Abstract
Fibrocytes derive from the bone marrow and are found in the circulation. They can be recruited to sites of injury and contribute to repair/remodeling. In vitro evidence suggests that fibrocytes may differentiate into fibroblasts to promote lung fibrosis. However, in vivo evidence for this is sparse. This review summarizes recent literature which may suggest that fibrocytes function to promote fibrosis via paracrine actions. In this way, secretion of growth factors, proteases and matricellular proteins may strongly influence the actions of resident epithelial and mesenchymal cells to promote repair and resolution or to tip the scale towards pathologic remodeling.
Keywords: bone marrow, mesenchymal cells, chemokines, periostin, growth factors
FIBROCYTES IN FIBROTIC LUNG DISEASES
Fibrocytes are bone marrow derived cells that circulate in peripheral blood and express both fibroblast and leukocyte markers [1–3]. They are adherent, can be cultured in vitro, and synthesize extracellular matrix (ECM) proteins such as collagen I, collagen III, and fibronectin [3]. In addition, fibrocytes express a variety of leukocyte markers such as CD45, CD13, CD34 and major histocompatibility antigens (MHC I and MHC II). There is significant controversy in the field on methods to identify a fibrocyte. A leukocyte marker, most commonly, CD45 is utilized but monocytes and macrophages may be able to phagocytose secreted ECM proteins such as type I collagen which is the most commonly used mesenchymal marker. Use of antibodies against procollagen for human studies may be better and is currently preferred. However, type I collagen is secreted in pro-form and processed into mature form in the extracellular space thus may still be potentially engulfed as procollagen [4]. This population comprises about 0.1 to 1% of human peripheral blood leukocytes in healthy volunteers [3,5]. Fibrocytes are known to express multiple chemokine receptors including CXCR4, CCR7 and CCR2 and these receptors can mediate migration of fibrocytes [6–9]. The activation of various receptor-mediated signaling pathways may affect fibrocyte function once localized to the area of injury or disease and and fibrocytes have been found in increased numbers in various forms of fibrotic lung disease in humans and animal models as summarized briefly below.
Asthma
Fibrocytes are found in remodeled airways of human asthmatic patients [10] and are localized to sites of airway smooth muscle bundles [11]. It is believed they play a pathogenic role in the disease as fibrocytes isolated from patients with chronic airway obstruction were easily differentiated into myofibroblasts in vitro following treatment with transforming growth factor (TGF-β1) [12]. There was also an association with increased levels of IL-4, IL-13, and IL-17A in the sputum of human asthmatics with elevated levels of circulating fibrocytes [13]. These factors may further influence the behavior of fibrocytes in situ as stimulation with IL-4 and IL-13 increased production of various ECM proteins. In contrast, fibrocytes proliferated, produced pro-inflammatory cytokines and increased α-smooth muscle actin (SMA) production in response to stimulation with IL-17A [13].
Systemic Sclerosis Interstitial Lung Disease (SSc-ILD)
The absolute number of fibrocytes in SSc-ILD patients is increased relative to controls [14]. Furthermore, SSc-ILD patients showed enhanced fibrocyte outgrowth from the peripheral blood, which may be related to increased expression of semaphorin-7a in SSc-ILD fibrocytes [15]. In lung tissue, numbers of fibrocytes are also increased in SSc-ILD patients compared to normal lungs [9]. It has been suggested that a specific defect in expression of caveolin noted in cells from SSc-ILD patients may lead to enhanced CXCR4 expression on fibrocytes. This in turn may explain the enhanced fibrocyte migration from the peripheral circulation into CXCL12-expressing tissue and explain the propensity for these patients to develop ILD [16].
Idiopathic Pulmonary Fibrosis (IPF)
In an initial small study, the percentage of circulating leukocytes with fibrocyte markers in patients with fibrotic lung disease was an order of magnitude higher than in normal patients (6–10% vs. 0.5%) [17], but these cells were largely negative for α-SMA [17]. The presence of fibrocytes in the lung tissue of patients with IPF has also been seen via immunohistochemistry [18]. In a larger study, the increased percentage of circulating fibrocytes was confirmed in IPF patients (2.72 ± 0.34%) compared to controls (1 ± 0.12%) and patients with acute exacerbations had the highest levels of all (14.51 ± 2.53%) compared to stable IPF patients or normal controls [19]. A correlation with increased fibrocytes in circulation and poor prognosis was also confirmed in a Japanese study looking at IPF and patients with collagen vascular disease associated lung fibrosis [20]. Fibrocytes from patients with IPF have been noted to produce increased levels of the profibrotic matricellular protein periostin when compared to normal controls (see below).[21]
Other lung diseases
Fibrocytes have also been noted in models of acute lung injury (ALI) [22],bronchiolitis obliterans [23] and sickle cell lung disease [24]. Increased fibrocyte numbers predicted lung transplant patients that would go on to develop bronchiolitis obliterans in a recent clinical study as well [25].
FIBROCYTE FUNCTIONS
The above studies suggest that fibrocytes correlate with worsened fibrotic outcomes. However, whether this is due to the differentiation of these cells into effector fibroblasts and their eventual secretion of ECM, their paracrine secretion of mediators to influence resident cells or both is unknown. The only study to definitively show that fibrocytes could promote lung fibrosis in vivo using an adoptive transfer design did not determine whether the effects were direct or paracrine [7]. While a fate-mapping strategy did suggest that up to 20% of S100A4-expressing fibroblasts from bleomycin-treated murine lungs could derive from hematopoietic precursors [26], these cells did not appear to become myofibroblasts. Furthermore, our own experiments looking for differentiation of labeled fibrocytes into fibroblasts in vivo have suggested that transferred fibrocytes do not lose expression of CD45 readily in vivo during the first week post-transfer (data not shown). Thus, we believe fibrocytes may play an active role in directing the remodeling process via paracrine actions. Fibrocytes are known to secrete a variety of growth and differentiation factors that may promote fibrogenesis including interleukin-β, tumor necrosis factor-α, CCL2, CCL3, CCL4, CXCL2, platelet derived growth factor (PDGF), transforming growth factor (TGF-β1), matrix metalloproteinases (MMP-1 and MMP-9), periostin and vascular endothelial growth factor (VEGF). They also can secrete a variety of ECM components including collagen 1, collagen 3 and fibronectin [3]. These factors may allow fibrocytes to have important impacts on resident lung cells during periods of injury and repair. It is also likely that a profibrotic environment may serve to cause fibrocyte proliferation and activation to further potentiate the repair and remodeling process. How selected mediators may act in autocrine and paracrine functions of fibrocytes is briefly summarized below and in Table 1.
Table 1.
Paracrine Mediators That Can Influence Fibrosis
| Mediator | Possible Modes of Action |
|---|---|
| Interleukin 1β |
|
| Tumor necrosis factor α |
|
| Platelet derived growth factor |
|
| Chemokines (e.g. CCL2, CCL12) |
|
| Transforming growth factor β |
|
| Matrix metalloproteinases (e.g. MMP9) |
|
| Periostin |
|
| Connective Tissue Growth Factor (CTGF) |
|
| Vascular endothelial growth factor (VEGF) |
|
| Cysteinyl leukotrienes |
|
| Extracellular Matrix Proteins (e.g. collagen, fibronectin) |
|
| Prostaglandin E2 |
|
TGF-βl
Numerous studies have demonstrated the capacity of fibrocytes to secrete TGF-β1 [2,27]. TGF-βl is one of the most well-studied inducers of pulmonary fibrosis [28]. It is a pleotropic cytokine involved in regulation of immunity, cancer, and fibrosis. It can act on many cell types to promote a pro-fibrotic phenotype. It is overexpressed in many fibrotic tissues and expression of active TGF-βl is sufficient to induce fibrosis in animal models [27,29]. TGF-β1 is secreted in latent form, and upon activation it binds to its cell surface receptors, leading to activation of canonical Smad-dependent and independent signaling pathways [30] and subsequent transcriptional activation allowing production of fibrotic matrix proteins. There are numerous therapeutic strategies that have been employed to inhibit the TGF-βl signaling pathway at multiple points in an effort to attenuate pulmonary fibrosis [31–37]. Expression of TGF-β1 is not limited to fibrocytes, but is also secreted by epithelial cells, T-cells, and monocytes/macrophages [38].
ECM and Proteases
Expression of fibrillar collagens, especially type I collagen is frequently used as part of the minimal definition of fibrocytes along with expression of CD45 [39]. The specific composition and structure of the ECM is critical for cell adhesion and migration during wound repair and fibrogenesis [40]. Collagen is important not only as a structural protein but also as signaling molecule. Type I and III collagens can initiate cell signaling by binding to a number of cell surface receptors including α1β1 and α2β1 integrins, discoidin domain receptor-2 (DDR-2) and several others [41]. Signaling through some of these receptors has been implicated in fibrogenesis [42]. Fibrocytes have also been shown to express prolyl 4-hydroxylase which is essential for the final stability of the collagen triple helix and for collagen cross-linking to form fibrils and fibers. Thus, fibrocytes likely contribute to the structure and rigidity of the collagen-rich fibrotic matrix which can influence cell behavior [38,43–45]. Fibronectin is another important fibrotic ECM protein produced by fibroblasts as well as fibrocytes [3,38]. Fibronectin has many functions during fibrogenesis, including promoting fibroblast recruitment and myofibroblast differentiation and binding to the latent TGF-βl complex enabling activation of latent TGF-βl [46,47].
Matrix metalloproteinases (MMPs) are endopeptidases that are involved in many pathophysiological processes. MMPs promote cell migration, proliferation and adhesion. Several MMPs (MMP-2, MMP-7 and MMP-9) are up-regulated in pulmonary fibrosis [48–50]. Fibrocyte expression of MMPs has been implicated in many aspects of cell migration and tissue remodeling [51–53]. In response to bleomycin, levels of MMP-9 are increased, and MMP-9 is believed to play a role in the mobilization of cells from the bone marrow to the lung [54]. Pulmonary fibrocytes have been shown to express MMP-9 and promote metastasis of lung cancer presumably via protease actions [55]. However, whether MMP-9 expression is protective or pathologic is uncertain. Overproduction of MMP-9 can lead to emphysematous changes in surfactant protein D−/− mice [56]. However, transgenic overexpression of MMP-9 in lung macrophages has also been shown to protect from bleomycin-induced lung fibrosis via a mechanism likely involving the destruction of profibrotic growth factors such as insulin-like growth factor binding protein-3 (IGFBP-3) [57]. Taken together, it is interesting to speculate that early post-injury recruitment of MMP-9-expressing fibrocytes may promote repair by enhancing cellular migration and access to growth factors. Unchecked expression of MMP-9 or persistent fibrocyte recruitment, however, may eventually tip the scale in favor of lung destruction. In this regard, it is interesting to note that in a model of collagen antibody-induced arthritis, adoptive transfer of fibrocytes worsened joint pathology and increased recruitment of neutrophils. This was associated with increased expression of MMPs by the transferred fibrocytes, again suggesting that proteases may play important roles in fibrocyte-mediated tissue remodeling [53].
Matricellular proteins
Fibrocytes also secrete several matricellular proteins which are signaling factors that are embedded within the ECM and regulate cell function and cell–matrix interactions, but do not contribute directly to the structural properties or organization of the ECM [58]. Connective tissue growth factor (CTGF) is a matricellular protein involved in wound healing, malignancy and fibrotic disease that enhances cell migration, proliferation and matrix deposition [59,60]. When induced by TGF-β1, CTGF interacts with and regulates the function of other growth factors, including TGF-βl and bone morphogenic protein 4 (BMP4) [61]. CTGF can bind to several different cell surface receptors activating intracellular signaling pathways leading to a fibrogenic phenotype. Overexpression of CTGF in vivo leads to pulmonary fibrosis in animal models [62–64] and inhibition of CTGF attenuates pulmonary fibrosis [65]. Fibrocytes derived from patients with fibrosis have increased expression of CTGF suggesting a critical function in the pathogenesis [27,66]. Inhibition of CTGF with a neutralizing antibody is currently under investigation as potential therapy for IPF.
Periostin is another matricellular protein that binds to matrix proteins and cellular receptors to affect cell function [67]. It has been found to be elevated in patients with IPF and predicts poor outcomes. Particularly fibrocytes from patients with IPF had increased production of periostin [21,68]. Animal modeling has demonstrated that depletion of periostin in circulating cells (presumably monocytes and fibrocytes) can protect the lung from bleomycin-induced fibrosis [21]. Additionally, blockade of cellular interactions with periostin can lessen fibrotic outcomes in vivo and limit mesenchymal cell wound closure in vitro [21]. Periostin also increases proliferation and collagen 1 expression in lung mesenchymal cells in a dose-dependent manner [21]. Periostin may crosslink collagen and stiffen the matrix created by fibroblasts, thereby activating the cells for further extracellular matrix production [69]. It has been speculated that stiffened lung matrices may promote ongoing fibroblast activation [70]. Periostin may also directly activate mesenchymal cells or induce TGF-β1 expression from these cells [71]. Finally, periostin has been shown to promote epithelial to mesenchymal transition in lung epithelial cells as well [71]. Thus, it is easy to see how recruitment of periostin-expressing fibrocytes could profoundly impact the pro-fibrotic potential of lung mesenchymal and epithelial cells.
Prostaglandins/Leukotrienes
Leukotrienes are eicosanoid factors produced by the oxidation of arachidonic acid that promote fibrosis by regulating fibroblast chemotaxis, proliferation, and collagen synthesis [72–74]. An imbalance between pro- and anti-fibrotic eicosanoids may be critical for progressive fibrosis. Patients with IPF have increased production of leukotrienes and decreased production of the anti-fibrotic prostaglandins including PGE2 [74,75]. Alveolar macrophages and fibrocytes vastly overproduce cysteinyl leukotrienes (cys LTs) when compared to fibroblasts [76]. Cys LT levels are elevated in IPF lungs due to macrophage production [74] and also possibly via increased fibrocyte recruitment. Fibrocytes express cysteinyl leukotriene receptors 1 and 2 and release of cys LTs have autocrine activities to promote fibrocyte proliferation and chemotaxis, at least in vitro [76]. In addition, release of cys LTs by fibrocytes would be expected to increase proliferation [77] and ECM synthesis by resident fibroblasts [78]. In this way, recruited fibrocytes could have profound effects on resident fibroblasts to promote lung remodeling. Zileuton, a 5-lipoxygenase inhibitor involved in leukotriene synthesis is currently under investigation as a potential therapy for IPF.
Other factors
Fibrocytes produce many other growth factors and cytokines that have been implicated in fibrosis, including tumor necrosis factor (TNF)α, platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) [51]. As with the above, expression of these factors are not unique to fibrocytes. The signaling pathways leading to expression of these factors may be different within fibrocytes versus other cell types potentially offering unique targets for intervention. Conversely, profibrotic cytokine expression by different cell types regulated by somewhat different signaling pathways may present a redundancy that impedes our ability to intervene. Inhibition of fibrosis will likely require an understanding of cytokine production by several different cell types.
FIBROCYTE PARACRINE TARGET CELL EFFECTS
Injury leads to an orchestrated series of events requiring an evolving makeup of cells, extracellular matrix proteins and secreted signaling factors in the extracellular space. These changes can be accomplished through rapid recruitment of cells into the microenvironment, transdifferentiation, proliferation, cell death and regulation of protein expression/secretion. The ability to recruit circulating fibrocytes to sites of injury enables rapid changes to the extracellular milieu influencing the behavior of structural cells during repair and fibrosis. Fibrocyte paracrine function is believed to play a central role in regulating all of these dynamic events through crosstalk with many other cells types.
Fibroblasts
Fibroblasts and myofibroblasts are thought to be the primary fibrogenic effector cells [79]. While fibrocyte to fibroblast/myofibroblast differentiation remains controversial [8,10,80] fibrocytes clearly secrete many factors which can affect fibroblast function [27]. Most of the factors are thought to favor fibroblast to myofibroblast differentiation and a fibrogenic phenotype in general. Fibrocytes secrete most if not all of the most well studied activators of fibroblast to myofibroblast differentiation, including TGF-β1, PDGF, leukotrienes, and CTGF [79]. These factors not only support myofibroblast transition with upregulation of α-smooth muscle actin and stress fiber formation but also lead to fibroblast migration and transcriptional activation of fibrotic matrix proteins such as type I collagen, type III collagen and fibronectin. The extent to which fibroblasts proliferate during fibrogenesis is also unclear but at least in vitro, many fibrocyte derived cytokines promote proliferation of cultured fibroblasts [81]. Thus fibrocyte paracrine action may be important in recruiting activated fibroblasts through chemotaxis, augmented proliferation and transcriptional activation. Production of these fibroblast activating factors are not unique to fibrocytes as they can be produced by macrophages, epithelial cells and fibroblasts. Indeed, one of the features of activated fibroblasts is production and release of profibrotic factors which may propagate progressive fibrosis. For example, TGF-β1 robustly induces fibroblast production of other profibrotic cytokines such as CTGF and periostin [82,83]. Fibrocytes, with shared features of leukocytes and fibroblasts have the ability to be quickly recruited from a circulating pool and secrete significant amounts of profibrotic factors in a targeted microenvironment. Fibrocyte production of these profibrotic factors may also be more dependent on inflammatory cytokines and chemokines suggesting a potential important function for fibrocytes in the transition from inflammation to early fibrogenesis [79].
Epithelial Cells
The function of epithelial cells during fibrogenesis is much less clear. The distal lung contains many different types of epithelial cells including type I and type II alveolar epithelial cells, club cells and small airway epithelial cells. Several reports have also suggested the existence of a bronchoalveolar epithelial cell [84,85]. During injury and repair/fibrosis there is evidence for epithelial cell death, proliferation and transdifferentiation [86]. All of these functions are potentially regulated by fibrocyte paracrine factors. Cell death is evident in fibrotic human tissue samples and in many animal models of fibrosis. Epithelial cell death is sufficient to induce fibrosis in animal models and inhibition of apoptosis abrogates lung fibrosis [87,88]. Whether this is due to loss of a constitutive anti-fibrotic function or through active release of profibrotic factors by the dying epithelial cell is unknown. Many secreted factors from fibrocytes have been shown to cause or augment epithelial cell death at least in vitro. For example, TGF-β1 has been shown to prominently promote lung epithelial cell death [89]. The balance between epithelial cell death and proliferation may ultimately dictate regeneration/restoration of homeostasis versus progressive fibrosis or sustained injury and death [86]. During regeneration, differentiation of epithelial stem cells to repopulate the lung epithelium is necessary [84,90–93]. Bronchoalveolar stem cells may be resistant to injury and differentiate into airway and alveolar epithelial cells. While factors regulating differentiation of adult lung epithelial progenitor cells are poorly defined, fibrocyte-derived factors such as fibroblast growth factor (FGF)7/keratinocyte growth factor (KGF) and prostaglandins likely play an important role [94–97]. Epithelial cells can also potentially differentiate toward a mesenchymal phenotype through a process of epithelial-mesenchymal transition (EMT) [98]. There is conceptual and experimental overlap between stem cell differentiation and EMT in which epithelial stem cell potency includes a mesenchymal phenotype [99,100]. Cell migration is a prominent mesenchymal phenotype and epithelial cell migration after injury has been well studied in vitro as a potential mechanism for repopulating the denuded basement membrane [101]. Indeed some studies have suggested a role for EMT transcription factors in regulating epithelial cell migration toward restoration of the denuded basement membrane [102]. Many factors produced by fibrocytes, TGF-β1, PDGF, CTGF and leukotrienes have been shown to induce or augment EMT in vitro. Many of the implicated fibrotic factors secreted by fibrocytes and other cells are not simply released into the milieu but remain attached to the ECM and may require cells in proximity or in contact for full signaling. There is strong evidence for fibrocyte/epithelial cell interaction in proximity. Fibrocytes quickly accumulate in the bronchoalveolar space after experimental fibrosis and are prominent in the bronchoalveolar space in patients with pulmonary fibrosis [7,103]. Extracellular matrix proteins such as fibrillar collagens and fibronectin, which are produced by fibrocytes themselves, act as signaling molecules by binding integrins and other cell surface receptors. Matricellular proteins such as CTGF and periostin activate cell signaling by binding many of the same cell surface receptors. Even classic pro-fibrotic growth factors such as TGF-β1 are largely attached to the ECM [104,105]. Thus, production of these factors in a precise niche and architecture by a rapidly mobile circulating cell likely fulfills this function in a unique way.
Inflammatory Cells
The prototypical pulmonary fibrotic disease, IPF, is notable for paucity of inflammatory cells. However, increased numbers of fibrocytes have also been identified in a number of other lung diseases in which fibrogenesis contributes significantly to the pathogenesis and organ dysfunction, including asthma and COPD. In these conditions, fibrocyte crosstalk with other cell types via antigen presentation may be critical to the pathogenesis. Several reports have demonstrated antigen presentation function of fibrocytes through expression of MHC class II complex molecules leading to T cell activation [1]. Fibrocytes express a number of cytokines that are regulators of leukocyte recruitment and activation. This is perhaps best exemplified in recent studies where fibrocytes were adoptively transferred into septic mice to improve outcomes. In these studies, transfer of fibrocytes was associated with reduced bacterial burden, increased T cell proliferation and alterations in cytokine expression that favored improved outcomes post-sepsis (increased IL-2 and IFNγ, yet diminished IL-6 and IL-10).[106] However, in IPF there is a notion of an imbalance favoring Th2 cytokines over Th1. In fact, the FITC model of lung fibrosis is known to be driven by Th2-mediated signaling, and adoptive transfer of fibrocytes can worsen fibrotic outcomes post-FITC, although it is not known whether this is due to augmentation of Th2 cytokines directly [107]. As a hybrid cell there is an opportunity for fibrocytes to possess a unique input/output signaling crosstalk with other cell types during inflammatory fibrosis. For example IL-1β (and other interleukins) strongly influence fibrocyte expression of inflammatory and profibrotic factors [2,13]. Direct cell-cell interaction may be important not only for antigen presentation but also for regulation of immune cell activation through other fibrocyte cell surface ligands [15].
Endothelial Cells
Angiogenesis and endothelial function, in general, may be important to the pathogenesis of pulmonary fibrosis [108]. There is a strong indication for aberrant vascular remodeling in IPF and inhibition of angiogenesis can attenuate lung fibrosis in animal models [109–111]. Several studies have indicated that fibrocytes can produce factors that support endothelial cell proliferation, migration and angiogenesis in vitro and in model systems in vivo. In addition there are several studies indicating a role for endothelial cell transition into a mesechymal phenotype presumably regulated by similar factors that regulate epithelial cell transition [112,113].
Expert commentary
Over the past two decades the existence of fibrocytes as a bone marrow-derived leukocyte/mesenchymal hybrid cell has been well established. Although expression of CD45 and type I collagen are often used as markers of fibrocytes, their primary function during fibrogenesis may be in their ability to be rapidly recruited to sites of injury and secrete profibrotic factors. Many, if not all of the factors secreted by fibrocytes can be secreted by other cell types. The unique functions of fibrocytes remain unknown. Fibrogenesis involves a complex cross-talk among many different cell types and fibrocytes may be positioned to orchestrate the fibrotic process through their paracrine functions (Figure 1).
Figure 1.
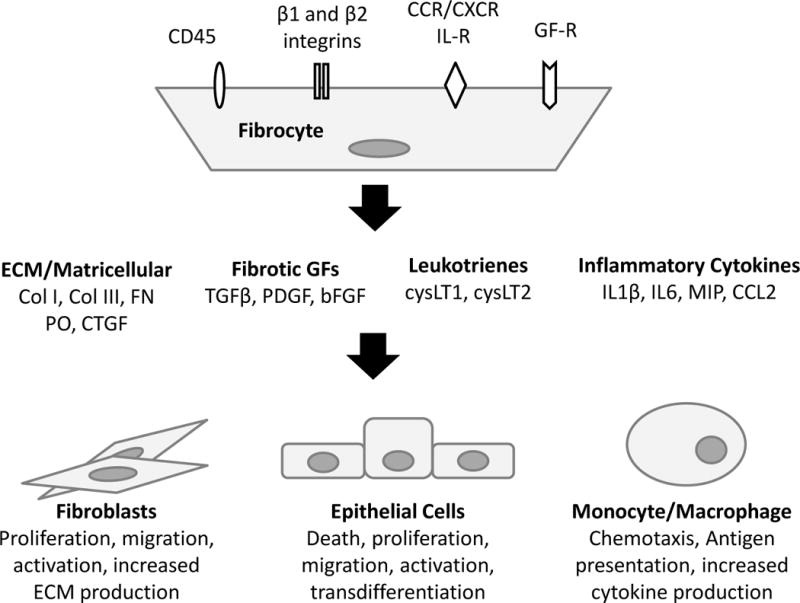
Proposed Paracrine Functions of Fibrocytes. Fibrocytes express a number of cell surface receptors which can regulate their recruitment and secretion of signaling factors. These factors can further regulate fibrocyte function and/or regulate the function of other cells in autocrine and paracrine fashion. Abbreviations: IL-R=interleukin receptor, GF-R=growth factor receptor, ECM=extracellular matrix, Col=collagen, FN=fibronectin, PO=periostin, CTGF=connective tissue growth factor, TGFβ=transforming growth factor β, PDGF=platelet derived growth factor, bFGF=basic fibroblast growth factor, cysLT= cysteinyl leukotrienes, IL1β=interleukin 1β, IL6=interleukin 6, MIP=macrophage inflammatory protein, CCL2=chemokine ligand 2.
Five year view
Many studies have identified fibrocytes in a variety of diseases and have identified the capacity of fibrocytes to express cytokines in vitro and in vivo. It remains unclear if fibrocytes have a unique essential function during the pathogenesis of disease. Fibrocytes possess a unique combination of features that certainly suggest a critical nonredundant paracrine function at an essential time and niche during fibrogenesis. Progress in newer techniques in generating chimera mice, such as adoptive transfer, bone marrow transplant and the cre-lox system, should enable animal models to study potential unique functions of fibrocytes and how activation and recruitment of these cells is regulated in vivo. As a circulating cell fibrocytes are an attractive potential biomarker. Better understanding of the regulation of fibrocyte function may lead to better translational studies beyond simply correlating fibrocyte numbers with disease states but also analyzing the fibrocyte activation state. Finally, a better understanding of fibrocyte function could lead to development of targeted therapy directed at activated fibrocytes.
Key Issues.
Fibrocytes are a unique cell type with features of circulating bone marrow derived leukocytes and fibroblasts. These cells have been identified by many groups, they express leukocyte and fibroblast markers and they are often found in increased numbers in a variety of fibrotic diseases.
Because fibrocytes are circulating cells found in the blood there is real potential for using fibrocytes as a biomarker for diagnosis, prognosis and response to specific treatments.
Fibrocytes can be recruited and activated by specific chemokines/cytokines and they can secrete a number of important profibrotic factors leading to recruitment and activation of other fibrogenic effector cells.
Fibrocyte-derived factors can influence the behavior of multiple cell types therefore they may have a large impact in orchestrating fibrogenesis.
Fibrocytes have a unique profile of receptors and secreted proteins suggesting that they may have a unique function during fibrogenesis and potentially offer a novel target for therapeutic intervention.
References
- 1.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94(12):6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 3.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20(1):33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 6.Moore BB, Kolodsick JE, Thannickal VJ, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–181. doi: 10.1165/rcmb.2005-0239OC. Epub 2006 Mar 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tourkina E, Bonner M, Oates J, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4(1):15. doi: 10.1186/1755-1536-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M, Sun G, Stacey M, Mori L, Mattoli S. Identification of Circulating Fibrocytes as Precursors of Bronchial Myofibroblasts in Asthma. J Immunol. 2003;170:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 11.Saunders R, Siddiqui S, Kaur D, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123(2):376–384. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178(6):583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 13.Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5(2):140–149. doi: 10.1038/mi.2011.60. [DOI] [PubMed] [Google Scholar]
- 14.Mathai SK, Gulati M, Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90(6):812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan Y, Reilkoff R, Peng X, et al. Role of semaphorin 7a signaling in transforming growth factor beta1-induced lung fibrosis and scleroderma-related interstitial lung disease. Arthritis Rheum. 2011;63(8):2484–2494. doi: 10.1002/art.30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tourkina E, Richard M, Oates J, et al. Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis. 2010;69(6):1220–1226. doi: 10.1136/ard.2009.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 18.Andersson-Sjoland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara A, Kobayashi H, Masuya M, et al. Correlation between circulating fibrocytes, and activity and progression of interstitial lung diseases. Respirology. 2012;17(4):693–698. doi: 10.1111/j.1440-1843.2012.02167.x. [DOI] [PubMed] [Google Scholar]
- 21.Naik PK, Bozyk PD, Bentley JK, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garibaldi BT, D’Alessio FR, Mock JR, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 2013;48(1):35–43. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris DA, Zhao Y, Lapar DJ, et al. Inhibiting CXCL12 blocks fibrocyte migration and differentiation and attenuates bronchiolitis obliterans in a murine heterotopic tracheal transplant model. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field JJ, Burdick MD, DeBaun MR, et al. The role of fibrocytes in sickle cell lung disease. PLoS One. 2012;7(3):e33702. doi: 10.1371/journal.pone.0033702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaPar DJ, Burdick MD, Emaminia A, et al. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Ann Thorac Surg. 1016;92(2):470–477. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanjore H, Xu XC, Polosukhin VV, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180(7):657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 28.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125(2):754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 30.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 31.Puthawala K, Hadjiangelis N, Jacoby SC, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177(1):82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis. 2013;5(1):48–73. doi: 10.3978/j.issn.2072-1439.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti-fibrotic therapies. Biochim Biophys Acta. 2013;1832(7):1088–1103. doi: 10.1016/j.bbadis.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 -an intimate relationship. Eur J Cell Biol. 2008;87(8–9):601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Iwamoto N, Distler JH, Distler O. Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials. Curr Rheumatol Rep. 2011;13(1):21–27. doi: 10.1007/s11926-010-0142-x. [DOI] [PubMed] [Google Scholar]
- 36.Arribillaga L, Dotor J, Basagoiti M, et al. Therapeutic effect of a peptide inhibitor of TGF-beta on pulmonary fibrosis. Cytokine. 2011;53(3):327–333. doi: 10.1016/j.cyto.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Horan GS, Wood S, Ona V, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 38.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36(4):598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Ogawa R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res. 2012;53(3):187–196. doi: 10.3109/03008207.2011.642035. [DOI] [PubMed] [Google Scholar]
- 41.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26(3):146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Olaso E, Arteta B, Benedicto A, Crende O, Friedman SL. Loss of discoidin domain receptor 2 promotes hepatic fibrosis after chronic carbon tetrachloride through altered paracrine interactions between hepatic stellate cells and liver-associated macrophages. Am J Pathol. 2011;179(6):2894–2904. doi: 10.1016/j.ajpath.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24(2):65–69. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 46.Fontana L, Chen Y, Prijatelj P, et al. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19(13):1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 47.Muro AF, Moretti FA, Moore BB, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(6):638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99(9):6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oikonomidi S, Kostikas K, Tsilioni I, Tanou K, Gourgoulianis KI, Kiropoulos TS. Matrix metalloproteinases in respiratory diseases: from pathogenesis to potential clinical implications. Curr Med Chem. 2009;16(10):1214–1228. doi: 10.2174/092986709787846587. [DOI] [PubMed] [Google Scholar]
- 50.Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW. Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. Am J Pathol. 1998;152(3):821–828. [PMC free article] [PubMed] [Google Scholar]
- 51.Hartlapp I, Abe R, Saeed RW, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15(12):2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-de-Alba C, Becerril C, Ruiz V, et al. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med. 2010;182(9):1144–1152. doi: 10.1164/rccm.201001-0028OC. [DOI] [PubMed] [Google Scholar]
- 53.Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of collagen antibody-induced arthritis. Arthritis Rheum. 2012;64(11):3583–3593. doi: 10.1002/art.34589. [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol. 2007;37(3):291–299. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Deventer HW, Wu QP, Bergstralh DT, et al. C-C chemokine receptor 5 on pulmonary fibrocytes facilitates migration and promotes metastasis via matrix metalloproteinase 9. Am J Pathol. 2008;173(1):253–264. doi: 10.2353/ajpath.2008.070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wert SE, Yoshida M, LeVine AM, et al. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci U S A. 2000;97(11):5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabrera S, Gaxiola M, Arreola JL, et al. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int J Biochem Cell Biol. 2007;39(12):2324–2338. doi: 10.1016/j.biocel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 59.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 60.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81(6):355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 61.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonnylal S, Shi-Wen X, Leoni P, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62(5):1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal. 2010;4(1):1–4. doi: 10.1007/s12079-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonniaud P, Margetts PJ, Kolb M, et al. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med. 2003;168(7):770–778. doi: 10.1164/rccm.200210-1254OC. [DOI] [PubMed] [Google Scholar]
- 65.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weng CM, Chen BC, Wang CH, et al. The ET Receptor Mediates Fibrocyte Differentiation in Chronic Obstructive Asthma: The Involvement of CTGF. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201301-0132OC. [DOI] [PubMed] [Google Scholar]
- 67.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 68.Okamoto M, Hoshino T, Kitasato Y, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37(5):1119–1127. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 69.Eckes B, Zweers MC, Zhang ZG, et al. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006;11(1):66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- 70.Tschumperlin DJ, Jones JC, Senior RM. The fibrotic matrix in control: does the extracellular matrix drive progression of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2012;186(9):814–816. doi: 10.1164/rccm.201208-1561ED. [DOI] [PubMed] [Google Scholar]
- 71.Sidhu SS, Yuan S, Innes AL, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107(32):14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ozaki T, Hayashi H, Tani K, Ogushi F, Yasuoka S, Ogura T. Neutrophil chemotactic factors in the respiratory tract of patients with chronic airway diseases or idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1992;145(1):85–91. doi: 10.1164/ajrccm/145.1.85. [DOI] [PubMed] [Google Scholar]
- 73.Wardlaw AJ, Hay H, Cromwell O, Collins JV, Kay AB. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol. 1989;84(1):19–26. doi: 10.1016/0091-6749(89)90173-5. [DOI] [PubMed] [Google Scholar]
- 74.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97(8):1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95(4):1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vannella KM, McMillan TR, Charbeneau RP, et al. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J Immunol. 2007;179(11):7883–7890. doi: 10.4049/jimmunol.179.11.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol. 1987;138(4):1190–1195. [PubMed] [Google Scholar]
- 78.Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988;27(8):2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- 79.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 80.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 81.Vancheri C. Idiopathic pulmonary fibrosis: an altered fibroblast proliferation linked to cancer biology. Proc Am Thorac Soc. 2012;9(3):153–157. doi: 10.1513/pats.201203-025AW. [DOI] [PubMed] [Google Scholar]
- 82.Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3(2):89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naik PK, Bozyk PD, Bentley JK, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046–1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 85.Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3(4):364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 87.Sisson TH, Mendez M, Choi K, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(3):254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal BD. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L143–151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- 89.Hagimoto N, Kuwano K, Inoshima I, et al. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol. 2002;168(12):6470–6478. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 90.Rawlins EL, Okubo T, Que J, et al. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb Symp Quant Biol. 2008;73:291–295. doi: 10.1101/sqb.2008.73.037. [DOI] [PubMed] [Google Scholar]
- 91.Gomperts BN, Strieter RM. Stem cells and chronic lung disease. Annu Rev Med. 2007;58:285–298. doi: 10.1146/annurev.med.58.081905.134954. [DOI] [PubMed] [Google Scholar]
- 92.Mason RJ, Williams MC, Moses HL, Mohla S, Berberich MA. Stem cells in lung development, disease, and therapy. Am J Respir Cell Mol Biol. 1997;16(4):355–363. doi: 10.1165/ajrcmb.16.4.9115744. [DOI] [PubMed] [Google Scholar]
- 93.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133(13):2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 94.Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann Surg. 2011;254(6):1066–1074. doi: 10.1097/SLA.0b013e3182251559. [DOI] [PubMed] [Google Scholar]
- 95.Schmeckebier S, Mauritz C, Katsirntaki K, et al. Keratinocyte growth factor and dexamethasone plus elevated cAMP levels synergistically support pluripotent stem cell differentiation into alveolar epithelial type II cells. Tissue Eng Part A. 2013;19(7–8):938–951. doi: 10.1089/ten.tea.2012.0066. [DOI] [PubMed] [Google Scholar]
- 96.Takase HM, Itoh T, Ino S, et al. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27(2):169–181. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savagner P, Kusewitt DF, Carver EA, et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202(3):858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 103.Borie R, Quesnel C, Phin S, et al. Detection of alveolar fibrocytes in idiopathic pulmonary fibrosis and systemic sclerosis. PLoS One. 2013;8(1):e53736. doi: 10.1371/journal.pone.0053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fontana L, Chen Y, Prijatelj P, et al. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19(13):1798. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 105.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 106.Nemzek JA, Fry C, Moore BB. Adoptive transfer of fibrocytes enhances splenic T-cell numbers and survival in septic peritonitis. Shock. 2013;40(2):106–114. doi: 10.1097/SHK.0b013e31829c3c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolodsick JE, Toews GB, Jakubzick C, et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172(7):4068–4076. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 108.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest. 2012;142(1):200–207. doi: 10.1378/chest.11-1962. [DOI] [PubMed] [Google Scholar]
- 109.Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3(4):357–363. doi: 10.1513/pats.200601-010TK. [DOI] [PubMed] [Google Scholar]
- 110.Burdick MD, Murray LA, Keane MP, et al. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171(3):261–268. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- 111.Wan YY, Tian GY, Guo HS, et al. Endostatin, an angiogenesis inhibitor, ameliorates bleomycin-induced pulmonary fibrosis in rats. Respir Res. 2013;14(1):56. doi: 10.1186/1465-9921-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hashimoto N, Phan SH, Imaizumi K, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43(2):161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]


