosci 8: 4379–4393, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3: a003947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielow ML, Gu GG, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci 15: 6085–6093, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouwens NW, Wilson RI. Signal propagation in Drosophila central neurons. J Neurosci 29: 6239–6249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Jiang SA, Campusano JM, Iniguez J, Su H, Hoang AA, Lavian M, Sun X, O'Dowd DK. Cav2-type calcium channels encoded by cac regulate AP-independent neurotransmitter release at cholinergic synapses in adult Drosophila brain. J Neurophysiol 101: 42–53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci 26: 265–272, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Whole cell recordings from brain of adult Drosophila. J Vis Exp 6: 248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20: 595–631, 1997 [DOI] [PubMed] [Google Scholar]
- Hirano Y, Fozzard HA, January CT. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol Heart Circ Physiol 256: H1478–H1492, 1989 [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem 5: 52–77, 1998 [PMC free article] [PubMed] [Google Scholar]
- King GF. Modulation of insect Ca(v) channels by peptidic spider toxins. Toxicon 49: 513–530, 2007 [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Andrade A, Allen SE. Alternative splicing: Functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim Biophys Acta 1828: 1522–1529, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26: 35–43, 2000 [DOI] [PubMed] [Google Scholar]
- Liu L, Johnson WA, Welsh MJ. Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc Natl Acad Sci USA 100: 2128–2133, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 98: 1422–1430, 2006 [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316: 440–443, 1985 [DOI] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452: 956–960, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 54: 89–103, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci 27: 1072–1081, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83: 117–161, 2003 [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem 278: 41099–41108, 2003 [DOI] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron 59: 311–321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Lance K, Levine RB, Duch C. Cav2 channels mediate low and high voltage-activated calcium currents in Drosophila motoneurons. J Physiol 590: 809–825, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci 16: 7868–7879, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 275: 3–26, 1994 [DOI] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science 240: 213–215, 1988 [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30: 505–533, 2007 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science 303: 366–370, 2004 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci 29: 163–201, 2006 [DOI] [PubMed] [Google Scholar]
- Worrell JW, Levine RB. Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol 100: 868–878, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle KM, Lu B, Pyfrom SC, Ben-Shahar Y. The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3 (Bethesda) 3: 441–450, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Feng G, Ren D, Eberl DF, Hannan F, Dubald M, Hall LM. Cloning and characterization of a calcium channel alpha 1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci 15: 1132–1143, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]