Abstract
Since the seminal studies of Otto Warburg in the 1920s, it has been widely recognized that cancers grow glycolytically, even in the presence of oxygen. This generates an abundance of protons in a gradient across most solid tumors with an acidic core and an alkaline rim. Whether and how this proton gradient may also serve in an autocrine fashion in these tumors is unclear. We demonstrate that human glioma cells form spheroids that act as a viable three-dimensional tumor model, forming physiologically relevant extracellular pH (pHe) and cell proliferation gradients. Using fluorescent cell cycle trackers, we determined that the rate of cell proliferation is directly dependent on pHe and that cells adjust their growth rate according to their position within the pH gradient. We further show that glioma cells sense pH via H+-sensitive K+ channels, which translate changes in pH into changes in membrane voltage. These channels are tonically active and blocked by acidic pHe, quinine, and ruthenium red. Blockade of this K+ conductance by acidic pHe or drug inhibition depolarized glioma cells and tumor spheroids and prevented their passage through the hyperpolarization-dependent G1-to-S phase cell cycle checkpoint, thereby inhibiting cell division. In this way, pHe directly determines the proliferative state of glioma cells.
Keywords: glioma, pH, cell cycle, potassium channel
over the past 100 years, certain hallmarks of cancer activity have been identified. These tend to be employed (to varying degrees) by cancers throughout the body. One such hallmark is increased glycolysis with increased tumor invasiveness, even in the presence of ample oxygen, termed the Warburg effect (12). The result of this phenomenon is fairly clear: a corresponding hyperacidity of the tumor interstitium due to increased proton production from glycolysis. Cancer cells must then develop mechanisms to overcome this inhospitable environment (11, 34).
Gliomas are archetypal of this effect. They represent an unfortunate intersection between destruction and prevalence, being both the most common and the most deadly primary brain cancer. Prognosis for a grade IV glioma, known as glioblastoma multiforme (GBM), is grim, with a median survival of 14 mo with the best current treatment (36). GBM lethality is mediated by a unique combination of rapid invasion and aggressive destruction of the surrounding brain (31). It has been demonstrated that, like other aggressive cancers, gliomas lean disproportionately on glycolytic mechanisms vs. oxidative phosphorylation for their ATP production (8, 27), which can lead to increased extracellular acidosis (12). Additionally, they possess other proton-extruding mechanisms, including the Na+/H+ antiporter NHE1 (23) and surface expression of a vacuolar H+-ATPase (29). The combination, then, of increased glycolysis, proton-extruding proteins, and, finally, tumor core necrosis leads to marked interstitial/extracellular pH (pHe) heterogeneity and hyperacidification (9, 10). This phenomenon is specific to or heightened in transformed cells vs. their nontransformed counterparts, as evidenced by the reversed intracellular pH (pHi)-pHe gradients of typical glioma cells (pHi 7.35, pHe 6.75) and astrocytes (pHi 7.0, pHe 7.30) (34).
Recent studies have shown that glial cells respond to and elicit changes in proton concentrations and brain function. For instance, glial cells in the brain stem are exquisitely sensitive to small alterations in pHe and, in turn, regulate breathing through ATP release (14). Additionally, pH is indirectly involved in depolarization-induced alkalinization, in which an increase in extracellular K+ ([K+]o) from neuronal activity leads to downstream astrocyte alkalinization and extracellular acidification, which then decreases neuronal excitability (28). Since glioma cells are exposed to even larger proton gradients in the tumor microenvironment, we reasoned that they too might rely on pHe as a signal.
There is some evidence to show that pHe can have a profound impact on glioma cell physiology. For instance, prior studies have shown that the acid-sensing ion channels (ASICs) are present in glioma cells and that these channels are involved in cell migration (30). C6 mouse glioma cells, used as a model of glial cells at large, swell upon extracellular acidification in a Na+-dependent manner, implicating NHE1 in postischemic brain edema (19). Next, VEGF expression is induced in cultured glioma cells upon application of acidic pHe (37). Finally, acidic pHe can promote a more stem-like phenotype for glioma cells in a reversible manner (17).
In light of these findings, we set out to examine the effect of pH on glioma physiology. We used glioma tumor spheroids, which were capable of reproducing the pHe heterogeneity previously described in vivo (9, 10), thus allowing us to map the dependence of a glioma cell's physiology on its microenvironment. We show that the interstitial spheroid pH can predict glioma cell proliferation and that changes in pH are sensed by ion channels that transduce pH changes to changes in membrane potential (Vm). We discuss how this serves as a feedback mechanism for these tumors to regulate their growth in a self-made acidic environment.
MATERIALS AND METHODS
Cell culture.
U251-MG and D54 human glioma cells were derived from World Health Organization grade IV GBM tumors. D54 cells were a gift from Dr. D. Bigner (Duke University). Cultured cortical astrocytes were obtained from postnatal day 5–8 mouse pups, passaged once, and then plated on coverslips. All cells were incubated in variants of DMEM-Ham's F-12 medium (DMEM/F-12; Invitrogen) with 7% FBS.
Tumor spheroid formation.
U251-MG human glioma cells were plated in 200 μl of DMEM/F-12 with 7% FBS at a starting concentration of 5 × 103–1.5 × 104 cells/well into agarose-coated 96-well plates. The plates were coated with autoclaved 1.5% (wt/vol) agarose-containing DMEM/F-12 (50 μl/well) and cooled until the agarose hardened. Initial spheroid formation occurred 2 days after the cells were plated; the spheroids were grown in an incubator in bicarbonate-buffered DMEM/F-12 at 37°C and 10% CO2 for ≥1 wk. After 1 wk, spheroid diameter was 100–500 μm.
Paraffin embedding.
Spheroids >1 wk old were collected and fixed with 4% paraformaldehyde in PBS, dehydrated in ethanol, and embedded in HistoGel blocks (Thermo Scientific) using cryomolds. HistoGel was processed to paraffin and then embedded in a paraffin block. Sections (7 μm) were cut using a Leica microtome and placed on positively charged glass slides (catalog no. 12-550-17, Fisher Scientific).
Immunocytochemistry.
The paraffin sections of tumor spheroid were deparaffinized using CitriSolv (catalog no. 22-143-975, Fisher Scientific), rehydrated, and washed with PBS. The spheroids were then blocked and permeabilized in PBS containing 0.3% Triton X-100 and 10% normal goat serum and then stained overnight at 4°C with rabbit anti-Ki67 antibody (1:1,000 dilution; catalog no. 15580, Abcam) in a 1:2 dilution of blocking buffer (BB) in PBS. After the slides were washed in PBS, they were stained with Alexa Fluor 488 goat anti-rabbit secondary antibody (1:500 dilution; catalog no. A-11008, Invitrogen) in the diluted BB for 2 h at room temperature, washed, and incubated for 5 min in PBS with 1 mg/ml 4′,6-diamidino-2-phenylindole (DAPI, 1:1,000 dilution; catalog no. 62248, Thermo Scientific) and then mounted with Aqua-Poly/Mount (catalog no. 18606, Polysciences) and a cover glass. Images were acquired using a Zeiss Axiovert 200M microscope with a ×20 air objective and Axiovision release 4.6 software. Ki67 staining was visualized using a FITC filter set, while DAPI staining was visualized using a DAPI filter set.
pH recordings of spheroids.
Spheroids >1 wk old were preincubated in sulfate- and phosphate-free (SPF) pH 6.0, 7.4, and 8.8 baths (for composition, see Electrophysiology) for 2 h at 37°C and then placed in a solution with the cell-impermeant pH indicator dye seminaphtharhodafluor-5F 5-(and 6-)carboxylic acid (SNARF-5F, 20 μM; catalog no. S-23922, Invitrogen) on the stage of a confocal microscope (model FV1000, Olympus) with 405-, 473-, and 559-nm diode lasers. After 30 min in this SNARF-5F solution, the fluorophore was excited with the 473-nm diode laser, and images were captured with two band-pass filters: 490–590 and 655–755 nm. The ratio of long to short wavelength provided the pH of the bath and spheroid interstitium and was calibrated using a linear regression of the pH 6.0, 7.4, and 8.8 baths (r2 = 0.9999).
Voltage dye recordings of spheroids.
Spheroids >1 wk old were preincubated in 2 μM bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3), catalog no. D8189, Sigma] and 2 μM CellTracker Orange (catalog no. C2927, Invitrogen) for 45 min at 37°C in SPF pH 7.4 bath (for composition see Electrophysiology) and washed once with SPF pH 7.4 bath. Spheroids were then placed in SPF pH 7.4 bath, excited at 473 nm [DiBAC4(3)] and 559 nm (CellTracker Orange), and captured using two band-pass filters: 490–540 and 575–675 nm. Results were normalized to the CellTracker Orange dye.
Confocal microscopy and image analysis.
Confocal images were captured with a confocal microscope (model FV1000, Olympus) using a ×20 air objective. Images were captured in z stacks of 5-μm section thickness through the spheroid with Olympus Fluoview ASW 3.1 and analyzed using WCIF ImageJ. For the pHe and Vm recordings, raw images were blurred with a 10-pixel-radius-mean filter, false-colored with ImageJ lookup tables, and saved as TIFF files. The Ki67-stained spheroids were analyzed by comparing the ratio of Ki67 to DAPI mean fluorescence intensity in the core and the rim of the tumor. Core and rim were delineated by marked changes in cellularity and laminar composition between the two phases and were selected via ImageJ as elliptical and annular sections, respectively.
Electrophysiology.
U251-MG and D54 human glioma cells were whole cell patch-clamped from 3- to 5-day-old coverslips using standard techniques. Patch pipettes were pulled to 3- to 5-MΩ resistance from thin-walled borosilicate glass (catalog no. TW150F-4, World Precision Instruments) using an upright puller (catalog no. PP-830, Narashige Instruments). Signals were captured with an Axopatch 200A amplifier (Molecular Devices), converted using Digidata 1320A (Molecular Devices), and recorded using pClamp 8 (Molecular Devices). Cells were 80% compensated for capacitance and series resistance but omitted if series resistance was >12 MΩ, which indicates poor whole cell access. Voltage-clamped cells were held at −40 mV until step protocols were administered in 20-mV increments. Current-clamp was used to monitor cell voltage in zero-current mode, without injection of additional current; changes in Vm were calculated using an average of at least two reversible barrel applications of either drug or different pHe bath and corrected for baseline drift over time. Standard pipette solution contained (in mM) 140 KCl, 10 EGTA, and 10 HEPES sodium salt and was adjusted to pH 7.2 with NaOH. SPF bath solutions consisted of (in mM) 130 NaCl, 5 KCl, 1 CaCl2, and either 15 MES + 15 HEPES (pH 6.0), 30 HEPES (pH 7.4), or 15 Tris + 15 HEPES (pH 8.8) and were adjusted to their respective pH with HCl or NaOH. Osmolarity for the pipette and bath solutions was 305 and 320 mosM, respectively, and was adjusted by addition of glucose.
Cell proliferation and viability.
For assessment of cell proliferation, D54 human glioma cells were plated at a starting concentration of 1 × 104 cells/well into 12-well plates in quadruplicate and incubated overnight at 37°C and 5% CO2 in DMEM/F-12 with 7% FBS. The first batch of cells was collected and counted using a Coulter Multisizer 3 counter (Beckman-Coulter). This was set as day 0, and the remaining plated cells were incubated in DMEM/F-12 with an additional 5.97, 18.88, and 59.72 mM NaHCO3 for pH 6.7, 7.2, and 7.7 media, respectively. The media were adjusted to 365 mosM using d-mannitol. Quinine and ruthenium red were applied at concentrations of 100 and 20 μM, respectively. The media were replaced daily to minimize changes in pH or drug potency.
For assessment of cell viability, D54 cells were plated in 10-cm dishes at 4 × 105 cells/dish in 10 ml of DMEM/F-12 with 7% FBS and allowed to adhere overnight. On the following day, the media were replaced with the respective pH- and drug-conditioned media and allowed to incubate at 37°C and 5% CO2 for the next 2 days. The cells were then collected and stained with Trypan blue, a diazo dye that enters cells with compromised plasma membranes. These cells were counted using a hemocytometer, and the percentage of viable cells was calculated for each condition.
Cell cycle.
D54 human glioma cells were plated in 10-cm dishes at 4 × 105–1 × 106 cells/dish in 10 ml of DMEM/F-12 with 7% FBS and allowed to adhere overnight. On the following day, the media were replaced with the respective pH- and drug-conditioned media and allowed to incubate at 37°C and 5% CO2 for the next 2 days. The cells were harvested and resuspended in cold PBS and then dehydrated with cold ethanol. The cells were spun down and resuspended in a propidium iodide (PI) staining solution (0.02 mg/ml; catalog no. P21493, Invitrogen), 0.2 mg/ml DNase-free RNase (catalog no. AM2286, Invitrogen), and 0.1% (vol/vol) Triton X-100 (catalog no. T-8787, Sigma) for 30 min. These preparations were analyzed using a cell sorter (model LSR II, BD Biosciences).
Statistics.
Statistics were performed using GraphPad Prism 5.0 and are shown as means ± SE; n ≥ 3 for all experiments.
RESULTS
Glioma cells organically evolve gradients of pHe and cell proliferation in vitro.
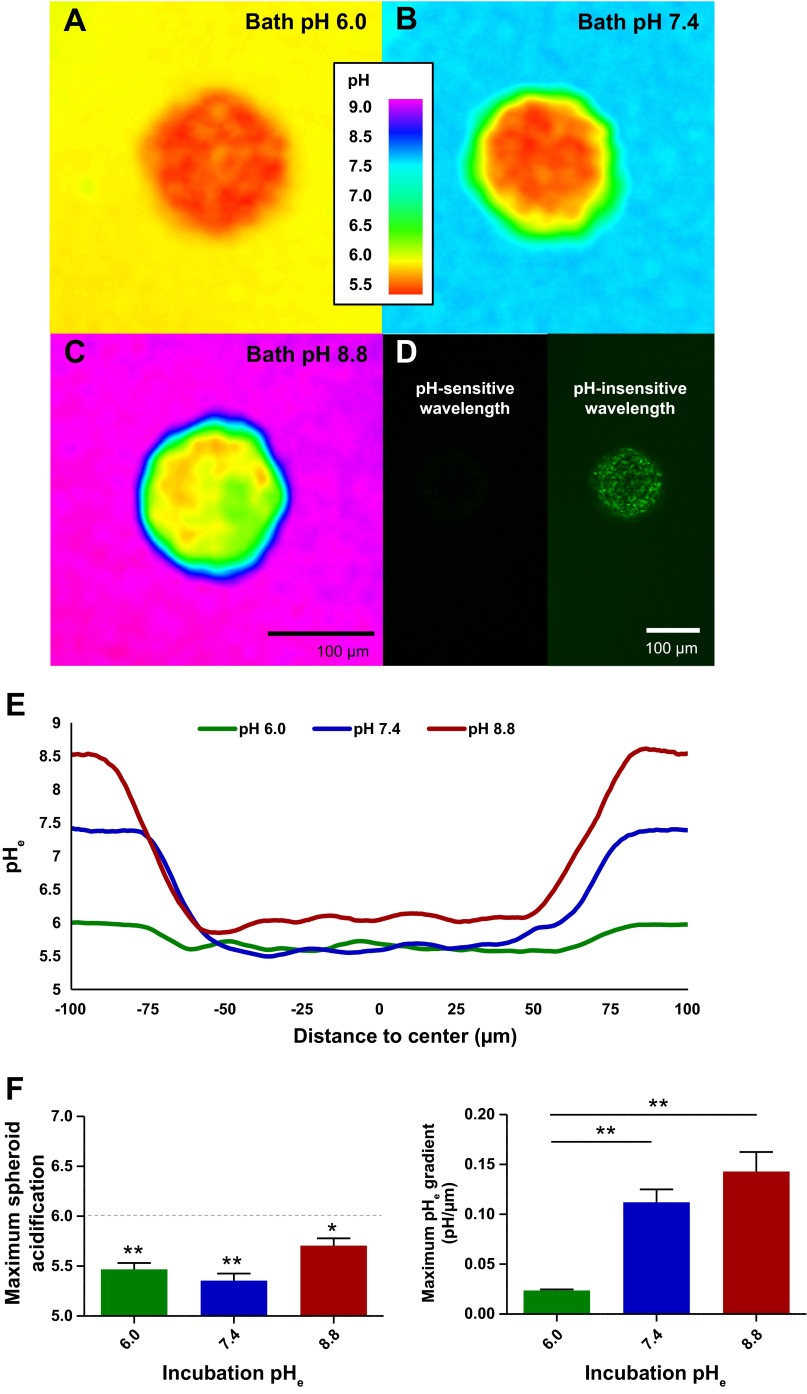
Prior studies demonstrated a proclivity of gliomas to acidify heterogeneously but, generally, with increased proton concentration toward the tumor core. The cause of this acidification is multifaceted and includes heterogeneous expression of acid extruders, poor access to nutrients, and a mix of cell populations (18). We wondered if tumor acidification could organically evolve from a clonal population of cells and in the presence of ample nutrients. Previous studies demonstrated a pHe gradient in rat gliomas in vivo (9, 10) and in glioma spheroids in vitro (1). To gain sufficient pHe resolution, we elected to use the cell-impermeant ratiometric pH indicator dye SNARF-5F (20 μM) to analyze spheroid pHe gradients. U251 human glioma cells, which quickly aggregate, were grown to form spheroids with ∼104 cells and analyzed via confocal microscopy for pHe differences. We compared pHe gradients from spheroids bathed for 2 h in acidic, neutral, and alkaline pH-buffered media to determine 1) if the core acidification was saturable and 2) how well the proton gradients dissipated (Fig. 1, A–C). In the span of the ∼100-μm-diameter spheroids, glioma cells experienced a wide range of proton concentrations, with an increased concentration focused in the core of the spheroids. All the spheroids acidified below pHe 6.0, regardless of incubating medium (Fig. 1, D–E; pHe 5.3–5.9, n = 3 each). Consequently, the proton concentration gradient was significantly steeper in the alkaline pH media solutions, where maximally there was an order-of-magnitude change in proton concentration every 7 μm into the spheroid core (Fig. 1F). Thus glioma cells in situ are capable of reconstituting and experiencing a wide range of proton concentrations over short lengths, and this pHe heterogeneity is slow to dissipate.
Fig. 1.
Quantification of tumor spheroid acidification. A–C: >1-wk-old U251 human glioma spheroids were preincubated and then placed for confocal imaging in pH 6.0, 7.4, and 8.8 baths with 20 μM cell-impermeant seminaphtharhodafluor-5F 5-(and-6-)-carboxylic acid (SNARF-5F) pH indicator dye. Representative z sections are shown at ×20 magnification through the middle of the spheroids. D: full diffusion of SNARF-5F in a representative pH 6.0-bathed spheroid. E: line graphs through the center of the representative spheroids show that they create standing extracellular pH (pHe) gradients that can acidify to ∼pHe 6.0 regardless of bathing medium. F and G: quantification of maximum acidity (1-tailed t-test for H0 pHe < 6.0, n = 3) and maximum gradient of acidification (1-way ANOVA with Tukey-Kramer post test, n = 3), respectively. *P < 0.05; **P < 0.01.
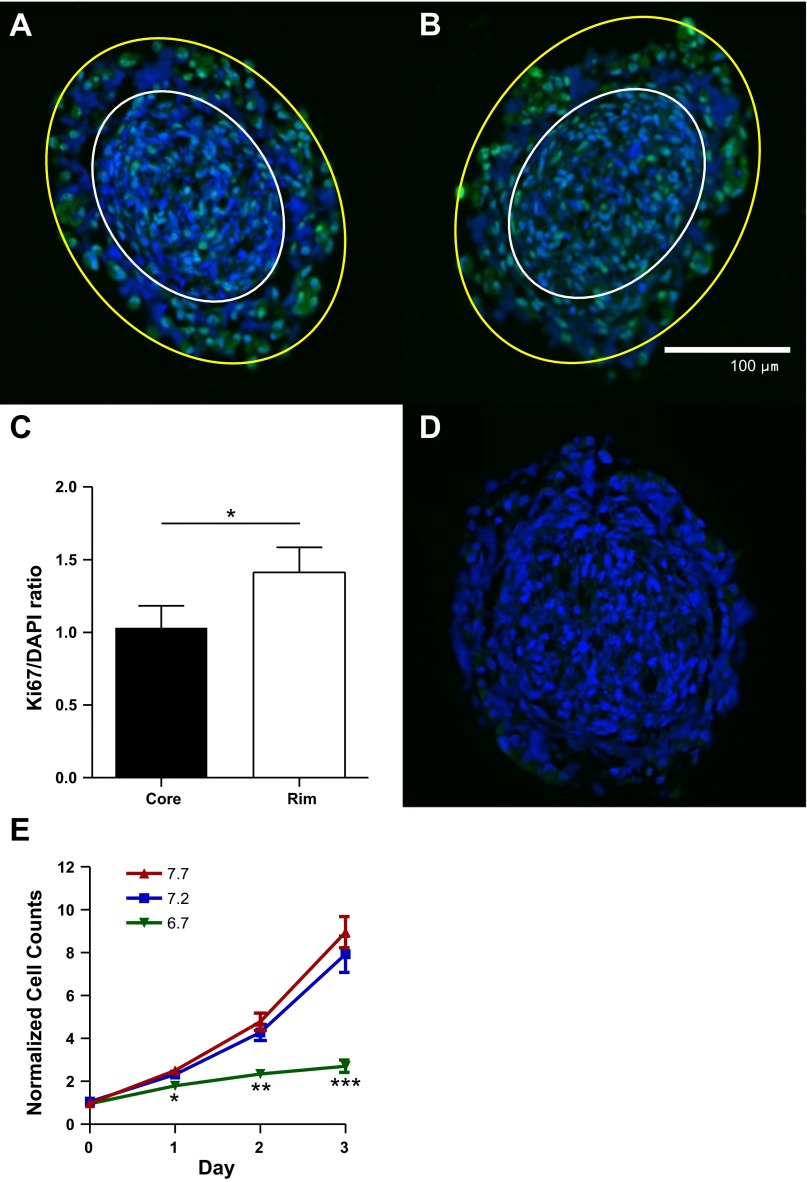
We further hypothesized that this acidification inversely corresponded to tumor cell proliferation. Paraffin sections of 1-wk-old tumor spheroids were immunostained for Ki67, a protein specifically expressed during all active phases of the cell cycle but absent at rest (the G0 phase). These spheroids demonstrated a rim of Ki67 expression (Fig. 2, A and B) that was decreased in the spheroid core relative to nuclear DAPI staining (Fig. 2C). Thus the cell proliferation gradient mimicked that of pHe within these spheroids, with greater proliferation and higher pHe away from the tumor core.
Fig. 2.
Human glioma spheroids evolve pHe and proliferation gradients. A and B: >1-wk-old U251 human glioma spheroids were embedded in paraffin, cross-sectioned, and stained for Ki67 (green) and 4′,6-diamidino-2-phenylindole (DAPI, blue). White outline encapsulates the core of the spheroid, while the area between yellow and white outlines encapsulates the spheroidal rim. Magnification ×20. C: Ki67 staining normalized to DAPI shows more proliferation of the rim glioma cells (2-tailed paired t-test, n = 7). D: control spheroid without Ki67 primary antibody. E: quantification of Coulter counter data, with cells cultured at varying pHe levels and media changed daily (2-tailed t-tests vs. pHe 7.2 as control, n ≥ 6). *P < 0.05; **P < 0.01; ***P < 0.001.
Glioma cells proliferate in alkaline pHe.
We next sought to directly test the relationship between cell proliferation and interstitial acidity. The functional consequence of protons on the many pH-dependent processes in glioma cells remains largely unknown. We cultured D54 human glioma cells, the growth curves of which have been well characterized (5, 16, 22), in a CO2/HCO3− media buffering system that set pHe to 6.7 (acidic), 7.2 (neutral), and 7.7 (alkaline). Glioma cells displayed a strong preference for alkaline pHe, even at supraphysiological levels (Fig. 2E). Cell counts using the Beckman Coulter counter revealed exponential growth at alkaline and neutral pHe and stagnation at acidic pHe. By day 3, there was no significant difference between cell counts at pHe 7.7 and 7.2 but 66% fewer cells in acidic than neutral pHe (n ≥ 6, P = 0.38 and P < 0.001, respectively). Thus extracellular proton concentrations control glioma cell growth.
pHe dependence of Vm in human glioma cells.
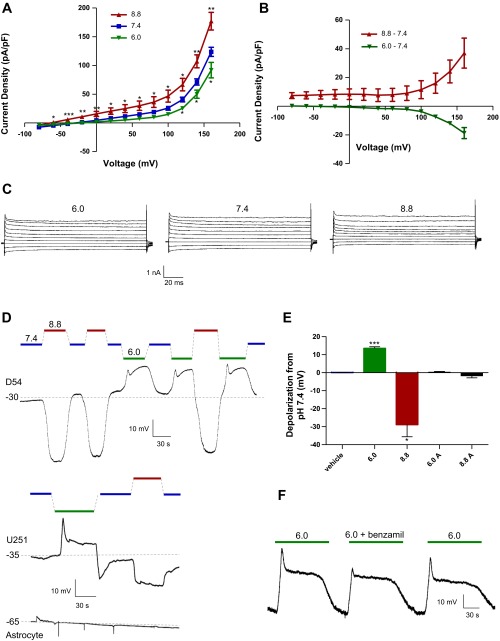
We hypothesized that pHe affects this glioma cell growth via a transmembrane chemosensor capable of translating extracellular protons to intracellular signaling and surmised that pHe-sensitive ion channels could act as this transducer. D54 glioma cells studied in whole cell patch-clamp mode showed large pHe-sensitive changes in current density and Vm (Fig. 3). Cells were placed in a pHe 7.4 bath with barrel-applied isotonic solutions of pHe 6.0, 7.4, and 8.8, buffered accordingly. In voltage-clamp mode (Fig. 3, A–C), current density decreased moderately with a switch from pHe 7.4 to 6.0 (−3.69 ± 0.34 pA/pF at +100 mV) and increased with a switch from pHe 7.4 to 8.8 (+11.5 ± 6.3 pA/pF at +100 mV). In current-clamp mode (Fig. 3, D–E), acidic pHe induced large depolarizations (+13.5 ± 0.92 mV), while alkaline pHe induced large hyperpolarizations (−28.8 ± 6.8 mV). These changes in Vm were sustained, reversible, and additive and occurred with or without physiological levels (60 nM) of Ca2+ in the pipette solution (data not shown). At pHe 6.0, there was an additional transient strong depolarization followed by a plateau phase; this transient depolarization is reflective of previously described ASICs (35) and was blocked by application of 10 μM benzamil (Fig. 3F), a potent amiloride analog and ASIC blocker, while the plateau phase was left untouched. We repeated these experiments in U251 glioma cells with similar effects (Fig. 3D). By contrast, cultured astrocytes did not show significant pHe-sensitive changes in Vm (+0.328 ± 0.34 and −1.67 ± 1.2 mV for acidic and alkaline pHe shifts, respectively; Fig. 3E). Thus pHe changes translate into changes in transmembrane current density of malignant, but not normal, glial cells.
Fig. 3.
Glioma cells display a pHe-sensitive whole cell conductance. A: voltage-clamp recordings from D54 human glioma cells in pHe 6.0, 7.4, and 8.8 bath solutions. B: pHe-sensitive currents after barrel application of pHe 6.0 or 8.8 from a control of pHe 7.4. C: representative traces of whole cell currents from D54 glioma cells in pHe 6.0, 7.4, and 8.8 bath solutions. D: whole cell current-clamp recordings from representative D54 and U251 human glioma cells, along with cultured cortical astrocytes (6.0A and 8.8A). E: quantification of pHe-sensitive current-clamp depolarizations and hyperpolarizations in D54 glioma cells and cortical astrocytes. F: representative current-clamp trace of D54 glioma cell with reversible acid-sensing ion channel blockade using 10 μM benzamil. *P < 0.05; **P < 0.01; ***P < 0.001.
pHe-modulated Vm in glioma cells is K+-dependent.
To determine the ion(s) responsible for these pHe-sensitive changes in glioma cells, we first repeated the current-clamp findings in normal (5 mM) and high (25 mM) [K+]o conditions (Fig. 4). We hypothesized that these biophysical changes were especially dependent on the Nernst potential for K+ (EK), with channels opening at high pHe (and driving the cell toward EK) and closing at low pHe (and driving the cell away from EK). Consistent with this hypothesis, the hyperpolarizations induced by high pHe were largely ablated by high [K+]o relative to the control baseline (−0.402 ± 2.0 mV; Fig. 4, A and B), which shifts EK from −87 to −49 mV, while the acidic pHe-dependent depolarizations remained intact (+20.22 ± 1.4 mV). We additionally stepped from each pHe condition to its respective high [K+]o (Fig. 4C) and found that the majority of the conductance at pHe 8.8 was K+-mediated (35.4 ± 1.3 mV depolarization) as opposed to that at pHe 6.0 (4.47 ± 0.30 mV depolarization, P < 0.001 vs. pHe 8.8). Thus extracellular protons evoke large shifts in the basal K+ permeability of glioma cells.
Fig. 4.
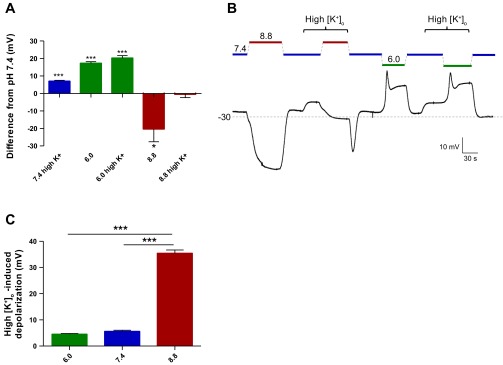
pHe-sensitive conductance is extracellular K+ concentration ([K+]o)-dependent. A: quantification of pHe-dependent changes in current-clamp mode in normal (5 mM) or high (25 mM) [K+]o in D54 glioma cells (n = 6, 2-tailed t-test vs. pHe 7.4 with normal [K+]o). B: representative current-clamp trace of D54 glioma cell switched to high-[K+]o pHe 6.0, 7.4, or 8.8 bath. C: depolarizations in current-clamp mode from each pHe condition induced by a step to high (25 mM) [K+]o (n = 5, 1-way ANOVA with Tukey-Kramer post test). *P < 0.05; ***P < 0.001.
pHe modulations of Vm are inhibited by K+ channel blockers.
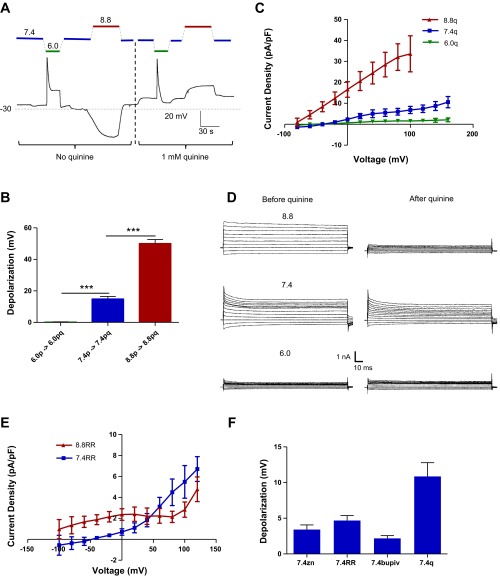
Next we sought to pharmacologically inhibit this pHe-sensitive permeability (Fig. 5). We assayed a variety of inhibitors previously known to inhibit pHe-sensitive K+ channels (21). We first tested drugs in current-clamp mode, where our most robust Vm response was from 1 mM quinine HCl (Fig. 5A). When applied in the bath, quinine completely prevented the acid-induced depolarizations and turned the alkaline-induced hyperpolarizations into slight depolarizations. To isolate quinine-sensitive current density and voltage changes, D54 glioma cells were preincubated in bath solutions of varying pHe for ≥2 h prior to recording. We captured larger pHe-sensitive currents in voltage-clamp mode (Fig. 5, C and D) at alkaline pHe (+1.56 ± 0.71 pA/pF at pHe 6.0 and +33.6 ± 8.6 pA/pF at pHe 8.8 at +100 mV). Additionally, quinine depolarized cells incubated in alkaline pHe to a far greater extent than those incubated in acidic pHe in current-clamp mode (Fig. 5B; +0.100 ± 0.23 mV at pHe 6.0 and +50.1 ± 2.62 mV at pHe 8.8), consistent with the hypothesis that this quinine-sensitive channel contributes to the pHe-sensitive Vm.
Fig. 5.
Inhibition of pHe-sensitive K+ conductance. A: current-clamp trace of D54 glioma cells tracking membrane potential (Vm) during modulation of pHe and 1 mM quinine via barrel application. B: quantification of quinine-induced depolarizations in cells bathed in pHe 6.0 (6.0p), pHe 6.0 + 1 mM quinine (6.0pq), pHe 7.4 (7.4p), pHe 7.4 + 1 mM quinine (7.4pq), pHe 8.8 (8.8p), and pHe 8.8 + 1 mM quinine (8.8pq). C: quinine-sensitive currents from voltage-clamp recordings of D54 glioma cells. D: representative voltage-clamp traces before and after 1 mM quinine application. E: ruthenium red (RR)-sensitive currents from voltage-clamp recordings of D54 glioma cells. F: drug-induced depolarizations from 100 μM zinc (zn), 200 μM ruthenium red, 1 mM bupivacaine (bupiv), and 1 mM quinine (q). ***P < 0.001.
However, we did not find that cells at pHe 6.0 were depolarized at baseline. In our current-clamp experiments where cells were preincubated at pHe 6.0 prior to patching, the average resting Vm was −39.83 ± 1.03 mV (n = 5); yet these cells were also completely resistant to subsequent quinine depolarization (Fig. 5B). It is therefore possible that some cells at this low pHe readjusted their Vm by closing Cl− conductance (ECl = 0 mV in our experiments) to compensate for the closed K+ channels, thus allowing them to maintain a less-depolarized Vm but still leaving them incapable of further subsequent depolarization.
This aforementioned conductance is markedly different from the previously described tonic ASIC current in D54 glioma cells (20), which was not significantly changed when the cells were incubated in different pHe baths. In conclusion, glioma cells express pHe-sensitive K+ currents that translate pH changes into changes in Vm, and these are blocked by quinine and acidic pHe.
Quinine depolarizes glioma spheroids.
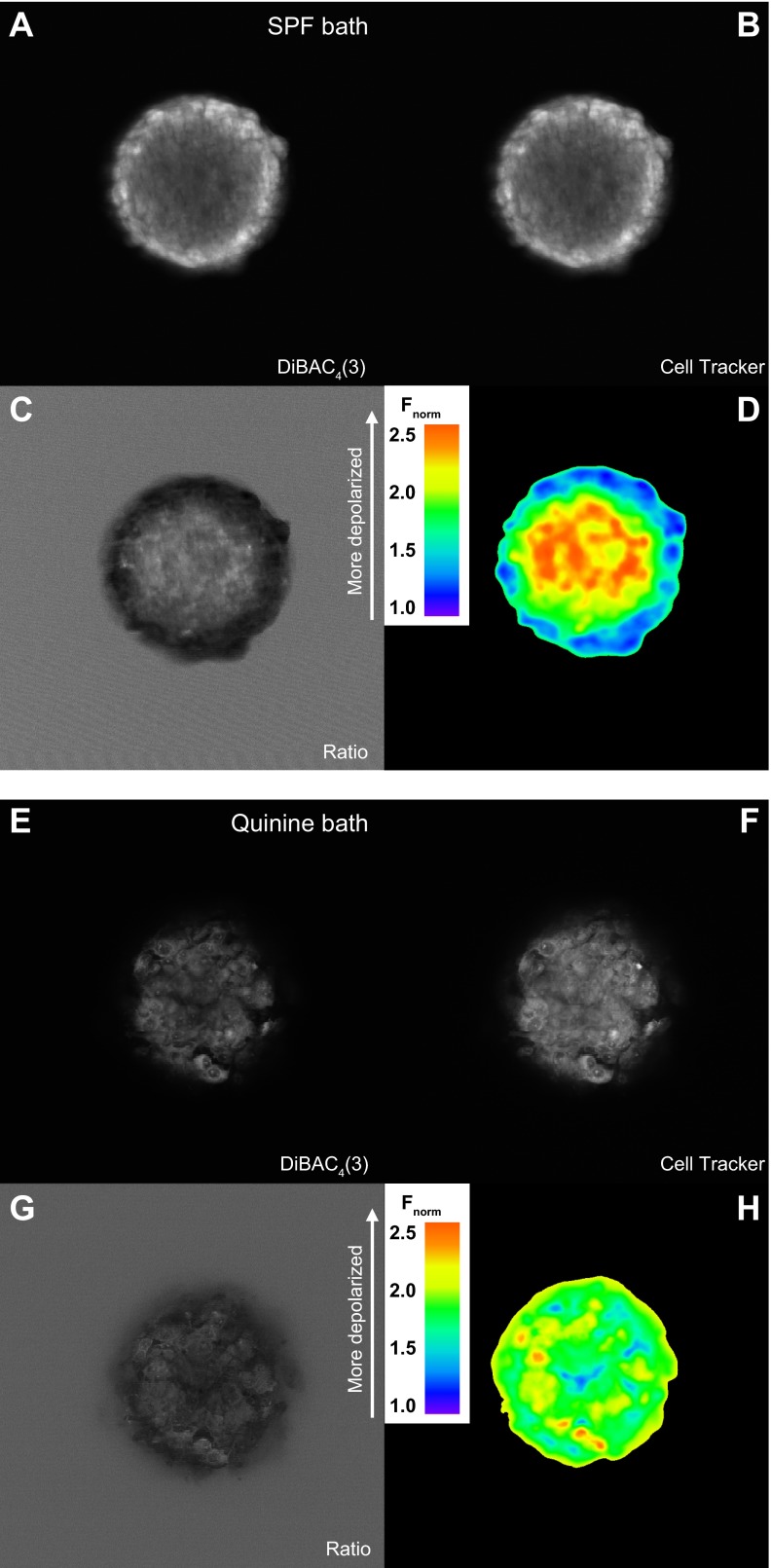
Since isolated glioma cells demonstrate a pHe dependence of Vm, we next tested if this held up when cells formed three-dimensional tumors (Fig. 6). U251 glioma spheroids were loaded with the slow-response voltage-sensitive dye DiBAC4(3). This bis-oxonol potentiometric dye enters and fluoresces within depolarized cells but is excluded from the mitochondrial membrane because of its negative charge. Additionally, it sacrifices speed for sensitivity, allowing even small changes in Vm to be accounted for. As this is a nonratiometric Vm indicator, we coloaded and normalized cells to the fluorescent labeler CellTracker Orange. Under control conditions and in the absence of any drug, we noticed a gradient of Vm that mimicked those of pHe and cell proliferation (Fig. 6, A–D); the rim of the spheroid displayed an alkaline environment rich in hyperpolarized and prolific cells, while the core contained cells of depolarized potentials and limited proliferation.
Fig. 6.
Quinine depolarizes glioma spheroids. A–D: confocal cross-section image of a U251 glioma spheroid coloaded with the voltage-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3); A] and the cell-tracking dye CellTracker Orange (B). Images were normalized to CellTracker Orange (Fnorm; C) and then falsely colored (D). E–H: confocal imaging of a U251 spheroid preincubated for 2 h with 1 mM quinine. E: voltage-sensitive channel; F: cell-tracker channel; G: normalization to cell-tracking dye; H: ratiometric coloring.
From here, we hypothesized that if this Vm gradient is caused by a pHe gradient, application of quinine should disrupt that relationship. We preincubated these spheroids for 2 h in 1 mM quinine HCl along with the coloaded Vm and cell-tracking dyes, allowing ample time for diffusion of drug beyond the tumor rim. Our prediction was that quinine would homogenize cellular Vm, clamping all quinine-affected cells to a similarly depolarized state in a manner similar to that observed in the current-clamp experiments (Fig. 5B). Consistent with this hypothesis, Fig. 6, E–H, shows a pronounced depolarization of the rim tumor spheroid cells, while the core cells remain similarly depolarized. This parallels quinine's effect on the monolayer glioma cells demonstrated previously: at acidic pHe, quinine shows little depolarization effect, and the magnitude of the effect grows in conjunction with a more alkaline pHe. Thus gliomas demonstrate exquisite sensitivity to the pHe environment directly surrounding them and modulate their Vm accordingly.
pHe-sensitive K+ conductance affects glioma cell proliferation.
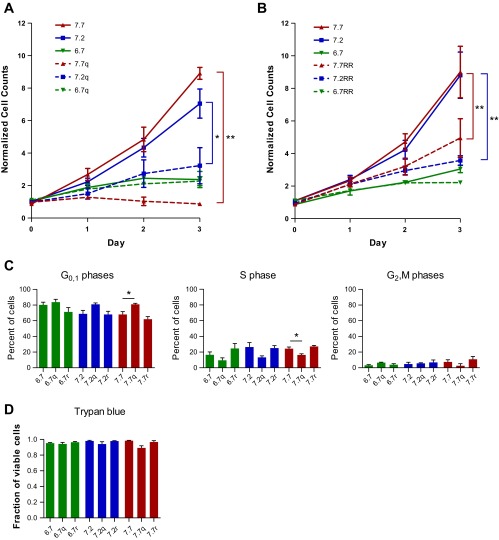
Given that the less-proliferating cells of the core sat at a more depolarized state and in a more acidic pHe environment, we wanted to establish a possible link between a glioma cell's pHe environment, its Vm, and its proliferative state. We repeated the Coulter counter studies with the additional manipulation of pharmacological inhibition from 100 μM quinine (Fig. 7). We hypothesized that, in acidic pHe conditions, cell proliferation should be low and quinine application should have no additional effect. On the other hand, in alkaline pHe conditions, cell proliferation should be high, but this should be blocked by the strong depolarization from quinine. Quinine was chronically applied, and media were replaced daily over the course of the experiment; consistent with our hypothesis, quinine showed a larger effect at higher pHe (Fig. 7A). At pHe 6.7, there was no significant decrease in cell counts at day 2 relative to control (95.7% of control, P = 0.90); in contrast, there was a significant reduction in cell counts at pHe 7.2 (45.8% of control, P < 0.05) and pHe 7.7 (9.7% of control, P < 0.01). We additionally used a second inhibitor from our screen of K+ channel blockers, ruthenium red (20 μM; Fig. 5F), which yielded pHe-sensitive results similar to those of quinine (Fig. 7B).
Fig. 7.
pHe-sensitive conductance modulates glioma cell cycle. A and B: Coulter counter results comparing drug inhibition of proliferation at pHe 6.7, 7.2, and 7.7. Drug conditions were compared with their respective pHe controls at day 2 (n ≥ 3; 2-sample t-tests). C: percentage of cells in different phases of the cell cycle as quantified by propidium iodide intensity via fluorescence-activated cell sorting. Cells were kept in 10-cm dishes for 2 days without media change. Drug conditions were compared with their drug-free counterparts (n ≥ 3; 2-sample t-tests). D: Trypan blue staining for cell viability after 2 days in each condition. Drug columns were compared with their respective drug-free pHe control (n = 3; 2-sample t-tests). Quinine (q) and ruthenium red (r) concentrations were 100 μM and 20 μM, respectively, for all experiments. Media were changed daily for the Coulter counter studies.
A decrease in cell count could be due to a decrease in cell proliferation or a decrease in cell viability. To separate these phenomena, we first tested cell viability with a Trypan blue stain (Fig. 7D). Trypan blue is a membrane-impermeant dye that is incorporated only in cells with compromised membranes. We found no significant difference in Trypan blue inclusion between control and drug conditions at any pHe (P > 0.05), indicating that the drugs might, instead, be having the bulk of their effect on cell proliferation. Thus the K+ channel inhibitors that demonstrated pHe-dependent depolarization of glioma cells also showed a pHe-dependent effect physiologically, with a greater growth inhibition at more alkaline pHe.
pHe-sensitive K+ conductance affects glioma cell G1-to-S phase transition.
Prior studies have especially implicated K+ channels as necessary for membrane hyperpolarization during the G1-to-S phase transition of the cell cycle (4, 26). We thus performed a complete cell cycle analysis using a PI stain and fluorescence-activated cell sorting. PI incorporates in DNA, and a cell's fluorescence intensity is dependent on the number of copies of DNA contained in that cell, thus allowing a more complete picture of cell cycle status from a larger population of cells. If this pHe-sensitive K+ conductance provided a necessary hyperpolarization from the G1 to the S phase, we would expect a clustering of cells in the G1 phase of the cell cycle with pharmacological inhibition, especially at higher pHe. In agreement with this hypothesis, we found that glioma cells were significantly increased in the G0/1 phase (81.0 ± 1.3% vs. 68.1 ± 3.4%, P < 0.05) and significantly decreased in the S phase at pHe 7.7 (16.4 ± 1.4% vs. 24.3 ± 2.1%, P < 0.05) in quinine vs. control conditions (Fig. 7C). In conclusion, the aforementioned pHe-sensitive, quinine-sensitive K+ glioma cell conductance specifically affects the G1-to-S phase transition of the glioma cell cycle.
DISCUSSION
In light of the widely held notion that cancers, in general, exhibit the Warburg effect (12), whereby tumors grow anaerobically and extrude lactate and protons even in the presence of O2, we asked whether this establishes a pHe gradient across a tumor mass and whether this could result in altered cell growth across the tumor.
Using glioma spheroids as a tumor model system, we were able to show that a homogeneous tissue mass, which presumably originated by clonal expansion from a single cell, is able to establish and maintain a pHe gradient where the core is highly acidic and the rim is alkaline. Importantly, we show that cells grown in this gradient adjust their rate of proliferation proportionally, with lowest growth in the acidic core and highest growth on the alkaline rim. Furthermore, using single-cell electrophysiology, we were able to identify a pHe-sensitive K+ conductance that likely acts as an environmental pH sensor, translating changes in pHe into changes in Vm.
Spheroids were preincubated in the bulk pHe solution 2 h prior to recording. It is likely that several factors are at play in the maintenance of this pH gradient: 1) cell coupling and bulk cellular presence forming a barrier for molecular clearance, 2) a gradient of protein expression compartmentalizing proton formation, uptake, and extrusion and maintaining a proton flux steady state (15), and 3) carbonic anhydrases acting as catalysts of “facilitated diffusion” by accelerating the clearance of acid from the spheroidal core (33). It is also very likely that these data would be recapitulated even in a bicarbonate-buffered environment. Work in colon carcinoma spheroids has demonstrated a role for carbonic anhydrase 9 in facilitating diffusion of the pH gradient within the tumor spheroids, and thus the most likely differences between a HEPES and a bicarbonate environment would be the rate of diffusion of protons and the subsequent steepness of the gradient (33). The actual formation of the spheroidal pHe gradient occurred in bicarbonate-buffered DMEM/F-12.
These findings are a significant extension of prior observations in the literature (1, 6, 32). The presence of pH gradients across gliomas has been previously shown; however, these studies often included inputs from the tumor cells themselves and the surrounding stroma or failed to provide the same cellular resolution of interstitial pH as our voltage-sensitive dye experiments. Furthermore, core tissue necrosis has often been ascribed to a loss of nutrient supply in the core (7). Our studies were carried out in the absence of vasculature, yet in the presence of ample media-derived nutrients; thus we can directly ascribe the graded growth pattern to the endogenously generated pH environment.
Acid-mediated depolarization of glioma cells might contribute to the current model of tumor core necrosis, which already includes nutritive exhaustion, cytokine release, and reactive oxygen species production (7). While these many mechanisms of core necrosis occur simultaneously in vivo, we show an acid-induced cell cycle arrest that is independent of oxygen and nutrient levels, as even in the presence of ample nutrients and cultured in a monolayer, glioma cells showed markedly attenuated growth at acidic pHe. Conversely, these cells are capable of thriving in supraphysiologically alkaline pHe conditions and may, in fact, rely on this alkaline pHe to allow them to hyperpolarize and pass the G1-to-S checkpoint of the cell cycle.
Our results are consistent with those from previous studies characterizing glioma cells as relatively depolarized compared with other glial cells (25). This is characteristic of highly proliferative cells; however, there still exist cell cycle checkpoints that require transient changes in Vm (2). As a result, it appears that glioma cells are depolarized en masse, yet they cannot proliferate if clamped to this depolarized potential. In this vein, we found an inhibitor of the pHe-dependent hyperpolarization in quinine that forcibly depolarized glioma cells in a pHe-dependent manner. Application of quinine in combination with a varied pHe allowed us to isolate the input of this pHe-sensitive K+ conductance from the other pHe-dependent cell cycle machinery at play. Incidentally, quinine has served as treatment for malaria for centuries, is inexpensive, and is well tolerated in high doses in humans, making it therapeutically relevant.
While our studies were able to unequivocally identify a K+ conductance as a pHe sensor for glioma cells, we were not able to definitively implicate any specific underlying channel(s). However, several candidates, especially members of the two-pore K+ channel family, fit our observation of a pHe-sensitive basal K+ conductance and will serve as targets in future studies (21). The most likely candidates given the sensitivities to quinine and ruthenium red are TASK-1 and TASK-3, both of which have been implicated in glioma cell physiology (24). Importantly, the kinetics, pharmacology, activation properties, and ion permeability of the K+ conductance observed in the present study are a poor fit for previously shown pHe-sensitive glioma channels, including ASICs and transient receptor potential (TRP) channels. Our resultant K+ conductance was not cation-nonspecific, unlike many of the pHe-sensitive ASICs (3). Additionally, K+ conductance was modulated by the same pathophysiological range of pHe created by the glioma cells and, unlike many of the ASICs and TRP channels, activation does not rely on extremely high acidity levels (pHe < 5.0) (13, 35). Finally, it is very possible that a residual conductance usually masked by the large quinine-sensitive basal current is unmasked in the quinine-bathed conditions. Glioma cells express many channels, and several of them, including ClC-3 channels, TRP channels, and P2X receptors, have demonstrated pH sensitivity (18).
Given the nature of our assay, we were able to only study tumor growth. In the brain, however, there exists a balance of growth and tumor invasion. Hence, it is possible that the pHe-dependent growth observed in the present study mediates an important switch between the growth and invasion phases of the tumor: tumor growth is favored by alkaline pHe, as observed, whereas tumor invasion may be aided by acidic pHe. Recent studies have highlighted the idea of “acid-mediated invasion” of solid tumors, with the most acidic tumor edge being the most predisposed to invasion (6). In our study, we sought to isolate the role of proton concentration in the tumor cell cycle independent of its role in tumor invasion. Future studies should replicate our findings in an environment where growth and invasion are amendable to study, for example, growth in cultured tissue slices.
Despite some of the limitations pointed out above, our findings suggest that gliomas possess an environmental sensor for pH and adjust their growth rate accordingly in a feedback manner. In light of this, it may be possible to disrupt this sensor, as we have done using quinine, and this, in turn, may be sufficient to disrupt tumor growth in vivo. Finally, while our studies were done in gliomas, it is possible that other solid tumors behave in the same way and that the pHe-dependent growth gradient observed in the present study may apply more broadly to other systemic cancers.
GRANTS
This research was funded by National Institutes of Health Grants R01 NS-031234 and R01 NS-036692 (to H. Sontheimer), P30 NS-047466 (to the UAB Neuroscience Molecular Detection Core), and 5P30 AR-048311 and 3P30 AI-027767-22S1 (to the UAB Comprehensive Flow Cytometry Core).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.H. and H.S. are responsible for conception and design of the research; A.H. and K.A.S. performed the experiments; A.H. and K.A.S. analyzed the data; A.H., K.A.S., and H.S. interpreted the results of the experiments; A.H. prepared the figures; A.H. drafted the manuscript; A.H. and H.S. edited and revised the manuscript; A.H., K.A.S., and H.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the University of Alabama at Birmingham (UAB) Neuroscience Molecular Detection Core, namely, Terry L. Lewis, Gretta Jordan, and Jamie McNaught, for help with spheroid paraffin embedding and sectioning. We also thank the UAB Comprehensive Flow Cytometry Core and Enid Keyser for help with cell cycle analysis.
REFERENCES
- 1.Acker H, Carlsson J, Holtermann G, Nederman T, Nylen T. Influence of glucose and buffer capacity in the culture medium on growth and pH in spheroids of human thyroid carcinoma and human glioma origin. Cancer Res 47: 3504–3508, 1987 [PubMed] [Google Scholar]
- 2.Becchetti A. Ion channels and transporters in cancer. I. Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol 301: C255–C265, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, McBain CJ, Gallo V. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci USA 99: 2350–2355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuddapah VA, Habela CW, Watkins S, Moore LS, Barclay TT, Sontheimer H. Kinase activation of ClC-3 accelerates cytoplasmic condensation during mitotic cell rounding. Am J Physiol Cell Physiol 302: C527–C538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, Gillies RJ. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73: 1524–1535, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta 1757: 1371–1387, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, Weil RJ, Nakano I, Sarkaria JN, Stringer BW, Day BW, Li M, Lathia JD, Rich JN, Hjelmeland AB. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci 16: 1373–1382, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Martin ML, Herigault G, Remy C, Farion R, Ballesteros P, Coles JA, Cerdan S, Ziegler A. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Res 61: 6524–6531, 2001 [PubMed] [Google Scholar]
- 10.Garcia-Martin ML, Martinez GV, Raghunand N, Sherry AD, Zhang S, Gillies RJ. High resolution pHe imaging of rat glioma using pH-dependent relaxivity. Magn Reson Med 55: 309–315, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer 8: 56–61, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4: 891–899, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Glitsch M. Protons and Ca2+: ionic allies in tumor progression? Physiology (Bethesda, MD) 26: 252–265, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grillon E, Farion R, Fablet K, De Waard M, Tse CM, Donowitz M, Remy C, Coles JA. The spatial organization of proton and lactate transport in a rat brain tumor. PLos One 6: e17416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas BR, Sontheimer H. Inhibition of the sodium-potassium-chloride cotransporter isoform-1 reduces glioma invasion. Cancer Res 70: 5597–5606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ 18: 829–840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honasoge A, Sontheimer H. Involvement of tumor acidification in brain cancer pathophysiology. Front Physiol 4: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakubovicz DE, Grinstein S, Klip A. Cell swelling following recovery from acidification in C6 glioma cells: an in vitro model of postischemic brain edema. Brain Res 435: 138–146, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem 284: 24526–24541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotshaw DP. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys 47: 209–256, 2007 [DOI] [PubMed] [Google Scholar]
- 22.McFerrin MB, Turner KL, Cuddapah VA, Sontheimer H. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am J Physiol Cell Physiol 303: C1070–C1078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am J Physiol Cell Physiol 278: C676–C688, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Meuth SG, Herrmann AM, Ip CW, Kanyshkova T, Bittner S, Weishaupt A, Budde T, Wiendl H. The two-pore domain potassium channel TASK3 functionally impacts glioma cell death. J Neurooncol 87: 263–270, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia 46: 63–73, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol 221: 1–6, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Oudard S, Boitier E, Miccoli L, Rousset S, Dutrillaux B, Poupon MF. Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res 17: 1903–1911, 1997 [PubMed] [Google Scholar]
- 28.Pappas CA, Ransom BR. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol 72: 2816–2826, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Philippe JM, Dubois JM, Rouzaire-Dubois B, Cartron PF, Vallette F, Morel N. Functional expression of V-ATPases in the plasma membrane of glial cells. Glia 37: 365–373, 2002 [PubMed] [Google Scholar]
- 30.Rooj AK, McNicholas CM, Bartoszewski R, Bebok Z, Benos DJ, Fuller CM. Glioma-specific cation conductance regulates migration and cell cycle progression. J Biol Chem 287: 4053–4065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem 105: 287–295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staub F, Baethmann A, Peters J, Weigt H, Kempski O. Effects of lactacidosis on glial cell volume and viability. J Cereb Blood Flow Metab 10: 866–876, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem 284: 20299–20310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11: 671–677, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci 12: 495–508, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Fukumura D, Jain RK. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: mechanism of low pH-induced VEGF. J Biol Chem 277: 11368–11374, 2002 [DOI] [PubMed] [Google Scholar]