Abstract
The lens is proposed to have an internal microcirculation system consisting of continuously circulating ionic fluxes that play an essential role in maintaining lens transparency. One of the key components of this system is the sodium leak conductance. Here we investigate the contribution of Cx46 hemichannels to the basal membrane permeability of peripheral fiber cells isolated from transgenic mouse lenses lacking Cx50 or both Cx50 and Cx46 (dKO) using the whole cell patch-clamp technique. Our results show that Cx46 hemichannels were largely closed at a resting voltage of −60 mV in the presence of millimolar divalent cation concentrations. However, even though the vast majority of these channels were closed at −60 mV, a small, persistent, inward current could still be detected. This current could be mostly blocked by exposure to 1 mM La3+ and was not observed in fiber cells isolated from dKO mouse lenses suggesting that it was due to Cx46 hemichannels. In addition, Cx50−/− fiber cells showed increased open channel noise and a depolarized resting potential compared with dKO fiber cells. Exposure of Cx50−/− fiber cells to La3+ hyperpolarized the resting potential to −58 mV, which is similar to the value of resting potential measured in dKO fiber and significantly reduced the open channel noise. In conclusion, these results suggest that Cx46 hemichannels may contribute to the sodium leak conductance in lens fiber cells.
Keywords: connexin, Cx46, lens, hemichannel
according to the fluid circulation system model proposed by Mathias (reviewed in Ref. 15), the lens has an internal circulatory system which plays a critical role in maintaining lens homeostasis. This circulatory system consists of continuously circulating sodium fluxes (and associated fluid), which flow into the lens along the narrow extracellular spaces between the fiber cells. As sodium moves toward the center of the lens, it is continuously entering the fiber cells through sodium leak channels. Once inside the fiber cell, sodium flows toward the surface of the lens via an intracellular pathway involving gap junctions. The surface epithelial cells contain large numbers of Na+-K+-ATPases that then pump sodium out of the lens.
Gap junctions play an important role in this circulation system. Gap junctions are composed of cell-to-cell channels that allow the flow of monovalent ions and other small molecules between neighboring cells. These channels are formed by the docking of two oligomeric subunits called hemichannels or connexons, each of which is located in the plasma membrane of closely apposed cells. Hemichannels can also exist as large, relatively nonselective ion channels in single plasma membranes (5). Vertebrate gap junctions are composed of a family of proteins called connexins with 21 human and 20 mouse members (31). Three connexins have been identified in the lens: Cx43, Cx50, and Cx46. These connexins show differences in their pattern of distribution within the lens. Cx43 is present only in the epithelium (20), whereas Cx46 is expressed exclusively in the fiber cells (21, 30). Cx50 is expressed in both the epithelium and in fiber cells (4, 24, 29).
Another important component of the lens microcirculation system is the sodium leak conductance. The molecular identity of this conductance is still unclear. However, one potential candidate is the undocked connexin hemichannel (16). There is increasing evidence that connexin hemichannels can open under certain physiological conditions without effecting cell viability. For example, Shahidullah et al. (26) showed that hypotonic stress caused the release of ATP in the porcine lens by a pathway that involves connexin and/or pannexin hemichannels. In addition to its role in healthy cells, aberrant hemichannel activity has been linked to several human hereditary disorders such as KIDS syndrome (9, 13, 17, 18) and cataracts (19, 23).
We previously showed that Cx50−/− fiber cells lacking Cx50 exhibited a large, nonselective current that was activated by reducing external divalent cations (7). This current was absent in fiber cells isolated from dKO mouse lenses lacking both Cx50 and Cx46 confirming that it was due to Cx46 hemichannels. Here, whole cell current-clamp recordings of freshly dissociated fiber cells from transgenic mouse lenses were used to investigate the hypothesis that Cx46 hemichannels may contribute to the sodium leak conductance observed in the intact lens. Our results suggest that Cx46 channels make a significant contribution to the resting membrane conductance of peripheral fiber cells even in the presence of extracellular divalent cations.
MATERIALS AND METHODS
Transgenic mice.
Cx50−/− mice were a generous gift from TW White. dKO mice were generated as previously described by Ebihara et al. (7). All of the mice were in a C57 genetic background. All animal husbandry and experimental procedures were approved by the Rosalind Franklin University Animal Care and Use Committee and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Dissociation of differentiating lens fiber cells.
Mice (4–8 wk old) were killed by CO2 asphyxiation and cervical dislocation. The lenses were dissected free from the extracted eyes and placed in M199 with Earles's balanced salts for 30 min. Typically, four lenses were used for each dissociation. We observed that it was important to keep the lens continuously submerged in fluid during and following removal from the eye to avoid damage to peripheral fiber cells. The lenses were then transferred to dissociation buffer (DB; in mM: 170 Na-gluconate, 4.7 KCl, 5 glucose, and 5 HEPES, pH 7.4). Adherent nonlens material was carefully removed from the lens with a pair of forceps. A posterior tear was made in the lens capsule, and the capsule was removed. The epithelial cells and strands of elongating fiber cells remained attached to the capsule. The capsule and adherent epithelial and fiber cells were incubated in DB containing 0.0625% collagenase (type IV; Worthington Biochemical, Lakewood, NJ) and 0.025% protease (type XXIV; Sigma-Aldrich, St. Louis, MO) at room temperature for 15 to 20 min. The capsule was washed once with DB and resuspended in DB. The epithelial and young fiber cells were then mechanically removed from the capsule by trituration with a fire-polished Pasteur pipette. The cells were then pelleted (1,000 g for 2 min) and resuspended in DB. The isolated cells were used immediately for patch-clamp and imaging experiments.
Electrophysiological recording and analysis.
Membrane currents were recorded in dissociated fiber cells using the whole cell patch-clamp technique as previously described by Ebihara et al. (7). The resistance of the patch pipettes was 5 to 10 MΩ when filled with standard internal solution. The internal solution contained the following (in mM): 140 CsCl or 140 KCl, 10 EGTA, 2 MgATP, 3 Na2ATP, 10 HEPES-Na, pH 7.4, and osmolarity 310–320 mosM. The standard extracellular solution was Na-gluconate Ringer that contained the following (in mM): 150 Na-gluconate, 4.7 KCl, 2 MgCl2, 5 glucose, 5 HEPES, pH 7.4, and osmolarity 310–320 mosM. The osmolarity was measured using a freezing point micro-osmometer (5004 Micro-osmette; Precision Systems, Natick, MA). All the membrane potentials in the graphs were corrected for liquid-junction potentials after the experiment using the “junction potential calculator” interface (Clampex version 10.2; Molecular Devices). The voltage-clamp protocols were not corrected for the liquid junction potential. They represent the command potentials that were applied to the patch pipette. Measurements of cell membrane parameters in response to 5-mV voltage-clamp pulses from −60 mV were performed using the Membrane test protocol in Clampex immediately following patch rupture. The data are given as means ± SE. N represents the number of fiber cells interrogated. Data were analyzed for their statistical significance using the unpaired Student's t-test or the one-sample t-test.
RESULTS
Our results are based on data from 29 Cx50−/− fiber cells and 13 dKO fiber cells lacking both Cx50 and Cx46 that had capacitances that ranged from 21.4 to 123 pF, which corresponded to cell lengths between 85 and 531 μm (Table 1). There was a linear relationship between cell length and cell capacitance (data not shown). All of the fiber cells had nuclei indicating that they were isolated from the outer cortex. The absence of Cx50 and Cx46 in the dKO fibers was confirmed by immunolocalization (7).
Table 1.
Membrane properties of isolated fiber cells from Cx50−/− and dKO mouse
| Cell Type | Cm, pF | Rm, MΩ | Raccess, MΩ | n |
|---|---|---|---|---|
| Cx50−/− | 66.45 ± 3.39 | 448.00 ± 36.984* | 17.88 ± 1.16 | 29 |
| dKO | 73.43 ± 10.1 | 712.08 ± 79.52 | 15.79 ± 0.97 | 12 |
Data represent means ± SE. Membrane properties of fiber cells were measured immediately following patch rupture at −60 mV in the presence of 0 Ca2+ and 2 mM Mg2+. Cm, membrane capacitance; Rm, membrane resistance; Raccess, access resistance; dKO, mouse lenses lacking both Cx50 and Cx46.
P < 0.001, compared with dKO cells by Student's t-test.
We used the whole cell patch-clamp technique to study the effects of divalent cations on the nonselective cation current in Cx50−/− fiber cells that we previously identified as the Cx46 hemichannel current based on a combination of patch-clamp and dye uptake experiments in Cx50−/− and dKO fiber cells (7). Figure 1A shows a representative family of current traces and corresponding steady-state current-voltage (I–V) relationship recorded from a Cx50−/− fiber in the presence of zero-added Ca2+ (0 Ca2+) and 2 mM Mg2+. The pipette solution contained CsCl internal solution to prevent contamination by potassium currents. Application of depolarizing voltage-clamp steps from a holding potential of −60 mV elicited a slowly activating current that reversed polarity from inward to outward at ∼−10 mV. The threshold for activation of the current was approximately −50 mV. Following repolarization to −60 mV, the current closed to a value close to zero over a time course of several hundred milliseconds. Superfusion of the fiber cell with a solution containing 1 mM Ca2+ and 1 mM Mg2+ resulted in a significant reduction in the size of the hemichannel current (Fig. 1B). Both the inward current and the outward current were reduced. Another effect of changing external calcium was to alter the kinetics of activation and deactivation. Increasing calcium caused a slowing of activation and an acceleration of deactivation. These effects were very similar to those previously described in Xenopus oocytes expressing rat Cx46 hemichannels (6, 22, 27).
Fig. 1.
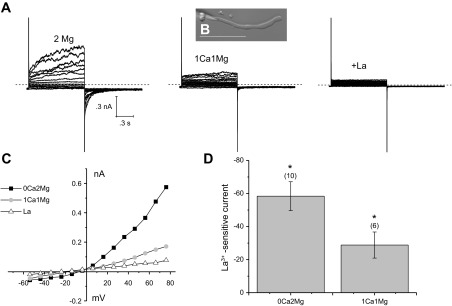
Effect of changing external calcium on membrane currents recorded from a Cx50−/− fiber cell using the whole cell patch-clamp technique. A: families of current traces were recorded from the fiber cell shown in B in the presence of 0 Ca2+ and 2 mM Mg2+ (left), after exposure to 1 mM Ca2+ and 1 mM Mg2+ (middle), and finally after exposure to 1 mM Ca2+, 1 mM Mg2+, and 1 mM La3+ (right). A series of depolarizing steps was applied in 10 mV increments between −60 and 80 mV from a holding potential of −60 mV. The intracellular pipette solution in these whole cell patch-clamp experiments contained 140 mM CsCl to block potassium currents. Dashed line represents zero current. Scale bar = 100 μm. C: current-voltage (I–V) relations obtained from the data shown in A. The current was measured at the end of the 8-s pulse and plotted as a function of voltage after correction for liquid junction potential. D: bar graph summarizing the La3+-sensitive current data at the holding potential in the presence of the indicated concentrations of Mg2+ and Ca2+. Data are means ± SE. Number of cells indicated by the values in parentheses. *P < 0.002, compared with the hypothesized population mean of 0.0 using the one-sample t-test.
To determine if the persistent inward current observed at negative membrane potentials was due to Cx46 hemichannels, we used the nonspecific hemichannel blocker, La+3 (3, 11). Application of 1 mM La3+ completely blocked the voltage- and time-dependent component of the current (Fig. 1C). It also reduced the inward holding current and baseline current fluctuations at −60 mV. By subtracting the current traces recorded in the presence of La3+ from the control current traces, La3+-sensitive currents could be isolated. The amplitude of the La3+-sensitive inward holding current measured in the presence of 1 mM Ca2+ and 1 mM Mg2+ ranged between −66 and −12 pA (n = 6). These results suggest that a small number of Cx46 hemichannels are active and contribute to the holding current and baseline current fluctuations at −60 mV even in the presence of normal concentrations of divalent cations.
To evaluate the relative contribution of potassium conductances and hemichannel conductances to the overall membrane conductance, we performed similar experiments using potassium chloride in the patch pipette. Application of 1 mM La3+ caused complete block of the Cx46 hemichannel current without appearing to affect the delayed rectifying K+ current indicating that its effects were reasonably specific for connexin hemichannels (Fig. 2). A similar effect was observed when we used 250 μM Gd3+ instead of 1 mM La3+ (data not shown).
Fig. 2.
La3+ blocks the Cx46 hemichannel current but not the potassium current. A: currents before and after the application of La3+ (1 mM) and the subtracted (La3+-sensitive) component recorded from a Cx50−/− fiber cell. Dashed line represents zero current level. B: I–V relations obtained from the data shown in A. The current was measured at the end of the 8-second pulse and plotted as a function of voltage after correction for liquid junction potential. C: bar graph summarizing the La3+-sensitive current data at −60 mV in the presence of the indicated concentrations of Mg2+ and Ca2+. Data are means ± SE. Number of cells indicated by the values in parentheses. *P < 0.005, compared with the hypothesized population mean of 0.0 using the one-sample t-test.
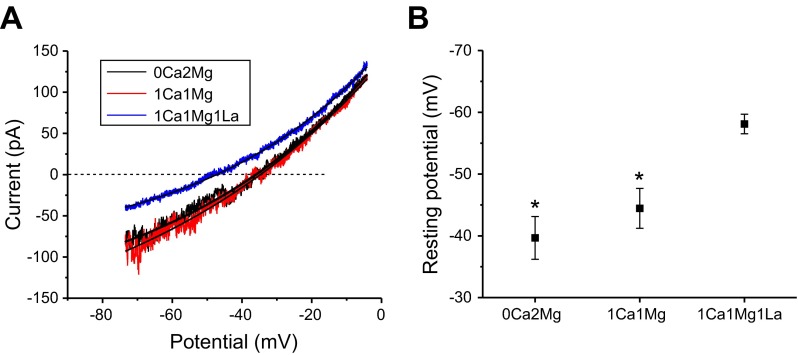
To investigate the effect of hemichannel conductances on the resting potential of the fiber cells, current recordings were obtained in response to 2-s voltage-clamp ramps from −80 to −10 mV from a holding potential of −60 mV, first in the presence of various concentrations of divalent cations and then after block of the hemichannel currents by La3+. Reversal potentials were measured by fitting the ramp currents to single exponentials and determining the zero crossing potential as illustrated in Fig. 3A. The results of these experiments are summarized in Fig. 3B. In the presence of 0 Ca2+ and 2 mM Mg2+, the mean resting potential was −39.7 ± 3.5 (n = 12). Perfusion with solutions containing 1 mM Ca2+ and 1 mM Mg2+ resulted in little or no change in resting potential. Subsequent perfusion with Na-gluconate Ringer containing 1 mM Ca2+ and 1 mM Mg2+ and 1 mM La3+ resulted in a significant shift in the resting potential toward the potassium equilibrium potential. The mean resting potential in the presence of La3+ was −58.1 ± 1.6 mV (n = 12). The deviation of the resting potential from the potassium equilibrium potential in the presence of La3+ can be accounted for by the pipette leak conductance. The resting membrane potential of the fiber cells did not depend on cell length. Fiber cells ranging from 50 to 600 μm showed very similar values for resting membrane potential. These results suggest that both potassium conductances and connexin hemichannel conductances contribute to the resting conductance of the peripheral fiber cells under physiological conditions.
Fig. 3.
Effect of La3+ on the resting membrane potential of Cx50−/− fiber cells. A: representative current responses to a voltage-clamp ramp protocol in the presence of 0 Ca2+, 2 Mg2+ (black current trace); 1 Ca2+, 1 Mg2+(red current trace); or 1 Ca2+, 1 Mg2+, 1 La3+(blue current trace). Smooth lines are exponential fits of the current response to determine the resting potential. B: plot summarizing the resting potential data determined using the voltage-ramp protocol shown in A after correction for liquid junction potential. Data are means ± SE (n = 12). *P < 0.001, compared with fiber cells treated with 1 mM La3+ by student's t-test.
Genetic ablation of Cx46 reduces the inward holding current and membrane noise under resting conditions.
To further examine the contribution of Cx46 hemichannels to the resting membrane conductance, we applied different concentrations of external divalent cations to fiber cells isolated from Cx50−/− and dKO mouse lenses and compared the effect that this had on the holding current measured at −60 mV. For these experiments, pipettes were filled with CsCl internal solution to minimize contamination by potassium channels. Figure 4A shows a representative example of an experiment performed on long fiber cell isolated from a Cx50−/− mouse lens that was continuously held at a holding potential of −60 mV. The bath solution initially contained 0 Ca2+ and 2 mM Mg2+. The fiber cell was then superfused with Na-gluconate Ringer containing no added divalent cations, which resulted a large increase in the inward holding current at −60 mV. This increase in holding current could be completely reversed by reperfusing the cell with Na-gluconate Ringer containing 2 mM Mg2+. Subsequent perfusion with Na-gluconate Ringer containing 1 Ca2+ and 1 mM Mg2+ caused a slight decrease in holding current.
Fig. 4.
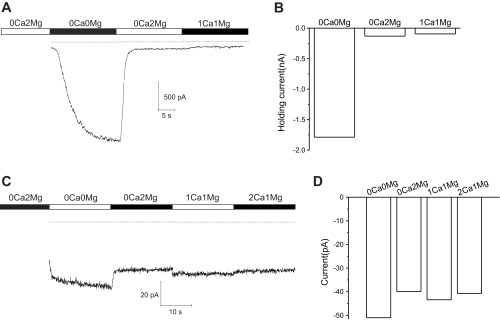
Cx50−/− fibers show a much larger increase in inward holding current in response to removal of external calcium than mouse lenses lacking both Cx50 and Cx46 (dKO) fibers. A: representative currents, obtained from a Cx50−/− fiber in response to changing external divalent cation concentrations to the indicated values. The fiber was continuously held at a holding potential of −60 mV throughout the experiment. The dashed line indicates zero current. B: bar graph summarizing the holding current data at −60 mV in the presence of different concentrations of Mg2+ and Ca2+ for the experiment shown in A. C: representative current response obtained from a dKO fiber at −60 mV in response to changing external divalent cations concentrations to the indicated values. D: bar graph summarizing the holding current data at −60 mV in the presence of different concentrations of Mg2+ and Ca2+ for the experiment shown in C.
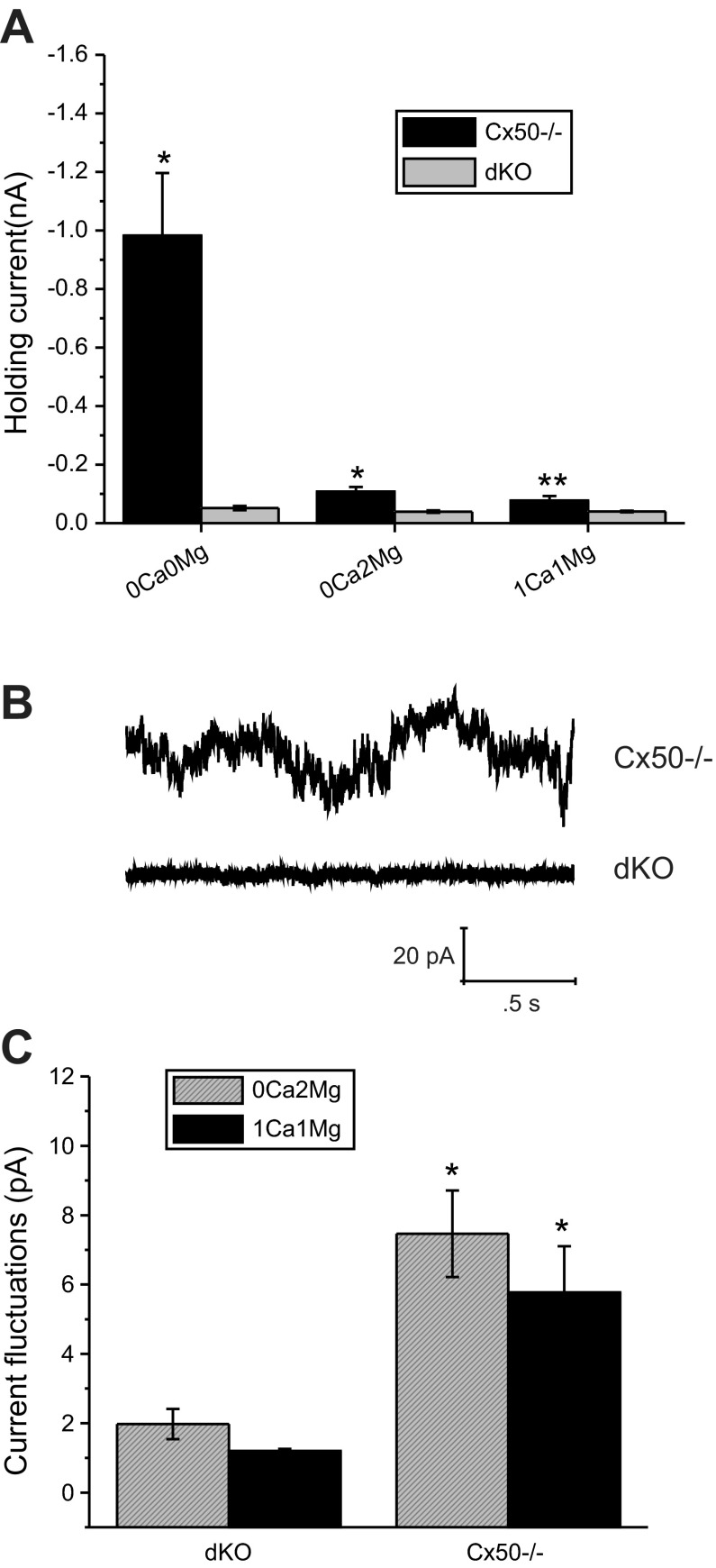
Figure 4B shows a representative example of an experiment performed on a fiber cell isolated from a dKO mouse lens using the protocol described above. Removal of external divalent cations caused a small but reproducible increase in the holding current at −60 mV that could be reversed by perfusing the cell with Na-gluconate Ringer containing 0 Ca2+ and 2 mM Mg2+. Subsequent perfusion with Na-gluconate Ringer containing 1 or 2 mM Ca2+ and 1 mM Mg2+ caused little or no change in the holding current. A population comparison of the holding current between Cx50−/− and dKO fibers (Fig. 5A) showed that in the absence of divalent cations, the mean inward holding current was ∼18 times larger in Cx50−/− cells than it was in dKO cells. A small but significant increase in inward holding current was also observed in the Cx50−/− cells in the presence of divalent cations. Cx50−/− fibers also showed a significantly increased level of baseline current fluctuations as illustrated in Fig. 5B, which shows sample current traces for representative fiber cells isolated from Cx50−/− and dKO lenses in the presence of 0 Ca2+ and 2 mM Mg2+. A population comparison of the current fluctuations showed an increase in mean current fluctuations in Cx50−/− fibers in the presence of divalent cations. These experiments suggest that although Cx46 hemichannels occurred less frequently in the presence of divalent cations, they were clearly present even at −60 mV.
Fig. 5.
dKO fibers show reduced inward holding current and basal current fluctuations at −60 mV. A: population response of holding current between Cx50−/− (black bars) and dKO fibers (gray bars). Data are means ± SE. Results are based on data obtained from 6 Cx50−/− fibers and 8 dKO fibers. *P < 0.001, compared with dKO by Student's t-test. **P < 0.01, compared with dKO by Student's t-test. B: sample current traces for representative fiber cells isolated from Cx50−/− and dKO lenses at −60 mV in the presence of 0 extracellular Ca2+ concentration and 2 mM extracellular Mg2+ concentration. C: population comparison of the basal current fluctuations at −60 mV showed an increase in mean current fluctuations in Cx50−/− fibers relative to dKO fibers in the presence of divalent cations. The size of the current fluctuations about the mean was quantified by calculating the standard deviation of a 1.5-s segment of the current trace. *P < 0.001, compared with dKO by unpaired Student's t-test.
Effect of La3+ on membrane conductances in dKO fibers.
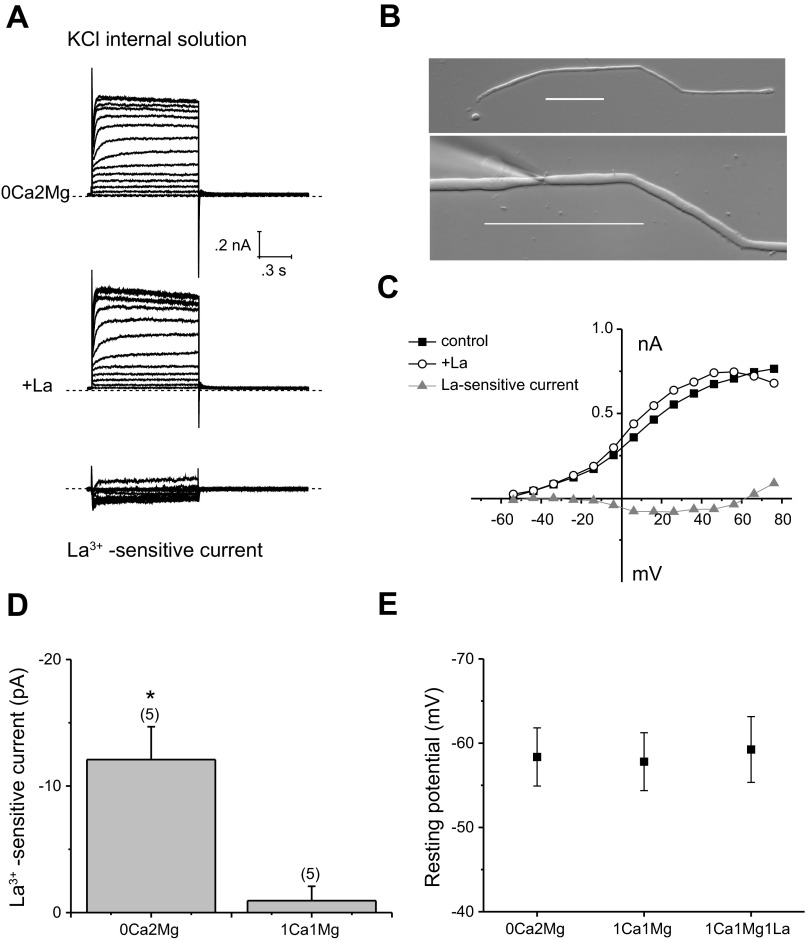
We were concerned that La3+ might be affecting other membrane channels in addition to connexin hemichannels. To test for this possibility, we examined the effect of La3+ on whole cell membrane currents in dKO fibers. Figure 6 shows whole cell currents recorded from a long, dKO fiber cell with KCl solution in the pipette. Under these conditions, the whole cell recording showed a prominent, delayed rectifying potassium current but lacked any indication of a connexin hemichannel current. Application of 1 mM La3+ had little or no effect on the membrane currents at potentials more negative than −20 mV. However, at more positive potentials, La3+ blocked a small, slowly activating inward current that appeared to reverse polarity at large, positive potentials. The voltage-gating properties of this current resembled those of the L-type calcium current, which is blocked by μM concentrations of La3+ (2, 12). Figure 6 summarizes the effect of La3+ on the amplitude of the holding current at −60 mV. No significant change in holding current was observed following application of La3+ in the presence of 1 mM [Ca2+]i. Furthermore, voltage-clamp ramp experiments showed that La3+ had no effect on the resting potential of the dKO fiber cells (Fig. 6).
Fig. 6.
La3+-sensitive currents in dKO fibers. A: currents before and after the application of La3+ (1 mM) and the subtracted (La3+-sensitive) component recorded from the dKO fiber cell shown in B. Dashed line represents zero current level. Scale bar = 100 μm. The internal pipette solution contained 140 mM KCl. The bath solution initially contained zero-added calcium and 2 mM Mg2+. C: I–V relations obtained from the data shown in A. The current was measured at the end of the 8-s pulse and plotted as a function of voltage after correction for liquid junction potential. D: bar graph summarizing the La3+ -sensitive current data at −60 mV in the presence of 0 Ca2+, 2 Mg2+ or 1 Ca2+, 1 Mg2+. Data are means ± SE. Number of cells indicated by the values in parentheses. *P < 0.01, compared with the hypothesized population mean of 0.0 using the one-sample t-test. E: plot summarizing the resting potential data after correction for liquid junction potential. Data are means ± SE (n = 5).
DISCUSSION
In this study, we addressed two questions. First, do Cx46 hemichannels contribute to the resting membrane conductance in Cx50−/− fiber cells? Second, can any additional sodium selective or nonselective cation conductances be detected by patch-clamp methods? Our results suggest that there are two main cation channels that contribute to the resting membrane conductance of the Cx50−/− fiber cells: potassium channels and Cx46 hemichannels. In the presence of normal concentrations of divalent cations, blocking Cx46 hemichannels using the nonspecific hemichannel blocker, La3+, resulted in a 20–30 pA reduction in the inward holding current at −60 mV (corresponding to 1 or 2 hemichannels) and a negative shift in the resting membrane potential toward the potassium equilibrium potential. A similar shift in the resting membrane potential was observed when connexin hemichannels were genetically deleted. Since the input conductance of fiber cells lacking connexin hemichannels was ∼1,400 pS, we would predict that if even 1 Cx46 hemichannel was open at −60 mV, it should be sufficient to cause an increase in input conductance and result in significant shift in the resting membrane potential as observed experimentally. In addition, we consistently observed that Cx50−/− fiber cells exhibited increased open channel noise at negative potentials compared with dKO fiber cells. This increase in open channel noise disappeared when the Cx46 hemichannel current ran down during prolonged patch-clamp experiments (data not shown) and was absent in Cx50−/− fibers treated with La3+ suggesting that it was due to the reopening of Cx46 hemichannels.
It has been proposed that a better candidate for the sodium leak conductance is a Gd3+-insensitive, La3+-sensitive conductance that is activated by cell shrinkage (10). However, we did not observe a significant La3+-sensitive, cation current in dKO mouse fibers at potentials more negative than −20 mV in the presence of millimolar Ca2+. Furthermore, the resting potential in the dKO fibers was insensitive to external divalent cations or La3+ suggesting that the effects of these agents in Cx50−/− fibers were primarily due to their blocking actions on Cx46 hemichannels. In addition, we did not observe cell shrinkage when dKO fibers were bathed in isotonic Na-gluconate Ringer. One source of variation between these studies could arise from species variations or alterations in the pattern of gene expression in transgenic mouse lenses lacking lens fiber connexins. Although we cannot completely rule out this possibility, the peripheral fiber cells isolated from the Cx50−/− and dKO lenses appeared to be remarkably normal in terms of their morphology in agreement with findings previously reported by Xia et al. (32) and White et al. (30). Furthermore, we did not detect any obvious functional differences between the dKO fibers and the Cx50−/− fibers except for the loss of connexin hemichannels.
Another potential source of variability comes from the methodology used to dissociate the fiber cells. In the studies reported by Gunning et al. (10), peripheral fiber cells were studied in the presence of the nonselective cation channel blocker Gd3+ to prevent fiber cell vesiculation and death. In contrast, we used lenses from transgenic mice that either lacked Cx50 or both Cx46 and Cx50 to reduce the size of the nonselective cation current activated during the dissociation process (7). Following cell dissociation, the fiber cells were maintained in Na-gluconate Ringer containing zero added calcium and variable concentrations of Mg2+ to prevent cell swelling and calcium loading. We also made several modifications in our dissociation protocol to improve the yield of healthy fiber cells. Instead of incubating the capsule in collagenase at 37°C, we incubated the capsule and adherent fiber cells in low concentrations of collagenase and protease at room temperature for 15 −20 min and then mechanically dislodged the fiber cells from the capsule by tituration. The main advantage of our approach is that it allows us to study connexin hemichannels, other mechanosensitive channels and calcium channels, all of which are blocked by Gd3+ (1, 2, 8, 14, 28).
In conclusion, our results suggest that Cx46 hemichannels may play an important role in the lens internal circulation system by allowing the entry of sodium from the extracellular space into the lens fiber cells. Much remains to be learned about how Cx46 hemichannels are regulated in the lens under both physiological and pathophysiological conditions. It is likely that many of the same factors that have been shown to regulate connexin hemichannels in other tissues (25) also regulate Cx46 hemichannels in the lens.
GRANTS
This work was supported by National Eye Institute Grant RO1-EY-10589 (to L. Ebihara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.E. conception and design of research; L.E., Y.K., and S.K. performed experiments; L.E., S.K., and J.-J.T. analyzed data; L.E. interpreted results of experiments; L.E. prepared figures; L.E. drafted manuscript; L.E., Y.K., S.K., and J.-J.T. edited and revised manuscript; L.E., Y.K., S.K., and J.-J.T. approved final version of manuscript.
REFERENCES
- 1.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 118: 2435–2440, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Beedle AM, Hamid J, Zamponi GW. Inhibition of transiently expressed low- and high-voltage-activated calcium channels by trivalent metal cations. J Membr Biol 187: 225–238, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Thiels M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99: 495–500, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahm R, van MJ, Prescott AR, Quinlan RA. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res 69: 45–56, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ebihara L. New roles for connexons. News Physiol Sci 18: 100–103, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol 102: 59–74, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebihara L, Tong JJ, Vertel B, White TW, Chen TL. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest Ophthalmol Vis Sci 52: 882–889, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol 185: 93–102, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol 293: C337–C345, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Gunning SJ, Chung KK, Donaldson PJ, Webb KF. Identification of a nonselective cation channel in isolated lens fiber cells that is activated by cell shrinkage. Am J Physiol Cell Physiol 303: C1252–C1259, 2012 [DOI] [PubMed] [Google Scholar]
- 11.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274: 236–240, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J Gen Physiol 95: 679–696, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JR, DeRosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J Invest Dermatol 129: 870–878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res 18: 558–565, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol 216: 1–16, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev 90: 179–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mese G, Sellitto C, Li L, Wang HZ, Valiunas V, Richard G, Brink PR, White TW. The Cx26–G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol Biol Cell 22: 4776–4786, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mhaske PV, Levit NA, Li L, Wang HZ, Lee JR, Shuja Z, Brink PR, White TW. The human Cx26-D50A and Cx26-A88V mutations causing keratitis-ichthyosis-deafness syndrome display increased hemichannel activity. Am J Physiol Cell Physiol 304: C1150–C1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minogue PJ, Tong JJ, Arora A, Russell-Eggitt I, Hunt DM, Moore AT, Ebihara L, Beyer EC, Berthoud VM. A mutant connexin50 with enhanced hemichannel function leads to cell death. Invest Ophthalmol Vis Sci 50: 5837–5845, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 116: 163–175, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115: 1077–1089, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfahnl A, Dahl G. Gating of Cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch 437: 345–353, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Ren Q, Riquelme MA, Xu J, Yan X, Nicholson BJ, Gu S, Jiang JX. Cataract-causing mutation of human connexin 46 impairs gap junction, but increases hemichannel function and cell death. PLoS One 8: e74732, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development 129: 167–174, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316: 2377–2389, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol 302: C1751–C1761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivas M, Kronengold J, Bukauskas FF, Bargiello T, Verselis V. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J 88: 1725–1739, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stout CE, Costantin JL, Naus CCG, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Tenbroek EM, Johnson R, Louis CF. Cell-to-cell communication in a differentiating ovine lens culture system. Invest Ophthalmol Vis Sci 35: 215–228, 1994 [PubMed] [Google Scholar]
- 30.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143: 815–25, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willecke K, Heynkes R, Dahl E, Stutenkemper H, Hennemann S, Jungbluth T, Suchyna T, Nicholson BJ. Mouse connexin37: cloning and functional expression of a gap junction gene highly expressed in lung. J Cell Biol 114: 1049–1057, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Ch Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res 83: 688–696, 2006 [DOI] [PubMed] [Google Scholar]