Abstract
Calcium dependent signaling is highly regulated in cardiomyocytes and determines the force of cardiac muscle contraction. The cardiac ryanodine receptors (RyR2) play important roles in health and disease. Modulation of RyR2 by phosphorylation is required for sympathetic regulation of cardiac function. Abnormal regulation of RyR2 contributes to heart failure, and atrial and ventricular arrhythmias. RyR2 channels are oxidized, nitrosylated, and hyperphosphorylated by protein kinase A (PKA) in heart failure, resulting in “leaky” channels. These leaky RyR2 channels contribute to depletion of calcium from the sarcoplasmic reticulum, resulting in defective cardiac excitation–contraction coupling. In this review, we discuss both the importance of PKA and calcium/calmodulin-dependent kinase II (CaMKII) regulation of RyR2 in health, and how altered phosphorylation, nitrosylation and oxidation of RyR2 channels lead to cardiac disease. Correcting these defects using either genetic manipulation (knock-in) in mice, or specific and novel small molecules ameliorates the RyR2 dysfunction, reducing the progression to heart failure and the incidence of arrhythmias.
Keywords: Ryanodine receptor, Calcium, Arrhythmia, Heart failure, Phosphorylation
1. The role of ryanodine receptors in the heart
Each calcium (Ca2+) release and reuptake cycle during each heart beat are initiated by the action potential (AP), an electrical signal that depolarizes the plasma membrane and the specialized invagination called the transverse-tubule (T-tubule). Voltage-gated Ca2+ channels on the T-tubule are activated by depolarization and allow a small amount of Ca2+ to enter the cell. The Ca2+ then activates ryanodine receptors (RyR2), which are embedded in the cardiac sarcoplasmic reticular (SR) membrane. Ca2+ is released from the SR via RyR2 raising the cytosolic [Ca2+] about ten-fold to ~1 µM. This process is termed Ca2+-induced Ca2+ release and is absolutely required for excitation–contraction (EC) coupling. Ca2+ binds to troponin C, which causes a conformational change exposing binding sites for myosin on the actin filaments. Myosin forms crossbridges with actin, which slide past each other shortening the sarcomere and causing cardiac muscle contraction. The Ca2+ is then pumped back into the SR by the sarcoplasmic/endoplasmic reticulum ATPase (SERCA2a) and exchanged across the plasma membrane for Na+ by the Na+/Ca2+ exchanger (NCX), lowering the cytosolic [Ca2+] to baseline levels of ~100 nM, thereby causing relaxation.
Ryanodine receptors exist as homotetrameric structures with at least two functional domains: the carboxy-terminus containing the transmembrane segments forming the Ca2+ conducting pore and the large amino-terminus (termed the “foot” structure), which contains modulatory binding sites [1,2]. Several modulatory elements are bound to it, including FK506 binding protein (aka calstabin), a member of the immunophilin family of cis-trans peptidyl-prolyl isomerases [3] (FKBP12 in skeletal muscle [4] and FKBP12.6 in cardiac muscle [5,6]), PKA and its anchoring protein, muscle A kinase anchoring protein (mAKAP), PP1 and PP2A [7]. FKBP12 (calstabin1) and FKBP12.6 (calstabin2), endogenous modulators of RyR1 and RyR2 respectively, have been shown to stabilize the channel complex, resulting in channels that demonstrate full conductance [5,8]. The recruitment of the kinase(s) and phosphatases into the complex is mediated through specific leucine zipper interactions on the anchoring proteins and RyR [9].
2. Ryanodine receptors: An important target of PKA and CaMKII
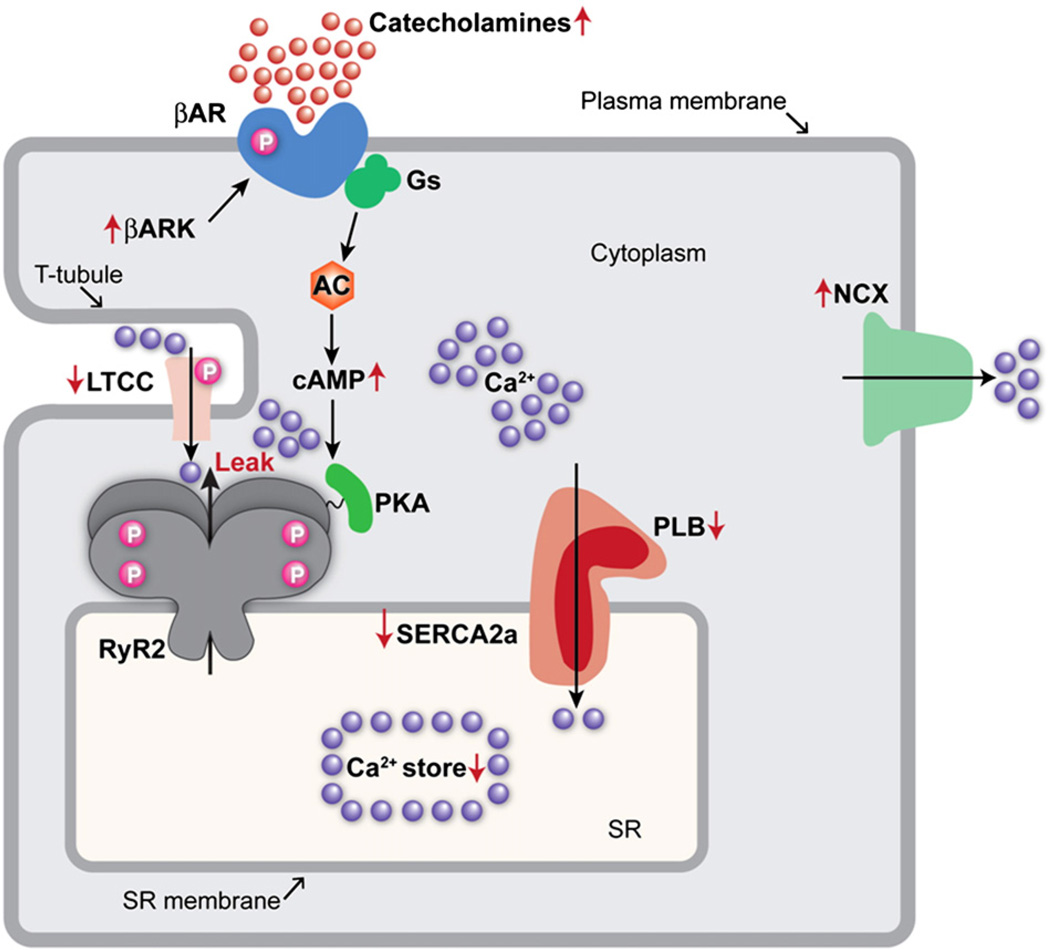
Epinephrine and norepinephrine bind to adrenergic receptors in cardiomyocytes, the activation of which augments inotropy, lusitrophy, and chronotropy [10]. These effects are correlated with [Ca2+]i. The pathway from β-AR, to Gαs to adenylyl cyclase (AC), to cAMP, leads to activation of PKA (Fig. 1). β-AR pathway has multiple targets, including Cav1.2, RyR2 and phospholamban, the regulator of SERCA (Fig. 1).
Fig. 1.
Ca2+ cycling in cardiac myocytes and regulation by PKA. Excitation–contraction (EC) coupling in the heart is initiated by depolarization of the transverse tubule (T-tubule) that activates voltage-gated l-type Ca2+ channels (LTCCs) in the plasma membrane. Ca2+ influx via LTCC triggers Ca2+ release from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR2). Catecholamine activation of β-ARs stimulates adenylyl cyclase (AC), and the generation of cyclic AMP (cAMP),which in turn activates protein kinase A (PKA). PKA phosphorylates several targets, augmenting the Ca2+ transient by increasing the activities of LTCC, RyR2 and SR Ca2+-ATPase (SERCA2a). Relaxation occurs after the Ca2+ is pumped out of the cytoplasm and into the SR by SERCA2a, which is regulated by phospholamban (PLB). In addition, Ca2+ is extruded from the cell by the sarcolemmal Na+/Ca2+ exchanger (NCX). Chronic activity of the sympathetic nervous system leads to hyperphosphorylation (indicated with the red ‘P’) of the β-adrenergic receptor, activation of β-AR kinase (βARK), and desensitization of β-ARs. In HF, RyR2 is hyperphosphorylated by PKA, leading to an increased open probability at low [Ca2+], consistent with leak of Ca2+ during diastole. The long-term effect of increased Ca2+ release and diastolic Ca2+ leak is depletion of SR Ca2+ stores. SERCA2a expression and activity is decreased in HF, which is linked to PLB hypophosphorylation. Arrows indicate increased or decreased expression or activity in HF.
PKA and CaMKII regulation of RyR2 channel function play important roles in modulating cardiac contractility and arrhythmogenesis. We have shown that there is single functional PKA phosphorylation site and a separate single functional CaMKII phosphorylation site on RyR2, and a single functional PKA phosphorylation site on RyR1 [11] yielding four sites for each type of phosphorylation on each homotetrameric channel. Although Ser2809 on canine and human RyR2 (Ser2808 in murine RyR2) was originally identified as a PKA and CaMKII phosphorylation site [12,13], site-directed mutagenesis studies and knock-in mice have shown that Ser2808 is exclusively phosphorylated by PKA [14], and Ser2815 (Ser2814 in murine RyR2) is phosphorylated by CaMKII [11,15,16]. Using site-directed mutagenesis to ablate the phosphorylation sites by substituting an alanine residue for the target serine residues, we showed that the channels can no longer be phosphorylated (RyR1-S2844A ablates PKA phosphorylation of RyR1, RyR2-S2808A ablates PKA phosphorylation of RyR2 and RyR2-S2814A ablates CaMKII phosphorylation of RyR2 [11]). Knock-in mice for each of these mutations confirmed that the respective channels have only a single PKA and CaMKII phosphorylation site [14,15]. Mice engineered with a RyR2-S2808A mutation have blunted inotropic and chronotropic responses to catecholamines [14,17,18]. Mice engineered with a RyR2-S2814A mutation have RyR2 channels that cannot be phosphorylated by CaMKII, and exhibit a blunted positive force frequency relationship [15]. Mice engineered with a phosphomimetic mutation (substituting an aspartic acid residue for serine) of the RyR2 PKA phosphorylation site showed an age dependent cardiomyopathy and arrhythmias [18].
Chen and colleagues proposed Ser2030 as the physiological PKA phosphorylation site on RyR2 [19]. In their initial report, however, phosphorylation of the Ser2030 had no functional effect on RyR2 (see Fig. 7 in [19]). The lack of a functional effect of phosphorylation of RyR2-Ser2030 has also been confirmed by the Marks laboratory [14].
Valdivia and colleagues developed their own RyR2-S2808A mouse and concluded that phosphorylation of Ser2808 plays no role in the cardiomyocyte response to β-adrenergic stimulation [20]. Curiously, significant blunting of the isoproterenol-stimulated Ca2+ transient and cell shortening (see Figs. 5C and 5E of Ref. [20]) was demonstrated in cardiomyocytes isolated from their RyR2-S2808A mice compared to WT controls when stimulated at 3 Hz. No data for the effects of stimulation at 3 Hz were provided in their paper [21], showing only the slower rates of stimulation where there is no blunting of the isoproterenol response. For technical reasons, it is not possible to pace isolated cardiomyocytes at the more physiological stimulation rate of approximately 10 Hz (HR = 600 bpm). Taken together, it appears that as the rate of stimulation is increased towards the physiological range, there is blunting of the response to β-adrenergic stimulation in the cardiomyocytes of S2808A knock-in mice.
The concept that activation of RyR2 and increases in the Ca2+ transient result in a sustained increased in cardiac contractility [17] has also been debated. Eisner and colleagues reported that modulation of RyR2 cannot cause a sustained increase in cardiac contractility because the EC coupling system adapts to increased SR calcium release via RyR2 and returns to baseline [22,23]. Eisner and colleagues activated RyR2 in cardiomyocytes using caffeine. They found that when caffeine is removed, the increase in the Ca2+ transient returns to normal, concluding that modulation of RyR has no maintained effect on systolic Ca2+. However, they also show that the fractional release (the fraction of the SR Ca2+ that is released via RyR2) is significantly increased when RyR2 is activated (see Fig. 5F in [23]). If one were to examine the physiological condition of the “fight-or-flight” response in which the trigger (l-type Ca2+ channel), the release channel (RyR2) and the SR Ca2+ reuptake pump (SERCA) are all activated coordinately (e.g. by PKA phosphorylation) then the increased fractional release of SR Ca2+ that Eisner and colleagues demonstrate would result in a maintained increase in systolic Ca2+ and cardiac contractility [17]. Furthermore, there is no physiological circumstance under which RyR2 would be activated in isolation as it is by caffeine application.
3. Role of calstabins (FBKPs) in modulating RyR2 function
We originally reported that calstabin1 (FKBP12) is a subunit of RyR1 [4] and that it is required to stabilize the closed state and prevent aberrant Ca2+ leak through the channel [8]. Many groups have also shown that calstabin2 (FKBP12.6), which is a subunit of RyR2 [5,24], plays an important physiological role in modulating RyR2 function [25–32].
Bers and colleagues recently reported that calstabin2 plays no significant role in cardiac physiology [33], which is in contrast to an earlier study in which the same group concluded that there is “an important SR-stabilizing effect of FKBP in intact rat ventricular myocytes” [34]. In the more recent study, the group used a fluorescent-labeled calstabin2 which had a binding affinity for RyR2 of 1 nM [33] that is more than two orders of magnitude higher than that of the native calstabin2 which is 160 nM as determined by Fleischer and colleagues using S35 labeled wild type calstabin2 [35]. Using this high affinity ligand to displace endogenous calstabin2, they estimated that ≤20% of the calstabin2 binding sites on RyR2 are occupied. They concluded that since most RyR2 channels do not have calstabin2 bound to them, calstabin2 cannot play an important physiological role. Fleischer and colleagues, however, using S35 labeled calstabin2 that had the normal binding affinity to RyR2, showed that 83% of the calstabin2 sites on RyR2 are occupied [36]. Thus, the abnormally high binding affinity of the fluorescent calstabin2 likely resulted in incorrect estimates of the native calstabin2 occupancy on RyR2.
We originally reported that PKA hyperphosphorylation of RyR2 causes depletion of calstabin2 from the RyR2 complex [7]. Subsequently, oxidation and nitrosylation of RyR2 were also shown to cause depletion of calstabin2 from the RyR2 complex and that the combination of all three processes (oxidation, nitrosylation and phosphorylation of Ser2808) depleted nearly all of the calstabin2 from the channel complex [17,18]. These observations have been supported by data from RyR2-S2808A mice that are protected from PKA phosphorylation induced depletion of calstabin2 and RyR2-S2808D mice that exhibit decreased levels of calstabin2 in the RyR2 complex [17,18] and confirmed by others [27,30].
Meissner and colleagues reported that PKA phosphorylation of RyR2 did not deplete calstabin from the channel, but the experimental conditions were not physiological, including a vast molar excess of calstabin which likely obscured the effect of PKA phosphorylation (see Fig. 1B in [37]). It is important to carefully control the stoichiometry of calstabin to RyR (1 calstabin to 1 RyR monomer) in order to reproduce the in vivo conditions. Moreover, it is important to control for the oxidative state of the channel. RyR channels are redox sensors with huge cytoplasmic domains containing more than 30 exposed free cysteines in each monomer (>120 per tetrameric channel) [38]. Some of the divergent results reported in the literature concerning the effects of PKA phosphorylation on calstabin binding to the channels are likely due to variations in the state of oxidation of the channels. For example when RyR2-S2808D channels are expressed in HEK cells there is progressive oxidation of the channel such that the binding of calstabin2 to the channel decreases each day the transfected cells are in culture [18]. This progressive oxidation is due to mitochondrial Ca2+ overload due to leaky RyR2 channels and ROS production, which oxidizes the channel [39].
4. Role of ryanodine receptors in heart failure
Congestive heart failure (HF), one of the leading causes of mortality and morbidity in the USA, is a complex syndrome, marked by reduced cardiac contractility and blunted adrenergic responsiveness. Patients with HF have chronic activation of the sympathetic nervous system resulting in a maladaptive attempt to improve cardiac function. β-adrenergic agonists or phosphodiesterase inhibitors, which are used to treat acute decompensated HF, increase contractility by increasing cAMP and increasing Ca2+ release, but also increase mortality. Blocking neurohormonal pathways is the current mainstay of HF therapy, but is limited by side effects and the requirement to carefully titrate the drugs [40].
Cardiac contractility is determined by the amplitude and kinetics of Ca2+ cycling. We reported that diastolic SR Ca2+ leak via RyR2 channels contributes to HF progression [7] and fatal ventricular arrhythmias [41–44]. Prior to the diastolic SR Ca2+ leak model of HF, the dogma was that HF was associated with SR Ca2+ overload. Subsequent to our initial report of the diastolic SR Ca2+ leak, both the SR Ca2+ depletion and the diastolic SR Ca2+ leak have been confirmed in HF [25,29,30,45,46].
The defective SR Ca2+ handling is characterized by leaky RyR2 channels due to stress induced dissociation of the stabilizing RyR2 subunit calstabin2, resulting in a diastolic SR Ca2+ leak, reduced SR Ca2+ content and decreased Ca2+ transient [7,18,41,47–49]. Compounding this problem is impaired SR Ca2+ uptake due to reduced activity of SERCA2a as a consequence of both reduced SERCA2a expression and increased inhibition of the pump by phospholamban [50], and enhanced Na+/Ca2+ exchanger (NCX) activity [51] (Fig. 1). Thus, these dysfunctional processes conspire to deplete the SR of Ca2+ and lead to impaired cardiac contractility [52]. Not surprisingly, therefore both the RyR2 leak and the impaired uptake have been targeted with novel therapeutics, which are now undergoing clinical testing in HF patients.
The HF-induced RyR2 Ca2+ leak is caused by the chronic hyperadrenergic state observed in HF patients, which in turn induces chronic PKA hyperphosphorylation of RyR2 at Ser2808, causing depletion of calstabin2 from the channel complex [7]. The term hyperphosphorylation describes RyR2 in which 3–4 of the four RyR2 monomers are PKA phosphorylated. PKA hyperphosphorylated/calstabin2 depleted channels are sensitized to cytosolic Ca2+ leading to inappropriate Ca2+ release during diastole, referred to as a diastolic SR Ca2+ leak. The HF RyR2 Ca2+ leak model of HF is supported by studies demonstrating that β-adrenergic stimulation causes depletion of calstabin2 from the RyR2 complex and that HF patients have RyR2 that are PKA hyperphosphorylated and calstabin2 depleted [27,28,30,31,53–56]. Furthermore, in HF RyR2 channels are nitrosylated and oxidized [18], and have diminished association with phosphatases [57] and PDE4D3 [49]. Reduced PDE4D3 and phosphatases results in elevated levels of cAMP near RyR2 [49] and a decreased rate of dephosphorylation [18,57]. Patients whose cardiac function has been normalized by treatment with left ventricular assist devices (LVADs) have reduced levels of circulating catecholamines [58] and reduced PKA phosphorylation of RyR2 and restoration of calstabin2 binding to RyR2 [7]. The discovery of the role of leaky RyR2 in HF also provides a mechanism to explain, at least in part, the therapeutic efficacy of β-AR blockers. β-blockers inhibit Ser2808 (Ser2809 in human RyR2) phosphorylation and indirectly prevent calstabin2 depletion from the RyR2 complex and reduce SR Ca2+ leak in HF patients [18,59,60].
Multiple animal models have been used to support a role for PKA hyperphosphorylation of RyR2 in HF progression [61]. Genetically altered mice harboring RyR2 that cannot be PKA phosphorylated (RyR2-S2808A), were protected against calstabin2 depletion from the RyR2 complex and HF progression 4 week post-MI [14]. PDE4D3 deficient mice develop an age-dependent cardiomyopathy and arrhythmias, RyR2 PKA hyperphosphorylation and calstabin2 depletion. Crossing the PDE4D3 deficient mice and RyR2-S2808A mice was protective [49]. Transgenic mice expressing a mutant calstabin2-D37V, which remains bound to PKA phosphorylated RyR2 channels, are also protected against post-MI HF [62].
Although there are numerous technical differences between the studies, the roles for PKA hyperphosphorylation, calstabin2 depletion and increased open probability of RyR2 channels in HF were challenged by Valdivia and colleagues [63]. For instance, we reported a difference in open probability between control and HF channels at diastolic [Ca2+] concentrations of 150 nM and not the 5 µM used by the Valdivia group. We examined RyR2 channels under conditions that correspond to diastole in the heart (low activating Ca2+) whereas Valdivia and colleagues used conditions that correspond to systole when the channels are normally open [63]. Valdivia and colleagues also reported that RyR2 from failing hearts are not PKA hyperphosphorylated and are not depleted of calstabin, based on experiments in which they do not normalize for the amount of RyR2 protein in each sample (see Fig.8A in [63]) and used centrifugation instead of co-immunoprecipitation to determine the components of the RyR2 complex. The presence of FKBP12 in their membrane fraction shows that there is contamination of the membrane fraction with cytosol since only FKBP12.6 and not FKBP12 binds to RyR2 [36]. Interestingly, Valdivia and colleagues showed that there is preservation of cardiac function in RyR2-S2808A mice compared to WT mice (see Supplemental Table 1, fractional shortening second line from the bottom of Ref. [20]). They showed no loss of function in the RyR2-S2808A mice 11 weeks after transverse aortic constriction (TAC) compared to a significant loss of function in WT controls ([WT: FS (%) 52.1 ± 3.2 to 42.6 ± 1.2, P < 0.05; RyR2-S2808A: 51.0 ± 2.6 to 51.9 ± 5.1, P = NS]) (Supplemental Table 1 in [20]). In summary, both our RyR2-S2808A mice and those of Valdivia and colleagues exhibit blunted responses to catecholamines and are protected against HF progression [14,17,18].
Chen has proposed that store overload induced calcium release (SOICR) explains why RyR2 channels are leaky [64]. SOICR likely does not constitute an independent phenomenon, however, but instead is a manifestation of two mechanisms: [1] Ca2+ waves are produced when sparks trigger additional sparks through CICR (Ca2+ induced Ca2+ release); and [2] SR [Ca2+], through its modulation of RyR gating, affects the probability that this occurs [65].
Alternative mechanisms have been proposed to explain SR Ca2+ leak in HF. CaMKIIδ levels are elevated in human HF [66] and there is an increase in CaMKII-dependent phosphorylation of RyR2 in HF. Mice expressing the CaMKII inhibitory peptide AC3-I are protected against HF leading to the proposal that CaMKII inhibitors may prevent HF progression [67]. Mice engineered with a RyR2-S2814A mutation were relatively protected from HF development after transverse aortic constriction compared with WT littermates [16]. These protective effects on cardiac contractility were not observed, however, after myocardial infarction in the S2814A mice [15]. The aortic banding model is a hypertrophy model in which cardiac function is initially increased and CaMKII phosphorylation of RyR2 is likely associated with the hypertrophy. The post-MI model is an ischemic dilated cardiomyopathy in which the RyR2 are PKA hyperphosphorylated and CaMKII phosphorylation of RyR2 is not elevated. RyR2 CaMKII phosphorylation is required, however, for the rate-related increase in contractility or Bowditch phenomenon [15].
5. Arrhythmias due to abnormal RyR2 function
Increased RyR2 activity has been shown to cause arrhythmias, particularly associated with increased catecholaminergic stimulation. This is best exemplified by catecholaminergic polymorphic ventricular tachycardia (CPVT), a rare inherited form of exercise-induced sudden cardiac death that occurs in individuals with structural normal hearts and normal ECGs. Mutations in RyR2 have been linked to CPVT [68,69]. RyR2 with CPVT mutations have reduced affinity for calstabin2, which results in leaky channels during exercise [44]. Calstabin2 deficient and haplo insufficient mice exhibit CPVT [44], but do not develop HF. Presumably the reason for the lack of cardiac dysfunction is that in contrast to the post-MI model where calstabin2 depletion from the RyR2 complex has been shown to promote HF progression, the calstabin2 deficient mice have otherwise normal cardiac function and are able to compensate for the loss of calstabin in the absence of a compromised ventricle (e.g. no MI). Calstabin2 deficient mice exhibit DADs and exercise-induced ventricular tachycardia (VT) [44] and RyR2 from calstabin2-deficient mice exhibit slightly increased open probability at baseline that increases substantially when the mice are exercised.
Early studies on canine atrial myocytes reported altered Ca2+ handling in cells isolated from the atria with atrial fibrillation [70]. Several studies have confirmed a role for RyR2 dysfunction in atrial fibrillation [71–75]. RyR2 from canine atria with atrial fibrillation are PKA phosphorylated, depleted of calstabin2 and have increased open probability at diastolic [Ca2+] [71]. Calstabin2-deficientmice have an increased incidence of atrial fibrillation [72]. Atrial fibrillation could be induced by intra-esophageal burst pacing protocol in 3 CPVT mouse models, RyR2-R2474S(+/−), RyR2-N2386I(+/−), RyR2-L433P(+/−), but not in WT mice [75]. Consistent with these in vivo results, there was a significant diastolic SR Ca2+ leak in atrial myocytes isolated from these CPVT mouse models.
Increased CaMKII activity and phosphorylation of RyR2 at Ser2814 may play a role in the pathogenesis of atrial fibrillation and ventricular arrhythmias. The RyR2-S2814A mice are protected from the development of atrial fibrillation [73,76] and pacing induced arrhythmias after transverse aortic constriction [77]. Conversely, mice engineered with a constitutively activated CaMKII phosphorylation site (S2814D) develop sustained ventricular tachycardia and sudden cardiac death with caffeine and epinephrine, or programmed electrical stimulation [77].
6. Fixing leaky RyR2 channels
The identification of the diastolic SR Ca2+ leak via RyR2 as a mechanism underlying HF progression and cardiac arrhythmias has led to novel therapeutic approaches. JTV-519 (K201), a 1,4-benzothiazepene, was noted to have effects on intracellular Ca2+ [78] and cardio-protective effects [79]. JTV-519 was found to inhibit Na+, Ca2+, and K+ currents [80,81]. Using a canine model of pacing induced HF Matsuzaki and colleagues reported that JTV-519 improved cardiac function [56]. Testing the drug in calstabin2-deficient mice showed that the ability of JTV-519 to prevent HF progression and fatal cardiac arrhythmias requires stabilization of the closed state of RyR2 by calstabin2 [42,82]. Moreover, JTV-519 had no effect on the gating properties of normal RyR channels and no effects in healthy dogs and mice [48].
We generated many derivatives of the 1,4-benzothiazepine JTV-519 and have developed a novel class of Ca2+ release channel stabilizers known as Rycals. An orally available Rycal, S107, improves skeletal muscle force generation and exercise capacity, reduces arrhythmias and improves muscle function in mice with Duchenne muscular dystrophy by reducing pathologic SR Ca2+ leak in cardiac and skeletal muscles [18,39,48,83–85]. Rycals are protective against post-MI HF progression [17,18] and suppressed VT/VF and sudden cardiac death in murine models of human CPVT. S107 also raises the seizure threshold in mice with leaky neuronal RyR2 channels and improves exercise capacity in mouse models of sarcopenia (age-related loss of muscle function) [39,41,43,48,49]. Leaky RyR2 channels in hippocampal neurons play a key role in stress-induced cognitive dysfunction. Treatment with S107 prevented stress-induced cognitive dysfunction in a murine model suggesting a novel mechanism and therapeutic approach to posttraumatic stress disorder [86].
7. Conclusions
It is evident that RyR channels play an important role in cardiac physiology and pathophysiology. Modulating their activity is important for flight-or-fight responses. Long-term activation of the channel, however, is detrimental, causing progression of HF and arrhythmogenesis. Limiting the diastolic leak using either genetic manipulation of RyR (alanine substitution of PKA phosphorylation site for instance) or Rycals yields clinical benefit in terms of cardiac function post-MI and incidence of arrhythmias (VT and atrial fibrillation) in mice. There are potentially other therapeutic targets in the key pathways that regulate Ca2+ handling in cardiac muscle. For example, the cardiac RyR2 is a macromolecular signaling complex with multiple enzymes and targeting proteins, each of which could be explored as a potential cause of and therapeutic target for HF.
Acknowledgments
Much of the work referred to in this review has been supported by the NHLBI, the Fondation Leducq, the Doris Duke Charitable Foundation and the Ellison Foundation.
Abbreviations
- AP
action potential
- Ca2+
Calcium
- RyR2
cardiac type 2 ryanodine receptor/Ca2+ release channel macromolecular complexes
- SR
sarcoplasmic reticulum
- SERCA2a
sarcoplasmic/endoplasmic reticulum ATPase
- T-tubule
transverse-tubule
Footnotes
Conflict of interest statement
ARM is a consultant for and owns shares in ARMGO Pharma Inc. a startup company developing RyR targeted therapeutics.
References
- 1.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 2.Zorzato F, Fujii J, Otso K, Phillips M, Green NM, Lai FA, et al. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]
- 3.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 4.Jayaraman T, Brillantes A-MB, Timerman AP, Erdjument-Bromage H, Fleischer S, Tempst P, et al. FK506 binding protein associated with the calcium release channel (ryanodine receptor) J Biol Chem. 1992;267:9474–9477. [PubMed] [Google Scholar]
- 5.Kaftan E, Marks AR, Ehrlich BE. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circ Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- 6.Timerman AP, Jayaraman T, Wiederrecht G, Onoue H, Marks AR, Fleischer S. The ryanodine receptor from canine heart sarcoplasmic reticulum is associated with a novel FK-506 binding protein. Biochem Biophys Res Commun. 1994;198:701–706. doi: 10.1006/bbrc.1994.1101. [DOI] [PubMed] [Google Scholar]
- 7.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 8.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 9.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 11.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 12.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 13.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 14.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, Dealmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, et al. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 20.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, et al. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, et al. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res. 2012;110:831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisner DA, Kashimura T, O'Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46:474–481. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522(Pt 2):259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timerman AP, Ogunbumni E, Freund E, Wiederrecht G, Marks AR, Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993;268:22992–22999. [PubMed] [Google Scholar]
- 25.Gellen B, Fernandez-Velasco M, Briec F, Vinet L, LeQuang K, Rouet-Benzineb P, et al. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation–contraction coupling. Circulation. 2008;117:1778–1786. doi: 10.1161/CIRCULATIONAHA.107.731893. [DOI] [PubMed] [Google Scholar]
- 26.Chelu MG, Danila CI, Gilman CP, Hamilton SL. Regulation of ryanodine receptors by FK506 binding proteins. Trends Cardiovasc Med. 2004;14:227–234. doi: 10.1016/j.tcm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 27.George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93:531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 28.Doi M, Yano M, Kobayashi S, Kohno M, Tokuhisa T, Okuda S, et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation. 2002;105:1374–1379. doi: 10.1161/hc1102.105270. [DOI] [PubMed] [Google Scholar]
- 29.Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L, et al. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ Res. 2001;88:188–194. doi: 10.1161/01.res.88.2.188. [DOI] [PubMed] [Google Scholar]
- 30.Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca(2+) leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 31.Ono K, Yano M, Ohkusa T, Kohno M, Hisaoka T, Tanigawa T, et al. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca(2+) release in heart failure. Cardiovasc Res. 2000;48:323–331. doi: 10.1016/s0008-6363(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 32.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 33.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010;106:1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCall E, Li L, Satoh H, Shannon TR, Blatter LA, Bers DM. Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circ Res. 1996;79:1110–1121. doi: 10.1161/01.res.79.6.1110. [DOI] [PubMed] [Google Scholar]
- 35.Jeyakumar LH, Ballester L, Cheng DS, McIntyre JO, Chang P, Olivey HE, et al. FKBP binding characteristics of cardiac microsomes from diverse vertebrates. Biochem Biophys Res Commun. 2001;281:979–986. doi: 10.1006/bbrc.2001.4444. [DOI] [PubMed] [Google Scholar]
- 36.Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, et al. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- 37.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 39.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dube P, Weber KT. Congestive heart failure: pathophysiologic consequences of neurohormonal activation and the potential for recovery: part II. Am J Med Sci. 2011;342:503–506. doi: 10.1097/MAJ.0b013e3182327527. [DOI] [PubMed] [Google Scholar]
- 41.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 43.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 44.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 45.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 46.Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson DC, Marks AR. Fixing ryanodine receptor Ca leak — a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech. 2010;7:e151–e157. doi: 10.1016/j.ddmec.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 51.Hobai IA, O'Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 52.Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation. 2010;121:822–830. doi: 10.1161/CIRCULATIONAHA.109.890954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogrodnik J, Niggli E. Increased Ca(2+) leak and spatiotemporal coherence of Ca(2+) release in cardiomyocytes during beta-adrenergic stimulation. J Physiol. 2010;588:225–242. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morimoto S, J OU, Kawai M, Hoshina T, Kusakari Y, Komukai K, et al. Protein kinase A-dependent phosphorylation of ryanodine receptors increases Ca2+ leak in mouse heart. Biochem Biophys Res Commun. 2009;390:87–92. doi: 10.1016/j.bbrc.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 55.Blayney LM, Jones JL, Griffiths J, Lai FA. A mechanism of ryanodine receptor modulation by FKBP12/12.6, protein kinase A and K201. Cardiovasc Res. 2010;85:68–78. doi: 10.1093/cvr/cvp273. [DOI] [PubMed] [Google Scholar]
- 56.Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 57.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J Biol Chem. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 58.Estrada-Quintero T, Uretsky BF, Murali S, Griffith BP, Kormos RL. Neurohormonal activation and exercise function in patients with severe heart failure and patients with left ventricular assist system. A comparative study. Chest. 1995;107:1499–1503. doi: 10.1378/chest.107.6.1499. [DOI] [PubMed] [Google Scholar]
- 59.Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 60.Reiken S, Gaburjakova M, Gaburjakova J, He KlKL, Prieto A, Becker E, et al. Beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 61.Kushnir A, Betzenhauser MJ, Marks AR. Ryanodine receptor studies using genetically engineered mice. FEBS Lett. 2010;584:1956–1965. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang F, Shan J, Reiken S, Wehrens XH, Marks AR. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci U S A. 2006;103:3456–3461. doi: 10.1073/pnas.0511282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 64.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sobie EA, Lederer WJ. Dynamic local changes in sarcoplasmic reticulum calcium: physiological and pathophysiological roles. J Mol Cell Cardiol. 2012;52:304–311. doi: 10.1016/j.yjmcc.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoch B, Haase H, Schulze W, Hagemann D, Morano I, Krause EG, et al. Differentiation-dependent expression of cardiac delta-CaMKII isoforms. J Cell Biochem. 1998;68:259–268. [PubMed] [Google Scholar]
- 67.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 69.Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, et al. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–2042. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 70.Sun H, Gaspo R, Leblanc N, Nattel S. Cellular mechanisms of atrial contractile dysfunction caused by sustained atrial tachycardia. Circulation. 1998;98:719–727. doi: 10.1161/01.cir.98.7.719. [DOI] [PubMed] [Google Scholar]
- 71.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 72.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, et al. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed after depolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A, et al. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, et al. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Oort RJ, Respress JL, Li N, Reynolds C, De Almeida AC, Skapura DG, et al. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55:932–938. doi: 10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaneko N, Matsuda R, Toda M, Shimamoto K. Inhibition of annexin V-dependent Ca2+ movement in large unilamellar vesicles by K201, a new 1,4-benzothiazepine derivative. Biochim Biophys Acta. 1997;1330:1–7. doi: 10.1016/s0005-2736(97)00132-6. [DOI] [PubMed] [Google Scholar]
- 79.Ito K, Shigematsu S, Sato T, Abe T, Li Y, Arita M. JTV-519, a novel cardioprotective agent, improves the contractile recovery after ischaemia-reperfusion in coronary perfused guinea-pig ventricular muscles. Br J Pharmacol. 2000;130:767–776. doi: 10.1038/sj.bjp.0703373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiriyama K, Kiyosue T, Wang JC, Dohi K, Arita M. Effects of JTV-519, a novel anti-ischaemic drug, on the delayed rectifier K+ current in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:646–653. doi: 10.1007/s002100000230. [DOI] [PubMed] [Google Scholar]
- 81.Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol. 1999;79:275–281. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- 82.Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci U S A. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fauconnier J, Meli AC, Thireau J, Roberge S, Shan J, Sassi Y, et al. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proc Natl Acad Sci U S A. 2011;108:13258–13263. doi: 10.1073/pnas.1100286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, et al. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150:1055–1067. doi: 10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]