Abstract
During development, Eph tyrosine kinase receptors and their ephrin ligands function as axon guidance molecules while, in adults, these molecules appear to be involved in the regulation of neural plasticity and emotion. The absence of EphA5 receptor mediated forward signaling may cause alterations in connectivity of neural networks and boundary formation during development, including central monoaminergic systems. In the present studies, we demonstrated altered aggressive responses by animals lacking functional EphA5 receptors. These behavioral changes were accompanied by altered concentrations of serotonin (5-HT) and the metabolite, 5-HIAA, in the hypothalamus. The changes of serotonin activity in hypothalamus also result in increase of body weight in EphA5 knockout mice. Furthermore, EphA5 knockout mice exhibited a significant decrease in activity levels following exposure to naïve intruders in their home cages. We conclude that the EphA5 receptor may be involved in mediation of aggressive behavior regulated, in part, by hypothalamic serotonin.
Keywords: Aggression, Body weight, Neurochemistry, Hypothalamus, Axon guidance
1. Introduction
The EphA5 receptor is a member of the Eph receptor tyrosine kinase family. Eph receptor tyrosine kinases and their corresponding ligands, ephrins, comprise the largest group of receptor tyrosine kinases with at least eight ligands in vertebrates (Wilkinson, 2001; Zhou, 1998). In the human genome, 13 Eph receptors have been identified and are found to be distributed in three separate chromosomes (Kullander et al., 2001). Based on the structural homology and the binding preference, ephrins are classified into two groups, ephrin-A and ephrin-B. Ephrin-A ligands generally bind to EphA receptors, whereas ephrin-B ligands bind to EphB receptors (Himanen et al., 2004). There are some exceptions to this general rule; e.g., the EphA4 receptor which can bind to both ephrin-A and ephrin-B ligands. A-type ephrins are anchored to themembrane through a glycosylpho-sphatidylinositol (GPI) linkage and B-type ephrins have both transmembrane and cytoplasmic regions (Flanagan and Van-derhaeghen, 1998; Gale et al., 1996).
The function of the EphA5 receptor is best characterized as an axon guidance molecule during neural development (Cheng et al., 1995; Yue et al., 2002). The EphA5 receptor and its ligand act as a repellant cue that prevents axons from entering inappropriate territories, thus restricting the cells to specific pathways during the migratory process (Wilkinson, 2001). During neural development, Eph receptors and their ligands are expressed in the projecting and target sites, respectively (Castellani et al., 1998; Gale et al., 1996; Gao et al., 1998a; Stein et al., 1999; Zhang et al., 1996). For example, in the case of hippo-camposeptal projections, EphA5 receptors are expressed in a gradient with the lateral hippocampus expressing low levels and the medial hippocampus expressing high levels of the receptor. At the target site, the lateral septum, ephrin-A5 is expressed with a complementary gradient such that the dorsomedial septum expresses low levels and the ventrolateral septum expresses high levels of this ligand (Zhang et al., 1996). During embryogenesis, the EphA5 transcript is highly expressed in the cortical plate (Castellani et al., 1998). It is also expressed in cortex, hippocampus, medial thalamus and the septum of the developing brain. This receptor is moderately expressed in other brain regions, including hypothalamus and amygdala (Gao et al., 1998a).
At the cellular level, the binding of ephrin-A5 with receptor-expressing neurons results in different consequences depending on the cell type. It has been demonstrated that this interaction causes inhibition of the neurite outgrowth of hippocampal, striatal, retinal, and cortical neurons, while it enhances the neurite outgrowth of sympathetic neurons and stimulates neurite sprouting of cortical neurons in vitro (Brownlee et al., 2000; Gao et al., 2000, 1998a, 1996). At the circuit level, overexpression of a truncated form of EphA5 receptor resulted in a miswiring of the hippocamposeptal pathway and corpus callosum connections in vivo (Yue et al., 2002). In particular, medial hippocampal neurons with high expression level of the EphA5 receptor projected to both the ventral and lateral part of the target site while lateral hippocampal neurons with relatively low EphA5 receptor expression did not exhibit any obvious alteration in their projection pattern. Taken together, the EphA5 receptor and its ligands serve as repulsive axon guidance cues in the developing brain. Their interaction triggers growth cone collapse and inhibits the neurite outgrowth in vitro. Further-more, abnormal expression of these molecules results in the disruption of axonal pathfinding and mid-line crossing in vivo (Henkemeyer et al., 1996; Hu et al., 2003; Yue et al., 2002).
Details of how the binding of the Eph receptors and their ligands inhibits neurite outgrowth are yet to be determined. Gale and Yancopoulos (1997) provided evidence for the collapse of actin cytoskeletal structure within the growth cone following the activation of ephrin-induced signaling. Signal transduction induced by Eph–ephrin binding requires the autophosphorylation of the Eph receptor (Drescher et al., 1995;Meima et al., 1997). This event occurs predominately based on the cell–cell contact. Soluble forms of ephrins can bind to Eph receptors, but do not trigger autophosphorylation unless the receptors are artificially assembled. Furthermore, this receptor–ligand system can activate the intracellular signaling pathways not only via the activation of Eph receptor, but also by clustering of ephrins. Recent studies have shown that reverse signaling induced by ephrin clustering affects commissural formation in the forebrain as well as angiogenic remodeling (Adams et al., 2001; Henkemeyer et al., 1996; Kullander et al., 2001).
The presence of a phosphorylated form of EphA5 receptor in the adult brain leads to the speculation about possible roles in synaptic plasticity (Gerlai et al., 1999). By infusing EphA5 receptor agonist/antagonist proteins into the hippocampus, Gerlai et al. (1999) showed that activation of the EphA5 receptor enhances hippocampal-dependent behavioral tasks whereas the inactivation of the EphA5 receptor impairs these functions. Specifically, animals exhibited elevated fear responses consequent to shock exposure following EphA5 receptor agonist infusion. These behavioral changes were also accompanied by alterations in long-term potentiation suggesting the role of EphA5 receptor in synaptic plasticity (Gao et al., 1998b). In addition, Halladay et al. (2004) showed that animals expressing a truncated EphA5 receptor exhibited learning deficits in striatal-dependent tasks. These behavioral changes were associated with changes in monoaminergic activities in striatum suggesting a possible role of EphA5receptor in striatal functions. Taken together, activation of EphA5 receptor and its ligand may be involved in synaptic plasticity in the adult nervous system.
The expression of EphA5 receptor is elevated in hippocampus, striatum, hypothalamus, and amygdale in the adult brain (Gerlai et al., 1999). In this study, we asked whether the absence of EphA5 receptor mediated forward signaling can affect brain neurochemistry and how the altered neurochemistry might affect the aggressive behaviors mediated by hypothalamus in adult animals. Offensive aggression was assessed using the resident–intruder paradigm. Offensive aggression is predominantly a testosterone-dependent behavior and is manifest as the attack behavior of a resident subject against an intruder (Wagner et al., 1979). This type of offensive aggression can also be modulated by serotonin activity and drugs (Chiavegatto et al., 2001; Fish et al., 1999; Lyons et al., 1999; Miczek et al., 1998). Genetic manipulations that target serotonin-related genes, and on genes that affect serotonin receptor numbers also can change this form of aggression in rodents (Chiavegatto et al., 2001; Chiavegatto and Nelson, 2003; Dulawa et al., 2000; Fischer et al., 2000; Liu et al., 2007; Nelson et al., 2006; Saudou et al., 1994; Schiller et al., 2006; Stork et al., 2000; Wersinger et al., 2007). We also assessed defensive aggression by using the target-biting paradigm. It has been shown that high serotonergic activity dampens both defensive aggression in animals and violent crime in humans and, conversely, reduced serotonergic activity is associated with high levels of aggression (Bioulac et al., 1980; Golden et al., 1991; Lidberg et al., 1985; Linnoila et al., 1983; Virkkunen et al., 1987).
Our results showed that EphA5 knockout mice exhibit an increase in shock-induced target-biting but a decrease in offensive aggression in the resident–intruder paradigm. The escalated levels of 5-HT and 5-HIAA found in hypothalamus may have contributed the decrease in offensive aggression in knockout mice. Interestingly, EphA5 knockout mice showed significantly higher bodyweight than the controls. This increase in body weight is likely attribute to the change in serotonin metabolism in hypothalamus. Moreover, EphA5 knockout mice exhibited decreased motor activity immediately following the resident–intruder test in the same context. We concluded that the absence of EphA5 receptor-induced signaling results in alterations of aggressive behaviors and these behavioral changes are accompanied by changes in serotonergic activity in the hypothalamus.
2. Results
2.1. Increased body weight in EphA5 knockout mice
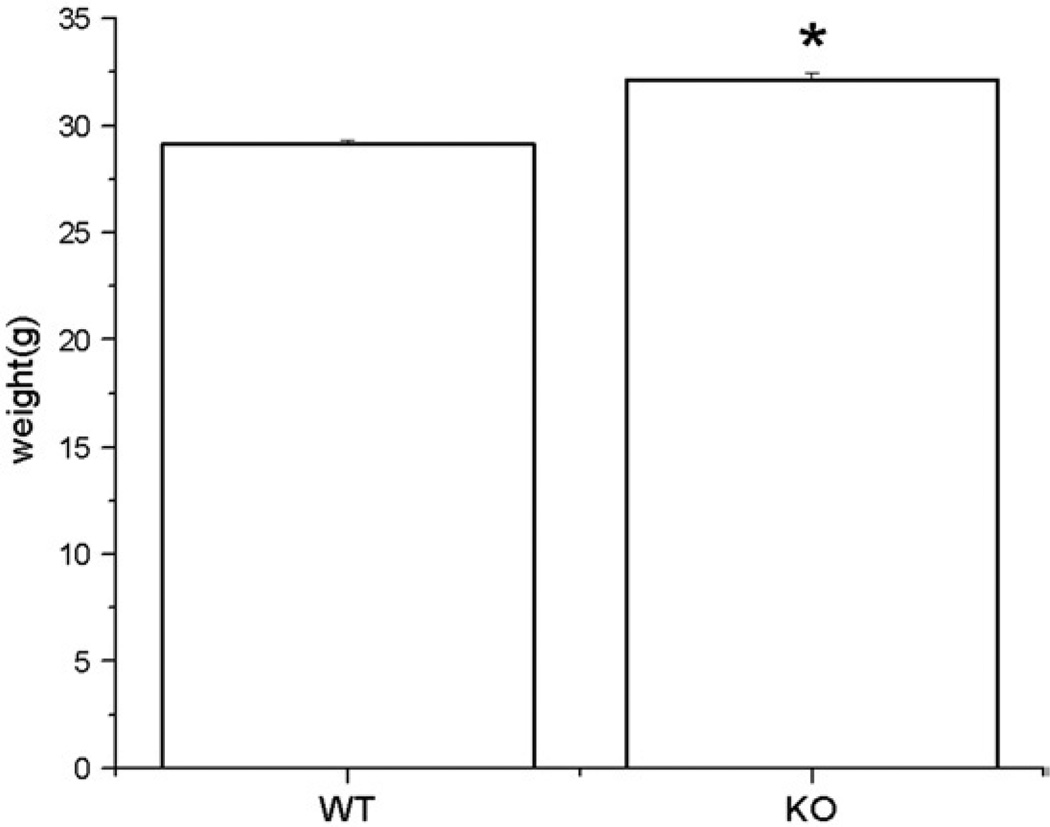
EphA5 knockout mice displayed significantly higher body weight compared to their wild-type littermates prior to any behavioral testing (F(1,94)=53.25, p<0.05), Fig. 1). The difference in body weight between the knockout mice and wild-type littermates was persistent throughout the entire behavioral tasks.
Fig. 1.
Shows the body weight of EphA5 mice. Asterisk indicates that the difference of body weight between groups is statistically significant (p<0.05). Data are given as mean±SEM.
2.2. Altered shock-induced target-biting in EphA5 knockout mice
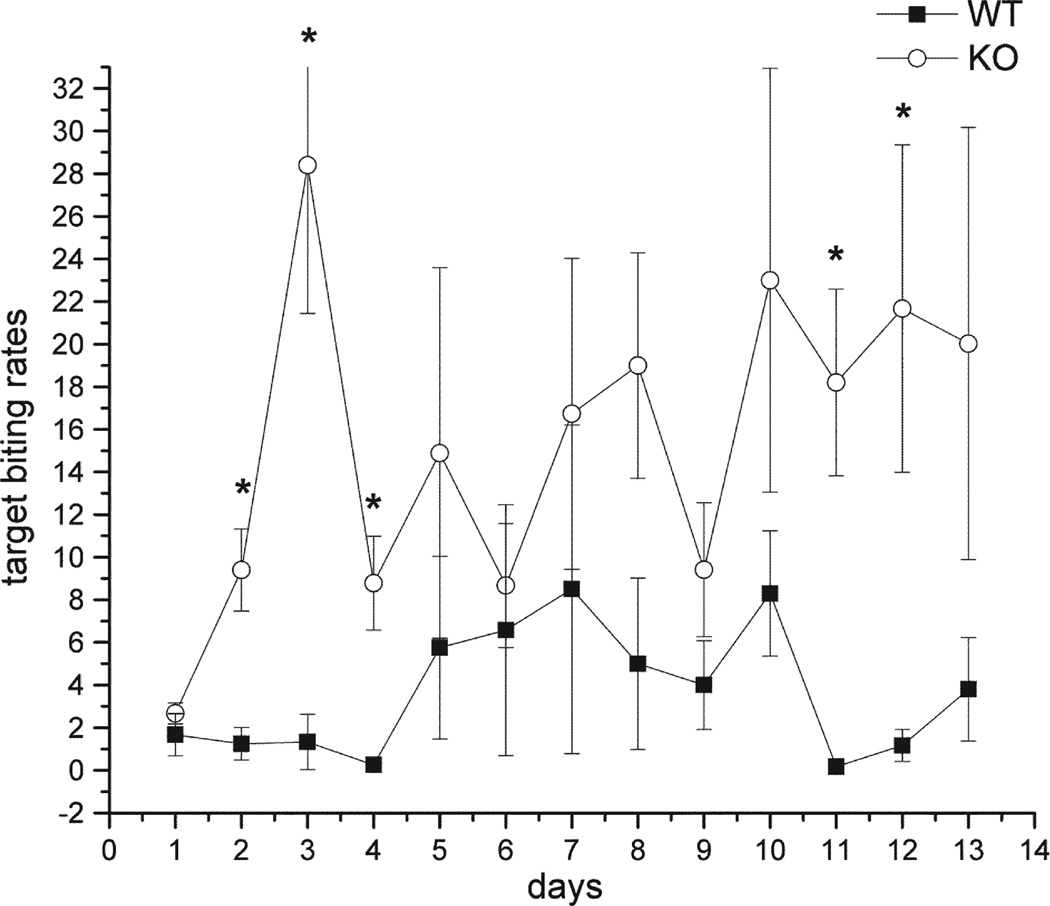
Under baseline conditions, mice exhibited three distinct rates of target-biting, a high post-shock rate (bin 1), an intermediate inter-shock interval rate, (bin2–7) and suppressed rate during the pre-shock tone (bin 8). EphA5 receptor knockout mice exhibited a higher target-biting frequency after the tail shock (post-shock interval) (F(2,5)=9.38, p<0.02) (Fig. 2). Furthermore, there was also a significant increase in target-biting during the inter-shock interval (bin2–bin7) in EphA5 knockout group compared to their wild-type littermates (F(2,5)=6.85, p<0.02). However, this increase in target-biting was not seen during the pre-shock interval in the EphA5 knockout mice, meaning that there was sufficient inhibition of target-biting associated with the presentation of tone in both knockout and wild-type mice. Post-hoc analysis revealed that there were significant differences in post-shock target-biting rates between knockout and wild-type mice on days 2, 3, 4, 11, and 12 (Fig. 2).
Fig. 2.
Tail shock-induced target-biting in the post-shock interval under baseline condition. Two groups are wild-type (■), and EphA5 knockout mice (○). Asterisks indicate the statistical difference in comparing the wild-type group (p<0.05). Data are given as mean±SEM.
2.3. Reduced resident–intruder aggression in EphA5 knockout mice
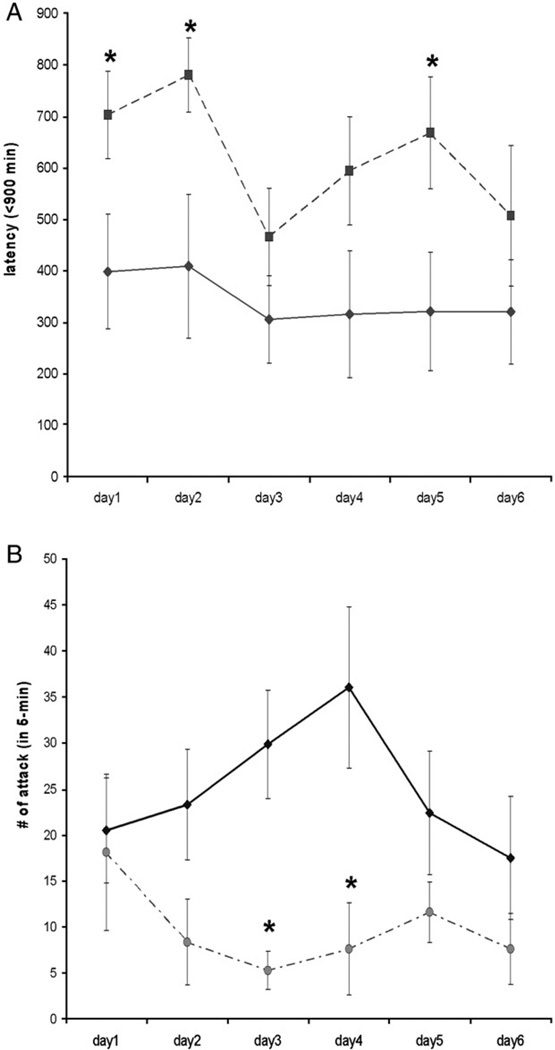
To investigate offensive aggression, we measured the attack behavior exhibited by the resident mice towards the intruder mice. In particular, the latency to the first attack and the total number of bites during testing was compared between the wild-type and the knockout mice. Wild-type mice attacked the naïve intruder with an average of 312.1±71.9 s to the first attack and emitted a total of 24.9±4.2 attacks per session over the 6-day observation period. As the testing continued, the frequency of attack behavior of EphA5 knockout animals was significantly lower than the wild-type littermates (F(1,21)=8.87, p<0.05). Likewise, EphA5 knockout mice displayed longer latencies to attack the intruder (F(1,21)=12.68, p<0.05) (Fig. 3A). Wild-type mice displayed fairly constant attack latencies over the course of testing (Fig. 3B). Finally, in addition to the territorial offensive attack, we also observed altered patterns of behavior exhibited by the EphA5 knockout mice during the course of testing. Encounters with naïve intruders caused a reduction in behavior in EphA5 knockout animals with these residents displaying less activity and initiating fewer encounters with intruders (such as sniffing or moving around the intruder in their home cages) (data not shown).
Fig. 3.
A and B. A: the total amount of attack from the resident animals against the intruders in entire 5 min across days. B: the time to initiate the first attack by the resident subjects in seconds. Solid line represents the control group whereas the dashed line is the EphA5 knockout group. Asterisks indicate the statistical difference in comparing the wild-type group (p<0.05). Data are given as mean±SEM.
2.4. No change in locomotor activity after experiencing intruder encounters in EphA5 knockout mice
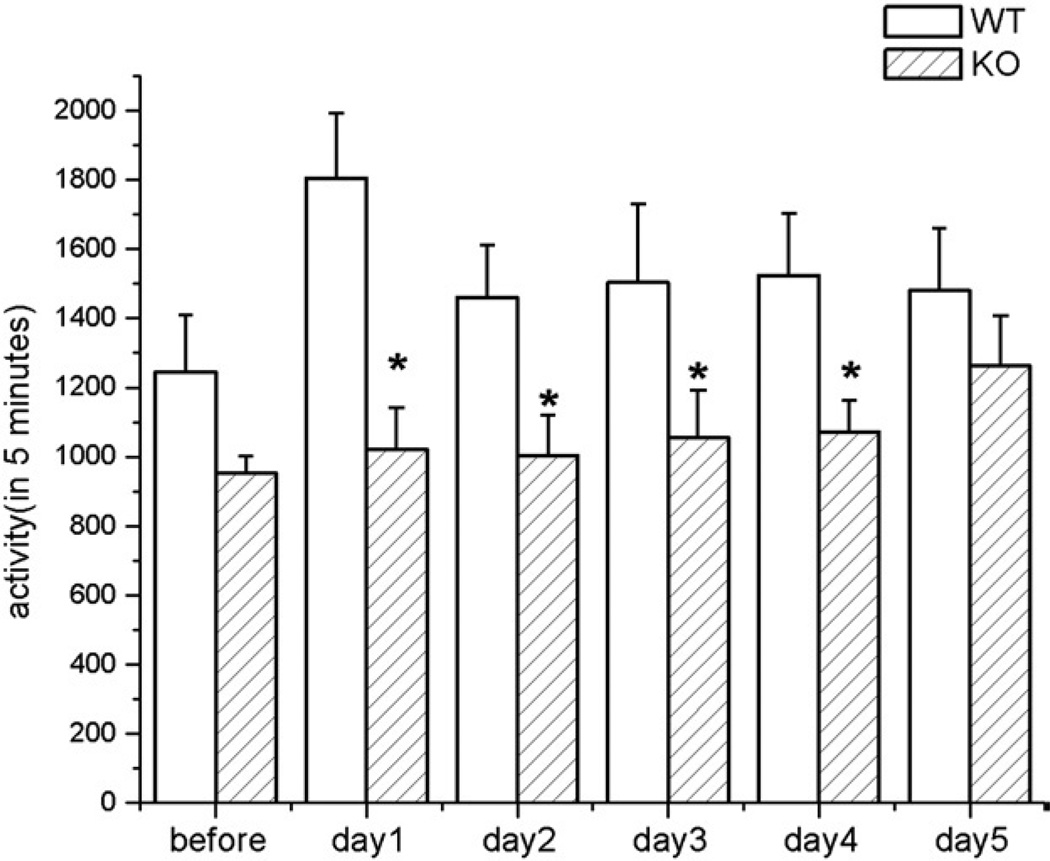
The overall activity level of EphA5 knockout animals was some-what less than the wild-type littermates prior to any behavioral testing (Fig. 4). However, activity counts began to diverge when locomotor activity tests followed immediately after the termination of the resident–intruder testing. EphA5 knockout mice did not display an immediate increase in activity following the resident–intruder testing. Rather the elevation of locomotor activity level was seen gradually over time (Fig. 4). EphA5 wild-type controls, on the other hand, displayed a significantly higher level of activity immediately after testing as compared to their activity level prior to the test (p<0.05). The difference in locomotor activity between EphA5 knockout and wild-type mice after resident–intruder testing was statistically significant (p<0.05) and this difference persisted for four days after the end of testing.
Fig. 4.
The general activity levels taken prior and after the behavioral testing. The open bar represents the EphA5 wild-type animals the diagonal-lined bar is the EphA5 knockout group. Asterisks indicate the statistical difference in comparing the wild-type group (p<0.05). Data are given as mean±SEM.
2.5. Alternations in neurochemistry in EphA5 knockout mice
Because of the significant difference in body weight between EphA5 knockout mice and their littermate controls, we included body weight as a covariant in the statistical analysis for our neurochemical data collected from hypothalamus. This was done so because of the importance of hypothalamus in regulation of food metabolism and body weight and their association with serotonin activity in this brain region (Gur et al., 2003; Svec et al., 2002). It is noteworthy that EphA5 knockout animals exhibited significant increases in serotonin levels in the hypothalamic region (F(2,13)=2.99, p<0.0315) as well as in dopamine levels (F(2,13)=0.0115, p<0.05). In contrast, the turnover ratios of dopamine (DOPAC/DA) (F(2,13)= 4.14, p<0.0406) and serotonin (5-HIAA/5-HT) (F(2,15)=0.05, p<0.05) were both significantly lower in knockout as compared to wild-type mice (Table 1). In the nucleus accumbens, knockout animals exhibited a 41% increase in dopamine levels, whereas dopamine turnover ratio (HVA/DA) was significantly reduced (F(1,15)=7.52, p<0.05). In addition, knockout animals showed approximately 40% decrease in 5-HIAA level and relatively lower 5-HT than the wild-type animals (Table 1). There were no changes in monoamines or monoaminergic turnover ratios in the hippocampus or frontal cortex (data not shown).
Table 1.
Neurotransmitter and metabolite levels in the brain regions of EphA5 knockout and wild-type animals
| DA | DOPAC | HVA | 5-HT | 5-HIAA | HVA/DA | 5-HIAA/5-HT | |
|---|---|---|---|---|---|---|---|
| Hypothalamus | |||||||
| WT | 0.184(0.035) | 0.154(0.041) | 0.290(0.013) | 0.110(0.043) | 0.178(0.04) | 1.95(0.32) | |
| KO | 0.339(0.05)* | 0.145(0.03) | 0.178(0.03) | 0.302(0.05)* | 0.287(0.051) | 1.09(0.24)* | |
| Nucleus accumbens | |||||||
| WT | 3.51(0.503) | 0.71(0.103) | 0.72(0.114) | 0.64(0.141) | 0.35(0.068) | 0.21(0.025) | |
| KO | 4.95(0.575) | 0.8(0.154) | 0.64(0.08) | 0.6(0.114) | 0.25(0.038) | 0.139(0.013)* | |
| Striatum | |||||||
| WT | 11.042(0.857) | 0.796(0.05) | 1.062(0.124) | 0.63(0.049) | 0.304(0.026) | ||
| KO | 12.374(1.408) | 1.036(0.137) | 1.123(0.125) | 0.601(0.068) | 0.283(0.025) | ||
| Hippocampus | |||||||
| WT | 0.051(0.005) | 0.028(0.007) | 0.027(0.005) | 0.495(0.183) | 0.419(0.037) | ||
| KO | 0.063(0.007) | 0.027(0.005) | 0.071(0.024) | 0.446(0.076) | 0.314(0.039) | ||
| Frontal cortex | |||||||
| WT | 0.859(0.428) | 0.289(0.115) | 0.277(0.069) | 0.246(0.028) | 0.176(0.022) | ||
| KO | 1.127(0.325) | 0.297(0.087) | 0.302(0.04) | 0.327(0.06) | 0.204(0.012) | ||
Values expressed as µg/g wet tissue. Numbers in parenthesis represent the standard error of the mean. Asterisks indicate the statistical significance comparing to the wild-type controls. p<0.05.
3. Discussion
Our observations demonstrate that EphA5 knockout mice have an increase in tail shock-induced target-biting in the target-biting paradigm, but a decrease in the number of bites and the latency of initiation the first attack in the resident–intruder test. EphA5 knockout mice did not show changes in their locomotor activity after encountering the intruders while their wild-type littermates exhibited significantly increased activity in their home cage after the encounter. At the neurochemical level, EphA5 mutants showed significantly elevated serotonin concentrations as well as the serotonin metabolite, 5-HIAA, in hypothalamus. When serotonin turnover ratio was used, EphA5 knockout animals exhibited a significant decrease in serotonin metabolism in the same region. Moreover, EphA5 knockout mice had significantly higher body weight than their wild-type littermates. Our results suggest the EphA5 receptor mediated forward signaling may account for these behavioral and neurochemical changes. The alterations of serotonin activity in hypothalamus may contribute to the increase of body weight in EphA5 knockout mice.
3.1. Defensive target-biting response in EphA5 animals
Our observations in the target-biting paradigm show that EphA5 knockout mice exhibited a significant increase in target-biting responses following the tail shock. This increase in target-biting rates was interpreted as an increase in defensive aggression (Carelli and Wagner, 1988; Miyakawa et al., 2001; Smoothy and Berry, 1984; Wagner and Carelli, 1987). It is likely that the absence of the EphA5 receptor forward signaling resulted in changes in the formation of neuronal patterning and connectivity among EphA5 receptor-expressing cells during development. The importance of EphA5 receptor forward signaling has been demonstrated in the mapping of retinotectal projections in mice (Feldheim et al., 2004). Halladay et al. (2004) further demonstrated that animals with a truncated EphA5 receptor could have deficits in striatum-dependent tasks. Nonetheless, the increased target-biting responses by EphA5 knockout mice in the post-shock and inter-shock intervals were specific toward the preceding tail shock, rather than a non-specific learning or general motor deficits. The target-biting rates in the pre-shock interval were similar in both knockout and wild-type animals, suggesting that there was sufficient acquisition of a relationship between the tone and the pending tail shock in these animals.
3.2. Offensive aggressive behavior in the resident–intruder paradigm
In the resident–intruder study, EphA5 animals displayed significantly reduced territorial behaviors directed against naïve intruders. The overall frequency of attack was reduced, as was the time to initiate the first attack. In some cases, the intruders actually approached the resident mouse and the fighting almost started instantly. Under these circumstances, EphA5 knockout mice engaged in fewer interactive behaviors with intruders and exhibited immobility. Furthermore, this immobility persisted in the absence of intruders for an additional four days as assessed in the post aggressive activity test. Overall, EphA5 knockout mice seemed to be less aggressive than the wild-type littermates, displaying fewer attacks and longer latencies to initiate attack. Consistently, EphA5 knockout mice showed immobility in their cages during and after the introduction of intruders. These results are consistent with findings from other reports that the non-aggressive mice showed increased immobility while they displayed long attack latency (Sluyter et al., 1996).
The aversive stimuli used in these two behavioral paradigms, tail shock and naive intruders, elicited strong aggression responses in the mice although these stimuli elicited different forms of aggression. When the animals were challenged by an intruder in the home cage, the initial territorial behavior of EphA5 knockout animals was not different from the wild-type control littermates, but started to decline after the second encounter with the intruder, and the decrease in this form of aggression persisted throughout the repeated assessment thereafter. Furthermore, EphA5 knockout animals engaged in decreased activity in their home cage evident in the later stages of testing. In contrast, the shock-induced target-biting was directed toward an inanimate object and was increased in the knockout mice. It is noteworthy that these behavioral changes in the EphA5 knockout animals are not related to non-specific motor function deficits. The EphA5 knockout animals displayed normal activity levels prior to the start of the aggression tests.
Another possibility that may count for the difference between the results from shock-induced attacks and resident–intruder attacks is that there is no need for visual sense in the shock-induced attacks, whereas the visual sense may play a role in resident–intruder attacks. It has been shown that the retinotectal mapping is altered in EphA5 knockout mice (Feldheim et al., 2004), and this may have detrimental effect on the visual capability of EphA5 knockout mice and reducing their attack behavior. However, the degree that the visual capability is affected by the slight alterations of retinotectal mapping remains to be investigated and we observe no gross behavior defects in these animals. Furthermore, the identification of intruder in the home cage can still be made with non-visual senses, such as olfactory or auditory senses (Guillot and Chapouthier, 1996).
3.3. Neurochemical differences
EphA5 knockout mice exhibited a significant decrement in serotonin turnover in the hypothalamus. This reduced serotonergic activity may account for our observations in the animals' responses following tail shock. The hypothalamus has been shown to play a central role in the mediation of aggressive behaviors in animals (Siegel et al., 1999). Neural circuits underlying aggression are centered in hypothalamus which interconnects with other regions in the brain, including periaqueductal gray (PAG), bed nucleus of the stria terminalis (BST), and amygdala (Delville et al., 2000; Gregg and Siegel, 2001). When a Syrian hamster encounters an intruder, c-Fos immunoreactivity was detected in these brain regions (Delville et al., 2000; Gregg and Siegel, 2001). The electrical stimulation in the ventral medial and intermediate hypothalamus elicits attacking behaviors in rats (Gregg and Siegel, 2001). It is plausible that the decrements of serotonin activity in hypothalamus may have an influential effect on the circuits responsible for the regulation of aggressive behavior in EphA5 knockout mice.
With respect to offensive aggression, the EphA5 knockout mice showed significantly elevated serotonin levels in hypothalamus compared to their wild-type littermates. The increase of serotonin in hypothalamus can explain the decrease in intruder attacks in knockout animals. Accumulated evidence has suggested an inverse relationship between serotonin level in cerebrospinal fluid (CSF) and brain with impulsivity, or escalated aggression, and suicide (Lee and Coccaro, 2001; van Praag, 1998). In rodents, some studies have shown a similar relationship with decreased serotonin and 5-HIAA levels associated with increased offensive aggression (Chiavegatto et al., 2001; Chiavegatto and Nelson, 2003; Ferrari et al., 2005; van der Vegt et al., 2003).
These functional changes in the EphA5 knockout mice were not related to motor deficits. Preliminary studies revealed that these mice animals responded to tone-cued stimuli at rates similar to the wild-type animals in the active-avoidance and passive-avoidance paradigms (data not shown). Furthermore, EphA5 knockout animals displayed normal performance in searching for a hidden platform in the Morris water maze.
A second important neurochemical change in EphA5 knockout mice was the alterations in dopamine and serotonin concentrations in the nucleus accumbens. We have demonstrated in our present study that EphA5 knockout mice had a substantial reduction in serotonin and metabolite (5-HIAA) levels compared to wild-type mice while they had a significant increase in dopamine turnover ratio (HVA/DA), and approximately 41%increase of dopamine level in this region. These data are consistent with a previous study using in vivo dialysis wherein mice displayed similar trends in dopamine and serotonin alteration 24 h following an aggressive confrontation (Ferrari et al., 2003). Our findings in EphA5 knockout mice show similar trends in dopamine and serotonin levels in the nucleus accumbens.
Finally, it appears likely that the abnormal neurochemical profile in the hypothalamic area may result in the increased body weight in the EphA5 knockout mice relative to their wild-type littermate controls. Previous studies have shown that the hypothalamus is essential for the regulation of energy balance, including body weight. Of importance, serotoninergic innervations mediate the control of food consumption, as well as physiological metabolism, and decreased serotonin neural activity has been implicated in an animal model of obesity (Gur et al., 2003; Svec et al., 2002). EphA5 knockout mice exhibited slightly lower activity levels relative to the control mice during the baseline condition. This may be attributed to the increase of their body weight. Thus, we concluded that the absence of EphA5 receptor mediated forward signaling might account for the altered serotonin concentration in hypothalamus, thus influencing offensive and defensive aggression in mice. Furthermore, the increased immobility response in the EphA5 knockout mice may be consequent to the absence of signaling regulated by EphA5 receptor.
4. Experimental procedures
4.1. Subjects and genotyping
EphA5 animals were obtained from Regeneron Pharmaceutical (Tarrytown, New York, USA). The generation of these knockout mice has been described previously (Feldheim et al., 2004). The line was maintained in our colony with EphA5 heterozygous knockout mice used for breeding. All mice were viable and fertile and appeared to be in good health. The genotype of mice was confirmed by polymerase chain reaction (PCR) of genomic DNA obtained from tails prior to the beginning of testing. The three primers used in PCR were: GCC-CGT TAT GAA AGT GCA TCT TTT CC, GCT-GGC GAA AGG GGG ATG TGC, and ACT GGC ATG GAA ATT GGC TCT GG. The 300-bp fragment was amplified from the knockout allele with two of the primers. The DNA polymerase, Taq, was purchased from Promega (Madison, WI). The temperature and timing used within each cycle were 94 °C/1’, 58°C/1’, and 74 °C/1’.
EphA5 knockout and wild-type mice were used for experiments in this study. All mice used for experiments were matched for age and body weight. Mice used as residents in the resident–intruder paradigm as well as those used in the target-biting experiment were individually housed in a pan cage (45 × 23 × 13 cm) with wood-chip bedding for 4–6 weeks prior to the beginning of behavioral testing. The colony room was a temperature- and humidity-regulated room and was maintained on a 12/12 h light/dark cycle (lights on at 7:30PM). All mice had had free access to food and water. All studies were approved by the Rutgers University Animal Care Committee.
4.2. Shock-induced target-biting in EphA5 knockout mice
There were 12 EphA5 knockout mice and 6 wild-type controls in this experiment. Mice were confined in an opaque, plastic cylinder (2.8 cm inner diameter; 9.8 cm long). Their tails were passed through a slot at the rear of the cylinder and taped in position with surgical tape. The cylinder was then placed in a larger outer chamber so that the leading edge of a bite target (model 278–1631 cable ties, Radio Shack) was within easy reach of the mouse. The target was attached to a model 16082 omni directional switch (Gerbrands, Arlington, MA, USA). The tail was rubbed with electrode paste and two brass bar electrodes (1.0 cm apart) placed on the tail approximately 1.5 cm from its base. A GE 1813 session light was mounted 1.0 cm above the target. Each chamber was equipped with an 8.0 cm loudspeaker mounted 10 cm above the target. A 2.0 mA, 0.15 s tail shock was delivered by an AC shock generator on a fixed-time, 2min schedule. A 2500 Hz, 70 dB tone preceded the shock for 15 s and terminated with its onset. Target bites over the 2-min trial were collected in eight 15-s bins, each accumulated over the session. A tail shock was present immediately following the termination of the eighth bin.
For the purpose of data analysis, three intervals, post-shock, pre-shock, and inter-shock, were defined with respect to delivery of the tail shock. The post-shock interval was the 15 s immediately following the tail shock (bin 1); the pre-shock interval was the 15 s prior to the shock delivery and marked by the presence of the tone (bin 8); the inter-shock interval included the intervening six bins (bin 2–6) averaged so as to be comparable to the post- and pre-shock intervals in length. The target-biting sessions lasted 20 min. Each mouse was repeatedly tested for 13 consecutive days in the same environmental context.
4.3. Resident–intruder attack in EphA5 knockout mice
In the resident–intruder paradigm and locomotor activity tests, 12 EphA5 homozygous knockout and 11 wild-type mice were used. In addition, CD1 mice were used as intruders. Resident–intruder studies were conducted by the introduction of a naïve intruder mouse into the home cage of the resident after which the attack behavior of the resident and intruder were recorded by a video camera and scored by observers blind to genotype. The resident mouse was allowed to encounter the intruder for up to 10 min. The latency to the first attack was recorded and was used as the first parameter. Afterwards, the animals were allowed to interact for 5 additional minutes before the intruder animal was removed from the resident’s cage. Over this 5-min session, the number of biting attacks was scored. Should no attack occur during the first 10-min period, a “0” was entered for attacks and “600 s” entered for latency.
4.4. Locomotor activity in EphA5 knockout mice
The locomotor activity of EphA5 animals was measured both prior to the start and after the resident–intruder testing. The home cage of each animal was placed into a photocell activity chamber (Columbus Instrument, Columbus, OH) for 5 min.
4.5. Brain chemistry
The EphA5 homozygous knockout and wild-type animals were sacrificed and brains dissected for portions of striatum, frontal cortex, hippocampus, and amygdala. The brain tissue was immediately stored in liquid nitrogen until assayed. The tissues were thawed, weighed, and ultrasonically homogenized in 0.3 ml of 0.4 N perchloric acid. After centrifugation at 16,000 ×g for 20 min at 4°C, the supernatants were stored at −80 °C until the neurochemical analysis was executed. 60 µl of brain tissue homogenates were injected onto an HPLC column BAS Bio-phase ODS 5 µm, 250/4.6 mm. The mobile phase consisted of filtered and degassed 14% methanol, 12 g citric acid, 19.525 g sodium phosphate dibasic, and 5 mg EDTA per liter. The flow rate was set at 0.7 ml/min. Dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) were electrochemically detected by a detector set at a range of 10nA (100nA for dopamine) and the sample was oxidized with a +0.72 V potential between the glassy carbon electrode and the Ag/AgCl reference electrode. The concentration was calculated by reference to external standards.
4.6. Data analysis
A one-way, repeated measures ANOVA was employed for the resident–intruder experiment and locomotor activity while a standard one-way ANOVA was used for analysis of brain neurochemistry in individual regions. Statistical analysis of target-biting was conducted by a one-way ANOVA with two repeated factors, interval and time. Post-hoc analysis, Tukey’s tests, were used to determine the level of significance with α = 0.05 accepted as statistical significance.
Acknowledgments
We would like to thank Dr. Z. Hu and Dr. B. Liou for training and assistance in genetic screening of mice and Dr. A. Halladay and L. Michna for training in behavioral paradigms. This work was supported by the RO1-DA11480, Johnson & Johnson, Busch Biomedical Research Grant, and Michael J. Fox foundation for Parkinson’s Research.
REFERENCES
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Bioulac B, Benezech M, Renaud B, Noel B, Roche D. Serotoninergic dysfunction in the 47, XYY syndrome. Biol. Psychiatry. 1980;15:917–923. [PubMed] [Google Scholar]
- Brownlee H, Gao PP, Frisen J, Dreyfus C, Zhou R, Black IB. Multiple ephrins regulate hippocampal neurite outgrowth. J. Comp. Neurol. 2000;425:315–322. doi: 10.1002/1096-9861(20000918)425:2<315::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wagner GC. The effects of repeated administration of fluprazine on target biting and intruder-evoked attacks. Psychopharmacology (Berl.) 1988;95:476–481. doi: 10.1007/BF00172958. [DOI] [PubMed] [Google Scholar]
- Castellani V, Yue Y, Gao PP, Zhou R, Bolz J. Dual action of a ligand for Eph receptor tyrosine kinases on specific populations of axons during the development of cortical circuits. J. Neurosci. 1998;18:4663–4672. doi: 10.1523/JNEUROSCI.18-12-04663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatto S, Nelson RJ. Interaction of nitric oxide and serotonin in aggressive behavior. Horm. Behav. 2003;44:233–241. doi: 10.1016/j.yhbeh.2003.02.002. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav. Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J. Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Palanza P, Parmigiani S, de Almeida RM, Miczek KA. Serotonin and aggressive behavior in rodents and nonhuman primates: predispositions and plasticity. Eur. J. Pharmacol. 2005;526:259–273. doi: 10.1016/j.ejphar.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur. J. Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Fischer HS, Zernig G, Schuligoi R, Miczek KA, Hauser KF, Gerard C, Saria A. Alterations within the endogenous opioid system in mice with targeted deletion of the neutral endopeptidase (‘enkephalinase’) gene. Regul. Pept. Lett. 2000;96:53–58. doi: 10.1016/s0167-0115(00)00200-7. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94,253. Psychopharmacology (Berl.) 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD. Ephrins and their receptors: a repulsive topic? Cell Tissue Res. 1997;290:227–241. doi: 10.1007/s004410050927. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Gao PP, Zhang JH, Yokoyama M, Racey B, Dreyfus CF, Black IB, Zhou R. Regulation of topographic projection in the brain: Elf-1 in the hippocamposeptal system. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11161–11166. doi: 10.1073/pnas.93.20.11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, Zhou R. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc. Natl. Acad. Sci. U. S. A. 1998a;95:5329–5334. doi: 10.1073/pnas.95.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WQ, Shinsky N, Armanini MP, Moran P, Zheng JL, Mendoza-Ramirez JL, Phillips HS, Winslow JW, Caras IW. Regulation of hippocampal synaptic plasticity by the tyrosine kinase receptor, REK7/EphA5, and its ligand, AL-1/Ephrin-A5. Mol. Cell. Neurosci. 1998b;11:247–259. doi: 10.1006/mcne.1998.0696. [DOI] [PubMed] [Google Scholar]
- Gao PP, Sun CH, Zhou XF, DiCicco-Bloom E, Zhou R. Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J. Neurosci. Res. 2000;60:427–436. doi: 10.1002/(SICI)1097-4547(20000515)60:4<427::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Shinsky N, Shih A, Williams P, Winer J, Armanini M, Cairns B, Winslow J, Gao W, Phillips HS. Regulation of learning by EphA receptors: a protein targeting study. J. Neurosci. 1999;19:9538–9549. doi: 10.1523/JNEUROSCI.19-21-09538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden RN, Gilmore JH, Corrigan MH, Ekstrom RD, Knight BT, Garbutt JC. Serotonin, suicide, and aggression: clinical studies. J. Clin. Psychiatry. 1991;52(Suppl):61–69. [PubMed] [Google Scholar]
- Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Chapouthier G. Olfaction, GABAergic neurotransmission in the olfactory bulb, and intermale aggression in mice: modulation by steroids. Behav. Genet. 1996;26:497–504. doi: 10.1007/BF02359754. [DOI] [PubMed] [Google Scholar]
- Gur E, Newman ME, Avraham Y, Dremencov E, Berry EM. The differential effects of food restriction on 5-HT1A and 5-HT1B receptor mediated control of serotonergic transmission in the hippocampus and hypothalamus of rats. Nutr. Neurosci. 2003;6:169–175. doi: 10.1080/1028415031000115936. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Tessarollo L, Zhou R, Wagner GC. Neurochemical and behavioral deficits consequent to expression of a dominant negative EphA5 receptor. Brain Res. Mol. Brain Res. 2004;123:104–111. doi: 10.1016/j.molbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hu Z, Yue X, Shi G, Yue Y, Crockett DP, Blair-Flynn J, Reuhl K, Tessarollo L, Zhou R. Corpus callosum deficiency in transgenic mice expressing a truncated ephrin-A receptor. J Neurosci. 2003;23:10963–10970. doi: 10.1523/JNEUROSCI.23-34-10963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons fromrecrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Coccaro E. The neuropsychopharmacology of criminality and aggression. Can. J. Psychiatry. 2001;46:35–44. doi: 10.1177/070674370104600106. [DOI] [PubMed] [Google Scholar]
- Lidberg L, Tuck JR, Asberg M, Scalia-Tomba GP, Bertilsson L. Homicide, suicide and CSF 5-HIAA. Acta Psychiatr. Scand. 1985;71:230–236. doi: 10.1111/j.1600-0447.1985.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Liu GX, Liu S, Cai GQ, Sheng ZJ, Cai YQ, Jiang J, Sun X, Ma SK, Wang L, Wang ZG, Fei J. Reduced aggression in mice lacking GABA transporter subtype 1. J. Neurosci. Res. 2007;85:649–655. doi: 10.1002/jnr.21148. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meima L, Kljavin IJ, Moran P, Shih A, Winslow JW, Caras IW. AL-1-induced growth cone collapse of rat cortical neurons is correlated with REK7 expression and rearrangement of the actin cytoskeleton. Eur. J. Neurosci. 1997;9:177–188. doi: 10.1111/j.1460-9568.1997.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology (Berl.) 1998;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Takao K, Niki H. Differential effect of Fyn tyrosine kinase deletion on offensive and defensive aggression. Behav. Brain Res. 2001;122:51–56. doi: 10.1016/s0166-4328(01)00171-1. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC, Chiavegatto S, Demas GE. Pleiotropic contributions of nitric oxide to aggressive behavior. Neurosci. Biobehav. Rev. 2006;30:346–355. doi: 10.1016/j.neubiorev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schiller L, Jahkel M, Oehler J. The influence of sex and social isolation housing on pre-and postsynaptic 5-HT1A receptors. Brain Res. 2006;1103:76–87. doi: 10.1016/j.brainres.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci. Biobehav. Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol. Biochem. Behav. 1996;54:113–116. doi: 10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Smoothy R, Berry MS. Effects of ethanol on murine aggression assessed by biting of an inanimate target. Psychopharmacology (Berl.) 1984;83:268–271. doi: 10.1007/BF00464792. [DOI] [PubMed] [Google Scholar]
- Stein E, Savaskan NE, Ninnemann O, Nitsch R, Zhou R, Skutella T. A role for the Eph ligand ephrin-A3 in entorhino-hippocampal axon targeting. J. Neurosci. 1999;19:8885–8893. doi: 10.1523/JNEUROSCI.19-20-08885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Svec F, Thompson H, Corll C, Porter J. Levels of hypothalamic neurotransmitters in lean and obese Zucker rats. Nutr. Neurosci. 2002;5:321–326. doi: 10.1080/1028415021000033785. [DOI] [PubMed] [Google Scholar]
- van der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav. Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- van Praag HM. Anxiety and increased aggression as pacemakers of depression. Acta Psychiatr. Scand., Suppl. 1998;393:81–88. doi: 10.1111/j.1600-0447.1998.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Nuutila A, Goodwin FK, Linnoila M. Cerebrospinal fluid monoamine metabolite levels in male arsonists. Arch. Gen. Psychiatry. 1987;44:241–247. doi: 10.1001/archpsyc.1987.01800150053007. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Beuving LJ, Hutchinson RR. Androgen-dependency of aggressive target-biting and paired fighting in male mice. Physiol. Behav. 1979;22:43–46. doi: 10.1016/0031-9384(79)90401-3. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Carelli RM. Effects of fluprazine (DU27716) and ethanol on target biting behavior and intruder-evoked attacks. Psychopharmacology (Berl.) 1987;91:193–197. doi: 10.1007/BF00217061. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat. Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Yue Y, Chen ZY, Gale NW, Blair-Flynn J, Hu TJ, Yue X, Cooper M, Crockett DP, Yancopoulos GD, Tessarollo L, Zhou R. Mistargeting hippocampal axons by expression of a truncated Eph receptor. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10777–10782. doi: 10.1073/pnas.162354599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Cerretti DP, Yu T, Flanagan JG, Zhou R. Detection of ligands in regions anatomically connected to neurons expressing the Eph receptor Bsk: potential roles in neuron-target interaction. J. Neurosci. 1996;16:7182–7192. doi: 10.1523/JNEUROSCI.16-22-07182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. The Eph family receptors and ligands. Pharmacol. Ther. 1998;77:151–181. doi: 10.1016/s0163-7258(97)00112-5. [DOI] [PubMed] [Google Scholar]