Abstract
Background:
Hyperinflation refers to a nonspecific increase in absolute lung volumes and has a poor prognosis in COPD. The relative contribution of increased airways resistance and increased parenchymal compliance to hyperinflation of each absolute lung volume is poorly understood. We hypothesized that increased residual volume (RV) and RV/total lung capacity (TLC) would be associated with reduced airway lumen dimensions, whereas increased functional residual capacity (FRC), TLC, and reduced inspiratory capacity (IC)/TLC would be associated with emphysema on CT scan. We examined whether clinical characteristics differed accordingly.
Methods:
The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study recruited smokers aged 50 to 79 years who were free of clinical cardiovascular disease. Gas trapping was defined as RV or RV/TLC greater than the upper limit of normal and hyperexpansion as FRC or TLC greater than the upper limit of normal or IC/TLC less than the lower limit of normal. Airway lumen diameters and percent emphysema < −950 Hounsfield units were quantified on CT images. Analyses were adjusted for age, sex, body size, race/ethnicity, education, and smoking.
Results:
Among 116 participants completing plethysmography, 15% had gas trapping, 18% has hyperexpansion, and 22% had both. Gas trapping was associated with smaller airway lumen diameters (P = .001), greater dyspnea (P = .01), and chronic bronchitis (P = .03). Hyperexpansion was associated with percent emphysema (P < .001), lower BMI (P = .04), and higher hemoglobin concentration (P = .001).
Conclusions:
Gas trapping and hyperexpansion on plethysmography were associated with distinct differences in lung structure and clinical characteristics. Absolute lung volumes should not be considered equivalent in their estimation of hyperinflation and provide insight into the extent of airway and parenchymal abnormalities in COPD.
COPD is characterized by airflow limitation that is not fully reversible and is a leading cause of morbidity and mortality.1,2 Hyperinflation in COPD is defined by an increase in absolute lung volumes3 and is believed to be partly due to inadequate emptying of the lungs as a result of increases in airways resistance, respiratory system compliance, or a combination of the two.3 Current guidelines do not specify which absolute lung volumes should be used to define hyperinflation.4
Specific lung volumes have been associated with different clinical outcomes in COPD.5‐9 For example, Martinez et al7 demonstrated increases in residual volume (RV) but not total lung capacity (TLC) to be associated with mortality independent of spirometric measures of airflow limitation. Our group observed increased RV and RV/TLC but not functional residual capacity (FRC), TLC, or inspiratory capacity (IC)/TLC to be associated with greater left ventricular mass independent of body size and traditional cardiac risk factors.9 Furthermore, interventions that alter airways resistance (eg, bronchoconstriction, bronchodilation) cause greater changes in RV than FRC or TLC.5,6,8 In contrast, obesity correlates better with FRC and TLC than RV, and weight loss improves FRC but not RV.10,11 These studies suggest heterogeneity across absolute lung volumes with respect to hyperinflation, but they did not examine the potential structural basis and clinical correlates of such heterogeneity.
Changes in airway caliber contribute directly to airways resistance,3,12 and emphysema alters parenchymal recoil in part by lack of elastin.13 Hence, a differential contribution of quantitative measures of airway dimensions14 and emphysematous destruction15 to specific lung volumes, as assessed on CT images, is likely. Some studies have reported simple correlations between specific lung volumes and CT imaging metrics of lung structure16‐19; however, detailed analyses are lacking.
The aims of the current study were to determine whether elevated RV and RV/TLC are associated with reduced airway lumen dimensions on CT imaging, whereas elevated FRC, TLC, and reduced IC/TLC are associated with emphysema. We then examined whether clinical characteristics differed accordingly.
Materials and Methods
Study Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study recruited patients with COPD and control subjects predominantly from MESA, a population-based prospective cohort study of subclinical atherosclerosis,20 and a separate, nonoverlapping lung cancer screening study.21 In addition, a small number of participants were recruited from the outpatient community at Columbia University Medical Center. Included participants were aged 50 to 79 years with a ≥ 10 pack-year smoking history. Exclusion criteria were clinical cardiovascular disease, asthma prior to age 45 years, prior lung resection, or cancer. The current report describes participants who were selected for and completed body plethysmography.
Study Oversight
Study procedures were approved by the institutional review board of Columbia University Medical Center (AAAD6395) and by the National Heart, Lung, and Blood Institute. Written informed consent was obtained from all participants.
Pulmonary Function Testing
Body plethysmography, single-breath diffusing capacity of lung for carbon monoxide divided by alveolar volume (Dlco/Va), and postbronchodilator spirometry were assessed with a V6200 Autobox (Sensormedics Corp), Autobox 220 Series instrument (Sensormedics Corp), and a dry-rolling-sealed spirometer (Occupational Marketing, Inc), respectively, following American Thoracic Society/European Respiratory Society recommendations and reported in liters at body temperature and pressure saturated.22‐24 Predicted spirometry values were calculated using reference equations by Hankinson et al.25 COPD status and severity were defined per American Thoracic Society/European Respiratory Society criteria.1 Predicted lung volume values and upper and lower limits of normal for each lung volume were calculated using reference equations for participants aged ≥ 65 years by Garcia-Rio et al26 and reference equations for participants aged < 65 years by Crapo et al.27
Gas trapping was defined as RV or RV/TLC above the upper limits of normal. Hyperexpansion was defined as FRC or TLC above the upper limits of normal or IC/TLC below the lower limit of normal.
Chest CT Image Acquisition and Analysis
Participants underwent full-lung thoracic CT imaging on a 64-slice helical scanner (Lightspeed VCT 64; GE Healthcare) (120 kVp, 200 mAs at 0.5 s, 0.75-mm slice thickness). Images were obtained at suspended full inspiration. Image attenuation and airway dimensions were assessed using Apollo software (VIDA Diagnostics, Inc)28 at a single reading center. Percent emphysema was defined as the percentage of total voxels within the lung field < −950 Hounsfield units (HU).29
The airway tree was identified by an automated region-growing technique, and all segmental bronchi were labeled anatomically. Subsegmental bronchi were further labeled along five prespecified paths: RB1, RB4, RB10, LB1+2, and LB10. Luminal diameter and wall thickness were measured perpendicular to the local long axis and averaged along the middle third of each labeled airway. Every scan underwent visual inspection by trained readers unaware of other participant information to confirm accuracy of automated airway labeling.
Anthropometry and Other Covariates
Height, weight, BMI, and blood hemoglobin concentration were measured according to MESA protocol.11 Age, sex, race/ethnicity, and education were self-reported, and dyspnea was assessed with the five-level (0-4) modified Medical Research Council dyspnea scale.30 Chronic bronchitis was self-reported and defined by the presence of cough and sputum for at least 3 months in each of 2 consecutive years.31 Oxygen saturation as measured by pulse oximetry (Spo2) (CMS-50F pulse oximeter; Contec Medical Systems Co, Ltd) was performed at rest while breathing ambient air. Smoking history was assessed by standard questionnaire, and current smoking status was confirmed by plasma cotinine levels (IMMULITE 2000 nicotine metabolite assay; Diagnostic Products Corp).
Statistical Analysis
Dichotomous variables are presented as proportions and continuous variables as mean ± SD unless otherwise indicated. Bivariate comparisons were tested by χ2, Fisher exact, or Student t tests as appropriate.
We assessed the association of each absolute lung volume with segmental and subsegmental airway lumen diameters or percent emphysema < −950 HU using linear regression models to adjust for age, sex, height, weight, BMI, race/ethnicity, educational attainment, and smoking status. Analyses for the airways included measures from all 18 segmental and 10 subsegmental bronchi (arising from RB1, RB4, RB10, LB1+2, and LB10) for each participant; generalized estimating equations were used to account for these multiple measures. Sex and current smoking status were included in the model because they have been shown to affect lung density.32‐34 Analyses were repeated for each absolute lung volume dichotomized above or below the relevant limit of normal and for the presence or absence of gas trapping and hyperexpansion by logistic regression. Secondary analyses assessed airway wall thickness (AWT) and the percent of AWT relative to total airway diameter (%AWT). Analysis using Dlco/Va % predicted was performed as an alternate measure of parenchymal destruction. Clinical characteristics of participants with COPD and gas trapping, hyperexpansion, or both were compared with those without COPD by logistic regression to adjust for age, sex, BMI, and smoking status.
A two-tailed P < .05 was considered to indicate statistical significance. Analyses were performed using SAS 9.3 (SAS Institute Inc) statistical software.
Results
Of 370 potential participants who were screened for plethysmography at one site, 132 were enrolled into the MESA COPD Study, and 116 completed the measures (e-Fig 1 (461.3KB, pdf) ). Enrolled participants were similar to those excluded except for differences in race/ethnic distribution, body weight, and smoking history (e-Table 1 (461.3KB, pdf) ).
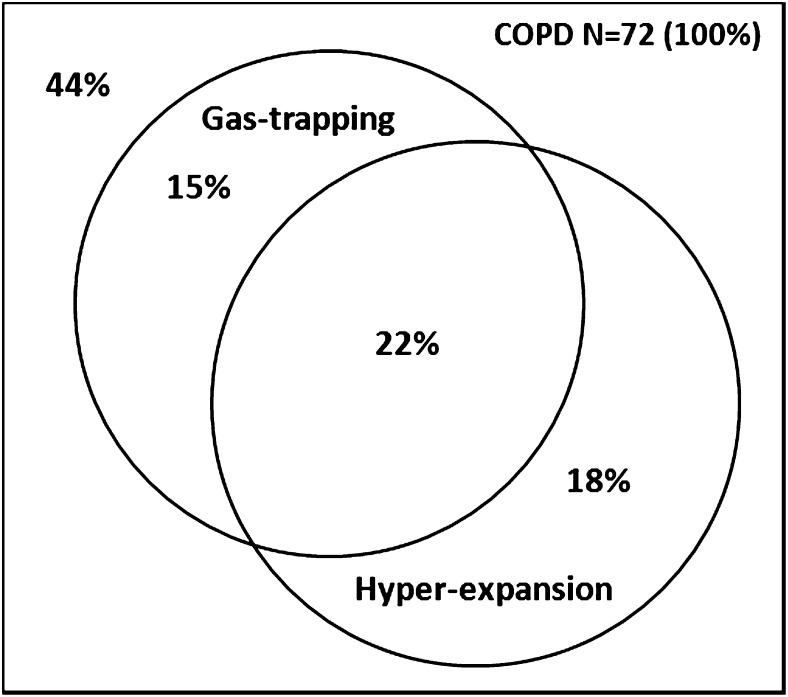
The mean age of the 116 included participants was 69 ± 6 years, and 57% were men. Clinical characteristics of these participants are summarized in Table 1. Seventy-two participants (62%) had COPD, which was predominantly of moderate severity. Thirty-eight participants with COPD (55%) had gas trapping, hyperexpansion, or both: 15% had isolated gas trapping, 18% had isolated hyperexpansion, and 22% had both gas trapping and hyperexpansion (Fig 1).
Table 1.
—Characteristics of the MESA COPD Study Participants With Plethysmography
| Characteristic | Value |
| No. participants | 116 |
| Age, y | 69 ± 6 |
| Male sex | 57 |
| Race/ethnic group | |
| White | 73 |
| Black | 19 |
| Other | 8 |
| Height, cm | 169 ± 10 |
| Weight, kg | 77 ± 19 |
| BMI, kg/m2 | 27 ± 6 |
| Cigarette smoking status | |
| Current | 37 |
| Former | 63 |
| Pack-y of smoking | 39 (26-56) |
| COPD | 62 |
| GOLD-COPD severity | |
| Mild | 33 |
| Moderate | 46 |
| Severe/very severe | 21 |
| Postbronchodilator spirometry | |
| FEV1, L | 2.15 ± 0.75 |
| FEV1 % predicted | 82 ± 25 |
| FEV1/FVC | 0.63 ± 0.14 |
| Plethysmography | |
| RV, L | 2.01 ± 0.75 |
| RV % predicted | 101 ± 33 |
| RV hyperinflationa | 12 |
| FRC, L | 3.34 ± 0.87 |
| FRC % predicted | 106 ± 23 |
| FRC hyperinflationa | 16 |
| IC, L | 2.30 ± 0.73 |
| IC % predicted | 93 ± 24 |
| IC hyperinflationb | 16 |
| TLC, L | 5.64 ± 1.27 |
| TLC % predicted | 101 ± 15 |
| TLC hyperinflationa | 8 |
| RV/TLC, L | 0.35 ± 0.10 |
| RV/TLC % predicted | 100 ± 28 |
| RV/TLC hyperinflationa | 18 |
| IC/TLC, L | 0.41 ± 0.08 |
| IC/TLC % predicted | 93 ± 24 |
| IC/TLC hyperinflationb | 20 |
| Dlco/Va, mL/min/mm Hg/L BTPS | 3.5 ± 0.8 |
| Dlco/Va % predicted | 72 ± 17 |
| Proportion with mMRC dyspnea ≥ 2 | 24 |
| Proportion with chronic bronchitis | 14 |
| Proportion with Spo2 < 95%c | 14 |
| Blood hemoglobin concentration, g/dL | 13.9 ± 1.2 |
| Percent emphysema < −950 HU | 1.8 (0.7-5.5) |
| Segmental airways | |
| No. airways quantified per participant | 18 (18-18) |
| Airway lumen diameter, mm | 4.4 (3.8-5.2) |
| AWT, mm | 1.5 (1.4-1.7) |
| %AWT | 25 (23-27) |
| Subsegmental airwaysd | |
| No. airways quantified per participant | 10 (10-10) |
| Airway lumen diameter, mm | 3.1 (2.6-3.8) |
| AWT, mm | 1.2 (1.0-1.3) |
| %AWT | 27 (25-29) |
Data are presented as mean ± SD, %, or median (first-third quartile) unless otherwise indicated. AWT = airway wall thickness; BTPS = body temperature and pressure, saturated; Dlco/Va = diffusing capacity of lung for carbon monoxide divided by alveolar volume; FRC = functional residual capacity; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HU = Hounsfield units; IC = inspiratory capacity; MESA = Multi-Ethnic Study of Atherosclerosis; mMRC = modified Medical Research Council; RV = residual volume; Spo2 = oxygen saturation as measured by pulse oximetry; TLC = total lung capacity.
Hyperinflation defined as values greater than the upper limit of normal.
Hyperinflation defined as values less than the lower limit of normal.
Spo2 measurements were available in 100 participants.
Subsegmental bronchial dimensions were quantified from branch descendants of five segmental airways (RB1, RB4, RB10, LB1+2, and LB10), yielding 10 subsegmental airway measurements per participant.
Figure 1.
Proportional Venn diagram depicting prevalence of gas trapping and hyperexpansion among Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study participants with COPD. Gas trapping was defined as residual volume or residual volume/total lung capacity greater than the upper limit of normal. Hyperexpansion was defined as functional residual capacity or total lung capacity greater than the upper limit of normal or inspiratory capacity/total lung capacity less than the lower limit of normal. Percentages do not sum to 100 because of rounding.
The median lumen diameter of segmental and subsegmental airways was 4.4 mm (first-third quartile, 3.8-5.2 mm) and 3.1 mm (first-third quartile, 2.6-3.8 mm), respectively, and the median percent emphysema < −950 HU was 1.8% (first-third quartile, 0.7%-5.5%) (Table 1). In bivariate analysis, segmental and subsegmental lumen diameters were significantly smaller in women than in men (P < .001). This association was no longer significant after additional adjustment for body height (P = .08).
Airway Dimensions and Gas Trapping
Smaller airway lumen diameters were independently associated with greater RV in unadjusted and fully adjusted models (Table 2). A 1-SD decrement in airway lumen diameter was associated with a 60-mL increase in RV (95% CI, 20-100 mL; P = .006). Smaller lumen diameters were also associated with significantly greater odds of RV exceeding the upper limit of normal (Table 3). In contrast, there was no evidence for an association of airway lumen diameters with FRC, TLC, and IC/TLC (Tables 2, 3). Additional adjustment for percent emphysema < −950 HU did not alter these associations appreciably (e-Table 2 (461.3KB, pdf) ).
Table 2.
—Differences in Absolute Lung Volumes by Airway Lumen Diameter
| Predicted Differences in Lung Volumes (95% CI) | |||||
| Variable | RV, mL | RV/TLC, % | FRC, mL | TLC, mL | IC/TLC, % |
| Per SD decrement in airway lumen diameters | 60 (20 to 100) | 0.7 (0.1 to 1.3) | 20 (−10 to 60) | 30 (−10 to 80) | −0.1 (−0.6 to 0.4) |
| P value | .006 | .02 | .24 | .17 | .81 |
Predicted mean differences were estimated with generalized estimating equations and were adjusted for age, sex, height, weight, BMI, race/ethnic group, level of education attained, and current smoking status. See Table 1 legend for expansion of abbreviations.
Table 3.
—Differences in Odds of Absolute Lung Volumes Being Above or Below the Limit of Normal by Airway Lumen Diameter
| OR (95% CI) for Lung Volumes Above or Below the Limit of Normal | |||||
| Variable | RV > ULN | RV/TLC > ULN | FRC > ULN | TLC > ULN | IC/TLC < LLN |
| Per SD decrement in airway lumen diameters | 1.3 (1.2-1.5) | 1.3 (1.1-1.5) | 0.9 (0.6-1.1) | 1.2 (0.9-1.6) | 0.9 (0.7-1.2) |
| P value | < .001 | .01 | .27 | .35 | .53 |
Predicted odds for the dichotomous term were estimated with generalized estimating equations. ULN and LLN were calculated using age-, sex-, and height-based reference equations; hence, logistic models were unadjusted. LLN = lower limit of normal; ULN = upper limit of normal. See Table 1 legend for expansion of other abbreviations.
The odds of gas trapping were significantly greater per SD decrement in segmental and subsegmental airway lumen diameters (OR, 1.3; 95% CI, 1.1-1.5; P = .003), without evidence of effect modification by sex (P for interaction = .24). In contrast, there was no evidence for an association between the odds of hyperexpansion and airway lumen diameters (OR, 1.1; 95% CI, 0.86-1.4; P = .35).
Similar to results for airway lumen diameter, greater %AWT was significantly associated with greater RV and RV/TLC but not FRC, TLC, or IC/TLC (e-Table 3 (461.3KB, pdf) ). AWT was not associated with lung volumes (e-Table 3 (461.3KB, pdf) ).
Emphysema and Hyperexpansion
Greater percent emphysema < −950 HU was associated with significantly higher FRC and TLC and reduced IC/TLC in addition to higher RV and RV/TLC (Table 4). Additional adjustment for segmental and subsegmental airway lumen diameters did not alter the pattern of association between percent emphysema < −950 HU and absolute lung volumes (e-Table 4 (461.3KB, pdf) ). Findings were similar when lung volumes were categorized above or below the relevant limit of normal (Table 5).
Table 4.
—Differences in Absolute Lung Volumes by Percent Emphysema < −950 HU
| Predicted Differences in Lung Volumes (95% CI) | |||||
| Variable | RV, mL | RV/TLC, % | FRC, mL | TLC, mL | IC/TLC, % |
| Per SD increment in percent emphysema < −950 HU | 250 (170 to 340) | 3.0 (1.7 to 4.5) | 250 (180 to 330) | 290 (190 to 390) | −1.0 (−2.2 to −0.5) |
| P value | < .001 | < .001 | < .001 | < .001 | .03 |
Predicted mean differences were estimated using generalized estimating equations and were adjusted for age, sex, height, weight, BMI, race/ethnic group, level of education attained, and current smoking status. See Table 1 legend for expansion of abbreviations.
Table 5.
—Differences in Odds of Absolute Lung Volumes Being Above or Below the Limit of Normal by Percent Emphysema < −950 HU
| OR (95% CI) for Lung Volumes Above or Below the Limit of Normal | |||||
| Variable | RV > ULN | RV/TLC > ULN | FRC > ULN | TLC > ULN | IC/TLC < LLN |
| Per SD increment in percent emphysema < −950 HU | 2.4 (1.1-5.1) | 2.1 (1.3-3.3) | 2.6 (1.5-4.6) | 2.3 (1.3-3.9) | 1.8 (1.0-3.2) |
| P value | .02 | .004 | < .001 | .002 | .04 |
The pattern of association observed between percent emphysema < −950 HU and absolute lung volumes was similar to that observed for Dlco/Va % predicted (e-Table 5 (461.3KB, pdf) ). The odds of hyperexpansion were significantly greater per SD increase in percent emphysema < −950 HU (OR, 2.2; 95% CI, 1.4-3.6; P = .001), as were the odds of gas trapping (OR, 2.1; 95% CI, 1.3-3.4; P = .002).
Clinical Characteristics of Gas Trapping and Hyperexpansion
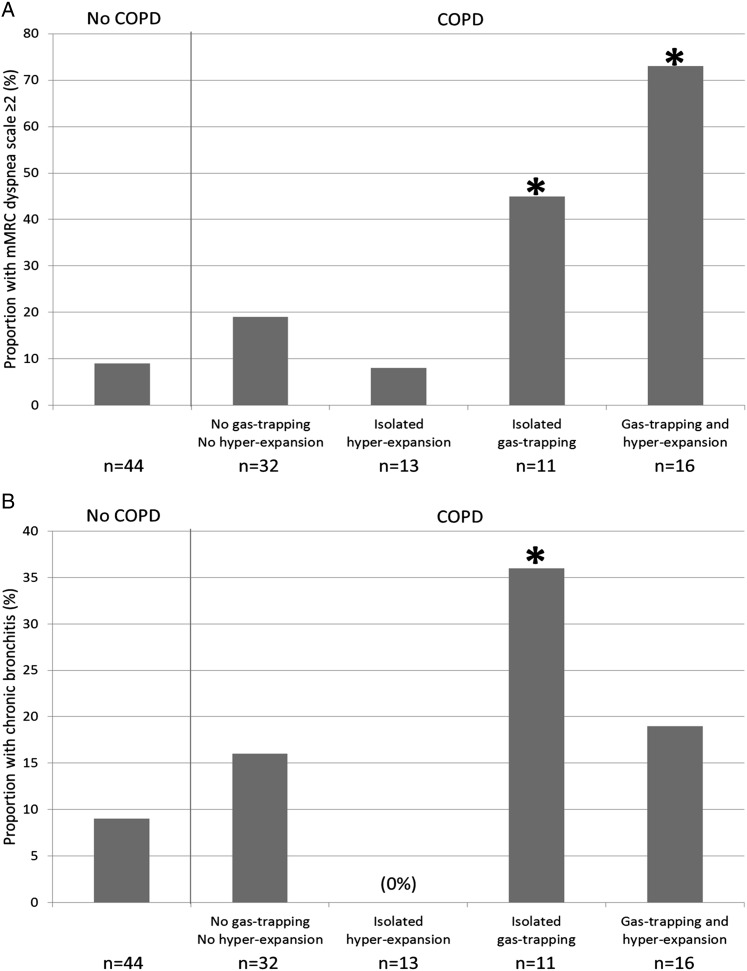
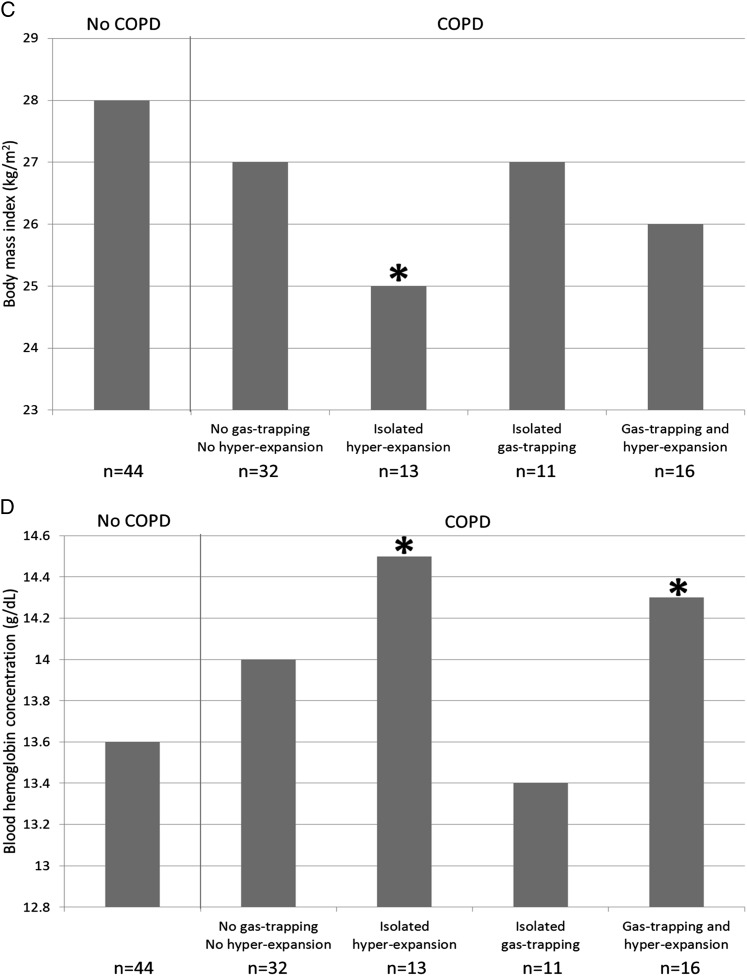
Compared with participants without COPD, those with gas trapping, hyperexpansion, or both were younger and trended toward higher prevalence of current smoking (Table 6). Participants with isolated gas trapping had smaller airway lumen diameters, greater dyspnea, and higher prevalence of chronic bronchitis compared with participants without COPD (Table 6, Figs 2A, 2B). Those with isolated hyperexpansion had lower BMI, lower Dlco/Va % predicted, higher percent emphysema < −950 HU, and higher hemoglobin concentration and trended toward lower Spo2 (Table 6, Figs 2C, 2D). Those with both gas trapping and hyperexpansion were more likely to be men and have severe COPD, smaller airway lumen diameters, greater percent emphysema < −950 HU, lower Dlco/Va % predicted, greater dyspnea, lower Spo2, and higher hemoglobin concentration (Table 6, Figs 2A, 2D). Finally, participants with COPD in the absence of gas trapping and hyperexpansion had milder COPD, lower Dlco/Va % predicted, and higher percent emphysema < −950 HU compared with control participants (Table 6). All associations remained statistically significant in models adjusting for age, sex, BMI, and current smoking status, except for the higher hemoglobin concentration among participants with both gas trapping and hyperexpansion (P = .10).
Table 6.
—Clinical Characteristics of MESA COPD Study Participants by COPD, Gas Trapping, and Hyperexpansion Status
| COPD | ||||||
| Characteristic | No COPDa | No Gas Trapping, No Hyperinflation | Isolated Hyperexpansion | Isolated Gas Trapping | Gas Trapping and Hyperexpansion | Global P Value |
| No. participants | 44 | 32 | 13 | 11 | 16 | … |
| Age, y | 70 ± 4 | 71 ± 5 | 65 ± 8 | 65 ± 9 | 66 ± 7 | .01 |
| P value | Reference | .88 | .01 | .04 | .03 | |
| Male | 43 | 63 | 77 | 45 | 75 | .09 |
| P value | Reference | .11 | .06 | 1.00 | .04 | |
| Race/ethnicity | .19 | |||||
| White | 73 | 81 | 85 | 55 | 63 | |
| P value | Reference | .56 | .48 | .29 | .66 | |
| Black | 14 | 9 | 15 | 45 | 38 | |
| P value | Reference | .73 | 1.00 | .05 | .09 | |
| Other | 14 | 3 | 0 | 0 | 0 | |
| P value | Reference | .23 | .32 | .33 | .18 | |
| BMI, kg/m2 | 28 ± 7 | 27 ± 5 | 25 ± 4 | 27 ± 6 | 26 ± 5 | .47 |
| P value | Reference | .58 | .04 | .70 | .16 | |
| Current smoker | 27 | 28 | 54 | 55 | 56 | .08 |
| P value | Reference | 1.00 | .10 | .15 | .06 | |
| Pack-y | 38 ± 19 | 45 ± 20 | 52 ± 27 | 42 ± 23 | 55 ± 36 | .24 |
| P value | Reference | .10 | .06 | .59 | .06 | |
| GOLD severity | … | |||||
| Mild | … | 53 | 46 | 9 | 0 | |
| Moderate | … | 44 | 38 | 82 | 31 | |
| Severe | … | 3 | 15 | 9 | 69 | |
| FEV1 % predicted | 101 ± 20 | 80 ± 15 | 81 ± 25 | 66 ± 12 | 47 ± 17 | < .001 |
| P value | Reference | < .001 | < .001 | < .001 | < .001 | |
| FVC % predicted | 100 ± 18 | 100 ± 15 | 107 ± 24 | 91 ± 13 | 76 ± 26 | .001 |
| P value | Reference | .97 | .30 | .06 | < .001 | |
| FEV1/FVC | 0.76 ± 0.04 | 0.60 ± 0.08 | 0.57 ± 0.09 | 0.56 ± 0.08 | 0.42 ± 0.10 | < .001 |
| P value | Reference | < .001 | < .001 | < .001 | < .001 | |
| Airway lumen diameter, mm | 4.2 (3.4-5.1) | 4.0 (3.2-4.8) | 4.1 (3.2-4.7) | 3.7 (2.9-4.6) | 3.9 (3.2-4.7) | < .001 |
| P value | Reference | .08 | .10 | .001 | .01 | |
| Dlco/Va % predicted | 80 ± 13 | 71 ± 14 | 61 ± 14 | 74 ± 20 | 38 ± 19 | < .001 |
| P value | Reference | .007 | .02 | .32 | < .001 | |
| Percent emphysema < −950 HU | 0.8 (0.4-1.3) | 3.0 (1.4-5.0) | 2.8 (2.1-7.9) | 1.1 (0.2-8.0) | 9.4 (7.4-25) | < .001 |
| P value | Reference | .001 | .001 | .29 | < .001 | |
| Proportion with mMRC dyspnea ≥ 2 | 9 | 19 | 8 | 45 | 73 | < .001 |
| P value | Reference | .31 | 1.00 | .01 | < .001 | |
| Proportion with chronic bronchitis | 9 | 16 | 0 | 36 | 19 | .003 |
| P value | Reference | .39 | .97 | .03 | .31 | |
| Proportion with Spo2 < 95% | 7 | 7 | 17 | 11 | 45 | .07 |
| P value | Reference | 1.00 | .60 | 1.00 | .01 | |
| Blood hemoglobin concentration, g/dL | 13.6 ± 1.2 | 14.0 ± 1.2 | 14.5 ± 0.8 | 13.4 ± 1.3 | 14.3 ± 1.3 | .04 |
| P value | Reference | .11 | .001 | .66 | .04 | |
Data are presented as mean ± SD, %, or median (first-third quartile) unless otherwise indicated. Gas trapping was defined as RV or RV/TLC > ULN. Hyperexpansion was defined as FRC or TLC > ULN or IC/TLC < LLN. See Table 1 and 3 legends for expansion of abbreviations.
Two and nine participants without COPD had isolated gas trapping and isolated hyperexpansion, respectively.
Figure 2.
Clinical characteristics of the MESA COPD Study participants by COPD, gas trapping, and hyperexpansion status. A, Dyspnea. B, Chronic bronchitis. C, BMI. D, Blood hemoglobin concentration. Gas trapping was defined as residual volume or residual volume/total lung capacity greater than the upper limit of normal. Hyperexpansion was defined as functional residual capacity or total lung capacity greater than the upper limit of normal or inspiratory capacity/total lung capacity less than the lower limit of normal. *P < .05 for comparison with control participants without COPD. mMRC = modified Medical Research Council. See Figure 1 legend for expansion of other abbreviation.
Discussion
Among current and former smokers with COPD, gas trapping on plethysmography was associated with smaller airway lumen diameters, greater dyspnea, and higher prevalence of chronic bronchitis. In contrast, hyperexpansion on plethysmography was associated with greater percent emphysema, lower BMI, and higher hemoglobin concentration. Presence of both gas trapping and hyperexpansion was associated with smaller airway lumen diameters and greater percent emphysema in addition to severe airflow obstruction, greater dyspnea, and higher hemoglobin concentration. Together, these findings demonstrate heterogeneity in COPD with respect to the structural and clinical correlates of hyperinflation.
COPD has long been recognized as a heterogeneous disorder of airways and parenchyma, with a common end result of reduced FEV1 and FEV1/FVC.31,35 The present findings suggest that gas trapping on plethysmography indicates significant contribution of airways narrowing, whereas hyperexpansion on plethysmography indicates emphysema. In support of these findings, a classic study of COPD subtypes by Burrows et al36 demonstrated that despite similar degrees of airflow obstruction, absence of roentgenologic emphysema is associated with an isolated increase in RV/TLC, whereas all lung volumes are higher in the presence of emphysema. Orlandi et al,17 reporting only on FRC, found a significant bivariate correlation with lung density but not airway dimensions. Hasegawa et al16 found unadjusted correlations of airway lumen area with % predicted RV and RV/TLC but not FRC or TLC. Their study did not assess, however, the independent contribution of percent emphysema and airway dimensions to lung volume hyperinflation or report clinical characteristics by pattern of lung volume hyperinflation.
Clinical characteristics differed according to the presence of gas trapping and hyperexpansion. Isolated gas trapping was associated with a higher prevalence of chronic bronchitis, a finding consistent with Burrows et al36 wherein the nonemphysematous inflammatory group had significantly greater sputum volume and higher prevalence of chronic cough. We also observed excess dyspnea with gas trapping independent of FEV1. Kim et al37 observed higher dyspnea among participants with chronic bronchitis that was also associated with greater percent airway wall area; however, the extent of emphysema was similar in the comparison group, and RV was not reported. The lack of dyspnea among participants with isolated hyperexpansion in the present study may reflect an earlier stage of lung disease because dyspnea was common among participants with both hyperexpansion and gas trapping. Alternatively, this finding may be due to partial misclassification of abnormal lung volumes based on reference equations or insufficient power.
Isolated hyperexpansion was associated with lower BMI and higher blood hemoglobin concentration. Consistent with these observations, Ogawa et al38 observed lower BMI among individuals with emphysema-dominant COPD; however, measures of hyperexpansion were not reported in their study. Early hematologic studies of emphysema reported mild or inconsistent increases in hemoglobin concentration, with some authors postulating a mechanism of depressed erythropoiesis due to chronic airways infection.39‐42 Although not directly tested in the present study, this hypothesis may be supported by the contrasting hemoglobin levels with isolated hyperexpansion (ie, emphysema-predominant COPD) and isolated gas trapping (ie, airways-predominant COPD).
Together, the observed clinical and structural correlates of hyperinflation suggest that therapies targeting the structural basis of gas trapping and hyperexpansion may improve clinical outcomes in COPD. Although not formally assessed in the present study, mechanisms by which emphysema may be associated with elevated FRC or TLC or reduced IC/TLC include diminished lung recoil, emphysema-mediated dynamic phenomena (eg, increased respiratory rate), and chest wall reconfiguration due to chronically elevated operating lung volumes.3,12 Contrary to our initial hypothesis, percent emphysema was also associated with RV and RV/TLC. This observation may result from gas trapping that arises from emphysema-associated reduction in driving pressure.43,44 Alternatively, this association may indicate that emphysematous loss of lung recoil and reduced small airway tethering beyond the scanner resolution may facilitate dynamic airway closure and contribute to gas trapping.45,46
Exercise-induced expiratory flow limitation is more common among healthy women than men, which has been postulated to be due to sex differences in airway lumen caliber.47,48 Although women had narrower airway lumens in the present study, sex did not modify the association between gas trapping and airway lumen size. Therefore, the physiologic consequence of gas trapping associated with COPD-related airway narrowing does not appear to be influenced by sex.
Gas trapping was associated with a greater %AWT, the equation for which includes airway lumen diameter. Gas trapping was not associated with AWT. These findings suggest that lumen size rather than wall thickness contributes to gas trapping in COPD and is consistent with Poiseuille’s law describing flow through a cylinder in which resistance is related to lumen radius to the fourth power.
Segmental and subsegmental airway dimensions were assessed in the present report, whereas prior studies based on pathologic specimens demonstrated airways < 2 to 3 mm in diameter as significant contributors to airways resistance in COPD.49 In support of the present analysis of comparatively proximal airways, prior studies have shown correlation between airway remodeling of large and small airways in COPD.50,51
Emphysema was estimated by densitometry, which can be affected by scanner model, protocol, depth of inspiration, and acute smoke exposure.32,52 However, all participants were imaged with the same scanner under the same protocol at TLC by trained technologists, and analyses were adjusted for cotinine-confirmed smoking status. Sensitivity analysis using diffusing capacity yielded similar results.
Conclusions
Absolute lung volumes commonly used as metrics of hyperinflation in COPD were differentially associated with lung structure and clinical characteristics. Gas trapping was independently associated with smaller segmental and subsegmental airway lumen diameters, greater dyspnea, and higher prevalence of chronic bronchitis. Hyperexpansion was associated with greater percent emphysema, lower BMI, and higher blood hemoglobin concentration. Absolute lung volumes provide insight into clinical COPD phenotypes and should not be considered equivalent in their estimation of hyperinflation.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Barr had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Smith: contributed to the study design, data analysis and interpretation, drafting of the manuscript, and revision and approval of the manuscript.
Dr Hoffman: contributed to the study design; data collection, analysis, and interpretation; and revision and approval of the manuscript.
Dr Basner: contributed to the study design, data collection and interpretation, and revision and approval of the manuscript.
Dr Kawut: contributed to the study design, data interpretation, and revision and approval of the manuscript.
Dr Kalhan: contributed to the study design, data collection and interpretation, and revision and approval of the manuscript.
Dr Barr: contributed to the study design; data collection, analysis, and interpretation; and revision and approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Smith is funded by the Fonds de la recherche en santé Québec. Dr Hoffman holds grants from the National Institutes of Health (NIH), Alpha-1 Foundation, and American Lung Foundation and is a founder of and shareholder in VIDA Diagnostics, Inc. Dr Basner holds grants from NIH and has received royalties from UpToDate, Inc. Dr Kawut holds grants from NIH. Dr Kalhan holds grants from NIH; has received royalties from UpToDate, Inc; and has consulted for Boehringer Ingelheim GmbH; Forest Laboratories, Inc; and Sunovion Pharmaceuticals Inc (formerly Elevation Pharmaceuticals). Dr Barr holds grants from NIH, Alpha-1 Foundation, and the US Environmental Protection Agency and has received royalties from UpToDate, Inc.
Role of sponsors: The protocols were approved by the institutional review boards of all collaborating institutions and by the National Heart, Lung, and Blood Institute (NHLBI). NHLBI staff monitored study performance routinely and participated in the internal review of the manuscript before submission. Fonds de la recherche en santé Québec had no role in the study design, collection or analysis of the data, or preparation of the manuscript.
Other contributions: The MESA Lung Study was designed by the study investigators.
Additional information: The e-Figure and e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- AWT
airway wall thickness
- Dlco/Va
diffusing capacity of lung for carbon monoxide divided by alveolar volume
- FRC
functional residual capacity
- HU
Hounsfield units
- IC
inspiratory capacity
- MESA
Multi-Ethnic Study of Atherosclerosis
- RV
residual volume
- Spo2
oxygen saturation as measured by pulse oximetry
- TLC
total lung capacity
Footnotes
Part of this work has been presented in abstract form at the American Thoracic Society International Conference, May 17-22, 2013, Philadelphia, PA.
Funding/Support: This study was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute [Grants R01-HL093081, R01-HL077612, and R01-HL075476; Contracts N01 HC 95159 through N01 HC 95165 and N01 HC 95169] and Fonds de la recherche en santé Québec.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365 [DOI] [PubMed] [Google Scholar]
- 2.Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary Data for 2010. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2012 [Google Scholar]
- 3.Bancalari E, Clausen J. Pathophysiology of changes in absolute lung volumes. Eur Respir J. 1998;12(1):248-258 [DOI] [PubMed] [Google Scholar]
- 4.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968 [DOI] [PubMed] [Google Scholar]
- 5.Rubinfeld AR, Pain MC. How mild is mild asthma? Thorax. 1977;32(2):177-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby JG, Juniper EF, Hargreave FE, Zamel N. Total lung capacity does not change during methacholine-stimulated airway narrowing. J Appl Physiol (1985). 1986;61(6):2144-2147 [DOI] [PubMed] [Google Scholar]
- 7.Martinez FJ, Foster G, Curtis JL, et al. ; NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deesomchok A, Webb KA, Forkert L, et al. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD. 2010;7(6):428-437 [DOI] [PubMed] [Google Scholar]
- 9.Smith BM, Kawut SM, Bluemke DA, et al. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127(14):1503-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106(5):651-660 [DOI] [PubMed] [Google Scholar]
- 11.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827-833 [DOI] [PubMed] [Google Scholar]
- 12.Leith DE, Brown R. Human lung volumes and the mechanisms that set them. Eur Respir J. 1999;13(2):468-472 [DOI] [PubMed] [Google Scholar]
- 13.Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev. 2009;89(3):759-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zach JA, Newell JD, Jr, Schroeder J, et al. ; COPDGene Investigators. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012;47(10):596-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med. 1999;159(3):851-856 [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309-1315 [DOI] [PubMed] [Google Scholar]
- 17.Orlandi I, Moroni C, Camiciottoli G, et al. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology. 2005;234(2):604-610 [DOI] [PubMed] [Google Scholar]
- 18.Arakawa H, Fujimoto K, Fukushima Y, Kaji Y. Thin-section CT imaging that correlates with pulmonary function tests in obstructive airway disease. Eur J Radiol. 2011;80(2):e157-e163 [DOI] [PubMed] [Google Scholar]
- 19.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3):1102-1108 [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881 [DOI] [PubMed] [Google Scholar]
- 21.Mesia-Vela S, Yeh CC, Austin JH, et al. Plasma carbonyls do not correlate with lung function or computed tomography measures of lung density in older smokers. Biomarkers. 2008;13(4):422-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338 [DOI] [PubMed] [Google Scholar]
- 23.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511-522 [DOI] [PubMed] [Google Scholar]
- 24.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735 [DOI] [PubMed] [Google Scholar]
- 25.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Chest. 2010;137(1):138-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Rio F, Dorgham A, Pino JM, Villasante C, Garcia-Quero C, Alvarez-Sala R. Lung volume reference values for women and men 65 to 85 years of age. Am J Respir Crit Care Med. 2009;180(11):1083-1091 [DOI] [PubMed] [Google Scholar]
- 27.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18(3):419-425 [PubMed] [Google Scholar]
- 28.Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):519-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653-657 [DOI] [PubMed] [Google Scholar]
- 30.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ. 1959;2(5147):257-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roisin RR, Vestbo J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (revised 2013). Global Initiative for Obstructive Lung Disease website. http://www.goldcopd.org. Accessed July 18, 2013
- 32.Ashraf H, Lo P, Shaker SB, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66(1):55-60 [DOI] [PubMed] [Google Scholar]
- 33.Camp PG, Coxson HO, Levy RD, et al. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136(6):1480-1488 [DOI] [PubMed] [Google Scholar]
- 34.Kim WJ, Silverman EK, Hoffman E, et al. ; NETT Research Group. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136(2):396-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrows B, Fletcher CM, Heard BE, Jones NL, Wootliff JS. The emphysematous and bronchial types of chronic airways obstruction. A clinicopathological study of patients in London and Chicago. Lancet. 1966;287(7442):830-835 [DOI] [PubMed] [Google Scholar]
- 36.Burrows B, Niden AH, Fletcher CM, Jones NL. Clinical types of chronic obstructive lung disease in London and in Chicago. A study of one hundred patients. Am Rev Respir Dis. 1964;90:14-27 [DOI] [PubMed] [Google Scholar]
- 37.Kim V, Han MK, Vance GB, et al. ; COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa E, Nakano Y, Ohara T, et al. Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64(1):20-25 [DOI] [PubMed] [Google Scholar]
- 39.Hume R. Blood volume changes in chronic bronchitis and emphysema. Br J Haematol. 1968;15(2):131-139 [DOI] [PubMed] [Google Scholar]
- 40.Baldwin ED, Cournand A, Richards DW., Jr Pulmonary insufficiency; a study of 122 cases of chronic pulmonary emphysema. Medicine (Baltimore). 1949;28(2):201-237 [DOI] [PubMed] [Google Scholar]
- 41.Wilson RH, Borden CW, Ebert RV. Adaptation to anoxia in chronic pulmonary emphysema. AMA Arch Intern Med. 1951;88(5):581-590 [DOI] [PubMed] [Google Scholar]
- 42.Fielding J, Zorab PA. Polycythaemia and Iron Deficiency in Pulmonary “Emphysema. Lancet. 1964;284(7354):284-286 [DOI] [PubMed] [Google Scholar]
- 43.Jain N, Covar RA, Gleason MC, Newell JD, Jr, Gelfand EW, Spahn JD. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 2005;40(3):211-218 [DOI] [PubMed] [Google Scholar]
- 44.Newman KB, Lynch DA, Newman LS, Ellegood D, Newell JD., Jr Quantitative computed tomography detects air trapping due to asthma. Chest. 1994;106(1):105-109 [DOI] [PubMed] [Google Scholar]
- 45.Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84(2):89-97 [DOI] [PubMed] [Google Scholar]
- 46.Petty TL, Silvers GW, Stanford RE. Radial traction and small airways disease in excised human lungs. Am Rev Respir Dis. 1986;133(1):132-135 [DOI] [PubMed] [Google Scholar]
- 47.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol (1985). 1998;84(6):1872-1881 [DOI] [PubMed] [Google Scholar]
- 48.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581(pt 3):1309-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355-1360 [DOI] [PubMed] [Google Scholar]
- 50.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142-146 [DOI] [PubMed] [Google Scholar]
- 51.Isajevs S, Taivans I, Svirina D, Strazda G, Kopeika U. Patterns of inflammatory responses in large and small airways in smokers with and without chronic obstructive pulmonary disease. Respiration. 2011;81(5):362-371 [DOI] [PubMed] [Google Scholar]
- 52.Bakker ME, Stolk J, Putter H, et al. Variability in densitometric assessment of pulmonary emphysema with computed tomography. Invest Radiol. 2005;40(12):777-783 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement