Abstract
KCNH2 encodes Kv11.1 and underlies the rapidly activating delayed rectifier K+ current (IKr) in the heart. Loss-of-function KCNH2 mutations cause the type 2 long QT syndrome (LQT2), and most LQT2-linked missense mutations inhibit the trafficking of Kv11.1 channels. Drugs that bind to Kv11.1 and block IKr (e.g., E-4031) can act as pharmacological chaperones to increase the trafficking and functional expression for most LQT2 channels (pharmacological correction). We previously showed that LQT2 channels are selectively stored in a microtubule-dependent compartment within the endoplasmic reticulum (ER). We tested the hypothesis that pharmacological correction promotes the trafficking of LQT2 channels stored in this compartment. Confocal analyses of cells expressing the trafficking-deficient LQT2 channel G601S showed that the microtubule-dependent ER compartment is the transitional ER. Experiments with E-4031 and the protein synthesis inhibitor cycloheximide suggested that pharmacological correction promotes the trafficking of G601S stored in this compartment. Treating cells in E-4031 or ranolazine (a drug that blocks IKr and has a short half-life) for 30 min was sufficient to cause pharmacological correction. Moreover, the increased functional expression of G601S persisted 4–5 h after drug washout. Coexpression studies with a dominant-negative form of Rab11B, a small GTPase that regulates Kv11.1 trafficking, prevented the pharmacological correction of G601S trafficking from the transitional ER. These data suggest that pharmacological correction quickly increases the trafficking of LQT2 channels stored in the transitional ER via a Rab11B-dependent pathway, and we conclude that the pharmacological chaperone activity of drugs like ranolazine might have therapeutic potential.
Keywords: potassium channel, trafficking, long QT syndrome
congenital long qt syndrome (LQTS) increases risk for polymorphic ventricular tachycardia (torsades de pointes), syncope, and sudden death. Type 2 LQTS (LQT2) is caused by loss-of-function mutations in KCNH2, and it is responsible for ∼30% of all LQTS cases (12, 20, 29). KCNH2 encodes the voltage-gated K+ channel α-subunit (Kv11.1) that conducts the rapidly activating delayed rectifier K+ current (IKr) in the heart. The majority of LQT2 mutations are missense, and studies suggest that up to 90% of these mutations inhibit the trafficking of Kv11.1 from the endoplasmic reticulum (ER) to the Golgi apparatus and cell surface membrane (3, 40).
An important finding is that drugs, which bind to Kv11.1 and block IKr, can act as pharmacological chaperones to correct the trafficking-deficient phenotype for most LQT2 mutations (pharmacological correction; 3, 41). This finding indicates that LQT2 mutations are promising targets for therapeutic intervention. Pharmacological chaperones bind to the high-affinity drug-binding domain and stabilize native-like Kv11.1 channel conformations that are suitable for ER export (16, 17, 19).
The ER is a reticular network that extends from the nucleus to the cell periphery. It contains a variety of domains or compartments that perform specific functions, including protein and lipid synthesis, Ca2+ storage, apoptosis signaling, quality control (QC), ER-associated degradation (ERAD), and ER export (38). LQT2 channels are stored in a microtubule-dependent ER compartment (34). The purpose of this study was to identify the microtubule-dependent ER compartment that selectively stores LQT2 channels and to determine whether pharmacological correction can increase the trafficking of the LQT2 channels that are stored in this compartment.1
MATERIALS AND METHODS
Clinical.
Twenty-eight patients heterozygous for LQT2 missense mutations G601S or A614V were identified (Table 1). Blood samples were obtained after signed written informed consent for genetic analyses and after approval by the local institutional ethics committees. Genomic DNA was isolated from blood leukocytes and mutational analyses were performed using standard methods.
Table 1.
Clinical characteristics of genotype-positive G601S and A614V patients
| Value | |
|---|---|
| Genotype-positive G601S families | |
| n | 3 |
| Genotype-positive subjects, n (female) | 9 (6) |
| Mean age ± SD, yr (female) | 47 ± 26 (36 ± 24) |
| Mean QTc ± SD, ms (female) | 460 ± 35 (465 ± 30) |
| Symptomatic, n (female) | 3 (3) |
| Genotype-positive A614V families | |
| n | 12 |
| Genotype-positive subjects, n (female) | 25 (16) |
| Mean age ± SD, yr (female) | 32 ± 18 (35 ± 20) |
| Mean QTc ± SD, ms (female) | 500 ± 50 (515 ± 55) |
| Symptomatic, n (female) | 14 (10) |
Symptomatic is defined as having experienced syncope, torsades, or sudden cardiac arrest/death. QTc is corrected QT interval.
Cell lines and drug exposure.
The human embryonic kidney 293 (HEK293) cell lines expressing wild-type Kv11.1 (WT-Kv11.1) or the trafficking-deficient LQT2 mutations G601S, A614V, and N629D were described previously (3, 13, 42). Cells were cultured at 37°C (5% CO2) in MEM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and geneticin. For some studies, we transiently expressed wild-type or dominant-negative mutant Rab11B (S25N) fused to green fluorescent protein (Rab11B-GFP or DN-Rab11B-GFP, respectively; kindly provided by Dr. Beate Schlierf, Institut für Biochemie, Universität Erlangen-Nürnberg, Erlangen, Germany) or Rab11B- and Rab11A-specific control-specific short hairpin RNA (shRNA) plasmids containing a GFP marker (SABiosciences, Frederick, MD) similar to that described previously (6, 15, 31). In addition, in some experiments, Chinese hamster ovary (CHO) cells were transfected with WT-Kv11.1 or G601S plasmid DNA (3 μg) using Superfect (Qiagen, Valencia, CA). For pharmacological studies, we used brefeldin a or bfa (10 μg/ml), nocodazole (20 μM), cycloheximide (20 μg/ml), E-4031 (10 μM), or ranolazine (50 μM) (Sigma-Aldrich, St. Louis, MO). Control studies were done in parallel with equivalent amounts of vehicle. The final DMSO concentrations were always <0.1%.
Immunocytochemistry and confocal imaging.
Immunocytochemistry and confocal imaging experiments were done similar to those previously described (34). Briefly, cells were plated in dishes containing collagen-coated coverslips. Cells were fixed with 4% buffered paraformaldehyde for 10 min, permeabilized with Triton X-100 (0.1%) for 10 min, and rinsed in 0.75% glycine buffer for 10 min to quench background fluorescence. The cells were incubated with 2 ml blocking solution (10% goat serum in PBS) for 1 h to block nonspecific binding sites and subsequently incubated with the appropriate primary antibodies: anti-Kv11.1 (1:1,000, Alomone Laboratories, Jerusalem, Israel), anti-Sec31a (1:100, BD Labs, Franklin Lakes, NJ), anti-ERGIC-53 (1:1,000, Alexis Biochemicals, Plymouth Meeting, PA), anti-Derlin-1 (1:100, Sigma-Aldrich), and anti-Bap31 (1:500, Abcam, Cambridge, MA) with blocking solution at room temperature. Excess antibody was washed off three times with blocking solution, and the cells were then incubated with the appropriate Highly Cross-absorbed Alexa Fluor antibodies (1:500, Invitrogen). After 1 h, the cells were washed four times with blocking solution alone. The coverslips were then mounted using ProLong Gold with DAPI mounting medium (Invitrogen). For Rab11B experiments, we prepared the cells as described above but utilized the fluorescence signal from the GFP tag for imaging analyses. Imaging was performed at the University of Kentucky Imaging Facility using a Leica TSP SP5 confocal microscope (Leica, Wetzler, Germany). Cells were randomly selected for imaging. Cells not expressing Kv11.1 did not show any immunostaining with anti-Kv11.1 (data not shown) nor any of the secondary antibodies alone (data not shown). Data are shown as representative z-scan images for each fluorescence signal. Overlapping signals of similar intensities for those colored as red and green are shown as yellow; red and purple are shown as pink; and green and purple are shown as white. Colocalization analyses were performed using ImageJ (http://rsbweb.nih.gov/ij/). The Costes' approach was used for correlation analyses between the two fluorescent signals to generate the Pearson coefficient (PC; 7, 11). The Costes' approach calculates threshold values to minimize the contribution of noise, and it utilizes statistical analysis to confirm colocalization based on image randomization. The closer the PC is to 1, the higher the degree of colocalization between the two signals. In our system, immunostaining the same primary antibody with different Alexa Fluor antibodies generated a mean PC of ∼0.8 (34). Based on our control data and the difficulty of interpreting PC values <0.5 (7), we interpret strong colocalization as having a mean PC value >0.65, moderate colocalization as having a mean PC value between 0.5 and 0.65, and weak colocalization as having a mean PC value <0.5.
Western blot analysis.
Western blot analyses were done as previously described (15, 34, 42). Briefly, cells were harvested with lysis buffer and equal masses of total protein were electrophoresed on an SDS-polyacrylamide gel, transferred electrophoretically to nitrocellulose membrane, and probed with anti-Kv11.1 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Na+-K+ ATPase (1:1,000, Abcam). The protein concentration was measured using the Bio-Rad DC protein assay. The Odyssey infrared imaging system (Li-Cor, Lincoln, NE) was used for antibody detection and quantification.
Electrophysiology.
Functional analysis of Kv11.1 current (IKv11.1) was done using standard whole cell patch-clamp technique as previously described (13). The external bath solution contained (in mM) 137 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4 with NaOH), and an internal pipette solution contained (in mM) 130 KCl, 1 MgCl2, 5 EGTA, 5 MgATP, and 10 HEPES (pH 7.2 with KOH). An Axopatch-200B patch-clamp amplifier (Axon Instruments, Union City, CA) was used to measure membrane currents and cell capacitance. The pipette resistances were 1–2 MΩ, and series resistance was compensated up to 95%. After intracellular access was obtained, only cells with seal resistances >1 GΩ were used for functional analyses. pCLAMP 10.0 computer software (Axon Instruments) was used to generate the voltage-clamp protocols, acquire current signals, and analyze the data. Origin 7.0 (Microcal, Northhampton, MA) was used for generating graphs. Cells were recorded within 1 h after removal from culture conditions.
Statistics.
Unpaired t-tests were performed on data sets with two groups. For data sets with more than two comparisons, an ANOVA was performed. To identify which experimental groups differed from control, post hoc Dunnett's tests were performed. Significance was reported at P < 0.05.
RESULTS
G601S is selectively stored in the Bap31 transitional ER compartment.
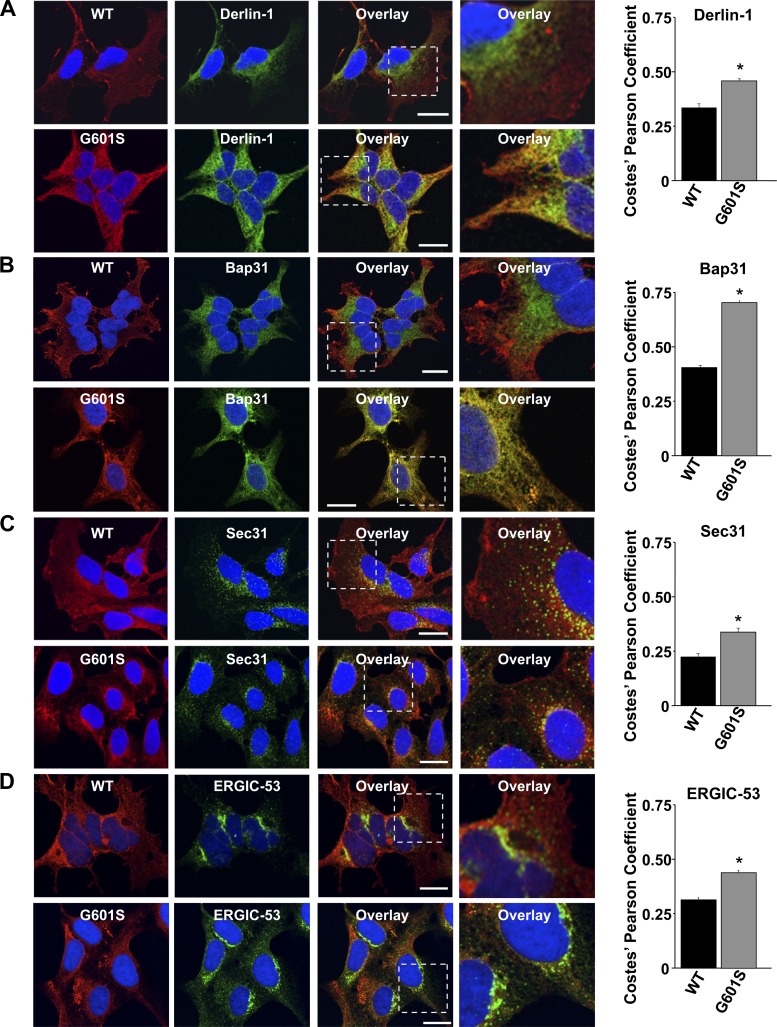
G601S is a trafficking deficient LQT2 mutation that is located in the pore-loop, and causes syncope and ventricular arrhythmias (Table 1) (2). We previously showed that G601S is selectively stored in a microtubule-dependent QC compartment in the ER (34). However, the identity of this compartment remained elusive. To clearly identify this ER compartment, we performed a series of confocal microscopy experiments that utilized cells stably expressing WT-Kv11.1 or G601S (Fig. 1). Cells were immunostained for Kv11.1 and several markers that label different parts of the ER network. Derlin-1 labels the ER that is responsible for retrotranslocation of ERAD substrates (22); Bap31 labels the transitional ER compartment (39); Sec31 labels ER exit sites (28); and ERGIC-53 labels the ER Golgi Intermediate Compartment (ERGIC) (32). We quantified colocalization by calculating the PC for the immunostaining of Kv11.1 and the different ER markers. The data show that WT-Kv11.1 weakly colocalized with the different ER markers, and G601S weakly colocalized with Derlin-1, Sec31, and ERGIC-53 (although the absolute PC values were slightly higher when compared with WT-Kv11.1). However, unlike WT-Kv11.1, G601S strongly colocalized with Bap31. Moreover, the selective colocalization between G601S and Bap31 was not cell-type dependent, because similar PC values between Bap31 and WT-Kv11.1 or G601S were found in CHO cells (PC = 0.39 ± 0.02, n = 10 images, and PC = 0.65 ± 0.03, n = 12 images, respectively, P < 0.05). These data suggest that G601S is selectively stored in the transitional ER.
Fig. 1.
G601S selectively colocalizes with Bap31. Shown are representative fluorescent images of cells expressing wild-type (WT)-Kv11.1 or G601S immunostained with anti-Kv11.1 (red, first column) and anti-Derlin-1 (A), anti-Bap31 (B), anti-Sec31 (C), or anti-ERGIC-53 (D) (green, second column). The overlay between anti-Kv11.1 and the different endoplasmic reticulum (ER) markers is shown in the third column. Scale bars, 10 μm. The fourth column shows a portion of the overlays (white dashed box, third column) in greater detail. Also shown are the corresponding Costes' Pearson coefficients (*P < 0.05 compared with WT-Kv11.1).
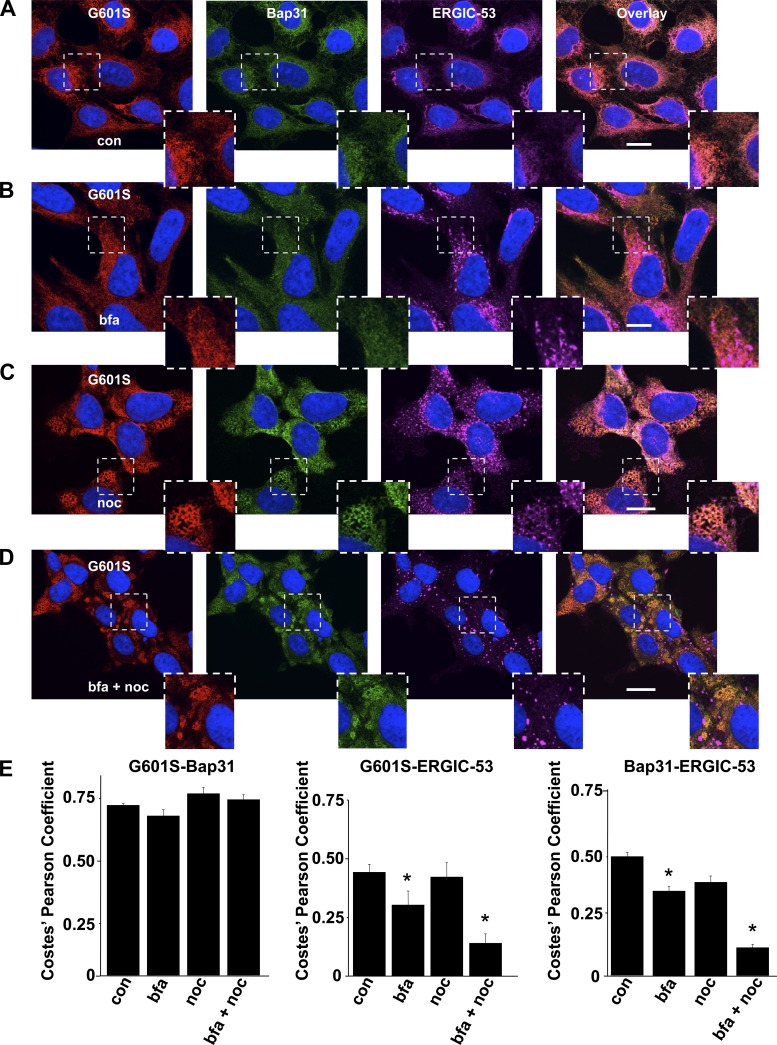
To confirm whether G601S and Bap31 colocalized in the transitional ER compartment, we tested whether the steady-state cellular distribution of G601S and Bap31 showed similar sensitivities to bfa, an inhibitor of COPI vesicular transport, and the microtubule depolymerizing agent nocodazole. We previously showed that the steady-state cellular distribution of G601S is insensitive to bfa but very sensitive to nocodazole (34). This is demonstrated in Fig. 2, which shows representative confocal images of cells expressing G601S immunostained for Kv11.1, Bap31, and ERGIC-53 in control conditions or after being treated with bfa, nocodazole, or bfa and nocodazole. The cells were immunostained with ERGIC-53 as a positive control, because the effects that bfa and nocodazole have on ERGIC-53 immunostaining are well documented (10, 23, 24, 34). Treating cells in bfa did not alter the steady-state cellular distribution of G601S or Bap31, but treating cells in nocodazole with or without bfa caused G601S and Bap31 to redistribute into peripherally located reticulated structures. Importantly, the PC for G601S and Bap31 did not decrease in any of the treatment conditions. Similar to previous reports, bfa increased the vesicular/juxtanuclear immunostaining of ERGIC-53; nocodazole caused ERGIC-53 to redistribute into a punctate pattern throughout the cell; and treating cells in bfa and nocodazole caused ERGIC-53 to concentrate in large spherical ERGIC structures that were clearly separate from G601S and Bap31 immunostaining. Together these data suggest that the microtubule-sensitive QC compartment that stores G601S is the transitional ER.
Fig. 2.
G601S and Bap31 show a similar sensitivity to brefeldin a (bfa) and nocodazole (noc). A–D: representative fluorescent images of cells expressing G601S immunostained with anti-Kv11.1 (red, first column), anti-Bap31 (green, second column), and anti-ERGIC-53 (purple, third column) in control conditions (A; n = 6 images), after being treated in bfa for 2 h (B; n = 6 images), noc for 2 h (C; n = 9 images), or bfa and noc (bfa + noc) for 2 h (D; n = 8 images). The fourth column shows the overlay. Scale bars, 10 μm. Each image shows a corresponding portion (white dashed box) in greater detail. E: mean Costes' Pearson coefficients for the colocalization of G601S and Bap31, G601S and ERGIC-53, or Bap31 and ERGIC-53 in control conditions (con) and after being treated in bfa for 2 h, noc for 2 h, or bfa + noc for 2 h (*P < 0.05 compared with control conditions).
E-4031 increases the trafficking of G601S from the transitional ER compartment.
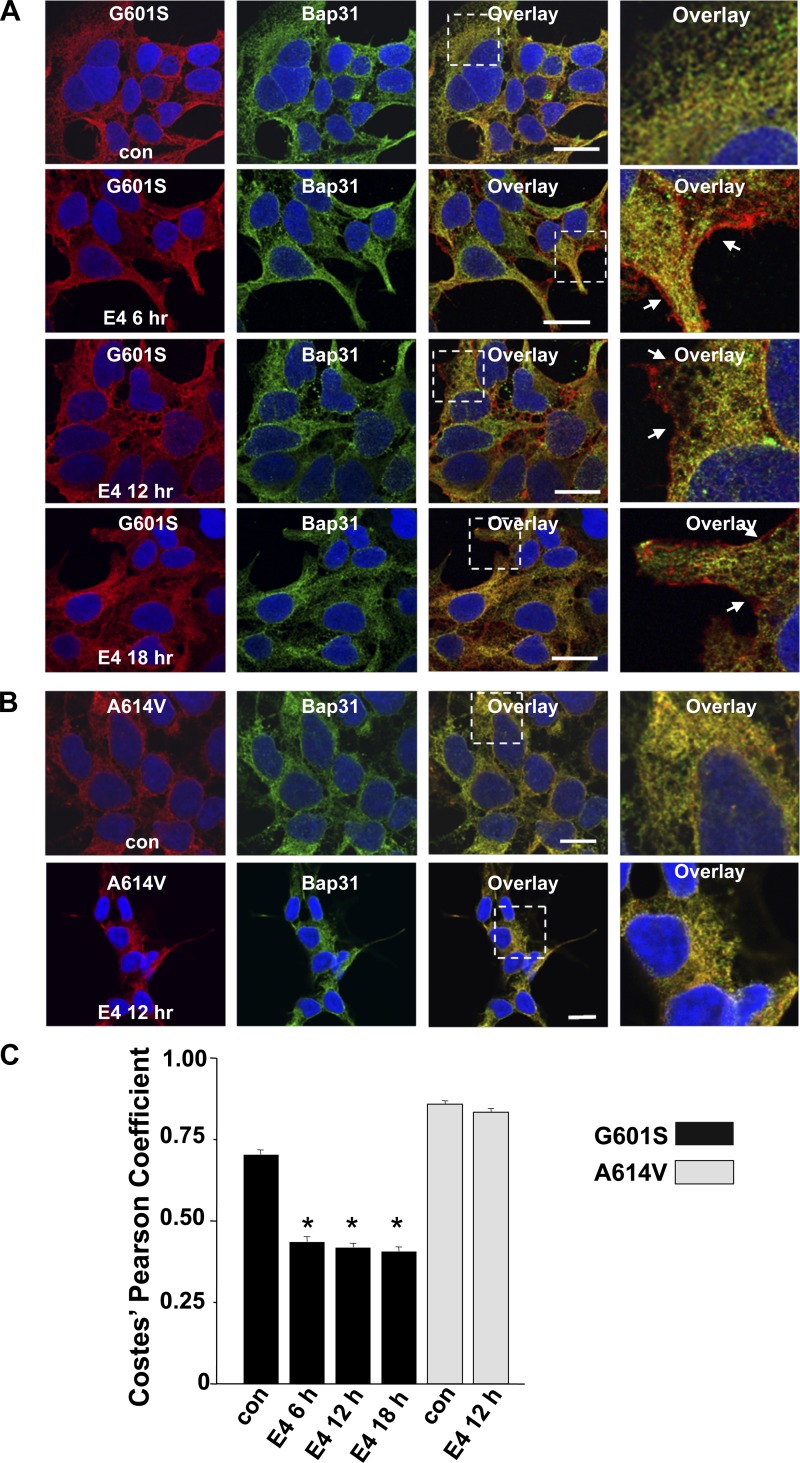
Previous studies show that the class III antiarrhythmic E-4031 can act as a pharmacological chaperone to increase the trafficking and functional expression of G601S (17, 41). We tested whether treating cells expressing G601S in E-4031 decreased the accumulation of G601S in the transitional ER. Figure 3A shows representative images of cells expressing G601S immunostained for Kv11.1 and Bap31 in control conditions or after treatment in E-4031 for 6, 12, or 18 h. Treating cells in E-4031 decreased G601S colocalization with Bap31 and increased G601S immunostaining at the cell surface membrane.
Fig. 3.
E-4031 treatment decreases G601S and Bap31 colocalization. A: representative fluorescent images of cells expressing G601S immunostained with anti-Kv11.1 (red, first column) and anti-Bap31 (green, second column) in control conditions (n = 30 images) and after being treated in E-4031 (E4) for 6 h (n = 8 images), 12 h (n = 30 images), or 18 h (n = 8 images). The third column shows the corresponding overlays. Scale bars, 10 μm. The fourth column shows a corresponding portion of the overlay (white dashed box) in greater detail. The arrows in the fourth column highlight the cell surface immunostaining of anti-Kv11.1. B: representative fluorescent images of cells expressing A614V immunostained with anti-Kv11.1 (red, first column) and anti-Bap31 (green, second column) in control conditions (n = 6 images) and after being treated in E-4031 for 12 h (n = 6 images). The third column shows the corresponding overlay. Scale bars, 10 μm. The fourth column shows a corresponding portion of the overlay (white dashed box) in greater detail. C: mean Costes' Pearson coefficients for the colocalization of G601S or A614V and Bap31 in cells for control conditions or after being treated in E-4031.
Similar effects were also seen with cells expressing the trafficking-deficient LQT2 mutation N629D, which also undergoes pharmacological correction with E-4031 (3). In control conditions, N629D strongly colocalized with Bap31 but this colocalization decreased after treatment in E-4031 for 12 h (PC = 0.69 ± 0.01, n = 8 images, PC = 0.53 ± 0.01, n = 8 images, respectively, P < 0.05). We also performed a negative-control experiment with cells expressing trafficking-deficient LQT2 mutation A614V (37). Similar to G601S, A614V is linked to LQT2 in several unrelated families (Table 1); however, unlike G601S, A614V does not undergo pharmacological correction with E-4031 (3). Immunostaining cells expressing A614V for Kv11.1 and Bap31 showed that A614V strongly colocalized with Bap31 in control conditions and in cells treated in E-4031 for 12 h (Fig. 3, B and C).
We wanted to determine what is happening to allow G601S export from the transitional ER following pharmacological correction. We previously showed that incorporating amino acid substitutions in the putative Kv11.1 drug-binding domain at Y652 prevents G601S misfolding (intragenic suppression; 14). We found that the Y652T substitution caused intragenic suppression of G601S (data not shown), and cells expressing Y652T or G601S/Y652T showed weak colocalization with Bap31 (PC = 0.46 ± 0.03, n = 13 images, and PC = 0.50 ± 0.04, n = 22 images, respectively). Based on these results, pharmacological correction likely causes conformational changes in the G601S to allow for its export from the transitional ER.
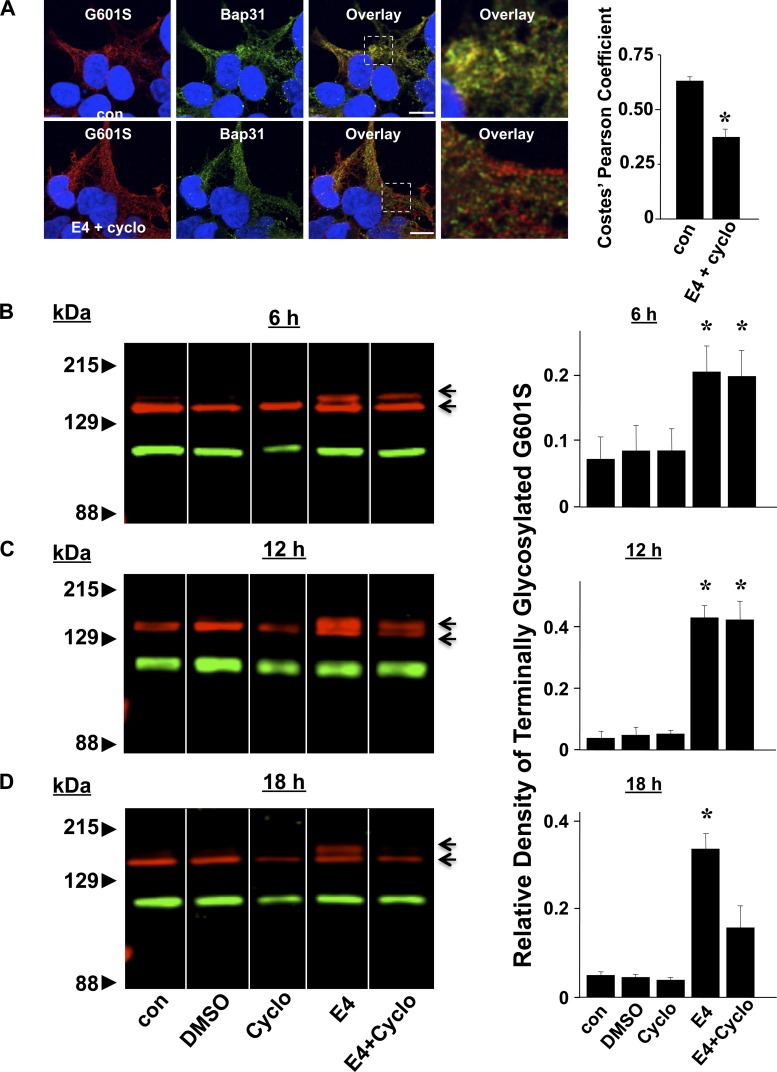
To test whether E-4031 actually increases the trafficking of G601S that is stored in the transitional ER to the cell surface membrane, we performed a series of pharmacological correction experiments in the presence of the protein synthesis inhibitor cycloheximide. Figure 4A shows representative images of cells expressing G601S immunostained for Kv11.1 and Bap31 in control conditions or after cycloheximide and E-4031 treatment for 6 h. Cycloheximide did not prevent the decrease in G601S and Bap31 colocalization following E-4031 treatment. WT-Kv11.1 is synthesized in the ER as a ∼135 kDa core-glycosylated N-linked glycoprotein, and it is processed by Golgi enzymes to generate the terminally glycosylated ∼155 kDa form (40, 42). Trafficking-deficient LQT2 mutations inhibit the formation of the terminally glycosylated Kv11.1, but the terminal glycosylation of LQT2 mutations is increased by treating cells in E-4031. We determined whether E-4031 increased the terminal glycosylation of G601S in cells treated with cycloheximide. Figure 4B shows Western blot analyses of cells expressing G601S in control conditions, after E-4031 treatment, or after treatment in cycloheximide and E-4031. Immunoblot analyses of cells expressing G601S in control conditions primarily showed the ER form of Kv11.1. Treating cells in E-4031 increased the fraction of the terminally glycosylated Kv11.1 (terminally glycosylated Kv11.1/total Kv11.1), and this was not prevented by cycloheximide treatment at time points <12 h. These data suggest that E-4031 initially causes pharmacological correction by facilitating the trafficking of G601S that is already stored in the transitional ER.
Fig. 4.
Pharmacological correction increases the terminal glycosylation of G601S in cells treated with cycloheximide. A: representative fluorescent images of cells expressing G601S immunostained with anti-Kv11.1 (red, first column) and anti-Bap31 (green, second column) in control conditions (n = 30 images) and after being treated in E-4031 plus cycloheximide (E4 + Cyclo) for 6 h (n = 8 images), 12 h (n = 30 images), or 18 h (n = 8 images). The third column shows the corresponding overlays. Scale bars, 10 μm. The fourth column shows a corresponding portion of the overlay (white dashed box) in greater detail. The mean Costes' Pearson coefficients are shown. B–D: representative immunoblots of cells expressing G601S (red) in control conditions and after being treated in DMSO, cycloheximide, E-4031, or E-4031 and cycloheximide for 6 h (B), 12 h (C), or 18 h (D). The blots were probed with anti-Kv11.1 (red) and Na+-K+-ATPase (green), which was used as a loading control. Also shown are the mean relative densities of the terminally glycosylated or mature G601S band in control conditions and treated in DMSO, cycloheximide, E-4031, or E-4031 and cycloheximide. To ensure that protein synthesis was inhibited before E-4031 treatment, the cycloheximide treatment preceded the E-4031 treatment by 30 min in all experiments. The immunoblots shown are all from the same blot with the same brightness and contrast ratios; however, some lanes were rearranged for presentation purposes.
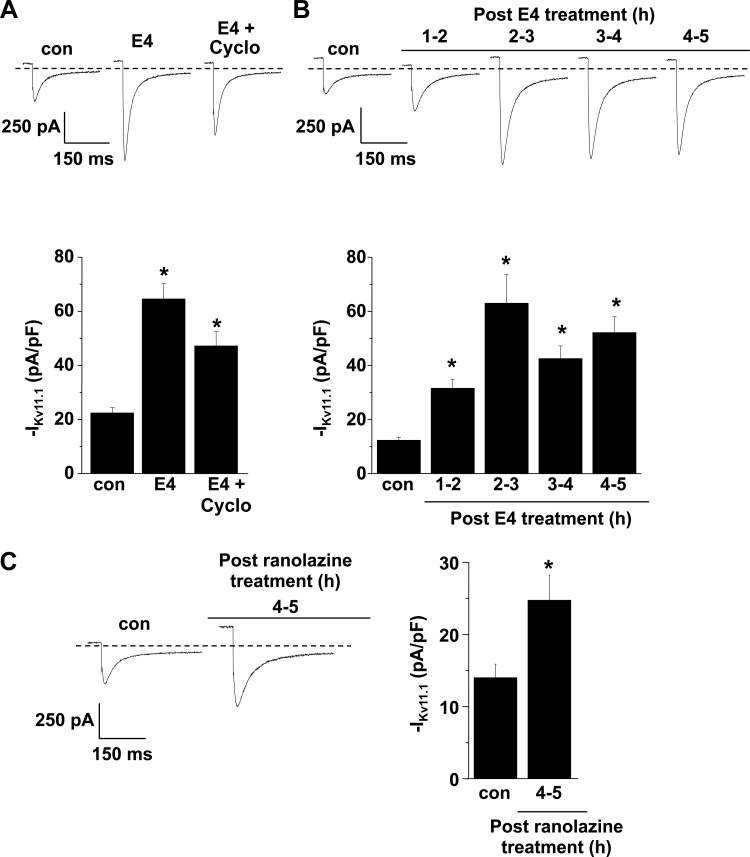
We also performed voltage-clamping experiments on cells expressing G601S and measured IKv11.1 in control conditions, following E-4031 treatment, or following cycloheximide and E-4031 treatment for 4–5 h. Similar to cells treated in E-4031, treating cells in cycloheximide and E-4031 showed an increase in IKv11.1 when compared with control (Fig. 5A). These data suggest that the initial increase in IKv11.1 following treatment in E-4031 primarily reflects the trafficking of G601S stored in the transitional ER.
Fig. 5.
Pharmacological correction of Kv11.1 current (IKv11.1) occurs in cells treated with cycloheximide and after 30 min. A: representative IKv11.1 traces recorded from cells expressing G601S in control conditions (n = 8 cells) and after E-4031 treatment without or with cycloheximide (E4 + Cyclo) for 4–5 h (n = 9 or 10 cells, respectively). IKv11.1 was recorded by stimulating cells with a maximally activating prepulse to 50 mV for 3 s, followed by a test pulse to −120 mV. The mean peak tail IKv11.1 measured during the test pulse in control cells and cells treated with E-4031 or E-4031 and cycloheximide is shown. To ensure that protein synthesis was inhibited before E-4031 treatment, cycloheximide treatment began 30 min before treatment in E-4031. B: representative IKv11.1 traces recorded from cells expressing G601S in control conditions (n = 12 cells) and 1–2 h (n = 10 cells), 2–3 h (n = 12 cells), 3–4 h (n = 10 cells), or 4–5 h (n = 13 cells) after treatment in E-4031 for 30 min. The mean peak tail IKv11.1 measured during the test pulse in control cells or at the different time points post 30 min E-4031 treatment is shown. C: representative IKv11.1 traces recorded from cells expressing G601S in control conditions (n = 8 cells) and 4–5 h (n = 12 cells) after treatment in ranolazine for 30 min. The mean peak tail IKv11.1 measured during the test pulse in control cells or 4–5 h following 30 min treatment in ranolazine is shown (*P < 0.05 compared with control conditions).
Since pharmacological correction increases the trafficking of G601S already stored in the transitional ER, we tested whether treating cells in E-4031 for a short amount of time was sufficient to increase the functional expression. Figure 5B shows that treating cells expressing G601S in E-4031 for only 30 min was sufficient to cause a ∼4–5 fold increase in IKv11.1 for up to 4–5 h after washout. In other words, prolonged or continual E-4031 treatment is not required for pharmacological correction of G601S. This implies that drugs, which have a short half-life, might be used as pharmacological chaperones. We tested whether treating cells expressing G601S in ranolazine, a drug that blocks IKr and is quickly metabolized by the liver (circulating half-life <2 h) (1, 27), could also increase the functional expression of G601S. Figure 5C shows that treating cells expressing G601S in ranolazine for 30 min increased IKv11.1 by 76% 4–5 h after drug washout.
The trafficking of G601S from the transitional ER compartment is dependent on Rab11B.
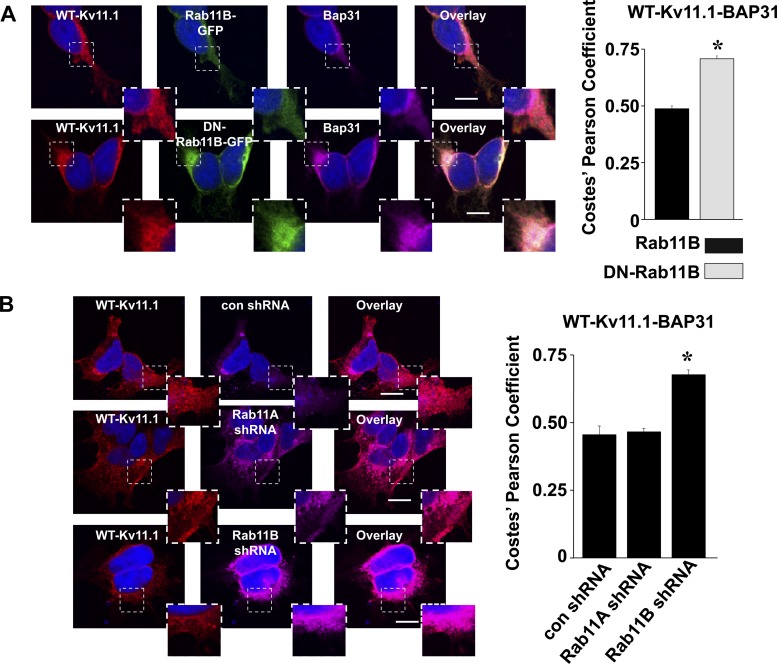
We next determined whether we could identify the trafficking pathway that underlies Kv11.1 export from the transitional ER. Rab11B is a small GTPase that regulates exocytosis and endocytosis (21, 31). We previously showed that DN-Rab11B (but not DN-Rab11A) mutations strongly inhibit the Golgi processing, cell surface expression, and functional expression of WT-Kv11.1 (15). The effect that DN-Rab11B has on WT-Kv11.1 trafficking is different from other cardiac ion channels, including Kir2.1 or Cav1.2 (6, 15). Since Rab11B uniquely regulates the trafficking of Kv11.1 early in the secretory pathway, we tested whether coexpressing WT-Kv11.1 with DN-Rab11B-GFP might cause WT-Kv11.1 to accumulate in the transitional ER. Figure 6A shows representative images of cells expressing WT-Kv11.1 and Rab11B-GFP or DN-Rab11B-GFP immunostained for Kv11.1 and Bap31. Coexpressing DN-Rab11B-GFP increased the colocalization between WT-Kv11.1 and Bap31. It should be noted that overexpressing DN GTPases might nonspecifically affect nucleotide exchange factors and impair the properties of other Rabs. To investigate the specificity of the DN-Rab11B effects, we next tested short hairpin RNA (shRNA)-mediated knockdown of Rab11B (and the closely related Rab11A as a negative control) (15). The selective shRNA-mediated knockdown of endogenous Rab11A or Rab11B using these plasmids was previously confirmed in HEK293 using Western blot analysis (6). Figure 6B shows representative images of GFP-positive cells transfected with control shRNA, Rab11A shRNA, or Rab11B shRNA immunostained for Kv11.1 and Bap31. Coexpressing Rab11B shRNA selectively increased the colocalization between WT-Kv11.1 and Bap31. We conclude that the trafficking of WT-Kv11.1 from the transitional ER is dependent on Rab11B.
Fig. 6.
Dominant-negative (DN)-Rab11B-green fluorescent protein (GFP) and Rab11B short hairpin (sh)RNA increases WT-Kv11.1 and Bap31 colocalization. A: representative fluorescent images of cells coexpressing WT-Kv11.1 and Rab11B-GFP or DN-Rab11B-GFP immunostained with anti-Kv11.1 (red, first column) and anti-Bap31 (purple, third column). GFP fluorescence (green, second column) and the corresponding overlays (fourth column) are also shown (n = 15 images). Each image shows a corresponding portion (white dashed box) in greater detail. Scale bars, 10 μm. Also shown are the mean Costes' Pearson coefficients for the colocalization of WT-Kv11.1 or Bap31 (*P < 0.05). B: representative fluorescent images of cells coexpressing WT-Kv11.1 and control shRNA (con shRNA), Rab11A shRNA, or Rab11B shRNA immunostained with anti-Kv11.1 (red, first column) and anti-Bap31 (purple, first column) (n = 11–13 images), and the corresponding overlays (third column). Each image shows a corresponding portion (white dashed box) in greater detail. Scale bars, 10 μm. Also shown are the mean Costes' Pearson coefficients for the colocalization of WT-Kv11.1 or Bap31 in each of the conditions (*P < 0.05).
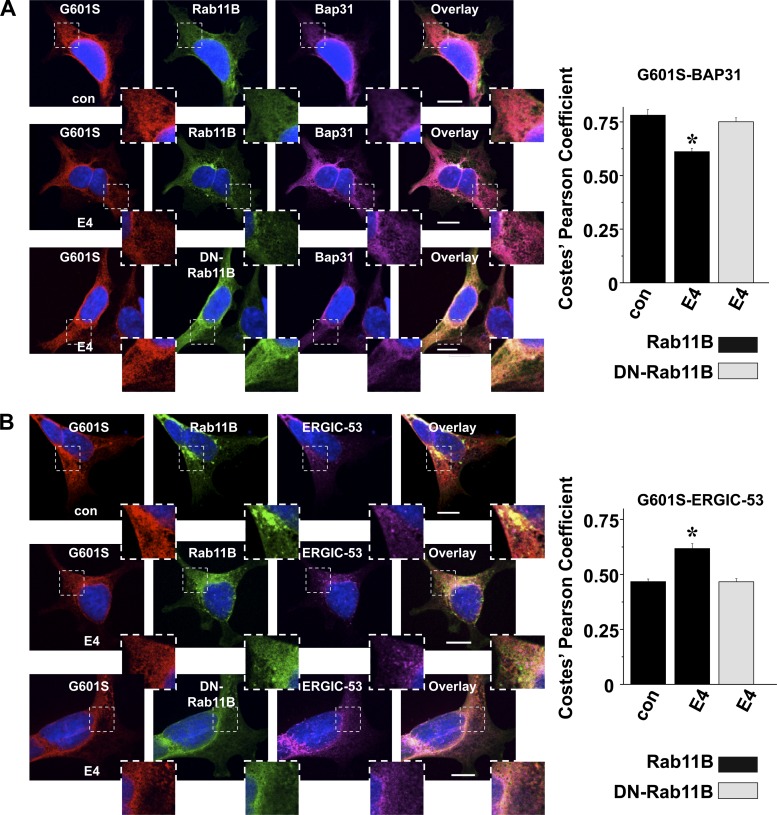
We also determined whether coexpressing DN-Rab11B-GFP prevented the pharmacological correction of G601S stored in the transitional ER. Figure 7A shows that coexpressing DN-Rab11B-GFP prevented the decrease in G601S and Bap31 colocalization following E-4031 treatment. We previously showed that pharmacological correction increases the colocalization of G601S and ERGIC-53 (34). Figure 7B shows that coexpressing DN-Rab11B-GFP prevented this too. Together these data suggest the pharmacological correction of G601S stored in the transitional ER is also dependent on Rab11B.
Fig. 7.
DN-Rab11B-GFP prevents the decrease in G601S and Bap31 colocalization and the increase in G601S and ERGIC-53 colocalization after E-4031 treatment. A: representative fluorescent images of cells coexpressing G601S and Rab11B-GFP or DN-Rab11B-GFP immunostained with anti-Kv11.1 (red, first column) and anti-Bap31 (purple, third column). The GFP fluorescence (green, second column) and the corresponding overlays (fourth column) for control cells or cells treated in E-4031 for 3 h (n = 9–11 images) are also shown. Scale bars, 10 μm. Each image shows a corresponding portion (white dashed box) in greater detail. Also shown at are the mean Costes' Pearson coefficients for the colocalization of G601S or Bap31 in control conditions or after being treated in E-4031 (*P < 0.05 compared with control conditions). B: representative fluorescent images of cells coexpressing G601S and Rab11B-GFP or DN-Rab11B-GFP immunostained with anti-Kv11.1 (red, first column) and anti-ERGIC-53 (purple, third column). The GFP fluorescence (green, second column) and the corresponding overlays (fourth column) for control cells or cells after being treated in E-4031 (E4) for 3 h (n = 10 images each) are also shown. Scale bars, 10 μm. Each image shows a corresponding portion (white dashed box) in greater detail. The graph shows the mean Costes' Pearson coefficient for the colocalization of G601S or ERGIC-53 in control conditions or after being treated in E-4031 (*P < 0.05 compared with control conditions).
DISCUSSION
A large number of congenital diseases are caused by mutations that result in protein misfolding, and cellular QC mechanisms in the ER prevent the trafficking of misfolded mutant proteins to their target membranes (4, 5, 16). Several studies show that high concentrations of chemicals, such as glycerol, can act as “chemical chaperones” to nonspecifically improve the folding and trafficking of mutant proteins (8, 30, 36, 41). More selective improvements in folding and trafficking can be achieved with pharmacological chaperones, which are drugs that selectively bind to the mutant protein (25, 41). Chemical and pharmacological chaperones likely improve mutant protein trafficking by increasing overall protein stability and/or shifting the equilibrium of mutant protein structure from misfolded to native-like (16). Zhou and colleagues (41) made the seminal discovery that drugs, which bind to Kv11.1 and block IKr, can act as pharmacological chaperones to improve the trafficking and functional expression for the LQT2 mutation N470D (41). These drugs are not likely to increase the overall stability of Kv11.1 because they do not improve the trafficking of WT-Kv11.1 and their effects are selective to a subset of trafficking-deficient LQT2 channels. Nonetheless, pharmacological chaperones appear to improve the trafficking for more than half of the trafficking-deficient LQT2 channels (3), and as such, the therapeutic potential to use pharmacological chaperones is high. Unfortunately, this approach is currently limited by the fact that these drugs block IKr.
We speculated that understanding the mechanism that underlies the trafficking-deficient LQT2 phenotype and pharmacological correction might provide new insight into the therapeutic potential for pharmacological chaperones. We previously demonstrated that a microtubule-dependent compartment in the ER negatively regulates trafficking-deficient LQT2 channels. We now show that this compartment is the transitional ER, and pharmacological correction initially increases the trafficking of the LQT2 channels that are stored in this compartment. This led us to test whether briefly exposing cells to the IKr blockers E-4031 or ranolazine could cause pharmacological correction of LQT2 channels. Indeed this was the case, and once the corrected LQT2 channels expressed at the cell surface, they remained functional for several hours after the drugs were washed out. Since pharmacological correction occurs relatively quickly and persists after drug washout, we propose that pharmacological chaperones with a short half-life might provide some therapeutic benefit. Moreover, ranolazine might be an ideal candidate drug. Not only is ranolazine quickly metabolized by the liver, but even when circulating levels are high it might not be proarrhythmic because it rapidly unblocks IKr during repolarization (27). Moreover, it has been shown to protect against acquired LQT2 arrhythmias by inhibiting the ryanodine receptors and the late cardiac Na+ current (18, 26). Whether or not ranolazine might be a practical therapeutic option for some LQT2 patients warrants further investigation.
We used a dominant negative strategy to show Rab11B is important for Kv11.1 trafficking from the transitional ER to the ERGIC. A number of studies show that Rab11B plays an important role in exocytosis, endocytosis, and protein recycling at the cell surface membrane (6, 9, 31, 33, 35). Studies suggest that Rab11B might play a unique role in regulating the cell surface expression of different ion channels, because coexpressing DN-Rab11B decreases the functional expression for the cystic fibrosis transmembrane conductance regulator, epithelial Na+ channel, and, to a lesser extent, Kir2.1 (9, 15, 33) but increases the functional expression of Cav1.2 (6).
In summary, we conclude that some LQT2 mutations are selectively stored in the transitional ER and that pharmacological correction increases the trafficking of these channels via a Rab11B-dependent pathway. Once the LQT2 mutations traffic, they can remain functionally active in the cell surface membrane for several hours even after the drug has been washed out. Although speculative, we suspect that drugs such as ranolazine might provide a therapeutic benefit for some LQT2 patients through its pharmacological chaperone activity.
GRANTS
This work was supported by the University of Kentucky Undergraduate Research and Creative Activities (eUreKa) Summer Research Grant (to P. S. Nataraj); American Heart Association predoctoral award PRE7370003 (to D. C. Bartos); National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL-60723 (to C. T. January); and NHLBI Grant R01-HL-087039 (to B. P. Delisle).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L.S. and B.P.D. conception and design of the research; J.L.S., A.R.R., P.S.N., E.A.S., and C.L.A. performed experiments; J.L.S., A.J.M., S.O., M.H., C.T.J., and B.P.D. analyzed data; J.L.S., A.R.R., P.S.N., D.C.B., C.T.J., and B.P.D. edited and revised the manuscript; J.L.S., A.R.R., P.S.N., D.C.B., E.A.S., A.J.M., S.O., M.H., C.L.A., C.T.J., and B.P.D. approved final version of the manuscript; D.C.B. and B.P.D. interpreted results of the experiments; B.P.D. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Timothy J. Kamp and Jabe M. Best (Univ. of Wisconsin, Madison) for technical assistance with the shRNA studies.
Footnotes
This article is the topic of an Editorial Focus by Ramon J. Ayon, Ruby A. Fernandez, and Jason X.-J. Yuan (5a).
REFERENCES
- 1.Abdallah H, Jerling M. Effect of hepatic impairment on the multiple-dose pharmacokinetics of ranolazine sustained-release tablets. J Clin Pharmacol 45: 802–809, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Akimoto K, Furutani M, Imamura S, Furutani Y, Kasanuki H, Takao A, Momma K, Matsuoka R. Novel missense mutation (G601S) of HERG in a Japanese long QT syndrome family. Hum Mutat Suppl 1: S184–S186, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113: 365–373, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Aridor M, Hannan LA. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1: 836–851, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Aridor M, Hannan LA. Traffic jams II: an update of diseases of intracellular transport. Traffic 3: 781–790, 2002 [DOI] [PubMed] [Google Scholar]
- 5a.Ayon RJ, Fernandez RA, Yuan JX. Mutant hERG channel traffic jam. Focus on “Pharmacological correction of long QT-linked mutations in KCNH2 (hERG) increases the trafficking of Kv11.1 channels stored in the transitional endoplasmic reticulum.” Am J Physiol Cell Physiol (August 28, 2013). 10.1152/ajpcell.00256.2013. [DOI] [PubMed] [Google Scholar]
- 6.Best JM, Foell JD, Buss CR, Delisle BP, Balijepalli RC, January CT, Kamp TJ. Small GTPase Rab11b regulates degradation of surface membrane L-type Cav1.2 channels. Am J Physiol Cell Physiol 300: C1023–C1033, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1: 117–125, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterworth MB, Edinger RS, Silvis MR, Gallo LI, Liang X, Apodaca G, Frizzell RA, Johnson JP. Rab11b regulates the trafficking and recycling of the epithelial sodium channel (ENaC). Am J Physiol Renal Physiol 302: F581–F590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell 7: 631–650, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86: 3993–4003, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80: 795–803, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Delisle BP, Anderson CL, Balijepalli RC, Anson BD, Kamp TJ, January CT. Thapsigargin selectively rescues the trafficking defective LQT2 channels G601S and F805C. J Biol Chem 278: 35749–35754, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Delisle BP, Slind JK, Kilby JA, Anderson CL, Anson BD, Balijepalli RC, Tester DJ, Ackerman MJ, Kamp TJ, January CT. Intragenic suppression of trafficking-defective KCNH2 channels associated with long QT syndrome. Mol Pharmacol 68: 233–240, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Delisle BP, Underkofler HA, Moungey BM, Slind JK, Kilby JA, Best JM, Foell JD, Balijepalli RC, Kamp TJ, January CT. Small GTPase determinants for the Golgi processing and plasmalemmal expression of human ether-a-go-go related (hERG) K+ channels. J Biol Chem 284: 2844–2853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem 277: 4989–4998, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Frommeyer G, Kaiser D, Uphaus T, Kaese S, Osada N, Rajamani S, Belardinelli L, Breithardt G, Eckardt L, Milberg P. Effect of ranolazine on ventricular repolarization in class III antiarrhythmic drug-treated rabbits. Heart Rhythm 9: 2051–2058, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Gong Q, Jones MA, Zhou Z. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J Biol Chem 281: 4069–4074, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapa S, Tester DJ, Salisbury BA, Harris-Kerr C, Pungliya MS, Alders M, Wilde AA, Ackerman MJ. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation 120: 1752–1760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khvotchev MV, Ren M, Takamori S, Jahn R, Sudhof TC. Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis. J Neurosci 23: 10531–10539, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilley BN, Ploegh HL. A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429: 834–840, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60: 821–836, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56: 801–813, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loo TW, Clarke DM. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J Biol Chem 272: 709–712, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Parikh A, Mantravadi R, Kozhevnikov D, Roche MA, Ye Y, Owen LJ, Puglisi JL, Abramson JJ, Salama G. Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm 9: 953–960, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajamani S, Shryock JC, Belardinelli L. Rapid kinetic interactions of ranolazine with HERG K+ current. J Cardiovasc Pharmacol 51: 581–589, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Salama NR, Chuang JS, Schekman RW. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell 8: 205–217, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem 271: 635–638, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Schlierf B, Fey GH, Hauber J, Hocke GM, Rosorius O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp Cell Res 259: 257–265, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri HP. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J Cell Sci 104: 671–683, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337–2350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JL, McBride CM, Nataraj PS, Bartos DC, January CT, Delisle BP. Trafficking-deficient hERG K channels linked to long QT syndrome are regulated by a microtubule-dependent quality control compartment in the ER. Am J Physiol Cell Physiol 301: C75–C85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokolowski S, Harvey M, Sakai Y, Jordan A, Sokolowski B. The large conductance calcium-activated K+ channel interacts with the small GTPase Rab11b. Biochem Biophys Res Commun 426: 221–225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest 101: 2257–2267, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Nagai R, Tomoike H, Takata S, Yano K, Yabuta K, Haneda N, Nakano O, Shibata A, Sawayama T, Kasai H, Yazaki Y, Nakamura Y. Four novel KVLQT1 and four novel HERG mutations in familial long-QT syndrome. Circulation 95: 565–567, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep 3: 944–950, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakana Y, Takai S, Nakajima K, Tani K, Yamamoto A, Watson P, Stephens DJ, Hauri HP, Tagaya M. Bap31 is an itinerant protein that moves between the peripheral endoplasmic reticulum (ER) and a juxtanuclear compartment related to ER-associated degradation. Mol Biol Cell 19: 1825–1836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem 273: 21061–21066, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem 274: 31123–31126, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J 74: 230–241, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]