Abstract
Background:
The increased prevalence of type 2 diabetes mellitus (T2DM) in countries endemic for TB poses a serious complication in the clinical management of this major infectious disease. Understanding the impact of T2DM on TB and the determinants of comorbidity is critical in responding to this growing public health problem with better therapeutic approaches. Here, we performed an exploratory study assessing a series of biologic parameters that could serve as markers of pathogenesis in TB with T2DM.
Methods:
Cross-sectional analyses of levels of heme oxygenase-1 (HO-1), acute phase proteins, tissue metalloproteinases, and tissue inhibitors of metalloproteinase (TIMPs) as well as cytokines and chemokines were performed in plasma samples from individuals with active pulmonary TB or with coincident TB and T2DM from South India.
Results:
Compared with patients with TB without diabetes, those with coincident T2DM exhibited increased Mycobacterium tuberculosis bacillary loads in sputum. Plasma levels of HO-1 but not of other acute phase proteins were higher in patients with TB and T2DM than in patients without diabetes, independent of bacillary sputum loads. HO-1 concentrations also positively correlated with random plasma glucose, circulating glycosylated hemoglobin, and low-density lipoprotein levels. Moreover, patients with coincident TB and T2DM exhibited increased plasma levels of TIMP-4 and elevated peripheral blood neutrophil counts, which, when considered together with HO-1, resulted in increased power to discriminate individuals with active TB with and without T2DM.
Conclusions:
Elevated plasma levels of HO-1 and TIMP-4 and peripheral blood neutrophil counts are potential single and combined markers of pathogenesis in TB and T2DM.
Type 2 diabetes mellitus (T2DM) and pulmonary TB are two of the most prevalent comorbid conditions in many parts of the world, and the convergence of these diseases appears to pose a serious threat to health care worldwide. Indeed, a variety of clinical and epidemiologic studies have identified T2DM as a risk factor for the development of active TB.1 Moreover, T2DM also appears to be associated with a greater severity of TB disease among the infected population and to have a detrimental effect on both disease presentation and response to treatment.2,3 Although the clinical and public health significance posed by the dual burden of TB and T2DM has been increasingly recognized, data examining the immunologic and metabolic basis of susceptibility to TB in patients with diabetes remain scarce. Although enhanced susceptibility to TB in patients with T2DM was initially attributed to a relative immunodeficiency among patients with diabetes, recent experimental and clinical studies are not consistent with this explanation. Indeed, increased levels of T helper cell 1, T helper cell 17, or both cytokines have been described in both TB-infected diabetic mice4 and humans with TB and diabetes.5,6
Heme oxygenase-1 (HO-1) is a major antioxidant highly expressed in lung tissue and is a key stress-response enzyme that degrades heme molecules, releasing free iron, carbon monoxide, and biliverdin.7 Recently, we have shown that HO-1 levels can distinguish latent or successfully treated TB from active disease with high accuracy8 and that HO-1 levels can also distinguish latent Mycobacterium tuberculosis (Mtb) infection from active disease in children.9 Interestingly, HO-1 levels have also been found to be elevated in T2DM, a finding attributed to an enhanced proinflammatory environment present in these individuals.10 Although HO-1 has been shown to be an important biomarker for TB severity, it has also been shown that other inflammatory markers, including acute phase reactants11 and matrix metalloproteinases (MMPs) and their endogenous inhibitors,12,13 can be used to distinguish patients with active TB.
Therefore, we hypothesized that measurement of HO-1 in combination with other markers might be used to detect increased comorbidity in TB with T2DM. To test this, we measured the levels of HO-1 and a variety of other markers in a group of pulmonary Mtb-infected individuals with and without T2DM. Our data reveal that elevated plasma levels of HO-1 and tissue inhibitor of metalloproteinase (TIMP)-4, in addition to increased absolute neutrophil counts (ANCs) in the blood, are potential markers of pathogenesis in TB with T2DM.
Materials and Methods
Study Population
We studied a group of 88 individuals with active pulmonary TB recruited from the TB Clinic at Stanley Medical Hospital, Chennai, India—the first consecutive 44 with diabetes and the first 44 without (Table 1). All individuals were sputum smear and culture positive. T2DM was diagnosed on the basis of glycosylated hemoglobin (HbA1c) levels and random blood glucose test, according to the American Diabetes Association criteria (all individuals with T2DM had HbA1c levels > 6.5% and random plasma glucose > 200 mg/dL). All individuals tested negative for HIV and were naive to antituberculous treatment. The two groups did not differ significantly in terms of radiologic extent of disease or site of disease as assessed by chest radiograph readings from three independent experts (data not shown). Anthropometric measurements and biochemical parameters, including plasma glucose, lipid profile, and HbA1c levels, were obtained using standardized techniques detailed elsewhere.14 Hematology was performed on all individuals using the Act-5 Diff-hematology analyzer (Beckman Coulter, Inc). The clinical databank used for the present study did not contain information on other comorbidities or medications used to treat diabetes and associated comorbid conditions. All individuals were examined as part of a clinical protocol approved by the National Institutes of Research in Tuberculosis Internal Ethical Committee (study ID: NCT01154959, project approval number NIRT-IEC: 2010002), and informed written consent was obtained from all participants.
Table 1.
—Characteristics of the Study Participants
| Parameter | TB (n = 44) | TB + Diabetes (n = 44) | Difference Between Groupsa | P Value |
| Age, y | 43.5 (20-70) | 45 (33-70) | 6.5 (3 to 11) | .002b |
| Men, No. (%) | 38 (86.4) | 31 (70.4) | 0.23 (−0.02 to 0.49) | .119 |
| BMI, kg/m2 | 22.2 (14.0-31.2) | 23.9 (19.6-33.4) | 1.7 (−0.4 to 3.1) | .111 |
| Systolic BP, mm Hg | 117 (90-169) | 115 (82-152) | −2.0 (−8.0 to 8.0) | .998 |
| Diastolic BP, mm Hg | 79 (40-112) | 70 (59-90) | −9.5 (−9.0 to 0.2) | .057 |
| Random plasma glucose, mg/dL | 101.5 (76-177) | 287 (200-653) | 180 (156 to 204) | < .001b |
| HbA1c, % | 5.6 (4.5-6.3) | 11.3 (8.0-16.2) | 5.3 (4.96 to 6.1) | < .001b |
| Total cholesterol, mg/dL | 182.5 (57-433) | 215 (124-259) | 30.5 (7.0 to 37) | .008b |
| HDL, mg/dL | 47 (34-86) | 38 (22-82) | −8.0 (−11 to −4) | < .001b |
| LDL, mg/dL | 112 (65-205) | 124 (47-185) | 13.5 (−3 to 24) | .119 |
| Triglycerides, mg/dL | 84.5 (57-433) | 182 (57-679) | 95.5 (47 to 104) | < .001b |
| RBC count, × 106/μL | 4.7 (2.9-8.0) | 4.6 (2.9-5.6) | −0.08 (−0.3 to 0.25) | .798 |
| Platelets, × 103/μL | 371 (94-666) | 411 (146-755) | 40 (−48 to 79) | .590 |
| WBC count, × 103 cells/μL | 10.3 (5.3-24.3) | 11.5 (7.3-20.4) | 1.2 (−0.1 to 2.6) | .053 |
| Neutrophils, × 103 cells/μL | 6.5 (2.0-21.2) | 7.8 (4.5-15.7) | 1.3 (0.5 to 2.3) | .041b |
| Eosinophils, × 103 cells/μL | 0.22 (0.06-1.0) | 0.25 (0.2-1.0) | 0.03 (−0.3 to 3.4) | .069 |
| Basophils, × 103 cells/μL | 0.08 (0.02-0.2) | 0.09 (0.4-0.3) | 0.12 (−0.01 to 0.27) | .203 |
| Monocytes, × 103 cells/μL | 0.8 (0.3-2.1) | 0.95 (0.3-1.9) | 0.12 (−0.03 to 0.26) | .125 |
| Lymphocytes, × 103 cells/μL | 1.8 (0.9-3.3) | 1.9 (0.98-4.3) | 0.09 (−0.05 to 0.49) | .100 |
| AFB sputum grade | 1.0 (1.0-2.0) | 2.0 (1.0-3.0) | 1.0 (0 to 1.0) | .005b |
Data represent medians and interquartile ranges, except for sex, which is displayed as % of individuals in each study group. Mann-Whitney tests were used to compare continuous variables between the groups, and percent of men was compared using the Fisher exact test (for sex). AFB = acid-fast bacillus; HbA1c = glycosylated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
The differences and interquartile ranges were calculated subtracting the median (or fraction) values assessed in the group of individuals with TB-diabetes comorbidity from those detected in the group of individuals with TB without diabetes.
P < .05.
Immunoassays
Levels of HO-1 (Enzo Life Sciences, Inc), apotransferrin and hepcidin (Uscn Life Science Inc), CD163, CD14, PD-1, interferon (IFN)-α, and IFN-β (R&D Systems, Inc) were measured using enzyme-linked immunosorbent assay kits. Levels of C-reactive protein (CRP), serum amyloid protein-A (SAA), haptoglobin, α2-macroglobulin, and several cytokines and chemokines were determined using a multiplex enzyme-linked immunosorbent assay system (Bio-Rad Laboratories, Inc). Levels of MMP-1, MMP-7, MMP-8, MMP-9, TIMP-1, TIMP-2, TIMP-3, and TIMP-4 were measured using a Luminex kit from R&D Systems, Inc.
Data Analysis
The median values with interquartile ranges were used as measures of central tendency. Continuous variables were compared using Mann-Whitney tests with Holm adjustment for multiple comparisons. The χ2 or Fisher tests were used to compare variables displayed as percentage. Spearman rank tests were used to assess correlations. Receiver operating characteristic (ROC) curves were used to test the power of each biomarker to distinguish TB with T2DM from TB with no T2DM cases. Multinomial logistic regression analyses adjusted for age, sex, BMI, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were performed to assess the OR of the associations between HO-1, TIMP-4, and/or ANC and TB with T2DM comorbidity. Three models of principal component analysis (PCA) were designed to assess how different combinations of candidate biomarkers contribute to the differentiation between TB with T2DM and TB without T2DM. In one model, only levels of HO-1, TIMP-4, and ANC were inputted. A second model inputted levels of several cytokines, chemokines, MMPs, and TIMPs, acute phase proteins, soluble markers of cellular activation, blood cell counts, and HO-1. A third model included all candidate biomarkers except for HO-1, TIMP-4, and ANC. An unsupervised two-way hierarchical cluster analysis (Ward method) using the same variables inputted in the different PCA models was used to specifically test whether the patients with TB with and without T2DM could be clustered separately. C-statistics ultimately tested the discriminatory power of each model illustrated in PCA and cluster analysis and compared with the performance of potential confounding factors (age, sex, BMI, total cholesterol, HDL, and triglycerides).
Results
Plasma Levels of HO-1, but Not of Acute Phase Proteins, Are Significantly Higher in Individuals With TB and T2DM
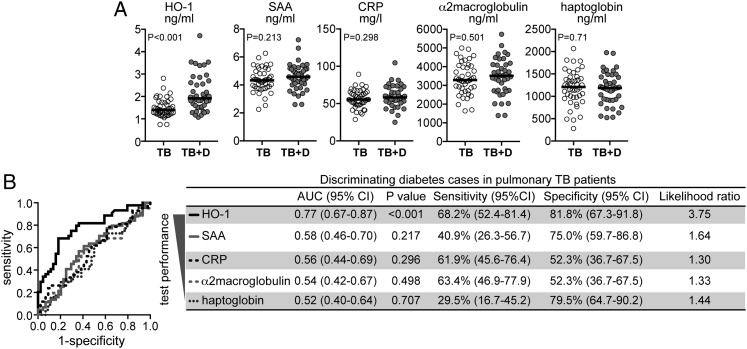
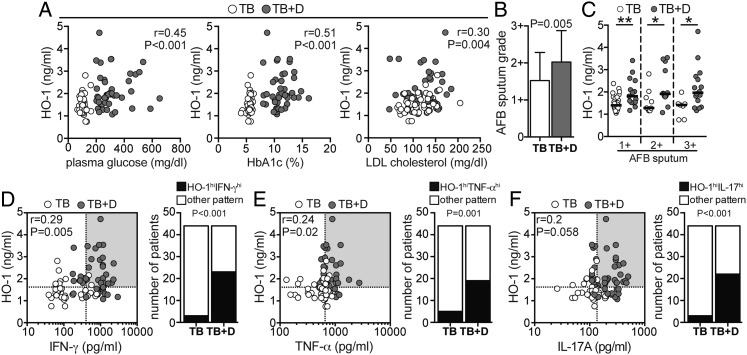
Individuals with coincident TB and T2DM were older and had higher concentrations of total cholesterol and triglycerides as well as lower HDL than patients with active TB without diabetes (Table 1). The two clinical groups were similar in sex, BMI, BP, and most of the hematologic parameters (Table 1). Since HO-1 has been previously shown to be an accurate marker for TB disease severity,8 we compared plasma HO-1 levels between individuals with TB and T2DM and TB and no T2DM. Individuals with TB and T2DM exhibited significantly higher levels of HO-1 than individuals with TB and no T2DM (Fig 1A). Because inflammation per se could cause nonspecific elevations of HO-1 in TB with T2DM, we measured the levels of other inflammatory markers (eg, SAA, CRP, α2-macroglobulin, and haptoglobin) in all individuals. Interestingly, plasma levels of all of these well-validated markers of inflammation did not differ between the study groups (Fig 1A). Thus, the ability of HO-1 to discriminate TB with T2DM from TB without T2DM was clearly higher than each of the other acute phase reactants tested (Fig 1B). Upon analysis of the entire study population, circulating levels of HO-1 positively correlated with parameters associated with severity and worse prognosis in diabetes, including levels of plasma glucose (Fig 2A), HbA1c (Fig 2A), and LDL (Fig 2A). Individuals with diabetes had higher acid-fast bacilli (AFB) sputum grades than patients without diabetes (Fig 2B, Table 1), suggesting that TB and T2DM comorbidity favors a reduced capacity to control Mtb infection. We then tested the potential influence of diabetes on HO-1 levels in patients with active TB by comparing individuals with diabetes with patients without diabetes matched for AFB sputum grade. We found that individuals with TB and T2DM displayed significantly higher concentrations of HO-1 than patients with TB and no T2DM independent of the AFB grade (Fig 2C). Interestingly, HO-1 levels positively correlated with plasma concentrations of IFN-γ (Fig 2D) and tumor necrosis factor (TNF)-α (Fig 2E) but not with IL-17A (Fig 2F). These three cytokines have been previously shown to be increased in individuals with TB and T2DM compared with those with TB and no T2DM.6 In addition, a significantly higher number of individuals presenting with simultaneously high values of HO-1 and elevated concentrations of IFN-γ, TNF-α, or IL-17A was detected in the TB with T2DM group (Figs 2D‐F). These results suggest that in TB with T2DM, pathways involving activation of HO-1 are involved.
Figure 1.
Plasma HO-1 levels are elevated in individuals with diabetes with active pulmonary TB. A, Plasma concentrations of HO-1, SAA, CRP, α2-macroglobulin, and haptoglobin from 44 individuals with active pulmonary TB without diabetes (TB) and 44 individuals with active pulmonary TB with diabetes (TB + D) were compared using Mann-Whitney tests. P values shown are adjusted for multiple measurements (Holm method). Bars represent median values. B, Receiver operating characteristic curves were used to assess the performance of HO-1 and the acute phase proteins to distinguish individuals with TB with diabetes from those with TB without diabetes (detailed C-statistic is shown on the left). AUC = area under the curve; CRP = C-reactive protein; HO-1 = heme oxygenase-1; SAA = serum amyloid protein-A.
Figure 2.
Plasma levels of HO-1 are associated with TB-diabetes comorbidity. A, Plasma concentrations of HO-1 were tested for correlations with glycemia (plasma glucose levels), percent of circulating HbA1c, and LDL cholesterol using Spearman rank tests. B, Mycobacterium tuberculosis quantitative bacillary sputum grade determined by AFB staining was compared between individuals with active TB with diabetes (TB + D) and without diabetes (TB) using the Mann-Whitney test. Data represent mean and SD. C, The study participants were stratified according to the quantitative bacillary sputum grade, and levels of HO-1 were compared between individuals with TB with and without diabetes with similar sputum grades using Mann-Whitney tests. Bars represent median values; *P < .05; **P < .01. D-F, Correlations between plasma levels of HO-1 and IFN-γ (D), TNF-α (E), or IL-17A (F) were assessed using Spearman tests. Graphs on the right show frequency of individuals displaying simultaneously values of HO-1 and IFN-γ, TNF-α, and IL-17A plasma concentrations higher than their respective medians in the study population. Data were analyzed using Fisher exact test. AFB = acid-fast bacillus; HbA1c = glycosylated hemoglobin; hi = higher than median values; IFN = interferon; LDL = low-density lipoprotein; TNF = tumor necrosis factor. See Figure 1 legend for expansion of other abbreviations.
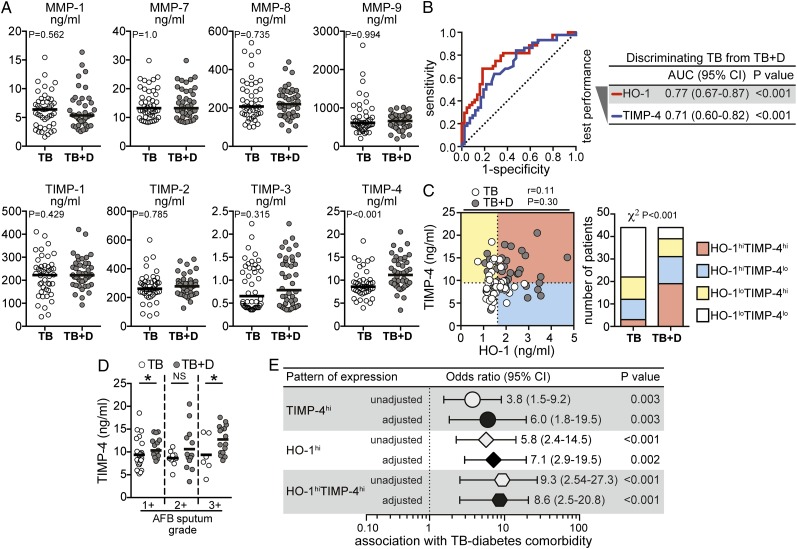
Individuals With TB and T2DM Exhibit Significant Elevations in Plasma Levels of TIMP-4
Increased levels of MMPs are associated with disease severity in TB.12,13 To test whether MMPs and TIMPs are also altered in TB with T2DM, we compared plasma levels of several of these enzymes in individuals with TB and T2DM and TB and no T2DM. Among all of these markers, TIMP-4 alone showed important differences between the groups. Individuals with TB and T2DM exhibited higher levels of TIMP-4 than individuals with TB and no T2DM (Fig 3A). The ability of TIMP-4 to discriminate between the two groups of patients was only slightly lower than HO-1 (Fig 3B). However, levels of HO-1 did not correlate with those of TIMP-4 (Fig 3C). Interestingly, the number of individuals exhibiting concomitantly high levels of HO-1 and TIMP-4 was dramatically increased in TB with T2DM (Fig 3C). When matched for AFB sputum grades, patients with diabetes still displayed higher values of TIMP-4 than patients without diabetes (Fig 3D). We next measured the associations between the presence of increased levels of TIMP-4, HO-1, or both, and the odds for having coincident TB and T2DM after adjustment for age, sex, BMI, total cholesterol, HDL, LDL, and triglycerides. Individuals with plasma concentrations of TIMP-4 above the median value established for the entire study population (9.5 ng/mL) were six times more likely to have coincident TB and T2DM than those with lower measurements (Fig 3E), whereas patients exhibiting HO-1 levels above the median value of study population (1.62 ng/mL) were approximately seven times more likely to have this comorbid condition than those with lower HO-1 values (Fig 3E). The strength of association with TB and T2DM comorbidity was considerably increased when the values of both TIMP-4 and HO-1 were elevated (Fig 3E).
Figure 3.
Plasma levels of TIMP-4 are elevated in individuals with active pulmonary TB with diabetes. A, Plasma concentrations of MMP-1, MMP-7, MMP-8, MMP-9, TIMP-1, TIMP-2, TIMP-3, and TIMP-4 were compared between individuals with active TB with diabetes (TB + D) and without diabetes (TB) using Mann-Whitney tests adjusted for multiple measurements (Holm method). Bars represent median. B, Receiver operating characteristic curves were used to test the performance of HO-1 or TIMP-4 to distinguish individuals with TB with and without diabetes. C, Levels of TIMP-4 and HO-1 were tested for correlation using the Spearman test. Dotted lines on the x-axis represent the median value of HO-1 within the total group of patients with pulmonary disease, and dotted lines on each y-axis indicate median values for TIMP-4. Patients were stratified in categories based on the values of HO-1 and/or TIMP-4 below or above their respective median values in the study population. The frequency of patients in each category was compared between the group of M tuberculosis-infected individuals with or without diabetes using the χ2 test. D, The study participants were stratified according to the quantitative bacillary sputum grade, and levels of TIMP-4 were compared between individuals with TB with and without diabetes with similar sputum grades by the Mann-Whitney test. Bars represent median; *P < .05. E, OR (multinomial logistic regression) adjusted for age, sex, BMI, total cholesterol, high-density lipoprotein (HDL), LDL, and triglycerides were calculated to assess the associations between levels of TIMP-4 and/or HO-1 above their respective median values in the study population and the presence of TB-diabetes comorbidity. Univariate analyses are also shown for comparison. lo = lower than median values; MMP = matrix metalloproteinase; NS = nonsignificant; TIMP = tissue inhibitor of metalloproteinase. See Figure 1 and 2 legends for expansion of other abbreviations.
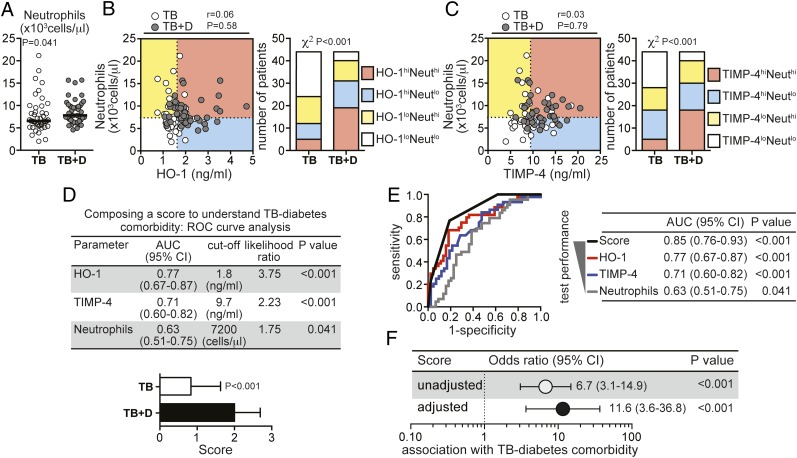
Individuals With TB and T2DM Exhibit Increased Absolute Peripheral Neutrophil Counts
ANCs in blood from TB with T2DM cases were significantly higher than in patients with TB and no T2DM (Fig 4A). The ANC did not correlate with either the plasma concentrations of HO-1 (Fig 4B) or TIMP-4 (Fig 4C). Nevertheless, individuals displaying concomitantly greater ANC and increased HO-1 or TIMP-4 levels were more frequent in the group of patients with TB and T2DM (Figs 4B, 4C). ANCs alone, however, were inferior to HO-1 or TIMP-4 in discriminating between patients with TB and T2DM and patients with TB and no T2DM (Fig 4D). Since these three variables (HO-1, TIMP-4, and ANC) were not correlated to each other and appeared to be independent markers of TB with T2DM, we hypothesized that a composite score using data from all three variables would increase the discrimination between the groups. ROC curve analyses established threshold levels from each variable with optimal discriminatory power. A score of 1 point was given to individuals presenting with values of either HO-1, TIMP-4, or ANC above their respective thresholds, resulting in a combined score ranging from 0 (no variables above their thresholds) to 3 (all variables above their thresholds). Using this approach, we found that patients with TB and T2DM displayed significantly higher scores than individuals with TB and no T2DM (Fig 4D). In addition, the score exhibited a better overall discriminatory performance compared with each individual variable (Fig 4E). As expected, the composite score revealed a dramatically high positive association with TB and T2DM (Fig 4F).
Figure 4.
Patients with TB-diabetes comorbidity exhibit increased peripheral blood neutrophil counts in addition to elevated plasma levels of HO-1 and TIMP-4. A, Neutrophil counts in the blood were compared between individuals with active TB with diabetes (TB + D) and without diabetes (TB) using Mann-Whitney tests. Bars represent median values. B, C, Neutrophil counts and HO-1 or TIMP-4 were tested for correlations using Spearman tests. Dotted lines on the x-axis represent the median values of HO-1 or TIMP-4 within the total group of patients with pulmonary disease, and dotted lines on each y-axis indicate median values for neutrophil counts. Patients were stratified in categories based on the values of the plasma markers and/or neutrophil counts below or above their respective median values in the study population. The frequency of patients in each category was compared between the group of M tuberculosis-infected individuals with or without diabetes using the χ2 test. D, ROC curve analysis established the cutoff values for HO-1, TIMP-4, and neutrophil counts with the highest likelihood ratio to infer occurrence of TB-diabetes comorbidity. These cutoff values were computed into a score according to the number of variables displaying values above their respective cutoff values. E, ROC curves compare the performance of the composite score in segregating patients with TB-diabetes comorbidity from those patients with TB without diabetes with the individual parameters. F, ORs per increase in 1 unit of the composite score adjusted for age, sex, BMI, total cholesterol, HDL, LDL, and triglycerides were calculated to assess the associations between the composite score and the presence of TB-diabetes comorbidity. Univariate analyses are also shown for comparison. Neut = neutrophil; ROC = receiver operating characteristic. See Figure 1‐3 legends for expansion of other abbreviations.
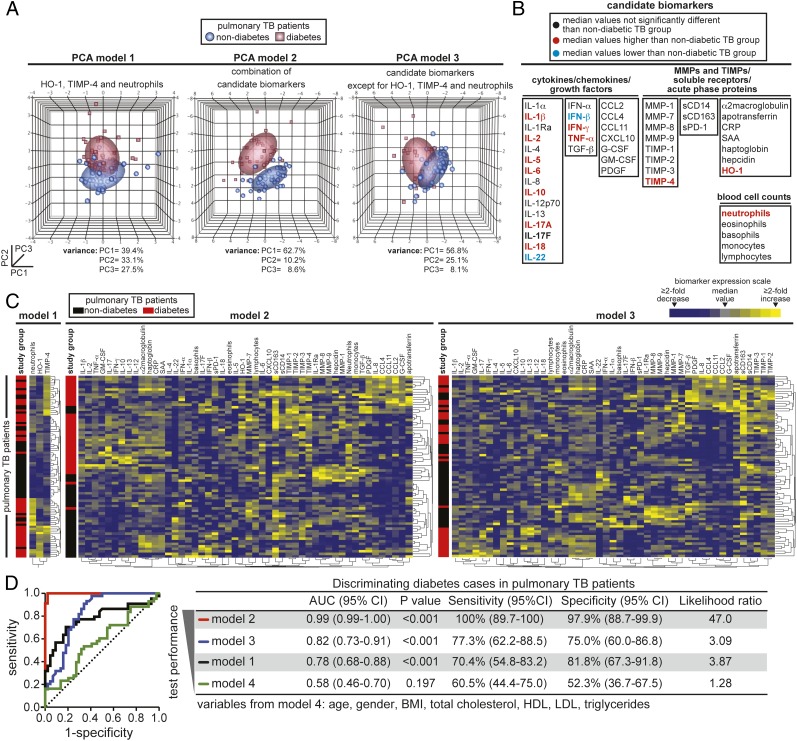
PCAs of the Proposed Pathogenic Markers for TB With T2DM
Having demonstrated that plasma levels of HO-1 and TIMP-4, and to a lesser extent ANC, contribute to the ability to discriminate TB with T2DM from TB without T2DM, we next used PCA models with different inputs to better define the role of these markers in the context of several other biomarkers. In the first PCA model (Fig 5A) in which only HO-1, TIMP-4, and ANC were used, we verified that patients from the two different groups clustered separately but with notable intersection. A second model included data from measurements of several other parameters (Fig 5B) and showed that the two clinical groups clustered separately with no intersection (Fig 5A). To verify the contribution of HO-1, TIMP-4, and neutrophil count in the context of all these other biomarkers, we designed a third model excluding just these three variables. With this modification, the intersection between the groups was dramatically increased (Fig 5A), implying that HO-1, TIMP-4, and ANC are indeed the critical components discriminating TB with T2DM from TB without T2DM in this larger panel. These results were reinforced by cluster analysis using the same combination of the markers from the PCA (Fig 5C). ROC curve analysis ultimately compared the overall performance of the diverse combination of candidate biomarkers and also combined potential confounding factors in distinguishing TB with T2DM from TB without T2DM (Fig 5D). Interestingly, when only information on age, sex, BMI, total cholesterol, HDL, LDL, and triglycerides were considered, the discriminatory performance was poor (area under the curve, 0.58; 95% CI, 0.46-0.70; P = .197) (Fig 5D). The best performance was achieved only when HO-1, TIMP-4, and ANC were considered in the context of several other candidate biomarkers. This approach confirmed that among several markers of inflammation, tissue remodeling, immune activation, and oxidative stress, HO-1, TIMP-4, and ANC are optimal for discrimination of TB with T2DM from TB without T2DM.
Figure 5.
PCA of candidate biomarkers for TB-diabetes comorbidity. A, Three PCA models computing data from plasma levels of several candidate biomarkers in combination with blood cell counts were used to assess if the clinical groups can be segregated. Normal contour ellipsoids were used to show limits of each clinical group (coverage area = 50%). The percentage of variance from the PCs shown in different three-dimensional graphs is described below each graph. B, All the biomarkers used in the PCA models are displayed. Variables are colored in red or blue if the levels in patients with diabetes and TB were significantly higher or lower (P < .05) than in patients with TB without diabetes, respectively, using Mann-Whitney tests after adjusting for multiple measurements (Holm method) (see Table 2 for details). C, Unsupervised clustering analyses (Ward method) used using the different combinations of candidate biomarkers shown in the diverse PCA models. A two-way clustering approach was used. Data on biomarkers were not normalized or transformed. Individuals with or without diabetes were listed in rows, and each biomarker was placed in a different column. The squares in the heat maps represent values below or above the median levels of a given biomarker in the study population (n = 48). D, ROC curve analyses were performed to estimate in a quantitative way the performance of the different combinations of biomarkers used in the PCA and cluster analyses in segregating patients with TB-diabetes comorbidity from those patients without diabetes with TB. A fourth model was included to assess potential influence of confounding variables. G-CSF = granulocyte colony stimulating factor; GM-CSF = granulocyte macrophage colony stimulating factor; PC = principal component; PCA = principal component analysis; PDGF = platelet-derived growth factor; sCD = soluble cluster of differentiation; sPD-1 = soluble Programmed Death-1; TGF = transforming growth factor. See Figure 1-4 legends for expansion of other abbreviations.
Table 2.
—Distribution of the Biomarkers Used in the Principal Component Analysis
| Biomarker | Units | TB (n = 44) | TB + Diabetes (n = 44) | Difference Between Mediansa | P Value |
| α2-Macroglobulin | ng/mL | 3,286 (2,748-4,024) | 3,513 (2,721-4,104) | 226.8 (−296 to 499) | .501 |
| Apotransferrin | ng/mL | 3.8 (2.6-6.5) | 4.1 (2.1-7.0) | 0.3 (−325 to 12.6) | .808 |
| CRP | mg/L | 55.4 (48.7-65.1) | 58.2 (52.8-69.9) | 2.8 (−2.6 to 8.16) | .298 |
| CCL2 | pg/mL | 313.0 (203.9-321.2) | 313.0 (239.3-427.2) | 0.0 (0.0 to 100.4) | .248 |
| CCL4 | pg/mL | 289.7 (165.2-362.7) | 224.2 (173.2-755.9) | −65.5 (−53.0 to 75.8) | .681 |
| CCL11 | pg/mL | 308.8 (250.1-378.4) | 304.7 (257.0-418.0) | −4.1 (−34.0 to 35.2) | .925 |
| CXCL10 | pg/mL | 10.6 (8.5-14.5) | 10.6 (9.1-13.9) | 0.0 (−1.46 to 1.6) | .866 |
| G-CSF | pg/mL | 61 (51.2-90.2) | 59.8 (46.2-92.5) | −1.2 (−9.8 to 9.5) | .853 |
| GM-CSF | pg/mL | 59.6 (54.4-70.1) | 62.7 (54.4-93.7) | 3.1 (−2.1 to 16.0) | .182 |
| Haptoglobin | ng/mL | 1,210 (977.8-1,421) | 1,185 (927.5-1,430) | −25.2 (−179 to 138) | .710 |
| Hepcidin | ng/mL | 87.9 (41.0-191.1) | 90.9 (30.7-226.7) | 3.0 (−4.0 to 33.1) | .811 |
| HO-1 | ng/mL | 1.4 (1.2-1.7) | 1.9 (1.6-2.6) | 0.5 (0.3 to 0.73) | < .001b |
| IFN-α | pg/mL | 11.3 (10.2-14.5) | 11.7 (10.9-12.9) | 0.4 (−0.9 to 0.8) | .820 |
| IFN-β | pg/mL | 10.3 (6.6-17.5) | 5.6 (5.1-6.8) | −4.7 (−7.4 to −1.9) | < .001b |
| IFN-γ | pg/mL | 102.7 (67.4-374.6) | 987.5 (431.6-1,179) | 884.8 (463 to 912) | < .001b |
| IL-1α | pg/mL | 1.2 (1.1-1.6) | 1.2 (1.2-1.6) | 0.0 (−0.09 to 0.17) | .833 |
| IL-1β | pg/mL | 103.0 (84.4-116.3) | 113.4 (99.5-164.5) | 10.4 (4.2 to 34.4) | .008b |
| IL-1Ra | pg/mL | 869.8 (659.0-1,274) | 873.4 (630.2-1,254) | 3.6 (−228 to 191) | .881 |
| IL-2 | pg/mL | 10.6 (6.6-17.4) | 15.7 (7.5-23.6) | 5.1 (0.5 to 7.3) | .025b |
| IL-4 | pg/mL | 0.3 (0.2-0.5) | 0.3 (0.3-0.5) | 0.01 (−0.04 to 0.1) | .337 |
| IL-5 | pg/mL | 0.3 (0.1-0.7) | 0.1 (0.1-0.2) | −0.2 (−0.6 to −0.03) | .003b |
| IL-6 | pg/mL | 583.5 (402.5-822.1) | 747.5 (561.1-1,027) | 164.0 (12.2 to 307) | .033b |
| IL-8 | pg/mL | 515.3 (330.0-989.1) | 418.7 (299.7-793.9) | −96.6 (−217 to 70.1) | .409 |
| IL-10 | pg/mL | 104.5 (81.3-136.0) | 168.5 (111.4-211.7) | 64.0 (30.2 to 82.1) | < .001b |
| IL-12p70 | pg/mL | 42.6 (34.9-52.3) | 44.6 (35.8-84.9) | 2.0 (0.0 to 15.5) | .099 |
| IL-13 | pg/mL | 53.0 (37.7-67.5) | 55.0 (47.1-86.0) | 2.0 (−2.4 to 17.2) | .128 |
| IL-17A | pg/mL | 116.4 (98.7-136.4) | 214.0 (132.6-310.3) | 97.6 (71.2 to 162.9) | < .001b |
| IL-17F | pg/mL | 112.6 (61.6-235.7) | 80.0 (66.0-116.5) | −32.6 (−62.7 to 2.2) | .075 |
| IL-18 | pg/mL | 7.9 (4.6-20.1) | 20.1 (12.8-30.2) | 12.2 (7.5 to 15.5) | < .001b |
| IL-22 | pg/mL | 30.3 (16.8-59.1) | 9.5 (7.9-12.0) | −20.8 (−27.6 to −13) | < .001b |
| MMP-1 | ng/mL | 6.4 (3.7-8.1) | 5.3 (4.2-7.5) | −1.1 (−1.7 to 836.9) | .562 |
| MMP-7 | ng/mL | 13.2 (10.2-18.8) | 13.2 (10.2-18.8) | 0.0 (−1.9 to 1.9) | .999 |
| MMP-8 | ng/mL | 207.7 (165.0-317.0) | 220.0 (161.4-285.8) | 12.3 (−44.0 to 33.0 | .735 |
| MMP-9 | ng/mL | 614.1 (483.9-868.4) | 657.6 (511.6-1,791.8) | 43.5 (−119 to 104) | .994 |
| PDGF | ng/mL | 4.8 (2.9-7.6) | 4.8 (2.6-7.9) | 0.0 (−1.7 to 1.3) | .713 |
| SAA | ng/mL | 4.3 (3.8-4.9) | 4.6 (4.1-51.2) | 0.3 (−0.1 to 0.6) | .213 |
| sCD14 | μg/mL | 8.5 (5.4-13.7) | 8.3 (4.9-12.1) | −0.2 (−3.0 to 1.5) | .602 |
| sCD163 | μg/mL | 2.0 (1.8-2.0) | 1.9 (1.7-2.0) | −0.1 (−0.15 to 0.02) | .168 |
| sPD-1 | pg/mL | 9.3 (5.4-16.8) | 9.0 (5.6-13.4) | −0.3 (−3.3 to 1.7) | .617 |
| TGF-β | pg/mL | 231.4 (182.0-340.9) | 210.4 (165.5-284.8) | −21.0 (−52.4 to 20.6) | .482 |
| TIMP-1 | ng/mL | 222.3 (156.7-259.6) | 221.7 (184.9-271.0) | −0.6 (−18.2 to 44.0) | .429 |
| TIMP-2 | ng/mL | 260.9 (229.7-309.7) | 277.6 (224.1-312.3) | 16.7 (−24.0 to 40.1) | .785 |
| TIMP-3 | ng/mL | 0.6 (0.4-1.3) | 0.8 (0.4-1.4) | 0.2 (−0.05 to 241.6) | .315 |
| TIMP-4 | ng/mL | 8.6 (7.4-10.1) | 11.1 (9.0-14.4) | 2.5 (0.9 to 3.8) | < .001b |
| TNF-α | pg/mL | 618.9 (383.0-686.0) | 690.3 (628.0-1038) | 71.4 (94.0 to 362.3) | < .001b |
Data are presented as median (interquartile range). Univariate comparisons using Mann-Whitney tests were used to compare the concentrations of each biomarker between the study groups. CRP = C-reactive protein; G-CSF = granulocyte colony stimulating factor; GM-CSF = granulocyte macrophage colony stimulating factor; HO-1 = heme oxygenase-1; IFN = interferon; MMP = matrix metalloproteinase; PDGF = platelet-derived growth factor; SAA = serum amyloid protein-A; sCD = soluble cluster of differentiation; sPD-1 = soluble Programmed Death-1; TGF = transforming growth factor; TIMP = tissue inhibitor of metalloproteinase; TNF = tumor necrosis factor.
The differences and interquartile ranges were calculated subtracting the median values assessed in the group of individuals with TB-diabetes comorbidity from those detected in the group of individuals with TB without diabetes.
P < .05 after adjustment for multiple measurements (Holm method).
Discussion
Prompt and accurate TB diagnosis is already a major issue,15 and the presence of diabetes adds complications in both the diagnostic and therapeutic aspects of TB control. Although there is growing evidence that diabetes increases susceptibility to TB,2,3,16 the host factors that influence this complex interaction are largely unexplored. In the present study, we examined some of the host factors that could potentially influence TB and T2DM comorbidity, focusing on parameters known to influence susceptibility to TB disease, disease severity, or response to treatment.
HO-1 promotes cytoprotection in diverse disease models including mycobacterial infection. We have previously shown that systemic levels of HO-1 are dramatically increased in individuals with active TB and particularly in those with bilateral lung lesions and elevated bacillary loads in the sputum.8 HO-1 is also known to be elevated in patients with T2DM and in individuals with impaired glucose regulation.10,17 Here, elevated plasma HO-1 concentrations positively correlated with HbA1c, suggesting association with a state of sustained hyperglycemia, which is known to be a major factor driving T2DM-related complications.18 Thus, HO-1 is a likely candidate for a molecule that may be involved in the interaction between TB and T2DM. Indeed, we found that HO-1 levels, by themselves, clearly discriminate patients with TB with T2DM from patients with TB without T2DM. Acute phase proteins are highly sensitive reactants induced during inflammatory responses and are known to be elevated in patients with TB.19 Notably, CRP has been proposed as a biomarker for active TB.20
Our findings have two important implications concerning the acute phase protein response in TB with T2DM. First, they reveal that diabetes has negligible effects in the acute inflammatory processes seen in TB, and second, they reveal that the increased levels of HO-1 in patients with TB and T2DM are not merely the consequence of a generalized elevation of acute phase reactants occurring when diabetes is superimposed on TB. Because HO-1 is a major antioxidant induced in responses to stress, we speculate that the higher values of this biomarker seen in TB with T2DM reflects the existence of greater oxidative stress in diabetic TB lesions, a hypothesis that should be addressed in future mechanistic studies.
The disease process in pulmonary TB involves enzymatic degradation of lung tissue by MMPs.12,21,22 This situation is reflected by the increased MMP levels in patients with TB, which correlate with disease severity.13 We have recently observed that plasma levels of MMPs and TIMPs are elevated in pediatric patients with TB compared with age-matched healthy children.9 Furthermore, T2DM by itself is known to affect expression of MMPs and TIMPs.23,24 However, our data clearly indicate that in the context of TB, further elevations in MMP levels are not a hallmark of TB with T2DM. TIMPs are endogenous regulators of MMP and modulate tissue pathology in inflammatory conditions. Our data reveal that TIMP-4 alone among the four recognized TIMPs is significantly elevated and serves as a powerful marker for TB with T2DM. Although TIMP-1 and TIMP-3 are believed to play protective roles in the development of diabetes,25,26 the function of TIMP-4 in either diabetes or TB remains unknown. Elevated levels of TIMP-4 could mean that either this factor plays a direct role in pathogenic processes associated with TB and T2DM comorbidity or it could be a more accurate readout of increased tissue damage than other tissue remodeling enzymes induced in stress responses. Notably, increased tissue expression of TIMP-4 has been recently linked to the progression of periodontal inflammation associated with T2DM.27 Additional studies will be necessary to clarify the role of TIMP-4 in pathologic processes in TB and T2DM comorbidity.
Neutrophils are a critical component of the innate immune response to TB and are believed to contribute to disease protection through oxidative killing of mycobacteria.28 These cells can also promote pathology in conditions of high bacterial load, which favor neutrophil accumulation,29,30 and a neutrophil-dominant interferon signature in whole blood has been shown to correlate with active TB disease.31 Therefore, our observation of peripheral neutrophilia in TB with T2DM is not surprising given that the same individuals display higher bacterial loads in sputum. Whether neutrophils directly contribute to the enhanced morbidity seen in patients with TB and T2DM remains to be determined.
It is possible that duration of TB disease could have an impact on the disease parameters, which may be reflected in changes in the levels of inflammatory markers such as those presented here. The present cross-sectional study recruited patients with TB at the time when disease became clinically evident, and we do not have reliable information on duration of disease prior to presentation for enrollment. This is a common problem in cross-sectional studies assessing patients with TB. The contribution is nonetheless important, since we identify three independent mechanisms of disease involving HO-1, TIMP-4, and neutrophils, respectively, in the TB-T2DM interaction. Regardless of mechanism, the findings from the present study strongly argue that certain parameters of TB severity are increased as a consequence of comorbid T2DM and correlate with poor glycemic control. Such differences would not easily be detected from chest radiograph readings alone, and indeed we could not find clear-cut differences in the radiographs between patients with diabetes and patients without diabetes in the population studied here.
In the present study, we also analyzed a variety of other factors known to play a role in either diabetes or TB. We assessed the importance of these multiple markers in discriminating individuals with TB and T2DM from individuals with TB and no T2DM using PCA and cluster analysis. Our results argue that although significant differences exist in other parameters between the TB with T2DM and TB without T2DM, it is HO-1 in conjunction with TIMP-4 and ANC that provides the most accurate combined parameter of TB and T2DM comorbidity.
Conclusions
This study highlights novel pathways that appear to be heavily influenced by the presence of T2DM in pulmonary TB. The observed association of these three different mechanistic pathways—one involving a cytoprotective enzyme, a second involving a regulator of tissue fibrosis, and the third involving the innate arm of the immune system—illustrates the complexity of the interaction between diabetes and TB, and we hope it will assist in the development of interventions for this growing comorbidity.
Acknowledgments
Author contributions: Drs Andrade and Babu take major responsibility for the integrity of the work and analyses.
Dr Andrade: contributed to conceiving and designing the research, analyzing the data, writing the manuscript, and reading and approving the final version of the manuscript.
Mr Pavan Kumar: contributed to conceiving and designing the research, performing the experiments, and reading and approving the final version of the manuscript.
Dr Sridhar: contributed to performing the clinical assessments and reading and approving the final version of the manuscript.
Dr Banurekha: contributed to performing the clinical assessments and reading and approving the final version of the manuscript.
Dr Jawahar: contributed to performing the clinical assessments and reading and approving the final version of the manuscript.
Dr Nutman: contributed to providing reagents/materials/analysis tools, writing the manuscript, and reading and approving the final version of the manuscript.
Dr Sher: contributed to writing the manuscript and reading and approving the final version of the manuscript.
Dr Babu: contributed to conceiving and designing the research, providing reagents/materials/analysis tools, writing the manuscript, and reading and approving the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank Michael Fay, PhD, and David Kugler, PhD, of the National Institute of Allergy and Infectious Diseases, National Institutes of Health for critical discussions on statistical analysis. We thank the staff of Department of Clinical Research and the Department of Social Work, National Institute for Research in Tuberculosis, especially R. Kalaiselvi, BA, and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study. We also thank R. Anuradha, MS; V. Gopinath, MS; and Jovvian George, MS, of the National Institutes of Health, International Center for Excellence in Research, for technical assistance.
Abbreviations
- AFB
acid-fast bacilli
- ANC
absolute neutrophil count
- CRP
C-reactive protein
- HbA1c
glycosylated hemoglobin
- HDL
high-density lipoprotein
- HO-1
heme oxygenase-1
- IFN
interferon
- LDL
low-density lipoprotein
- MMP
matrix metalloproteinase
- Mtb
Mycobacterium tuberculosis
- PCA
principal component analysis
- ROC
receiver operating characteristic
- SAA
serum amyloid protein-A
- T2DM
type 2 diabetes mellitus
- TIMP
tissue inhibitor of metalloproteinase
- TNF
tumor necrosis factor
Footnotes
Dr Andrade and Mr Pavan Kumar contributed equally to the work.
Funding/Support: This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184(11):6275-6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47(5):634-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208(5):739-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61(2):748-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade BB, Pavan Kumar N, Mayer-Barber KD, et al. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS ONE. 2013;8(5):e62618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavan Kumar N, Anuradha R, Andrade BB, et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20(5):704-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao W, Rong S, Zhang M, et al. Plasma heme oxygenase-1 concentration in relation to impaired glucose regulation in a non-diabetic Chinese population. PLoS ONE. 2012;7(3):e32223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Beer FC, Nel AE, Gie RP, Donald PR, Strachan AF. Serum amyloid A protein and C-reactive protein levels in pulmonary tuberculosis: relationship to amyloidosis. Thorax. 1984;39(3):196-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkington P, Shiomi T, Breen R, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121(5):1827-1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ugarte-Gil CA, Elkington P, Gilman RH, et al. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS ONE. 2013;8(4):e61333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deepa M, Pradeepa R, Rema M, et al. The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I). J Assoc Physicians India. 2003;51:863-870 [PubMed] [Google Scholar]
- 15.Wallis RS, Kim P, Cole S, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13(4):362-372 [DOI] [PubMed] [Google Scholar]
- 16.Reed GW, Choi H, Lee SY, et al. Impact of diabetes and smoking on mortality in tuberculosis. PLoS ONE. 2013;8(2):e58044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SW, Yeung VT, Benzie IF. Heme oxygenase microsatellite polymorphism, oxidative stress, glycemic control, and complication development in type 2 diabetes patients. Free Radic Biol Med. 2012;53(1):60-63 [DOI] [PubMed] [Google Scholar]
- 18.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11(5):343-354 [DOI] [PubMed] [Google Scholar]
- 20.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS ONE. 2011;6(1):e15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundararajan S, Babu S, Das SD. Comparison of localized versus systemic levels of Matrix metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and cytokines in tuberculous and non-tuberculous pleuritis patients. Hum Immunol. 2012;73(10):985-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J. 2011;38(2):456-464 [DOI] [PubMed] [Google Scholar]
- 23.Hsieh HL, Lin CC, Hsiao LD, Yang CM. High glucose induces reactive oxygen species-dependent matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Mol Neurobiol. 2013;48(3):601-614 [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Guo S, Yao F, Zhang Y, Li T. Increased ratio of serum matrix metalloproteinase-9 against TIMP-1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complications. 2013;27(4):380-382 [DOI] [PubMed] [Google Scholar]
- 25.Han X, Sun Y, Scott S, Bleich D. Tissue inhibitor of metalloproteinase-1 prevents cytokine-mediated dysfunction and cytotoxicity in pancreatic islets and beta-cells. Diabetes. 2001;50(5):1047-1055 [DOI] [PubMed] [Google Scholar]
- 26.Federici M, Hribal ML, Menghini R, et al. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest. 2005;115(12):3494-3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung HY, Kim YG, Park JW, Suh JY, Lee JM. The expression of a nitric oxide derivative, tissue inhibitors of metalloproteinase-3, and tissue inhibitors of metalloproteinase-4 in chronic periodontitis with type 2 diabetes mellitus. J Periodontal Implant Sci. 2013;43(2):87-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33(1):14-25 [DOI] [PubMed] [Google Scholar]
- 29.Eruslanov EB, Lyadova IV, Kondratieva TK, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73(3):1744-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nandi B, Behar SM. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208(11):2251-2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973-977 [DOI] [PMC free article] [PubMed] [Google Scholar]