Abstract
Recently, we screened several KV channels for possible dependence on plasma membrane phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). The channels were expressed in tsA-201 cells and the PI(4,5)P2 was depleted by several manipulations in whole-cell experiments with parallel measurements of channel activity. In contrast to reports on excised-patches using Xenopus laevis oocytes, we found only KV7, but none of the other tested KV channels, to be strongly dependent on PI(4,5)P2. We now have extended our study to KV1.2 channels, a KV channel we had not previously tested, because a new published study on excised patches showed regulation of the voltage-dependence of activation by PI(4,5)P2. In full agreement with those published results, we found a reduction of current amplitude by ~20% after depletion of PI(4,5)P2 and a small left shift in the activation curve of KV1.2 channels. We also found a small reduction of KV11.1 (hERG) currents that was not accompanied by a gating shift. In conclusion, our whole-cell methods yield a PI(4,5)P2-dependence of KV1.2 currents in tsA-201 cells that is comparable to findings from excised patches of Xenopus laevis oocytes. We discuss possible physiological rationales for PI(4,5)P2 sensitivity of some ion channels and insensitivity of others.
Keywords: Voltage-gated potassium channel; phosphatidylinositol 4,5-bisphosphate; KV1.2
Introduction
Here we revisit the regulation of voltage-gated potassium (KV) channels by plasma membrane phosphoinositide phospholipids. Although not in high abundance, the phosphoinositides of eukaryotic biological membranes regulate many membrane proteins through protein-lipid interaction domains.1-3
At the plasma membrane, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) is the dominant phosphoinositide. It enhances the activity of many ion channels, and, for some channels, is necessary for activity.3-6 Thus, it is obligatory for function of all 5 members of the KV7 channel family and of nearly all inward rectifiers and TRP channels.3,7-9 In recent reviews, the number of ion channels said to be regulated by PI(4,5)P2 has grown so large (> 80,3,6) that one might anticipate that all plasma membrane channels are sensitive. However, using whole-cell recording and enzymatic methods to deplete endogenous PI(4,5)P2, our laboratory failed to find PI(4,5)P2 sensitivity in several channels. For example, we found that only 4 out of 8 tested voltage-gated calcium (CaV) channel subtypes were significantly depressed when PI(4,5)P2 levels were enzymatically lowered, and some of these sensitive CaV channels became nearly insensitive when coexpressed with a different CaVβ subunit.10,11
Recently, we screened for PI(4,5)P2 sensitivity of 8 voltage-gated potassium (KV) channels from the KV1, 2, 3, and 4 families, again using whole-cell methods and enzyme recruitment.12 Three of the channels we tested, KV1.1, 1.4, and 3.4, had been studied before in excised patches from Xenopus laevis oocytes.13 The authors had reported interesting changes of current kinetics and amplitude when exogenous brain PI(4,5)P2 was applied to the cytoplasmic face. Thus, we assumed our screen would identify many lipid-sensitive channels, yet we saw no sensitivity to PI(4,5)P2 depletion for any of them (KV1.1, 1.3, 1.4, 1.5, 2.1, 3.4, 4.2, and 4.3). For large test depolarizations, neither the current amplitude nor the gating kinetics were changed. In the same study, we did confirm that our methods easily resolved the well-known lipid sensitivity of KV7.1, 7.2, and 7.3 and Kir2.1 channels. Subsequently, using mostly different approaches, Rodriguez-Menchaca et al. reported that KV1.2 channels are sensitive to PI(4,5)P2 depletion.14 They found a ~30% decrease in current amplitude from Xenopus oocytes by depleting excised patches of PI(4,5)P2 and a restoration of the original current amplitude by perfusing PI(4,5)P2 onto the inside-out patches. They recognized a dual effect of depleting PI(4,5)P2: First, a decrease of maximum open probability and, second, a left-shift of ~14 mV in the voltage dependence of the activation curve. This result was not in contradiction to ours since we had not tested KV1.2 channels in our screen. Nevertheless, we were stimulated by this new work to check whether our whole-cell assay system, which had given negative results with other channels, would confirm PI(4,5)P2 sensitivity of KV1.2.
Results
As in our previous paper,12 the experimental design was to study ion channels transfected in mammalian tsA-201 cells by whole-cell voltage clamp. Plasma membrane phosphoinositides were depleted by 2 enzymatic maneuvers: (1) by stimulating a G protein coupled receptor (GPCR) coupled to phospholipase C (PLC), and (2) by using chemical dimerization to recruit lipid phosphatases to the plasma membrane.
PI(4,5)P2 dependence of KV1.2 channels
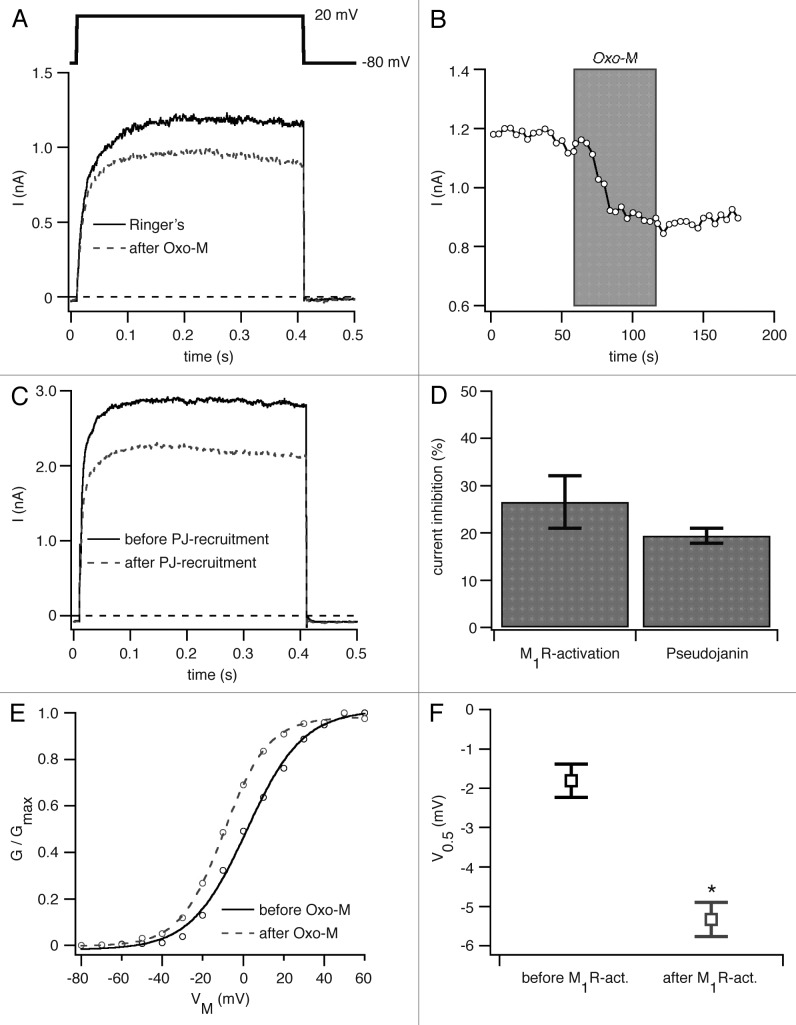
We first tested PI(4,5)P2 depletion by PLC. KV1.2 channels were co-expressed with PLC-coupled M1 muscarinic receptors (M1R) in tsA-201 cells, and depolarizing pulses to 20 mV elicited outward K+ currents (Fig. 1A). Application of the muscarinic agonist oxotremorine methiodide (Oxo-M) led to a clear decrease in the current amplitude (Fig. 1A and B) on average by 27 ± 6% (n = 5, Fig. 1D). Activation of phospholipase C (PLC) is a complex stimulus. It generates several intracellular signals including depletion of PI(4,5)P2, rise of cytoplasmic inositol trisphosphate (Ins[1,4,5]P3) and calcium, production of diacylglycerol (DAG) and activation of protein kinase C (PKC).
Figure 1. PI(4,5)P2 dependence of KV1.2 channels. (A) KV1.2 channels and M1R were transiently expressed in tsA-201 cells. Currents were recorded in the whole-cell configuration (pulse protocol shown above current traces). Figure shows current traces before (solid black) and after (dashed gray) application of 10 µM Oxo-M. (B) Time course of KV1.2 mediated currents at +20 mV for the experiment shown in (A). (C) Current traces of KV1.2 channels coexpressed with pseudojanin-YFP and LDR-CFP before (solid black) and after (dashed gray) addition of rapamycin. (D) Percentage inhibition of KV1.2 channels by activation of M1R or by recruitment of pseudojanin to the plasma membrane. (E) Representative G-V curve generated from test pulses to membrane potentials of -80 to +60 mV from a holding potential of -80 mV before (solid black) and after (dashed gray) activation of M1R by Oxo-M application. (F) Voltage of half-maximal activation (V0.5) for KV1.2 channels before and after M1R-activation. *p < 0.05.
To check whether the effects were really due to PI(4,5)P2 depletion, we turned to recruitment of the lipid phosphatase pseudojanin (PJ) to the plasma membrane as another tool to deplete PI(4,5)P2. Pseudojanin is an engineered fusion protein containing a rapamycin-binding domain (FKBP) and 2 lipid phosphatase domains in tandem (derived from Inp54p and Sac1 enzymes), which dephosphorylate PI(4,5)P2 at the 5-position (Inp54p) and PI(4)P at the 4-position (Sac1) to yield phosphatidylinositol (PI).12,15 Addition of the membrane-permeable drug rapamycin dimerizes the FKBP domain with the coexpressed membrane anchor Lyn-FRB-CFP, thus recruiting the pseudojanin phosphatases to the plasma membrane.16 This dimerization strategy depletes PI(4,5)P2 at the plasma membrane without generating downstream signaling molecules like Ins(1,4,5)P3 or DAG. As the FKBP-rapamycin-FRB complex is very stable, the recruitment of pseudojanin to the plasma membrane is irreversible and results in a lasting depletion of PI(4,5)P2.12,15,16 Rapamycin addition to cells coexpressing KV1.2, pseudojanin, and LDR-CFP resulted in a clear 19 ± 2% decrease in current amplitude (n = 5, Fig. 1C and D). These experiments show that KV1.2 channel current is PI(4,5)P2 sensitive as previously reported.14
We next asked whether the voltage-dependence of activation can be shifted by turning on PLC. We coexpressed KV1.2 with M1R and measured the conductance-voltage (G-V) relation before and after activation of M1R (Fig. 1E). There was a small, but significant left shift in the normalized activation curve by 3.5 ± 0.4 mV (n = 5) (Fig. 1F). Thus, we confirm the observations of Rodriguez-Menchaca et al.14
PI(4,5)P2 dependence of hERG channel activation
We and others have reported a change in the voltage-dependence of activation of erg channels if PI(4,5)P2 levels are altered.17,18 Bian et al. reported a left shift of about ~19 mV for the activation of hERG channels upon dialysis of 10 µM PI(4,5)P2 into the cells via the patch pipette,18 while we found a right shift of about ~5 mV in the activation curve for rat erg1 channels upon depletion of PI(4,5)P2 by activating M1R.17 We decided to extend our previously published recordings on rat erg1 channels to hERG channels to test whether we would observe a similar right shift in the voltage-dependence.
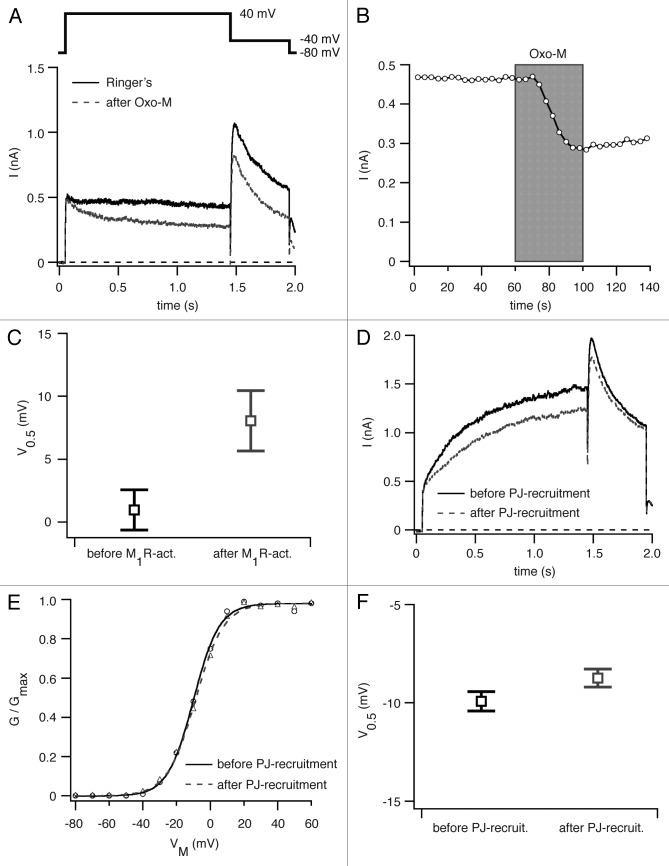
We co-expressed hERG and M1R in tsA-201 cells and measured current amplitudes before and after M1R activation. After addition of Oxo-M we observed an inhibition of hERG mediated current of 31 ± 7% (n = 5) (Fig. 2A and B), in good agreement with our work on rat erg1 channels after M1R activation.17 We next asked whether this decrease in current amplitude is accompanied by a change in the activation curve for hERG channels. Again, we co-expressed hERG channels with M1R and measured G-V curves before and after addition of Oxo-M to deplete PI(4,5)P2. We detected a right shift of the activation curve of about ~7 mV (n = 5), which correlates very well with the 5 mV right shift Hirdes at al. had observed for rat erg1 channels (Fig. 2C).17
Figure 2. Voltage-dependence of activation of hERG channels before and after PI(4,5)P2 depletion. (A) Figure shows current traces for hERG channels expressed in tsA-201 cells together with M1R before (solid black) and after (dashed gray) application of 10 µM Oxo-M. Pulse protocols as shown above current traces. (B) Time course of hERG channel mediated current at +40 mV from the experiment shown in (A). (C) Voltages of half-maximal activation (V0.5) of hERG channels before and after M1R-activation. (D) Current traces for hERG channels expressed together with pseudojanin-YFP (PJ) and LDR-CFP before (solid black) and after (dashed gray) recruitment of PJ to the plasma membrane by rapamycin-application. (E) Representative G-V curve of hERG channels generated from test pulses to membrane potentials of -80 to +60 mV from a holding potential of -80 mV before (solid black) and after (dashed gray) recruitment of PJ to the plasma membrane. (F) Voltages of half-maximal activation (V0.5) for hERG channels before and after PJ-recruitment.
Our next step asked whether the observed right shift in the activation curve is caused by the depletion of PI(4,5)P2 at the plasma membrane or by signaling pathways downstream of PI(4,5)P2 hydrolysis, such as activation of protein kinases. Unlike KV1.2 channels, it had been shown for hERG channels that activation of PKC leads to a right shift of the activation curve.19 We expressed hERG channels together with pseudojanin-YFP and LDR-CFP in tsA-201 cells and applied rapamycin to induce translocation of pseudojanin-YFP to the plasma membrane. Recruiting PJ to deplete PI(4,5)P2 led to a significant decrease (15 ± 1%, n = 5) of hERG mediated current amplitude (Fig. 2D) but, in the same cells, no significant shift in the voltage-dependence of activation (Fig. 2E and F). We conclude from this result that a depletion of PI(4,5)P2 does not alter the voltage-dependence of activation of hERG channels and that our finding of a right shift in the activation curve after M1R-activation should be attributed to other signals downstream of PI(4,5)P2 cleavage by PLC.
Discussion
We now review 2 broad questions briefly: (1) Are KV channels sensitive to plasma membrane PI(4,5)P2; and (2) is there a physiological benefit from such sensitivity or insensitivity?
For excitable cells, the KV channels whose PI(4,5)P2 sensitivity is best studied are the KV7 (KCNQ) family.7,20,21 It is widely accepted that the 5 members of this family absolutely require PI(4,5)P2 to function. They bind PI(4,5)P2 with low enough affinity that when the lipid is depleted enzymatically by 90–95% either by PLC or by 5-phosphatases, the current falls by 80–95%. In addition, KV1.2 channels have clear PI(4,5)P2 sensitivity. In whole-cell experiments, currents decrease and gating is shifted in response to M1R or voltage-sensing phosphatase activation, and in excised patches, the same effects are induced by rundown, by anti-PI(4,5)P2 antibodies, and by blocking lipid kinases, and current is restored by application of PI(4,5)P2.14,22,23 With KV1.2 however, rather than eliminating current, PI(4,5)P2 depletion modulates channel properties more gently, reducing the amplitude by 25–30% and shifting gating. Possibly with a more severe elimination of PI(4,5)P2, the channel could be shown to require the lipid absolutely, but the published experiments and ours never attenuate the current by as much as 50%. Similarly, with hERG (KV11) channels we found a small depression of current (15%) but in this case no shift of gating attributable to PI(4,5)P2 depletion.
Lipid effects on other members of the KV1, 2, 3, and 4 families are less pronounced. In the simple whole-cell recording tests we did with KV1.1, 1.3, 1.4, 1.5, 2.1, 3.4, 4.2, and 4.3, neither the current amplitude at large depolarizations nor the kinetics were sensitive to enzymatic depletion of PI(4,5)P2.12 We did not check for possible gating shifts at intermediate voltages, but we would say at least that these channels do not require physiological levels of PI(4,5)P2 to function. This relative insensitivity of so many KV channels was unexpected since earlier reports with membrane patches excised from Xenopus oocytes had indicated a striking loss of open-channel inactivation of KV1.1 (with KVβ1.1), 1.4, and 3.4 channels when brain PI(4,5)P2 was added to the patch.13 Is this a discrepancy? Probably not, since our experiments involved enzymatic removal of PI(4,5)P2 in whole-cell recording of mammalian cells, whereas the others used addition of PI(4,5)P2 to membrane patches excised from oocytes. We would suggest that these 3 responding KV channels may have little sensitivity to membrane PI(4,5)P2 in the physiologically accessible range. The application of additional brain PI(4,5)P2 to the cytoplasmic face might be able to force the membrane phosphoinositide level into a higher non-physiological range or might expose the channels to lipid micelles and solvents that had other actions. However such speculations are untested.
Hence, we suggest that besides the very strongly PI(4,5)P2-dependent KV7 channels, very few KV channels, namely KV1.2 and KV11.1 channels so far, show significant physiological sensitivity to changes in PI(4,5)P2 levels below the endogenous resting level. This brings us to the question, what are physiological benefits of such sensitivity or insensitivity?
We envision 2 broad physiological advantages for PI(4,5)P2 sensitivity of ion channels. (1) Sensitivity specifically to PI(4,5)P2 allows ion channel function and cell excitability to be modulated in response to receptor activation of PLC, and (2) as first suggested by Hilgemann,24 a requirement for particular phosphoinositides could keep ion channels silenced or at low activity during trafficking whenever they are not in an appropriate membrane compartment.
On the first advantage, regulation by PLC, we are already well informed since this mechanism involves modulation of ion channels at the plasma membrane and classical electrophysiology. KCNQ2/KCNQ3 channels gate slowly. They are already partly open at typical resting potentials and are non-inactivating. Muscarinic cholinergic stimulation of superior cervical ganglion cells results in a profound suppression of KCNQ2/KCNQ3-mediated resting currents because PLC is activated and PI(4,5)P2 becomes depleted. The cells depolarize a little, become more excitable, and fire repetitively to long depolarizing stimuli.21 A similar story could be developed for KATP channels, found in many cells. These inward rectifier channels (Kir6) are PI(4,5)P2 sensitive25 and regulate the cell resting potential and excitability as well. We can suggest that in excitable cells expressing PI(4,5)P2-sensitive channels regulating the resting potential, it is still appropriate that each action potential repolarize quickly. Despite increased excitability, a reserve of potassium channel activity is needed. For that reason, the PI(4,5)P2 insensitivity of e.g., KV1.1/KVβ1.1, KV1.5/KVβ1.3, KV3, and KV4, which are rapidly-gating, repolarizing channels13,26,27 might be adaptive to maintain action potential repolarization. Similarly, a loss of inactivation gating of KV1.1/KVβ1.1 channels as well as of KV3.4 channels with M1R stimulation12,28 could add repolarizing reserve. In these latter cases, the effect is not from depletion of PI(4,5)P2 but probably rather by channel phosphorylation stimulated by diacylglycerol.12,28,29 Finally, a loss of PI(4,5)P2 increases the availability of KV1.2 channels by shifting their voltage-dependence of activation to the left and would increase their contribution to repolarization.
How might cardiac KV7.1/KCNE1 and hERG channels fit into this interpretation? Both have some PI(4,5)P2 sensitivity.12,17,18,20 In the human heart, these channels are the molecular basis for the 2 most important repolarizing currents, IKs and IKr.30 Therefore, a decrease in PI(4,5)P2 could prolong the cardiac action potential considerably. Indeed, mutations in the genes for KV7.1 and hERG have been associated with a loss of PI(4,5)P2 binding and critical pathological conditions like long QT syndrome and cardiac arrhythmias.3,18,20 The heart seems to avoid this potentially serious problem in a different way. It has PI(4,5)P2 at the plasma membrane to keep the channels active, but the endogenous PLC-coupled receptors do not deplete the PI(4,5)P2 very much.24 Apparently, PI(4,5)P2 resynthesis can keep up with PI(4,5)P2 breakdown in cardiac cells. In conclusion, PI(4,5)P2 regulates the activity of voltage-gated potassium channels in different tissues by different mechanisms. They allow dynamic regulation of cellular excitability through slowly-gating channels like KCNQ2 and KCNQ3 in neurons while preserving the activity of action-potential-repolarizing fast KV channels of neurons and the slow KV7.1 and hERG channels in the heart.
The second potential advantage of PI(4,5)P2 sensitivity concerns silencing during trafficking of channels in intracellular membranes, a subject we know less about. We would need further information about the physiology of intracellular compartments. What are the ion gradients across their membranes? What is their membrane potential? Is this electrical potential important for the compartment's function? Does it change during cellular activities? From such information we might be able to deduce which ion channels would be good to silence and which to promote as they traffic through that compartment. Phosphoinositides would be likely candidates to accomplish such regulation since each compartment has different lipids.1 We already know of compartments with lumens that are acidic or have high sodium or calcium concentrations, and we know of stimuli that release stored calcium. This knowledge probably only scratches the surface of a much fuller understanding that will eventually emerge about compartmental electrophysiology.
We envision several possible outcomes of these inquiries. Internal membranes may have membrane potentials more positive than the resting potential of the plasma membrane. If so, ion channels with voltage-dependent inactivation might already be inactivated during internal trafficking, and that category of channels would not need a lipid-based mechanism to ensure lack of activity. In addition, when we understand the membrane potential of a compartment, we should recognize some transiting ion channels that would be compatible with that membrane potential and others that are not that must be kept silent there by some mechanism.
In sum, we propose hypotheses for why some channels are sensitive and some channels are not sensitive to the lipid PI(4,5)P2.
Materials and Methods
Cell culture and plasmids
All experiments were performed in tsA-201 cells cultured at 37°C and 5% CO2 in DMEM (Invitrogen) supplemented with 10% FBS (PAA) and 0.2% penicillin/streptomycin (Invitrogen). Transient transfection of cells was performed as previously described.12
The following plasmids were generously given to us: M1R (M1 muscarinic receptor) –YFP from Neil Nathanson (University of Washington); pseudojanin-YFP from Gerald Hammond and Robin Irvine (University of Cambridge); KV1.2 from Diomedes Logothetis (Virginia Commonwealth University); LDR (Lyn11-targeted FRB)-CFP from Tamas Balla (National Institute of Health); and hERG from Olaf Pongs (University of Hamburg).
Electrophysiology
Whole-cell recordings were performed as previously described.12
Data analysis and statistics
Data analysis was performed using Igor Pro (Wavemetrics) and Excel (Microsoft). Statistical data are presented as mean ± SEM unless otherwise stated. The Student t-test was used to test for statistical significance. We considered p-values of < 0.05 as significant.
Acknowledgments
We thank all colleagues who have generously provided us with plasmids (see Material and Methods). We are also thankful to all members of the Hille laboratory and many members of the Department of Physiology and Biophysics at the University of Washington for discussions and experimental advice, and to Lea M Miller for technical help. This study was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award R01 NS08174 (BH) and the Alexander von Humboldt-Foundation (MK).
Glossary
Abbreviations:
- FKBP
FK506-binding protein
- FRB
rapamycin-binding domain of mTOR
- GPCR
G-protein coupled receptor
- KV channel
voltage-gated potassium channel
- LDR
Lyn11-targeted FRB
- M1R
M1 muscarinic (acetylcholine) receptor
- Oxo-M
oxotremorine methiodide
- PI(4,5)P2
phosphatidylinositol 4, 5-bisphosphate
- PJ
pseudojanin recruitable phosphatase
- PLC
phospholipase C
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/25816
References
- 1.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 2.Varnai P, Thyagarajan B, Rohács T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–82. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logothetis DE, Petrou VI, Adney SK, Mahajan R. Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch. 2010;460:321–41. doi: 10.1007/s00424-010-0828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–8. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–95. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Menchaca AA, Adney SK, Zhou L, Logothetis DE. Dual Regulation of Voltage-Sensitive Ion Channels by PIP2. Front Pharmacol. 2012;3:170. doi: 10.3389/fphar.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–20. doi: 10.1016/S0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Craciun LC, Mirshahi T, Rohács T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–75. doi: 10.1016/S0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 9.Rohács T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J Biol Chem. 1999;274:36065–72. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- 10.Suh BC, Leal K, Hille B. Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 2010;67:224–38. doi: 10.1016/j.neuron.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh BC, Kim DI, Falkenburger BH, Hille B. Membrane-localized β-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc Natl Acad Sci U S A. 2012;109:3161–6. doi: 10.1073/pnas.1121434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse M, Hammond GRV, Hille B. Regulation of voltage-gated potassium channels by PI(4,5)P2. J Gen Physiol. 2012;140:189–205. doi: 10.1085/jgp.201210806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–70. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Menchaca AA, Adney SK, Tang Q-Y, Meng X-Y, Rosenhouse-Dantsker A, Cui M, Logothetis DE. PIP2 controls voltage-sensor movement and pore opening of Kv channels through the S4-S5 linker. Proc Natl Acad Sci U S A. 2012;109:E2399–408. doi: 10.1073/pnas.1207901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond GRV, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337:727–30. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods. 2005;2:415–8. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirdes W, Horowitz LF, Hille B. Muscarinic modulation of erg potassium current. J Physiol. 2004;559:67–84. doi: 10.1113/jphysiol.2004.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian JS, Kagan A, McDonald TV. Molecular analysis of PIP2 regulation of HERG and IKr. Am J Physiol Heart Circ Physiol. 2004;287:H2154–63. doi: 10.1152/ajpheart.00120.2004. [DOI] [PubMed] [Google Scholar]
- 19.Thomas D, Zhang W, Wu K, Wimmer AB, Gut B, Wendt-Nordahl G, Kathöfer S, Kreye VA, Katus HA, Schoels W, et al. Regulation of HERG potassium channel activation by protein kinase C independent of direct phosphorylation of the channel protein. Cardiovasc Res. 2003;59:14–26. doi: 10.1016/S0008-6363(03)00386-9. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Zaydman MA, Wu D, Shi J, Guan M, Virgin-Downey B, Cui J. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc Natl Acad Sci U S A. 2011;108:9095–100. doi: 10.1073/pnas.1100872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DA, Hughes SA, Marsh SJ, Tinker A. Regulation of M(Kv7.2/7.3) channels in neurons by PIP2 and products of PIP2 hydrolysis: significance for receptor-mediated inhibition. J Physiol. 2007;582:917–25. doi: 10.1113/jphysiol.2007.132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falkenburger BH, Jensen JB, Hille B. Kinetics of M1 muscarinic receptor and G protein signaling to phospholipase C in living cells. J Gen Physiol. 2010;135:81–97. doi: 10.1085/jgp.200910344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol. 2010;135:99–114. doi: 10.1085/jgp.200910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilgemann DW. On the physiological roles of PIP2 at cardiac Na+ Ca2+ exchangers and KATP channels: a long journey from membrane biophysics into cell biology. J Physiol. 2007;582:903–9. doi: 10.1113/jphysiol.2007.132746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–9. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 26.Rudy B, McBain CJK. KV3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–26. doi: 10.1016/S0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura TY, Coetzee WA, Vega-Saenz De Miera E, Artman M, Rudy B. Modulation of KV4 channels, key components of rat ventricular transient outward K+ current, by PKC. Am J Physiol. 1997;273:H1775–86. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 28.Ritter DM, Ho C, O’Leary ME, Covarrubias M. Modulation of KV3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J Physiol. 2012;590:145–61. doi: 10.1113/jphysiol.2011.218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covarrubias M, Wei A, Salkoff L, Vyas TB. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron. 1994;13:1403–12. doi: 10.1016/0896-6273(94)90425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyders DJ. Structure and function of cardiac potassium channels. Cardiovasc Res. 1999;42:377–90. doi: 10.1016/S0008-6363(99)00071-1. [DOI] [PubMed] [Google Scholar]