Abstract
Background
Outcomes data comparing endoscopic eradication therapies (EET) and esophagectomy are limited in patients with early esophageal cancer (EC).
Objective
To compare overall survival and EC-related mortality in patients with early EC treated with EET and esophagectomy.
Design and setting
Population-based study
Patients
Patients with early EC (stage T0 and T1) were identified from the SEER database (1998–2009). Demographics, tumor specific data and survival were compared. Cox proportional hazards regression models were used to evaluate association between treatments and EC-specific mortality.
Intervention
EET and esophagectomy
Main outcome measurements
(i) Mid (2-years) and long-term (5-years) overall survival and esophageal cancer specific mortality, (ii) Outcomes based on histology and stage and (iii) treatment patterns and predictors of cancer-specific mortality.
Results
430 (21%) and 1,586 (79%) underwent EET and esophagectomy, respectively. There was no difference in the 2-year (EET: 10.5% vs. esophagectomy: 12.7%, p=0.27).and 5-year (EET: 36.7% vs. esophagectomy: 42.8%, p=0.16) EC related mortality rates between the two groups. EET patients had higher mortality rates due to non-EC causes (5-years: 46.6% vs. 20.6%, p<0.001). Similar results were noted when comparisons were limited to patients with T0 and T1a disease and EAC. There was no difference in EC-specific mortality in the EET compared to surgery group [HR: 1.4 (95% CI 0.9–2.03)]. Variables associated with mortality were older age, year of diagnosis, radiation therapy, higher stage and ESCC.
Limitations
Comorbidities, recurrence rates not available.
Conclusions
This population-based study demonstrates comparable mid and long-term EC- related mortality in patients with early EC undergoing EET and surgical resection.
Keywords: Esophageal cancer, esophageal adenocarcinoma, endoscopic eradication therapies, esophagectomy, survival, mortality
INTRODUCTION
Esophageal cancer incidence continues to increase faster than almost any other cancer in the Western World.1, 2 Despite the recent advances, patients with esophageal cancer have a dismal 5-year survival rate of 16.8% (all stages).3 Early esophageal cancer, defined as disease limited to the mucosa or submucosa, constitutes approximately 20% of all cases of esophageal cancer.4, 5 Given the high tumor-free survival rates, esophagectomy has been the standard treatment for patients with early esophageal cancer to which all other therapies are compared. 6–10 Esophagectomy in patients in whom the cancer has not yet penetrated the muscularis mucosa is associated with 5-year survival rates as high as 90%.11,9 However, esophagectomy for early esophageal cancer is associated with an overall operative mortality rate of 2% and major morbidity rate of up to 10%, even in high volume and centers with multi-disciplinary care.11, 12
Based on a growing body of literature suggesting favorable outcomes compared to esophagectomy, endoscopic eradication therapies have gained gradual acceptance and are endorsed in society guidelines, especially in the field of Barrett’s related neoplasia.4, 9, 12–16 The basic premise of endoscopic eradication therapies is that patients with cancer limited to the mucosa have a very low risk (0–2%) of lymph node metastasis.17 On the other hand, most experts agree that patients with submucosal, poorly differentiated cancer, size > 2 cm, lymphatic or venous infiltration should generally be referred for surgical resection given the high risk of lymph node metastasis - at least 20%).18,17 Although data suggests that endoscopic eradication therapies are highly effective, studies comparing endoscopic eradication therapies with surgical resection are limited.4, 9, 10 Unfortunately, no randomized controlled trial that may provide conclusive evidence for which of these two treatments is superior has been conducted nor are any such trials expected in the foreseeable future.
The aims of this study were to use the Surveillance Epidemiology and End Results (SEER) database: (i) to compare mid (2-years) and long-term (5-years) overall survival and esophageal cancer specific mortality in patients with early esophageal cancer treated with endoscopic eradication therapies and esophagectomy, (ii) to compare outcomes (esophageal cancer-free survival) based on histology (EAC) and stage and (iii) to evaluate the treatment patterns and independent associations of treatment received with cancer-specific mortality.
METHODS
Data source
The SEER Program is an on-going contract-supported program of the National Cancer Institute (NCI) to collect population-based cancer incidence, individual patient and tumor characteristics, initial treatment and follow-up survival data from U.S. cancer registries (http://www.seer.cancer.gov). Last expanded in 2010, SEER-18 includes 18 cancer registries that cover approximately 28% of the U.S. population.19 Registries include the Alaska Natives, Metropolitan Atlanta, Greater California, Los Angeles, San Francisco-Oakland, San Jose-Monterey, as well as Connecticut, New Jersey, Detroit (Metropolitan), Iowa, Kentucky, Utah, Louisiana, New Mexico, Rural Georgia, Greater Georgia, Seattle (Puget Sound), and Hawaii. The SEER data contain de-identified patient data and, therefore, this study was exempted from Institutional Review Board review by the Office of Human Subject Research at the National Institutes of Health.
Study population
The study population was comprised of patients with a first primary esophageal cancer [International Classification of Diseases for Oncology (ICD-O-3 codes): C150–C155, C158–C159)], microscopically confirmed, and diagnosed from 1998 – 2009. All histologies were included. Patients diagnosed with early esophageal cancer, as defined by the modified American Joint Committee on Cancer (AJCC) criteria, were included for analysis. SEER extent of disease codes were used to identify patients with early esophageal cancer (00 – carcinoma in situ, invasive tumor confined to: 10 – mucosa, 11 – lamina propria, 12 – muscularis mucosae and 16 – submucosa). Patients with advanced stage disease [invasion of muscularis propria and beyond (codes: 20, 30, 40, 60, 65, 80, 85) or lymph node metastases], those that did not receive any endoscopic eradication therapies or surgical treatment, coded as unknown for extension of tumor or metastasis (code 99) and those with a diagnosis only provided by death certificate were excluded.
Variable definitions
Demographic, treatment, and survival information were extracted. Early disease as defined by extent of disease variables was categorized as stage T0 (carcinoma in situ), T1a (invasive tumor confined to mucosa, lamina propria, muscularis mucosae), or T1b (submucosa). Stage T1 encompasses both T1a and T1b disease. The SEER surgery codes used are similar to those based on the American College of Surgeons Commission on Cancer's Facility Oncology Registry Data System surgery codes, with supplementary annotations from the previous version of the SEER Program Code Manual (7). Surgery codes were categorized as endoscopic resection (with or without ablation therapy) or esophagectomy (partial or total). Variables analyzed also included: age at diagnosis (year), sex, race (white, non-white), tumor histology (squamous cell carcinoma, adenocarcinoma, other), tumor stage (T0, T1a, T1b), tumor size, tumor grade (well, moderately, poorly, or undifferentiated), radiation therapy (yes, no), SEER site (Northeast, Midwest, South, West), and year of diagnosis.
Statistical analyses
The frequencies and percentages for categorical variables, and the means and standard deviations for continuous variables, were calculated to characterize the treatment groups. Demographic features were compared between treatment groups by univariate analyses using t- tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. For individuals with sufficient follow-up, we compared esophageal cancer-specific 2- and 5-year survival rates between endoscopic eradication therapies and surgical resection.
Overall 2-year and 5-year survival was defined as the proportion of patients alive among all patients (alive or dead) with at least 2 years and 5 years of follow-up data, respectively. Esophageal cancer-specific survival was defined as the proportion of patients alive among all included patients (alive or dead from recurrent esophageal cancer). Stratified survival distributions were estimated and plotted using the Kaplan-Meier technique and log-rank tests were used to assess heterogeneity in survival curves.
Multivariable Cox proportional hazards regression models were used to study the independent association of the type of treatment received with esophageal cancer-specific survival or overall survival. Esophageal cancer-specific survival included patients with a cause of death originating from esophageal cancer while all other deaths were right censored. In contrast, in the overall survival analysis all patient deaths were considered events. All potential covariates were entered into a multivariable Cox proportional hazards regression model which was then reduced by using stepwise backward elimination with the significance threshold for retention in the model set at α = 0.10. In the multivariable Cox proportional hazards regression model, the maximum partial likelihood method was used to estimate the Hazard Ratio (HR) for each variable and the 95% confidence interval (CI). The HR ratio for indicator variables is interpreted as the ratio of the estimated hazard for those with a value of 1 to the estimated hazard for those with a value of 0 (controlling for all other covariates). For the outcome, all HRs are the estimated hazard for esophagectomy (1) compared with the estimated hazard for endoscopic resection (0). The proportional hazards assumption was assessed through visual inspection of log-log plots of survival against analysis time as well as through linear regression of the scaled Schoenfeld residuals 20 against analysis time. The Kaplan-Meier product limit method was used to analyze overall survival and the log-rank test was used to compare overall survival between patients treated with endoscopic eradication therapies and esophagectomy. All data analyses were performed using STATA 11.0 21 and SAS 9.2. 22 Data are presented as counts (percentages), means (standard deviations), or HRs (95% confidence intervals) where appropriate. Differences between treatment groups were considered to be statistically significant at P<0.05.
RESULTS
A total of 2,016 patients with early esophageal cancer undergoing endoscopic eradication therapies (n=430, 21.3%) and esophagectomy (n=1586, 78.7%) between 1998 and 2009 meeting inclusion criteria were identified. Patients with nodal metastasis were excluded from this analysis (n=259). Table 1 summarizes the baseline characteristics and cancer-related variables in the two groups. The vast majority of cases were White men and overall histological distribution was: EAC – 1,567 (77.7%), ESCC – 311 (13.3%), and others – 179 (9%). The distribution based on stage of disease was as follows: Stage T0 – 357 (17.7%), Stage T1a – 935 (46.3%), and Stage T1b – 724 (36%). The type and distribution of endoscopic eradication therapies are summarized in Supplementary Table 1. Endoscopic mucosal resection (EMR) was the predominant treatment modality (n=295, 68.6%) followed by EMR with ablation (n=45, 10.4%). Surgical techniques in the esophagectomy group are also highlighted in Supplementary Table 1.
Table 1.
Baseline characteristics and outcomes of patients with early esophageal cancer by treatment
| Variable | Endoscopic eradication therapies (n=430) | Esophagectomy (n=1586) | P-value |
|---|---|---|---|
|
| |||
| Mean age (yrs, SD) | 70.5 (10.3) | 63.4 (9.8) | <0.001 |
|
| |||
| Men (n, %) | 335 (77.9) | 1346 (84.9) | 0.001 |
|
| |||
| Whites (n, %) | 401 (93.3) | 1463 (92.2) | 0.496 |
|
| |||
| Stage (n, %) | |||
| Stage T0 | 142 (33.0) | 215 (13.6) | <0.001 |
| Stage T1a | 232 (54.0) | 703 (44.3) | |
| Stage T1b | 56 (13.0) | 668 (42.1) | |
|
| |||
| Histology (n, %) | |||
| Adenocarcinoma | 320 (74.4) | 1247 (78.6) | 0.118 |
| Squamous cell | 70 (16.3) | 200 (12.6) | |
| Others | 40 (9.3) | 139 (8.8) | |
|
| |||
| Histology grade: Well-differentiated | 48/166 (28.9) | 189/1133 (16.7) | 0.001 |
|
| |||
| Mean tumor size (mm, SD) | 16.5 (16.0) | 21.6 (19.7) | 0.022 |
|
| |||
| Radiation therapy (n,%) | 34 (8.0) | 198 (12.6) | 0.008 |
|
| |||
| SEER Site | |||
| Northeast | 100 (23.3) | 305 (19.2) | 0.108 |
| Midwest | 54 (12.6) | 202 (12.7) | |
| South | 63 (14.7) | 299 (18.9) | |
| West | 213 (49.5) | 780 (49.2) | |
|
| |||
| Mean follow-up within SEER (months, SD) | 34.4 (29.3) | 48.5 (36.0) | <0.001 |
|
| |||
| Patients with 2-year follow-up (n,%)1 | 306 (71.1%) | 1313 (82.8%) | <0.001 |
| 2-year overall survival | 240 (78.4%) | 1075 (81.8%) | 0.165 |
| 2-year EC related mortality | 32 (10.5) | 167 (12.7) | 0.278 |
| 2-year other cause mortality | 34 (11.1%) | 71 (5.4%) | <0.001 |
|
| |||
| Patients with 5-year follow-up (n,%)2 | 150 (34.9) | 724 (45.7) | 0.009 |
| 5-year overall survival | 25 (16.7) | 265 (36.6) | <0.001 |
| 5-year EC related mortality | 55 (36.7) | 310 (42.8) | 0.164 |
| 5-year other cause mortality | 70 (46.6) | 149 (20.6) | <0.001 |
397 patients censored (19.7% with <2 yrs. follow-up)
1142 patients censored (56.6% with <5 yrs. follow-up)
Outcomes between endoscopic eradication therapies and esophagectomy in early esophageal cancer
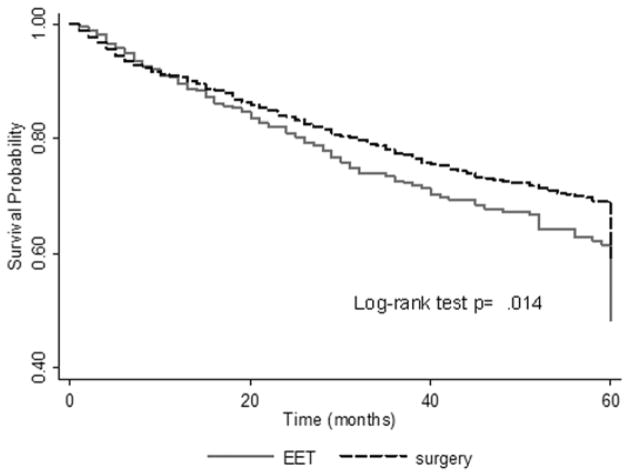
As shown in Table 1, patients in the endoscopic eradication therapies group were older compared with the esophagectomy group (mean age: 70.5 years vs. 63.4 years, p<0.001). A significantly higher proportion of patients with Stage T0 and T1a underwent endoscopic eradication therapies compared with esophagectomy (p<0.001). Patients in the endoscopic eradication therapies group were less likely to receive radiation therapy (8% vs. 12.6%, p<0.001), more likely to harbor smaller overall tumors (mean size: 17 mm vs. 23.2 mm, p=0.001) and have tumors with well-differentiated histology (27.5% vs. 14.8%, p=0.001). The mean follow-up in months was shorter in the endoscopic eradication therapies group (34.4 vs. 48.5 months, p<0.001). In individuals with at least 2 years of follow-up, there was no difference in 2-year overall survival between the two groups (endoscopic eradication therapies: 78.4% vs. esophagectomy: 81.8%, p=0.17). Although the proportion of deaths attributed to esophageal cancer was comparable between the two groups (10.5% vs. 12.7%, p=0.27), patients receiving endoscopic eradication therapies were more likely to die of other causes (predominantly heart disease) compared with patients receiving esophagectomy (11.1% vs. 5.4%, p<0.001). In individuals with at least 5 years of follow-up, esophageal cancer specific mortality was comparable between the two groups (endoscopic eradication therapies: 36.7% vs. esophagectomy: 42.8%, p=0.16). Patients in the endoscopic eradication therapies group were more likely to die of non-esophageal cancer causes (46.6% vs. 20.6%, p<0.001) (Table 1). Overall survival by the Kaplan-Meier’s estimate was higher in the esophagectomy group (log-rank test, p=0.014) (Figure 1). Excluding patients who received radiation (n=232), similar results were noted. There were no differences between the two groups with regards to 2-year and 5-year esophageal cancer specific mortality (p=0.34 and p=0.25, respectively). Also, similar results were noted when patients with T0 disease were excluded from the analysis.
Figure 1.
Overall survival by the Kaplan-Meier’s estimate in endoscopic eradication therapies and esophagectomy groups
Comparison of endoscopic eradication therapies and esophagectomy outcomes limited to patients with stage T0 and T1a esophageal cancer
Similar to the overall results, there was no difference between the two groups with regards to the 2-year overall survival (endoscopic eradication therapies: 79.6% vs. esophagectomy: 84%, p=0.1). Higher mortality related to other causes was noted in the endoscopic eradication therapies arm compared to esophagectomy (10.4% vs. 5%, p=0.002) with no difference in esophageal cancer specific mortality (10% vs. 11.1%, p=0.64). Similar results were noted when 5-year esophageal cancer specific mortality was compared between the two groups (Table 2).
Table 2.
Outcomes of patients with early esophageal cancer limited to stage T0 and T1a by treatment
| Variable | Endoscopic eradication therapies (n=374) | Esophagectomy (n=918) | P-value |
|---|---|---|---|
|
| |||
| Patients with 2-year follow-up (n,%)1 | 269 (71.9) | 760 (82.8) | <0.001 |
| 2-year overall survival | 214 (79.6) | 638 (84.0) | 0.101 |
| 2-year EC related mortality | 27 (10.0) | 84 (11.1) | 0.645 |
| 2-year Other cause mortality | 28 (10.4) | 38 (5.0) | 0.002 |
|
| |||
| Patients with 5-year follow-up (n,%)2 | 129 (34.5) | 389 (42.4) | 0.009 |
| 5-year overall survival | 21 (16.3) | 159 (40.9) | <0.001 |
| 5-year EC related mortality | 49 (38.0) | 145 (37.3) | 0.885 |
| 5-year other cause mortality | 59 (45.7) | 85 (21.9) | <0.001 |
263 patients censored (28.6% with <2 yrs. follow-up)
774 patients censored (84.3% with <5 yrs. follow-up)
Outcomes between endoscopic eradication therapies and esophagectomy in patients with esophageal adenocarcinoma
Of the 1,567 patients with early EAC, 320 (20.4%) patients underwent endoscopic eradication therapies and 1247 (79.6%) underwent esophagectomy. As highlighted in Table 3, patients undergoing endoscopic eradication therapies were older (mean age 70.1 vs. 63.2 years, p<0.001) and more likely to be diagnosed with stage T0 (28.3% vs. 13.1%, p<0.001) and T1a disease (58.8% vs. 43.5%, p<0.001) compared to those undergoing esophagectomy. There was no difference between the two groups with regards to the overall 2-year survival (p=0.07). Comparable esophageal cancer related mortality was noted between the two groups (p=0.22) whereas mortality related to other causes was significantly higher in the endoscopic eradication therapies group (13.5% vs. 5.7%, p<0.001). Similar results were noted in patients with at least 5 years of follow-up (Table 3). Similar results, with regards to 2-year and 5-year esophageal cancer related mortality, were noted when patients with T0 disease were excluded from this analysis.
Table 3.
Baseline characteristics and outcomes of patients with early esophageal adenocarcinoma by treatment
| Variable | Endoscopic eradication therapies (n=320) | Esophagectomy (n=1247) | P-value |
|---|---|---|---|
|
| |||
| Mean age (yrs, SD) | 70.1 (10.3) | 63.4 (9.8) | <0.001 |
|
| |||
| Men (n, %) | 262 (81.9) | 1110 989.0) | 0.001 |
|
| |||
| Whites (n, %) | 312 (97.5) | 1196 (95.9) | 0.183 |
|
| |||
| Stage (n, %) | |||
| Stage T0 | 92 (28.8) | 186 (14.9) | <0.001 |
| Stage T1a | 189 (59.1) | 570 (45.7) | |
| Stage T1b | 39 (12.2) | 491 (39.4) | |
|
| |||
| Histology grade: Well-differentiated | 36/119 (30.3) | 159/859 (18.5) | <0.001 |
|
| |||
| Mean tumor size (mm, SD) | 14.4 (10.3) | 20.6 (18.2) | 0.009 |
|
| |||
| Radiation therapy (n,%) | 24 (7.6) | 138 (11.2) | 0.062 |
|
| |||
| SEER Site | |||
| Northeast | 74 (23.1) | 248 (19.9) | 0.040 |
| Midwest | 44 (13.8) | 161 (12.9) | |
| South | 38 (11.9) | 231 (18.5) | |
| West | 164 (51.3) | 607 (48.7) | |
|
| |||
| Mean follow-up within SEER (months, SD) | 33.7 (28.7) | 48.5 (35.7) | <0.001 |
|
| |||
| Patients with 2-year follow-up (n,%)1 | 224 (70.0) | 1020 (81.8) | <0.001 |
| 2-year survival | 176 (78.6) | 852 (83.5) | 0.076 |
| 2-year EC related mortality | 18 (8.0) | 110 (10.8) | 0.220 |
| 2-year other cause mortality | 30 (13.4) | 58 (5.7) | <0.001 |
|
| |||
| Patients with 5-year follow-up (n,%)2 | 105 (32.8) | 525 (42.1) | 0.003 |
| 5-year survival | 16 (15.2) | 201 (38.3) | <0.001 |
| 5-year EC related mortality | 32 (30.5) | 210 (40.0) | 0.067 |
| 5-year other cause mortality | 57 (54.3) | 114 (21.7) | <0.001 |
323 patients censored (20.6% with <2 yrs. follow-up)
937 patients censored (59.8% with <5 yrs. follow-up)
Comparison of endoscopic eradication therapies and esophagectomy in patients with stage T0 and T1a esophageal adenocarcinoma
Limiting the analyses to patients with Stage 0 and 1a, there were 1,037 patients with early EAC [Stage T0 – 278 (26.8%), Stage T1a – 759 (73.2%)]. A total of 281 (27%) and 756 (73%) underwent endoscopic eradication therapies and esophagectomy, respectively. Although esophageal cancer related mortality was comparable between the two groups (p=0.67), mortality related to other causes was higher in the endoscopic eradication therapies group (12.6% vs. 5.5%, p=0.001). Similar results were noted when 5-year survival rates were compared between the two groups (Table 4).
Table 4.
Outcomes in patients with early esophageal adenocarcinoma limited to stage T0 and T1a by treatment
| Variable | Endoscopic eradication therapies (n=281) | Esophagectomy (n=756) | P-value |
|---|---|---|---|
|
| |||
| Patients with 2-year follow-up (n,%)1 | 199 (70.8) | 621 (82.1) | <0.001 |
| 2-year survival | 158 (79.4) | 531 (85.5) | 0.041 |
| 2-year EC related mortality | 16 (8.0) | 56 (9.0) | 0.672 |
| 2-year other cause mortality | 25 (12.6) | 34 (5.5) | 0.001 |
| Patients with 5-year follow-up (n,%)2 | 91 (32.4) | 294 (38.9) | 0.054 |
| 5-year survival | 13 (14.3) | 128 (43.5) | <0.001 |
| 5-year EC related mortality | 29 (31.9) | 99 (33.7) | 0.749 |
| 5-year other cause mortality | 49 (53.9) | 67 (22.8) | <0.001 |
271 patients censored (20.9% with <2 yrs. follow-up)
652 patients censored (62.9% with <5 yrs. follow-up)
Comparison of endoscopic eradication therapies and esophagectomy in patients with T1a and T1b esophageal cancer
There were no differences in the 2-year and 5-year esophageal cancer related mortality (p=0.18 and 0.27, respectively) when the analysis was limited to patients with T1a esophageal cancer (n=935). Similar results were noted when outcomes were compared in patients with EAC (n=759) and non-EAC (n=176) cases. Comparable 2-year and 5-year esophageal cancer related mortality rates (p=0.8 and p=0.2, respectively) were noted when endoscopic eradication therapies were compared to surgery in patients with T1b esophageal cancer (n=724), including subgroup of patients with EAC (n=530) and non-EAC (n=194).
Predictors of esophageal cancer-free survival
Results of the Cox proportional hazards regression models showed that the HR for esophageal cancer-free survival and overall survival in the endoscopic eradication therapy group was not different from the surgical resection group (HR 1.42, 95% CI 0.99–2.03, p=0.06 and HR 1.01, 95% CI 0.81–1.26, p=0.88, respectively). The significant variables associated with increased mortality included age at diagnosis, exposure to radiation therapy, increasing stage of disease (stage T1a and T1b) and year of diagnosis whereas tumor histology of EAC was associated with improved survival compared to ESCC (Table 5). Similar results were noted when models were limited to Stage T0 and T1a cancer cases with no difference in the HR between the two groups for esophageal cancer-free survival and overall survival (HR 1.18, 95% CI 0.78–1.8, p=0.43 and HR 0.81, 95% CI 0.62–1.05, p=0.12, respectively) (Table 5). When the survival analysis was limited to early EAC (stage T0, T1a and T1b) or early EAC limited to stage T0 and T1a, treatment modality was not a predictor for esophageal cancer-free survival (data not shown).
Table 5.
Cox proportional regression analysis (multivariate): Esophageal Cancer-Specific Mortality
| Variable | Hazard Ratio (95% CI) | P value |
|---|---|---|
|
| ||
| Model 1: EC overall | ||
| EET vs. Surgery | 1.42 (0.99, 2.03) | 0.054 |
| Age at diagnosis | 1.04 (1.03, 1.06) | <0.001 |
| Histology | ||
| Adenocarcinoma | Reference | |
| Squamous cell histology | 1.47 (1.11, 1.93) | 0.007 |
| Other histology | 1.79 (1.28, 2.52) | 0.001 |
| Stage | ||
| Stage 0 | Reference | |
| Stage 1A | 2.56 (2.19, 5.51) | <0.001 |
| Stage 1B | 3.47 (2.19, 5.51) | <0.001 |
| Year of diagnosis | 0.95 (0.91, 0.99) | 0.026 |
| Radiation | 3.04 (1.03, 1.06) | 0.003 |
| SEER Region | ||
| Northeast | 2.00 (1.26, 3.17) | 0.003 |
| Midwest | Reference | |
| West | 2.63 (1.66, 4.16) | <0.001 |
| South | 1.98 (1.31, 3.01) | 0.001 |
|
| ||
| Model 2: EC Stage T0 and T1a | ||
| EET vs. Surgery | 1.18 (0.78, 1.80) | 0.437 |
| Age at diagnosis | 1.04 (1.02, 1.06) | <0.001 |
| Histology | ||
| Adenocarcinoma | Reference | |
| Squamous cell histology | 1.71 (1.15, 2.54) | 0.008 |
| Other histology | 1.74 (1.06, 2.86) | 0.028 |
| Stage | ||
| Stage 0 | Reference | |
| Stage 1A | 2.49 (1.56, 3.99) | <0.001 |
| Year of diagnosis | 0.94 (0.88, 0.99) | 0.032 |
| Radiation | 4.45 (3.12, 6.35) | <0.001 |
| SEER Region | ||
| Northeast | 2.39 (1.21, 4.72) | 0.012 |
| Midwest | 2.60 (1.30, 5.20) | |
| West | Reference | 0.007 |
| South | 2.09 (1.11, 3.93) | 0.022 |
Proportion of patients undergoing endoscopic eradication therapies and esophagectomy (time- trend analysis)
Figure 2 graphs the proportion of patients receiving endoscopic eradication therapies out of the total patients treated with endoscopic eradication therapies and esophagectomy. These data suggest a significant increase in the proportion of patients with esophageal cancer undergoing endoscopic eradication therapies and subsequent decline in the proportion of patients undergoing surgical resection (p for trend <0.001) (Supplementary Table 2). A similar stage-specific increase in the number of patients undergoing endoscopic eradication therapies was noted along with a decline in surgical resection (Stage 0, p for trend <0.001; Stage 1a, p for trend <0.001; and Stage 1b, p for trend = 0.006) (Figure 2 and Supplementary Table 2).
Figure 2.
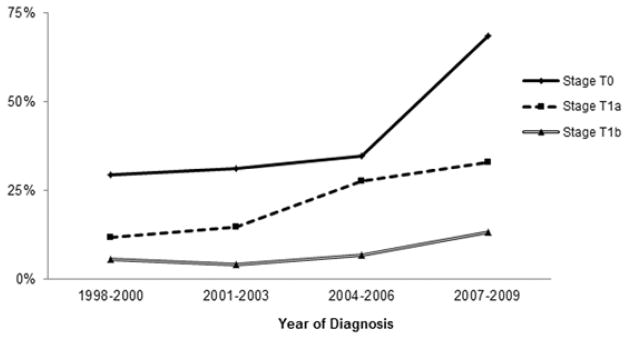
Proportion of esophageal cancer treated by endoscopic eradication therapies by stage and time
DISCUSSION
Based on favorable safety profiles and oncologic outcomes, endoscopic eradication therapy is becoming increasingly popular worldwide. Endoscopic eradication therapy of Barrett’s related high-grade dysplasia has been endorsed in recent guidelines and several large cohort studies have demonstrated favorable outcomes with endoscopic eradication therapies in patients with Barrett’s related intramucosal cancer.9, 13, 15, 23
Results of this large population-based study that identified 2,016 patients with early esophageal cancer from the SEER database demonstrate comparable 2-year and 5-year esophageal cancer-specific survival rates between patients undergoing endoscopic eradication therapies and surgical resection. Similar long term results were noted when analyses were limited to stage T0 and T1a. Cox proportional hazards regression models showed that the HR for esophageal cancer-free survival in the endoscopic eradication therapy group was not different from the esophagectomy group (HR 1.42, 95% CI 0.9–2.03). Similar results were noted when modeling was limited to Stage 0 and 1a (HR 1.18, 95% CI 0.78–1.8). This study demonstrates comparable 2-year and 5-year esophageal cancer related survival in patients with EAC.
Studies comparing survival outcomes between endoscopic eradication therapies and esophagectomy in patients with early esophageal cancer are limited.4, 9, 10 Previously, Das et al in a similar study compared outcomes between these two treatment modalities and reported comparable cancer-free survival rates in the two groups. This study was limited by the small sample size of patients with mucosal esophageal cancer undergoing endoscopic eradication therapies, the inability to study outcomes specific to EAC, and the lack of overall survival analyses. Prasad et al compared long-term outcomes in 178 patients with mucosal EAC treated endoscopically (n=132, 74%) and surgically (n=46, 26%) at a single tertiary care referral center. There was no difference in the cumulative mortality and overall survival between the two groups.9 Similar results were reported comparing endoscopic eradication therapy to esophagectomy in patients with Barrett’s related HGD.23,10 Our population-based study overcomes the main limitation of generalizability of these studies that were based on single expert centers with expert endoscopists and surgeons.
Our results highlight not only the increasing use of endoscopic eradication therapies in the management of patients with early esophageal cancer (overall and stage specific) (Figure 2), but also the intrinsic differences in patient characteristics undergoing endoscopic eradication therapies and esophagectomy. Patients in the endoscopic eradication therapies group were frequently older and more likely to demonstrate well-differentiated tumor histology, whereas patients in the esophagectomy group were more likely to show larger tumors and receive radiation therapy. Age and higher comorbidity is the most likely explanation for poorer overall 5-survival and higher mortality rates due to other non-esophageal cancer causes in the endoscopic eradication therapies group.
Results of the Cox proportional hazards modeling showed that the age at diagnosis, exposure to radiation therapy, increasing stage of disease (stage T1a and T1b) and year of diagnosis were all variables associated with increasing mortality whereas tumor histology of EAC was associated with improved survival compared with ESCC. Similar results were noted when modeling was limited to Stage T0 and T1a cases. Increasing stage (Stage T1a and T1b compared to Stage T0) as a predictor of mortality in this study is consistent with the recent AJCC Cancer Staging report on esophageal and esophagogastric junction cancers.5
There are several limitations of this study that merit discussion. The SEER database does not provide information on comorbidities, which may introduce potential selection bias. However, given the older age and higher non-esophageal cancer related deaths in the endoscopic eradication therapy group it is logical to conclude that patients with more comorbidities are more likely to undergo endoscopic eradication therapies. Similar findings were previously observed by Prasad et al that reported patients referred to endoscopic eradication therapy were either poor surgical candidates or did not wish to undergo surgery.9 It should be noted that despite the potential for bias against endoscopic eradication therapies because of higher co-morbidity and older age, there was no difference in esophageal cancer-related mortality between the two groups. Lack of confirmation of the final diagnosis by expert GI pathologists is a limitation of this study. This database does not capture pre-treatment staging data as assessed by endoscopic ultrasonography. Another limitation is that the SEER database reports only the first therapy and does not report on the number of patients that fail endoscopic eradication therapy and subsequently undergo surgical resection or the number of patients with recurrent cancers post endoscopic eradication therapies. Given the comparable esophageal cancer-free survival rates, the lack of data on incomplete resection and local recurrence should not impact our overall results. Similarly, the SEER database does not include detailed information on the use of chemotherapy in these patients. Data on procedural complications related to esophagectomy and endoscopic eradication therapies were not available in this database. The authors acknowledge that radiation therapy is not indicated for this group of patients and is not the standard of care. The reason for this finding is unclear and may be related to other co-existing comorbidities and individualized treatment decisions. It is unlikely that this finding represents a subset of patients downstaged following neoadjuvant chemoradiation as the SEER database only captures pre-treatment staging information. Exclusion of patients who received radiation therapy had no impact on overall results. 12 Although results of this study demonstrate comparable 5-year esophageal cancer-specific survival, these results should be interpreted with caution given the large number of patients undergoing endoscopic eradication therapies who were censored for this endpoint. Endoscopic eradication therapies in this database predominantly included EMR and/or PDT with grouping of other thermal ablation techniques. Hence, this study does not allow evaluation of specific endoscopic therapies. We acknowledge that the use of PDT has declined dramatically with the advent of radiofrequency ablation especially given the availability of data from a randomized controlled trial, better tolerability, durability and ease of application.14, 24 Consistent with other reports,9, 10 EMR was the predominant modality for treatment in this study.
Strengths of this study include the use of a large population-based database with data that reports cumulative experiences from multiple institutions across the country (academic and community) and thus provides “real life” and more generalizable data compared to results from a single tertiary care institution. This study includes the largest cohort of patients with early esophageal cancer undergoing endoscopic eradication therapy and surgery to date. The SEER database have been well established and include population-based case identification with associated procedures, detailed review of medical records, accurate data on pathology and tumor staging, rigorous data collection procedures and high quality control standards. This ensures a high accuracy of available data and excellent patient follow-up.4 These data validate our current practice of performing endoscopic eradication therapy for HGD and mucosal cancer (EMR and/or mucosal ablation). Esophagectomy should still be considered the treatment of choice for early esophageal cancer that extends into the submucosa (T1b),12 although there may be a role for endoscopic eradication therapies in selected patients with T1b disease.25 Future studies should evaluate long-term survival data (5-year survival) in patients undergoing endoscopic eradication therapies with newer techniques such as radiofrequency ablation, identify T1b cancer patients who may be able to undergo appropriate endoscopic eradication therapies with expanded indication and achieve comparable outcomes with esophagectomy. While this and other recent studies clearly establish the role of endoscopic eradication therapies for patients with stage T0 and T1a esophageal cancer, prospective trials should focus on identifying patient and provider specific determinants of optimal outcomes, address quality of life and cost-effectiveness of treatment options and address appropriate surveillance protocols to detect and manage recurrences.
In conclusion, our results from this population-based study demonstrate comparable esophageal cancer related mortality rates in patients with early esophageal cancer undergoing endoscopic eradication therapies and surgical resection. In the absence of randomized controlled trial data, these results provide confidence that endoscopic eradication therapies are a reasonable alternative to esophagectomy for the treatment of early esophageal cancer.
Supplementary Material
Acknowledgments
No funding was obtained or provided for this study.
ABBREVIATIONS
- EAC
esophageal adenocarcinoma
- ESCC
esophageal squamous cell carcinoma
- SEER
Surveillance, Epidemiology, and End Results
- NCI
National Cancer Institute
- HGD
high-grade dysplasia
- AJCC
American Joint Committee on Cancer
- ICD
International Classification of Diseases for Oncology
- HR
hazard ratio
- CI
confidence interval
Footnotes
No writing assistance was provided for this manuscript.
Results of this study will be presented in part at the AGA Late Breaking Abstract Oral Presentation, Digestive Disease Week 2013, Orlando.
Disclosures: Sachin Wani, MD is supported by the AGA Takeda Research Scholar Award in GERD and Barrett’s esophagus. Jennifer Drahos, PhD, MPH and Michael B. Cook, PhD are supported by the Intramural Program of the National Institutes of Health. None of the other authors have any disclosures relevant to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–70. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340–5. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 5.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 6.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–73. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice TW, Blackstone EH, Goldblum JR, et al. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001;122:1077–90. doi: 10.1067/mtc.2001.113749. [DOI] [PubMed] [Google Scholar]
- 8.Oh DS, Hagen JA, Chandrasoma PT, et al. Clinical biology and surgical therapy of intramucosal adenocarcinoma of the esophagus. J Am Coll Surg. 2006;203:152–61. doi: 10.1016/j.jamcollsurg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology. 2009;137:815–23. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 11.Wani S, Early D, Edmundowicz S, et al. Management of high-grade dysplasia and intramucosal adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol. 2012;10:704–11. doi: 10.1016/j.cgh.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143:336–46. doi: 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 15.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 17.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol. 2012;107:850–62. doi: 10.1038/ajg.2012.78. quiz 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271–8. doi: 10.1097/SLA.0b013e3181fbad42. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2009), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012 Based on the November 2011 submission.
- 20.Schoenfeld D. Partial Residuals for The Proportional Hazards Regression Model. Biometrika. 1982;69:239–241. [Google Scholar]
- 21.StataCorp LP. Stata 11. College Station, Tex: StataCorp LP; 2009. [Google Scholar]
- 22.SAS Institute Inc. SAS 9.2. Cary, NC: SAS Insitute Inc; [Google Scholar]
- 23.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2007;132:1226–33. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011;141:460–8. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manner H, Pech O, Heldmann Y, et al. Efficacy, Safety, and Long-term Results of Endoscopic Treatment for Early-stage Adenocarcinoma of the Esophagus With Low-risk sm1 Invasion. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2012.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.