Abstract
Conventional MHC class Ia-restricted CD8+ T cells play a dominant role in the host response to virus infections but recent studies indicate that T cells with specificity for nonclassical MHC class Ib molecules may also participate in host defense. To investigate the potential role of class Ib molecules in anti-viral immune responses, Kb−/−Db−/−CIITA−/− mice lacking expression of MHC class Ia and class II molecules were infected with LCMV. These animals have a large class Ib-selected CD8+ T cell population and they were observed to mediate partial (but incomplete) virus clearance during acute LCMV infection as compared to Kb−/−Db−/−®2M−/− mice that lack expression of both MHC class Ia and class Ib molecules. Infection was associated with expansion of splenic CD8+ T cells and induction of granzyme B and IFN© effector molecules in CD8+ T cells. Partial virus clearance was dependent on CD8〈®+ cells. In vitro T cell re-stimulation assays demonstrated induction of a population of ®2M-dependent, MHC class Ib-restricted CD8+ T cells with specificity for viral antigen(s) and a yet to be defined nonclassical MHC molecule(s). MHC class Ib-restricted CD8+ T cell responses were also observed after infection of Kb−/−Db−/− mice, despite the low number of CD8+ T cells in these animals. Long term infection studies demonstrated chronic infection and gradual depletion of CD8+ T cells in Kb−/−Db−/−CIITA−/− mice, demonstrating that class Ia molecules are required for viral clearance. These findings demonstrate that class Ib-restricted CD8+ T cells have the potential to participate in the host immune response to LCMV.
Keywords: MHC, T cells, viral, antigen presentation
Introduction
The mouse genome encodes a large number of nonclassical MHC class I (class Ib) molecules, including many with unknown functions (1, 2). The majority of mouse MHC class Ib genes are encoded at the H2-Q, T, and M region at the telomeric end of the MHC region (1). In general, class Ib genes show little polymorphism relative to the highly polymorphic classical MHC class I genes, H2-K, -D, and –L. Protein folding and expression in most cases requires assembly with β2-microglobulin (®2M). The MHC class Ib tissue distribution and expression levels vary considerably (3). The majority of Class Ib molecules have lower expression level on the cell surface compared to class Ia molecules. Nevertheless, a population of CD8+ T cells can be selected by the Class Ib molecules expressed on hematopoietic cells and thymic epithelia cell in the thymus; the Class Ib restricted CD8+ T cells selected by the hematopoietic cells tends to have a more activated phenotype (4, 5). Some class Ib proteins, including CD1d and MR1, are encoded outside of the MHC. CD1d presents lipid antigens to CD8-negative natural killer T (NKT) cells (6–8). MR1 controls the development and activation of mucosal-associated invariant T (MAIT) cells in a commensal bacteria dependent way, probably through presentation of yet undefined ligands (9, 10). NKT and MAIT express highly restricted TCR repertoires with invariant TCRα chains and restricted TCR Vβ repertoires and they have a capacity to be stimulated rapidly to produce cytokines through a “memory-like” response.
The H2-Q, -T, and –M regions of C57BL/6 mice encode >20 predicted protein-coding class Ib molecules. Several of these have been shown to function in the immune system. Qa-1 (encoded by H2-T23) regulates natural killer (NK) cell activation by serving as the ligand for the CD94/NKG2A inhibitory receptor (11–13). This class Ib molecule predominantly assembles with a peptide derived from the leader sequence of class Ia molecules, H2-D and L. However, Qa-1 also has the capacity to present a variety of self and foreign peptides to TCRαβ T cells (14–18). Qa-2 (encoded by H2-Q6, -Q7, -Q8, and -Q9) assembles with a highly diverse repertoire of endogenous peptides sharing a common peptide-binding motif (19) and functions as an antigen-presenting molecule in tumor immunity (20). H2-M3 selectively binds and presents N-formylated bacterial and mitochondrial peptides to CD8+ T cells (2, 21).
Various studies have implicated class Ib-restricted CD8+ T cells in the host response to intracellular bacterial pathogens. Both Qa-1- and H2-M3-restricted T cell responses can be demonstrated in mice after infection with Listeria monocytogenes (LM) (22–26). The H2-M3-restricted T cell response to LM is characterized by a rapid primary response and relatively little memory response (27, 28). Impaired early bacterial clearance in H2-M3-deficient mice demonstrates a non-redundant role for H2-M3 in LM infection (29). The higher bacterial burden in Kb−/−Db−/−M3−/− mice compared to Kb−/−Db−/− mice at day 7 post LM infection suggests that H2-M3 has protective function against LM infection (30). H2-M3-restricted T cell responses can also be demonstrated in Chlamydia pneumoniae, Mycobacterium tuberculosis, and Salmonella enterica infections (31–34).
Recently, evidence has been published demonstrating that class Ib-restricted CD8+ T cells can also participate in the immune response to viruses. Braaten, et al. demonstrated that class Ib-restricted CD8+ T cells effectively control chronic γ-herpesvirus 68 (γHV68) infections in class Ia-deficient Kb−/−Db−/− mice (35). Virus infection was not controlled in ®2M-deficient animals, whereas it was controlled equivalently in B6 and B6.Kb−/−Db−/− mice. Although the class Ib specificity of the T cells was not identified, a requirement for CD1d was excluded. Swanson et al. demonstrated that Qa-2 (Q9)-restricted CD8+ T cells clear mouse polyoma virus (PyV) infection and check persistent viral replication in Kb−/−Db−/− mice with efficiency similar to that observed in B6 mice (36). The T cell response was observed to be primarily focused on a nonamer peptide from the VP2 capsid protein containing the previously defined Qa-2 peptide-binding motif. These two studies raise the possibility that class Ib molecules might participate in the host response to other virus infections.
A relatively small number of CD8+ T cells are selected by class Ib molecules in the thymus and these cells are prevented from undergoing extensive homeostatic expansion in peripheral lymphoid organs by a numerical excess of conventional class Ia- and class II-restricted T cells. However, class Ib-restricted T cells expand to relatively large populations in the secondary lymphoid organs of Kb−/−Db−/−CIITA−/− mice that lack conventional T cells selected by MHC class Ia or class II molecules (37). These mice provide an interesting model to study the potential role of class Ib-restricted T cells in virus infections. Lymphocytic choriomeningitis virus (LCMV) is a prototype arenavirus that has been studied extensively in its natural rodent host to investigate immune responses in the setting of acute or chronic virus infection. Viral clearance in acute LCMV Armstrong infections is dependent on CD8+ T cells and ®2M (38). H2-Db- and Kb-restricted CD8+ T cells with defined LCMV peptide specificity markedly expand during acute infection to represent as much or more than 70% of the total CD8+ T cells in the spleen at day 8 post infection in B6 mice (39). In the present study, the T cell response to acute LCMV Armstrong infection was evaluated in Kb−/−Db−/−CIITA−/− and Kb−/−Db−/− mice. The results demonstrate that LCMV can elicit β2M-dependent, class Ib-restricted CD8+ T cells that have a capacity to mediate partial virus clearance during the acute phase of virus infection. However, these T cells are insufficient to clear virus and prevent chronic virus infection.
Materials and Methods
Mice
C57BL/6, CIITA−/−, ®2M−/−, and B6.K1(Qa-2−/−) mice were obtained from Jackson Laboratory. The CIITA−/− mice were backcrossed with C57BL/6 mice for 7 generations (Jackson Lab). Kb−/−Db−/− mice were backcrossed to C57BL/6 mice for 10 generations and were obtained from Taconic Farm (40). Kb−/−Db−/−CIITA−/− mice and Kb−/−Db−/−®2M−/− mice were generated by crossing Kb−/−Db−/− mice with either CIITA−/− mice or ®2M−/− mice (37). B6.129S6-H2-T23tm1Cant/J mice, which are Qa-1−/−, were a gift from Dr. Harvey Cantor (Harvard). CD1d−/− mice were a gift from Dr. Luc Van Kaer (Vanderbilt University). H2-M3−/− mice were a gift from Dr. Chyung-Ru Wang (Northwestern University). 8–12 weeks old mice were used in the experiments. Both sex mice were used in the LCMV clearance assay; the mice were sex-matched in other experiments. Mice were housed in a specific pathogen-free facility at the University of Utah and were handled according to the IACUC policies.
Cell lines
Vero cells and BHK-21 cells were cultured in DMEM media supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin and 292 µg/ml L-glutamine (Invitrogen). Mouse primary cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 292 µg/ml L-glutamine, 100 µM non-essential amino acids, 1 mM sodium pyruvate, and 55 µM 2-mercaptoethanol (Invitrogen). All the cells are maintained in humidified 37°C, 5% CO2 incubator.
Virus and infection
LCMV-Armstrong virus was a gift from Dr. Matthew Williams (University of Utah). Virus was maintained in BHK-21 cells, and the titer was obtained by Vero cell plaque assay (41). Briefly, 6×105/well Vero cells were seeded in 6-well plate (Corning Costar) 1 day before the plaque assay, so that they would form confluent monolayers; virus stock were 10-fold serially diluted in DMEM media, and 200 µl of the diluted virus was added on top of the Vero cell monolayer; the plate was incubated for 1 hour before the addition of 0.5% agarose in 199 media (Invitrogen); 4 days later, 0.5% agarose in 199 media containing neutral red was added to the plate, and the plaque number was counted the following day. The infection of mice was achieved by intraperitoneal inoculation with LCMV-Armstrong virus in a dose of 2×105 PFU/mouse. At certain time points after the infection, mouse tissues were harvested for the desired experiments. Tissues collected for plaque assay were weighed and homogenized in DMEM media, followed by 10-fold serial dilutions and incubation with vero cell monolayers to determine virus titers as described (41).
Antibodies and flow cytometry
Mouse-specific TCR® (H57–597), CD4 (GK1.5, RM4–5), CD8〈 (53-6.7), IFN© (XMG1.2), Granzyme B (16G6) monoclonal antibodies, and TCR V® panel antibodies conjugated with FITC, PE, PerCP, APC, PerCP-Cy5.5, PE-Cy5, PE-Cy7, pacific blue or Alexafluor488 fluorophore were purchased from BD bioscience or eBiosience. Suspended single cells were stained with antibodies for detecting surface proteins in 4°C for 20 min. If necessary, intracellular staining was done after surface staining by the BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit according to the manufacture’s instructions. Cells were fixed by 1% PFA after the staining. The fluorescence was detected by FACS Canto II machine (BD bioscicence) and the data was analyzed by Flowjo software (Tree Star).
The MR1 blocking antibody (26.5) used in in vitro stimulation assay was a gift from Dr. Ted Hansen (Washington University School of Medicine).
CD8+ T cell depletion
Mice were intraperitoneally injected with 300 µg CD8 monoclonal antibody (2.43) at day -3, -2, -1 before the LCMV infection, and at day 6 after the LCMV infection. The depletion efficiency was ≥95% based on analysis by flow cytometry.
In vitro restimulation assay
Peritoneal macrophages or bone marrow-derived macrophages were used as antigen presenting cells in the assay. To harvest the peritoneal macrophages, mice were i.p. injected with 100 µg ConA; 3 days after the injection, peritoneal exudates were harvested and 4×105 cells were plated in one well of the 96-well plate; 2 hours after incubation, non-adherent cells were washed away and the remaining macrophage cells were either mock-treated or infected with LCMV-Armstrong for 1 day. Bone marrow cells were harvested from the femur and tibia and cultured in media containing L929 supernatant for 6 days; the non-adherent cells were removed and the remaining adherent cells, which were >99% F4/80+, were harvested and re-plated in 1×105/well as antigen presenting cells; the cells were either mock-treated or infected with LCMV-Arm for 1 day before the addition of responding cells. Spleens from LCMV infected mice were harvested at day 8 post the LCMV infection. Single splenocyte suspensions were made, and CD8+ T cells were enriched using a CD8a+ T cell isolation kit (Miltenyi Biotec), and further purified by staining with CD8 antibody (53-6.7), followed by FACS sorting to get high purified CD8+ T cells, if necessary. 1×105 enriched CD8+ T cells were added as responding cells to the prepared antigen presenting macrophages. The MR1 blocking antibody was added to the antigen presenting macrophages at 5 µg/ml 1 hour before the addition of the responding cells. The cells were co-cultured for 20 hours, 10µg/ml Brefeldin A was added to the cells for additional 4 hours, and the cells were harvested for analysis of IFNγ production by flow cytometry.
Results
Partial clearance of LCMV Armstrong in mice lacking conventional MHC class Ia and class II molecules
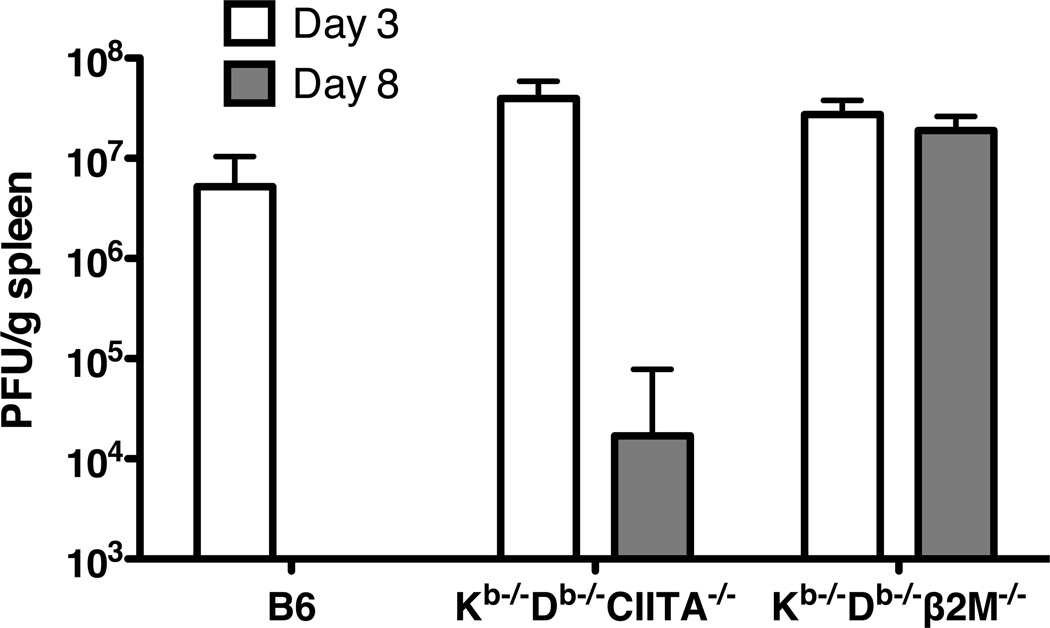
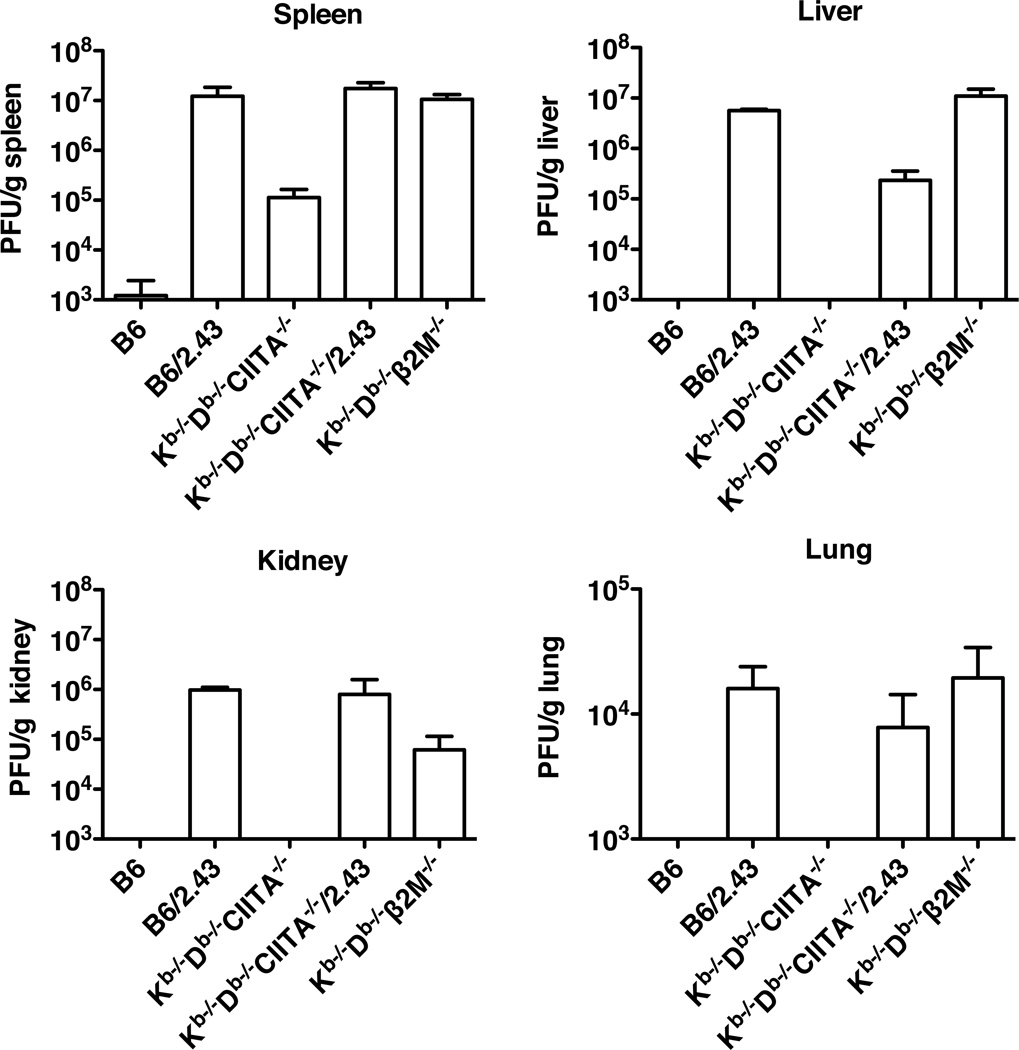
Previous studies have demonstrated that CD8+ T cells and ®2M are required to clear virus during acute LCMV Armstrong infections (38). In B6 mice, the CD8+ T cell response is predominantly focused on three immunodominant peptides presented by H2-Db and an additional epitope presented by H2-Kb (39). We used Kb−/−Db−/−CIITA−/− mice to evaluate the capacity of MHC class Ib-restricted T cells to recognize LCMV antigens and substitute for conventional CD8+ T cells in controlling acute infection. Kb−/−Db−/−CIITA−/− mice, which express class Ib molecules but not class Ia or class II MHC proteins (37), were observed to have lower virus burden and to partially clear LCMV by day 8 post infection as compared to Kb−/−Db−/−®2M−/− mice, which lack expression of both class Ib and class Ia molecules (Fig.1). Viral titers in the spleens of Kb−/−Db−/−CIITA−/− mice were generally reduced 2–3 logs as compared to ®2M-deficient animals. By contrast, B6 mice consistently cleared virus to undetectable levels at day 8 or 12 post infection. These results raised the possibility that T cells with specificity for class Ib molecules may have a capacity to contribute to the host immune response in acute LCMV infection.
Figure 1.
Partial control of LCMV infection in Kb−/−Db−/−CIITA−/− mice. B6, Kb−/−Db−/−CIITA−/−, Kb−/−Db−/−β2M−/− mice were injected i.p. with 2×105 PFU/mouse LCMV-Armstrong. Day 3 and day 8 post infection, virus titers in spleen tissues were measured by plaque assay. 3–4 mice were included in each group; the data represent one of two independent experiments.
Expansion of CD8+ TCRαβ T cells during acute viral infection
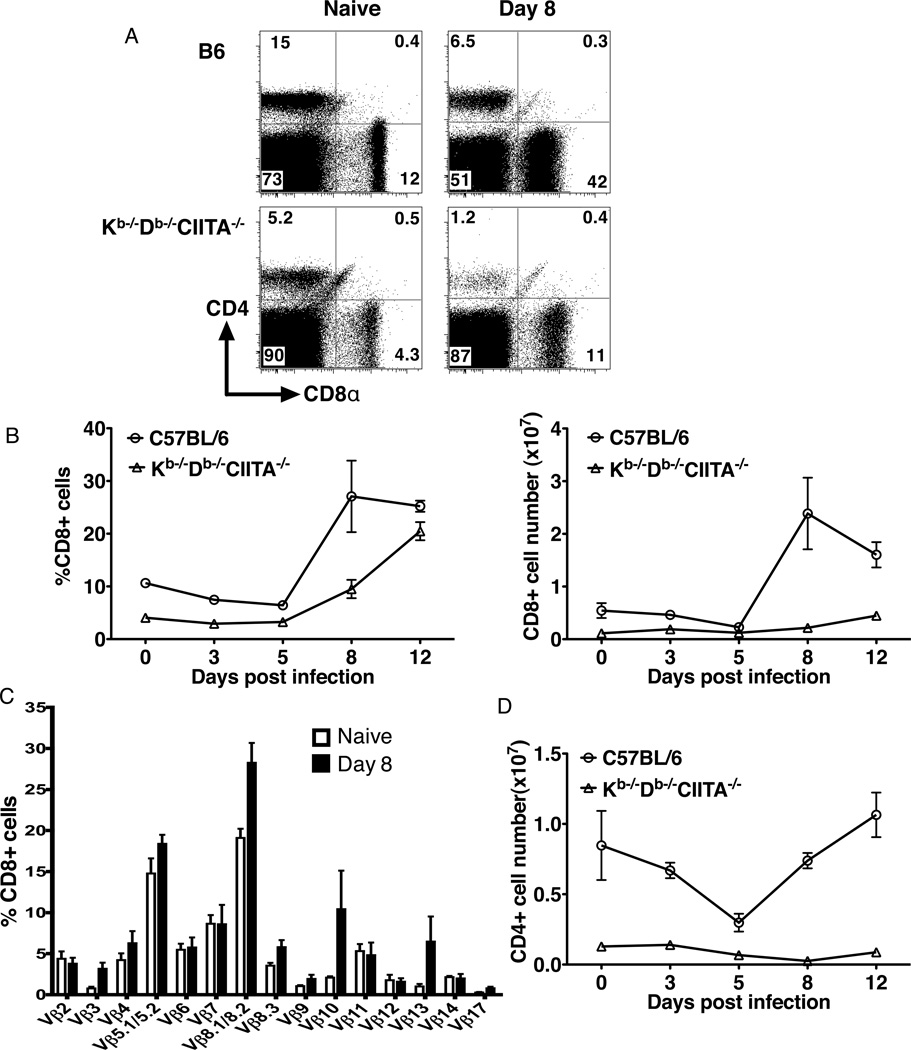
Kb−/−Db−/−CIITA−/− mice have CD1d-restricted CD8- NKT cells, in addition to an expanded population of CD8+ T cells with specificity for class Ib molecules. Similar to B6 mice, an expansion in the percent and total number of CD8+ T cells in the spleen was observed in class Ia-deficient mice during acute LCMV infection (Fig.2A, B), but the total number of CD8+ T cells per spleen was considerably greater in B6 animals before and after infection. CD8+ T cells from class Ia-deficient mice express a diverse TCR repertoire as judged by Vβ chain usage (37, 42). A similarly diverse Vβ repertoire was observed in the CD8+ T cell populations in the spleens of Kb−/−Db−/−CIITA−/− mice 8 days post infection (Fig.2C). However, the post-infection repertoire was skewed as compared to uninfected animals, with increases in the fraction of CD8+ T cells expressing Vβ8, Vβ10, and Vβ13. Despite undetectable expression of MHC class II molecules in Kb−/−Db−/−CIITA−/− mice, a substantial population of CD4+ T cells is present in the peripheral lymphoid organs in these animals (42). As with the CD8+ T cells in these mice, it is likely that the CD4+ T cells have a history of homeostatic expansion. A substantial fraction of the CD4+ T cells appear to represent NKT cells with specificity for CD1d (data not shown). No expansion of CD4+ T cells was observed in Kb−/−Db−/−CIITA−/− mice after acute LCMV infection. Indeed, the absolute number for CD4+ T cells was decreased in spleens after infection (Fig.2D), which may reflect a reduced number of CD1d-restricted NKT cells upon acute LCMV infection (43, 44). Thus, CD8+ T cells selectively undergo expansion during acute LCMV infection of Kb−/−Db−/−CIITA−/− mice.
Figure 2.
CD8+ T cells in Kb−/−Db−/−CIITA−/− mice undergo expansion after LCMV infection. B6 and Kb−/−Db−/−CIITA−/− mice were inoculated i.p. with 2×105 PFU/mouse LCMV-Armstrong. At day 3, 5, 8, 12 post infection, splenocytes were harvested for flow cytometry analysis and enumeration. (A) Representative flow cytometry of CD4+ and CD8+ cells in spleens from naïve and day 8 LMCV infected mice. (B) The percentage and absolute number of CD8+ cells in LCMV-Arm infected mice at different days post infection were analyzed. (C) TCR V® usage in splenic CD8+ T cells from naïve and 8 day LCMV infected Kb−/−Db−/−CIITA−/− mice was determined by flow cytometry. (D) The absolute number of CD4+ cells in the LCMV-Arm infected mice spleen at different days post infection as determined by flow cytometry. These results are representative of at least two independent experiments.
Effector phenotype in virus-expanded CD8+ T cells
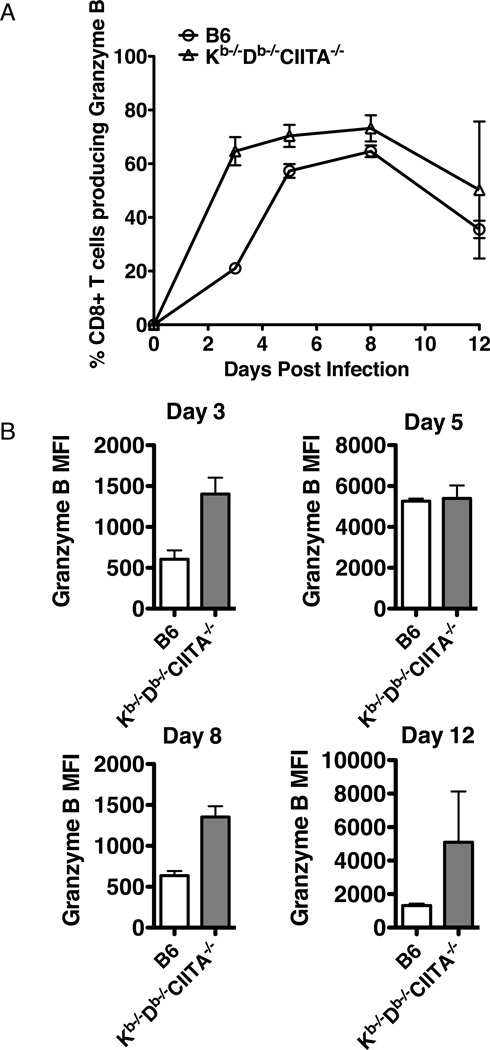
Most of the CD8+ T cells in naïve Kb−/−Db−/−CIITA−/− mice have a memory-like surface phenotype, CD44high, Ly6chigh, β7 integrinlow, and CD122high, reflecting a history of homeostatic expansion (37). This phenotype makes it complicated to evaluate the T cells for effector differentiation after infection. To assess the differentiation state of the virus-expanded CD8+ T cells in Kb−/−Db−/−CIITA−/− mice, we used granzyme B expression as a marker of cells with effector function (45, 46). During the course of an acute LCMV-Armstrong infection, CD8 T cells from Kb−/−Db−/−CIITA−/− mice robustly produced granzyme B throughout the infection and the fraction of CD8+ T cells expressing granzyme B was greater than or equal to that observed in B6 mice (Fig.3A). No granzyme B expression was observed in CD8+ cells from Kb−/−Db−/−CIITA−/− mice prior to infection. The expression level in individual cells exceeded that observed in CD8+ T cells from B6 mice as measured by mean fluorescence intensity in flow cytometric analysis at several time points (Fig.3B). These data suggest that a majority of CD8+ T cells in Kb−/−Db−/−CIITA−/− mice acquire effector function early in the course of LCMV-Armstrong infection and this is sustained for at least 12 days post infection.
Figure 3.
Early and robust LCMV-specific granzyme B production by CD8 T cells during LCMV infection in Kb−/−Db−/−CIITA−/− mice. Splenocytes from LCMV-infected B6, and Kb−/−Db−/−CIITA−/− mice were stained on the surface for CD8〈 and TCR®, then intracellularly for granzyme B and analyzed by flow cytometry. (A) The percentage of granzyme B producing CD8α+TCRβ+ splenocytes from B6 and Kb−/−Db−/−CIITA−/− mice at day 3, 5, 8 and 12 post LCMV-Arm infection. (B) The mean fluorescence intensity (MFI) of granzyme B expression on the CD8〈+TCR®+ cells at day 3, 5, 8 and 12 post infection.
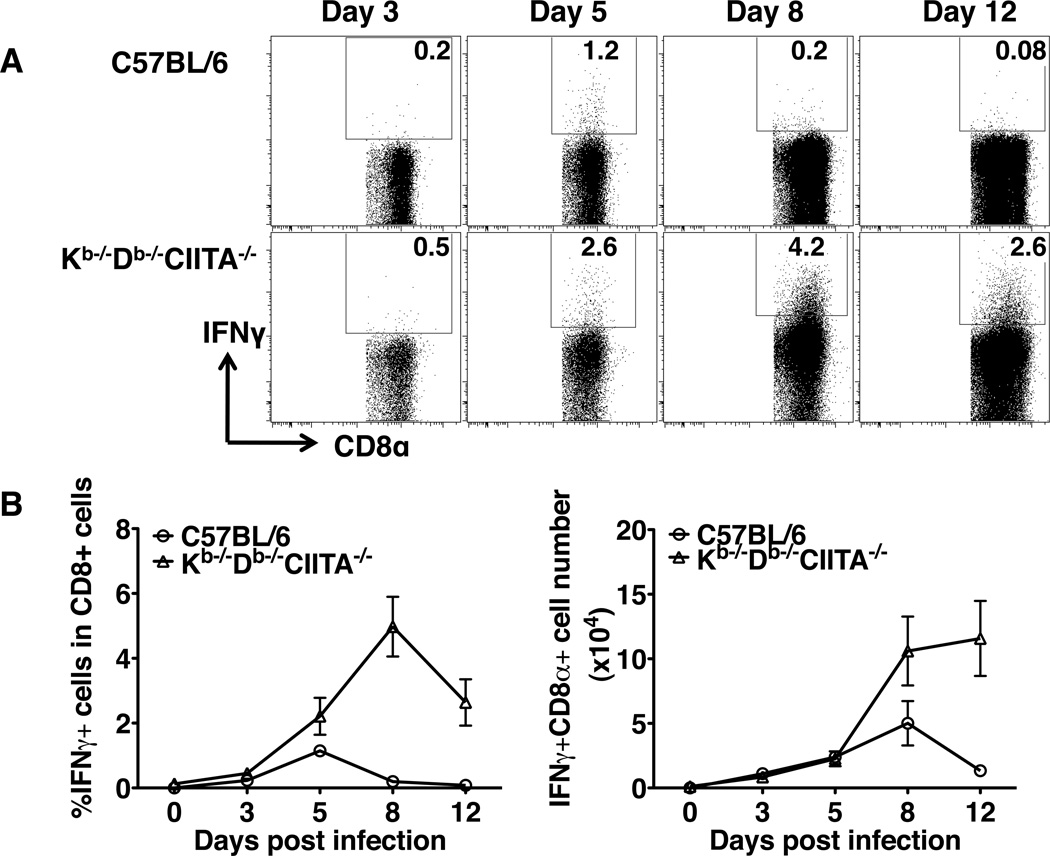
As an additional indicator of effector function, ex vivo production of IFNγ by CD8+ T cells in response to LCMV infection was assessed. At various time points after infection, B6 and Kb−/−Db−/−CIITA−/− mice splenocytes were harvested and permeabilized to evaluate the production of IFN©. A fraction of CD8+ T cells in Kb−/−Db−/−CIITA−/− mice produced IFNγ by day five post LCMV infection (Fig.4A,B). While the peak of the B6 CD8+ T cell IFNγ response was observed at day five post infection, the fraction of IFNγ producing CD8+ T cells continued to expand during the time points analyzed in Kb−/−Db−/−CIITA−/− mice. Similar results were observed with animals injected with brefeldin A 6 hours prior to analysis to inhibit cellular cytokine secretion (47) (data not shown). The continued production of IFNγ in these animals might reflect an ongoing response to viral antigens given that these animals do not fully clear infection. Overall, the results indicate that CD8+ T cells in Kb−/−Db−/−CIITA−/− mice acquire effector functions during acute LCMV infection.
Figure 4.
Substantial ex vivo IFNγ production by CD8+ cells from LCMV-infected mice. (A) B6 and Kb−/−Db−/−CIITA−/− mice were infected with 2 × 105 PFUs LCMV-Armstrong and, at days 3, 5, 8, and 12, spleens were harvested and analyzed for surface CD8〈 and intracellular IFN© by flow cytometry. (B) The percentage and total number of IFN© producing CD8α+ spleen lymphocytes was determined for B6, and Kb−/−Db−/−CIITA−/− mice at various time points post infection.
Requirement for CD8+ cells in controlling virus replication in Kb−/−Db−/−CIITA−/− mice
Depletion experiments were performed to evaluate the role of CD8+ T cells in controlling acute LCMV infection in Kb−/−Db−/−CIITA−/− mice. Mice were injected with CD8 antibody specific for the CD8〈® heterodimer to selectively deplete CD8αβ T cells but not CD8α-expressing dendritic cells or CD8αα-expressing CD8 T cells. Depletion was greater than ninety-five percent in these experiments (data not shown). Based on plaque forming assays, virus was cleared to undetectable levels in the spleen, liver, kidney, and lung of B6 mice at day 8 post infection (Fig.5). As observed previously, virus was still present in the spleens of Kb−/−Db−/−CIITA−/− mice, but titers were reduced 2–3 logs relative to Kb−/−Db−/−β2M−/− mice. In other tissues, virus was reduced to undetectable levels in Kb−/−Db−/−CIITA−/− but not in Kb−/−Db−/−β2M−/− mice. Depletion of CD8+ T cells resulted in complete reversal of virus clearance in B6 and Kb−/−Db−/−CIITA−/− mice. Titers in the organs of CD8-depleted animals were similar to those observed in Kb−/−Db−/−β2M−/− mice. These results suggest that CD8+ T cells are essential for the partial viral clearance observed in mice lacking conventional MHC class Ia- and class II-restricted T cells.
Figure 5.
Control of LCMV-Arm infection in B6 and Kb−/−Db−/−CIITA−/− mice is dependent on CD8+ cells. B6, Kb−/−Db−/−CIITA−/−, and Kb−/−Db−/−β2M−/− mice were either mock treated or i.p. inoculated with CD8-specific antibody (clone 2.43) at day -3, -2, -1 and day 6 of LCMV-Arm infection to deplete the CD8+ T cells. Spleen, liver, kidney, and lung tissues were harvested for plaque assay at day 8 post LCMV-Arm infection.
Direct evidence for a class Ib-restricted CD8+ T cell response to LCMV
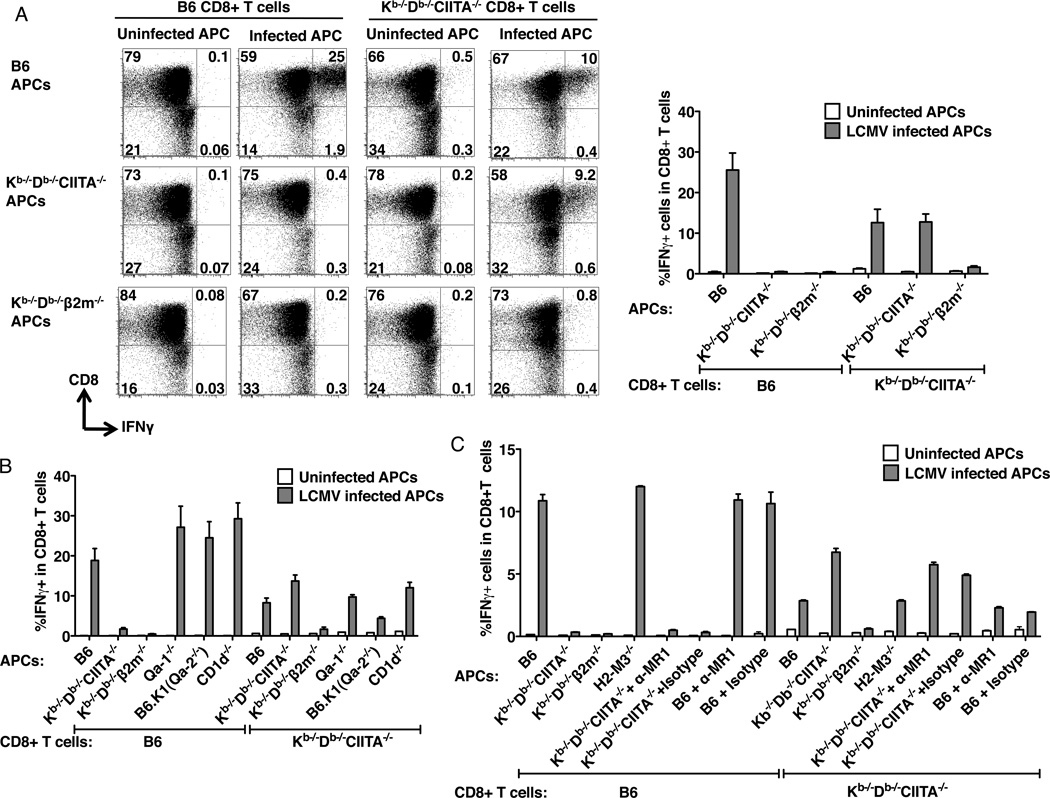
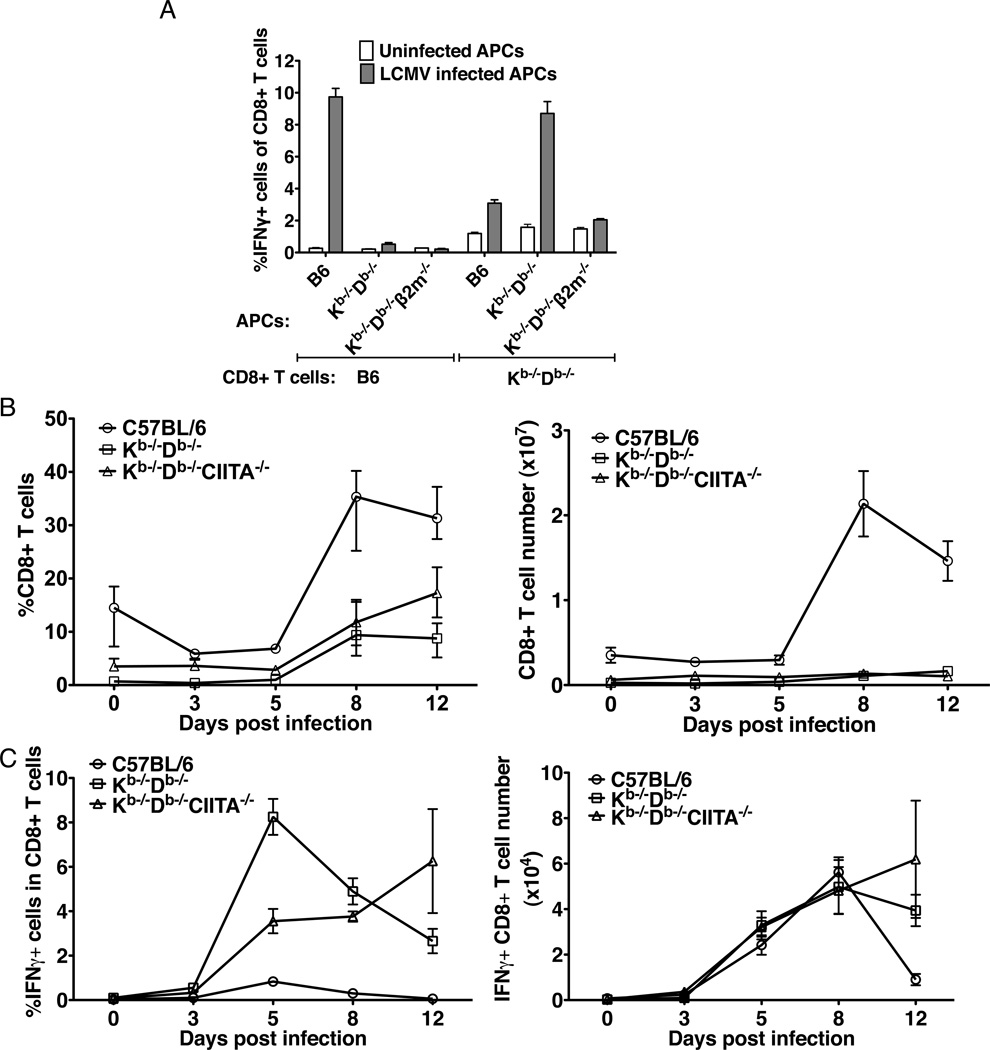
Under certain conditions granzyme B and IFNγ production can be induced directly in memory phenotype CD8+ T cells by cytokines such as IL-15 in the absence of TCR-mediated antigen recognition (48). Given the memory-like phenotype of CD8+ T cells in Kb−/−Db−/−CIITA−/− mice, we could not exclude the possibility that expansion of CD8+ T cells and acquisition of effector functions were induced through nonspecific bystander mechanisms as opposed to direct antigen recognition by class Ib-restricted T cells. In vitro assays were performed to investigate the specificity of CD8+ T cells from LCMV-infected Kb−/−Db−/−CIITA−/− mice. Initial experiments demonstrated that CD8+ T cells from LCMV-infected B6 mice produce IFN© during 6 hour co-culture assays with LCMV-infected syngeneic macrophages, measured by intracellular cytokine staining and flow cytometry. However, under identical conditions, no response was observed in T cells from LCMV-infected Kb−/−Db−/−CIITA−/− mice (data not shown). A variety of experimental parameters were analyzed and we found that in vitro responses were observed if the culture period was extended. A large fraction of CD8+ T cells purified from 8 day post infection Kb−/−Db−/−CIITA−/− mice produce IFN© after an 24 h culture with LCMV-infected but not uninfected syngeneic macrophages (Fig.6A). LCMV-immune CD8+ T cells from B6 mice respond in vitro to infected macrophages from B6 but not Kb−/−Db−/−CIITA−/− or Kb−/−Db−/−β2M−/− mice, indicating that the T cell response in these animals is dominated by class Ia-restricted T cells. By contrast, CD8+ T cells from infected Kb−/−Db−/−CIITA−/− mice respond equally well to infected peritoneal macrophages from B6 and Kb−/−Db−/−CIITA−/− mice. However, they do not respond to infected macrophages from Kb−/−Db−/−β2M−/− mice. These results provide direct evidence that LCMV infection induces a population of antigen-specific, ®2M-dependent MHC class Ib-restricted CD8+ T cells in mice lacking conventional class Ia and class II molecules.
Figure 6.
MHC class Ib-restricted CD8+ T cells from LCMV-infected Kb−/−Db−/−CIITA−/− mice. (A) Peritoneal macrophages were harvested from ConA inoculated B6, Kb−/−Db−/−CIITA−/−, or Kb−/−Db−/−®2M−/− mice as a source of antigen presenting cells (APCs). APCs were either uninfected or infected with LCMV-Arm for 1 day. At day 8 post LCMV-infection, splenocytes were harvested from B6 or Kb−/−Db−/−CIITA−/− mice and CD8+ T cells were enriched and co-cultured with APC for 1 day, followed by analysis of IFN© production by flow cytometry. Left: Representative flow cytometry plots showing the production of IFN© by cultured CD8+ T cells. Right: The percentage of IFN©+ CD8+ T cells. T cells were obtained from 3 mice for each group. The data represent one of three independent experiments. (B) B6, Kb−/−Db−/−CIITA−/−, Kb−/−Db−/−β2m−/−, Qa-1−/−, B6.K1(Qa2−/−), and CD1d−/− bone marrow-derived macrophages were used as APCs. CD8+ T cells enriched from day 8 infected mice were co-cultured with the LCMV uninfected or infected APCs for 1 day, and the production of IFN© was measure by flow cytometry. (C) B6, Kb−/−Db−/−CIITA−/−, Kb−/−Db−/−β2m−/−, and H2-M3−/− bone marrow-derived macrophages were used as APCs. MR1 blocking antibody was added as indicated to the B6 or the Kb−/−Db−/−CIITA−/− APCs in 5µg/ml to block the MR1 molecule. CD8+ T cells enriched from day 8 infected mice were co-cultured with the LCMV uninfected or infected APCs for 1 day, and the production of IFNγ was measure by flow cytometry.
To further study the MHC restriction of the CD8+ T cells in the Kb−/−Db−/−CIITA−/− mouse, macrophages derived from the bone marrow of Qa-1−/−, B6.K1(Qa-2−/−), CD1d−/−, H2M3−/− mice were employed as the antigen presenting cells in the in vitro restimulation assay (Fig. 6B,C). MR1 antibody (clone 26.5) was also used in the in vitro restimulation assay to block the MR1 molecule (Fig.6C). Although a lower response was observed when the Kb−/−Db−/−CIITA−/− CD8+ T cells were stimulated by the LCMV infected Qa-2−/− macrophages, the response is not fully reduced. There was no significant difference observed between the Kb−/−Db−/−CIITA−/− CD8+ T cells response to the other Ib deficient macrophages and the control macrophages. Blocking MR1 didn't inhibit the response. No dominant MHC Class Ib restricted response was found. It is possible that the polyclonal anti-LCMV CD8+ T cell response might be restricted by multiple MHC class Ib molecules in Kb−/−Db−/−CIITA−/− mice, or the T cells may have specificity for a yet to be defined MHC class Ib molecule.
Response of Kb−/−Db−/− mice to the LCMV infection
Kb−/−Db−/− mice, deficient in MHC class Ia but not class II molecules, have normal numbers of CD4+ T cells and very few CD8+ T cells. Although few in number, the CD8+ T cells in these animals are thought to be selected to by MHC class Ib molecules, and we were interested in determining whether a class Ib-restricted T cell response would be observed after LCMV infection. Indeed, the CD8+ T cells from day 8 infected Kb−/−Db−/− mice respond to syngenic LCMV infected macrophages, but not to the Kb−/−Db−/−β2m−/− macrophages (Fig.7A). Consistent with a specific immune response, the fraction and absolute number of spleen CD8+ T cells expanded after acute LCMV infection (Fig.7B). A marked expansion of CD8+ T cells with effector phenotype was also observed as measured by ex vivo IFNγ production (Fig.7C) and granzyme B (data not shown).
Figure 7.
Class Ib-restricted T cells are induced in LCMV-Arm infected Kb−/−Db−/− mice. (A) B6 and Kb−/−Db−/− CD8+ T cells from day 8 infected mice were restimulated with B6, Kb−/−Db−/− or Kb−/−Db−/− β2m−/− bone marrow-derived macrophages which were uninfected or infected with LCMV. (B) The percentage and number of spleen CD8+ T cells in naïve and day 3, 5, 8, 12 LCMV-Arm infected B6, Kb−/−Db−/− and Kb−/−Db−/−CIITA−/− mice. (C) The IFN©+ CD8+ T cell percentage and number in the naïve, day 3, 5, 8, 12 LCMV-Arm infected B6, Kb−/−Db−/− and Kb−/−Db−/−CIITA−/− mice spleen. The data represent one of two independent experiments.
Chronic virus infection in Kb−/−Db−/−CIITA−/− mice
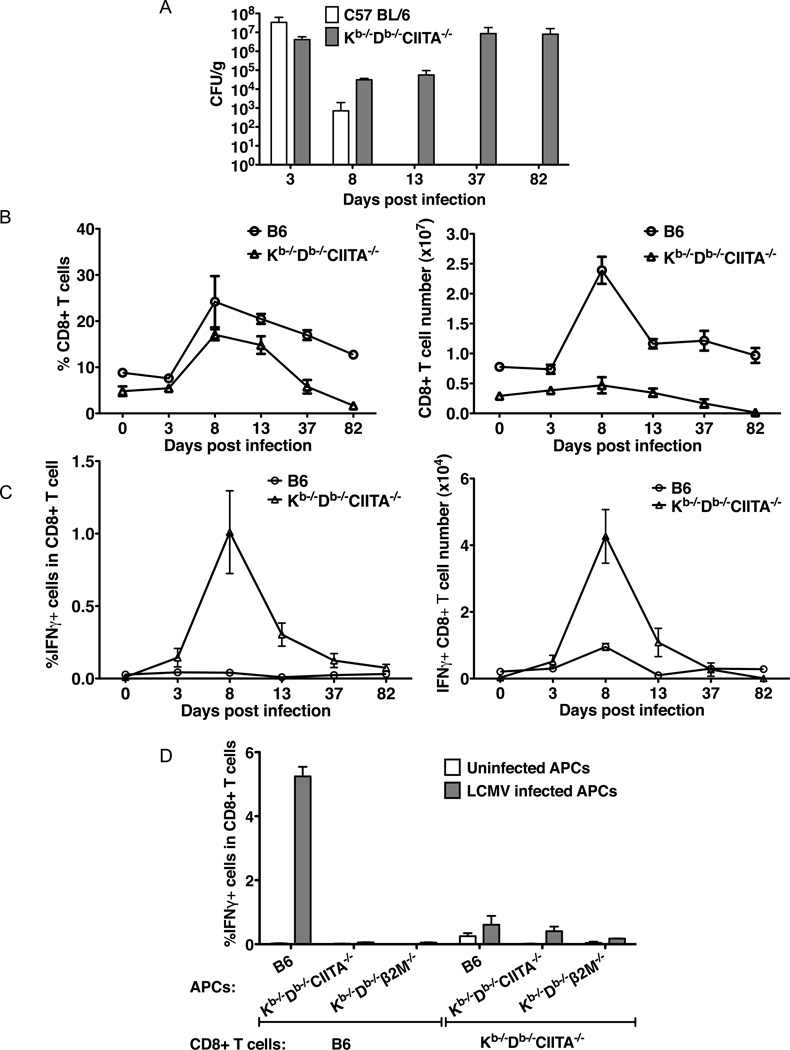
Analysis of the acute phase of virus infection demonstrated that substantial titers of virus were present in spleen at day 8 (and 12) post infection in Kb−/−Db−/−CIITA−/− mice. Long term infection experiments were performed to determine if virus is eventually cleared in these animals. As shown in Fig.8A, continued viral replication occurs after the initial period of infection such that very high viral titers are present at day 37, similar to titers present at day 3 post infection. Thus the class Ib-restricted CD8+ T cells in Kb−/−Db−/−CIITA−/− mice are insufficient to clear LCMV, and a state of chronic infection is established. The vast majority of chronically infected Kb−/−Db−/−CIITA−/− mice survive for at least 80 days. However, spleen CD8+ T cells and IFNγ-producing T cells continuously decline in number (Fig.8B,C) such that they are almost undetectable by day 82 post infection. Little or no response is detected with T cells from Kb−/−Db−/−CIITA−/− mice collected at late time points after infection based on in vitro restimulation experiments (Fig.8D). The observed depletion of CD8+ T cells during the course of chronic infection in Kb−/−Db−/−CIITA−/− mice may result from chronic activation induced cell death combined with a very low thymic output of class Ib-restricted T cells.
Figure 8.
Long-term infection. Groups of B6 and Kb−/−Db−/−CIITA−/− mice were infected with 2×105 PFU LCMV-Arm. At day 3, 8, 13, 37, 82 post infection, a minimum of 3 mice of each strain were characterized to determine the virus titer in the spleen tissue, and the CD8+ T cell expansion and INFγ production were determined. (A) Virus in spleen was measured by plaque assay. (B) The kinetics of spleen CD8+ T cell expansion and contraction. (C) The kinetics of ex vivo IFNγ production by CD8+ T cells. (D) In vitro restimulation of CD8+ T cells from day 49 infected mice. The CD8+ T cells from day 49 infected mice were co-cultured with infected or uninfected B6, Kb−/−Db−/−CIITA−/−, Kb−/−Db−/−®2m−/−bone marrow-derived macrophages for 1 day, and the IFN© production was measured by flow cytometry.
Discussion
In the present study, Kb−/−Db−/−CIITA−/− mice lacking conventional class Ia and class II MHC molecules were used to investigate the potential for class Ib-selected T cells to mount an antigen-specific effector response to LCMV infection. These animals were observed to mediate partial but incomplete virus clearance during acute LCMV Armstrong infection. However, clearance is incomplete and a chronic infection follows. Infection was associated with an initial expansion of splenic CD8+ T cells and induction of granzyme B and IFN© effector molecules in this population of cells, followed by a continuous decline and ultimate depletion of CD8+ T cells. CD8-depletion experiments demonstrated that partial viral clearance in the early phases of infection was dependent on CD8αβ+ cells. In vitro T cell stimulation assays demonstrated the induction of a population of LCMV-specific, MHC class Ib-restricted CD8+ T cells in both Kb−/−Db−/−CIITA−/− and Kb−/−Db−/− animal strains.
The most definitive evidence for a class Ib-restricted T cell response to LCMV comes from in vitro assays demonstrating cytokine production by T cells cultured with LCMV-infected antigen presenting cells. Antigen presentation to T cells from Kb−/−Db−/−CIITA−/− and Kb−/−Db−/− mice required β2M but not Kb or Db class Ia molecules. This contrasts with the in vitro recall response of T cells from B6, which is clearly dominated by class Ia-restricted T cells, with very little response induced by Kb−/−Db−/−CIITA−/− or Kb−/−Db−/− APC. A key element required to establish an assay for detecting an in vitro response of LCMV-immune T cells from Kb−/−Db−/−CIITA−/− mice was the requirement for a longer culture period. Very strong IFNγ responses were observed by intracellular staining after 6 h of co-culture of CD8+ T cells from LCMV-immune B6 mice with infected APC. Under the same conditions, no response was observed with T cells from Kb−/−Db−/−CIITA−/− mice. However, a substantial fraction of T cells were observed to produce IFNγ after 24 h of culture. The delayed kinetics of cytokine production could reflect inherent differences in the function of class Ib- and class Ia-restricted T cells, delayed kinetics of class Ib ligand generation in infected APC, or some form of early CD8+ T cell exhaustion in Kb−/−Db−/−CIITA−/− mice (49, 50), making the T cells more resistant to re-stimulation in vitro.
Kb−/−Db−/−CIITA−/− mice have markedly expanded numbers of peripheral CD8+ T cells compared to Kb−/−Db−/− mice because they lack conventional class II-restricted CD4+ T cells that compete for homeostatic factors and inhibit the peripheral expansion of the relatively small number of CD8+ T cells selected in the thymus by class Ib molecules in Kb−/−Db−/− mice (37). CD8+ T cells in Kb−/−Db−/−CIITA−/− mice have a memory-like phenotype as a result of their history of extensive homeostatic expansion, and thus they may be more sensitive to stimulation than the small population of class Ib-selected CD8+ T cells present in mice bearing conventional class Ia molecules. CD8+ T cells in Kb−/−Db−/− mice also have a memory-like phenotype that probably results from some degree of homeostatic expansion that may still occur in an environment with excess CD4+ T cells. Alternatively, it is possible that class Ib-selected T cells acquire a memory-like phenotype as a consequence of alternative developmental pathways in the thymus (4, 51). Our results indicate that class Ib-restricted T cell responses to LCMV are induced in both Kb−/−Db−/−CIITA−/− and Kb−/−Db−/− mice, despite differences in the extent of homeostatic expansion of class Ib-restricted CD8+ T cells, and the expression of MHC class II molecules and the presence of CD4+ T cells. However, the potential participation of class Ib-restricted T cells in the response to LCMV infection in wild-type B6 mice remains to be explored.
In contrast to B6 mice, Kb−/−Db−/−CIITA−/− mice do not clear the acute LCMV Armstrong infection. Virus clearance in B6 mice is absolutely dependent on CD8+ T cells and it does not require CD4+ T cells (38). The partial control of virus observed at day 8 post LCMV infection in Kb−/−Db−/−CIITA−/− mice is also dependent on the CD8+ T cells. Our results demonstrate that class Ib-restricted CD8+ T cells in Kb−/−Db−/−CIITA−/− mice are less effective than conventional CD8+ T cells in controlling LCMV infection. It is possible that clearance would be observed if greater numbers of class Ib-restricted T cells were present. Alternatively, differences in the effector function, ligand density, or the (remaining) expansion potential of class Ib-restricted CD8+ T cells may preclude viral clearance. In addition, continuous activation and expansion of a relatively small number of precursor cells in the setting of high viral replication may make this unconventional population of T cells more susceptible to exhaustion and depletion (52, 53).
The H2-M3-restricted CD8+ T cell response in acute infection with Listeria monocytogenes is observed to occur more rapidly than the class Ia-restricted T cell response and the class Ib-restricted T cells appear to comprise a major fraction of the responding T cell population during acute infection (21, 27). By contrast, memory development is defective in the H2-M3-restricted response, potentially a result of inefficient competition with class Ia-restricted T cells for interaction with dendritic cells during secondary infection (27, 28, 55). T cells with specificity for H2-M3 represent only a small fraction of total antigen-specific cells in recall responses. Thus, in this model, class Ib-restricted T cells appear to function as a bridge between innate and adaptive immunity in acute infection, with little contribution to immunological memory. In LCMV-infected Kb−/−Db−/−CIITA−/− mice, we observed early expression of granzyme B in CD8+ T cells. IFNγ production in vivo appeared to have similar early kinetics relative to T cells from B6 mice, but with a progressive increase until at least day 12 post infection, long after the disappearance of IFNγ-producing T cell in B6 animals. The continued production of IFNγ may reflect an ongoing T cell response to residual virus in the mutant mice. A determination of whether class Ib-restricted T cells participate in the early stages of the host response to LCMV in mice expressing a normal complement of MHC molecules will require identification of the specificity of the class Ib-restricted T cell response.
Braaten, et al. were first to report that class Ib-restricted CD8+ T cells can effectively control a chronic viral infection (35). Kb−/−Db−/− but not ®2M-deficient mice were observed to control γ-herpesvirus 68 (γHV68) infection through a mechanism dependent on TCRαβ+ CD8+ T cells. This was associated with a marked increase in CD8+ T cells in the spleen at day 42 post infection. The expanded CD8+ T cells acquired an effector/memory phenotype based on cell surface phenotype and the capacity to produce IFNγ and TNFα after in vitro stimulation with PMA and ionomycin. A dramatic skewing the TCR Vβ repertoire of CD8+ T cells was observed in Kb−/−Db−/− mice after γHV68 infection, with >70% of cell expressing Vβ4. Despite highly restricted Vβ utilization, analysis of CDR3 length demonstrated considerable diversity in the responding T cell repertoire. Analysis of TCR Vβ utilization in CD8+ T cells from LCMV-infected Kb−/−Db−/−CIITA−/− mice demonstrated some skewing in the Vβ repertoire as compared to uninfected mice, but the repertoire remained very diverse in the virus-expanded T cells, in contrast to the results in Kb−/−Db−/− mice with γHV68 (35). This might indicate that the class Ib-restricted T cell response to LCMV is more diverse in its specificity as compared to the response to γHV68.
The recent study by Swanson et al. was the first to define the specificity of class Ib-restricted immune response to a virus (36). In contrast to ®2M-deficient animals, Kb−/−Db−/− mice were observed to rarely develop tumors after infection with oncogenic mouse polyoma virus. Like class Ia-expressing B6 mice, Kb−/−Db−/− mice were able to clear acute PyV infection and to check persistent viral infection. In contrast to our results with LCMV, no appreciable increase in the number of CD8+ T cells was detected in spleens until 25 days post infection. Indeed, antigen specific T cells were observed to slowly accumulate until 30–70 d post infection. The induction of IFNγ production by in vitro peptide stimulation of polyclonal CD8+ T cells from PyV-infected Kb−/−Db−/− mice was difficult to demonstrate (36). It is possible that cytokine responses would be observed in this system after longer restimulation cultures, similar to our findings with class Ib-restricted T cells induced by LCMV. CD8+ T cell clones and MHC tetramers were used to demonstrate that the class Ib-restricted T cell response to PyV is highly focused on a single epitope from the VP2 capsid protein presented by Q9 (Qa-2). T cells with this specificity represented 40% of the CD8+ T cells in the spleen at day 80 post infection. Despite a capacity to control PyV infection, the class Ib-restricted T cells showed a pronounced functional impairment, possibly resulting from persistent infection, chronic stimulation, and partial CD8+ T cell exhaustion.
The specificity of the class Ib-restricted CD8+ T cell response to LCMV identified in the current study was studied using MHC cass Ib deficient mice and an MR1 blocking antibody. Antigen-specific, class Ib-restricted CD8+ T cell responses have been demonstrated for Qa-1, Qa-2, and H2-M3. The results using APC from Qa-1-deficient donor mice suggest that Qa-1 does not play a significant role in this response. H2-M3, with its strong preference for binding N-formylated bacterial and mitochondrial peptides, seems an unlikely candidate for presentation of viral epitopes. It is notable, however, that recognition of a nonformylated influenza virus hemagglutinin peptide by H2-M3-restricted cytotoxic T cells has been described (56–58). Nevertheless, there was no significant reduction observed in the restimulation response when the Kb−/−Db−/−CIITA−/− CD8+ T cells was co-cultured with infected H2M3−/− APCs. Responses were slightly reduced with APC from Qa-2-deficient B6.K1 mice, but we cannot rule out the potential impact of differential expression of other molecules in this case. No effect was observed with an MR1 blocking antibody, although we still cannot absolutely rule out participation of this molecule. The murine H2-Q, -T, and –M regions of C57BL/6 mice encode >20 predicted protein-coding class Ib molecules. Several, including thmymic leukemia antigen (TL, encoded by T3/T18d) and T10/T22 do not bind peptides (59, 60). TL interacts with high affinity to CD8αα and regulates the function of CD8 αα + intraepithelial lymphocytes (61). T22 is a ligand for a population of TCRγδ T cells (60, 62). However, a large number of class Ib molecules with potential antigen presentation function remain to be characterized. While class Ib-selected T cells represent a relatively small fraction of the total CD8+ TCRαβ repertoire, there is growing evidence for their potential participation in the immune response to a diverse array of microbial pathogens.
Acknowledgements
The authors are grateful to Dr. Matthew Williams for critical reagents and advice, and Dr. Atilla Kumanovics for helpful discussions. We thank David Coe and Hu Dai for excellent technical assistance.
Abbreviations
- APC
antigen presenting cell
- ®2M
®2-microglobulin
- ConA
concanavalin A
- LCMV
lymphocytic choriomeningitis virus
- LM
Listeria monocytogenes
- NKT
natural killer T
- PyV
polyoma virus
Footnotes
This work was supported by National Institute Health Grants AI33614 and AI20554.
Disclosures
The authors have no financial conflicts of interest
References
- 1.Kumanovics A, Takada T, Lindahl KF. Genomic organization of the mammalian MHC. Annu Rev Immunol. 2003;21:629–657. doi: 10.1146/annurev.immunol.21.090501.080116. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 3.Howcroft TK, Singer DS. Expression of nonclassical MHC class Ib genes: comparison of regulatory elements. Immunol Res. 2003;27:1–30. doi: 10.1385/IR:27:1:1. [DOI] [PubMed] [Google Scholar]
- 4.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H, Bediako Y, Xu H, Choi HJ, Wang CR. Positive selecting cell type determines the phenotype of MHC class Ib-restricted CD8+ T cells. Proc Natl Acad Sci U S A. 2011;108:13241–13246. doi: 10.1073/pnas.1105118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zajonc DM, Kronenberg M. Carbohydrate specificity of the recognition of diverse glycolipids by natural killer T cells. Immunol Rev. 2009;230:188–200. doi: 10.1111/j.1600-065X.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 7.Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 9.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraft JR, Vance RE, Pohl J, Martin AM, Raulet DH, Jensen PE. Analysis of Qa-1(b) peptide binding specificity and the capacity of CD94/NKG2A to discriminate between Qa-1-peptide complexes. J Exp Med. 2000;192:613–624. doi: 10.1084/jem.192.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambayashi T, Kraft-Leavy JR, Dauner JG, Sullivan BA, Laur O, Jensen PE. The nonclassical MHC class I molecule Qa-1 forms unstable peptide complexes. J Immunol. 2004;172:1661–1669. doi: 10.4049/jimmunol.172.3.1661. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan BA, Kraj P, Weber DA, Ignatowicz L, Jensen PE. Positive selection of a Qa-1-restricted T cell receptor with specificity for insulin. Immunity. 2002;17:95–105. doi: 10.1016/s1074-7613(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 15.Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, Soloski MJ. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 16.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WL, Zhang L, Liang B, Saenger Y, Li J, Chess L, Jiang H. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci U S A. 2007;104:20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira CC, van Veelen PA, Querido B, de Ru A, Sluijter M, Laban S, Drijfhout JW, van der Burg SH, Offringa R, van Hall T. The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigen-processing defects. J Exp Med. 2010;207:207–221. doi: 10.1084/jem.20091429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabaczewski P, Chiang E, Henson M, Stroynowski I. Alternative peptide binding motifs of Qa-2 class Ib molecules define rules for binding of self and nonself peptides. J Immunol. 1997;159:2771–2781. [PubMed] [Google Scholar]
- 20.Chiang EY, Stroynowski I. The role of structurally conserved class I MHC in tumor rejection: contribution of the Q8 locus. J Immunol. 2006;177:2123–2130. doi: 10.4049/jimmunol.177.4.2123. [DOI] [PubMed] [Google Scholar]
- 21.Colmone A, Wang CR. H2-M3-restricted T cell response to infection. Microbes Infect. 2006;8:2277–2283. doi: 10.1016/j.micinf.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Bouwer HG, Bai A, Forman J, Gregory SH, Wing EJ, Barry RA, Hinrichs DJ. Listeria monocytogenes-infected hepatocytes are targets of major histocompatibility complex class Ib-restricted antilisterial cytotoxic T lymphocytes. Infect Immun. 1998;66:2814–2817. doi: 10.1128/iai.66.6.2814-2817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaman MS, Perarnau B, Lindahl KF, Lemonnier FA, Forman J. Response to Listeria monocytogenes in mice lacking MHC class Ia molecules. J Immunol. 1999;162:5429–5436. [PubMed] [Google Scholar]
- 24.Bouwer HG, Barry RA, Hinrichs DJ. Lack of expansion of major histocompatibility complex class Ib-restricted effector cells following recovery from secondary infection with the intracellular pathogen Listeria monocytogenes. Infect Immun. 2001;69:2286–2292. doi: 10.1128/IAI.69.4.2286-2292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenz LL, Dere B, Bevan MJ. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity. 1996;5:63–72. doi: 10.1016/s1074-7613(00)80310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulden PH, Fischer P, 3rd, Sherman NE, Wang W, Engelhard VH, Shabanowitz J, Hunt DF, Pamer EG. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity. 1996;5:73–79. doi: 10.1016/s1074-7613(00)80311-8. [DOI] [PubMed] [Google Scholar]
- 27.Kerksiek KM, Busch DH, Pilip IM, Allen SE, Pamer EG. H2-M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J Exp Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton SE, Porter BB, Messingham KA, Badovinac VP, Harty JT. MHC class Ia-restricted memory T cells inhibit expansion of a nonprotective MHC class Ib (H2-M3)-restricted memory response. Nat Immunol. 2004;5:159–168. doi: 10.1038/ni1026. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H, Choi HJ, Xu H, Felio K, Wang CR. Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3. J Immunol. 2010;186:489–498. doi: 10.4049/jimmunol.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tvinnereim A, Wizel B. CD8+ T cell protective immunity against Chlamydia pneumoniae includes an H2-M3-restricted response that is largely CD4+ T cell-independent. J Immunol. 2007;179:3947–3957. doi: 10.4049/jimmunol.179.6.3947. [DOI] [PubMed] [Google Scholar]
- 32.Chun T, Serbina NV, Nolt D, Wang B, Chiu NM, Flynn JL, Wang CR. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J Exp Med. 2001;193:1213–1220. doi: 10.1084/jem.193.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi T, Yamada H, Yajima T, Wajjwalku W, Hara T, Yoshikai Y. H2-M3-restricted CD8+ T cells induced by peptide-pulsed dendritic cells confer protection against Mycobacterium tuberculosis. J Immunol. 2007;178:3806–3813. doi: 10.4049/jimmunol.178.6.3806. [DOI] [PubMed] [Google Scholar]
- 34.Ugrinovic S, Brooks CG, Robson J, Blacklaws BA, Hormaeche CE, Robinson JH. H2-M3 major histocompatibility complex class Ib-restricted CD8 T cells induced by Salmonella enterica serovar Typhimurium infection recognize proteins released by Salmonella serovar Typhimurium. Infect Immun. 2005;73:8002–8008. doi: 10.1128/IAI.73.12.8002-8008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braaten DC, McClellan JS, Messaoudi I, Tibbetts SA, McClellan KB, Nikolich-Zugich J, Virgin HW. Effective control of chronic gamma-herpesvirus infection by unconventional MHC Class Ia-independent CD8 T cells. PLoS Pathog. 2006;2:e37. doi: 10.1371/journal.ppat.0020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson PA, 2nd, Pack CD, Hadley A, Wang CR, Stroynowski I, Jensen PE, Lukacher AE. An MHC class Ib-restricted CD8 T cell response confers antiviral immunity. J Exp Med. 2008;205:1647–1657. doi: 10.1084/jem.20080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jay DC, Reed-Loisel LM, Jensen PE. Polyclonal MHC Ib-restricted CD8+ T cells undergo homeostatic expansion in the absence of conventional MHC-restricted T cells. J Immunol. 2008;180:2805–2814. doi: 10.4049/jimmunol.180.5.2805. [DOI] [PubMed] [Google Scholar]
- 38.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 40.Perarnau B, Saron MF, San Martin BR, Bervas N, Ong H, Soloski MJ, Smith AG, Ure JM, Gairin JE, Lemonnier FA. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur J Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laouini D, Casrouge A, Dalle S, Lemonnier F, Kourilsky P, Kanellopoulos J. V beta T cell repertoire of CD8+ splenocytes selected on nonpolymorphic MHC class I molecules. J Immunol. 2000;165:6381–6386. doi: 10.4049/jimmunol.165.11.6381. [DOI] [PubMed] [Google Scholar]
- 43.Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, Brutkiewicz RR. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–10754. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y, Roberts TJ, Wang CR, Cho S, Brutkiewicz RR. Long-term loss of canonical NKT cells following an acute virus infection. Eur J Immunol. 2005;35:879–889. doi: 10.1002/eji.200425495. [DOI] [PubMed] [Google Scholar]
- 45.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Bannard O, Kraman M, Fearon D. Pathways of memory CD8+ T-cell development. Eur J Immunol. 2009;39:2083–2087. doi: 10.1002/eji.200939555. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 48.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 52.Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 54.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 55.Kerksiek KM, Ploss A, Leiner I, Busch DH, Pamer EG. H2-M3-restricted memory T cells: persistence and activation without expansion. J Immunol. 2003;170:1862–1869. doi: 10.4049/jimmunol.170.4.1862. [DOI] [PubMed] [Google Scholar]
- 56.Milligan GN, Flaherty L, Braciale VL, Braciale TJ. Nonconventional (TL-encoded) major histocompatibility complex molecules present processed viral antigen to cytotoxic T lymphocytes. J Exp Med. 1991;174:133–138. doi: 10.1084/jem.174.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byers DE, Lindahl K. Fischer. H2-M3 presents a nonformylated viral epitope to CTLs generated in vitro. J Immunol. 1998;161:90–96. [PubMed] [Google Scholar]
- 58.Byers DE, Lindahl KF. Peptide affinity and concentration affect the sensitivity of M3-restricted CTLs induced in vitro. J Immunol. 1999;163:3022–3028. [PubMed] [Google Scholar]
- 59.Weber DA, Attinger A, Kemball CC, Wigal JL, Pohl J, Xiong Y, Reinherz EL, Cheroutre H, Kronenberg M, Jensen PE. Peptide-independent folding and CD8 alpha alpha binding by the nonclassical class I molecule, thymic leukemia antigen. J Immunol. 2002;169:5708–5714. doi: 10.4049/jimmunol.169.10.5708. [DOI] [PubMed] [Google Scholar]
- 60.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien YH. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 61.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 62.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]