Abstract
In human populations, cigarettes and alcohol generally serve as gateway drugs, which people use first before progressing to marijuana, cocaine or other illicit substances. To understand the biological basis of the gateway sequence of drug use, we developed an animal model in mice and focused on the effects of nicotine on subsequent responses to cocaine. We found that pretreatment of mice with nicotine increased the response to cocaine as assessed by both addiction-related behaviors and synaptic plasticity in the striatum, a brain region critical for addiction-related reward. Locomotor sensitization was increased by 98%, conditioned place preference was increased by 78%, and cocaine-induced reduction in long-term potentiation (LTP) was enhanced by 24%. The responses to cocaine were altered only when nicotine was administered first, and nicotine and cocaine were then administered concurrently. Reversing the order of drug administration was ineffective. Cocaine had no effect on nicotine induced behaviors and synaptic plasticity. Nicotine primed the response to cocaine by enhancing its ability to induce transcriptional activation of the FosB gene through inhibiting histone deacetylase, causing global histone acetylation in the striatum. We tested this conclusion further with a histone deacetylase inhibitor and found that it similarly simulated the actions of nicotine on cocaine by priming the response to cocaine, and enhancing FosB gene expression and LTP depression in the nucleus accumbens. Conversely, in a genetic mouse model of Rubinstein Taybi’s syndrome, characterized by reduced histone acetylation, the effects of cocaine on LTP were diminished. We achieved a similar effect pharmacologically by infusing a low-dose of theophylline, an activator of histone deacetylase, into the nucleus accumbens. These data from mice prompted an analysis of epidemiological data, which indicated that most cocaine users initiate cocaine use after the onset of smoking while actively smoking and that initiating cocaine use after smoking increases the risk of becoming dependent on cocaine, consistent with our data in mice. If our findings in mice apply to humans, a decrease in smoking rates in young people could also lead to a decrease in cocaine addiction.
In the general population in the United States and in other Western societies, there is a well-defined sequence of drug usage in which the use of tobacco or alcohol precedes the use of marijuana, which in turn precedes the use of cocaine and other illicit drugs (1–3). For example, in 2009 in the United States (4), among adults aged 18–34 who had used cocaine at least once, 90.4% had smoked cigarettes before they began to use cocaine, 4.7% began using both drugs at the same age, 2.4% used cocaine first, and 2.5% had never smoked. Epidemiological studies have established the sequence of use among different substances and specified their association, but these studies cannot establish cause and effect nor identify the underlying cellular and molecular mechanisms.
To address the mechanisms by which gateway drugs exert their effects, we developed an animal model in mice to explore the gateway sequence of drug use at behavioral, electrophysiological, and molecular genetic levels. In mice, one can readily control the order of use between drugs so that this order is the only determinant of outcome; other factors, such as the relative availability of substances, which may be important in human populations, are irrelevant. We first tested mice behaviorally to examine how sequential administration of nicotine and cocaine alters locomotor sensitization and conditioned place preference, two addiction-related behaviors that are modulated by drugs of abuse largely through effects on the striatum, where most drugs of abuse exert their addictive effects (5–7). The nucleus accumbens (NAc) in the ventral striatum is critical for reward and addiction and is a site of convergence and integration of rewarding input from dopaminergic neurons in the ventral tegmental area (VTA) and glutamatergic input from the amygdala and the prefrontal cortex. The core of the NAc is made up primarily of GABAergic inhibitory spiny neurons. The NAc sends inhibitory feedback to the dopaminergic cells of the VTA. Reduction of excitatory input to the NAc is thought to decrease inhibitory output from the NAc to the VTA and thereby contribute, through disinhibition, to the increased reward and enhanced locomotion activation observed after cocaine administration (8).
We used sequential drug administration to examine three physiological and molecular markers of the priming effects of nicotine on cocaine and of cocaine on nicotine in the NAc of the striatum: synaptic plasticity, transcription of FosB, an immediate response gene implicated in addiction to many drugs of abuse and the response to other rewarding stimuli, and the involvement of histone acetylation on chromatin structure.
The molecular pathways involved in addiction share some of the same molecular logic and even some of the same molecular steps encountered in long-term memory storage (9–11). In snails, flies and mice, long-term memory storage depends on modifications of chromatin structure and the consequent activation of a downstream cascade of gene expression, leading to growth and maintenance of new synaptic connections (9–14). This cascade is initiated in part by the acetylation of histone tails by the CREB binding protein (CBP), a histone acetyltransferase (15). The addiction process is also mediated through histone acetylation of the promoter of the transcription factor FosB. Accumulation of this transcription factor is a crucial step in the formation of addiction to most drugs of abuse and has been used as a molecular marker for these processes. We hypothesized that nicotine may exert a priming effect in the gateway sequence of drug use by increasing FosB gene expression in response to cocaine through increased histone acetylation (16). Such alteration would enhance the response to succeeding stimulation with cocaine by increasing access of transcription factors to the promoters of certain genes, for example FosB.
Results
Chronic pretreatment with nicotine increases behavioral responses to cocaine
We developed a behavioral paradigm to examine the interaction between drugs by sequentially administering nicotine and cocaine to C57BL/6J mice and examining locomotor sensitization (Fig. 1, A to C) and CPP (Fig. 1D), two widely used behavioral correlates of addictive behavior (17–22).
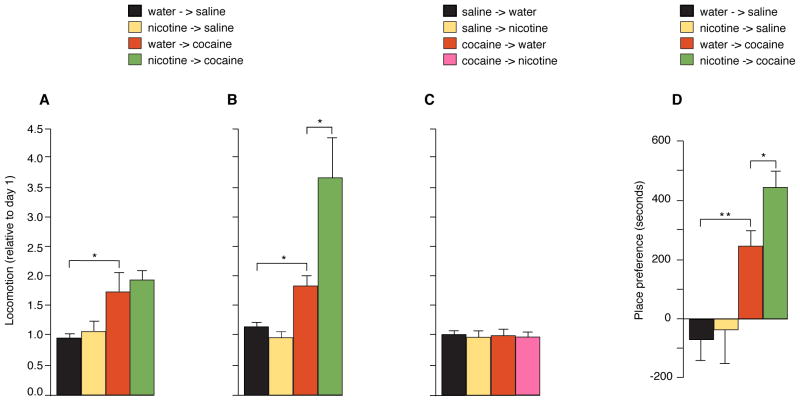
Fig. 1.
Nicotine priming enhances cocaine-induced behavioral endpoints, sensitization and conditioned place preference (CPP). For sensitization, we treated mice with nicotine (50 μg/ml) in the drinking water either for 24 hours (Fig. 1A) or 7 days (Fig. 1B). For the subsequent 4 days, the mice were treated with a single cocaine injection per day (20 mg/kg), with continued exposure to nicotine in the drinking water (n = 10–15 per group). Data expressed as total distance traveled on day 4 compared with day 1. (A) Lack of effect of 24 hours nicotine treatment on cocaine-induced locomotion. (B) Effect of 7 days of nicotine treatment on cocaine-induced locomotion. (C) Lack of effect of 7 days of cocaine treatment on nicotine-induced locomotion. (D) Effect of nicotine pretreatment on CPP. After 7 days of exposure to nicotine, mice were conditioned to either side of the place preference chamber with cocaine or saline. Preference scores were calculated subtracting the time spent in the cocaine-paired side after conditioning to the time before conditioning (n = 8 per group). Data represent mean ± SEM.
Locomotor sensitization is a robust and readily assayed effect of psychostimulants. It measures the animal’s gradually increasing behavioral and motivational response to a fixed drug dose, assayed as an increase in locomotor activity (23). Sensitization is very long lasting and is thought to result from long-term plastic changes in the striatum. We treated mice with nicotine (50 μg/ml) in the drinking water either for 24 hours (Fig. 1A) or 7 days (Fig. 1B). For the subsequent 4 days, mice were treated with a single cocaine injection per day (20 mg/kg), with continued exposure to nicotine in the drinking water. Mice treated with nicotine (50 mg/ml) showed the same levels of locomotion (that is, no increase in locomotion compared to day 1) as water controls. Mice treated only with cocaine showed a 58% increase in locomotion (sensitization) compared with controls (from 1.17 ± 0.06 to 1.85 ± 0.12, *p < 0.05). Mice treated with nicotine for 7 days followed by cocaine showed a significant enhancement of 98% in locomotor activity compared with mice treated with cocaine alone (from 1.85 ± 0.12 to 3.67 ± 0.64, *p < 0.05) (Fig. 1B). By contrast, mice pretreated for only 1 day with nicotine showed no increase in locomotor response to cocaine administered for the next 4 days above that were caused by cocaine treatment alone (Fig. 1A). There was also no increase in the locomotor response when the protocol was reversed, and mice were pretreated with cocaine for 7 days followed by concurrent cocaine and nicotine administration for 4 days (Fig. 1C).
Conditioned place preference (CPP) is a more naturalistic model of addictive behavior than sensitization. CPP measures an animal’s preference for a particular place in its environment, which develops as that place becomes consistently associated with a rewarding stimulus and assumes some of the rewarding effects of the stimulus (cocaine). We compared CPP in mice treated with nicotine in the drinking water (50 μg/ml) for 7 days and then 4 days of cocaine with mice exposed to cocaine alone for 7 days. Cocaine alone increased place preference by 223% compared with saline control (from 76.47 ± 4.18 to 247.1 ± 52.9 s, P <0.05) (Fig. 1D). As with sensitization, mice pretreated with nicotine showed a 78% further increase in preference for the cocaine-coupled chamber compared with mice treated only with cocaine, with no prior exposure to nicotine (from 247.1 ± 52.9 sec to 441.2 ± 64.7sec, *p < 0.05) (Fig. 1D).
Nicotine priming enhances the depression of long-term synaptic potentiation (LTP) induced by cocaine
We next explored the consequences of sequential drug administration for reward-related synaptic plasticity by examining LTP in the core of the NAc in the ventral striatum. As noted above, the NAc plays a critical role in addiction. To simulate the sequential drug administration conditions of the behavioral experiments, we treated the mice with nicotine in water (10 μ/ml) for 7 days and administered cocaine (30 mg/kg) on the 8th day, ten minutes before the mice were sacrificed. In brain slices taken from the striatum, we measured LTP of the excitatory synapses (in the cortico-accumbens pathway) in the NAc core (Fig. 2A) known to be reduced in response to chronic cocaine administration (23). This reduction presumably disinhibits the dopaminergic cells of VTA, which enhances reward. Similarly, we found a reduction in LTP after a single cocaine injection. This reduction in LTP was markedly enhanced by pretreatment of mice with nicotine for 7 days. The reduction started immediately after the high-frequency stimulation (HFS) and persisted for up to 180 min compared with cocaine alone (control, 129 ± 6%; cocaine alone, 112 ± 5%; 7 days of nicotine + cocaine, 88 ± 6%; P < 0.05 compared with cocaine alone; Fig. 2, B and C). Nicotine alone did not produce significant changes in LTP (7 days of nicotine, 137 ± 7%).
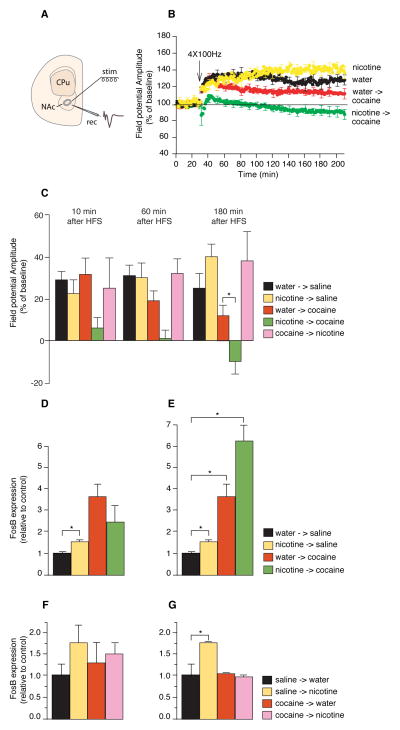
Fig. 2.
Synaptic plasticity and gene expression responses are enhanced by pretreatment with nicotine followed by cocaine, but not the reverse. (A) A schematic illustration of stimulation (stim) and recording (rec) sites in the coronal slices. (B) LTP measured 180 min after high-frequency stimulation (HFS). Experimental groups include control (water followed by saline; n = 6), 7 days of nicotine (n = 6), single injection of cocaine (n = 6), and 7 days of nicotine followed by a single cocaine injection (n = 9). (C) Time course of change in LTP for all groups. An additional experimental group is included (7 days of cocaine treatment followed by 24 hours of nicotine; pink, n = 5). (D to G) Real-time PCR measurements of FosB expression (control normalized to 1.0). Data represent means ±SEM. (D) Twenty-four hours of nicotine followed by cocaine (n = 9 per group). (E) Seven days of nicotine followed by cocaine (n = 9 per group). (F) A single injection of cocaine followed by 24 hours of nicotine (n = 5 in each group). (G) Seven days of cocaine followed by 24 hours of nicotine (n = 7 in each group). *P < 0.05.
Treatment with cocaine alone for 7 days or cocaine treatment for 7 days followed by 24 hours of nicotine treatment did not alter LTP (7 days cocaine 137 ± 7%, 7 days cocaine followed by 24 hours nicotine 138 ± 4 %, Fig. 2, B and C). Thus, as in the behavioral experiments, the cocaine-induced synaptic plasticity (i.e., decreased LTP) in the NAc is enhanced after priming with nicotine, suggesting that the disinhibition of VTA and consequently the rewarding properties of cocaine, are increased by nicotine priming.
FosB expression is increased by sequential drug administration
Because an important step in the molecular sequence leading to the expression of the addictive phenotype in mice is increased FosB expression in the striatum (24), we asked whether the nicotine-induced enhancements of sensitization, conditioned place preference, and reduction in LTP in response to cocaine correlate with changes in FosB expression in the striatum.
Using real-time RT-PCR to measure FosB RNA levels, we found that both 24 hours and 7 days of administration of nicotine (10 μg/mg) in the drinking water was associated with 50% and 61% increases in FosB expression (control set at one, increase of 1.5 ± 0.22 after 24 hours nicotine, and 1.61 ± 0.16 after 7 days nicotine, *p < 0.05, Fig. 2, D and E). We then administered a single cocaine (30 mg/kg) injection after 7 days of pretreatment with nicotine and observed a 74% further increase in FosB expression compared with mice treated with cocaine alone without prior exposure to nicotine (cocaine alone 3.5 ± 0.69, nicotine 7 days followed by cocaine 6.12 ± 0.8, *p < 0.05, Fig. 2E). As in the behavioral and physiological experiments, 24 hours of nicotine pre-treatment produced quite different results from the 7 days treatment and did not lead to an increased FosB response to cocaine (Fig. 2D). The fact that 7 days but not 24 hours of nicotine pre-exposure increased FosB response to cocaine suggested that the effect of long-term nicotine treatment on cocaine response is not merely due to the short-term interaction between the acute effects of the two drugs but rather to a metaplastic effect: chronic nicotine administration produces changes in gene expression in the brain so that, following nicotine, the brain responds differently to cocaine with long term synaptic changes.
Cocaine treatment does not enhance FosB expression induced by nicotine
We next asked whether the effect of nicotine on gene expression was also unidirectional, that is: does nicotine pretreatment followed by cocaine increase the response to cocaine whereas the reverse order of drug treatment does not? To address this question, we gave cocaine (30 mg/kg) in two protocols: for 24h or 7 consecutive days followed by 24 hours of treatment with nicotine. We found that 7 days of cocaine treatment blunted rather than amplified the effect of nicotine on FosB expression in the striatum when compared to the response to nicotine alone. The increase in FosB expression seen after nicotine treatment alone was not seen when nicotine administration followed either single injection or 7 day cocaine pretreatment (Fig. 2, F and G). The effects on FosB expression are unidirectional; nicotine primes the cocaine response, but cocaine does not prime the nicotine response.
Chronic nicotine induces hyper-acetylation of histones H3 and H4 at the FosB promoter
Cocaine treatment causes alterations in chromatin structure in the striatum: an acute cocaine injection increases acetylation of histone H4 but not H3 at the FosB promoter (14, 15). We therefore asked whether nicotine enhances FosB expression in the striatum by altering chromatin structure at the FosB promoter and, if so, does it magnify the effect of cocaine? In principle, nicotine-induced histone modifications could open chromatin structure, creating a permissive environment for gene transcription that could enhance the expression of FosB to subsequent administration of cocaine. We therefore examined acetylation of both histone H3 and H4 locally at the FosB promoter. We found that after 7 days of nicotine treatment (10 μg/ml), acetylation of both histone H3 and histone H4 was increased by 34% and 39%, respectively (control set at 1, 7 days nicotine acH3 1.34 ± 0.06, 7 days nicotine acH4 1.40 ± 0.24, *p < 0.05, Fig. 3, A and B). By contrast, cocaine alone increased acetylation of histone H4 (by 44%) (control set at 1, cocaine alone acH4, 1.44 ± 0.14, *p<0.05) but not of histone H3. Moreover, a single injection of cocaine (30 mg/kg) administered after 7 days of nicotine treatment did not increase acetylation of histone H4 further. The finding that nicotine causes acetylation of both H4 and H3 lysine residues suggests that nicotine may enhance the action of cocaine on FosB expression in the striatum by acetylating a wider range of histone lysine residues than cocaine. Broad acetylation in promoter regions is often associated with a cumulative effect that leads to increased transcription and is correlated with an increase in the total number of lysine residues that are acetylated in the histone N terminal tails of different promoters (25).
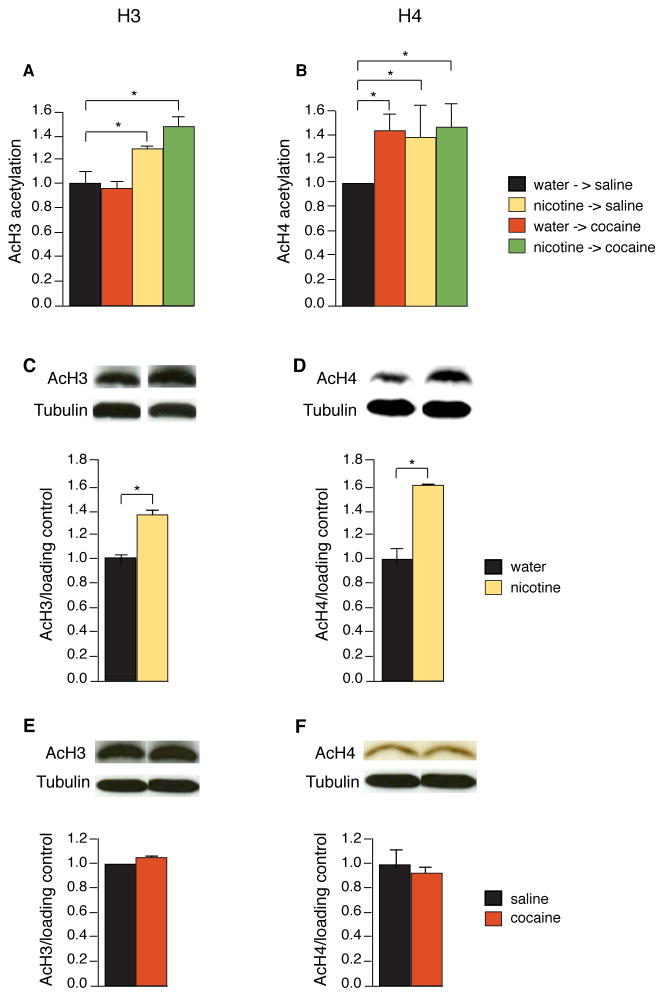
Fig. 3.
Nicotine, but not cocaine, induces histone hyperacetylation in the striatum. (A and B) Chromatin immunoprecipitation assays for acetylation for (A) histone H3 (K9) and (B) H4 (K5 to K16) at the FosB promoter were performed in animals treated with acute cocaine (30 mg/kg), 7 days of nicotine (10 mg/ml), and 7 days of nicotine followed by acute cocaine (control set at 1, n = 3 to 6 per group). Data represent means ± SEM. (C to F) After 7 days of nicotine or cocaine exposure, protein extracted from striatal lysates was incubated with antibodies detecting histone H3 (K9) (C and E) and histone H4 (K5 to K16) (D and F) tail modifications. *P < 0.05
7- day nicotine treatment increases histone H3 and H4 acetylation at promoters in the striatum
The ability of nicotine to produce robust acetylation at the FosB promoter suggested that nicotine-induced enhancement of acetylation could be occurring not only locally, at the FosB promoter of the NAc, but more widely at the promoters of other genes expressed in the striatum. To explore this idea, we used immunoblotting and examined the extent of chromatin modifications in the whole striatum of mice chronically treated with nicotine. We found that 7 days of nicotine treatment (10 μg/ml) increased histone H3 and H4 acetylation by 32% and 61% in the whole striatum, compared with controls treated with water, much as it did at the FosB promoter (control set at 1, acH3 1.32 ± 0.02, acH4 1.61 ± 0.01 *p<0.05, Fig. 3, C and D). By contrast, 7 days of cocaine administration by itself produced no increase in acetylation of histone H3 and H4 in the whole striatum, measured 24 hours after the last cocaine administration (Fig. 3, E and F).
7 days of nicotine treatment reduces histone deacetylase (HDAC) activity in the striatum
Does the hyperacetylation produced by nicotine result from activation of one or more acetylases or from the inhibition of deacetylases? To address this question, we assayed HDAC activity directly in the nuclear fraction of cells in the striatum. We found that there was a 28% reduction in HDAC of mice treated for 7–10 days with nicotine (10 mg/μl) (control set at 100%, nicotine 72 ± 6.0%, *p < 0.05, Fig. 4A). When we reversed the order of drug administration and treated the mice with cocaine (30 mg/kg) for 7 days, we found no decrease in HDAC activity in the nuclear fraction (Fig. 4B). Thus, the increase in histone acetylation after nicotine treatment seems to result from reduced HDAC activity in the striatum. These results suggest that nicotine affects histone acetylation by inhibiting HDAC activity.
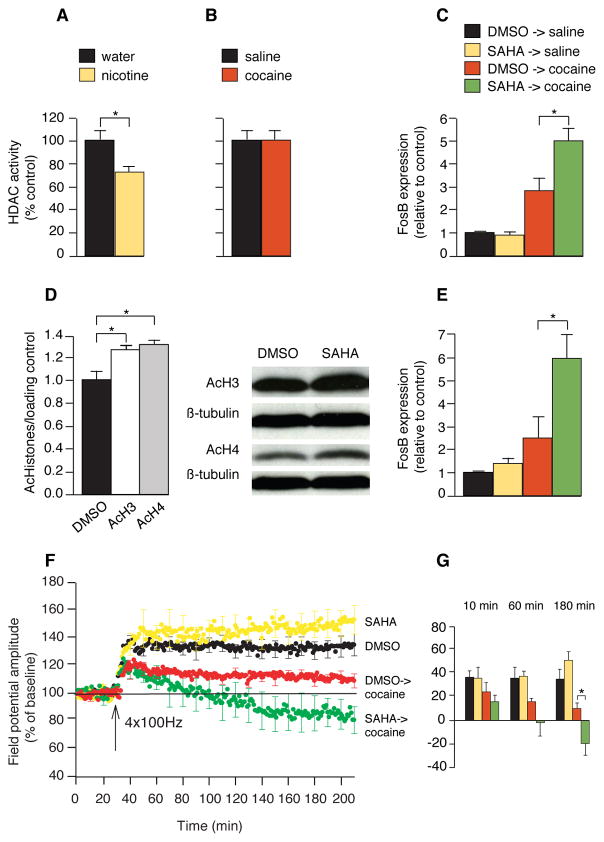
Fig. 4.
Nicotine inhibits HDAC activity and its effects are mimicked by an acetylase inhibitor. (A-B) In the nuclear fraction, an HDAC activity assay that detects deacetylation of lysine residues was performed in mice treated with nicotine (10 μg/ml) for 7 – 10 days (A) and cocaine (30 mg/kg) for 7 days (B) (n = 5–7 in each group). Data represent means ± SEM. (C) Real-time RT-PCR measuring FosB expression in animals treated with systemic SAHA (25 mg/kg i.p.) followed by cocaine compared with controls (n = 5–7 in each group). (D) Immunoblots of histone H3 and H4 acetylation after SAHA treatment (n = 3–4 in each group). (E) Real-time RT-PCR measuring FosB expression in animals treated with local SAHA (100 μM) infused to the NAc for 7 days followed by cocaine (30 mg/kg) compared with controls (n = 4–7 in each group). (F) LTP measurement in mice treated with SAHA (25 mg/kg) followed by cocaine (30 mg/kg). SAHA was given 2 hours before cocaine or saline injections (n = 5 to 6). Field potential amplitudes were measured in the core of the NAc. (G) Histogram summary of time course of SAHA and cocaine effects on LTP. *P < 0.05.
An HDAC inhibitor mimics the action of nicotine in enhancing cocaine responses
As an independent test of the finding that nicotine produces its effect on cocaine responses by inhibiting histone deacetylases, we asked whether we could simulate the effect of nicotine by specifically inhibiting deacetylases with the histone deacetylases inhibitor suberoyl ailide hydroxamine acid (SAHA). We administered SAHA (25 mg/kg intraperitoneally), instead of nicotine, 2 hours before cocaine treatment (30 mg/kg) and observed a 71% increase in FosB expression in the pretreated mice compared to mice treated with cocaine alone (control set at 1.0; cocaine alone, 2.8 ± 0.59; SAHA followed by cocaine, 4.8 ± 0.54; *P < 0.05; Fig. 4C). This effect of SAHA was similar in magnitude to the enhanced FosB expression induced by cocaine when pretreated by nicotine. SAHA alone caused global histone acetylation in the striatum, as did nicotine (*p < 0.05, Fig 4D). This result is consistent with our earlier finding that SAHA caused a marked increase in FosB expression when given before an acute cocaine injection compared with the effect of cocaine alone (15). To be certain that SAHA exerts its effect on gene transcription specifically in the NAc, we infused SAHA (100 μM) locally into the NAc for 7 days and then treated with cocaine (30 mg/kg). Using real-time RT-PCR, we found that a single cocaine injection after local pretreatment with SAHA for 7 days resulted in 142% more FosB mRNA expression than without pretreatment with SAHA (Fig. 4E).
We next asked whether pretreatment with SAHA substitutes for nicotine in producing an effect on cocaine-induced synaptic plasticity, similar to the effect of nicotine on FosB expression. We administered SAHA (25 mg/kg i.p.) 2 hours prior to cocaine administration and measured field potential amplitudes in the core of the NAc. SAHA pretreatment fully simulated nicotine pretreatment and induced a greater reduction in LTP in the core cells of the NAc than did cocaine alone (control 135 ± 7%; cocaine alone 109 ± 5%; SAHA followed by cocaine 81 ± 9%, *p < 0.05) consistent with the idea that the mechanism of action mediating the greater reduction of LTP after chronic nicotine treatment is an increase in histone acetylation in the striatum. Similar to nicotine alone, SAHA alone did not cause a depression in LTP but rather tended to facilitate it (though without statistical significance, SAHA alone 150 ± 10%; Fig. 4, E and F). The overall effects of SAHA and nicotine were quantitatively and qualitatively similar. SAHA, like nicotine, is known to enhance the behavioral effects of cocaine (26), but the electrophysiological effects of the drugs are unknown. These results support our experimental findings that nicotine inhibits histone deacetylases.
Hypoacetylated chromatin attenuates the response to cocaine
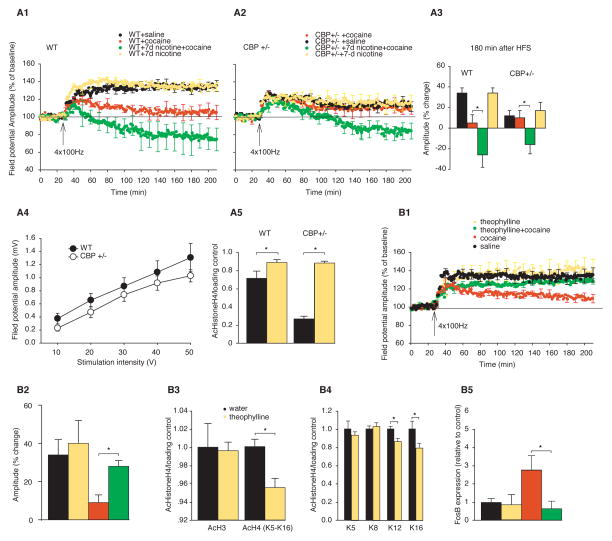
To test further the idea that histone acetylation and deacetylation are key molecular mechanisms for the effect of nicotine on the response to cocaine, we conducted two sets of experiments, one genetic and one pharmacological. To determine the impact of decreased acetylation on the response to cocaine, we first used a genetic approach by studying genetically modified mice with Rubinstein-Taybi syndrome. These mice are in a hypoacetylated state because they lack one functional allele of the histone acetyl transferase CBP. Since SAHA helps restore some of the memory deficits in these mice (12), we wondered whether nicotine, which acts like SAHA, would restore some of these memory deficits as well. We found that in the control group, CBP haploinsufficient mice showed impaired LTP (Fig. 5A, 1–4). Similar to CBP+/− saline controls, a single injection of cocaine had no effect on LTP in the mutant mice. By contrast, the wild-type littermates treated with cocaine showed the expected reduction in LTP compared with wild-type saline controls (wild-type control 135 ± 7%; cocaine 105 ± 8%; nicotine followed by cocaine 74 ± 13%, **p < 0.01). Treatment with 7 days nicotine rescued some of the impaired response to cocaine in CBP haploinsufficient mice, which exhibited a reduction of LTP in response to cocaine (CBP+/− mice control 112 ± 6%; cocaine 110 ± 7%; 84 ± 9%, Fig. 5A, 1–3). There was no difference in the input-output curve between CBP+/− mice and their wild-type littermates (Fig. 5A 4).
Fig. 5.
The priming effect of nicotine on cocaine in CBP+/− mice and mice treated with theophylline. (A1–A2) LTP measurement in CBP+/− mice and wild-type littermate controls treated with 7 days of nicotine; acute cocaine; and 7 days of nicotine followed by cocaine injection (n = 5–8). (A3) Histogram summary of changes in LTP amplitude at 180 min after HFS in different groups of mice (as shown in A1–A2). (A4) Input-output curve comparing CBP+/− mice and their wild-type littermates. (A5) Histone H4 (K5–16) tail acetylation in striatal lysates of CBP+/− mice and wild-type littermates after 7 days of nicotine exposure (n=4 per group). (B1) LTP measurement in mice treated with theophylline for 7 days; mice treated with theophylline followed by acute cocaine; mice treated with cocaine alone; and controls (n = 6–10 in each group). (B2) Histogram summary of changes in LTP amplitude at 180 min after HFS in different groups of mice (as shown in B1). (B3 and B4) Immunoblots of striatal lysates from mice treated for 7 days with theophylline (200 mg/liter in drinking water) probed with antibodies against acetylated histone H3 (K9) and acetylated histone H4 (K5 to K16) (B3), and antibodies against specific acetylated lysine residues on histone H4 (K5, K8, K12, and K16) (B4) (n = 4 per group). (B5) Real-time PCR measuring FosB expression in animals treated with local theophylline (0.2 mM) infused into the NAc for 7 days compared with controls (n = 5 per group). Groups as shown in B1. *P < 0.05.
Using immunoblots, we then examined the level of acetylated histones in the striatum. We found that CBP mutant mice had a 49% reduction in histone H4 acetylation in the striatum compared with wild-type controls (control 0.71 +/− 0.09, CBP+/− mice 0.35 ± 0.02). After 7 days of nicotine treatment, histone H4 acetylation in the mutant mice increased to values comparable to those in wild-type mice exposed to nicotine (wild-type nicotine 0.94 ± 0.03; CBP+/− nicotine 0.93 ± 0.02; *p < 0.05, Fig. 5A 5).
We then used a low-dose of theophylline, a histone deacetylase (HDAC) stimulator, to achieve a hypoacetylated state by activating histone deacetylases. At low doses, theophylline is a relatively specific HDAC stimulator, binding to allosteric sites on the enzymes (most likely HDAC1 and HDAC3). At higher doses, it has the opposite effect: it is an HDAC and phosphodiesterase inhibitor and an adenosine receptor blocker (27,28). After determining the optimal dose for HDAC activation, we used theophylline to achieve a hypoacetylated state. We hypothesized that hypoacetylation would attenuate the action of cocaine in wild-type mice and thereby produce an effect that would be opposite to that of SAHA and nicotine. We first investigated the effect of theophylline on LTP and then on histone acetylation and FosB mRNA expression in the NAc. After treatment with low-dose theophylline (200 mg/ml) for 7 days, there was no difference between the theophylline-treated group and water controls: both groups showed a comparable increase in LTP. However, the theophylline-treated mice exhibited an attenuated LTP reduction in response to cocaine treatment (30 mg/kg) (controls 135 ± 8%; theophylline 140 ± 12%; cocaine 109 ± 4%; theophylline followed by cocaine 128 ± 3%, *p < 0.05, Fig. 5B, 1 and 2). Theophylline treated mice also showed a reduction in levels of acetylated histone H4 (Fig. 5B 3) specifically in acH4(K12) and acH4(K16) (control set at one; acH4(K12) 0.80 ± 0.05; acH4(K16) 0.078 ± 0.06, *p < 0.05, Fig. 5B 4).
To stimulate HDAC selectively in the NAc, we next infused theophylline directly into NAc and examined its effect on FosB transcription. We observed that mice treated locally for 7 days with theophylline (0.2 μM) in the NAc and then injected with cocaine (30 mg/kg) for two hours exhibited no increase in FosB mRNA expression in response to cocaine, although cocaine alone increased FosB expression by 176%. Rather they exhibited the same levels of FosB mRNA in the NAc, as did controls (control set at 1; theophylline alone 0.82 ± 0.22; cocaine 2.76 ± 0.64; theophylline followed by cocaine 0.66 ± 0.12, *p < 0.05, Fig. 5B 5). These data support the idea that a hypoacetylated histone state, caused either by a genetic mutation or pharmacologically by local HDAC activation, causes a reduction in FosB expression as well as a reduction in the depression of LTP in response to cocaine. By contrast, nicotine decreases HDAC activity, and thereby increases global histone acetylation in the striatum, creating an environment primed for the induction of gene expression. The acetylated chromatin induced by nicotine exposure allows greater FosB gene expression in response to cocaine injection than FosB gene expression after cocaine alone. This model is illustrated in Fig. 6.
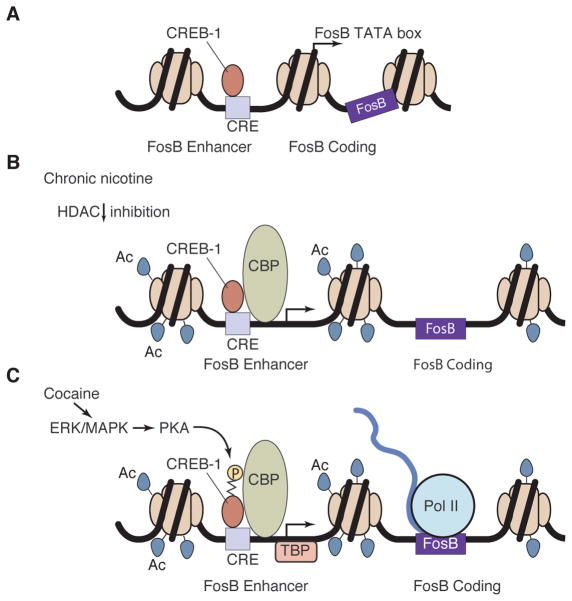
Fig. 6.
A molecular model for the nicotine-cocaine gateway sequence of drug usage. (A) FosB promoter region at baseline. (B) Acetylation of the promoter region of FosB after 7 days of nicotine exposure. (C) FosB expression in response to cocaine with previous nicotine exposure. TBP, TATA box–binding protein.
The priming effect of nicotine requires concurrent administration of cocaine
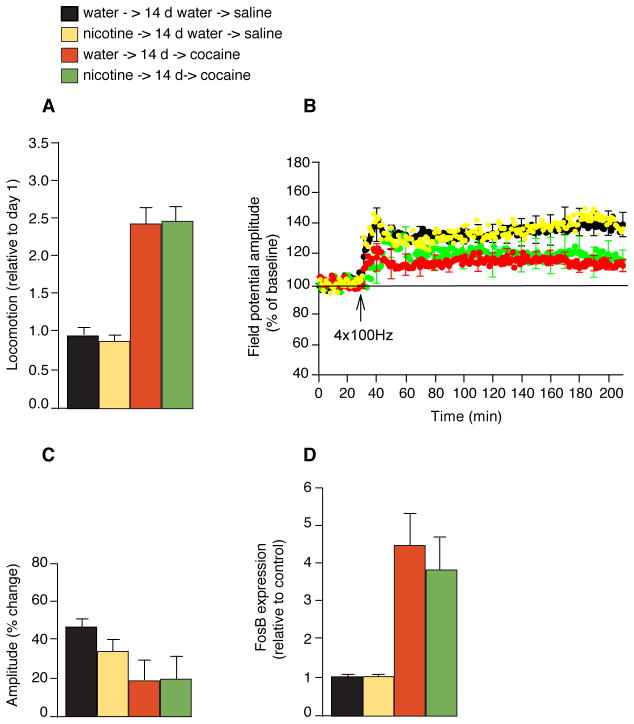
To investigate further the duration of the priming effect of nicotine, we repeated some of the behavioral, electrophysiological, and gene expression studies but administered cocaine (the dosages for nicotine and cocaine were 10 mg/ml and 30 mg/kg for the biochemical and electrophysiological studies; dosages were 50 mg/ml and 20 mg/kg for behavioral studies) 14 days after stopping 7 days of nicotine treatment (10 or 50 mg/ml). We saw no enhancement of the locomotor effect of cocaine (sensitization) in contrast to the increase in sensitization we observed when we gave nicotine for 7 days prior to and then concurrently with cocaine (compare Fig. 7A with Fig. 1B). This pattern was also observed in physiological experiments. The magnitude of LTP in the mice that received cocaine 14 days after cessation of nicotine treatment did not differ from that of LTP in the cocaine alone group (Fig. 7, B and C). Similarly, the expression of FosB after cocaine administration was the same in mice exposed to nicotine 14 days earlier and in mice without previous nicotine exposure (Fig. 7D). These findings indicate that the priming effect of nicotine does not occur unless nicotine is given repeatedly and in close conjunction with cocaine.
Fig. 7.
Priming effect of nicotine on cocaine-induced responses requires continuous nicotine administration. (A) Behavioral assay for locomotor sensitization to 4 days of cocaine (20 mg/kg) following 14 days of water after 7 days of nicotine treatment (50 μg/ml); compared with 4 days of cocaine treatment with no prior nicotine; 14 days of water after 7 days of nicotine treatment; and controls (n = 12–15). (B) LTP measurement in mice treated with a single cocaine injection 14 days after 7 days of nicotine; single cocaine injection without prior nicotine treatment; 14 days of water following 7 days of nicotine; and controls (n = 6–7). (C) Histogram of the effect of nicotine cessation on LTP amplitude 180 min after HFS. (D) Real-time PCR measuring FosB expression in the striata of mice pretreated with nicotine for 7 days followed by water for 14 days, and then treated with a single cocaine injection; cocaine with no previous nicotine exposure; nicotine for 7 days, followed by 14 days of water; and controls (n = 3 to 4 in each group). Data represent means ± SEM.
From animal models to human epidemiology
Our results in mice showing that priming of cocaine responses by nicotine requires more than one day of treatment plus concurrent exposure to nicotine with the first exposure to cocaine stimulated us to return to human populations and ask two questions. What is the smoking status of cocaine users at the time they start using cocaine? Does the smoking pattern at the time of cocaine initiation affect the likelihood of moving on to regular cocaine use?
To address these questions, we reexamined existing data from a small longitudinal cohort of former New York State high school students whom we had followed from ages 15.7 to 34.2 and from whom we had obtained detailed monthly drug use histories (29). From these data, we identified months of use and non-use of cigarettes and cocaine. Of the cocaine users who initiated cocaine after smoking, 81.2% did so in a month when they were actively smoking and only 18.8% in a month when they were not smoking. Thus, the majority of cocaine users smoked cigarettes before they started using cocaine, and, in addition, they started using cocaine while they were actively smoking cigarettes. Our animal observations suggest that this pattern of cocaine use onset while actively smoking might result in enhanced effects of cocaine and therefore be associated with the highest rates of cocaine dependence.
To examine this hypothesis, we tested the consequences of the sequential order of drug use for using cocaine regularly by analyzing data from the National Epidemiological Study of Alcohol Related Consequences (NESARC) (30), a cohort representative of the U.S. population. We compared the rates of lifetime cocaine dependence among three groups: those who had started to use cocaine after they had started to smoke and before they had stopped smoking, but in whom the smoking status at time of cocaine use onset could not be determined, including a few individuals who had started to use both drugs at the same age; those who had started cocaine use before beginning to smoke; and those who had ever smoked fewer than 100 cigarettes, including none. We found that the rate of cocaine dependence was the highest among cocaine users who initiated cocaine after having smoked (20.2%). The rates of dependence were much lower among those who initiated cocaine before smoking (6.3%) or who had ever smoked less than 100 cigarettes, including none (10.2%) (p < 0.001).
Discussion
Many drugs of abuse, including heroin, LSD, marijuana, and the two drugs that we have studied here, nicotine and cocaine, exert their addictive effects in part by increasing levels of dopamine in the ventral striatum (31). Dopamine leads to the synthesis of cAMP and the activation of extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK), which activates cyclic AMP dependent protein kinase (PKA) in the spiny stellate cell (32–34). PKA, in turn, phosphorylates CREB, a transcription factor that binds to the CRE-containing promoter of FosB. Upon phosphorylation, CREB recruits CBP, a transcription factor that is also a histone acetyl transferase, which acetylates lysine residues in the N terminal domains of histone proteins. Acetylation of these lysine residues decreases the positive charges of histones and their binding to DNA and opens up the promoter region. As a result, the promoter becomes more accessible to RNA polymerase, enabling its activity and thereby the transcription of the FosB gene, and its splice variant FosB, which is implicated in addiction. FosB is characterized by a particularly long half-life and its accumulation has been shown to be critical for the formation of addiction in animal studies. This process, the increase in histone acetylation at the FosB promoter and the facilitation of the FosB mRNA expression, is in turn opposed by the activity of histone deacetylases that typically restore the acetylation levels to baseline (13, 14).
We have found that nicotine by itself induces only a small increase in FosB expression in the striatum. However, nicotine also inhibits histone deacetylase, leading to a more widespread acetylation of histones at a larger number of amino acids in the striatum than does cocaine alone, thereby creating an environment that is in principle primed for the induction of a number of genes. We find that this ability of nicotine to hyperacetylate chromatin widely by inhibiting histone deacetylases is not shared by cocaine, which causes a more local and transient acetylation at the FosB promoter but not throughout the whole striatum. When a second drug of abuse, in this case cocaine, is given to animals after nicotine exposure, the higher histone acetylation levels lead to greater activation of FosB and, likely, other genes.
To test this idea further, we substituted a histone deacetylase inhibitor, SAHA, for nicotine and found that SAHA phenocopied the priming effects of nicotine on FosB expression and LTP measurements. These results suggest a model whereby nicotine exerts it priming effect on cocaine through its inhibition of histone deacetylase and provides a potential molecular explanation for the unidirectional sequence of drug use of nicotine on cocaine observed both in human populations and in our animals. Moreover, we observed the priming effect of nicotine only when cocaine administration partially overlapped with nicotine exposure, suggesting that HDAC inhibition by nicotine depends on continuous nicotine intake. This is supported by our epidemiological data, which similarly show that, in human populations, most individuals start using cocaine while they are concurrently using nicotine, possibly enhancing the physiological effects of cocaine. These studies raise an interesting question that can now be explored in animal models: Is the hyperacetylation produced by nicotine also a molecular explanation of drug action shared by the two other gateway drugs, alcohol and marijuana? Is there a single mechanism for all gateway sequences or does each sequence utilize a distinct mechanism?
HDAC activators may be of potential clinical utility in the treatment of addiction, since they could decrease FosB expression in response to cocaine. Modifying HDAC activators so that they target the striatum specifically would be particularly desirable, since systemic treatments with HDAC activators or HAT inhibitors are likely to have cognitive and other deleterious effects.
Our results, which show how nicotine acts as a gateway drug on the brain, an effect that is likely also to occur when nicotine exposure is from passive and non-smoked forms, emphasize the need for developing more effective public health prevention programs for all products that contain nicotine, especially those targeted toward young people. Our data suggest that effective interventions would not only prevent smoking and its negative health consequences but could also decrease the risk of progression to chronic illicit drugs. A combined molecular and translational epidemiological approach of the sort we outline here can address a family of questions emerging from human epidemiological research. In addition to the gateway sequence of drug use discussed here, similar approaches could illuminate the differences in developmental outcome depending on age of exposure to a particular substance, the consequences of prenatal drug exposure for the behavior and drug taking propensity of the offspring, and the vulnerability of adolescents to addiction.
Supplementary Material
Acknowledgments
We thank Ronald Breslow for supplying us with SAHA.
Funding: This work has been supported by NIH grant # 5 R01 DA024001 (Kandel, E., Kandel, D. and Levine, A., Multiple Principle Investigators); and K 5 DA00081 (Kandel, D.).
Footnotes
Materials and Methods; References.
Author contributions: A.L. contributed to planning and executing all experiments and writing of the manuscript, Y.H. performed electrophysiology experiments, B.D. performed the SAHA and theophylline treatment experiments and helped editing the manuscript, D.D.P. helped with the sensitization experiments, E.A.G. performed the CPP experiments and helped with the manuscript, S.X. assisted with all biochemical and molecular biology experiments, D.Y. performed genotyping for CBP mice, C.S. performed the analysis of the epidemiological data. D.B.K. and E.R.K. participated in planning and overseeing the experimental work and writing and editing the manuscript.
Competing interests: There are no competing interests.
References and notes
- 1.Kandel DB. Stages in adolescent involvement with drugs. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- 2.Kandel DB, editor. Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. Cambridge Univ. Press; Cambridge, England: 2002. pp. 3–15. [Google Scholar]
- 3.Yamaguchi K, Kandel DB. Patterns of drug use from adolescence to young adulthood: II. Sequences of progression. Am J Public Health. 1984;74:668–672. doi: 10.2105/ajph.74.7.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Survey on Drug Use and Health, 2009 [Computer file], ICPSR29621-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2010. Nov 16, [Google Scholar]
- 5.Thomas MJ, Berrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature Neuroscience. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 6.Clarke PB. Dopaminergic mechanisms in the locomotor stimulant effects of nicotine. Biochem Pharmacol. 1990;40:1427. doi: 10.1016/0006-2952(90)90436-o. [DOI] [PubMed] [Google Scholar]
- 7.Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;18:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 8.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 9.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 10.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ, Malenka RC. The addicted brain. Sci Am. 2004;290:78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- 12.Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci. 2005;27:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestler EJ, Barrot M, Self OW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;25, 1104:2–6. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004;25:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Desai RI, Terry P. Evidence of cross-tolerance between behavioural effects of nicotine and cocaine in mice. Psychopharmacology. 2003;166:111–119. doi: 10.1007/s00213-002-1319-4. [DOI] [PubMed] [Google Scholar]
- 19.James-Walke NL, Williams HL, Taylor DA, McMillen BA. Periadolescent nicotine exposure produces sensitization to reinforcement by diazepam in the rat. Neurotoxicol Teratol. 2007;29:31–36. doi: 10.1016/j.ntt.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Klein LC. Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine responses in adult male and female rats. Exp Clin Psychopharmacol. 2001;9:251–261. doi: 10.1037//1064-1297.9.3.251. [DOI] [PubMed] [Google Scholar]
- 21.McMillen BA, Davis BJ, Williams HL, Soderstrom K. Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. Eur J Pharmacol. 2005;509:161–164. doi: 10.1016/j.ejphar.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 22.McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nestler EJ. Transcriptional mechanisms of addiction: role of deltaFosB. Philos Trans R Soc London, B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renthal W, Maze I, Krishnan V, Covington HE, III, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;8:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Ito KK, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, Barnes PJ. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci USA. 2002;99:8921–8926. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–818. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- 29.Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- 30.Grant BF, Moore TC, Kaplan KD. Source and accuracy statement: Wave I National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- 31.Salas R, De Biasi M. Opposing actions of chronic stress and chronic nicotine on striatal function in mice. Neurosci Lett. 2008;25:32–34. doi: 10.1016/j.neulet.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;10:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 34.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;10:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.