Abstract
Aims
A few case series in adults have described the characteristics of epithelioid glioblastoma (e-GB), one of the rarest variants of this cancer. We evaluated clinical, radiological, histological, and molecular characteristics in the largest series to date of paediatric e-GB.
Methods
Review of clinical characteristics and therapy, imaging studies, and histology was performed in patients younger than 22 years with e-GB seen at our institution over 15 years. Sequencing of hotspot mutations and FISH of relevant genes were undertaken.
Results
Median age at diagnosis of six patients was 7.6 years. Tumours originated in the cerebral cortex (n=2) or diencephalon (n=4). Three patients presented with acute, massive haemorrhage and three had leptomeningeal dissemination at diagnosis. Paediatric e-GB had the typical histological characteristics seen in adult tumours. Universal immunoreactivity for INI1 and lack of diverse protein expression were seen in all cases. One tumour had a chromosome 22q loss. Three tumours (50%) harboured a BRAF: p.V600E. One thalamic tumour had an H3F3A p.K27M. All patients received radiation therapy with (n=3) or without chemotherapy (n=3). All patients experienced tumour progression with a median survival of 169 days. One patient with non-metastatic disease had early leptomeningeal progression. Two patients had symptomatic tumour spread outside the central nervous system (CNS) through a ventriculo-peritoneal shunt. One additional patient had widespread metastases outside the CNS identified at autopsy.
Conclusions
Paediatric e-GBs are rare cancers with an aggressive behaviour that share histological and genetic characteristics with their adult counterparts. BRAF inhibition is a potential treatment for these tumours.
Keywords: BRAF, epithelioid, glioblastoma, paediatric, rhabdoid
Introduction
Glioblastoma (World Health Organization [WHO] grade IV), one of the most malignant central nervous system (CNS) cancers in children and adults, is characterized by architectural diversity and cellular heterogeneity [1]. Although several uncommon variants of glioblastoma, each with an idiosyncratic morphology, have been described on the basis of these diverse histological characteristics [2–11], the 2007 WHO classification only recognizes two of them: giant-cell glioblastoma and gliosarcoma [1].
Two of the rarest variants of glioblastoma display cells with an epithelioid or rhabdoid phenotype and have very similar histological characteristics [12–16]. Both variants display round or oval cells with a distinct cell membrane, laterally positioned nucleus, and eosinophilic cytoplasm [12–16]. Although one study proposed that focal loss of INI1 immunoreactivity in rhabdoid areas and diverse protein expression should differentiate rhabdoid glioblastoma (r-GB) from epithelioid glioblastoma (e-GBs) [15], several other reports used the terms ‘epithelioid’ and ‘rhabdoid’ interchangeably [12, 13, 17–21]. e-GB is also distinct from the exceptionally rare true epithelial glioblastoma described in adults, which is a tumour with epithelial differentiation in the form of squamous nests and acinar structures [14]. Atypical teratoid/rhabdoid tumour (AT/RT) is an important differential diagnosis alongside e-GB, particularly in children. To complicate matters, several instances of AT/RT arising within previously diagnosed low-grade gliomas have been described [22–26].
We report the clinical, radiological, histological, and molecular characteristics in the largest series to date of paediatric e-GBs. We showed that children with e-GB experienced an aggressive course, despite the use of intensive therapy, with a high prevalence of haemorrhagic episodes and tumour dissemination in the leptomeninges and outside the CNS.
Patients and Methods
Once Institutional Review Board approval was obtained, we retrospectively identified in our institutional Neuro-Oncology and Pathology databases from January 1st, 1997 until December 31st, 2012 all patients younger than 22 years with newly diagnosed high-grade glioma that displayed cells with epithelioid or rhabdoid morphology. Upon central pathology review, we selected only those patients whose tumours met the diagnostic criteria for glioblastoma and harboured a significant epithelioid component. Furthermore, these tumours had to fulfill the immunohistochemical criteria for e-GB as previously described [15]. Information about clinical characteristics and therapy was abstracted from the patients’ medical records. Central radiological review of pertinent CT and MRI scans at diagnosis and during therapy was performed. All patients had at least a T2- and a T1-weighted brain MRI sequence before and after administration of contrast at diagnosis available for analysis.
Standard histological review, immunohistochemical studies, and interphase fluorescence in situ hybridization (iFISH) were performed in 5-µm sections of formalin-fixed, paraffin-embedded (FFPE) tissue. Samples from diagnosis and an episode of progressive disease 3 months later were obtained in one case. Immunohistochemical assessment employed antibodies to BRG1 (SMARCA4 gene product), INI1 (SMARCB1 gene product), GFAP, synaptophysin, NFP, EMA, cytokeratins (AE1/3 & CAM 5.2 antibodies), desmin, smooth muscle actin, melan A, and P53.
Dual-colour iFISH was done using standard methods as previously described [27]. Probes were derived from BAC clones (BACPAC Resources, Oakland, CA), labeled with either AlexaFluor-488 or Rhodamine fluorochromes, and validated on normal control metaphase spreads to confirm chromosomal location. The following target genes were probed relative to controls (BAC clones in parentheses): PDGFRA (RP11-231C18 + RP11-601I15) with 4p12 control (CTD-2057N12 + CTD-2588A19); EGFR (RP11-148P17 + RP11-1083E20) with 7q31.2 control (RP11-460J21 + CTB-133K23); MET (RP11-163C9) with 7p11.2 control (RP11-251I15); CDKN2A (RP11-149I2 on 9p21) with NOTCH1 (RP11-370H5 + RP11-1008C19 on 9q34.3); PTEN (CTD-2553L21) with 10p11.2 control (RP11-254A5 + RP11-322I2); SMARCB1 (CTD-2034E7 + CTD-2355C2) with 22q13.3 control (RP3-402G11).
Analyses of BRAF: p.V600E, H3F3A p.K27M and H3F3A p.G34, HIST1H3B p.K27M, IDH1 p.R132, and IDH2 p.R172 were performed in DNA extracted from FFPE scrolls using the Maxwell® 16 Plus LEV DNA purification kit (Promega, Madison, WI) according to the manufacturer’s instructions. DNA was then eluted in molecular grade DNase/RNase-free water (Ambion, Foster City, CA) and quantified using PicoGreen (Invitrogen, Carlsbad, CA). Polymerase chain reaction (PCR) experiments used previously published primers and GoTaq® Long PCR Master Mix (Promega, Madison, WI) [28–30]. Direct sequencing of PCR products was performed using BigDye version 3.1 and a 3730XL DNA analyzer (Applied Biosystems, Foster City, CA). Results were screened using CLC Main Workbench sequence analysis software version 6.0.2 (CLC bio, Cambridge, MA).
Results
Six patients that met our diagnostic criteria were identified. Four (2%) of 199 patients with newly diagnosed high-grade glioma treated at our institution during the study period had an e-GB. In addition, we consulted on two additional patients with the same diagnosis during the study period. The median age at diagnosis of all 6 patients was 7.6 years. (Table 1) The median interval from onset of symptoms to radiological diagnosis was 2.5 weeks (range, 1 day to 3 months). Histological diagnosis was delayed by 7 weeks in patient 2, who presented with an acute intra-tumoural and intra-ventricular haemorrhage. Patient 6 had Li-Fraumeni syndrome (heterozygous germline TP53 mutation at IVS3-2 A>C).
Table 1.
Clinical and radiological characteristics at diagnosis, pattern of tumor recurrence, and survival of patients with epithelioid glioblastoma
| Patient # |
Age at Diagnosis (years) |
Gender / Race | Primary Tumor Location |
Intra-Tumoral Hematoma at Diagnosis |
Metastatic Disease at Diagnosis |
Site of First Tumor Progression |
Tumor Spread Outside the CNS |
Survival (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | 10.7 | F / Caucasian | Thalamus | YES | No | Outside the CNS | YES | 144 |
| 2 | 11.3 | M / AA | Temporal lobe | YES | YES¶ | Leptomeningeal | YES‡ | 118 |
| 3§ | 10.2 | F / AA | Bi-thalamic | No | YES¶ | Outside the CNS | YES | 42 |
| 4 | 3.5 | M / Mixed race | Frontal Lobe | YES | YES¶ | Leptomeningeal | No | 194 |
| 5 | 3.5 | F / Caucasian | Hypothalamic | No | No | Leptomeningeal | No | 230 |
| 6§ | 5 | M / Caucasian | Thalamus | No | No | Local | No | 290 |
CSF positive for malignant cells and tumor nodule in cauda equina (patient 2), CSF positive for malignant cells only (patient 3), and multiple foci of metastatic spread in the brain (patient 4);
Tumor spread outside the CNS was documented at autopsy;
Patients 3 and 6 were only seen as consultation
Abbreviations: M, male; F, female; AA, African-American; CNS, central nervous system; CSF, cerebrospinal fluid
Radiological Characteristics at Diagnosis
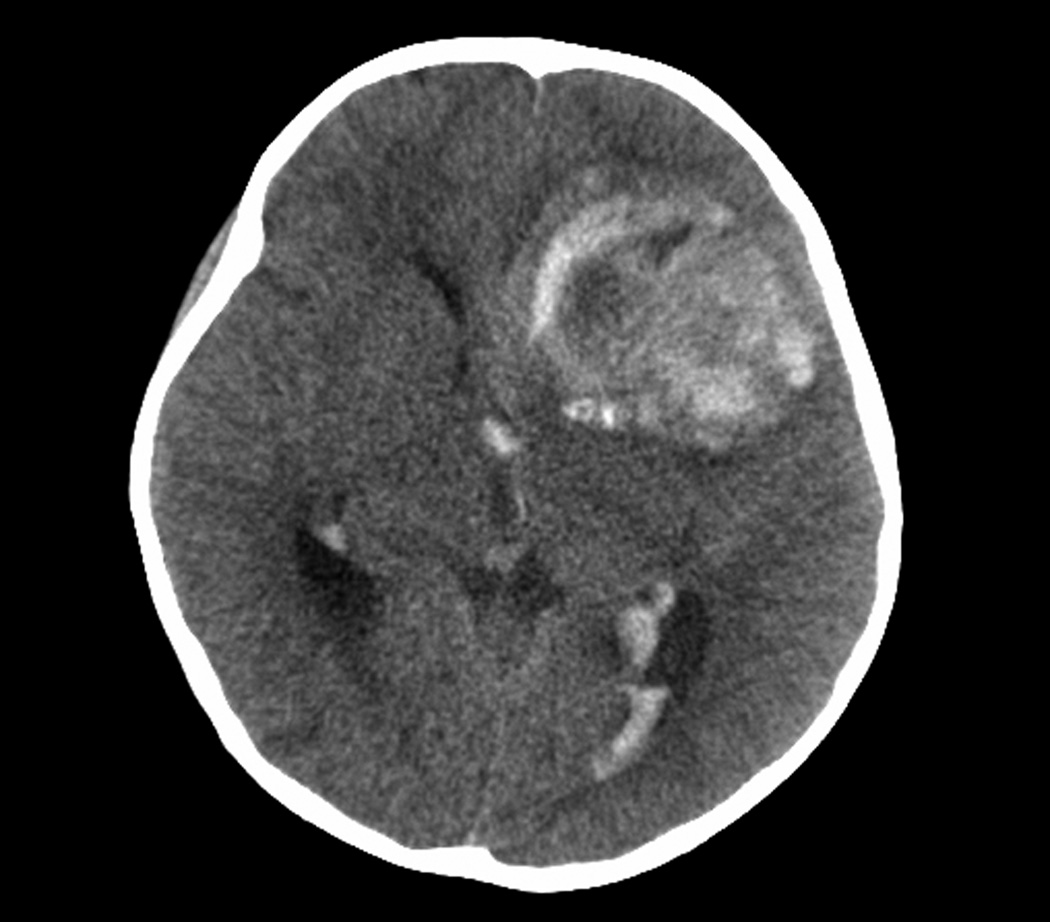
Two tumours originated in the cerebral cortex and four in the diencephalon. (Table 1) Three patients had an acute, symptomatic intra-tumoural and intra-ventricular hemorrhage at diagnosis. (Figure 1) Three patients had disseminated leptomeningeal tumour at diagnosis. All tumours had varying degrees of enhancement with gadolinium. Five evaluable patients had tumours with restricted diffusion.
Figure 1.
Intra-tumoral hematoma and spread of hemorrhage inside the ventricular system at diagnosis in patient 4
Patient 3 had long-lasting radiological abnormalities preceding the diagnosis of e-GB. She was diagnosed with hydrocephalus and required a ventriculo-peritoneal (VP) shunt at the age of 1 month. A brain MRI obtained at 3 years of age disclosed an abnormal area in the posterior left thalamus, which was isointense on T-1 and hypointense on T-2 weighted MRI. A brain CT obtained at 7 years of age demonstrated that this same area was heavily calcified. A brain CT obtained at the time of diagnosis of e-GB showed these same calcifications within the tumour.
Treatment after Diagnosis
Patients 1 and 5 received local radiation therapy (RT; total dose of 59.4 Gy). Patient 1 received erlotinib (Tarceva, OSI Pharmaceuticals, Melville, NY; Roche, Basel, Switzerland; Genentech, South San Francisco, CA) concurrently with RT [31]. Patient 5 received vorinostat (Zolinza, Merck Sharp & Dohme, Whitehouse Station, NJ) concurrently with RT, followed by a combination of temozolomide (Temodar, Merck Sharp & Dohme, Whitehouse Station, NJ) and bevacizumab (Avastin, Genentech, South San Francisco, CA) after completion of RT. Patients 2 and 4, who had leptomeningeal dissemination at diagnosis, underwent a near-total resection of the primary tumour, followed by craniospinal RT (local tumour doses of 54 and 55.8 Gy, respectively; craniospinal dose between 30.6 and 39.6 Gy). Patient 4 received oral etoposide after completion of RT. Patient 3 received only local RT despite the presence of tumour cells in the cerebrospinal fluid because of her poor clinical condition. Patient 6 initially underwent surgical resection alone, while waiting for the confirmation of his diagnosis. Local tumour progression was diagnosed 97 days after surgery, at which time he underwent a second tumour resection followed by craniospinal RT.
Radiological Characteristics at Progression
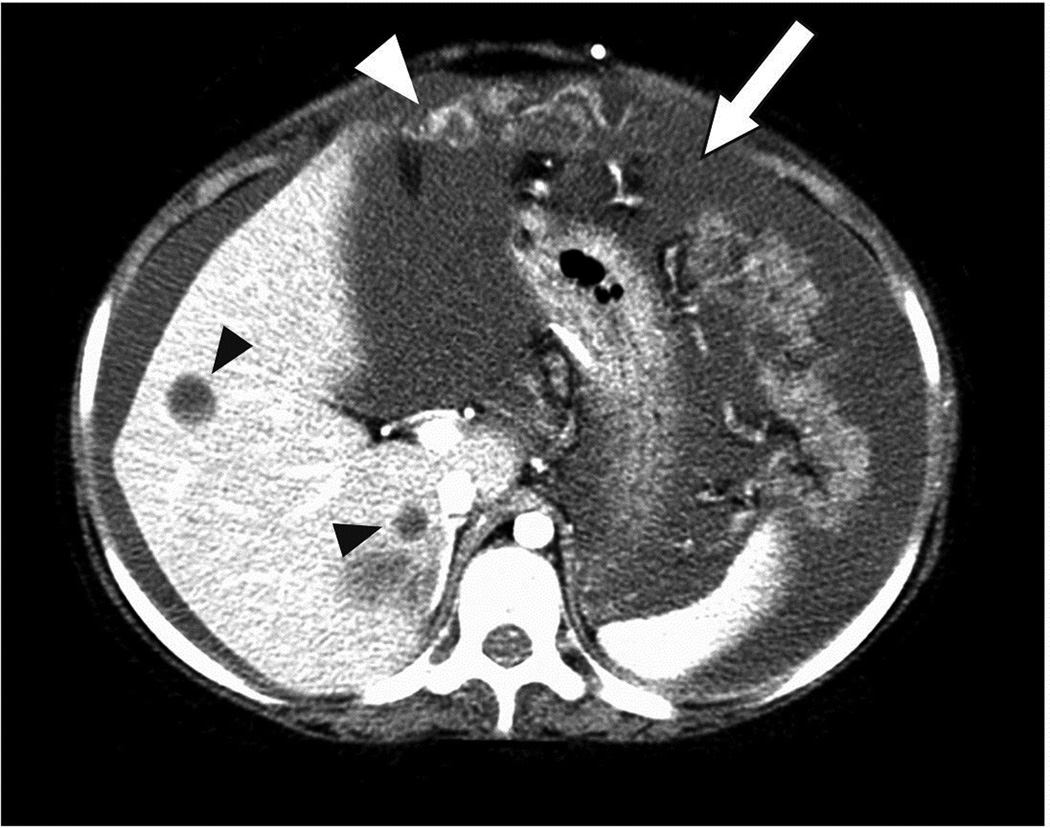
Patients 1 and 3 experienced disease progression at sites outside the CNS, while receiving local RT; histological confirmation was obtained in both cases. (Table 1) Patient 1 developed tumour spread in the scalp near the site of VP shunt insertion. Patient 3, who also had a VP shunt, was diagnosed with neoplastic ascites, peritoneal tumour deposits, and multiple liver metastases. (Figure 2) Patients 2 and 4 experienced rapid progression of their leptomeningeal disease despite the use of craniospinal RT. Patient 5 developed tumour dissemination in the leptomeninges during chemotherapy and within 3 months of completing local RT. Patient 6 experienced massive local tumour progression following surgery alone. He developed leptomeningeal spread of the tumour shortly after completion of craniospinal RT. Patients 1 and 4 experienced further intra-tumoural haemorrhage after tumour progression.
Figure 2.
Neoplastic ascites (white arrow) and peritoneal tumor deposits (white arrowhead), and multiple liver metastases (black arrowheads) as the sites of first progression in patient 3
Histopathological Evaluation
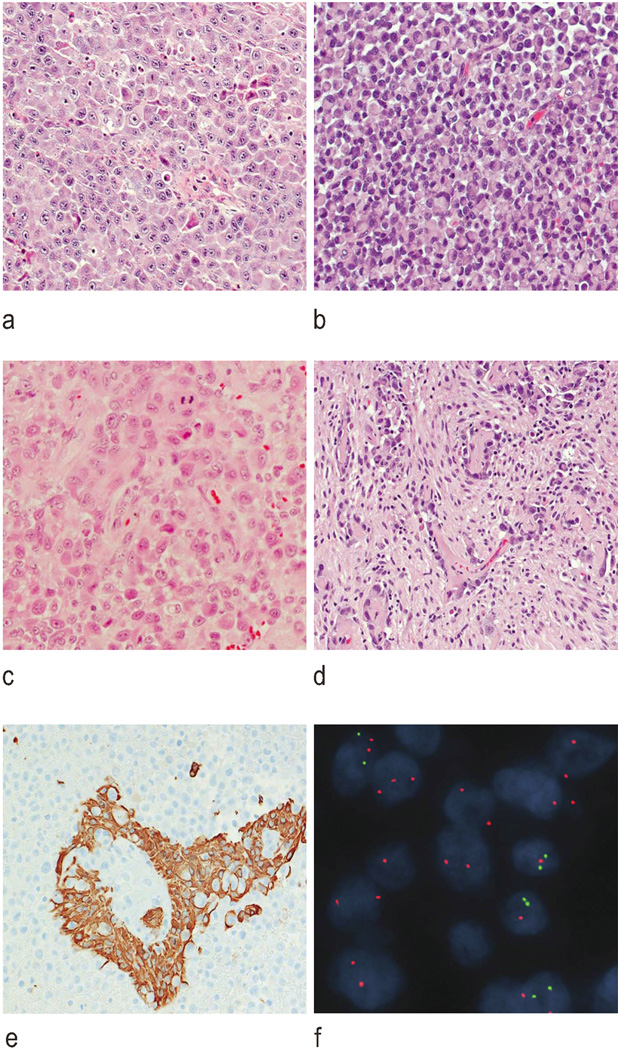
While all e-GBs contained areas of pleomorphic cells with an astrocytic phenotype, they were dominated by groups of relatively monomorphic cells with distinct plasma membranes and eccentrically placed nuclei. Varying subtly from region to region, the cytology could be interpreted as blending epithelioid, rhabdoid, and gemistocytic phenotypes. (Figure 3) Mitotic figures were readily found among the tumour cells, and microvascular proliferation and necrosis were further histological features. The vasculature in these tumours appeared indistinguishable from that of a classic glioblastoma. All tumours contained at least a few small foci of haemosiderin-laden macrophages, suggesting pre-operative intra-tumoural haemorrhage. One e-GB contained two small areas with a gliosarcomatous phenotype, but no tumour contained foci of epithelial metaplasia, and lipidization was never a prominent cytological feature. Whereas the tumour initially resected from patient 1 harboured areas with astrocytic and epithelioid phenotypes, the tumour obtained at progression contained only the epithelioid component. Immunohistochemistry showed that all e-GBs contained a few foci of GFAP-positive cells. (Figure 3) Immunoreactivities for INI1 and BRG1 were universal in all e-GBs. The tumour from patient 1 contained rare cytokeratin-positive cells. Otherwise, all e-GBs failed to express synaptophysin, NFP, EMA, desmin, smooth muscle actin, and melan A. Only one tumour (patient 1) showed strong and widespread nuclear immunoreactive for P53.
Figure 3.
Histopathological characteristics of epithelioid glioblastoma (e-GB) in children. Several cytological phenotypes characterize these tumors, including epithelioid (a), rhabdoid (b), and astrocytic/gemistocytic (c). e-GBs resemble classic glioblastomas in some areas (d; all H&E, ×200). Immunoreactivity for GFAP was variable in tumor cells (e; anti-GFAP antibody, ×200). Homozygous deletion of CDKN2A was evident in two patients (f; iFISH: green and red fluorochromes targeted CDKN2A and the control locus on 9q, respectively)
Molecular Characteristics
Molecular analysis revealed that three of six e-GBs (50%) contained a BRAF: p.V600E mutation. (Table 2) Two of these tumours were thalamic and one originated in the cerebral cortex. One of the thalamic tumours (patient 1) also harbored an H3F3A p.K27M mutation, which was present at diagnosis and recurrence. No mutations were detected at H3F3A p.G34, HIST1H3B p.K27M, IDH1 p.R132, and IDH2 p.R172. iFISH analysis revealed EGFR amplification in one case. (Table 3) The tumour from patient 4 showed homozygous deletion of CDKN2A. None of the cases with BRAF: p.V600E showed concomitant homozygous deletion of CDKN2A. The tumour of patient 6 showed hemizygous deletion of PTEN. Monosomy 22q, as indicated by loss of one probe signal at both the SMARCB1 locus and at 22q13.3, was detected in the tumour from patient 2.
Table 2.
Analysis of hotspot mutations in epithelioid glioblastoma
| Patient # |
Surgery | Single Nucleotide Variations | |||||
|---|---|---|---|---|---|---|---|
| BRAF: p.V600E |
H3F3A p.K27M |
H3F3A p.G34 |
HIST1H3B p.K27M |
IDH1 p.R132 |
IDH2 p.R172 |
||
| 1 | 1 | + | + | − | − | − | − |
| 2 | + | + | − | − | − | − | |
| 2 | + | − | − | − | − | − | |
| 3 | + | − | − | − | − | − | |
| 4 | − | − | − | − | − | − | |
| 5 | − | − | − | − | − | − | |
| 6 | − | − | − | − | − | − | |
Abbreviations: +, mutation present; −, mutation absent
Surgery 1 and 2 indicate tumor resected at diagnosis and at the time of progression, respectively
Table 3.
FISH analysis of copy number gains and losses in patients with epithelioid glioblastoma
| Patient # |
Surgery | Copy Number Abnormalities | |||||
|---|---|---|---|---|---|---|---|
| PDGFRA (4q12) | EGFR (7p12) | MET (7q31) | CDKN2A (9p21) | PTEN (10q23) | SMARCB1 (22q11) | ||
| 1 | 1 | normal | polysomy 3–4 | polysomy 3–4 | normal | normal | normal |
| 2 | polysomy 3–4 | polysomy 3–7 | polysomy 3–7 | polysomy 3–4 | polysomy 3–4 | polysomy 3–4 | |
| 2 | normal | polysomy 3–4 | polysomy 3–4 | normal | normal | monosomy | |
| 3 | normal | polysomy 3–4 | polysomy 3–4 | polysomy 3–4 | polysomy 3–4 | polysomy 3–4 | |
| 4 | polysomy 3–4 | amplified | hemizygous deletion | homozygous deletion | normal | normal | |
| 5 | normal | polysomy 3–4 | polysomy 3–4 | polysomy 3–4 | monosomy | normal | |
| 6 | polysomy 3–4 | polysomy 3–4 | polysomy 3–4 | monosomy | hemizygous deletion | normal | |
Surgery 1 and 2 indicate tumor resected at diagnosis and at the time of progression, respectively
Outcome
All patients died of disease progression after a median survival of 169 days (range, 42 to 290 days). (Table 1) Whole-body autopsy was obtained after death in patient 2. Patient 4 underwent a brain-only autopsy. Patient 2 had residual tumour in the primary cerebral site and leptomeninges, and widespread involvement of all parenchymal organs outside the CNS and of mediastinal and abdominal lymph nodes. Patient 4 had extensive tumour involvement of the brain and subarachnoid space and a large area of recent intra-tumoural hemorrhage.
Discussion
Only a few case series have previously described e-GB, a very rare variant of glioblastoma [12, 14–16]. Although most patients have been adults, three children with e-GB were included in one of these studies [16]. Here, we describe a very aggressive disease course in the largest series to date of paediatric e-GB. Three of our patients (patients 1, 2, and 4) presented acutely because of massive intra-tumoural and intra-ventricular hemorrhage, and three patients (patients 2, 3, and 4) had leptomeningeal tumour dissemination at diagnosis. Two patients with non-metastatic tumours (patients 1 and 5) had either tumour progression outside the CNS or leptomeningeal dissemination during or shortly after completion of RT. Two of our patients developed further intra-tumoural hemorrhage at progression.
Although glioblastoma in children remains a deadly cancer, this pattern of aggressive tumour behaviour has rarely been reported in previous studies. Spontaneous, symptomatic haemorrhage was found in less than 10% of paediatric high-grade gliomas, including glioblastoma [32, 33]. Leptomeningeal dissemination at diagnosis was described in approximately 5% of high-grade gliomas in children [34], and only a few case reports described dissemination of glioblastoma through a VP shunt outside the CNS in children [35]. Except for patients with e-GBs, we have not observed any association between intra-tumoural haemorrhage and leptomeningeal spread in other patients with nonepithelioid glioblastoma at our institution.
It is possible that the frequent occurrence of intra-ventricular haemorrhage in our patients could partly account for their predisposition to leptomeningeal dissemination at diagnosis. The spread of tumour outside the CNS in patient 3 could also be due to a decision not to deliver craniospinal RT at the consulting institution.
Unlike e-GB in adults which arises predominantly in the cerebral cortex [14, 16], two-thirds of our patients had tumours originating in the diencephalon. One of our patients had imaging abnormalities many years before the diagnosis of e-GB to suggest that the tumour might have undergone malignant transformation.
We have shown that the histological characteristics of e-GB in children are akin to those previously described in adults [12, 14–16]. Histological evaluation at recurrence in one of our patients showed selection of the epithelioid phenotype. Similar to e-GB in adults, our patients’ tumours consistently expressed INI1 [14, 15]. GFAP was only focally expressed. Importantly, all tumours lacked a polyphenotypic expression pattern by immunohistochemistry [14, 15]. Replicating a recent study of mostly adult patients [16], our molecular analysis showed that BRAF: p.V600E is also frequent in paediatric e-GBs. Interestingly, we identified an H3F3A p.K27M mutation in the thalamic tumour of one patient at diagnosis and in the distant recurrence. This histone mutation was previously shown to occur predominantly in midline paediatric glioblastomas, particularly thalamic tumours [29, 37]. Of all chromosome copy number gains and losses analyzed, only a lower prevalence of EGFR amplification seems to distinguish paediatric and adult e-GBs [14, 16]. Unlike e-GB in adults [16], one of our patients had a tumour with a monoallelic chromosome 22q loss which included the SMARCB1 locus.
r-GB, another extremely rare variant of glioblastoma, is an important differential diagnosis of e-GB [15]. Despite the report of immunohistochemical differences between these two entities [15], specifically focal loss of INI1 expression in rhabdoid areas and the expression of multiple proteins from different histogenetic lineages in r-GB, several recent studies described patients with r-GBs whose tumours met the diagnostic criteria for e-GB [17–21].
Other tumours that enter the differential diagnoses of e-GB in adults are metastatic carcinomas and melanomas, but these are less relevant for children [14]. The distinction between e-GB and AT/RT is critical in the paediatric age group and should be more straightforward now that a universal lack of INI1 expression can be demonstrated in the tumour cells of an AT/RT. However, at least two patients in the current study (patients 4 and 6), who were diagnosed before the widespread availability of INI1 immunohistochemistry, were initially referred for treatment with a presumptive diagnosis of AT/RT.
Recent studies have unveiled key molecular abnormalities in paediatric glioblastomas [29, 36, 37]. BRAF: p.V600E mutation has been reported in a subset of other, more common, paediatric high-grade gliomas [38]. One pre-clinical study showed that cells lines and orthotopic xenografts of high-grade glioma harboring a BRAF: p.V600E were sensitive to an oral BRAF inhibitor [39]. Likewise, BRAF inhibition may also be a promising therapy for a subset of children with e-GB.
Acknowledgements
We thank Charlene Henry and the technical staff of Anatomic Pathology, St. Jude Children’s Research Hospital, for their help with immunohistochemistry.
Funding: This work was supported by the United States National Institutes of Health Cancer Center Support (CORE) Grant P30 CA21765, by Musicians Against Childhood Cancer (MACC), by the Noyes Brain Tumor Foundation, and by the American Lebanese Syrian Associated Charities (ALSAC).
List of abbreviations
- WHO
World Health Organization
- CNS
central nervous system
- r-GB
rhabdoid glioblastoma
- e-GB
epithelioid glioblastoma
- AT/RT
atypical teratoid/rhabdoid tumour
- iFISH
interphase fluorescence in situ hybridization
- FFPE
formalin-fixed, paraffin-embedded
- PCR
polymerase chain reaction
- VP
ventriculo-peritoneal
- RT
radiation therapy
Footnotes
Author’s contributions
AB and DWE – study design
AB, RGT, NS, PK Jr, AG, and DWE – collection of cases and data
RGT, JD, RL, DWE – molecular analysis of tumor samples
DWE – histopathological review
AB, NS – radiological review
AB, DWE – manuscript preparation
AB, RGT, NS, PK Jr, JD, RL, AG, DWE – review of the manuscript
Conflict of Interests: The authors have no conflicts of interest to declare in regards to this study.
This work was presented at the 2013 Pediatric Neuro-Oncology Basic and Translational Research Conference, Society of Neuro-Oncology, Fort Lauderdale, May 16–17, 2013.
References
- 1.Kleihues P, Burger PC, Aldape KD, Brat DJ, Biernat W, Bigner DD, Nakazato Y, Plate KH, Giangaspero F, von Deimling A, Ohgaki H, Cavenee WK. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Webster WK, editors. WHO Classification of Tumours of the Central Nervous System. 4th edn. Lyon: IARC Press; 2007. pp. 33–49. [Google Scholar]
- 2.Kepes JJ, Fulling KH, Garcia JH. The clinical significance of“adenoid” formations of neoplastic astrocytes, imitating metastatic carcinoma, in gliosarcomas. A review of five cases. Clin Neuropathol. 1982;1:139–150. [PubMed] [Google Scholar]
- 3.Kornfeld M. Granular cell glioblastoma: a malignant granular cell neoplasm of astrocytic origin. J Neuropathol Exp Neurol. 1986;45:447–462. [PubMed] [Google Scholar]
- 4.Mørk SJ, Rubinstein LJ, Kepes JJ, Perentes E, Uphoff DF. Patterns of epithelial metaplasia in malignant gliomas. II. Squamous differentiation of epithelial-like formations in gliosarcomas and glioblastomas. J Neuropathol Exp Neurol. 1988;47:101–118. doi: 10.1097/00005072-198803000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Burger PC, Pearl DK, Aldape K, Yates AJ, Scheithauer BW, Passe SM, Jenkins RB, James CD. Small cell architecture--a histological equivalent of EGFR amplification in glioblastoma multiforme? J Neuropathol Exp Neurol. 2001;60:1099–1104. doi: 10.1093/jnen/60.11.1099. [DOI] [PubMed] [Google Scholar]
- 6.Kraus JA, Lamszus K, Glesmann N, Beck M, Wolter M, Sabel M, Krex D, Klockgether T, Reifenberger G, Schlegel U. Molecular genetic alterations in glioblastoma with oligodendroglial component. Acta Neuropathol. 2001;101:311–320. doi: 10.1007/s004010000258. [DOI] [PubMed] [Google Scholar]
- 7.Brat DJ, Scheithauer BW, Medina-Flores R, Rosenblum MK, Burger PC. Infiltrative astrocytomas with granular cell features (granular cell astrocytomas): a study of histopathologic features, grading, and outcome. Am J Surg Pathol. 2002;26:750–757. doi: 10.1097/00000478-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ozolek JA, Finkelstein SD, Couce ME. Gliosarcoma with epithelial differentiation: immunohistochemical and molecular characterization. A case report and review of the literature. Mod Pathol. 2004;17:739–745. doi: 10.1038/modpathol.3800109. [DOI] [PubMed] [Google Scholar]
- 9.Perry A, Aldape KD, George DH, Burger PC. Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer. 2004;101:2318–2326. doi: 10.1002/cncr.20625. [DOI] [PubMed] [Google Scholar]
- 10.Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131:397–406. doi: 10.5858/2007-131-397-G. [DOI] [PubMed] [Google Scholar]
- 11.Perry A, Miller CR, Gujrati M, Scheithauer BW, Zambrano SC, Jost SC, Raghavan R, Qian J, Cochran EJ, Huse JT, Holland EC, Burger PC, Rosenblum MK. Malignant gliomas with primitive neuroectodermal tumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol. 2009;19:81–90. doi: 10.1111/j.1750-3639.2008.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenblum MK, Erlandson RA, Budzilovich GN. The lipid-rich epithelioid glioblastoma. Am J Surg Pathol. 1991;15:925–934. doi: 10.1097/00000478-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt-Ashmead J, Kleinschimdt-DeMasters BK, Hill DA, Mierau GW, McGavran L, Thompson SJ, Foreman NK. Rhabdoid glioblastoma. Clin Neuropathol. 2001;20:248–255. [PubMed] [Google Scholar]
- 14.Rodriguez FJ, Scheithauer BW, Giannini C, Bryant SC, Jenkins RB. Epithelial and pseudoepithelial differentiation in glioblastoma and gliosarcoma: a comparative morphologic and genetic study. Cancer. 2008;113:2779–2789. doi: 10.1002/cncr.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinschmidt-DeMasters BK, Alassiri AH, Birks DK, Newell KL, Moore W, Lillehei KO. Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI-1 but not claudin 6. Am J Surg Pathol. 2010;34:341–354. doi: 10.1097/PAS.0b013e3181ce107b. [DOI] [PubMed] [Google Scholar]
- 16.Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. 2013;37:685–698. doi: 10.1097/PAS.0b013e31827f9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lath R, Unosson D, Blumbergs P, Stahl J, Brophy BP. Rhabdoid glioblastoma: a case report. J Clin Neurosci. 2003;10:325–328. doi: 10.1016/s0967-5868(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel J, Silva R, Pimentel T. Primary malignant rhabdoid tumors of the central nervous system: considerations about two cases of adulthood presentation. J Neurooncol. 2003;61:121–126. doi: 10.1023/a:1022135518846. [DOI] [PubMed] [Google Scholar]
- 19.Babu R, Hatef J, McLendon RE, Cummings TJ, Sampson JH, Friedman AH, Adamson C. Clinicopathological characteristics and treatment of rhabdoid glioblastoma. J Neurosurg. 2013 May 3; doi: 10.3171/2013.3.JNS121773. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.He MX, Wang JJ. Rhabdoid glioblastoma: case report and literature review. Neuropathology. 2011;31:421–426. doi: 10.1111/j.1440-1789.2010.01166.x. [DOI] [PubMed] [Google Scholar]
- 21.Momota H, Iwami K, Fujii M, Motomura K, Natsume A, Ogino J, Hasegawa T, Wakabayashi T. Rhabdoid glioblastoma in a child: case report and literature review. Brain Tumor Pathol. 2011;28:65–70. doi: 10.1007/s10014-010-0010-4. [DOI] [PubMed] [Google Scholar]
- 22.Allen JC, Judkins AR, Rosenblum MK, Biegel JA. Atypical teratoid/rhabdoid tumor evolving from an optic pathway ganglioglioma: case study. Neuro Oncol. 2006;8:79–82. doi: 10.1215/S1522851705000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chacko G, Chacko AG, Dunham CP, Judkins AR, Biegel JA, Perry A. Atypical teratoid/rhabdoid tumor arising in the setting of a pleomorphic xanthoastrocytoma. J Neurooncol. 2007;84:217–222. doi: 10.1007/s11060-007-9361-z. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt-DeMasters BK, Birks DK, Aisner DL, Hankinson TC, Rosenblum MK. Atypical teraroid/rhabdoid tumor arising in a ganglioglioma: genetic characterization. Am J Surg Pathol. 2011;35:1894–1901. doi: 10.1097/PAS.0b013e3182382a3f. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Nishihara H, Katoh M, Yoshinaga T, Mahabir R, Kanno H, Kimura T, Tanino M, Ikeda J, Sawamura Y, Nagashima K, Tanaka S. Case of atypical teratoid/rhabdoid tumor in an adult, with long survival. Brain Tumor Pathol. 2011;28:71–76. doi: 10.1007/s10014-010-0008-y. [DOI] [PubMed] [Google Scholar]
- 26.Endo S, Terasaka S, Yamaguchi S, Ikeda H, Kato T, Kobayashi H, Tanaka S, Houkin K. Primary rhabdoid tumor with low grade glioma component of the central nervous system in a young adult. Neuropathology. 2013;33:185–191. doi: 10.1111/j.1440-1789.2012.01336.x. [DOI] [PubMed] [Google Scholar]
- 27.Langdon JA, Lamont JM, Scott DK, Dyer S, Prebble E, Bown N, Grundy RG, Ellison DW, Clifford SC. Combined genome-wide allelotyping and copy number analysis identify frequent genetic losses without copy number reduction in medulloblastoma. Genes Chromosomes Cancer. 2006;45:47–60. doi: 10.1002/gcc.20262. [DOI] [PubMed] [Google Scholar]
- 28.Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW, Jones TA, Aarum J, Dalton J, Bailey S, Chaplin T, Carter RL, Gajjar A, Broniscer A, Young BD, Ellison DW, Sheer D. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 29.Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Zhang J, Baker SJ., St Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broniscer A, Baker SJ, Stewart CF, Merchant TE, Laningham FH, Schaiquevich P, Kocak M, Morris EB, Endersby R, Ellison DW, Gajjar A. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res. 2009;15:701–707. doi: 10.1158/1078-0432.CCR-08-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982;10:437–444. doi: 10.1227/00006123-198204000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Dropcho ED, Wisoff JH, Walker RW, Allen JC. Supratentorial malignant gliomas in childhood: a review of fifty cases. Ann Neurol. 1987;22:355–364. doi: 10.1002/ana.410220312. [DOI] [PubMed] [Google Scholar]
- 34.Benesch M, Wagner S, Berthold F, Wolff JE. Primary dissemination of high-grade gliomas in children: experiences from four studies of the Pediatric Oncology and Hematology Society of the German Language Group. J Neurooncol. 2005;72:179–183. doi: 10.1007/s11060-004-3546-5. [DOI] [PubMed] [Google Scholar]
- 35.Newton HB, Rosenblum MK, Walker RW. Extraneural metastases of infratentorial glioblastoma multiforme to the peritoneal cavity. Cancer. 1992;69:2149–2153. doi: 10.1002/1097-0142(19920415)69:8<2149::aid-cncr2820690822>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 36.Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Frühwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Dürken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jäger N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Frühwald MC, Roggendorf W, Kramm C, Dürken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzweski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley MY, Yu M, Fisher PG, Rowitch DH, Ford JM, Berger MS, Ji H, Gutmann DH, James CD. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolaides TP, Li H, Solomon DA, Hariono S, Hashizume R, Barkovich K, Baker SJ, Paugh BS, Jones C, Forshew T, Hindley GF, Hodgson JG, Kim JS, Rowitch DH, Weiss WA, Waldmann TA, James CD. Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res. 2011;17:7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]