Abstract
This study investigated the effects of obesity and ambient temperature on physiological responses and markers of oxidative stress to submaximal exercise in obese and lean people. Sixteen healthy males were divided into an obese group (n=8, %fat: 27.00±3.00%) and a lean group (n=8, %fat: 13.85±2.45%). Study variables were measured during a 60 min submaximal exercise test at 60% VO2max in a neutral (21±1°C) and a cold (4±1°C) environment. Heart rate, blood lactate, rectal temperature, serum levels of malondialdehyde (MDA) and superoxide dismutase (SOD) were measured at rest, during exercise and in recovery. Heart rate of both groups was significantly lower (P<0.05) in the cold than the warm environment, but there were no significant differences between the two groups. Serum SOD activity increased to a significantly greater extent (P<0.05) in the cold than the neutral environment, and remained elevated for longer during exercise in the obese group than the lean group. Serum MDA level during submaximal exercise was not significantly different between conditions or groups. Cold stress in exercise may challenge antioxidant defence mechanisms in obese subjects, but lipid peroxidation remains unchanged.
Keywords: oxidative stress, cold, obese, physiological response, exercise
INTRODUCTION
Exercise can produce an imbalance between free radicals and reactive oxygen species (ROS) and antioxidants, resulting in oxidative stress. Despite a large number of existing studies, there is a great deal of controversy over whether oxidative stress and subsequent damage are truly associated with exercise. Exercise-induced oxidative stress is potentially influenced by exercise intensity and duration, subject's physical capacity, and environmental conditions [20]. Strenuous outdoor cold-weather training is associated with increased oxidative stress [33]. Intensive voluntary short-term cold exposure during ice-bathing also induces oxidative stress [27]. Winter swimming has been suggested to contribute to increased free radical formation and accelerated lipid peroxidation [26].
Obese individuals may increase radical production via increased exercise load from increased weight bearing during exercise [1], and they have demonstrated decreased antioxidant defence function such as a reduction in erythrocyte antioxidant enzyme activities [19]. Evidence of obesity-induced oxidative stress has been accumulating in previous studies [14, 35]. Nieman et al. [17] reported that oxidative burst activity was slightly elevated in obese subjects, and Bouloumie et al. [4] reported that elevated leptin levels of the obese may stimulate intracellular production of free radicals. Subsequently, obese populations have been shown to be vulnerable to oxidative stress. Vincent et al. [34] suggested acute exercise induced greater oxidative stress levels in obese young adults as compared to non-obese ones. Acute intensive exercise can generate damaging oxidative stress that may exacerbate complications of obesity. Moreover, there is evidence to suggest that oxidative stress has a causative role in reduced immune function and obese subjects show a depressed immune function [25].
Obesity and cold conditions may act synergistically to enhance oxidative stress during submaximal exercise. Despite a large number of existing studies, there is a great deal of controversy over whether oxidative stress and subsequent damage are truly associated with exercise. Therefore the purpose of the present study was to determine whether alterations in serum level of malondialdehyde (MDA, lipid peroxidation marker), activity of superoxide dismutase (SOD, primary detoxification enzyme), and physiological responses were different between obese and lean individuals following 1 hour of submaximal exercise in cold versus neutral conditions.
MATERIALS AND METHODS
Subjects
Subjects were 16 healthy male students, and were divided into an obese group (≥25% n=8) and lean group (<20%, n=8) based on percentage of body fat. All participants had not experienced exercise training in cold or neutral conditions for at least one year. Characteristics of the subjects are shown in Table 1. The study protocol was approved by the Institutional Review Board of Keimyung University. Informed consent was obtained from all the study participants.
TABLE 1.
CHARACTERISTICS OF SUBJECTS
| Group | Age | Height | Body weight | Body fat | VO2max | Peak | 60%VO2max | ||
|---|---|---|---|---|---|---|---|---|---|
| (years) | (cm) | (kg) | (%) | (mL·kg-1·min-1) | HR (beats·min-1) | kp | HR (beats·min-1) | kp | |
| Lean | 21.34 | 173.05 | 68.35 | 13.85 | 36.75 | 192.30 | 4.50 | 142.50 | 2.65 |
| 1.15 | 1.35 | 2.00 | 2.45 | 2.55 | 2.50 | 0.25 | 2.50 | 0.25 | |
| Obese | 22.45 | 172.95 | 80.15* | 27.00* | 30.10* | 193.45 | 4.00 | 141.45 | 2.50 |
| 1.10 | 1.95 | 2.33 | 3.0 | 2.25 | 2.65 | 0.25 | 3.55 | 0.25 | |
Note: Values are mean and SD
P<0.05 Compared to lean group, kp: kilopond, HR: heart rate
Experimental procedure
The percentage of body fat was estimated from the skinfold thickness of triceps, suprailiac crest, and thigh by Jackson's [10] and Siri's [28] equations. Skinfold thickness was measured using skinfold calipers (Skyndex, USA). In preliminary testing, a graded maximal exercise test was used for determination of VO2max. Subjects were asked to refrain from vigorous exercise 24 hours prior to testing. Subjects started cycling at 60 rpm at 0.5 kilopond (kp) for 2 min. Thereafter, the intensity increased 0.5 kp every 2 min with fixed 60 rpm until the subjects became exhausted. VO2 was measured from samples of expired air samples taken until volitional fatigue. A Quinton metabolic measurement system (Quinton Inc. Seattle, WA, USA) and accompanying software was used for measurement of VO2, VCO2, ventilation (VE), and respiratory exchange ratio (RER). Heart rate was taken during each stage through continuous direct 12-lead EKG monitoring. VO2max was determined as the highest VO2 that showed a plateau in spite of an increased exercise intensity attained during the test. Study variables were measured during 60 min submaximal exercise at 60% VO2max in neutral (ambient temperature: 21±1°C, relative humidity 65±2%) and cold conditions (ambient temperature: 4±1°C, relative humidity 30±1%). To minimize the effects of circadian rhythm, all exercise bouts including preliminary testing were performed between 2:00 and 5:00 pm, and at the same time of day for each trial. Trials were separated by 1 week to ensure complete recovery between trials, and were randomized in a counterbalanced order, with each subject serving as his own control. For the comparison of basic physiological responses, heart rate, blood lactate, and rectal temperature were measured at rest and six times at 10-min intervals during exercise and at 5, 10, 15, 30 min during the recovery phase after exercise. A flexible intravenous catheter (Venflon 1.2, BOC health care, Helsing , Sweden) was inserted for blood collection. After the subjects rested in bed for 30 min in either neutral or cold conditions for familiarization, the first blood sample was drawn at rest. After the resting blood sample, the subjects performed 5 min of warm-up cycling at 30-45% of VO2max, immediately followed by 60 min at the subjects’ predetermined kp corresponding to 60% VO2max from preliminary testing in cold or neutral conditions, appropriately. The same clothing was worn in each exercise testing session.
Heart rate was measured using a heart rate monitor (Polar Electro OY PE3000, Finland), and blood lactate was measured from the fingertip by a lactate analyzer (YSI 1500, USA). Rectal temperature was measured using a thermistor (YSI 402, USA) inserted 5 cm past the anal sphincter. Serum levels of malondialdehyde (MDA) and superoxide dismutase (SOD) activity were measured as indicators of lipid peroxidation and antioxidant status, respectively. Blood samples for determination of serum MDA and SOD activity were collected at rest, six times during exercise (10 min intervals), and in the recovery phase (15, 30 min). Serum level of MDA was spectrophotometrically estimated according to Janero's method [12] by thiobarbituric acid-reactivity of the MDA-586 kit (BIOXYTECH, USA). SOD activity was spectrophotometrically determined by the SOD-525 kit (BIOXYTECH, USA) that is based on the SOD-mediated increase in the rate of autoxidation of 5,6,6a,11b-tetrahydro -3,9,10-trihydroxybenzo[c]fluorene (BXT-01050) in aqueous alkaline solution [16]. Measurements of MDA and SOD were all done in duplicate, and the reproducibility calculated as the coefficient of variation for MDA and SOD was 4.65% and 6.10%, respectively.
Statistics
Values are reported as the mean and standard deviation. Significant differences of dependent variables were analyzed using a two-factor (ambient temperature×group) repeated-measures ANOVA and a three-factor (during exercise time×ambient temperature×group) repeated-measures ANOVA, and Bonferroni t-tests were used for post hoc analysis of the comparison between groups with similar time or between different ambient temperature with similar group . P<0.05 was considered statistically significant.
RESULTS
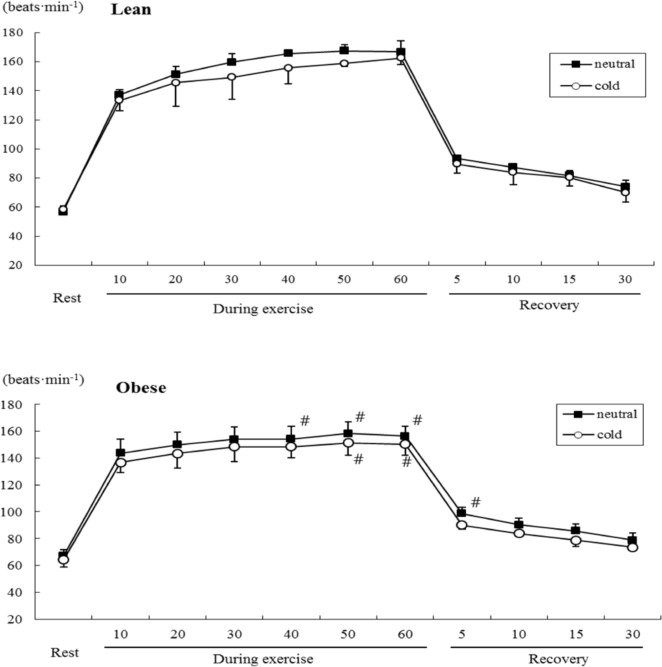
Heart rate increased during exercise (Figure 1), and was significantly lower in the cold than the neutral environment (temperature main effect, F=7.466, P<0.05) in both groups (Table 2). Heart rate responses also demonstrated a significant interaction (F=12.589, P<0.05) of group and time (Table 2), and heart rate changed in both groups over time (P<0.05, Figure 1).
FIG. 1.
COMPARISON OF HEART RATE DURING SUBMAXIMAL EXERCISE BETWEEN OBESE AND LEAN GROUPS
Note: # P<0.05 Compared to lean group, significant main effect by 3-way MANOVA : Temperature
TABLE 2.
THREE-FACTOR REPEATED MEASURES ANOVA RESULTS FOR PHYSIOLOGICAL PARAMETERS AND SERUM LEVELS DURING EXERCISE
| Variables | Main Effect | Interaction | |||||
|---|---|---|---|---|---|---|---|
| Time | Te | Group | T×G | T×Te | Te×G | T×G×Te | |
| Heart rate | P<0.05 | P<0.05 | NS | P<0.05 | NS | NS | NS |
| Lactate | P<0.05 | NS | NS | P<0.05 | NS | NS | NS |
| Rectal Te | P<0.05 | NS | NS | P<0.05 | NS | NS | P<0.05 |
| MDA | P<0.05 | NS | NS | NS | NS | NS | P<0.05 |
| SOD | P<0.05 | P<0.05 | P<0.05 | NS | P<0.05 | NS | P<0.05 |
Note: Te: temperature, T: time, G: group
Blood lactate concentration in both groups tended to be lower in the cold than the neutral conditions, but these differences did not reach statistical significance (Figure 2).
FIG. 2.
COMPARISON OF BLOOD LACTATE LEVELS DURING SUBMAXIMAL EXERCISE BETWEEN OBESE AND LEAN GROUPS
Note: * P<0.05 Compared to neutral
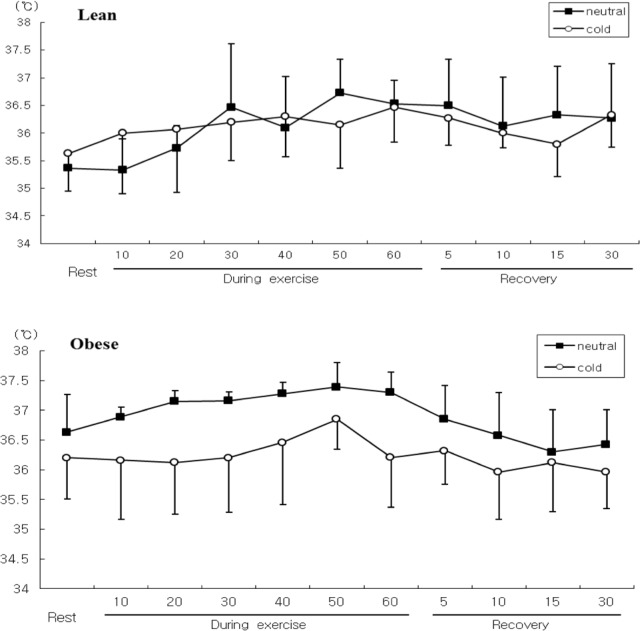
Rectal temperature also tended to be lower in the cold than the neutral conditions, but these differences were not significant between conditions or groups . However, there was a significant time×temperature×group interaction for rectal temperature (F=3.012, P<0.05). Rectal temperature was higher in the obese group than the lean group in neutral conditions, and the pattern of changes with time in the obese group was different between conditions (Figure 3).
FIG. 3.
COMPARISON OF RECTAL TEMPERATURE DURING SUBMAXIMAL EXERCISE BETWEEN OBESE AND LEAN GROUPS.
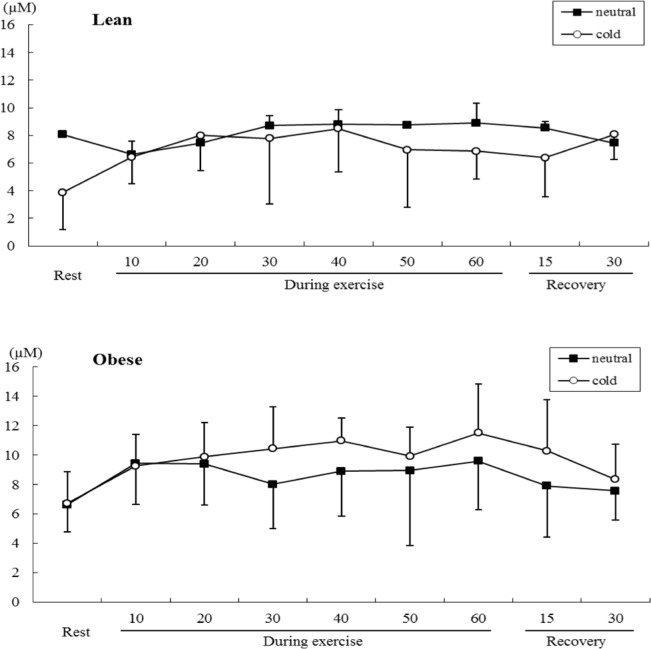
Serum MDA level changed in both groups over time during submaximal exercise (time main effect, F=7.801, P<0.05), but there no statistically significant differences between conditions or groups.
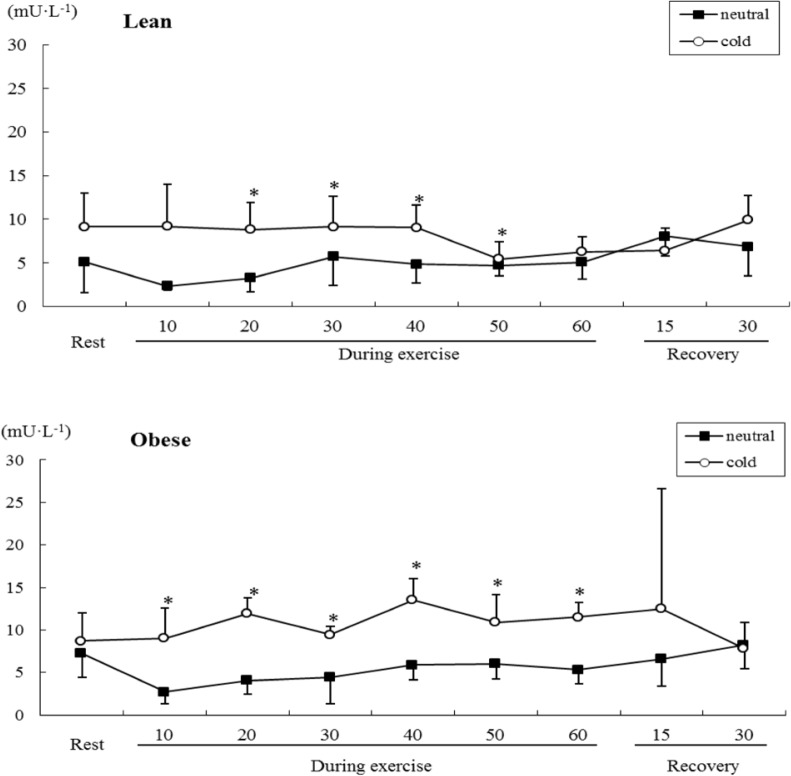
Changes in serum SOD activity were significantly different between conditions (F=33.270, P<0.05) and groups (F=4.524, P<0.05). There was also a significant time×temperature×group interaction effect (F=2.518, P<0.05). Serum SOD activity increased to a significantly (P<0.05) greater extent in the cold than the neutral conditions in both groups, and remained elevated for longer during exercise in the obese group than the lean group (Figure 5).
FIG. 4.
COMPARISON OF SERUM MDA LEVELS DURING SUBMAXIMAL EXERCISE BETWEEN OBESE AND LEAN GROUPS.
FIG. 5.
COMPARISON OF SERUM SOD ACTIVITIES DURING SUBMAXIMAL EXERCISE BETWEEN OBESE AND LEAN GROUPS
Note: * P<0.05 Compared to neutral
DISCUSSION
The acute exercise effects on metabolic function depend on a complex interaction among factors related to exercise mode, intensity and duration, in addition to environmental conditions. In the cold, oxygen uptake and cardiovascular function during exercise are different compared to exercise in warm conditions, but these responses are also dependent on exercise intensity and duration [38]. In the present study, both obese and lean groups exhibited a lower heart rate in the cold than the neutral conditions during submaximal exercise. Heart rates are lower as a result of parasympathetic activation of the baroreflex receptor during exercise in cold as compared to warm conditions [30]. Parkin et al. [21] reported a lower tendency of heart rate without significance during fatiguing submaximal exercise in ambient temperature of 3°C as compared to 20°C. This blunting is more pronounced during the latter stages of exercise. In the present study this blunting of the heart rate was more evident in the obese group than the lean group. Exercise during cold exposure suppresses increase in body temperature, and increases blood flow to the skin to prevent a decrease in skin temperature. This effect is greater in individuals with less subcutaneous fat of the insulation function [39]. In cold conditions, the general vasoconstriction elevates central venous pressure, which ultimately leads to a decrease in heart rate [8]. We supposed that non-obese subjects showed a lower response of vasoconstriction as compared to obese subjects in exercise during cold exposure.
Submaximal exercise during cold exposure is accompanied by higher blood lactate concentrations as a result of lower aerobic metabolism and reduced clearance of lactate [29]. However, in the present study blood lactate concentration in both groups tended to be lower in the cold than the neutral conditions. These unexpected results could be due to lower peripheral circulation following vasoconstriction [7], greater activation of the sympatho-adrenal system [3], and greater lipid metabolism than glucose metabolism during exercise in cold as compared to warm conditions [31, 11]. Peripheral vasoconstriction decreases peripheral blood flow and reduces convective heat transfer from the body's core to skin during cold exposure. Consequently, heat loss is reduced and core temperature is maintained. Moreover, metabolic heat production can be high enough during exercise to compensate for increased heat loss and allow core temperature to be maintained during exercise in cold conditions. Therefore, metabolic heat production likely has a greater influence on core temperature during exercise in cold than warm conditions. Layden et al. [15] found heat production to be sufficient for maintaining core temperature during exercise for 90 min at 64% VO2max in cold conditions. Body composition can also influence core temperature in ambient conditions via differences in metabolic responses, such as catecholamine-mediated responses and mobilization of free fatty acids. Contaldo et al. [5] reported that cold-induced metabolic responses were low in obese as compared to lean subjects. In extreme cold stress situations when skin blood flow is minimal, fat mass is known to be important in preventing a decrease in core temperature [37]. Van Ooijen et al. [32] suggest that even in mild cold, the fatness of a subject might play a role in insulation. In the present study obese subjects showed a higher rectal temperature than lean subjects during exercise in neutral conditions. Therefore, extra subcutaneous fat in obese subjects may prevent heat loss, resulting in higher core temperature than lean individuals during exercise in neutral conditions.
Obese individuals may increase muscular work from carrying excessive weight, and this could subsequently increase radical production through increased oxidative phosphorylation and electron leakage in the electron transport system [1]. Several metabolic parameters may predispose the obese individual to oxidant stress, including increased oxygen demand for muscle metabolism, decreased antioxidant function, and increased lipid level. Therefore, obese individuals may be at greater risk of health complications associated with oxidative stress [9]. Plasma lipids are oxidized at a greater rate in obese subjects compared with non-obese subjects [22]. Vincent et al. [34] suggested that obesity exacerbated the elevated lipid peroxidation levels after exercise regardless of exercise modality, and aged obese women were at a greater risk for oxidative stress compared with their non-obese counterparts [36].
This study expected to find a significant increase in serum MDA concentration as a marker of lipid peroxidation [13] in obese subjects after exercise while breathing cold air. The results showed that although serum MDA concentration tended to be higher during exercise in the cold versus warm conditions, the differences did not reach statistical significance. In agreement with this investigation, previous studies by Alessio et al. [1] and Quandry et al. [24] have also found no alteration in MDA levels. Quandry et al. [24] suggested that the blood oxidative stress response was an effect of exercise intensity rather than absolute metabolic workload. This concept may highlight the importance of regular exercise training in preventing activity-related oxidative stress, because blood oxidative stress – as indicated by increased plasma MDA concentration – occurred at maximal-intensity exercise, but was not observed after submaximal intensity exercise [24].
Interestingly, serum SOD activity increased to a significantly greater extent (P<0.05) in the cold than the neutral conditions, and remained elevated for longer during exercise in the obese group than the lean group. This finding suggests that obesity and a cold air environment challenge antioxidant defences during submaximal exercise. Antioxidant enzymes such as SOD provide the primary defence against ROS generated during exercise, and this enzyme activity is known to increase in response to exercise [2]. An acute bout of maximal exercise has been shown to increase SOD activity, and this activation of SOD was proposed to result from increased free radical production during exercise [1]. In contrast, Ohno et al. [18] did not find an increase of SOD activity during submaximal exercise below the lactate threshold. Enzymatic antioxidant activity increase at first for adaptation followed by decrease as utilization during exercise [6], the remaining elevation of serum SOD activity for longer duration indicates the challenge of antioxidant defence in obese subjects. The present findings indicated increased SOD activity in obese individuals during submaximal exercise in the cold. Osorio et al. [20] suggested that oxidative stress is higher during exercise in the cold compared to warm or hot conditions. The interaction of exercise with cold induced significant oxidative stress. In the present study, increased SOD activity of the obese is partial evidence of oxidative stress during submaximal exercise in cold conditions. Although we should have analysed the isoforms of SOD for the exact exercise-induced oxidative stress [23], this study only analysed serum SOD activity. Therefore we could not confirm whether this was associated with oxidative damage to cells.
CONCLUSIONS
In conclusion, the blunting of heart rate was more evident in the obese group than the lean group during exercise in cold conditions. Blood lactate concentration and rectal temperature in both groups tended to be lower in the cold than the neutral conditions. As obese subjects had a greater extent and longer duration of SOD activity in cold conditions, cold weather exercise may challenge antioxidant defence mechanisms, while lipid peroxidation remains unchanged. Further work is warranted to study the interaction between obesity and exercise-induced oxidative stress in cold ambient conditions.
REFERENCES
- 1.Osorio R.A.L, Christofani J.S, Almeida V.D, Russo A.K, Picarro I.C. Reactive oxygen species in pregnant rats: effect of exercise and thermal stress. Com. Biochem. Physiol. Part C. 2007;135:89–95. doi: 10.1016/s1532-0456(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 2.Venditti P, De Rosa R, Caldarone G, Di Meo S. Functional and biochemical characteristics of mitochondrial fractions from rat liver in cold-induced oxidative stress. Cell Mol. Life Sci. 2004;61:3104–3116. doi: 10.1007/s00018-004-4308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siems W.G, van Kuijk F.J.G.M, Maass R, Brenke R. Uric acid and glutathione levels during short-term whole body cold exposure. Free Rad. Biol. Med. 1994;16:299–305. doi: 10.1016/0891-5849(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 4.Siems W.G, Brenke R, Sommerburg O, Grune T. Improved antioxidative protection in winter swimmers. Int. J. Spt. Med. 1999;92:193–198. doi: 10.1093/qjmed/92.4.193. [DOI] [PubMed] [Google Scholar]
- 5.Alessio H.M, Hagerman A, Fulkerson B, Ambrose J, Rice R, Wiley R. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med. Sci. Sports Exerc. 2000;32:1576–1581. doi: 10.1097/00005768-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Olusi S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotective enzymes in humans. Int. J. Obes. 2002;26:1159–1164. doi: 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- 7.Konukoglu D, Serin O, Ercan M, Turhan M.S. Plasma homocysteine levels in obese and non-obese subjects with or without hypertension: its relationship with oxidative stress and copper. Clin. Biochem. 2003;36:405–408. doi: 10.1016/s0009-9120(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 8.Vincent H.K, Taylor A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obe. 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 9.Nieman D.C, Henson D.A, Nehlsen-Cannarella S.L, Ekkens M, Utter A.C. Influence of obesity on immune function. JADA. 1999;99:294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 10.Bouloumie A, Marumo T, Lafontan M, Buse R. Leptin induces oxidative stress in human endothelial cells. FASEBJ. 1999;13:1231–1238. [PubMed] [Google Scholar]
- 11.Vincent H.K, Morgan J.W, Vincent K.R. Obesity exacerbates oxidative stress levels after acute exercise. Med. Sci. Sports Exerc. 2004;36:772–779. doi: 10.1249/01.mss.0000126576.53038.e9. [DOI] [PubMed] [Google Scholar]
- 12.Samartin S, Chandra R.K. Obesity, overnutrition and the immune system. Nutrition Research. 2001;21:243–262. [Google Scholar]
- 13.Jackson A.S, Pollock M.L, Ward A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980;12:175–182. [PubMed] [Google Scholar]
- 14.Siri W.E. Body Composition from Fluid Spaces and Density: Analysis of Methods. In: Brozek J, Henschel A, editors. Techniques for Measuring Body Composition. Washington DC: National Academy of Sciences National Research Council; 1961. pp. 223–244. [Google Scholar]
- 15.Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 16.Nebot C, Moutet M, Huet P, Xu J.Z, Yadan J.C, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autooxidation of a tetracyclic catechol. Analytical Biochemistry. 1993;214:442–451. doi: 10.1006/abio.1993.1521. [DOI] [PubMed] [Google Scholar]
- 17.Young A.J. Energy substrate utilization during exercise in extreme environments. In: Pandolf KB, Holloszy JO, editors. Exercise Sports Science Review. Baltimore: Williams and Wilkins; 1990. pp. 65–117. [PubMed] [Google Scholar]
- 18.Therminarias A, Flore P, Oddou-Chirpaz M.F, Pellerei E, Quirion A. Influence of cold exposure on blood lactate response during incremental exercise. Eur. J. Appl. Physiol. 1989;58:411–418. doi: 10.1007/BF00643518. [DOI] [PubMed] [Google Scholar]
- 19.Parkin J.M, Carey M.F, Zhao S, Febbraio M.A. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J. Appl. Physiol. 1999;86:902–908. doi: 10.1152/jappl.1999.86.3.902. [DOI] [PubMed] [Google Scholar]
- 20.Young A.J, Castellani J.W. Exertion-induced fatigue and thermoregulation in the cold. Comparative Biochem. Physiol. 2001;128:769–776. doi: 10.1016/s1095-6433(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 21.Hannah J.N, McHill P, Sinclair J.D. Human cardiorespiratory responses to acute cold exposure. Clin. Experimental Pharmacol. Physiol. 1975;2:229–238. doi: 10.1111/j.1440-1681.1975.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 22.Stainsby W.N, Brooks G.A. Control of lactic acid metabolism in contracting muscles during exercise. Exer. Spt. Sci. Rev. 1990;18:29–64. [PubMed] [Google Scholar]
- 23.Flore P, Therminarias A, Oddou-Chirpaz M.F, Quirion A. Influence of moderate cold exposure on blood lactate during incremental exercise. Eur. J. Appl. Physiol. 1992;64:213–217. doi: 10.1007/BF00626283. [DOI] [PubMed] [Google Scholar]
- 24.Bergh U, Harley H, Lansberg L, Eckblom B. Plasma norepinephrine concentration during submaximal and maximal exercise at lowered skin and core temperatures. Acta. Physiol. Scand. 1979;106:383–384. doi: 10.1111/j.1748-1716.1979.tb06417.x. [DOI] [PubMed] [Google Scholar]
- 25.Tipton M.J, Franks E.M, Mereilly G.S, Mekjavic L.B. Substrate utilization during exercise and shivering. Eur. J. Appl. Physiol. 1997;76:103–108. doi: 10.1007/s004210050220. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs I, Martineau L, Vallerand A.L. Thermoregulatory thermogenesis in humans during cold stress. Exerc. Sport Sci. Rev. 1994;22:221–250. [PubMed] [Google Scholar]
- 27.Layden J.D, Patterson M.J, Nimmo M.A. Effect of reduced ambient on fat utilization during submaximal exercise. Med. Sci. Sports Exerc. 2002;34:774–799. doi: 10.1097/00005768-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Contaldo F, Scalfi L, Coltordi A, Lanzilli A. Reduced cold-induced thermogenesis in familial human obesity. Klin. Wochenschr. 1986;64:177–180. doi: 10.1007/BF01713459. [DOI] [PubMed] [Google Scholar]
- 29.Webb P. Temperature of skin, subcutaneous tissue, muscle and core in resting men in cold, comfortable and hot conditions. Eur. J. Appl. Physiol. 1992;64:471–476. doi: 10.1007/BF00625070. [DOI] [PubMed] [Google Scholar]
- 30.Van Ooijen A.M.J, Van Marken Lichtenbelt W.D, van Steenhoven A.A, Westerterp K.R. Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol. Behav. 2004;82:545–553. doi: 10.1016/j.physbeh.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Higdon J.V, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler. Thromb Vasc. Biol. 2003;23:365–367. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- 32.Pipek R, Dankner G, Ben-Amotz A, Aviram M, Levy Y. Increased plasma oxidizability in participants with severe obesity. J. Nutr. Environ. Med. 1996;6:267–272. [Google Scholar]
- 33.Vincent H.K, Vincent K.R, Bourguignon C, Braith R.W. Obesity and postexercise oxidative stress in older women. Med. Sci. Sports Exerc. 2005;37:213–219. doi: 10.1249/01.mss.0000152705.77073.b3. [DOI] [PubMed] [Google Scholar]
- 34.Kayatekin B.M, Gonenc S, Acikgoz O, Uysal N, Dayi A. Effects of sprint exercise on oxidative stress in skeletal muscle and liver. Eur. J. Appl. Physiol. 2002;87:141–144. doi: 10.1007/s00421-002-0607-3. [DOI] [PubMed] [Google Scholar]
- 35.Quindry J.C, Stone W.L, King J, Broeder C.E. The effects of acute exercise on neutrophils and plasma oxidative stress. Med. Sci. Sports Exerc. 2003;35:1139–1145. doi: 10.1249/01.MSS.0000074568.82597.0B. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee A.K, Mandal A, Chanda D, Chakraborti S. Oxidants, antioxidants and physical exercise. Mol. Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 37.Ohno H, Sato Y, Yamashita K, Doi R, Arai K, Kando T, Taniguchi N. The effect of brief physical exercise on free radical scavenging enzyme systems in human red blood cells. Can. J. Physiol. Pharmacol. 1986;64:1263–1265. doi: 10.1139/y86-213. [DOI] [PubMed] [Google Scholar]
- 38.Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Medicine. 2006;36:327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 39.Powers S.K, Jackson M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]