Abstract
Background:
The nature of bronchial mucosal inflammation and its physiologic and clinical significance in rhinovirus-induced asthma exacerbations is unclear. We investigated bronchial mucosal inflammatory response and its association with physiologic and clinical outcomes in an experimental model of rhinovirus-induced asthma exacerbations.
Methods:
We used immunohistochemistry methods to detect phenotypes of inflammatory cells infiltrating the bronchial mucosa before and after experimental rhinovirus infection in 10 subjects with asthma and 15 normal subjects.
Results:
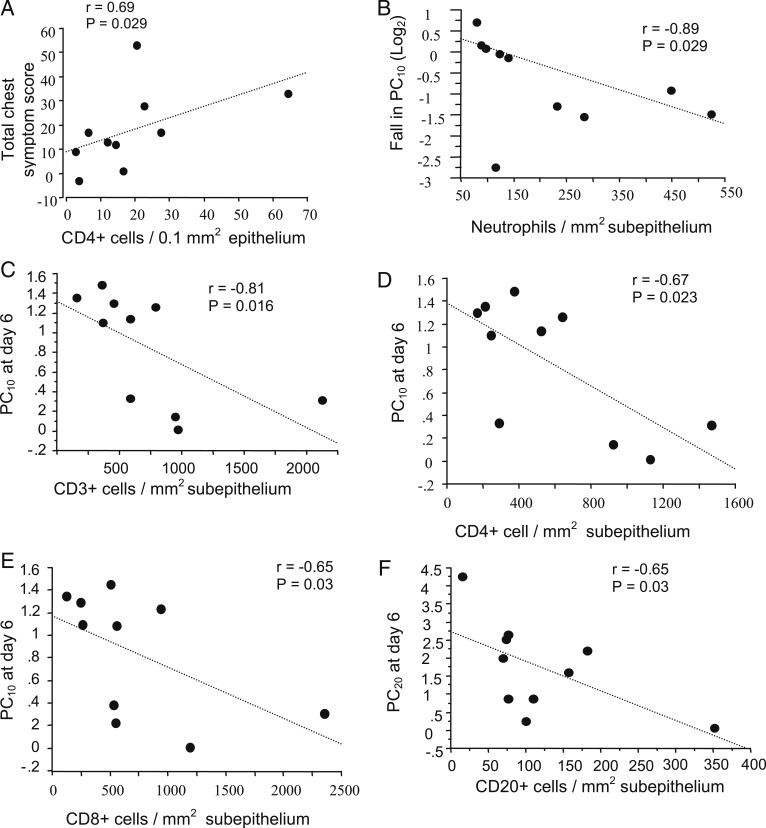
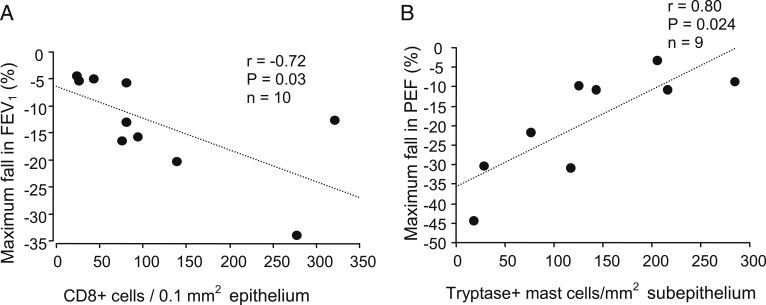
Compared with baseline, rhinovirus infection significantly increased the number of epithelial (P = .005) and subepithelial (P = .017) neutrophils in subjects with asthma only and subepithelial CD68+ macrophages in both subjects with asthma (P = .009) and normal subjects (P = .018) but more so in those with asthma (P = .021). Numbers of CD45+, CD68+, and CD20+ cells; neutrophils; and eosinophils at day 4 postinfection were positively associated with virus load (r = 0.50-0.72, P = .016-0.03). At acute infection in subjects with asthma, CD4+ cells correlated with chest symptom scores (r = 0.69, P = .029), the fall in the 10% fall in FEV1 (PC10) correlated with neutrophils (r = −0.89, P = .029), the PC10 correlated inversely with CD4+ (r = −0.67, P = .023) and CD8+ cells (r = −0.65, P = .03), the 20% fall in FEV1 was inversely associated with CD20+ cells (r = −0.65, P = .03), and higher epithelial CD8+ cell counts were significantly associated with a greater maximum fall in FEV1 (r = −0.72, P = .03), whereas higher subepithelial mast cell counts were significantly associated with a lower maximum percent fall in peak expiratory flow (r = 0.8, P = .024).
Conclusions:
In subjects with asthma, rhinovirus infection induces bronchial mucosal neutrophilia and more severe monocyte/macrophage infiltration than in normal subjects. Airway neutrophils, eosinophils, and T and B lymphocytes during infection are related to virus load and physiologic and clinical severity, whereas mast cells are related to greater lung function.
Rhinoviruses (RVs) are the major cause of acute exacerbations of asthma.1‐3 Human experimental RV infection in volunteers with mild asthma is associated with augmented physiologic and inflammatory responses to allergen challenge,4,5 reductions in peak expiratory flow (PEF)6 and FEV1,7 and increases in bronchial reactivity.8 In agreement, our own previously reported study showed that experimental RV16 infection in asthma induced significant increases in bronchial reactivity, lower respiratory tract symptoms, and lung function impairment and that significant changes in these outcomes did not occur in normal subjects.9
Regarding RV-induced airway inflammation, two previous studies of experimental RV infection reported increases in submucosal CD3+ lymphocytes and eosinophils in asthmatic and normal groups combined10 and in subjects with asthma alone.11 The increased number of mucosal CD3+ cells was accompanied by an increase in airway responsiveness and correlated positively with cold symptoms.10,11 However, it is unclear whether RV-induced bronchial mucosal cellular inflammatory responses differ between subjects with and without asthma.
In this article, we extend our previous work9 to compare the nature of the bronchial mucosa inflammatory response to experimental RV infection in subjects with asthma and in normal healthy subjects and explore its physiologic and clinical significance in asthma. We hypothesized that the bronchial mucosal cellular inflammatory response to RV infection will be exaggerated and of a distinctive predominant inflammatory cell phenotype in subjects with asthma compared with normal healthy subjects and that the inflammatory cells recruited to the bronchial mucosa will be associated with virus load and increased clinical symptoms, airways responsiveness, and airflow obstruction associated with an exacerbation.
Materials and Methods
Subjects
We extended our previously reported investigation of the same 10 subjects with atopic asthma and 15 subjects without atopic asthma (Table 1).9 These subjects were human RV16 neutralizing antibody seronegative. This study was conducted in accordance with the amended Declaration of Helsinki. All subjects gave written informed consent, and the study protocol was approved by the St. Mary’s NHS Trust Research Ethics Committee (99/BA/345). See e-Appendix 1 (376KB, pdf) for other details.
Table 1.
—Baseline Demographic Data
| Subjects | Male (Female) Sex | Age, y | Serum IgE, U/mL | FEV1 % Predicted | Positive Skin Pick Test | Histamine PC20, mg/mL |
| Normal (n = 15) | 8 (7) | 27 ± 2.3 | 27 ± 8.0 | 104 ± 3.3 | 0 | 20 ± 2.5 |
| With asthma (n = 10) | 2 (8) | 23 ± 1.4 | 241 ± 49.8a | 106 ± 4.4 | 9 | 3 ± 0.7a |
Data are presented as counts or mean ± SEM. PC20 = 20% fall in FEV1.
P < .0001 vs normal (Student t test).
Experimental Infection With Human RV16
Subjects were administered 10,000 tissue-culture infective dose 50% of RV16 on day 0 by nasal spray.9 See e-Appendix 1 (376KB, pdf) for virologic confirmation of RV16 infection.
Bronchoscopy and Clinical Data
Bronchial biopsy specimens were taken 14 days before infection (baseline), at day 4 (acute infection), and at 6 weeks postinfection (convalescence). For details about the physiologic and clinical data obtained, see e-Appendix 1 (376KB, pdf) .
Immunohistochemistry
CD45+ pan-leukocyte and inflammatory cells, eosinophils, neutrophils, mast cells, and CD3+, CD4+, CD8+, and CD20+ cells were immunostained using previously described methods.12 For details on the immunostaining method, see e-Appendix 1 (376KB, pdf) .
Quantification
The areas of epithelium and subepithelium in bronchial biopsy specimens were assessed using NIH Image, version 1.55 software (US National Institute of Mental Health). The inflammatory cells were counted using a light microscope. The data for cell counts were expressed as the number of positive cells per square millimeter of the subepithelium and per 0.1 mm2 epithelium. For details on the counting methods, see e-Appendix 1 (376KB, pdf) .
Statistical Analysis
Within-group differences in cell counts between baseline and infection were assessed with Wilcoxon matched pairs test. Mann-Whitney U test was used to compare differences between groups. Spearman rank correlation was used to test for correlations between the numbers of phenotypes of inflammatory cells and physiologic and clinical data. P < .05 indicated significance. For further details on the statistical analyses, see e-Appendix 1 (376KB, pdf) .
Results
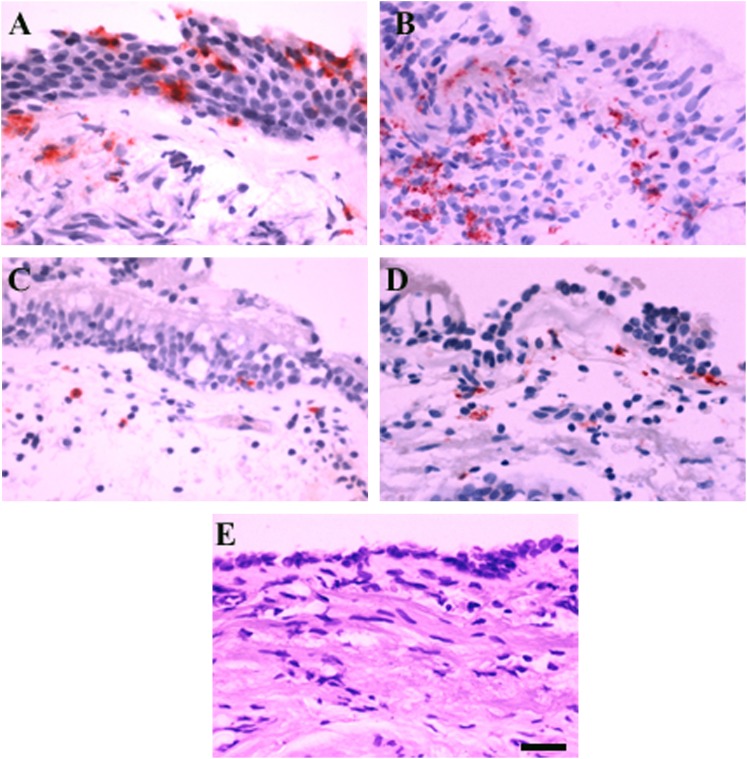
Inflammatory cells were present in both the bronchial epithelial and the bronchial subepithelial compartments. Representative photographs are shown in Figures 1A‐E. Elastase-positive neutrophils (Fig 1A) and CD68+ monocytes/macrophages (Fig 1B) appeared to be more frequent in the bronchial mucosa of subjects with asthma during acute infection compared with baseline (Figs 1C, 1D). Application of irrelevant antibodies for the inflammatory cell markers was negative (Fig 1E).
Figure 1.
Immunohistochemistry-stained cells are seen as red fuchsin positivity. A and B, Subject with asthma at day 4 rhinovirus 16 infection demonstrating more elastase-positive neutrophils (A) and CD68+ monocytes/macrophages (B) in bronchial epithelium and subepithelium. C and D, Subject with asthma at baseline showing fewer neutrophils (C) and CD68+ cells (D). E, Negative control sample shows an absence of signal. Scale bar = 20 μm for all images.
Absolute Counts of Inflammatory Cells
Total Leukocytes:
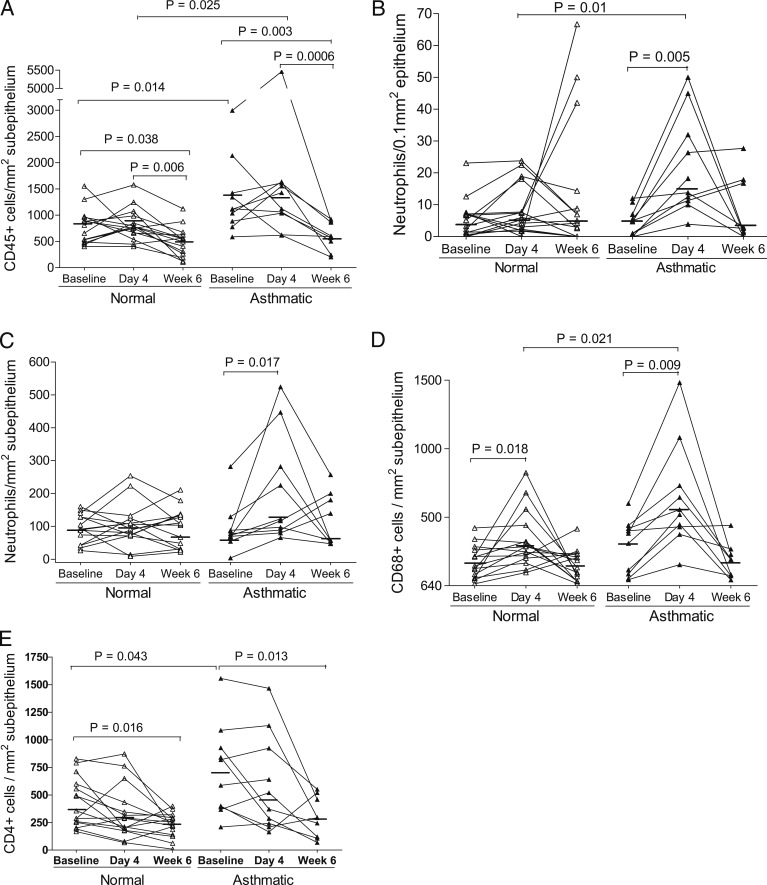
The number of subepithelial CD45+ cells in subjects with asthma was significantly greater at both baseline (P = .014) and day 4 infection (P = .025) (Fig 2A) than in normal subjects at the same time points. Compared with their respective baseline and day 4 values, the number of subepithelial CD45+ cells was significantly less at week 6 postinfection in both normal subjects (P = .038 and P = .006) and subjects with asthma (P = .003 and P = .0006) (Fig 2A). There were no significant differences in counts of epithelial CD45+ cells within and between groups.
Figure 2.
Cell counts in bronchial biopsy specimens of subjects with and without asthma at baseline and days 4 and 6 of rhinovirus 16 infection. A, CD45+ cells. B, Epithelial neutrophils. C, Subepithelial neutrophils. D, CD68+ cells. E, CD4+ cells. Data are presented as the number of positive cells per square millimeter of subepithelium or per 0.1 mm2 of epithelium. △ and ▲ show individual counts, and horizontal bars show median values (Wilcoxon matched pairs and Mann-Whitney U tests).
Granulocytes:
Compared with baseline, the number of neutrophils in both epithelium (P = .005) (Fig 2B, Table 2) and subepithelium (P = .017) (Fig 2C) of the subjects with asthma but not of normal subjects were significantly increased at day 4. At this time point, there was a significantly greater number of epithelial neutrophils in the subjects with asthma (P = .01) (Fig 2B). EG2+ eosinophil counts were not significantly increased from baseline in either group after infection (Table 2). Compared with normal subjects, subjects with asthma had a significantly higher number of epithelial eosinophils at baseline (P = .011), day 4 (P = .046), and week 6 (P = .01) (Table 2) and showed trends toward a greater number of subepithelial eosinophils at baseline (P = .07) and day 4 (P = .09), which became statistically significant at week 6 (P = .03) (Table 2).
Table 2.
—Counts of Phenotype of Inflammatory Cells Infiltrating Epithelial and Subepithelial Areas in Bronchial Mucosa of Subjects With and Without Asthma
| Asthma | Normal | |||||
| Group and Cells | Baseline | D 4 | Wk 6 | Baseline | D 4 | Wk 6 |
| Epithelium | ||||||
| CD45+ | 126 (41-434) | 164 (93-1,600) | 111 (62-597) | 109 (42-254) | 152 (84-394) | 113 (27-205) |
| Neutrophil elastase positive | 4.9 (0-12) | 16.1a (4-50) | 2.6 (0-28) | 5.2 (0-23) | 6 (0-24) | 5 (6-67) |
| EG2+ | 8.4b (0-160) | 2.4c (0-53) | 1.2d (0-4) | 0 (0-8) | 0 (0-9) | 0 (0-2) |
| Tryptase positive | 6 (0-15) | 7c (0-16) | 11d (3-24) | 2 (0-41) | 2 (0-7) | 5 (0-13) |
| CD68+ | 14 (3-67) | 28 (1-640) | 24 (7-70) | 22 (0-78) | 30 (5-93) | 36 (4-133) |
| CD3+ | 88 (20-145) | 104 (44-452) | 100a (81-393) | 97 (40-187) | 99 (38-239) | 125 (34-267) |
| CD4+ | 13 (2-38) | 15 (3-65) | 10 (2-55) | 11 (0-26) | 7 (1-35) | 5 (0-27) |
| CD8+ | 64 (11-194) | 80 (23-321) | 77 (48-186) | 79 (3-152) | 70 (20-148) | 76 (17-134) |
| CD20+ | 2 (0-7) | 1 (0-4) | 1 (0-3) | 0 (0-12) | 1 (0-15) | 0 (0-2) |
| Subepithelium | ||||||
| EG2+ | 84 (0-572) | 55 (4-717) | 26d (1-133) | 18 (0-214) | 9 (0-202) | 5 (0-50) |
| Tryptase positive | 142 (78-252) | 121 (8-285) | 95 (16-243) | 183c,d (81-342) | 92 (20-335) | 58 (16-290) |
| CD3+ | 803 (292-1,280) | 587 (159-2,127) | 458 (241-1,406) | 527 (225-1,091) | 434 (91-1,261) | 440 (99-1,373) |
| CD8+ | 712 (129-1,451) | 536 (120-2,354) | 457 (136-1,103) | 407 (155-792) | 418 (114-1,003) | 306 (41-623) |
| CD20+ | 53 (17-322) | 88 (15-351) | 71 (10-275) | 51 (0-386) | 58 (4-379) | 14 (0-98) |
Data are presented as median (range) of positive cell counts per 0.1 mm epithelium and per square millimeter subepithelium.
P = .005 and .023 vs asthma at baseline, respectively.
P = .011 vs normal at baseline.
P = .036, .046, and .049 vs normal on d 4, respectively.
P = .006, .01, and .03 vs normal at wk 6, respectively.
Macrophages:
Compared with baseline, the number of subepithelial CD68+ cells in both normal subjects (P = .018) and subjects with asthma (P = .009) were significantly increased at day 4 of acute infection (Fig 2D). There was a significantly greater number of subepithelial CD68+ cells present in subjects with asthma than in normal subjects at day 4 (P = .021). No significant differences in counts of epithelial CD68+ cells were found within and between groups (Table 2).
Mast Cells:
There were no significant differences within the subjects with asthma in epithelial and subepithelial mast cell counts from baseline to day 4 or week 6 (Table 2). The number of subepithelial mast cells of normal subjects at baseline was unexpectedly higher than at day 4 (P = .036) and week 6 (P = .006) (Table 2). The number of epithelial tryptase-positive mast cells was significantly greater in subjects with asthma than in normal subjects at day 4 (P = .049) and week 6 (P = .01) (Table 2).
Lymphocytes:
In subjects with asthma only, the number of epithelial CD3+ cells increased at week 6 from baseline (P = .023) (Table 2). Unexpectedly, significant decreases in subepithelial CD4+ counts from baseline were seen at week 6 in both normal subjects (P = .016) and subjects with asthma (P = .013) (Fig 2E). Compared with normal subjects, subjects with asthma had a significantly greater number of subepithelial CD4+ cells at baseline (P = .043). There were no significant differences in epithelial CD4+, CD8+, or CD20+ cell counts and in subepithelial CD3+, CD8+, or CD20+ cell counts in either group after infection (Table 2).
Changes in Numbers of Inflammatory Cells
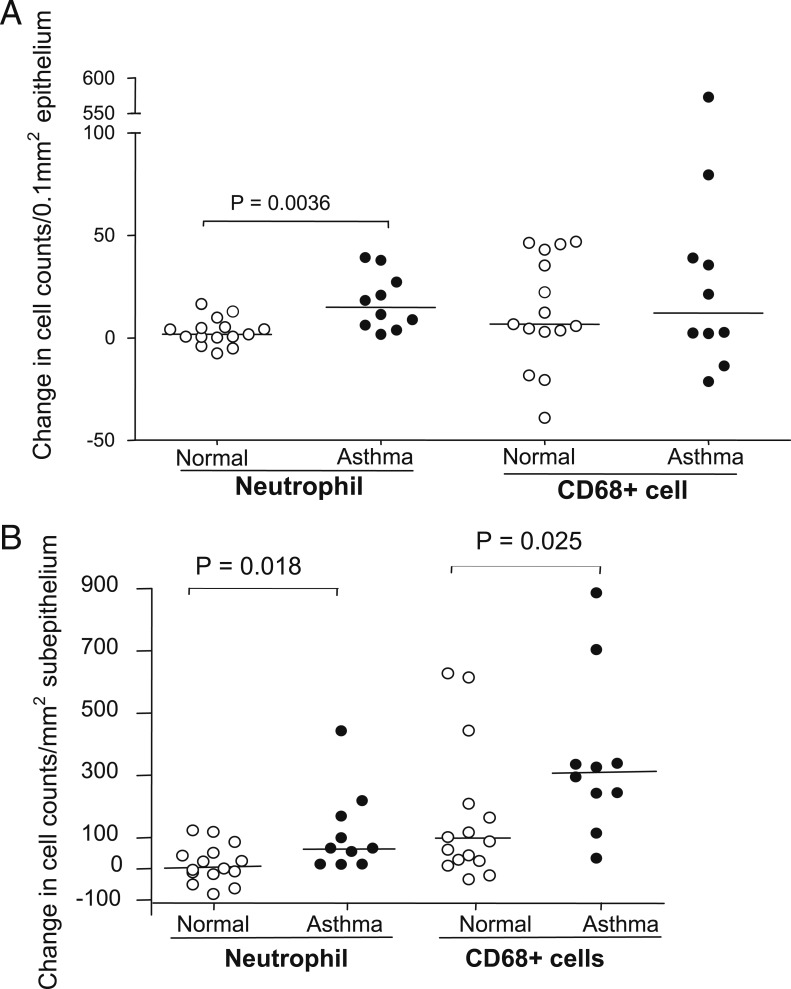
To investigate differences in inflammatory responses of subjects with and without asthma to RV infection, the magnitude of the changes of inflammatory cell counts from baseline to infection was compared. The changes in numbers of both epithelial (P = .0036) and subepithelial (P = .018) neutrophils from baseline to day 4 infection in subjects with asthma were significantly greater than those in normal subjects (Figs 3A, 3B). The changes in subepithelial CD68+ cell counts from baseline to day 4 infection in subjects with asthma were also significantly greater than those in normal subjects (P = .025) (Fig 3B). No significant differences with respect to changes in numbers of other inflammatory cell phenotypes were seen between groups from baseline to day 4 or week 6.
Figure 3.
A and B, Changes in counts of epithelial (A) and subepithelial (B) neutrophils and CD68+ cells from baseline to d 4 postinfection in bronchial biopsy specimens from subjects with and without asthma. Data are presented as change in the number of positive cells per 0.1 mm2 of epithelium or per square millimeter of subepithelium. ○ and ● show individual changes in counts, and horizontal bars show median values (Mann-Whitney U test).
Associations
Virus Load:
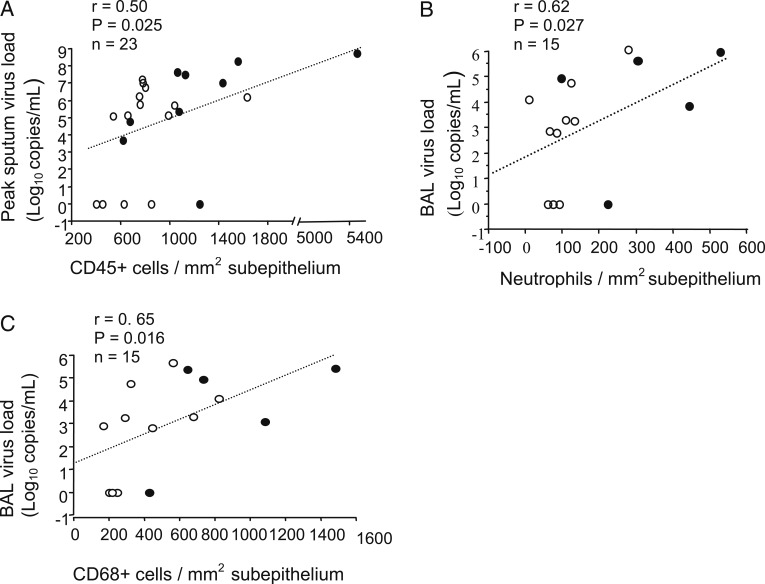
In all subjects at day 4, the number of subepithelial CD45+ cells was associated with peak sputum virus load (r = 0.5, P = .025) (Fig 4A), and subepithelial neutrophils (r = 0.62, P = .027) (Fig 4B), EG2+ eosinophils (r = 0.64, P = .016), and CD68+ cells (r = 0.65, P = .016) (Fig 4C) correlated with BAL virus load.
Figure 4.
A-C, Correlations between the numbers of subepithelial CD45+ cells (A) at d 4 and peak sputum virus load at d 3 after infection and neutrophils (B) and CD68+ cells (C) at d 4 and BAL virus load at d 4 in all subjects with asthma (●) and normal subjects (○) (Spearman rank correlation).
Clinical Symptoms and Airway Hyperresponsiveness:
At day 4, epithelial CD4+ counts in subjects with asthma correlated with total chest symptom scores recorded during the 2-week postinfection period (r = 0.69, P = .029) (Fig 5A). Those with high subepithelial neutrophil counts had a significantly larger fall in the 10% fall in FEV1 (PC10) from baseline to day 6 postinfection (r = 0.89, P = .029) (Fig 5B). The PC10 at day 6 correlated inversely with subepithelial CD3+ (r = −0.81, P = .016) (Fig 5C), CD4+ (r = −0.67, P = .023) (Fig 5D), and CD8+ (r = −0.65, P = .03) (Fig 5E) cells, and the 20% fall in FEV1 at day 6 was inversely associated with subepithelial CD20+ cells (r = −0.65, P = .03) (Fig 5F).
Figure 5.
A-F, In subjects with asthma alone, correlations between total chest symptom score d 0 to 14 after the rhinovirus 16 infection period and counts of epithelial CD4+ cells (A), between fall in PC10 (log2) and counts of subepithelial neutrophils at d 4 (B), between PC10 at d 6 and counts of subepithelial CD3+ (C), CD4+ (D), and CD8+ (E) cells at d 4; and between PC20 at d 6 and counts of subepithelial CD20+ cells at d 4 (F) (Spearman rank correlation, n = 10 for all). PC10 = 10% fall in FEV1; PC20 = 20% fall in FEV1.
Lung Function:
At day 4, higher epithelial CD8+ cell counts in subjects with asthma were significantly associated with a greater maximum fall in FEV1 (percent fall from baseline) (r = −0.72, P = .03) (Fig 6A). Of interest, higher subepithelial mast cell counts were significantly associated with a lower maximum percent fall in PEF (r = 0.8, P = .024) (Fig 6B). In all subjects at day 4, higher epithelial (r = 0.64, P = .03) and subepithelial (r = −0.53, P = .01) CD4+ cell counts were significantly associated with a greater maximum fall in FEV1.
Figure 6.
A, B, In subjects with asthma at d 4 after rhinovirus infection, correlations between maximum fall in FEV1 (%) and counts of epithelial CD8+ cells (A) and between maximum fall in PEF (%) and counts of subepithelial tryptase-positive mast cells (B) (Spearman rank correlation). PEF = peak expiratory flow.
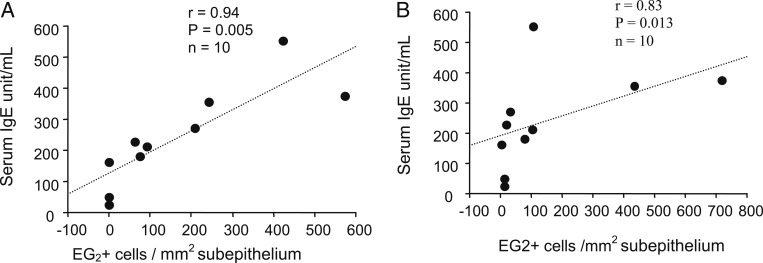
Serum IgE:
The levels of serum IgE were significantly correlated with subepithelial EG2+ eosinophils in normal subjects at day 4 (r = 0.64, P = .017) and in subjects with asthma at both baseline (r = 0.94, P = .005) and day 4 (r = 0.83, P = .013) (Figs 7A, 7B). There were no significant associations between serum IgE level and other phenotypes of inflammatory cells.
Figure 7.
A, B, Correlations between serum IgE levels and counts of subepithelial EG2+ eosinophils in asthma at baseline (A) and d 4 after rhinovirus infection (B) (Spearman rank correlation).
Discussion
We demonstrated that compared with normal subjects, subjects with asthma and RV infection had an exaggerated bronchial mucosal inflammatory response particularly characterized by increased numbers of neutrophils and CD68+ monocytes/macrophages. Significant correlations were found between CD45+ cells, neutrophils, and CD68+ cells and virus load and between neutrophils and T and B lymphocytes and airway hyperresponsiveness, chest symptoms, and reduced lung function, whereas mast cells were related to better lung function.
Previous experimental studies have shown that RV16 infection is associated with a significant increase in peripheral blood and BAL neutrophil counts in allergic asthma.13 In sputum, virus infection in asthma exacerbations has been associated with increased numbers of neutrophils and elevation of both neutrophil and eosinophil degranulation products, and cell lysis has been related to clinical severity.14 Increased numbers of neutrophils have been detected in the bronchial mucosa of subjects with severe asthma experiencing naturally occurring exacerbations.15 To our knowledge, however, no attempt has been made to determine whether RV infection alone can initiate bronchial mucosal neutrophilia, and its physiologic and clinical outcomes are unknown.
We reported in a previous publication on the clinical study from which the present biopsy specimens were collected increased numbers of BAL eosinophils during acute infection in the asthmatic group relative to the normal group, with similar, but nonsignificant trends for neutrophils.9 However, significant increases in BAL or induced sputum inflammatory cells from baseline to acute RV infection within the asthma group were not detected. Consistent with the BAL results, we found increased epithelial eosinophil and neutrophil counts in subjects with asthma vs normal subjects during acute RV infection. In addition, we demonstrated significant increases in the number of both epithelial and subepithelial neutrophils from baseline to acute infection in the asthma group, which were significantly higher than that in the normal group. We did not find increased numbers of neutrophils at week 6, which suggests that virus-induced airway neutrophilic inflammation in patients with mild asthma occurs only during the virus-induced acute asthma exacerbation and not during convalescence. Thus, in contrast to normal subjects, acute experimental RV16 infection selectively increases bronchial mucosal neutrophils in atopic asthma, and the higher numbers of neutrophils are related to the severity of infection and airway hyperresponsiveness. These data suggest that virus infection is one of the major etiologies inducing neutrophilic asthma. Because neutrophils play an important role in innate immune responses to clear virus, they can cause destruction of lung tissues through release of their proteases and other mediators.16 Therefore, the balance between the protective and pathologic effects of neutrophils in the course of virus-induced asthma exacerbations needs to be clarified when drug treatment to control neutrophilic inflammation17 is studied in the future.
Whereas we consider that asthma severity and treatment affect the magnitude of the inflammatory response to RV infection, the nature of the inflammation is more influenced by the time at which the biopsy specimens are taken in relation to the onset of RV infection. With respect to the latter, we believe that the results should not have been affected by the subjects with mild asthma studied who had not taken inhaled or oral corticosteroids.
The effect of RV infection on sputum and BAL CD68+ cells in subjects with and without asthma was not investigated in our previously reported study.9 Seymour et al18 reported an increase in the number of macrophages seen in bronchial biopsy specimens of normal subjects in response to experimental RV16 infection, but subjects with asthma were not investigated. In contrast, the present study shows that acute RV infection increases the number of subepithelial CD68+ cells of subjects with and without asthma. Notably, the changes in levels observed in the asthma group from baseline to acute infection were significantly greater than those in the normal group. Other work has suggested that impaired macrophage production of T-helper 1-related cytokines is strongly implicated in asthma exacerbation pathogenesis.19,20 Therefore, further investigation is needed to discern their role in asthma exacerbations induced by RV infection.
Airway mast cells are key in the immediate allergic response and are long associated with the pathogenesis of stable asthma.21,22 Using chloroacetate esterase to identify lysosomal granules in mast cells, Lozewicz et al23 found increased mast cell numbers in endobronchial biopsy specimens from subjects with atopic asthma compared with normal subjects. However, the numbers of tryptase-positive mast cells, tryptase-positive and chymase-negative mast cells, and tryptase- and chymase-positive mast cells in bronchial mucosa of subjects with symptomatic atopic asthma was not greater than that in normal subjects.24,25 We did not find a difference in the number of tryptase-positive mast cells in the bronchial mucosa between subject groups at baseline, which is consistent with these previous reports.24,25 Although there were no changes in numbers of epithelial mast cells within either group during RV infection, we detected a greater number of epithelial mast cells in asthma day 4 and week 6 compared with normal day 4 and week 6, implying that asthma susceptibility to immediate allergic response could be greater after RV infection. The numbers of subepithelial mast cells declined between baseline and acute infection and further declined between acute infection and convalescence in normal subjects. This finding appears to be inconsistent with an earlier study in which the number of tryptase-positive mast cells in bronchial submucosa declined between the infection and convalescent periods, but no initial increase was seen between baseline and infection.26 Of note, we detected in the present study that in subjects with asthma, those with the highest subepithelial mast cell counts had the lowest maximum percent falls in PEF during acute infection. In this regard, the findings support the results described previously in which the numbers of mast cells in severe asthma correlated with better rather than worse lung function, suggesting that recruitment of mast cells, particularly to the distal lung, may be protective for lung function in severe asthma.27
The biologic mechanism for a protective role remains unclear. Rauter et al28 notably demonstrated that β-tryptase, a major protease released during mast cell activation, cleaves IgE. They suggested that IgE cleavage by effector cell proteases is a natural mechanism for controlling allergic inflammation. The role of mast cells in the pathogenesis of virus-induced asthma exacerbations clearly merits further study.
We demonstrate for the first time, to our knowledge, in subjects with asthma that epithelial CD4+ cell counts at day 4 of infection correlates with total chest symptom scores, and the number of bronchial mucosal CD3+, CD4+, and CD8+ T and CD20+ B lymphocytes are associated with increased airway hyperresponsiveness. Additionally, the subjects with asthma with high epithelial CD8+ counts had significantly greater maximum falls in FEV1. The findings suggest the involvement of both T and B lymphocytes in the severity of asthma exacerbations induced by virus infection. The absence of increased counts of CD3+ and CD8+ T lymphocytes on day 4 of acute RV infection in subjects with asthma alone is not in keeping with increased subepithelial CD3+ and CD8+ cells at day 6 reported previously.11 This inconsistency may be due to differences in timing of the biopsies, as the present specimens were taken at day 4 vs day 6. The reductions in subepithelial CD4+ counts from baseline to convalescence may be due to a decrease in blood CD4+ cell counts during acute infection,9 which could be a result of less recruitment of CD4+ cells into the lung.
IgE plays a critical role in eosinophil recruitment into the airways after antigen provocation.29 Zambrano et al30 demonstrated that patients with mild asthma with high levels of IgE had significantly greater lower respiratory tract symptom scores during the initial 4 days of infection than those with low IgE levels. These patients also had higher total blood eosinophil counts at baseline. Building on these findings, we demonstrate that subjects with asthma with high levels of serum IgE at both baseline and day 4 have significantly greater numbers of submucosal EG2+ eosinophils. These findings imply that patients with atopic asthma with high levels of serum IgE could have more EG2+ eosinophils in their airway and experience more severe illness during acute RV infection. Anti-IgE therapy,31,32 therefore, may be useful for preventing exacerbations of asthma triggered by RV infection.
We acknowledge that an atopic nonasthma group was not included in the present study. Thus, the differences observed between the normal and asthma groups may have been partly due to changes attributable to atopy vs asthma. Previous work reported that compared with normal subjects without atopy, nasal biopsy specimens of subjects with atopy during experimental RV16 infection did not show increases in infiltration of inflammatory cells (including neutrophils).33 Another study showed no difference in changes of bronchial mucosa inflammatory cell counts between normal subjects and subjects with atopic rhinitis experiencing naturally occurring common cold virus infection.34 However, to answer this question directly, bronchial mucosa inflammatory responses to experimental RV16 infection in subjects with atopy but not asthma requires further investigation.
We also acknowledge that we have investigated relatively small numbers of subjects, albeit in some detail, and that a large number of statistical tests were carried out; thus, the study may have failed to detect some genuine observations due to limitations of statistical power. However, we consider its strengths to include the comprehensive and meticulous immunochemical approach and rigorous design of the human challenge model, permitting accurate sampling before and during infection of a uniform nature and known time of onset. Despite these caveats, novel data and interesting observations have emerged.
In conclusion, RV16 infection induced a more severe neutrophil and monocyte/macrophage-rich inflammatory response in the bronchial mucosa of subjects with asthma compared with normal subjects similarly infected. Numbers of mucosa neutrophils, monocytes/macrophages, EG2+ eosinophils, and CD4+, CD8+, and CD20+ cells in subjects with asthma during acute infection were related to virus load, increasing airway reactivity, and worsening of asthma symptoms and lung function, whereas tryptase-positive mast cells were related to better lung function. These data add to our understanding of the pattern of inflammatory response occurring during RV-induced experimental exacerbations of asthma and, as such, could have an impact on the design of future treatment modalities.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Drs Zhu and Message had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Zhu: contributed to the counting, data analyses, drafting of the manuscript, and review of the manuscript.
Dr Message: contributed to the subject recruitment, bronchial biopsy specimen and relevant clinical data collection, and writing and review of the manuscript.
Dr Qiu: contributed to the immunohistochemical staining and writing and review of the manuscript.
Dr Mallia: contributed to the subject recruitment, bronchial biopsy specimen and relevant clinical data collection, and writing and review of the manuscript.
Dr Kebadze: contributed to the viral detection and writing and review of the manuscript.
Dr Contoli: contributed to the subject recruitment, bronchial biopsy specimen and relevant clinical data collection, and writing and review of the manuscript.
Dr Ward: contributed to the study design and writing and review of the manuscript.
Dr Barnathan: contributed to the study design and writing and review of the manuscript.
Dr Mascelli: contributed to the study design and writing and review of the manuscript.
Dr Kon: contributed to the study design and writing and review of the manuscript.
Dr Papi: contributed to the study design and writing and review of the manuscript.
Dr Stanciu: contributed to the study design and writing and review of the manuscript.
Prof Jeffery: contributed as the co-principal investigator, to the direction of all histologic aspects of the study, and to the writing and review of the manuscript.
Prof Johnston: contributed as the principal investigator, to the direction of all aspects of the study, and to the writing and review of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Contoli received grants from AstraZeneca and Chiesi Farmaceutici SpA and participated as a paid speaker for AstraZeneca, Boehringer Ingelheim GmbH, Chiesi Farmaceutici SpA, Menarini, Merck Sharp & Dohme Corp, and Novartis Corp. Dr Papi consulted to AstraZeneca, Chiesi Farmaceutici SpA, GlaxoSmithKline plc, and Zambon Co SpA. Prof Jeffery has acted as a consultant to GlaxoSmithKline plc and Argenta Discovery 2009 Limited. Prof Johnston consulted to Boehringer Ingelheim GmbH, Centocor Inc, Chiesi Farmaceutici SpA, GlaxoSmithKline plc, Grünenthal, Novartis Corp, Sanofi Pasteur SA, and Synairgen plc and received a grant from Centocor Inc. Drs Zhu, Message, Qiu, Mallia, Kebadze, Ward, Barnathan, Mascelli, Kon, and Stanciu have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- PC10
10% fall in FEV1
- PEF
peak expiratory flow
- RV
rhinovirus
Footnotes
Drs Zhu and Message contributed equally to this work.
Funding/Support: This work was supported by a Medical Research Council Clinical Research Fellowship and a British Medical Association HC Roscoe Fellowship (to Dr Message), British Lung Foundation/Severin Wunderman Family Foundation Lung Research Programme [Grant P00/2], Asthma UK [Grants 02/027 and 05/067], Wellcome Trust [Grants 063717 and 083567/Z/07/Z], the National Institute for Health Research Biomedical Research Centre and Clinical Lecturer funding schemes, Centocor Inc, Medical Research Council Centre [Grant G1000758], and European Research Council FP7 Advanced [Grant 233015] (to Prof Johnston).
References
- 1.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307(6910):982-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128(6):1165-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83(1):1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94(6):2200-2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardin PG, Fraenkel DJ, Sanderson G, van Schalkwyk EM, Holgate ST, Johnston SL. Peak expiratory flow changes during experimental rhinovirus infection. Eur Respir J. 2000;16(5):980-985 [DOI] [PubMed] [Google Scholar]
- 7.Grünberg K, Timmers MC, de Klerk EP, Dick EC, Sterk PJ. Experimental rhinovirus 16 infection causes variable airway obstruction in subjects with atopic asthma. Am J Respir Crit Care Med. 1999;160(4):1375-1380 [DOI] [PubMed] [Google Scholar]
- 8.Cheung D, Dick EC, Timmers MC, de Klerk EP, Spaan WJ, Sterk PJ. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152(5):1490-1496 [DOI] [PubMed] [Google Scholar]
- 9.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105(36):13562-13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151(3 pt 1):879-886 [DOI] [PubMed] [Google Scholar]
- 11.Grünberg K, Sharon RF, Sont JK, et al. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med. 2001;164(10):1816-1822 [DOI] [PubMed] [Google Scholar]
- 12.O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155(3):852-857 [DOI] [PubMed] [Google Scholar]
- 13.Jarjour NN, Gern JE, Kelly EA, Swenson CA, Dick CR, Busse WW. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105(6pt 1):1169-1177 [DOI] [PubMed] [Google Scholar]
- 14.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19(1):68-75 [DOI] [PubMed] [Google Scholar]
- 15.Qiu Y, Zhu J, Bandi V, Guntupalli KK, Jeffery PK. Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax. 2007;62(6):475-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56(6):672-686 [DOI] [PubMed] [Google Scholar]
- 17.Nagarkar DR, Wang Q, Shim J, et al. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol. 2009;183(10):6698-6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour ML, Gilby N, Bardin PG, et al. Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects. J Infect Dis. 2002;185(4):540-544 [DOI] [PubMed] [Google Scholar]
- 19.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023-1026 [DOI] [PubMed] [Google Scholar]
- 20.Laza-Stanca V, Message SD, Edwards MR, et al. The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. PLoS Pathog. 2011;7(7):e1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaya M, Sasaki H. Rhinovirus and asthma. Viral Immunol. 2003;16(2):99-109 [DOI] [PubMed] [Google Scholar]
- 22.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328(4):257-265 [DOI] [PubMed] [Google Scholar]
- 23.Lozewicz S, Gomez E, Ferguson H, Davies RJ. Inflammatory cells in the airways in mild asthma. BMJ. 1988;297(6662):1515-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley BL, Azzawi M, Jacobson M, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88(4):661-674 [DOI] [PubMed] [Google Scholar]
- 25.Djukanović R, Wilson JW, Britten KM, et al. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990;142(4):863-871 [DOI] [PubMed] [Google Scholar]
- 26.Thomas LH, Fraenkel DJ, Bardin PG, Johnston SL, Holgate ST, Warner JA. Leukocyte responses to experimental infection with human rhinovirus. J Allergy Clin Immunol. 1994;94(6):1255-1262 [DOI] [PubMed] [Google Scholar]
- 27.Balzar S, Chu HW, Strand M, Wenzel S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am J Respir Crit Care Med. 2005;171(5):431-439 [DOI] [PubMed] [Google Scholar]
- 28.Rauter I, Krauth MT, Westritschnig K, et al. Mast cell-derived proteases control allergic inflammation through cleavage of IgE. J Allergy Clin Immunol. 2008;121(1):197-202 [DOI] [PubMed] [Google Scholar]
- 29.Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996;183(4):1303-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambrano JC, Carper HT, Rakes GP, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111(5):1008-1016 [DOI] [PubMed] [Google Scholar]
- 31.D’Amato G, Perticone M, Bucchioni E, Salzillo A, D’Amato M, Liccardi G. Treating moderate-to-severe allergic asthma with anti-IgE monoclonal antibody (omalizumab). An update. Eur Ann Allergy Clin Immunol. 2010;42(4):135-140 [PubMed] [Google Scholar]
- 32.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Immunohistochemical analysis of nasal biopsies during rhinovirus experimental colds. Am J Respir Crit Care Med. 1994;150(4):1130-1136 [DOI] [PubMed] [Google Scholar]
- 34.Trigg CJ, Nicholson KG, Wang JH, et al. Bronchial inflammation and the common cold: a comparison of atopic and non-atopic individuals. Clin Exp Allergy. 1996;26(6):665-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement