Abstract
Ultra-endurance competitions are becoming increasingly popular but there is limited research on female participants. The purpose of this study was to examine changes in estrogen and the IGF-I system in women after an ultra-marathon. Six pairs of pre- and post- menopausal women were matched for race finish times;mean finish time was 20 hours. Blood samples were drawn 24 hours before the race, at the finish, and 24 hours into recovery. Samples were analysed for estradiol, total IGF-I, IGFBP-1, and intact IGFBP-3. There was a significant increase in estradiol following the race in both groups (P < 0.05). Total IGF-I decreased after the race (P < 0.01) and remained lower in recovery. IGFBP-1 increased after the race (P < 0.001) but returned to pre-race levels after 24 hours, while intact IGFBP-3 was significantly lower post-race and in recovery (P < 0.001). Postmenopausal women had significantly lower estradiol at baseline, but there were no other group differences. These results demonstrate that among recreational female runners, an ultra-marathon is associated with IGF system changes that are consistent with an energy-deficient, catabolic state. Further research is needed to confirm the effect of these endocrine changes on health and performance.
Keywords: ultra-endurance, exercise, IGF-I, IGFBP-1, IGFBP-3, estrogen
INTRODUCTION
Ultra-endurance competition is defined as any event that exceeds 6 hours in duration [31], but can range from 50 km road races to multi-day events covering thousands of kilometers over varying terrain. Numerous studies have documented the physiological stress of ultra-endurance activity, either in the form of competition or military exercises, and it has been associated with a significant endocrine response [1, 15, 23]. However, the majority of studies have focused on young males and women are underrepresented in the literature, despite the fact that increasing numbers of women are participating in these long-duration events.
Ultra-marathons were once thought to be reserved for elite or “extreme” athletes, but now these events attract recreational athletes of all ages. In most cases the combination of long distance, rough terrain, and elevation changes results in a lower pace than a conventional footrace and significant portions of the race may be covered by walking as opposed to running, particularly among recreational athletes. Furthermore, food and water are typically freely available. Taken together, these factors may suggest that the physiological stress of these events is minimal for non-competitive athletes. One goal of this study was to document the endocrine response to an ultra-marathon among recreational female participants.
The insulin-like growth factor I (IGF-I) system can provide an indication of physiological strain, negative energy balance, and fatigue [6, 20, 23]. IGF-I is a polypeptide that plays a critical role in the growth and development of many tissues and is associated with numerous health and exercise outcomes [26]. The actions of IGF-I are mediated by a family of IGF binding proteins that regulate the bioavailability of IGF-I in circulation. A negative energy balance, either from prolonged exercise, caloric restriction, or both, results in lower bioavailable IGF-I [8, 20]. While this may be an adaptive response that diverts substrate from growth processes to acute energy demands [8], the importance of the IGF system for musculoskeletal integrity among women should also be considered [24]. The bioavailability of IGF-I is influenced by female sex steroids [9, 30] and given this, one might expect to find different results in postmenopausal women with low circulating estrogen, yet to our knowledge no studies have examined the hormonal response to ultra-endurance exercise specifically in postmenopausal women. Thus, the second objective of this study was to compare circulating concentrations of estradiol and components of the IGF-I system between pre- and post-menopausal women before and after completing an ultra-marathon.
MATERIALS AND METHODS
Participants
Twenty-six women (7 postmenopausal) who were registered to compete in an ultra-marathon volunteered for this study. Complete data were collected from 25 women, and following the race pairs of pre- and post-menopausal women were matched based on race finish times to control for differences in performance and exercise intensity. Ultimately the matching process yielded six pairs of women and so the final sample size was 12. Menopause was defined as an absence of menses for at least 12 months. Although it was not possible to control for phase of menstrual cycle, all the premenopausal women were in the follicular phase of their cycle (day 1 to 13), based on self-reported menstrual cycles. All participants were screened for contraindications and exclusion criteria included: use of HRT or hormone contraceptives, amenorrhea, or any injuries or medical conditions that would prohibit normal training or active participation in the testing protocols. The procedures were reviewed and approved by the Institutional Human Subject Research Committee and met the ethical standards of the Helsinki Declaration. All participants gave written informed consent.
Procedures
This was a field-based observational study conducted using a real ultra-marathon competition. The event was an off-road race over primarily single-track dirt paths with 1300 metres of elevation change over 1 lap of the course (∼50km). The race has 3 possible distances: 50km, 100km, and 100 mile, which require 1, 2 or 3 laps respectively. Four pairs of women completed 100km and 2 pairs completed 50km. The event has a maximum time limit of 35 hours, with a 100 km median finishing time of approximately 19 hours for men and 22 hours for women and a 50km median finishing time of 8 hours for men and 9.5 hours for women. There are 5 aid stations over the course and participants consumed food and drink ad libitum. The average daytime temperature on race day was 20.6°C with a high of 27.5°C and no precipitation.
At least one week prior to the event each participant was assessed for cardiorespiratory fitness and relative body fat. The day prior to the event a resting blood sample was drawn and in order to minimize the effects of circadian changes in hormones, the time of the sampling was adjusted to approximate each participant's anticipated finish times the next day. Participants were advised to avoid strenuous exercise for 24 hours, food for 3 hours, and caffeine for 4 hours, prior to the pre-race blood draw. A second blood sample was drawn at the finish line, and a third sample was drawn approximately 24 hours after the event, as close as possible to the same time of day as the previous samples.
Determination of Cardiorespiratory Fitness and Anthropometric Measurements
Anthropometric measures included height and weight, and relative fat was determined using the Jackson-Pollock 7 -site skinfold protocol [12]. Peak oxygen uptake was determined using a graded exercise test on a treadmill. The speed remained constant throughout the test while the grade was increased 2% every 2 minutes until volitional fatigue. Oxygen consumption was measured using the Vista Mini CPX open-circuit spirometry system and the TurboFit version 5.09 software (VacuMed, Ventura, CA).
Blood Samples
Blood samples were drawn from an antecubital arm vein using standard venipuncture procedures. Pre-race and 24 hour recovery samples were collected in the lab and post-race samples were collected at a medical station at the finish line within 15 minutes of finishing the race. All blood samples were analysed for hematocrit and then allowed to clot on ice for one hour before centrifugation. Aliquots of serum were stored at -80°C until assayed.
Endocrine measurements
Blood samples were analysed for estradiol and for circulating components of the IGF-I system including total IGF-I, IGFBP-1 and IGFBP-3. It has been suggested that what are often explained as exercise-induced increases in IGFBP-3 are actually increases in IGFBP-3 fragments that result from proteolysis [1, 27]. To address this issue, we measured intact or functional IGFBP-3 using a ligand-binding immunoassay. The assay uses biotinylated IGF-I as the ligand which will bind to intact IGFBP-3 in diluted samples. By utilizing a strepavidin-peroxidase conjugate, only complexes of IGFBP-3/biotinylated IGF-I are involved in signal generation and only functional IGFBP-3 is measured. An assay based on similar principles has been shown to provide an accurate assessment of intact IGFBP-3 among a variety of patient populations and controls [16].
Samples were analysed using ELISA for: estradiol (DIAsource; Nivelles, Belgium) total insulin-like growth factor I (DIAsource;Nivelles, Belgium), bioactive IGFBP-3 (Mediagnost, Reutlingen, Germany), and IGFBP-1 (ALPCO Diagnostics;Salem, USA). Assay sensitivities were: 5 pgml–1 for estradiol, 1.1 ngml–1 for IGF- I, 0.18 ngml–1 for bioactive IGFBP-3, and 0.5 µgL–1 for IGFBP-1. Samples were analysed in duplicate and all samples from a given subject were assayed in the same run to minimize the effects of inter-assay variation. Average intra-assay variation was: 4.9% for estradiol, 2.8% for IGF- I, 6.5% for IGFBP-3 and 8.0% for IGFBP-1.
Statistical Analysis
Examination of skewness, kurtosis, and histograms of all measures demonstrated that the concentrations of estradiol and IGFBP-1 violated the assumption of a normal distribution. Subsequently, the estradiol and IGFBP-1 were transformed to meet the assumptions of the analysis using square root and log transformations, respectively. The transformed values were used for analysis, however untransformed data are presented in tables and figures. Differences in baseline hormone concentrations between groups were examined using independent sample t-tests and repeated measures ANOVA was used to determine if there were differences in hormone and binding protein concentrations across time and between groups. Spearman's Rho correlations were used to examine relationships among hormone concentrations. Molar volume ratios of IGF-I to IGFBP-3 were also compared across time and between groups. The molar volume ratios were calculated according to the procedure outlined by Nindl et al. [22]. The molecular masses used were 7.5kDa for IGF-I and 40kDa for IGFBP-3 [19], and the sample volumes used were 25µL and 10µL respectively. All statistical analyses were performed using SPSS version 15 software and statistical significance was set at p < 0.05.
RESULTS
Participants
Participant characteristics are shown in Table 1. The participants were healthy recreational runners with a range of experience in long-distance running. Three participants were running their first ultra and the others had completed anywhere from 2 to 7 previous events. The self-reported maximum weekly training distance reached during preparation for the race ranged among participants between 30 and 100 kilometers, with an average of approximately 50 km. Finish times ranged from 140 to 172% of the winning time in each event. There were no significant differences between the two groups in relative body fat, cardiorespiratory fitness, or race finish times.
TABLE 1.
PARTICIPANT CHARACTERISTICS. MEAN ± SD (RANGE)
| Pre-menopausal (N = 6) | Post-menopausal (N = 6) | |
|---|---|---|
| Age (years) | 37.0 ± 5.4 (30-44) | 57.2 ± 3.8 (53-62) |
| BMI (kg∙m–2) | 23.6±3.1 (20.4-25.5) | 23.8± 2.3 (20.7-26.7) |
| Fat (%) | 26.0±6.5 (16.7-33.4) | 25.4 ± 5.5 (19.4-33.8) |
| VO2peak (ml∙kg–1∙min–1) | 55.1 ± 5.0 (N = 4) (48.5-60.1) | 51.0±2.6 (N = 4) (48.8-54.7) |
| Race finish time (hours) | 19.2± 7.7 (9.1-26.5) | 20.8±8.4 (9.5-26.7) |
| Average pace (km∙h–1) | 4.57 ± .67 (3.8-5.5) | 4.21 ± .64 (3.7-5.3) |
Changes in Plasma Volume
We assessed changes in hematocrit and calculated relative change in plasma volume according to the procedures of Van Beaumont 29]. Hematocrit decreased significantly across time in both groups (P < 0.05) and 10 of the 12 participants had a decrease in hematocrit from the pre-race to finish line samples. Average hematocrit values for premenopausal women were 39.7±2.9, 37.2±4.2, and 33.9±2.1 for pre-race, post-race, and recovery samples, respectively. For postmenopausal women hematocrit values were 39.5±2.9, 36.3±7.0, and 35.3±7.1. The average change in plasma volume from pre-race to post-race was 12.5 ± 17% for premenopausal women and 18.0±25.4% for postmenopausal women. There was no significant difference in hematocrit between groups.
Changes in Hormone and Binding Protein Concentrations
Hormone concentrations were corrected for changes in plasma volume [29] and both corrected and uncorrected values were analysed. The relative changes in hormone concentrations are provided for both corrected and uncorrected values, however all statistical analyses and graphs presented used uncorrected data, as it is the concentration of hormone the target tissues are exposed to that is likely most relevant. Analysis with corrected or uncorrected data did not change which main effects were significant at P < 0.05.
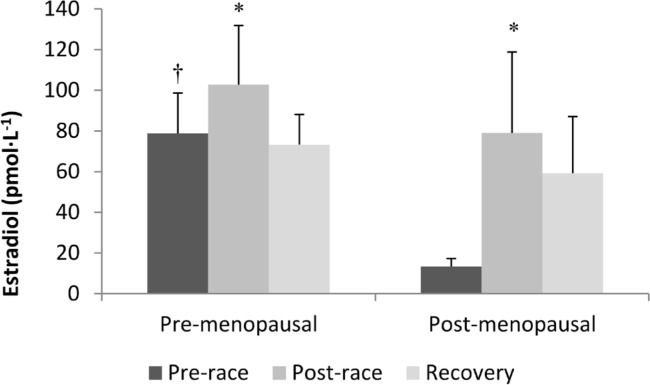
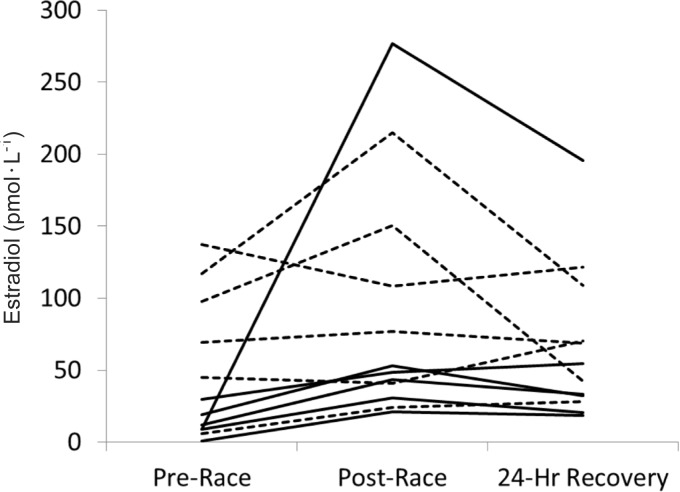
Before the race, estradiol was significantly higher in the pre-menopausal women (P < 0.05). Estradiol increased, on average, 97% (139% with plasma volume correction) from pre-race to post-race (P < 0.05) (Figure 1), with 10 of the 12 participants having a greater than 10% increase and 9 of 12 having a greater than 50% increase (Figure 2). 24-hour recovery estradiol was not significantly different than pre-race or post-race samples. There was no significant group effect, however, there was a trend towards a significant group by time interaction (P = 0.09).
FIG. 1.
SERUM ESTRADIOL CONCENTRATIONS BEFORE, AFTER, AND 24 HOURS AFTER AN ULTRA-MARATHON IN PRE- AND POST-MENOPAUSAL WOMEN.
Note:Data are expressed as means ± SEM. Black bars and pre-race values, dark grey bars are immediately post-race values and light grey bars are 24 hour recovery values. † significantly different than pre-race value in post-menopausal women (P < 0.05). * significantly different than pre-race value (P < 0.05).
FIG. 2.
INDIVIDUAL PARTICIPANT CHANGES IN CIRCULATING ESTRADIOL BEFORE, AFTER, AND 24 HOURS AFTER AN ULTRA-MARATHON.
Note: Broken lines are pre-menopausal women and solid lines are post-menopausal women.
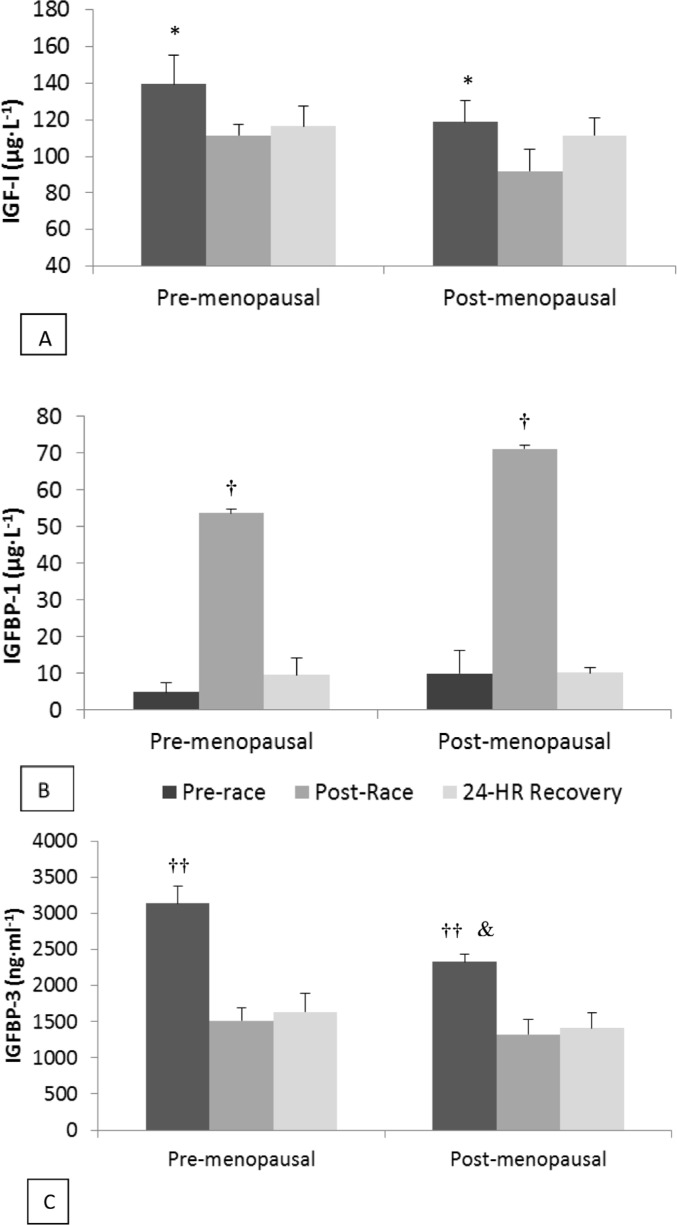
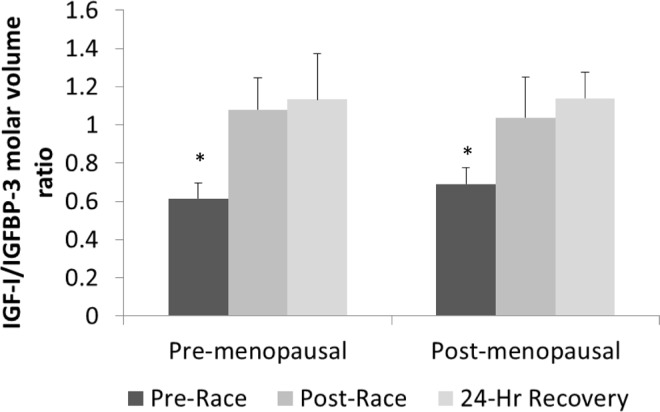
Figure 3 shows the pre- and post-race concentrations of IGF-I, IGFBP-3, and IGFBP-1 in both groups. Circulating IGF-I decreased by 21% (-9.9% with plasma volume correction) after the race compared to pre-race values (P < 0.01) and remained significantly lower than pre-race concentrations after 24 hours of recovery (P < 0.05). There was no difference in IGF-I between groups, although there was a significant correlation between pre-race concentrations of estradiol and IGF-I (ρ = 0.685, P < 0.05) and a negative correlation between estradiol and IGFBP-1 (ρ= -0.678, P < 0.05). Circulating concentrations of IGFBP-1 increased substantially (∼700%;650% with plasma volume correction) after the race in both groups (P < 0.001) but had returned to pre-race levels after 24 hours of recovery. Before the race, intact IGFBP-3 was significantly higher in the pre-menopausal group compared to the post-menopausal group (Figure 3, P < 0.05). IGFBP-3 was significantly lower after the race in both groups (P < 0.001), with an average decrease of 48% (41% with plasma volume correction) and concentrations remained lower than pre-race concentrations after 24 hours of recovery (P < 0.001). The molar volume ratio of IGF-I to intact IGFBP-3 was significantly greater immediately after the race and 24 hours after the race compared to the pre-race ratio (P < 0.01), with no difference between groups (Figure 4). The group x time interaction was not statistically significant for any component of the IGF system.
FIG. 3.
SERUM IGF-I (PANEL A), IGFBP-1 (PANEL B) AND INTACT IGFBP-3 (PANEL C) CONCENTRATIONS BEFORE, AFTER, AND 24 HOURS AFTER AN ULTRA-MARATHON IN PRE- AND POST-MENOPAUSAL WOMEN.
Note:Data are expressed as means ± SEM. Black bars and pre-race values, dark grey bars are immediately post-race values and light grey bars are 24 hour recovery values.
* Significantly greater than post-race (P < 0.01) and recovery (P < 0.05)
† Significantly greater than pre-race and 24 hour recovery (P < 0.001)
†† Significantly greater than post-race and 24 hour recovery (P < 0.01).
& Significantly lower than corresponding value in pre-menopausal women (P < 0.05)
FIG. 4.
MOLAR VOLUME RATIONS OF IGF-I TO INTACT IGFBP-3 BEFORE, AFTER, AND 24 HOURS AFTER AN ULTRA-MARATHON IN PRE- AND POST-MENOPAUSAL WOMEN.
Note:Data are expressed as means ± SEM. Black bars and pre-race values, dark grey bars are immediately post-race values and light grey bars are 24 hour recovery values.
* Significantly lower than post-race and 24 hour recovery values (P < 0.01).
DISCUSSION
Ultra-endurance exercise is associated with large energy demands [17, 31]. While evidently “extreme” events, they increasingly attract runners of all ages and competitive levels. This is the first study to explore the hormonal response to ultra-endurance exercise among both pre- and post-menopausal women. The main findings of the study were 1) circulating components of the IGF-I system changed significantly following the race in a pattern consistent with previous studies of elite athletes and male military personnel 2) estradiol was dramatically increased after the race even among postmenopausal women and 3) there were no significant differences in the IGF-I response between women with differing menstrual status and estrogen at baseline.
This is the first study to examine the response of estradiol among postmenopausal women during ultra-endurance exercise. Both groups showed a significant increase in estradiol immediately following the race with a mean increase of 97%. The relative increase from baseline was greater among postmenopusal women and as a result, there was no significant difference between groups in the post-race estradiol concentrations, despite postmenopausal women having significantly lower levels at baseline.
Previous studies have shown an increase in estradiol in women following prolonged running [2]. Copeland et al. [3] found increased estradiol after both endurance and resistance exercise in women across a wide age range and Kemmler et al. [14] reported an increase in estradiol among postmenopausal women after 60 minutes of exercise. However, all of these studies employed an exercise stimulus that was significantly shorter than the present study. In contrast, Berg et al. [1] found a decrease in estradiol among premenopausal women after ultra-endurance exercise, such that levels were undetectable after a 6 day adventure race. Thus, the large increase we observed among women in response to an ultra-marathon is unique –particularly among postmenopausal women. It is interesting to note that several studies have shown similarly large increases in estradiol among males following prolonged exercise [7, 18]. Taken together with the present findings, it appears that individuals with low circulating estradiol at baseline may demonstrate the greatest increases in response to prolonged exercise. This is supported by Bonen and Keizer [2] who observed the greatest increase in estradiol following a marathon in an amenorrheic female subject.
The mechanism of increased estradiol is not known. Changes in plasma volume could play a role, although the majority of participants displayed a hemodilution at the end of the race, which should decrease the total concentration. Decreased splanchnic blood flow during exercise is known to decrease the metabolic clearance of steroids [13], however, in the present study postmenopausal women had low levels of estradiol prior to the race so it is unlikely that a diminished clearance can fully explain the large increases we observed. Increases among individuals with low baseline levels of estradiol, such as postmenopausal women or men [7, 18], suggest an increased secretion of steroids, however, the source may differ between pre- and post-menopausal women. Changes in estrogen and testosterone in response to exercise have been shown to occur independently of changes in gonadotropins and it has been suggested that sympathetic stimulation of the adrenal glands is the primary source of exercise-induced increases in sex steroids [2].
The implications of the elevated concentrations of estradiol are unclear. Decreased estrogen after menopause is associated with metabolic dysfunction that leads to numerous pathological changes including central obesity and insulin resistance [28]. Thus, increased estrogen could be beneficial and in the present study postmenopausal women still had estradiol concentrations in the pre-menopausal range 24 hours after the race. Further study of the possible health implications is warranted.
Estrogen may also impact performance in long duration events, as a number of studies have shown that estradiol can enhance fat oxidation and consequently diminish hepatic glycogen use [11]. This effect is believed to explain gender differences in fuel oxidation, with women utilizing fat to a greater extent than men during endurance exercise [10]. Greater fat oxidation could improve performance during prolonged endurance exercise, and the increased estradiol observed in the present study may be beneficial. Unfortunately the timing of the samples does not allow us to determine at what point during the race estradiol concentrations began to increase. Further research is needed to confirm the effects of prolonged exercise on estrogen concentrations among hypoestrogenic individuals and the associated effects on health or performance.
The IGF system typically responds positively to short duration exercise with transient increases in circulating IGF-I, IGFBP-3, and IGFBP-1 [4, 25]. However, prolonged exercise and a negative energy balance appear to have the opposite effect, resulting in lower levels of bioavailable IGF-I [8, 20]. The present results confirm these findings and demonstrate that a prolonged trail race, even at a low to moderate pace among recreational runners, is associated with diminished IGF-I, diminished functional IGFBP-3, and increased IGFBP-1 in female runners. It is interesting that the changes in IGF-I did not differ between pre-and post-menopausal women, despite evidence that sex-steroids can influence the IGF system [9, 30]. The lack of group difference in IGF-I or related binding proteins after prolonged exercise may be explained by the fact that estradiol increased in postmenopausal women after the race and reached pre-menopausal concentrations. Waters et al. [30] found that amenorrheic women had the same exercise-induced changes in GH or IGFBP-1with or without estrogen replacement, thus it is possible that the relationship between estrogen and the GH/IGF-I axis is uncoupled during exercise.
Circulating total IGF-I decreased an average of 21% after the race and after 24 hours of recovery remained 11% lower than baseline with no difference between pre-menopausal or postmenopausal runners. This is consistent with the 15% decrease in total IGF-I observed after a 60 km Nordic ski race in young men [21]. The IGF-I system is believed to provide an important measure of the metabolic strain that results from high levels of physical activity combined with restriction of energy and sleep [23, 26]. While elevated IGF-I may be beneficial from the perspective of tissue growth [25], during prolonged endurance exercise lower levels of bioavailable IGF-I may be advantageous as the insulin-like activity of IGF-I could hinder lipid mobilization and have a hypoglycemic effect. Thus, lower levels of IGF-I may represent an adaptive response to the caloric deficit of intense, prolonged exercise [8].
It is also important to consider the IGF-I binding proteins as they affect IGF-I bioavailability. The majority (∼75%) of IGF-I circulates bound in a ternary complex with IGFBP-3 and an acid-labile subunit, and this complex cannot cross the capillary endothelium, thus providing circulating reservoir of IGF-I and prolonging its half-life [19]. Studies of the effects of exercise on IGFBP-3 have produced mixed results [5, 27], with conclusions hampered by the fact that many immunoassays designed to measure IGFBP-3 also detect fragments of BP-3 that result from proteolysis. There is always some degree of IGFBP-3 proteolysis in circulation, however, it is markedly increased in catabolic states such as severe illness, surgery, late pregnancy and possibly intense exercise [16]. Proteolysis may generate IGFBP-3 fragments of 30, 20 and 15 kDa, and these fragments have limited ability to bind and sequester IGF-I. Thus, proteolysis could result in increased IGF-I bioavailability. To address this issue, we measured intact or functional IGFBP-3 using a ligand-binding immunoassay. We observed a 50% decrease in intact IGFBP-3 following the race, which suggests the race was associated with increased proteolysis. Berg et al. [1] found a 26% increase in IGFBP-3 proteolysis after ultra-endurance running and our results suggest that 9 to 26 hours of trail running may also increase IGFBP-3 proteolysis.
Gravholt et al. [9] found IGFBP-3 proteolysis was greater in women with Turner syndrome who have hypoestrogenism and in the present study we found the pre-race concentrations of IGFBP-3 were significantly lower in postmenopausal compared to premenopausal women. Gravholt et al. [9] reported that that treating Turner syndrome patients with sex steroids normalized the proteolysis, so it is possible that the similar estradiol levels that were achieved over the course of the race may explain the similar concentrations of intact IGFBP-3 in both groups of women after the race.
Although both IGF-I and IGFBP-3 decreased, the decrease in BP-3 was greater so the molar ratio of total IGF-I to bioactive IGFBP-3 increased from pre- to post-race and remained significantly elevated in recovery. If the ratio of total IGF-I to IGFBP-3 is a biomarker of IGF-I bioavailability as suggested by Nindl et al. [22], then IGF-I actions would have been increased in the present study. Berg et al. [1] suggested that increased IGF-I bioavailability may be important for protecting against lean tissue loss during prolonged exercise. A combination of increased estradiol and bioavailable IGF-I could have positive effects on musculoskeletal integrity among postmenopausal women.
IGF-I is also regulated by IGFBP-1, which plays an important role in glucose homeostasis and is inversely proportional to insulin concentrations. It is thought to inhibit IGF-I actions by sequestering free IGF-I [19]. IGFBP-1 consistently increases in response to prolonged exercise and Nindl et al. [25] determined that IGFBP-1 is the component of the IGF system most sensitive to physiological strain. In the present study we observed an 8-fold increase in circulating IGFBP-1 after prolonged exercise in women. This effect was transient and IGFBP-1 levels had returned to baseline levels after 24 hours of recovery.
This study has several limitations which should be noted. The study was a field-based observational study and thus there was no control over exercise intensity, or food and water intake. To address the issue of intensity we matched pairs of women for race finish time and assumed they completed the event at a similar pace, however, it is possible that there were variations in pace and breaks at aid stations that contributed to overall finish time and as such average pace could only be estimated. It is also likely that as a result of differences in intake and output the overall energy balance varied among the participants. If we assume the IGF system provides a marker of fatigue and energy balance then the similar responses we observed among pre- and post-menopausal women suggest that energy balance between groups was also similar. Finally it is important to note that 2 of the matched pairs completed a shorter distance race than 4 pairs. Although this is a limitation in the study, the matching of the pairs of pre- and post-menopausal women completing each distance allowed us to examine the overall effect of prolonged trail running in both groups, thus demonstrating the endocrine response to ultra-endurance exercise in a population that is underrepresented in the literature.
CONCLUSIONS
Prolonged endurance events are increasing in popularity among recreational athletes of all ages. In some cases with non-competitive athletes the race may be completed at a relatively slow pace, which perhaps suggests there is less physiological impact. Despite this, the observed changes in circulating components of the IGF system in the present study were consistent with previous studies of elite athletes completing an adventure race [1] and young males completing prolonged military field exercises [23]. The IGF-I system provides a marker of general condition and energy balance, thus, the present results suggest that among women athletes ultra-endurance competition is associated with significant perturbation to physiological systems. Further research is needed to determine the health and performance effects of these endocrine changes.
REFERENCES
- 1.Berg U, Enqvist J.K, Mattsson C.M, Carlsson-Skwirut C, Sundberg C.J, Ekblom B, Bang P. Lack of sex differences in the IGF-IGFBP response to ultra endurance exercise. Scand. J. Med. Sci. Sports. 2008;18:706–714. doi: 10.1111/j.1600-0838.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonen A, Keizer H.A. Pituitary, ovarian, and adrenal hormone responses to marathon running. Int. J. Sports Med. 1987;8(Suppl 3):161–167. doi: 10.1055/s-2008-1025723. [DOI] [PubMed] [Google Scholar]
- 3.Copeland J.L, Consitt L.A, Tremblay M.S. Hormonal responses to endurance and resistance exercise in females aged 19-69 years. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B158–B165. doi: 10.1093/gerona/57.4.b158. [DOI] [PubMed] [Google Scholar]
- 4.Copeland J.L, Heggie L. IGF-I and IGFBP-3 during continuous and interval exercise. Int. J. Sports Med. 2008;29:182–187. doi: 10.1055/s-2007-965114. [DOI] [PubMed] [Google Scholar]
- 5.Dall R, Lange K.H, Kjaer M, Jorgensen J.O, Christiansen J.S, Orskov H, Flyvbjerg A. No evidence of insulin-like growth factor-binding protein 3 proteolysis during a maximal exercise test in elite athletes. J. Clin. Endocrinol. Metab. 2001;86:669–674. doi: 10.1210/jcem.86.2.7180. [DOI] [PubMed] [Google Scholar]
- 6.Elloumi M, El Elj N, Zaouali M, Maso F, Filaire E, Tabka Z, Lac G. IGFBP-3, a sensitive marker of physical training and overtraining. Br. J. Sports Med. 2005;39:604–610. doi: 10.1136/bjsm.2004.014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsburg G.S, O'Toole M, Rimm E, Douglas P.S, Rifai N. Gender differences in exercise-induced changes in sex hormone levels and lipid peroxidation in athletes participating in the Hawaii Ironman triathlon. Ginsburg-gender and exercise-induced lipid peroxidation. Clin. Chim. Acta. 2001;305:131–139. doi: 10.1016/s0009-8981(00)00427-7. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Merino D, Chennaoui M, Drogou C, Guezennec C.Y. Influence of energy deficiency on the insulin-like growth factor I axis in a military training program. Horm. Metab. Res. 2004;36:506–511. doi: 10.1055/s-2004-825730. [DOI] [PubMed] [Google Scholar]
- 9.Gravholt C.H, Frystyk J, Flyvbjerg A, Orskov H, Christiansen J.S. Reduced free IGF-I and increased IGFBP-3 proteolysis in Turner syndrome: modulation by female sex steroids. Am. J. Physiol. Endocrinol. Metab. 2001;280:E308–E314. doi: 10.1152/ajpendo.2001.280.2.E308. [DOI] [PubMed] [Google Scholar]
- 10.Horton T.J, Pagliassotti M.J, Hobbs K, Hill J.O. Fuel metabolism in men and women during and after long-duration exercise. J. Appl. Physiol. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 11.Isacco L, Duche P, Boisseau N. Influence of hormonal status on substrate utilization at rest and during exercise in the female population. Sports Med. 2012;42:327–342. doi: 10.2165/11598900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Jackson A, Pollock M.L. Practical assessment of body composition. Physician Sportsmedicine. 1985;13:76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 13.Keizer H.A, Poortman J, Bunnik G.S. Influence of physical exercise on sex-hormone metabolism. J. Appl. Physiol. 1980;48:765–769. doi: 10.1152/jappl.1980.48.5.765. [DOI] [PubMed] [Google Scholar]
- 14.Kemmler W, Wildt L, Engelke K, Pintag R, Pavel M, Bracher B, Weineck J, Kalender W. Acute hormonal responses of a high impact physical exercise session in early postmenopausal women. Eur. J. Appl. Physiol. 2003;90:199–209. doi: 10.1007/s00421-003-0874-7. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer W.J, Fragala M.S, Watson G, Volek J.S, Rubin M.R, French D.N, Maresh C.M, Vingren JL, Hatfield D.L, Spiering B.A, Yu-Ho J, Hughes S.L, Case H.S, Stuempfle K.J, Lehmann D.R, Bailey S, Evans D.S. Hormonal responses to a 160-km race across frozen Alaska. Br. J. Sports Med. 2008;42:116–120. doi: 10.1136/bjsm.2007.035535. [DOI] [PubMed] [Google Scholar]
- 16.Lassarre C, Duron F, Binoux M. Use of the ligand immunofunctional assay for human insulin-like growth factor ((IGF) binding protein-3 (IGFBP-3) to analyze IGFBP-3 proteolysis and igf-i bioavailability in healthy adults, GH-deficient and acromegalic patients, and diabetics. J. Clin. Endocrinol. Metab. 2001;86:1942–1952. doi: 10.1210/jcem.86.5.7467. [DOI] [PubMed] [Google Scholar]
- 17.Lucas S.J, Anglem N, Roberts W.S, Anson J.G, Palmer C.D, Walker R.J, Cook C.J, Cotter J.D. Intensity and physiological strain of competitive ultra-endurance exercise in humans. J. Sports Sci. 2008;26:477–489. doi: 10.1080/02640410701552872. [DOI] [PubMed] [Google Scholar]
- 18.Malarkey W.B, Hall J.C, Rice R.R, Jr, O'Toole M.L, Douglas P.S, Demers L.M, Glaser R. The influence of age on endocrine responses to ultraendurance stress. J. Gerontol. 1993;48:M134–M139. doi: 10.1093/geronj/48.4.m134. [DOI] [PubMed] [Google Scholar]
- 19.Mohan S, Baylink D.J. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J. Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 20.Nemet D, Connolly P.H, Pontello-Pescatello A.M, Rose-Gottron C, Larson J.K, Galassetti P, Cooper D.M. Negative energy balance plays a major role in the IGF-I response to exercise training. J. Appl. Physiol. 2004;96:276–282. doi: 10.1152/japplphysiol.00654.2003. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen U.N, Mougin F, Simon-Rigaud M.L, Rouillon J.D, Marguet P, Regnard J. Influence of exercise duration on serum insulin-like growth factor and its binding proteins in athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1998;78:533–537. doi: 10.1007/s004210050456. [DOI] [PubMed] [Google Scholar]
- 22.Nindl B.C, Kraemer W.J, Marx J.O, Arciero P.J, Dohi K, Kellogg M.D, Loomis G.A. Overnight responses of the circulating IGF-I system after acute, heavy-resistance exercise. J. Appl. Physiol. 2001;90:1319–1326. doi: 10.1152/jappl.2001.90.4.1319. [DOI] [PubMed] [Google Scholar]
- 23.Nindl B.C, Alemany J.A, Kellogg M.D, Rood J, Allison S.A, Young A.J, Montain S.J. Utility of circulating IGF-I as a biomarker for assessing body composition changes in men during periods of high physical activity superimposed upon energy and sleep restriction. J. Appl. Physiol. 2007;103:340–346. doi: 10.1152/japplphysiol.01321.2006. [DOI] [PubMed] [Google Scholar]
- 24.Nindl B.C, Pierce J. R, Durkot M.J, Tuckow A.P, Kennett M.J, Nieves J.W, Cosman F, Alemany J.A, Hymer W.C. Relationship between growth hormone in vivo bioactivity, the insulin-like growth factor-I system and bone mineral density in young, physically fit men and women. Growth Horm. IGF Res. 2008;18:439–445. doi: 10.1016/j.ghir.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Nindl B. C, Alemany J.A, Tuckow A.P, Kellogg M.D, Sharp M.A, Patton J.F. Effects of exercise mode and duration on 24-h IGF-I system recovery responses. Med. Sci. Sports Exerc. 2009;41:1261–1270. doi: 10.1249/MSS.0b013e318197125c. [DOI] [PubMed] [Google Scholar]
- 26.Nindl B.C, Pierce J.R. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med. Sci. Sports Exerc. 2010;42:39–49. doi: 10.1249/MSS.0b013e3181b07c4d. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz A.J, Brasel J.A, Hintz R.L, Mohan S, Cooper D.M. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 1996;81:3492–3497. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- 28.Spangenburg E.E, Wohlers L.M, Valencia A.P. Metabolic dysfunction under reduced estrogen levels: looking to exercise for prevention. Exerc. Sport Sci. Rev. 2012;40:195–203. doi: 10.1097/JES.0b013e31825eab9f. [DOI] [PubMed] [Google Scholar]
- 29.Van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. J. Appl. Physiol. 1972;32:712–713. doi: 10.1152/jappl.1972.32.5.712. [DOI] [PubMed] [Google Scholar]
- 30.Waters D.L, Dorin R.I, Qualls C.R, Ruby B.C, Baumgartner R.N, Robergs R.A. Estradiol effects on the growth hormone/insulin-like growth factor-1 axis in amenorrheic athletes. Can. J. Appl. Physiol. 2003;28:64–78. doi: 10.1139/h03-006. [DOI] [PubMed] [Google Scholar]
- 31.Zaryski C, Smith D.J. Training principles and issues for ultra-endurance athletes. Curr. Sports Med. Rep. 2005;4:165–170. doi: 10.1097/01.csmr.0000306201.49315.73. [DOI] [PubMed] [Google Scholar]