Abstract
Iron homeostasis is achieved by regulating the intestinal absorption of the metal and its recycling by macrophages. Iron export from enterocytes or macrophages to blood plasma is thought to be mediated by ferroportin under the control of hepcidin. Although ferroportin was identified over a decade ago, little is understood about how it works. We expressed in Xenopus oocytes a human ferroportin-enhanced green fluorescent protein fusion protein and observed using confocal microscopy its exclusive plasma-membrane localization. As a first step in its characterization, we established an assay to detect functional expression of ferroportin by microinjecting oocytes with 55Fe and measuring efflux. Ferroportin expression increased the first-order rate constants describing 55Fe efflux up to 300-fold over control. Ferroportin-mediated 55Fe efflux was saturable, temperature-dependent (activation energy, Ea ≈ 17 kcal/mol), maximal at extracellular pH ≈ 7.5, and inactivated at extracellular pH < 6.0. We estimated that ferroportin reacts with iron at its intracellular aspect with apparent affinity constant < 10−7 M. Ferroportin expression also stimulated efflux of 65Zn and 57Co but not of 64Cu, 109Cd, or 54Mn. Hepcidin treatment of oocytes inhibited efflux of 55Fe, 65Zn, and 57Co. Whereas hepcidin administration in mice resulted in a marked hypoferremia within 4 h, we observed no effect on serum zinc levels in those same animals. We conclude that ferroportin is an iron-preferring cellular metal-efflux transporter with a narrow substrate profile that includes cobalt and zinc. Whereas hepcidin strongly regulated serum iron levels in the mouse, we found no evidence that ferroportin plays an important role in zinc homeostasis.
Keywords: cobalt transport, enterocytes, iron overload, iron transport, macrophages, membrane transport, trace metal nutrition, Xenopus laevis oocyte, zinc transport
systemic iron homeostasis is achieved primarily by regulating the intestinal absorption of the metal and its recycling by macrophages (reviewed in Ref. 17). The main control point is thought to be ferroportin (Fpn), a transporter mediating the export of iron from enterocytes or macrophages to the extracellular fluid and blood plasma. Highlighting the essential roles of ferroportin in iron homeostasis, mice lacking ferroportin died during embryonic development. If rescued by the selective expression of ferroportin in tissues mediating materno-embryonic iron transfer, mice survived but accumulated iron in macrophages, enterocytes, and hepatocytes and developed iron-restricted anemia (12). Intestine-specific inactivation of ferroportin in mice, beginning at age 7 days, resulted in a severe anemia that could be corrected by parenteral iron administration, revealing a critical role for ferroportin in the iron-absorptive pathway (12). Plasma-membrane expression of ferroportin is subject to regulation by hepcidin (reviewed in Refs. 17, 18, 37, 48). This hormone, produced predominantly in the liver, is bound by ferroportin, an event that triggers the internalization and degradation of ferroportin (39). Some human mutations in ferroportin cause impaired ferroportin delivery to the plasma membrane or decreased iron transport and result in macrophage iron retention whereas others render ferroportin insensitive to hepcidin and cause systemic iron overload (4, 13, 14, 24, 36, 42, 48).
Although ferroportin was identified over a decade ago (1, 11, 31), very little is known about how it works. As a first step in exploring its functional properties and molecular mechanisms, we established an assay to detect the functional expression of ferroportin in RNA-injected Xenopus oocytes, and directly examined its iron-transporting properties.
Iron loading was observed to induce increased ferroportin mRNA levels and plasma-membrane expression of ferroportin in macrophages and lung epithelial cells (3, 10, 45, 51). Ferroportin expression in macrophages was also responsive to loading with other metals (including cadmium, copper, and zinc) (7, 45), and manganese exposure stimulated ferroportin expression in HEK293T cells and mouse brain (52), raising the possibility that ferroportin activity may protect against metal-induced cytotoxicity. These observations have prompted us to test the hypothesis that ferroportin is a cellular exporter of a broad range of transition metals that includes cadmium, copper, iron, manganese, and zinc.
MATERIALS AND METHODS
Reagents and media.
Reagents were obtained from Sigma-Aldrich (St. Louis, MO) or Research Products International (Prospect, IL) unless otherwise indicated. Human ceruloplasmin was obtained from EMD Biosciences (Billerica, MA), and human hepcidin (25-amino acid hormone) was obtained from Peptides International (Louisville, KY).
Modified Barth's medium (MBM) contained 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.75 mM CaCl2, 0.66 mM NaNO3, and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), buffered to pH 7.5 by using 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris base). Calcium-free MBM was prepared by equimolar substitution of CaCl2 with additional MgSO4.
In transport assays, except as otherwise indicated in Fig. 2, Xenopus oocytes were incubated in a medium (hereinafter “standard efflux medium”) of composition 100 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.5 mM bathophenanthroline disulfonic acid (BPS), 1 mM nitrilotriacetic acid (NTA) trisodium salt, and 50 μg/ml apotransferrin (ApoTf) (R&D Systems, Minneapolis, MN), buffered by using 0–5 mM 2-(N-morpholino)ethanesulfonic acid (MES) and 0–5 mM N′,N′-diethylpiperazine (DEPP) (GFS Chemicals, Columbus, OH) to obtain pH 5.0–8.5 as indicated.
Fig. 2.
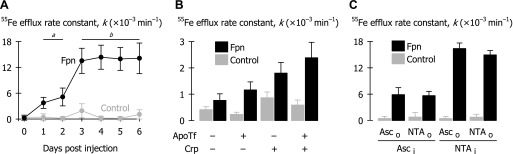
Optimization of assay conditions for the detection of ferroportin-mediated 55Fe efflux from Xenopus oocytes. A: first-order 55Fe efflux rate constants (k) were measured in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) from 0 to 6 days postinjection with Fpn-EGFP RNA by injecting oocytes with 50 nl of 5 μM 55Fe (n = 11–15 per group). Two-way ANOVA revealed an interaction (P < 0.001); within Fpn, all time points differed from one another (P < 0.001) except those enclosed by the top bars: aP = 0.33, bP ≥ 0.79. (Data in A are from the same oocyte preparation as used in Fig. 1; oocytes were injected on successive days and all assays, microscopy conducted on a single day.) B: effects of withholding (−) or adding (+) 50 μg/ml apotransferrin (ApoTf) and 200 U/ml ceruloplasmin (Crp) to efflux medium [pH 7.0, without bathophenanthroline disulfonic acid (BPS)] on Fe efflux from control oocytes and oocytes expressing Fpn-EGFP injected with 50 nl of 2 mM 55Fe, 1 mM l-ascorbic acid (n = 6–12 per group). Three-way ANOVA revealed that the addition of ApoTf (interaction Fpn × ApoTf, P < 0.001) or Crp (interaction Fpn × Crp, P < 0.001) each increased k in a ferroportin-specific manner but that their effects did not depend on the presence of the other (no three-way interaction, P = 0.35). C: effects of injectate (i) and extracellular (o) l-ascorbic acid and nitrilotriacetic acid (NTA) on Fe efflux from control oocytes and oocytes expressing Fpn-EGFP (n = 9–15 per group). Oocytes were injected with 50 nl of 5 μM 55Fe in injectate containing either 5 mM l-ascorbic acid (Asci) or NTA (NTAi) and incubated in efflux media containing either 5 mM l-ascorbic acid (Asco) or NTA (NTAo). Three-way ANOVA revealed a main effect of expression (P < 0.001); two-way ANOVA within Fpn indicated that k differed according to injectate chelater used (P < 0.001) but not extracellular chelater (P = 0.13). Data from A, B, and C are from three independent preparations.
Expression of human ferroportin in Xenopus oocytes.
We performed laparotomy and ovariectomy on adult female Xenopus laevis frogs (Nasco, Fort Atkinson, WI) under 3-aminoethylbenzoate methanesulfonate anesthesia (0.1% in 1:1 water/ice, by immersion) following a protocol approved by the University of Cincinnati Institutional Animal Care and Use Committee. Ovarian tissue was isolated and treated with 3 mg/ml collagenase A (Roche Diagnostics, Indianapolis, IN) in calcium-free MBM for 3 h, after which defolliculate oocytes (Dumont stage V-VI) were selected and stored at 17°C in standard MBM with 10 mg/l gentamicin.
We expressed in Xenopus oocytes the human Fpn isoform 1A (the product of the human SLC40A1 gene) tagged at its COOH terminus with the enhanced green fluorescent protein, thus Fpn-EGFP. Fpn-EGFP cDNA (39) was subcloned into the pOX(+) oocyte expression plasmid vector (29) between EcoRI and NotI under the SP6 promoter. We linearized Fpn-EGFP-pOX(+) using SnaBI (New England Biolabs, Ipswich, MA) and synthesized RNA in vitro using the mMESSAGE mMACHINE SP6 RNA polymerase kit (Applied Biosystems-Ambion, Austin, TX) according to the manufacturers' protocols. Oocytes were injected with ∼50 ng of Fpn-EGFP RNA (at 1 μg/μl in water) and incubated in MBM with 10 mg/l gentamicin for up to 6 days before being used in functional assays. “Control” oocytes were noninjected.
Live-cell imaging of Fpn expression by confocal microscopy.
We monitored the time course of Fpn-EGFP expression and its subcellular distribution by using live-cell imaging of RNA-injected Xenopus oocytes 0–6 days postinjection (dpi). Oocytes in MBM were mounted on the Zeiss LSM 710 META confocal laser-scanning microscope. EGFP was visualized by using the C-45 Apochromat ×10/0.45 W objective, exciting at 488 nm, and detecting fluorescence in the range 505–530 nm with optical slice of 11.4 μm approximately bisecting the oocyte.
Radiotracer assays.
We used 55Fe (added as FeCl3) at final specific activity 0.33–2.0 GBq/mg, 59Fe (added as Fe(SO4)2) at final specific activity 1.6 GBq/mg, 57Co (added as CoCl2) at final specific activity 180–230 GBq/mg, and 54Mn (added as MnCl2) at final specific activity 2.9 GBq/mg, obtained from Perkin-Elmer Life Science Products (Boston, MA); 109Cd (added as CdCl2) at final specific activity 57 MBq/mg and 65Zn (added as ZnCl2) at final specific activity 2.2 GBq/mg, obtained from the National Laboratory (Oak Ridge, TN); 64Cu (added as CuCl2) at final specific activity 4.2–32 MBq/mg, obtained from Washington University in St. Louis (St. Louis, MO).
To measure metal-ion efflux from oocytes expressing ferroportin, we adopted an approach we have used previously to assay amino-acid-dependent 86Rb efflux from native oocytes (27) and [3H]glutamate efflux from oocytes expressing the vesicular glutamate transporters (VGLUT) (28). We injected control oocytes and oocytes expressing Fpn-EGFP (4 or 5 dpi except as indicated in Fig. 2A) with 50 nl of metal radionuclide (*M, injectate concentration as indicated in figure legends) in vehicle of the following composition (except as noted below): 250 mM KCl, 5 mM NTA (added as nitrilotriacetic acid trisodium salt), and buffered to pH ≈ 7.0 by MES (∼1 mM). As exceptions to this, NTA and MES were replaced by l-ascorbic acid and DEPP where annotated “Asci” (Fig. 2, B and C) and NTA and MES were replaced by l-histidine and DEPP in solutions containing 64Cu (Figs. 3 and 5B). After injection, oocytes were incubated in ice-cold MBM for 1–10 min before the start of the efflux assay.
Fig. 3.
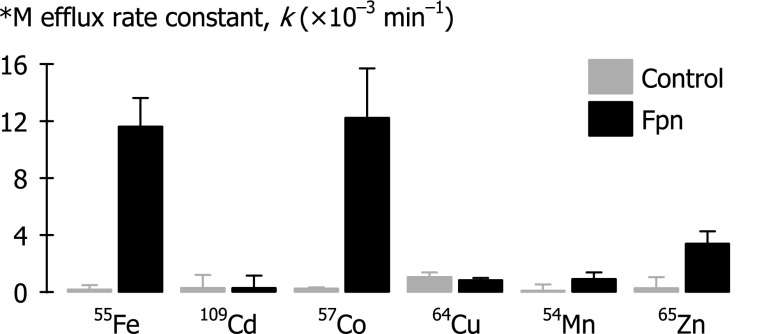
Initial screen for metal substrates of ferroportin. First-order rate constants (k) of radiotracer metal (*M) efflux were measured over 30 min in control oocytes and oocytes expressing Fpn-EGFP (injectate [*M] = 5 μM; n = 8–16 per group). Two-way ANOVA revealed an interaction (P < 0.001); Fpn differed from control for 55Fe, 57Co, and 65Zn (P < 0.001), but not for 109Cd (P = 0.98), 64Cu (P = 0.71), or 54Mn (P = 0.11).
Fig. 5.
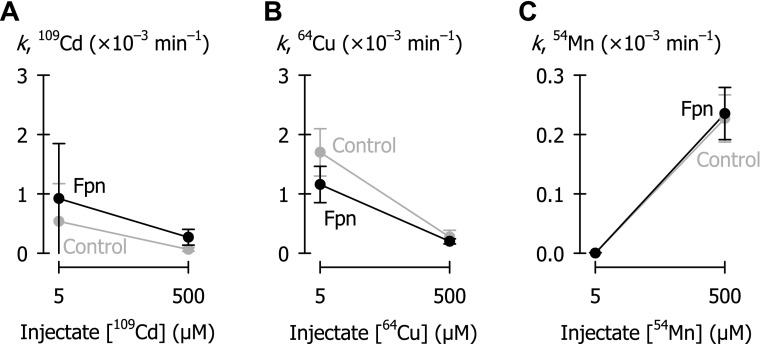
Metal efflux in control oocytes (gray) and oocytes expressing ferroportin (black) as a function of metal injectate (50 nl) concentration. A: 109Cd (n = 11–16 per group); in two-way ANOVA, Fpn did not differ from control (P = 0.18). B: 64Cu (n = 10–20 per group); two-way ANOVA revealed an interaction (P < 0.008); Fpn did not differ from control at injectate [64Cu] of 500 μM (P = 0.53), and Fpn was lower than control at 5 μM injectate (P < 0.001). C: 54Mn (n = 11–16 per group); in two-way ANOVA, Fpn did not differ from control (P = 0.60).
To initiate the efflux assay, we placed oocytes in standard efflux medium at pH 7.5 unless otherwise indicated. Oocytes were then transferred individually in 200 μl of medium to flat-bottomed wells of a 96-well plate and incubated 30 min (except as indicated in Fig. 4, A and B) at 22–24°C (except as indicated in Figs. 6C, 7B, 8B). To terminate efflux, we removed 150 μl of medium from each well for liquid-scintillation counting (0.75 × Nt). The corresponding oocytes were rinsed twice in ice-cold medium, solubilized with 5% SDS, and counted (N0 − Nt) using Scintisafe-30% liquid-scintillation cocktail (Fisher Scientific, Pittsburgh, PA). We fit our data by Eq. 1 to obtain the first-order rate constant (k) for metal radionuclide (*M) efflux for individual oocytes; N0 is counts per minute (cpm) in the oocyte at time 0, and Nt is cpm in the medium at time t = 30 min (or other endpoints as indicated in Fig. 4, A and B).
| (1) |
Fig. 4.
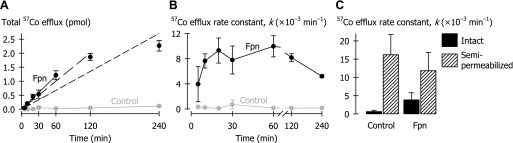
Kinetics of ferroportin-mediated metal efflux. Oocytes were injected with 50 nl of 50 μM 57Co. A: total 57Co efflux (in pmoles per oocyte) as a function of time in control oocytes (gray) and oocytes expressing Fpn-EGFP (black). Data from oocytes expressing Fpn-EGFP were fit by linear regression (y-intercept constrained at 0) over the following ranges of time points: 0–240 min (short dashed black line), root-mean-square error (RMSE) = 0.32, adjusted r2 = 0.86, P < 0.001; 0–120 min (long dashed black line), RMSE = 0.12, adjusted r2 = 0.97, P < 0.001; 0–60 min (solid black line), RMSE = 0.055, adjusted r2 = 0.99, P < 0.001; and 0–30 min (line not shown for clarity), RMSE = 0.063, adjusted r2 = 0.93, P < 0.001. B: first-order rate constants (k) for 57Co efflux as a function of time in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) (n = 8–12 per group), from the same experiment as that depicted in A. Two-way ANOVA revealed an interaction (P < 0.001); Fpn differed from control at all time points (P < 0.001). Within Fpn, 120- and 240-min time points differed from 60 min (P ≤ 0.008). C: first-order rate constants (k) for 57Co efflux (over 30 min) in intact (solid bars) or semipermeabilized (hatched bars) control oocytes and oocytes expressing Fpn-EGFP (n = 20–30 per group). Oocytes were pretreated for 30 min in MBM containing 1% dimethyl sulfoxide and 0.1% bovine serum albumin, without or with 100 μg/ml amphotericin B as permeabilizing agent. Two-way ANOVA revealed an interaction (P < 0.001); intact Fpn differed from intact control (P = 0.008); within Fpn, intact differed from semipermeabilized (P < 0.001). (Data in C are from an oocyte preparation independent of that used in A and B).
Fig. 6.
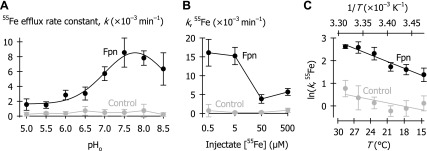
Functional characteristics of Fpn-mediated 55Fe efflux. A: first-order rate constants (k) of 55Fe efflux as a function of extracellular pH (pHo) in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) injected with 50 nl of 500 μM 55Fe (n = 10–16 per group). Two-way ANOVA revealed an interaction (P < 0.001). Data for oocytes expressing Fpn were fit by a 4-parameter Gaussian function (Eq. 2) in which a = (6.9 ± 0.5) × 10−3 min−1, b = 0.8 ± 0.1, pHoptm = 7.8 ± 0.1, and kmin = (1.6 ± 0.4) × 10−3 min−1 (r2 = 0.98, P < 0.001). B: 55Fe efflux rate constants (k) in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) as a function of injectate 55Fe concentration (n = 10–15 per group). Two-way ANOVA revealed an interaction (P < 0.001); Fpn differed from control at every concentration (P < 0.001). Within Fpn, all concentrations differed from one another (P ≤ 0.003) except 0.5 cf. 5 μM (P = 0.18). C: 55Fe efflux rate constants (k) as a function of temperature (T) in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) (injectate [55Fe] = 5 μM) (n = 10–12 per group). Data were fit by Eq. 3 for Fpn: activation energy (Ea) = 17.3 ± 1.1 kcal/mol, lnA = 31.5 ± 1.9 (r2 = 0.79, P < 0.001, n = 67); and control: Ea = 8.4 ± 1.8 kcal/mol, lnA = 14.5 ± 3.1 (r2 = 0.25, P < 0.001, n = 67). Ea differed between Fpn-EGFP and control (P < 0.001).
Fig. 7.
Functional characteristics of Fpn-mediated efflux of 57Co and 65Zn. A: 57Co efflux rate constants (k) in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) as a function of injectate 57Co concentration (n = 11–16 per group). Two-way ANOVA revealed an interaction (P < 0.001); Fpn differed from control at every concentration (P < 0.001). B: 57Co efflux rate constants (k) as a function of temperature in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) (n = 10–12 per group) (injectate [57Co] = 0.5 μM). Data were fit by Eq. 3 for Fpn: Ea = 20.7 ± 1.4 kcal/mol, lnA = 37.0 ± 2.3 (r2 = 0.77, P < 0.001, n = 68); and control: Ea = 27.9 ± 6.3 kcal/mol, lnA = 45.7 ± 10.7 (r2 =0.24, P < 0.001, n = 62). Ea did not differ between Fpn and control (P = 0.25). C: 65Zn efflux rate constants (k) in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) as a function of injectate 65Zn concentration (n = 10–18 per group). Two-way ANOVA revealed an interaction (P < 0.001); Fpn differed from control at every concentration (P < 0.001) except 0.5 μM (P = 1.0). D: 65Zn efflux rate constants (k) as a function of temperature in control oocytes (gray) and oocytes expressing Fpn-EGFP (black) (n = 9–12 per group) (injectate [65Zn] = 50 μM). Data were fit by Eq. 3 for Fpn: Ea = 20.5 ± 1.8 kcal/mol, lnA = 35.7 ± 3.1 (r2 = 0.67, P < 0.001, n = 65); and control: Ea = 6.9 ± 2.9 kcal/mol, lnA = 10.6 ± 4.9 (r2 =0.080, P < 0.021, n = 66). Ea differed between Fpn and control (P < 0.001).
Fig. 8.
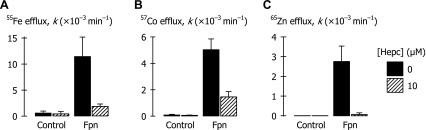
Effects of hepcidin ferroportin-mediated metal efflux in oocytes. *M efflux rate constants (k) were measured in control oocytes and oocytes expressing Fpn-EGFP following 24-h preincubation in MBM plus 100 μM cycloheximide, 0.03% DMSO without (solid bars) or with 10 μM hepcidin (Hepc; hatched bars). A: effect of hepcidin treatment on 55Fe efflux rate constants (injectate [55Fe] = 5 μM) (n = 8–12 per group); two-way ANOVA revealed an interaction (P < 0.001). B: effect of hepcidin treatment on 57Co efflux rate constants (injectate [57Co] = 500 μM) (n = 14–17 per group); interaction (P < 0.001). C: effect of hepcidin treatment on 65Zn efflux rate constants (injectate [65Zn] = 50 μM) (n = 13–14 per group); interaction (P < 0.001).
pH-dependence of iron efflux.
First-order rate constants of 55Fe efflux as a function of pH were fit by a 4-parameter Gaussian function (Eq. 2) in which pHoptm is the optimal pH (i.e., pH at which k is maximal), a = (kmax − kmin), and b is the standard deviation, which defines the width of the bell-shaped relationship such that k is half-maximal at pH = (pHoptm − b) and at pH = (pHoptm + b) (i.e., the inflection points).
| (2) |
Temperature dependence of iron, cobalt, and zinc efflux.
We controlled incubation temperature by mounting a sealed 96-well plate in a water bath during the efflux period. We monitored actual temperature by using the TA-29 thermistor positioned in the efflux medium within the well and recorded from the CL-100 controller (both Warner Instruments, Hamden, CT). Metal efflux rate constants (k) obtained over the approximate range 15–30°C were fit by a modified Arrhenius function (Eq. 3) in which Ea is the Arrhenius activation energy, A is the y-intercept, R is the universal gas constant (1.987 cal·mol−1·K−1), and T is the absolute temperature.
| (3) |
Measurement of serum iron and zinc in mice treated with hepcidin.
We examined the effect of hepcidin administration on serum iron and zinc levels in mice by using a protocol approved by the University of California, Los Angeles Office of Animal Research Oversight. Male C57BL/6 mice aged 7–8 wk were given intraperitoneally 50 μg of human hepcidin in 100 μl water, or water alone. We obtained sera 3–4 h after injection from terminal blood samples drawn by cardiac puncture in mice under isoflurane anesthesia. We analyzed serum zinc and iron concentrations by using colorimetric assays (Iron Assay Kit, Sekisui Diagnostics, Lexington, MA; Quantichrom Zinc Assay Kit, Bioassay Systems, Hayward, CA).
Statistical and regression analyses.
We performed statistical analyses by using SigmaPlot (version 12; Systat Software) with critical significance level α = 0.05. We have presented our data as means and standard deviation (SD) for n independent observations and used parametric tests in their analysis. Oocyte metal-ion efflux rate constant data were fit by Eqs. 1–3 using the least-squares method of regression analysis, the results of which are expressed as the estimates of fit parameters ± standard error (SE); r2 is the regression coefficient and P then describes the significance of the fit. To test between-group comparisons and comparisons of fit parameters (Eq. 2), we performed Student's unpaired t-test, or one-way or multivariate analysis of variance (ANOVA) followed by pairwise multiple comparisons by using the Holm-Šidák test.
RESULTS
Expression and localization of EGFP-tagged human ferroportin in oocytes.
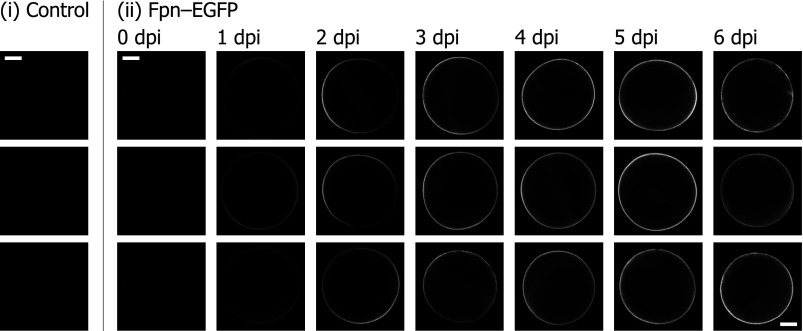
We expressed EGFP-tagged human ferroportin (Fpn-EGFP) in RNA-injected oocytes and examined the localization and time course of protein expression by using confocal laser-scanning microscopy (Fig. 1). No autofluorescence or reflectance was apparent in control (noninjected) oocytes under the conditions described. We observed faint fluorescence at or near some portions of the plasma membrane of oocytes injected with Fpn-EGFP RNA as early as 1 dpi. The fluorescence signal at the oocyte perimeter intensified at 2–3 dpi predominantly on the animal pole of the oocytes (the location of the animal pole was determined by visual inspection). The fluorescence signal extended around the entire oocyte perimeter by 4 dpi, peaked in intensity around 4–5 dpi, and did not increase further by 6 dpi. Over this time course, we did not observe any intracellular fluorescence, indicating that nascent Fpn-EGFP is directed to the plasma membrane rather than accumulating in intracellular stores.
Fig. 1.
Live-cell imaging of enhanced green fluorescent protein-tagged ferroportin (Fpn-EGFP) expression in Xenopus oocytes. Control oocytes (i) and oocytes injected with Fpn-EGFP RNA at 0–6 days postinjection (dpi; ii) in modified Barth's medium (MBM) were mounted on the confocal laser-scanning microscope. Each image is of a separate oocyte with optical slice ≈ 11.4 μm roughly bisecting the oocyte, collected using identical settings (pinhole, intensity, and gain). Scale bars, 0.2 μm.
Radiotracer efflux assay to detect functional expression of human Fpn in RNA-injected Xenopus oocytes.
We established an assay to detect the functional expression of Fpn in RNA-injected Xenopus oocytes. Expression of Fpn-EGFP stimulated by up to 300-fold the first-order rate constants (k) describing 55Fe efflux compared with control oocytes.
Using the same oocyte preparation in which we monitored the time course of fluorescence associated with Fpn-EGFP expression (Fig. 1), we observed a modest increase in 55Fe efflux from oocytes expressing Fpn-EGFP compared with control oocytes at 1–2 dpi in the presence of NTA, BPS, and apotransferrin (Fig. 2A). Fpn-EGFP expression more robustly stimulated 55Fe efflux rate constants at 3–6 dpi.
In an independent preparation, 55Fe efflux rate constants in oocytes expressing Fpn were increased relative to control oocytes under all conditions tested (NTA was present in all efflux media) (Fig. 2B). Addition of human ApoTf or bovine ceruloplasmin to the efflux medium further increased the 55Fe efflux rate constants in oocytes expressing Fpn. Since these conditions may mimic plasma—ceruloplasmin is a soluble plasma ferroxidase and transferrin, the plasma iron carrier—these observations are consistent with a role for Fpn in the cellular export of iron prior to its oxidation and handoff to transferrin.
Fpn-mediated 55Fe efflux rate constants were higher when we included 5 mM NTA in the injectate than when we included 5 mM l-ascorbic acid (Fig. 2C). This observation should not be interpreted to indicate the oxidation state of the transported form of iron since we cannot predict whether the radioactive iron was in the ferrous or ferric form on its arrival at the plasma membrane. In both cases, Fpn-mediated 55Fe efflux rate constants in efflux media containing NTA did not differ from those in efflux media containing l-ascorbic acid (Fig. 2C).
We subsequently included BPS, a potent transition-metal chelating agent, in the efflux medium to ensure adequate sequestration of the metal. When the media contained ApoTf, inclusion of BPS had no appreciable effect on 55Fe efflux when oocytes were injected with 50 nl of 5 μM 55Fe, but it modestly accelerated efflux when we used higher injectate concentrations (50–500 μM 55Fe) (data not shown). We found no isotope effect on ferroportin efflux activity. The first-order rate constants for ferroportin-mediated iron efflux did not depend on whether we used 55Fe or 59Fe (data not shown), an observation that validates the use of either radioisotope in assaying ferroportin activity in vitro or in vivo.
Based on these several observations, subsequent experiments were performed under assay conditions chosen to ensure the highest efflux activity relative to control (i.e., maximize the signal-to-noise ratio), specifically: 1) assays were performed at 4–5 dpi, 2) radiotracer metal injectate solutions contained NTA, and 3) standard efflux medium contained BPS, NTA, and ApoTf. We elected not to use ceruloplasmin in subsequent experiments since its inclusion offered only limited improvement of the assay; ceruloplasmin increased the 55Fe efflux rate constant but decreased to fourfold the signal-to-noise ratio compared with fivefold in its absence (both in the presence of ApoTf, Fig. 2B).
Screening for metal substrates of ferroportin.
As an initial screen for metal substrates of ferroportin, we measured radiotracer metal (*M) efflux over 30 min in control oocytes and oocytes expressing ferroportin. Fpn expression stimulated the efflux of 55Fe, 57Co, and 65Zn, but not 109Cd, 64Cu, or 54Mn (Fig. 3).
In an effort to validate our assay, we examined 1) the kinetics of Fpn-mediated metal efflux and 2) the effect of semipermeabilization of the plasma membrane. We chose to monitor cobalt efflux since the rate constant of 57Co efflux was similar to that of 55Fe (Fig. 3) and cobalt is less prone to redox changes under the conditions presented than is iron. We found that cumulative (total) 57Co efflux was linear up to 1 h, but was no longer linear at 2 or 4 h (Fig. 4A). We found that measuring efflux at time points from 10 min (but not 5 min) provided reliable estimates of k (Fig. 4B); however, k was underestimated at 120 min and 240 min relative to 60 min. In all subsequent experiments, we measured metal efflux over 30 min.
In an independent preparation in which Fpn expression also stimulated 57Co efflux from intact cells (Fig. 4C), we found that the efflux rate constants were considerably higher in Fpn-expressing cells that had been semipermeabilized by using amphotericin B. This observation indicates that cellular efflux of the metal is the rate-limiting step described by the efflux rate constant in intact cells and not, for example, the dissociation of metal from intracellular proteins. Therefore, measurement of metal efflux up to 60 min from oocytes expressing Fpn provides a valid estimate of the rate of transport (efflux) mediated by Fpn.
To determine whether differences in metal-binding affinities of Fpn or intracellular metal-binding proteins could account for our failure to observe efflux of 109Cd, 64Cu, or 54Mn at injectate *M concentration of 5 μM (Fig. 3), we further examined efflux of 109Cd, 64Cu, and 54Mn over a broader concentration range. In the case of each radiotracer injected at 0.05 μM, the radioactivity (cpm) appearing in the efflux medium was below the limit of detection, so data are not shown. Fpn expression (in preparations independent of that used in Fig. 3) did not stimulate efflux of 109Cd, 64Cu, and 54Mn when these metals were injected at either 5 μM or 500 μM (Fig. 5).
Functional characteristics of ferroportin-mediated iron efflux.
Fpn-mediated 55Fe efflux rate constants were dependent on extracellular pH (Fig. 6A). Data were fit by a Gaussian function from which we predicted optimal pH (pHoptm) of 7.8 ± 0.1 (SE) and an inflection point (midpoint) at pH 7.0 ± 0.1.
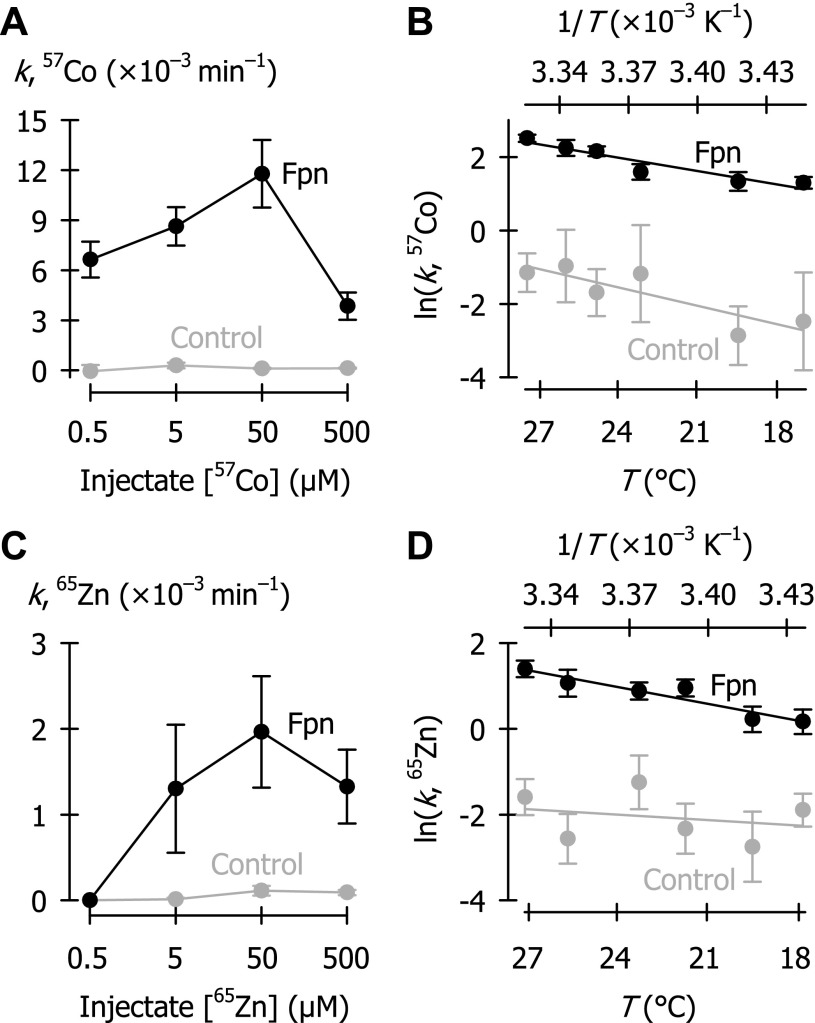
We measured 55Fe efflux rate constant as a function of injectate iron concentration over the range 0.5–500 μM. Fpn expression stimulated 55Fe efflux at all concentrations tested, and 55Fe efflux rate constants were maximal at 0.5–5 μM injectate 55Fe concentration (Fig. 6B). That the 55Fe efflux rate constants were lower at elevated injectate 55Fe concentrations (50, 500 μM) demonstrates that Fpn-mediated iron efflux is saturable.
Fpn-mediated 55Fe efflux was strongly temperature-dependent over the range 15–29°C (Fig. 6C). From the rate constants of 55Fe efflux in oocytes expressing Fpn, we estimated Arrhenius activation energy (Ea) of 17.3 ± 1.1 kcal/mol.
Functional characteristics of Fpn-mediated cobalt and zinc efflux.
Fpn expression increased the 57Co efflux rate constant at all injectate concentrations tested relative to control (Fig. 7A); the maximum stimulation was observed when injectate 57Co concentration was 50 μM. Fpn-mediated 57Co efflux was strongly temperature-dependent over the range 17–28°C, with Ea = 20.7 ± 1.4 kcal/mol (Fig. 7B).
Fpn expression stimulated the efflux rate constants for 65Zn at injectate concentrations of 5–500 μM, with a peak around 50 μM (Fig. 7C); however, we detected no Fpn-mediated 65Zn efflux when 65Zn injectate concentration was only 0.5 μM. 65Zn efflux was strongly temperature-dependent over the range 18–27°C, with Ea = 20.5 ± 1.8 kcal/mol (Fig. 7D).
The Arrhenius activation energies (Ea) of metal efflux in oocytes expressing Fpn did not differ among the three metals: iron, cobalt, and zinc (P = 0.18). The metal efflux rate constants for 55Fe or 57Co in Fpn-expressing oocytes were generally similar whereas 65Zn efflux rate constants were considerably lower (see Figs. 3, 6, and 7).
Effects of hepcidin on ferroportin-mediated metal efflux in oocytes.
Treatment of oocytes for 24 h with 10 μM hepcidin plus 100 μM cycloheximide (the latter was used to inhibit protein synthesis de novo) inhibited Fpn-mediated 55Fe efflux by 87% ± 12% (SE) compared with cycloheximide alone (Fig. 8A). This same hepcidin treatment also inhibited Fpn-mediated 57Co efflux by 71% ± 5% (SE) and abolished 65Zn efflux in oocytes expressing Fpn (Fig. 8, B and C). In an independent preparation (data not shown), we observed greater than 60% inhibition of Fpn-mediated 55Fe efflux following 24 h of treatment with as little as 100 nM hepcidin (P < 0.001).
Effect of hepcidin treatment on serum iron and zinc levels in the mouse.
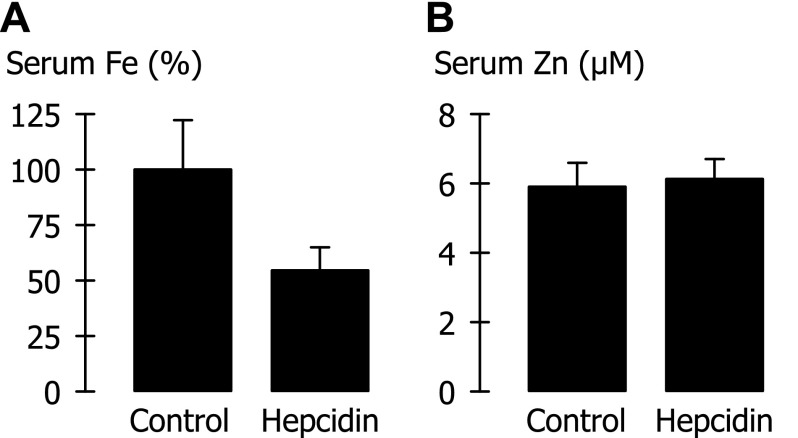
Our observations that Fpn could mediate zinc efflux in the oocyte system and that such activity was hepcidin-sensitive prompted us to examine whether hepcidin treatment in mice could decrease serum zinc levels. We observed a marked hypoferremia in mice within 4 h of administering hepcidin (Fig. 9A). (Normalizing serum iron by the mean control serum iron in each of four trials was necessary to account for the high between-trial variability in serum iron of control animals.) In those same animals, however, hepcidin treatment had no effect on serum zinc levels (Fig. 9B).
Fig. 9.
Effect of hepcidin treatment on serum iron and zinc levels in the mouse. Iron and zinc levels were measured in sera obtained from blood drawn 3–4 h after administration of hepcidin or vehicle only (Control). A: serum iron (Fe), normalized by the mean control serum iron in each of four trials. Mean serum iron in control mice was 32.4 μM (SD 11.7 μM, n = 16). Hepcidin treatment (n = 16) differed from control (P < 0.001). B: serum zinc (Zn) did not differ between control (n = 14) and hepcidin treatment (n = 15) (P = 0.34).
DISCUSSION
We have examined the functional properties of ferroportin-mediated metal export by expressing ferroportin in RNA-injected Xenopus oocytes and measuring radiotracer metal efflux. Our results provide direct evidence of ferroportin-mediated cellular efflux of iron, cobalt, and zinc. Ferroportin is thought to deliver iron to transferrin subsequent to iron oxidation by the membrane-bound ferroxidase hephaestin (in the enterocyte) or the plasma or membrane-bound ferroxidase forms of ceruloplasmin (2, 5, 6, 15, 35, 48). We found that ferroportin iron-efflux activity per se does not require transferrin or ferroxidase since we could measure ferroportin activity in the absence of these proteins (provided that the efflux medium contained an iron chelator).
Rate constants describing iron efflux were maximal at extracellular pH 7.5–7.8, and efflux was inactivated at extracellular pH < 6.0. We therefore predict that acidemia or interstitial acidosis should modestly inhibit ferroportin activity whereas alkalemia or alkalosis should modestly accelerate ferroportin-mediated iron efflux. The extracellular pH range over which ferroportin activity varies in vitro points to the possibility that ferroportin activity could be regulated by extracellular pH via an effect on exofacial histidine residues since 1) the observed optimal pH coincides with the isoelectric point (pI) for histidine of 7.6 and 2) the inflection point at pH 7.0 is close to the expected pKa2 for histidine. Whereas pKa2 = 6.0 for histidine in solution, its pKa2 can be as high as 7.0 in proteins. For example, the 12 titratable histidine residues in transferrin have average pKa2 = 6.6 (53). Ferroportin contains a histidine-rich region in its putative second extracellular loop (17).
The iron-efflux rate constant was maximal when the oocyte injectate 55Fe concentration was 0.5 μM in a volume of 50 nl (see Fig. 6B). Assuming an oocyte volume of 0.5–1.0 μl (8, 19), this corresponds to an optimal intracellular 55Fe concentration in the order of 5 × 10−8 M to 1 × 10−7 M; however, the effective free concentration is expected to be lower as a result of binding by intracellular proteins, e.g., ferritin, ovalbumin. (We attempted to minimize the effects of intracellular binding proteins by ensuring that only a short time, i.e., 1–10 min, elapsed between radiotracer injection and the start of the efflux assay, and by limiting the efflux assay to within the linear phase.) The optimal intracellular 55Fe concentration in our assay therefore provides an estimate of the upper limit of the apparent affinity constant for iron at the internal aspect of ferroportin, i.e., K0.5Fe ≤ 10−7 M. (At intracellular concentrations greater than K0.5Fe, k is expected to decrease as the transporter approaches saturation.) Likewise (by using data in Figs. 7, A and C) we estimated apparent affinity constants (K0.5Co, K0.5Zn) for 57Co and 65Zn of ≤ 5 × 10−6 M. Whereas the efflux rate constant for 57Co was similar to that for 55Fe, the efflux rate constant for 65Zn was much lower (Fig. 3). Comparison of the ratios Vmax/K0.5 (i.e., “specificity constant”) for each transported substrate permits a description of a transporter's substrate selectivity. Assuming that the binding capacity of intracellular proteins (ferritin, ovalbumin, metallothionein, etc.) is roughly similar for each of the metals then, by substituting for Vmax the maximum observed efflux rate constant k (from Fig. 3), we judge the order of ferroportin substrate selectivity to be as follows: Fe > Co > Zn.
We found that ferroportin does not transport cadmium, copper, or manganese. Our observation that ferroportin does not transport cadmium is consistent with the finding that cadmium does not exit renal tubular cells towards the extracellular fluid or blood plasma despite the expression of ferroportin on basolateral membranes (50). Cadmium is thought to gain entry to tubular cells 1) by megalin-dependent endocytosis of cadmium-metallothionein followed by transport of cadmium into the cytoplasm by divalent metal-ion transporter-1 (DMT1), or 2) by apical uptake of cadmium by DMT1 or the Zrt-/Irt-like metal-ion transporters ZIP14, ZIP8, and its accumulation in tubular cells leads to nephropathy (16, 20, 43, 47, 49).
Whereas we found that manganese is not a ferroportin substrate, one group of investigators has reported that ferroportin can also function as a manganese exporter (30). Those investigators measured 54Mn efflux from RNA-injected oocytes over 4 h, well beyond the limit (1 h) of linear 57Co efflux that we observed for oocytes expressing ferroportin (see Fig. 2A). In that same study, investigators expressed metal export as a flux (pmol/4 h); however, a flux is only meaningful when the cis-concentration of the substrate is known and remains unchanged during the time course of the assay (we have described efflux instead as a first-order rate constant). Both of these maneuvers are likely to underestimate iron efflux relative to manganese efflux. In addition, three other observations in the study just cited create some doubt on the interpretation that ferroportin transports manganese: 1) the 2-h accumulation of 1 μM 54Mn did not differ between oocytes expressing DMT1 alone and DMT1 plus ferroportin (DMT1 was used to load the oocyte with 54Mn); 2) efflux of ∼16 μM 54Mn from oocytes expressing Fpn was cis-inhibited by a sixfold excess concentration of iron by only ∼15% whereas such an excess should afford a much stronger inhibition if the transporter were shared by both metals (46) (the effect of iron in control oocytes was not shown); and 3) 54Mn efflux from oocytes expressing ferroportin was trans-inhibited by unlabeled iron or manganese whereas unlabeled substrates sharing a transporter system are expected to trans-stimulate radiotracer flux (44, 46). Nevertheless, in 2 out of a total of 11 independent trials (data not shown), and only when we injected 0.5 mM 54Mn, we found the 54Mn efflux rate constant (k) to be significantly (P < 0.05) higher in ferroportin-expressing oocytes than in control oocytes (in another trial at 0.5 mM, ferroportin was significantly lower than control). Since the rate of 2 out of 11 trials exceeded the expected false positive rate of 5% (we set α = 0.05), we considered whether the absolute difference in k was meaningful. The average increase in k (0.5 mM 54Mn injectate) was 0.37 × 10−3 min−1, from which we might estimate a specificity constant (Vmax/K0.5) for manganese some three orders of magnitude lower than that for iron. That is, ferroportin expression produced no meaningful increase in the magnitude of the manganese flux, and the predicted apparent affinity of ferroportin for manganese was extremely low compared with iron, such that any ferroportin-mediated manganese transport should be trivial at most. Manganese should therefore not be considered a physiological substrate of ferroportin. The possibility that ferroportin might transport manganese under conditions of cellular manganese loading would require further testing.
Ferroportin-mediated iron efflux was strongly temperature-dependent. The activation energy (Ea) of 17 kcal/mol lies within the range of Ea (roughly 10–40 kcal/mol) commonly observed for membrane transporters, i.e., uniporters, symporters, or antiporters (26), whose transport cycles comprise several conformation changes. In contrast, ion channels are thought to undergo fewer conformational changes than do transporters, and generally exhibit Ea < 10 kcal/mol. Supporting the transporter model of ferroportin, metal effluxes appeared to be saturable processes (Figs. 6B and 7A).
The observation that the activation energies of metal efflux in oocytes expressing ferroportin did not differ between iron, cobalt, and zinc strengthens the conclusion that the observed stimulation of cobalt and zinc transport was specific to ferroportin. Further confirming this specificity, treatment of oocytes with the iron-regulatory hormone hepcidin inhibited the efflux of all three metals in oocytes expressing ferroportin but not in control oocytes. Binding of hepcidin by ferroportin triggers the internalization and degradation of ferroportin (39).
Whereas the cobalt-containing cobalamin vitamers are essential micronutrients in humans, the absorption and metabolic needs of free cobalt are trivial. Whether ferroportin could play any role in mammalian Co metabolism is therefore unclear. Mutations in the plant ferroportin homologs (FPN1 and FPN2, which are expressed in the stele and root of Arabidopsis thaliana) disrupt metabolism both of iron and cobalt, suggesting that both metals are substrates of the plant ferroportins (34).
Our finding that ferroportin could mediate hepcidin-sensitive zinc efflux in vitro prompted us to examine a role for ferroportin in zinc metabolism. Whereas investigators have attributed the hypozincemia of inflammation to the interleukin-6 (IL-6)-dependent upregulation of ZIP14 in the liver (25), we considered that an effect of hepcidin on ferroportin expression could additionally contribute to the hypozincemia since IL-6 strongly induces hepcidin production (38, 40). Whereas hepcidin administration in mice resulted in a marked hypoferremia within 4 h, we observed no effect of hepcidin on serum zinc levels in those same animals. Therefore it is unlikely that ferroportin-mediated zinc efflux contributes appreciably to zinc homeostasis.
The liver represents the largest single pool of zinc that can rapidly exchange with plasma (i.e., within hours) (22). Ferroportin is expressed at relatively low levels in mammalian hepatocytes (11), and zinc transporter-1 (ZnT1) probably accounts for the greater fraction of zinc efflux from liver. ZnT1 is also found on the basolateral membrane of enterocytes and, like ferroportin (45), is responsive to the metal-regulatory transcription factor-1 (MTF-1) (9, 23, 41). That hepcidin treatment had no effect on serum zinc suggests that the contribution of ferroportin to zinc efflux from hepatocytes and enterocytes is at best modest compared with that of ZnT1. Unlike iron homeostasis, the zinc economy also depends on rapid alterations in zinc secretion into the intestine, leading to changes in net zinc excretion (21).
Conclusions.
Functional expression in Xenopus oocytes reveals that ferroportin is an iron-preferring cellular metal-efflux transporter that is sensitive to changes in extracellular pH and temperature. Cobalt and zinc count among its narrow substrate profile; however, whereas hepcidin strongly regulated serum iron levels in the mouse, we found no evidence to suggest that ferroportin participates significantly in zinc homeostasis.
GRANTS
This study was supported by Public Health Service Grants R01 DK080047 (to B. Mackenzie), R01 DK082717 (to E. Nemeth), and P30 DK078392 (Digestive Health Center, Cincinnati Children's Hospital and University of Cincinnati) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and by the University of Cincinnati (to B. Mackenzie). The production of copper-64 at Washington University-St. Louis School of Medicine is supported by the U.S. Department of Energy (DoE), Nuclear Physics Isotope Program. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the DoE, NIDDK, or the National Institutes of Health.
DISCLOSURES
T. Ganz and E. Nemeth are shareholders and scientific advisors of Intrinsic LifeSciences and Merganser Biotech.
AUTHOR CONTRIBUTIONS
C.J.M., T.G., E.N., and B.M. conception and design of research; C.J.M., A.S., T.G., E.N., and B.M. performed experiments; C.J.M., A.S., T.G., E.N., and B.M. analyzed data; C.J.M., A.S., T.G., E.N., and B.M. interpreted results of experiments; C.J.M., A.S., and B.M. prepared figures; C.J.M. and B.M. drafted manuscript; C.J.M., A.S., T.G., E.N., and B.M. approved final version of manuscript; A.S., T.G., E.N., and B.M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank John H. Alexander, BS, Sarah R. Anthony, BS, and Carl N. Chotas, MS (University of Cincinnati), and Eileen Fung, PhD, Bo Qiao, MD, and Emilio Ramos, PhD (David Geffen School of Medicine at UCLA) for help in the laboratory. This work was presented in part at Experimental Biology, Anaheim, California, 24–28 April, 2010 (32) and at the International BioIron Congress, Vancouver, British Columbia, Canada, 22-26 May 2011 (33).
REFERENCES
- 1.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275: 19906–19912, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Anderson GJ, Frazer DM, McKie AT, Vulpe CD. The ceruplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol Dis 29: 367–375, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camaschella C, Poggiali E. Rare types of genetic hemochromatosis. Acta Haematol 122: 140–145, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Attieh ZK, Dang T, Huang G, van der Hee RM, Vulpe C. Decreased hephaestin expression and activity leads to decreased iron efflux from differentiated Caco2 cells. J Cell Biochem 107: 803–808, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab 2: 309–319, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chung J, Haile DJ, Wessling-Resnick M. Copper-induced ferroportin-1 expression in J774 macrophages is associated with increased iron efflux. Proc Natl Acad Sci USA 101: 2700–2705, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey JL, Davidson N, Lester HA, Brecha N, Quick M. Protein kinase C modulates the activity of a cloned γ-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J Biol Chem 269: 14759–14767, 1994 [PubMed] [Google Scholar]
- 9.Cousins RJ. Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res 80: 243–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 106: 3979–3984, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403: 776–781, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1: 191–200, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Drakesmith H, Schimanski LM, Ormerod E, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, Bastin JM, Cowley D, Chinthammitr Y, Robson KJ, Townsend AR. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood 106: 1092–1097, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fernandes A, Preza GC, Phung Y, De Domenico I, Kaplan J, Ganz T, Nemeth E. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood 114: 437–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals 16: 9–40, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Fujishiro H, Yano Y, Takada Y, Tanihara M, Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 4: 700–708, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Ganz T. Systemic iron homeostasis. Physiol Rev 93: 1721–1741, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Ganz T, Nemeth Iron Imports E., IV Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 290: G199–G203, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Goldin AL. Expression of ion channels in Xenopus oocytes. In: Expression and Analysis of Recombinant Ion Channels: From Structural Studies to Pharmacological Screening, edited by Clare JJ, Trezise DJ. Weinheim: Wiley-VCH Verlag, 2006 [Google Scholar]
- 20.Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25: 643–655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr 130: 1360S–1366S, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Leggett RW. A biokinetic model for zinc for use in radiation protection. Sci Total Environ 420: 1–12, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis 35: 33–46, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodish H, Berk A, Kaiser CA, Krieger M, Bretscher A, Ploegh H, Amon A, Scott MP. Transmembrane transport of ions and small molecules. In: Molecular Cell Biology. New York: W. H. Freeman and Company, 2013 [Google Scholar]
- 27.Mackenzie B, Harper AA, Taylor PM, Rennie MJ. Na+/amino acid coupling stoichiometry of rheogenic system B0,+ transport in Xenopus oocytes is variable. Pflügers Arch 426: 121–128, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie B, Illing AC, Morris MEK, Varoqui H, Erickson JD. Analysis of a vesicular glutamate transporter (VGLUT2) supports a cell-leakage mode in addition to vesicular packaging. Neurochem Res 33: 238–247, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem J 403: 59–69, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta 1818: 651–657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5: 299–309, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Mitchell CJ, Shawki A, Nemeth E, Ganz T, Mackenzie B. Functional expression in Xenopus oocytes reveals that human ferroportin is an iron exporter shared with zinc (Abstract). FASEB J 24: 1017.–3., 2010 [Google Scholar]
- 33.Mitchell CJ, Shawki A, Nemeth E, Ganz T, Mackenzie B. Functional expression in Xenopus oocytes reveals that human ferroportin is an iron exporter shared with zinc (Abstract). Am J Hematol 86: E87, 2011 [Google Scholar]
- 34.Morrissey J, Baxter IR, Lee J, Li L, Lahner B, Grotz N, Kaplan J, Salt DE, Guerinot ML. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21: 3326–3338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostad EJ, Prohaska JR. Glycosylphosphatidylinositol-linked ceruloplasmin is expressed in multiple rodent organs and is lower following dietary copper deficiency. Exp Biol Med 236: 298–308, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Nemeth E, Ganz T. Hepcidin and iron-loading anemias. Haematologica 91: 727–732, 2006 [PubMed] [Google Scholar]
- 37.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr 26: 323–342, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 122: 78–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflügers Arch 447: 744–751, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Schimanski LM, Drakesmith H, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, Bastin JM, Cowley D, Chinthammitr Y, Robson KJ, Townsend AR. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood 105: 4096–4102, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H+-coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Curr Top Membr 70: 169–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein WD. Transport and Diffusion Across Cell Membranes. Orlando, FL: Academic, 1986 [Google Scholar]
- 45.Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood 116: 4657–4664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Winkle LJ. Biomembrane Transport. San Diego, CA: Academic, 1999 [Google Scholar]
- 47.Vesey DA. Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol Lett 198: 13–19, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Wessling-Resnick M. Iron imports. III. Transfer of iron from the mucosa into circulation. Am J Physiol Gastrointest Liver Physiol 290: G1–G6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolff NA, Abouhamed M, Verroust PJ, Thévenod F. Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured renal proximal tubule cells. J Pharmacol Exp Ther 318: 782–791, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Wolff NA, Liu W, Fenton RA, Lee WK, Thévenod F, Smith CP. Ferroportin 1 is expressed basolaterally in rat kidney proximal tubule cells and iron excess increases its membrane trafficking. J Cell Mol Med 15: 209–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang F, Wang X, Haile DJ, Piantadosi CA, Ghio AJ. Iron increases expression of iron-export protein MTP1 in lung cells. Am J Physiol Lung Cell Mol Physiol 283: L932–L939, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Yin Z, Jiang H, Lee ESY, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem 112: 1190–1198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue KT, Lee M, Zheng J, Callender R. The determination of the pKa of histidine residues in proteins by Raman difference spectroscopy. Biochim Biophys Acta 1078: 296–302, 1991 [DOI] [PubMed] [Google Scholar]