Abstract
Auditory brain stem circuits rely on fast, precise, and reliable neurotransmission to process auditory information. To determine the fundamental role of myelination in auditory brain stem function, we examined the evoked auditory brain stem response (ABR) from the Long Evans shaker (LES) rat, which lacks myelin due to a genetic deletion of myelin basic protein. In control rats, the ABR evoked by a click consisted of five well-defined waves (denoted waves I–V). In LES rats, waves I, IV, and V were present, but waves II and III were undetectable, indicating disrupted function in the earliest stages of central nervous system auditory processing. In addition, the developmental shortening of the interval between waves I and IV that normally occurs in control rats was arrested and resulted in a significant increase in the central conduction time in LES rats. In brain stem slices, action potential transmission between the calyx of Held terminals and the medial nucleus of the trapezoid body (MNTB) neurons was delayed and less reliable in LES rats, although the resting potential, threshold, input resistance, and length of the axon initial segment of the postsynaptic MNTB neurons were normal. The amplitude of glutamatergic excitatory postsynaptic currents (EPSCs) and the degree of synaptic depression during high-frequency stimulation were not different between LES rats and controls, but LES rats exhibited a marked slow component to the EPSC decay and a much higher rate of presynaptic failures. Together, these results indicate that loss of myelin disrupts brain stem auditory processing, increasing central conduction time and reducing the reliability of neurotransmission.
Keywords: auditory brain stem, MNTB principal neuron, calyx of Held synapse, myelin, auditory neuropathy, ABR
myelin is a critical cellular element that increases axonal conduction velocity. Defects in central myelination are associated with a number of neurodegenerative diseases, including multiple sclerosis (MS) (Utzschneider et al. 1994). In addition to an increase in the central conduction time, demyelination is associated with hearing disorders (Noffsinger et al. 1972; Peyvandi et al. 2010), but it is unclear which aspects of neurotransmission are altered in the demyelinated auditory system.
The auditory system depends on highly precise and rapid neurotransmission to compare submillisecond differences in the timing of binaural signals to compute sound source localization (Carr et al. 2001). For this, auditory pathways in the brain stem rely on very fast, precise, and reliable relay nuclei in the cochlear nucleus and superior olivary complex (Oertel and Young 2004; Trussell 1999). Neurotransmission at these nuclei undergoes a series of morphological and biophysical changes near the onset of hearing. Among these changes, there is massive myelination of auditory axons, which is predicted to increase conduction velocity and thus reduce central conduction time in the processing of auditory information.
It is essential to understand the importance of myelination for auditory processing because the loss of temporal resolution of auditory information is an important cause of hearing disorders (Hill et al. 2004; Moore 2000). Approximately 7% of patients with demyelinating diseases such as MS show hearing loss with normal cochlear function (Lewis et al. 2010; Noffsinger et al. 1972; Peyvandi et al. 2010). However, this number may underestimate the true incidence of hearing impairment, since up to 40% of MS patients with normal audiograms complain of hearing difficulty with even a modest level of background noise (Lewis et al. 2010). In addition, ∼20% of these patients are unable to identify correctly the direction to a sound source (Noffsinger et al. 1972). Although estimates of the prevalence vary, there is a clear consensus that hearing loss due to MS is a significant clinical problem. Typically, MS patients present sensorineural bilateral high-frequency hearing loss, whereas cochlear function is usually not affected (Peyvandi et al. 2010). Such auditory pathology is characteristic and common to auditory neuropathy (or auditory asynchrony), in which afferent conduction is disrupted within the auditory pathway or at synapses in the auditory brain stem, or both (Rance 2005; Starr et al. 1996).
Recent studies have proposed that synaptic alteration mediated by progressive demyelination is the major determinants of neurodegenerative diseases (Centonze et al. 2009). Moreover, evidence suggests that pre- and postsynaptic mechanisms are likely to contribute to axonal damage during neuronal degeneration that accompanies inflammatory disorders such as MS (Cianfoni et al. 2007; Rossi et al. 2009; Srinivasan et al. 2005). This is consistent with studies reporting that glutamate levels are increased in the brains of patients with MS and in animal models of experimental autoimmune encephalomyelitis (Cianfoni et al. 2007; Rossi et al. 2009; Srinivasan et al. 2005). However, inflammation pathology is a complex process mediated by many factors aside from loss of myelin. To isolate the effect of myelin loss from other factors, we studied the effect of dysmyelination on in vivo auditory brain stem responses (ABRs) and auditory signal transmission at the level of the single synapse using the Long Evans shaker (LES) rat, which has a genetically determined loss of myelin basic protein (MBP) in the central nervous system (CNS) and consequently produces little or no myelin in the CNS.
MATERIALS AND METHODS
Animals.
LES rats (from Dr. J. M. Kwiecien, McMaster University, Ontario, Canada) between the ages of postnatal day 15 (P15) and P30 were used in accordance with protocols approved by the University of Texas Health Science Center, San Antonio Institutional Animal Care and Use Committee protocols. Animals were maintained as a heterozygous stock. A fraction of offspring from the heterozygous animals exhibited the LES phenotype and could be easily identified by gross movement disorders (a distinguishable tremor) starting around 2 wk following birth (Delaney et al. 1995; O'Connor et al. 1999). The LES phenotype is autosomal recessive, present only in animals that are homozygous for the MBP mutation, whereas heterozygous and wild-type animals show no symptoms (Kwiecien 2010; Kwiecien et al. 1998; O'Connor et al. 1999; Smith et al. 2013). We observed no difference in MBP staining and the ABRs between heterozygous and wild-type rats (at P23; data not shown), and thus both were used as controls.
Auditory brain stem responses.
Rats were anesthetized with 4% isoflurane and maintained with 2% isoflurane during recording (1 l/min O2 flow rate). The lack of a withdrawal reflex in response to a toe pinch was taken as evidence for adequate anesthesia. ABR recordings were carried out in a sound attenuation chamber (Med Associates, Albans, VT). Body temperature was controlled with a heating pad on the underside of the rat that maintained body temperatures between 36 and 37°C. Recording electrodes were subdermal sterile stainless steel electrodes placed on top of the head (active), ipsilateral mastoid (reference), and contralateral mastoid (ground). The electrical potential differences between the vertex and the mastoid electrodes were amplified and filtered (100–5,000 Hz), and a recording window of 10 ms starting at click sound stimulation onset was distally sampled at 40-μs intervals. Acoustic stimuli were generated using the Auditory Evoked Potentials Workstation [Tucker-Davis Technologies (TDT), Alachua, FL]. Stimulus intensity was calibrated with a condenser microphone. ABR recordings were performed with both click and tone burst stimuli. Closed-field click recordings were taken separately for each ear using a series of square waves (0.1-ms duration) through TDT Multi-Field Magnetic Speakers. Sound stimuli traveled through plastic tubing (Tygon; 3.2-mm outer diameter). Sound intensities ranged from 90 to 20 dB in 5-dB decrements with a repetition rate of stimuli of 16/s, and responses to 512 sweeps were averaged. The lowest sound intensity that produced a reproducible waveform was interpreted as the threshold. Free-field tone pip stimuli ABR recordings (3-ms duration, 0.2-ms rise-fall time, 16 stimuli/s) were taken at frequencies of 4, 8, 12, and 16 kHz at sound intensity levels of 90–20 dB in decrements of 10 dB. TDT Multi-Field Magnetic Speakers were placed 10 cm away from the ear canal of interest.
Slice preparation.
Transverse brain stem slices (200 μm thick) were prepared from LES rat pups at P15–P18. After rapid decapitation, the brain stem was quickly removed from the skull and immersed in ice-cold low-calcium artificial cerebrospinal fluid (aCSF) containing the following (in mM): 125 NaCl, 2.5 KCl, 3 MgCl2, 0.1 CaCl2, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 0.4 ascorbic acid, 3 myo-inositol, and 2 Na-pyruvate, pH 7.3–7.4 when bubbled with carbogen (95% O2-5% CO2; osmolarity 310–320 mosM). After being cut, the brain stem slices were transferred to an incubation chamber containing normal aCSF bubbled with carbogen and maintained at 35°C for 30–45 min and thereafter at room temperature. The normal aCSF was the same as the slicing aCSF, but with 1 mM MgCl2 and 2 mM CaCl2.
Electrophysiology
Whole cell patch-clamp recordings were performed in normal aCSF at room temperature (22–24°C) using an EPC-10 amplifier controlled by Pulse software (HEKA Elektronik, Lambrecht/Pfalz, Germany). For voltage-clamp recordings of excitatory postsynaptic currents (EPSCs), the pipette solution contained (in mM) 110 CsCl, 20 tetraethylammonium (TEA)-Cl, 5 Na2-phosphocreatine, 10 HEPES, 4 Mg-ATP, 10 mM EGTA, and 0.3 GTP, pH adjusted to 7.3 with CsOH. For current-clamp recordings of action potential (AP), the pipette solution contained (in mM) 130 K-gluconate, 20 KCl, 5 Na2-phosphocreatine, 10 HEPES, 4 Mg-ATP, 5 mM EGTA, and 0.3 GTP, pH adjusted to 7.3 with KOH. This pipette solution is expected to generate liquid junction potential of 11 mV; membrane potentials were not corrected for this constant liquid junction potential. Patch electrodes had resistances of 2.5–3 MΩ. For voltage-clamp experiments, series resistance (Rs) was <10 MΩ and compensated 80%. Current-clamp recordings were continued only if the initial uncompensated Rs was <20 MΩ. AP and EPSC recordings were performed in comparable age groups of control and LES rats in the range of P16–P20. The presynaptic axons of the calyx of Held terminal were stimulated with a bipolar platinum-iridium electrode (Frederick Haer, Bowdoinham, ME) placed near the midline spanning the afferent fiber tract of the medial nucleus of the trapezoid body (MNTB). An Iso-Flex stimulator driven by a Master 10 pulse generator (A.M.P.I., Jerusalem, Israel) delivered 100-μs pulses at 1.2 times threshold (<15 V constant voltage). Signals were filtered at 2.9 kHz and acquired at a sampling rate of 10–50 μs. Data were analyzed off-line and presented using Igor Pro (Wavemetrics, Lake Oswego, OR). Differences were considered statistically significant when P values were <0.05 by the Student's t-test. Data are means ± SE.
Immunohistochemistry.
Brain stem slices (100 μm thick) were prepared as for electrophysiology but subsequently fixed with 4% (wt/vol) paraformaldehyde in phosphate buffer solution (PBS) for 30 min. Free-floating sections were blocked in 4% goat serum and 0.3% Triton X-100 in PBS for 1 h. For double immunohistochemistry, slices were incubated with mouse monoclonal anti-ankyrin G (1:100; Santa Cruz) and guinea pig polyclonal anti-vesicular glutamate transporter 1 (1:3,000; Chemicon) overnight at 4°C. Antibody labeling was reported by incubation with different Alexa dye-conjugated secondary antibodies (1:500; Invitrogen) for 2 h at room temperature. Slices were mounted onto Superfrost slides in photobleaching-protective medium (Vectashield; Vector Laboratories, Burlingame CA). Stained slices were viewed with laser lines at 488 (for green) and 633 nm (for red) using a ×40 oil-immersion objective on a confocal laser scanning microscope (LSM-510; Zeiss).
RESULTS
Delayed and distorted ABR waveforms in dysmyelinated LES rats.
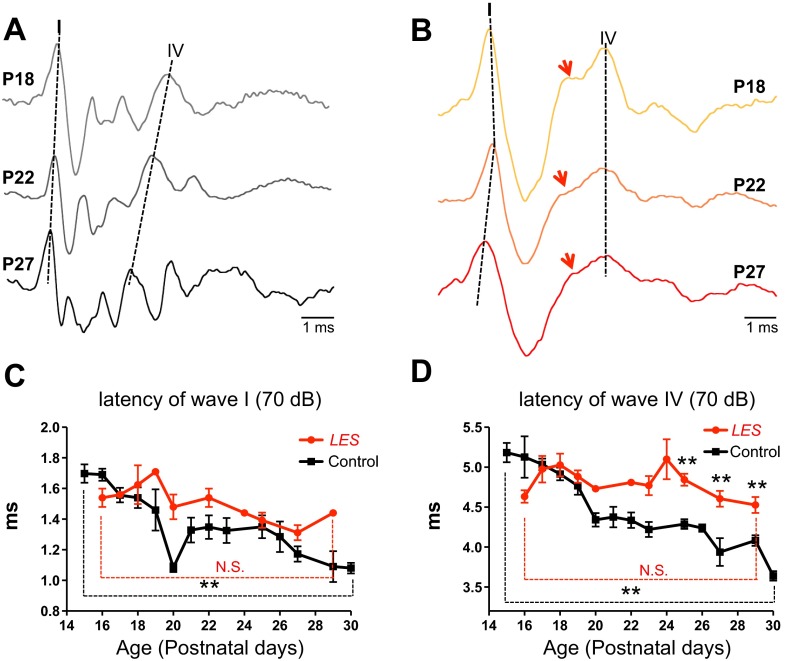
We examined the ABR in LES rats (P15–P30) in response to click stimulation to localize hearing dysfunction within early auditory processing pathways. There was no significant difference in the ABR threshold to a click stimulus between control and LES rats (36 ± 3.5 dB in control and 37 ± 4.9 dB in LES rats, n = 19 and 11, respectively, P > 0.05). In both control and LES rats, ABR waveforms peaks increased in latency and decreased in amplitude with decreasing sound intensity from 90 to 40 dB (Fig. 1, A and B). In control rats, the ABR waveform consisting of five distinct peaks (herein referred to as waves I–V) during the 6 ms following a click stimulus was similar to that described by others (Roncagliolo et al. 2000). In contrast, the ABR from LES rats showed distorted waveforms. Waves I, IV, and V were clearly present, but waves II and III were undetectable in LES rats even at the highest stimulation intensity tested (90 dB), although wave IV exhibited an inflection on its rising phase that may represent fusion with a delayed wave III (Fig. 1B). In addition to the loss of waves II and III, the latencies of waves I and IV were markedly increased in LES rats (1.23 ± 0.05 ms in LES vs. 0.82 ± 0.02 ms in control at 90 dB and 1.6 ± 0.03 ms in LES vs. 1.35 ± 0.02 ms in control at 70 dB for wave I, and 4.52 ± 0.07 ms in LES vs. 3.89 ± 0.09 ms in control at 90 dB and 4.89 ± 0.07 ms in LES vs. 4.48 ± 0.07 ms in control at 70 dB for wave IV, respectively, P < 0.01; Fig. 1C). Loss of waves II and III and increased latencies for waves I and IV strongly suggest that dysmyelination in the LES rats causes major defects in signal transmission in the auditory brain stem.
Fig. 1.
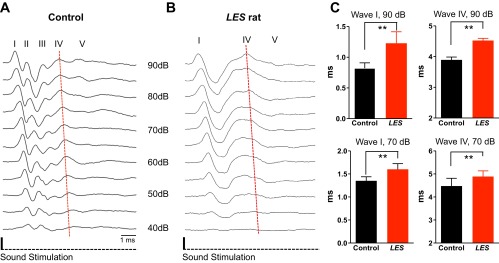
Auditory brain stem responses (ABR) from Long Evans shaker (LES) rats showed loss of central waves and significantly increased latency. A and B: representative ABR recordings using click stimuli in control (A) and LES rats (B) at postnatal day 20 (P20). Roman numerals identify waves I–V in control and waves I and IV in the LES rat. Red dashed lines indicate the peak of wave IV along different sound intensities. C: summary of the latencies of waves I and IV (the time from initiation of sound stimulation to peak of wave) at 90 and 70 dB in control and LES rats (**P < 0.01). Values are means ± SE. Latencies were measured the time from the initial to the peak of waves.
Increased ABR threshold for high-frequency tone stimulation in LES rats.
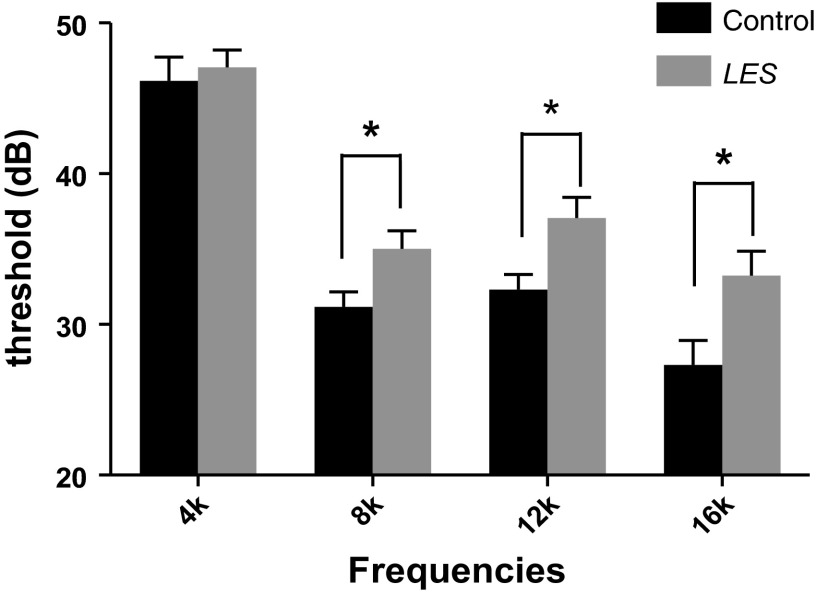
We next examined the frequency dependence of ABR threshold using tone stimulus at a range of frequencies (4, 8, 12, and 16 kHz) with varying sound intensities (from 90 to 20 dB). For low sound frequency stimulation (4 kHz), ABR threshold was similar in control and LES rats (46 ± 1.5 dB in control vs. 47 ± 1.1 dB in LES). At higher frequencies, threshold decreased in both animals but was significantly higher in LES rats at 8 kHz (31 ± 0.9 dB in control vs. 35 ± 1.2 dB in LES, P < 0.05), 12k Hz (32 ± 1.0 dB in control vs. 37 ± 1.3 dB in LES, P < 0.05), and 16k Hz (27 ± 1.5 dB in control vs. 33 ± 1.5 dB in LES, n = 28 and 36, respectively, P < 0.05; Fig. 2). We also recorded ABRs at frequencies of 32 and 64 kHz, but at these higher frequencies ABR threshold was greatly increased to 70–90 dB and was not different between control and LES rats (data not shown). These results indicate that LES rats have a frequency-dependent hearing impairment and thus could be a useful model to study MS-associated hearing disorders or auditory neuropathy in which the cochlear amplification function of outer hair cells is intact but afferent conduction is disrupted in particular at high-frequency sound transmission (Peyvandi et al. 2010; Rance 2005; Starr et al. 1996).
Fig. 2.
Higher ABR threshold in LES rats in response to high-frequency sound stimulation in LES rats. ABR thresholds at frequencies of 4, 8, 12, and 16 kHz were recorded using tone stimuli in control and LES rats (at P18 to P30). Thresholds at 8-, 12-, and 16-kHz stimuli in LES rats are significantly higher than in control (*P < 0.05). Values are means ± SE.
Myelination is required for the developmental decrease in the central conduction time of the auditory pathway.
Brain stem auditory pathways undergo a series of changes following the onset of hearing that reduce the central conduction time (Rusu and Borst 2011; Taschenberger and von Gersdorff 2000). These changes include massive myelination as well as changes in ion channels and synaptic structure that shorten the AP and reduce synaptic delays (Crins et al. 2011; Taschenberger and von Gersdorff 2000). To determine the functional importance of postnatal myelination in brain stem auditory circuits, we examined the developmental profile of the ABR during the first 2 wk after hearing onset, from P15 to P30. During this period, the differences in the ABR waveform between control and LES rats became progressively more pronounced (Fig. 3, A and B). Waves II and III remained undetectable in LES rats up to P30. In controls, the latencies to waves I and IV dramatically decreased during postnatal development from P15 to P30 (from 1.65 ± 0.125 ms at P15 to 1.03 ± 0.04 ms at P30 for wave I, and from 5.18 ± 0.121 ms at P15 to 3.64 ± 0.06 ms at P30 for wave IV, n = 4, P < 0.01; Fig. 3, A, C, and D). This developmental decrease in latency was greatly attenuated in LES rats; the latency to wave I decreased from slightly 1.54 ± 0.06 ms (P16) to 1.31 ± 0.05 ms (P27), whereas the latency of wave IV remained constant (4.6 ± 0.78 ms at P16 and 4.6 ± 0.09 ms at P27, n = 4, P > 0.05; Fig. 3, B–D). Thus latencies to wave IV were significantly longer in the LES than in control rats at P25–P29 (Fig. 3D). Because of the developmental decrease in latencies, the central conduction time (CCT), defined as the time between wave I and IV peaks, decreased during development in control rats. In contrast, the CCT did not decrease in LES rats. At P29, CCT was 2.78 ± 0.09 ms (n = 4) in control and 3.15 ± 0.08 ms in LES rats (n = 4, P < 0.01). These results indicate that myelination is the major determinant for the developmental decrease in central conduction time that occurs in the first 2 wk after hearing onset.
Fig. 3.
Arrested development of the ABR waveform in LES rats. A and B: ABR click recordings at 70 dB from control and LES rats at P18, P22, and P27. Controls show a clear reduction in the latencies of waves I and IV during postnatal development (A). LES rats show a slight developmental reduction in the latency of wave I, with no significant change in the latency of wave IV (B). Note the absence of wave II and III in LES rats. Arrows indicate the inflection on the rising phase of wave IV. C and D: summary of postnatal changes in latencies of waves I and IV in control (black) and LES rats (red; **P < 0.01). Dashed lines indicate the peaks of waves I and IV along postnatal ages. N.S. indicates statistical nonsignificance (P > 0.05).
Broader AP waveforms and neurotransmission failures in the MNTB of LES rats.
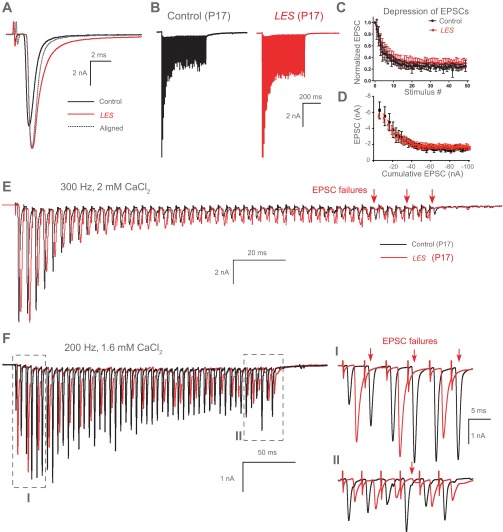
The ABR from LES rats indicated a loss of wave III and a significant increase in the latency to wave IV, which arises from activity in the superior olivary complex (SOC) and nucleus of the lateral lemniscus (Hall 2007). The SOC is a group of nuclei responsible for sound localization requiring high temporal fidelity of impulse propagation (Carr et al. 2001). We thus examined neurotransmission in vitro using brain stem slices containing the SOC. We recorded the AP from MNTB neurons located in the SOC during afferent fiber simulation of the presynaptic axons that lead to the calyx of Held nerve terminal. We placed the bipolar afferent fiber stimulator on the midline of brain stem slices, ∼300–400 μm from the MNTB. In both control and LES rats at P16–P18, low-frequency synaptic stimulation (0.2 Hz) led to the generation of a fast, overshooting AP in postsynaptic neurons (Fig. 4A). There was no significant difference in the resting potential (−76 ± 3.1 mV in control and −75 ± 1.5 mV in LES, P > 0.05) or AP amplitude (109 ± 2.9 mV in control and 100 ± 3.4 mV in LES). However, AP half-width was larger in the LES rats at comparable ages (582 ± 20.8 and 457 ± 14.5 μs, n = 10 and 3, respectively, P < 0.01) and had a larger slow afterdepolarization (ADP; Fig. 4A). A second difference in transmission at low frequency in LES rats was a nearly twofold longer AP latency, measured as the time between presynaptic stimulation and the peak of postsynaptic action potential (LES: 1.75 ± 0.22, n = 7; control: 0.89 ± 0.07 ms, n = 3, P < 0.05; Fig. 4A).
Fig. 4.
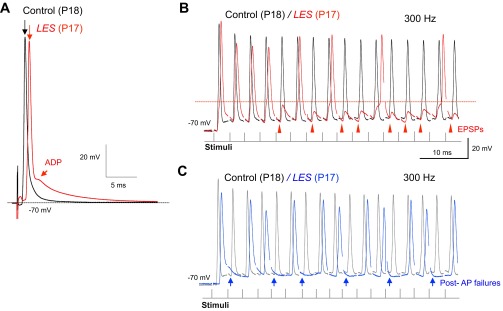
Altered action potential (AP) waveform and pre- and postsynaptic AP failures in the medial nucleus of the trapezoid body (MNTB) from LES rats. A: postsynaptic APs evoked by afferent fiber stimulation in the MNTB neurons from control and LES rats (at P18 and P17, respectively). Top arrows indicate the peak of APs showing that AP from LES rat is strongly delayed. Bottom arrow indicates the afterdepolarization (ADP) in neurons from the MNTB of LES rat. B: AP trains recorded in response to repetitive stimulation at 300 Hz from control (black trace) and LES (red trace) rats. Recordings from LES rat showed postsynaptic AP failures caused by excitatory postsynaptic potentials (EPSPs), which did not reach threshold, indicated by the red horizontal line. C: LES rats showed frequent presynaptic AP and EPSP failures at 300 Hz (blue arrows indicate presynaptic AP and EPSP failures).
During acoustic stimulation in vivo, the relay synapse formed by the calyx of Held and the MNTB neuron transmits hundreds of impulses per second with only occasional failures (Hermann et al. 2007; Kadner et al. 2006; Kopp-Scheinpflug et al. 2003; Lorteije et al. 2009; Smith et al. 1998; Sommer et al. 1993). To test the role of myelin on the performance of the MNTB under high-frequency stimulation, we stimulated the presynaptic axons at 100–300 Hz. The MNTB neurons from P16–P18 control rats followed trains of up to 300 Hz with no failures (n = 3). In contrast, postsynaptic recordings in the MNTB from LES rats at comparable ages exhibited frequent AP failures (n = 7). We observed two types of failures: postsynaptic AP failures [i.e., failure of the excitatory postsynaptic potential (EPSP) to generate an AP; Fig. 4B] and presynaptic AP failures (i.e., failure to generate an EPSP, presumably due to failure of the presynaptic AP; Fig. 4C).
Slower and less reliable synaptic transmission in the MNTB of LES rats.
The failure of the EPSP to generate a postsynaptic AP in MNTB neurons from LES rats during high-frequency stimulation could be due to altered release of glutamate or alterations in postsynaptic excitability. To test the first possibility, we measured evoked EPSCs from postsynaptic neurons in the MNTB in control and LES rats at P17–P20 under voltage clamp. The amplitude and 10–90% rise time of the EPSCs were not different in control and LES rats (amplitude: 4.8 ± 0.89 and 5.0 ± 0.85 nA, respectively, n = 5; rise time: 0.28 ± 0.01 and 0.35 ± 0.04 ms, n = 7 and 5, respectively, P > 0.05). The EPSC decay from control rats could be well fit as a single exponential with a time constant tau (τ) = 0.52 ± 0.03 ms (n = 5). In contrast, the EPSC decay from LES rats had two kinetic components. The fast component (τ = 0.66 ± 0.08 ms, n = 5, P > 0.05 compared with control) accounted for 95 ± 1.2% of the EPSC amplitude. The slow phase of EPSC decay had a time constant of tau (τ) = 7.43 ± 0.90 ms. Finally, the latency from stimulation to the EPSC in MNTB from LES rats was strongly increased compared with control (0.89 ± 0.05 ms for control and 1.27 ± 0.14 ms for LES, n = 4, P < 0.05; Fig. 5A).
Fig. 5.
Delayed onset and failures of excitatory postsynaptic currents (EPSCs) in dysmyelinated synapses. A: representative traces of single EPSCs recorded during afferent fiber stimulation in the MNTB from control (black) and LES (red) rats. Dotted line indicates EPSC of control aligned to that of LES synapse to show the decay kinetics of EPSCs from control and LES synapses. B: representative traces of EPSCs produced by a train of stimuli (100 Hz, 500 ms) from control (P17, black) and LES (P17, red). C: normalized amplitude of EPSCs is summarized for control and LES synapses (100 Hz). D: plot of EPSCs against cumulative EPSCs in control (black) and LES (red). E: EPSC train at 300 Hz in control and LES synapses in normal external solution (2 mM CaCl2). A number of EPSC failures are present in the late end of the train (arrows). F: EPSC train at 200 Hz with external recording solution containing 1.6 mM CaCl2 to reduce the depression of EPSCs. A longer delayed onset and failures of EPSCs are shown in the LES synapse. Inset: expanded time scale of boxed areas of the trace (top, initial EPSCs in train; bottom, later EPSCs in train). Note the increased delay of EPSC onset and failures of EPSCs (red arrows).
Next, we tested how dysmyelination affects high-frequency synaptic transmission in the auditory brain stem. During a train of stimuli at 100 Hz, the amplitude of EPSCs displayed strong depression, falling to about 20% of the initial amplitude near the end of the train in both control and LES rats (Fig. 5B). There was no significant difference in the degree of depression between control and LES rats (n = 4; Fig. 5C). To determine the size of the neurotransmitter pool available for evoked release, we plotted the EPSC amplitudes during a train versus their cumulative amplitude (Fig. 5D). By adjusting a line to the linear portion of these data (second to fourth EPSC) and extrapolating to the x-axis, we measured the total equivalent EPSC available for release at the beginning of the train (Elmqvist and Quastel 1965; Kushmerick et al. 2006). This analysis revealed no significant difference for cumulative EPSC pool size (control: 33 ± 3.7 nA; LES: 41 ± 6.6 nA on cumulative EPSC, n = 4, P = 0.3458). The results suggest that the pool of neurotransmitter available for release at the beginning of a train is similar in control and LES rats.
We next used higher stimulation frequencies and observed that whereas MNTB neurons from control rats could routinely follow 200- to 300-Hz stimulation, neurons from LES rat showed synaptic transmission failures at these higher stimulation frequencies. All recordings of EPSCs trains from LES slices showed EPSCs failures at the end of the train at 300 Hz (n = 7), whereas recordings from control rats did not show any EPSC failures (n = 4; Fig. 5E). To examine whether strong depression of EPSCs leads to failure at the end of the train in LES recordings, we slightly decreased extracellular Ca2+ concentration from 2 to 1.6 mM. This decrease in extracellular Ca2+ concentration reduced the amplitude and the degree of depression of EPSCs in control and LES rats, but the LES rats still showed failures of EPSCs at 200 Hz (Fig. 5F).
Central dysmyelination did not affect the intrinsic firing properties of the MNTB neurons in auditory brain stem.
We next examined whether dysmyelination impacts the intrinsic firing properties of the MNTB neuron recorded under current clamp. Resting membrane potentials were −77.3 ± 1.12 and −75.6 ± 1.49 mV in control and LES neurons at P16–P18 (n = 12 and 8, respectively, P = 0.36). Current injections with durations ranging from 2 to 50 ms and amplitudes from −100 to +500 pA evoked passive membrane responses and postsynaptic APs (Fig. 6, A–C). AP thresholds were similar in control and LES neurons (−48 ± 2.mV and −47 ± 2.2 mV, n = 12 and 8, respectively). Short depolarizing current injection (500 pA, 2 ms) evoked a single AP with amplitude of 109 ± 2.3 mV and half-width of 0.66 ± 0.03 ms in control (n = 12) and amplitude of 111 ± 3.1 mV and half-width of 0.66 ± 0.05 ms in LES rats (n = 8). Thus, unlike the case for synaptic activation, there was no significant difference in the AP waveforms of the MNTB neurons evoked by current injection in control and LES rats (Fig. 6, D and E). To study the firing pattern of the MNTB neurons, we stimulated the neurons with long depolarizing current steps (50–100 ms). In most recordings, MNTB neurons from both control and LES rats fired only a single AP in response to long suprathreshold current injections (500 pA, 50–100 ms; Fig. 6, B and C). The input resistance of MNTB neurons, obtained by hyperpolarizing current injections (−50 to −100 pA), exhibited a trend toward lower values in LES rats, but this difference was not statistically significant (control: 195 ± 15 MΩ, n = 10; LES: 164 ± 18.7 MΩ, n = 6, P = 0.21; Fig. 6H). These results indicate that axonal dysmyelination had little effect on the intrinsic excitability of MNTB neurons.
Fig. 6.
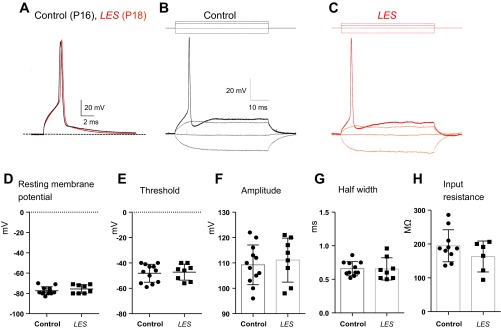
Dysmyelination did not change the intrinsic firing properties of the MNTB neuron. A: single AP evoked by a single step-current injection (500 pA, 2 ms) from postsynaptic principal neurons in control (P16, black trace) and LES rats (P18, red trace). B and C: representative traces of APs evoked by depolarizing currents (−50 to 200 pA) in control (black trace; B) and LES rats (red trace; C). D–H: summaries of resting membrane potential (D), threshold of AP (E), amplitude of AP (F), half-width of AP (G), and input resistance (H).
Structure of initial segments in dysmyelinated MNTB neurons.
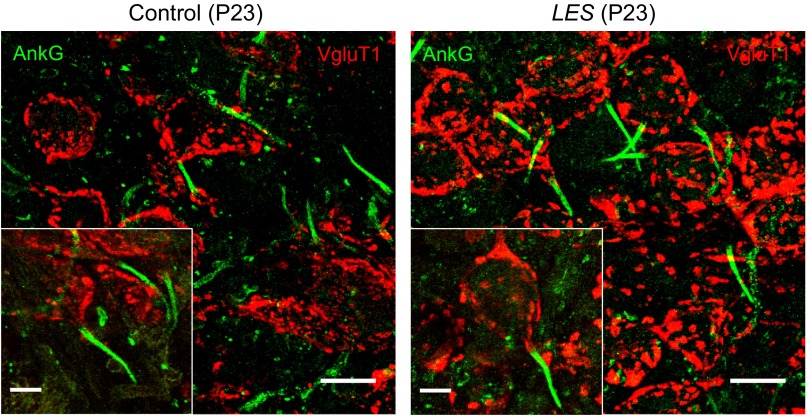
We compared the structure of axon initial segments of the MNTB neurons in control and LES rats using immunostaining. In control and LES rat brain stem slices (at P23), we detected the presence of ankyrin-G, which is markedly expressed at the axon initial segments in both control and LES rats (Fig. 7). The pattern of ankyrin-G expression we observed is similar to that reported for Na+ channel expression in the MNTB (Leão et al. 2005), as expected because ankyrin-G anchors Na+ channels to the initial segment (Bennett and Lambert 1999). The length and thickness of ankyrin-G signal were similar in control and LES, indicating that myelin loss did not affect the structure of axon initial segments of the MNTB. Thus immunostaining suggests that dysmyelination does not critically affect the structure of the axon initial segments in the auditory nervous system.
Fig. 7.
Expression of ankyrin-G (AnkG) in the LES rat brain stem. Fixed slices of auditory brain stem containing the MNTB were stained for AnkG and vesicular glutamate transporter 1 (VgluT1). In expanded images (insets, bottom left), AnkG was located at axon initial segments of principal neurons in the MNTB (green) in control and LES auditory brain stems. VgluT1 is stained for the calyx of Held terminals in the MNTB (red). Both control and LES rats were at P23. Scale bar = 20 and 10 μm for main images and insets, respectively.
DISCUSSION
To determine the cellular mechanisms of hearing disorders associated with central myelin loss, we studied the consequence of central dysmyelination using in vivo ABR recordings and in vitro single-cell recordings from the MNTB neuron in LES rats that lack functional MBP. The waves in the ABRs reflect synchronized firing at different stages of early auditory processing. We observed a loss of ABR waves and delayed and unreliable neurotransmission in the MNTB. These results indicate that central myelination is one of key components to achieve synchrony of the synaptic potentials and/or tract discharges in the auditory pathway.
In vivo ABRs in the dysmyelinated auditory brain stem.
During the first 2 wk after the onset of hearing, the ABR waveforms in control rats rapidly decreased in latency, reflecting an increase in conduction velocity along the auditory tracts and an increase in amplitude, similar to that described for another rodent model (Roncagliolo et al. 2000). In contrast, LES rats showed lower amplitude and longer latency of the ABR waves, and the developmental decrease in latencies was dramatically arrested in LES rats (Fig. 3B).
Wave I is due to the activities of the cochlea, spiral ganglion, and peripheral auditory nerve, and waves II and III are due to the activity of the cochlea nucleus and the SOC in the central auditory nerve (Hall 2007). We observed an increase in the latency even for the first wave of the ABR. A similar observation was made for the ABR recorded from the myelin-deficient (md) rat (Ito et al. 2004). These results suggest that central demyelination affects the timing of signal transmission even along the peripheral auditory pathway. One of the most striking differences in the ABR from LES rats was the loss of waves II and III, which could not be clearly distinguished at any age of the animal up to P30 or stimulation intensity up to 90 dB (Figs. 1 and 3). A similar defect was observed in the taiep rat, which is a neurological mutant characterized by progressive dysmyelination of the CNS and loss of waves II and III by P60 (Roncagliolo et al. 2000). However, the LES rats did not show detectable wave II and III in ABRs from the end of the second week of life (P14), just after hearing onset. Previous study suggests that dys- or demyelination induce the loss of synchrony of APs along the auditory fibers (Roncagliolo et al. 2006) and lead to the widening and merging of waves I and II and waves III and IV (Roncagliolo et al. 2000). Alternatively, loss of waves II and III could be due to altered synaptic activity in the cochlear nucleus, where these waves are generated (Shaw 1988). Latencies of all waves were significantly longer in LES than control rats (Figs. 1 and 3). In addition, during the post-hearing development, the latencies of waves I and IV and central conduction time decreased in control rats. This developmental decrease in central conduction time of the auditory pathway appears arrested in LES rats, because the time from peak I to IV progressively increased from P15 to P30 (Fig. 3).
Altered neurotransmission in the MNTB.
What is the underlying cause of the alterations in the ABR including the deterioration of waves II and III and a significantly increased latency of wave IV? We investigated whether these alterations were due to changes in intrinsic excitability of neurons in auditory tracts or changes in neurotransmission at SOC synaptic relays (Shaw 1988).
Passive and active membrane properties such as resting potential, input resistance, and AP threshold were quite similar for MNTB neurons of control and LES rats. In addition, APs generated by somatic current injections did not differ in terms of maximum overshoot or half-width. Thus lack of myelin does not measurably affect the intrinsic properties of the MNTB neuron.
A second possible cause for postsynaptic failures during high-frequency stimulation is depression of neurotransmitter release. EPSC amplitudes were similar in control and LES rats, as was the degree of depression of EPSC amplitudes during high-frequency stimulation. Using the initial phase of EPSC depression curves, we estimated the total pool of neurotransmitter available and again observed no significant difference. Thus reduced glutamate release does not cause postsynaptic failures in the LES MNTB.
In contrast to AP generation by current injection, during synaptic activation APs from LES rats were broader than those from controls and presented a pronounced ADP (Fig. 4A). Consistent with this finding, EPSCs from LES rats exhibited a slow component of decay not observed in controls. Although the amplitude of the slow component is only about 5% of the total EPSC, due to its long time constant it makes a significant contribution to the total EPSC charge integral and may generate the prominent ADP observed in the AP from LES rats (Johnston et al. 2009), unlikely the presynaptic ADP in the calyx of Held terminal, which is generated by a Na+ conductance (Kim et al. 2010). During high-frequency stimulation, the broader AP and ADP may interfere with the removal of Na+ channel inactivation and may activate low-threshold K+ channels, both of which would extend the refractory period of the MNTB neuron thus leading to postsynaptic failures during high-frequency stimulation.
We found a close association between the delayed ABR waves and delayed synaptic transmission in the auditory brain stem (Figs. 4 and 5). In vivo recordings from LES rats showed a higher threshold for ABR generation at high-frequency sound stimulations (8–16 kHz). In vitro whole cell recording from the MNTB neurons showed a number of failures of EPSCs and APs in high-frequency train in LES rats (Figs. 4 and 5). This result indicates that central dysmyelination leads to the failure of synaptic transmission and deteriorates sound transmission at high frequencies. One caveat in a direct comparison of the in vivo ABR data and the in vitro brain slice electrophysiology experiments is that the latter were performed at room temperature, and temperature had a strong influence on synaptic transmission at the MNTB (Kim and von Gersdorff 2012; Kushmerick et al. 2006; Renden and von Gersdorff 2007). The effects of temperature on quantal size, synaptic vesicle recycling, and resting potential are unlikely to affect our conclusions because temperature will presumably affect both normal and LES neurons. In a previous study, raising temperature from 24 to 37°C increased the ability of both control and LES MNTB neurons to follow high-frequency stimulation but did not eliminate the differences between the two genotypes in high-frequency firing (Kim et al. 2013).
Clinical implications.
A typical hearing loss symptom reported in ∼90% of patients with MS is sensorineural bilateral high-frequency hearing loss, whereas cochlear function is usually not affected (Peyvandi et al. 2010). Such auditory pathology is a characteristic common to auditory neuropathy (or auditory asynchrony), in which the cochlear amplification function of outer hair cells is normal but afferent conduction is disrupted within the auditory pathway itself, at synapses in the auditory brain stem, or both (Rance 2005; Starr et al. 1996). This abnormality in timing and synchronicity of firing of neurons in auditory pathways can cause problems in understanding speech in auditory neuropathy patients (Zeng et al. 2005). Recent studies suggest that auditory neuropathy is closely related to disrupted neuronal synchrony resulting perhaps from impaired hair cell ribbon synapses, or myelin damage, or a reduction of functioning fibers (Oertel 2005; Rance 2005). Results from this study indicate that central dysmyelination decreases the temporal fidelity of action potential propagation and synaptic transmission and thus impairs the transmission and processing of information in auditory brain stem pathways.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grant R03 DC011140 (to J. H. Kim) and American Heart Association Grant 11BGIA7430033 (to J. H. Kim). CK is supported by the Brazilian funding agencies CNPq, FAPEMIG, and CAPES.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.K., K.T., and J.H.K. performed experiments; S.E.K. and J.H.K. analyzed data; S.E.K. and J.H.K. interpreted results of experiments; S.E.K. and J.H.K. prepared figures; S.E.K., C.K., and J.H.K. edited and revised manuscript; S.E.K., K.T., C.K., and J.H.K. approved final version of manuscript; C.K. and J.H.K. drafted manuscript; J.H.K. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Jacek M. Kwiecien for providing the LES rat breeding pairs and for tips on how to care for the rats and breed them, and we also appreciate Jose C. Granados for technical assistance.
REFERENCES
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol 28: 303–318, 1999 [DOI] [PubMed] [Google Scholar]
- Carr CE, Soares D, Parameshwaran S, Perney T. Evolution and development of time coding systems. Curr Opin Neurobiol 11: 727–733, 2001 [DOI] [PubMed] [Google Scholar]
- Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D'Amelio M, Cavallucci V, Martorana A, Bergamaschi A, Cencioni MT, Diamantini A, Butti E, Comi G, Bernardi G, Cecconi F, Battistini L, Furlan R, Martino G. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29: 3442–3452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfoni A, Niku S, Imbesi SG. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. Am J Neuroradiol 28: 272–277, 2007 [PMC free article] [PubMed] [Google Scholar]
- Crins TT, Rusu SI, Rodríguez-Contreras A, Borst JG. Developmental changes in short-term plasticity at the rat calyx of Held synapse. J Neurosci 31: 11706–11717, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KH, Kwiecien JM, Wegiel J, Wisniewski HM, Percy DH, Fletch AL. Familial dysmyelination in a Long Evans rat mutant. Lab Anim Sci 45: 547–553, 1995 [PubMed] [Google Scholar]
- Elmqvist D, Quastel DM. A quantitative study of end-plate potentials in isolated human muscle. J Physiol 178: 505–529, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW. New Handbook of Auditory Evoked Responses. Boston, MA: Pearson, 2007 [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol 98: 807–820, 2007 [DOI] [PubMed] [Google Scholar]
- Hill PR, Hartley DE, Glasberg BR, Moore BC, Moore DR. Auditory processing efficiency and temporal resolution in children and adults. J Speech Lang Hear Res 47: 1022–1029, 2004 [DOI] [PubMed] [Google Scholar]
- Ito T, Tokuriki M, Shibamori Y, Saito T, Nojyo Y. Cochlear nerve demyelination causes prolongation of wave I latency in ABR of the myelin deficient (md) rat. Hear Res 191: 119–124, 2004 [DOI] [PubMed] [Google Scholar]
- Johnston J, Postlethwaite M, Forsythe ID. The impact of synaptic conductance on action potential waveform: evoking realistic action potentials with a simulated synaptic conductance. J Neurosci Methods 183: 158–164, 2009 [DOI] [PubMed] [Google Scholar]
- Kadner A, Kulesza RJ, Jr, Berrebi AS. Neurons in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat may play a role in sound duration coding. J Neurophysiol 95: 1499–1508, 2006 [DOI] [PubMed] [Google Scholar]
- Kim JH, Kushmerick C, von Gersdorff H. Presynaptic resurgent Na+ currents sculpt the action potential waveform and increase firing reliability at a CNS nerve terminal. J Neurosci 30: 15479–15490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, von Gersdorff H. Suppression of spikes during posttetanic hyperpolarization in auditory neurons: the role of temperature, Ih currents, and the Na+-K+-ATPase pump. J Neurophysiol 108: 1924–1932, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Renden R, von Gersdorff H. Dysmyelination of auditory afferent axons increases the jitter of action potential timing during high-frequency firing. J Neurosci 33: 9402–9407, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signaling in KCNA1-null mice: an electrophysiological study in vivo. J Neurosci 23: 9199–9207, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Gersdorff H. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci 26: 1366–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien JM, O'Connor LT, Goetz BD, Delaney KH, Fletch AL, Duncan ID. Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat. J Neurocytol 27: 581–591, 1998 [DOI] [PubMed] [Google Scholar]
- Kwiecien JM. Cellular compensatory mechanisms in the CNS of dysmyelinated rats. Comp Med 60: 205–217, 2010 [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci 25: 3724–3738, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MS, Lilly DJ, Hutter MM, Bourdette DN, McMillan GP, Fitzpatrick MA, Fausti SA. Audiometric hearing status of individuals with and without multiple sclerosis. J Rehabil Res Dev 47: 669–678, 2010 [DOI] [PubMed] [Google Scholar]
- Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci 29: 13770–13784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR. Auditory neuroscience: is speech special? Curr Biol 10: R362–R364, 2000 [DOI] [PubMed] [Google Scholar]
- Noffsinger D, Olsen WO, Carhart R, Hart CW, Sahgal V. Auditory and vestibular aberrations in multiple sclerosis. Acta Otolaryngol Suppl (Stockh) 303: 1–63, 1972 [PubMed] [Google Scholar]
- O'Connor LT, Goetz BD, Kwiecien JM, Delaney KH, Fletch AL, Duncan ID. Insertion of a retrotransposon in Mbp disrupts mRNA splicing and myelination in a new mutant rat. J Neurosci 19: 3404–3413, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci 27: 104–110, 2004 [DOI] [PubMed] [Google Scholar]
- Oertel D. Importance of timing for understanding speech Focus on “perceptual consequences of disrupted auditory nerve activity”. J Neurophysiol 93: 3044–3045, 2005 [DOI] [PubMed] [Google Scholar]
- Peyvandi A, Naghibzadeh B, Ahmady Roozbahany N. Neuro-otologic manifestations of multiple sclerosis. Arch Iran Med 13: 188–192, 2010 [PubMed] [Google Scholar]
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif 9: 1–43, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol 98: 3349–3359, 2007 [DOI] [PubMed] [Google Scholar]
- Roncagliolo M, Benítez J, Eguibar JR. Progressive deterioration of central components of auditory brainstem responses during postnatal development of the myelin mutant taiep rat. Audiol Neurootol 5: 267–275, 2000 [DOI] [PubMed] [Google Scholar]
- Roncagliolo M, Schlageter C, León C, Couve E, Bonansco C, Eguibar JR. Developmental impairment of compound action potential in the optic nerve of myelin mutant taiep rats. Brain Res 1067: 78–84, 2006 [DOI] [PubMed] [Google Scholar]
- Rossi S, Furlan R, De Chiara V, Musella A, Lo Giudice T, Mataluni G, Cavasinni F, Cantarella C, Bernardi G, Muzio L, Martorana A, Martino G, Centonze D. Exercise attenuates the clinical, synaptic and dendritic abnormalities of experimental autoimmune encephalomyelitis. Neurobiol Dis 36: 51–59, 2009 [DOI] [PubMed] [Google Scholar]
- Rusu SI, Borst JG. Developmental changes in intrinsic excitability of principal neurons in the rat medial nucleus of the trapezoid body. Dev Neurobiol 71: 284–295, 2011 [DOI] [PubMed] [Google Scholar]
- Shaw NA. Effect of electroconvulsive shock on the slow components of the brain stem auditory evoked potential. Exp Neurol 100: 242–247, 1988 [DOI] [PubMed] [Google Scholar]
- Smith CM, Mayer JA, Duncan ID. Autophagy promotes oligodendrocyte survival and function following dysmyelination in a long-lived myelin mutant. J Neurosci 33: 8088–8100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol 79: 3127–3142, 1998 [DOI] [PubMed] [Google Scholar]
- Sommer I, Lingenhohl K, Friauf E. Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp Brain Res 95: 223–239, 1993 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 128: 1016–1025, 2005 [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain 119: 741–753, 1996 [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162–9173, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol 61: 477–496, 1999 [DOI] [PubMed] [Google Scholar]
- Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci USA 91: 53–57, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol 93: 3050–3063, 2005 [DOI] [PubMed] [Google Scholar]