Abstract
Vascular calcifications and bone health seem to be etiologically linked via common risk factors such as aging and subclinical chronic inflammation. Epidemiologic studies have shown significant associations between low bone mineral density (BMD), fragility fractures and calcifications of the coronary arteries and the abdominal aorta. In the last decade, high-resolution peripheral quantitative computed tomography (HR-pQCT) has emerged as in-vivo research tool for the assessment of peripheral bone geometry, density, and microarchitecture. Although vascular calcifications are frequently observed as incidental findings in HR-pQCT scans, they have not yet been incorporated into quantitative HR-pQCT analyses. We developed a semi-automated algorithm to quantify lower leg arterial calcifications (LLAC), captured by HR-pQCT. The objective of our study was to determine validity and reliability of the LLAC measure.
HR-pQCT scans were downscaled to a voxel size of 250 µm. After subtraction of bone volumes from the scans, LLAC were detected and contoured by a semi-automated, dual-threshold seed-point segmentation. LLAC mass (in mg hydroxyapatite; HA) was calculated as the product of voxel-based calcification volume (mm3) and mean calcification density (mgHA/cm3)/1000. To determine validity, we compared LLAC to coronary artery calcifications (CAC), as quantified by multi-detector computed tomography (MDCT) and Agatston scoring in forty-six patients on chronic hemodialysis. Moreover, we investigated associations of LLAC with age, time on dialysis, type-2 diabetes mellitus, history of stroke, and myocardial infarction. In a second step, we determined intra- and inter-reader reliability of the LLAC measure.
In the validity study, LLAC were present (>0 mgHA) in 76% of patients, 78% of patients had CAC (>0 mgHA). Median LLAC was 6.65 (0.08 – 24.40) mgHA and median CAC as expressed by Agatston score was 266.3 (15.88 – 1877.28). We found a significant positive correlation between LLAC and CAC (rho=0.6; p<0.01). Dialysis patients with type-2 diabetes mellitus (DM; 35%) and history of stroke (13%) had higher median LLAC than patients without those conditions (DM 20.0 fold greater, p=0.006; Stroke 5.1 fold greater, p=0.047;). LLAC was positively correlated with time on dialysis (rho=0.337, p=0.029), there was a trend towards a positive association of LLAC and age (rho=0.289, p=0.053). The reliability study yielded excellent intra- and inter-reader agreement of the LLAC measure (intra-reader ICC=0.999, 95% CI=0.998–1.000; inter-reader ICC=0.998, 95% CI=0.994–0.999).
Our study indicates that the LLAC measure has good validity and excellent reliability. The use of HR-pQCT for the simultaneous evaluation of arterial calcifications, peripheral bone geometry, bone density, and bone microarchitecture should facilitate future research on osteo-vascular interactions and potential associations with cardiovascular events.
Keywords: HR-pQCT, Lower Leg Arterial Calcifications, Quantification, Agatston-Score
Introduction
Vascular calcifications represent significant morphologic sequelae of atherosclerosis and other vascular pathologies such as Mönckeberg’s sclerosis. A strong body of evidence supports a positive association between coronary artery calcifications (CAC) and morbidity and mortality resulting from cardiovascular events [1, 2]. Moreover, vascular calcifications appear to be linked with poor bone health. Researchers have found remarkable associations between CAC, abdominal aortic calcifications (AAC), lower bone mineral density (BMD) [3, 4], greater bone loss over time [5, 6], and fragility fractures [7, 8]. Men with higher serum levels of bone resorption markers exhibit a higher risk for cardiovascular events [9]. Vice versa, higher serum osteocalcin levels were shown to be associated with lower progression of abdominal aortic calcifications and lower ten-year mortality [10]. Circulating osteoblast-like cells appear to be crucially involved in the formation of calcified atherosclerotic plaques [11]. Pivotal regulators of bone metabolism such as RANKL/OPG, FGF-23/Klotho, Dkk-1, and Fetuin A seem to be able to modulate vascular calcifications [12–15]. With osteoporosis and atherosclerosis further sharing a multitude of etiologic risk factors such as dependence on sex hormones, kidney function, oxidative stress, and pro-inflammatory changes of the immune system, interdisciplinary research that targets osteo-vascular interactions seems crucial [16]. In chronic kidney disease, the co-occurrence of vascular pathologies and skeletal disease has been recognized as an important disease sub-entity and is therefore distinctively referred to as ‘chronic kidney disease – mineral bone disorder’ (CKD-MBD) [17].
Dual-energy x-ray absorptiometry (DXA) and central quantitative computed tomography (QCT) can be used to simultaneously quantify BMD and AAC [18, 19]. Although DXA is considered as current clinical gold standard for the diagnosis of osteoporosis and the assessment of fracture risk, this technique has certain limitations. Not all patients that sustain fragility fractures have osteoporotic BMD, moreover DXA is incapable of directly quantifying bone microarchitecture which is an important co-determinant of bone strength. In the last two decades, high-resolution imaging techniques such as high-resolution peripheral quantitative computed tomography (HR-pQCT) and high resolution magnetic resonance imaging (HR-MRI) have emerged as non-invasive options of measuring bone microarchitecture in research settings [20–23].
Inspecting HR-pQCT scans of elderly diabetic patients, users have noticed and reported vascular calcifications as incidental, secondary findings [24, 25]. In spite of growing interest in osteo-vascular interactions, HR-pQCT has not been used to measure vascular calcifications. Therefore, we developed a semi-automated method to quantify vascular calcifications of the ultradistal lower extremities captured by HR-pQCT and tested validity and reliability of this measure. The specific objective of our validity study was to compare vascular calcifications of the lower legs (LLAC) as determined by HR-pQCT with coronary artery calcifications (CAC) as quantified by multidetector computed tomography (MDCT). Moreover, we investigated the associations of LLAC with age, time on dialysis, type-2 diabetes mellitus, history of stroke and myocardial infarction. The specific objective of the reliability study was to determine intra-operator and inter-operator agreement of this novel technique.
Methods
Study Design
We conducted two studies in two independent groups of patients. The validity study included forty-six patients and compared arterial calcifications in the lower leg to arterial calcifications in the coronary arteries and clinical subject characteristics based on medical records. The reliability study tested intra- and inter-operator agreement of the LLAC measure.
Participants of Validity Study
We included adults aged twenty-one years and older with chronic kidney disease treated with hemodialysis since vascular calcifications are highly prevalent in this distinct group of patients. Specifically, we recruited forty-six patients from the Hemodialysis Unit of the Division of Nephrology and Dialysis, Medical University of Vienna, who agreed to undergo HR-pQCT scanning and MDCT scanning. Twenty participants were female, the remaining twenty-six participants were male. The mean age of patients was fifty-nine years, ranging from thirty-two to eighty-nine years. The vast majority of patients were Caucasian, one patient was Asian. The study protocol was approved by the Ethics Committee of the Medical University of Vienna. All patients gave written informed consent.
Participants of Reliability Study
We included fourteen postmenopausal women with lower leg arterial calcifications (LLAC) who were participants in a case-control study investigating the effect of type-2 diabetes on bone microarchitecture assessed by HR-pQCT, conducted at the University of California, San Francisco [26]. The mean age of patients was sixty-four years, ranging from fifty-seven years to seventy-five years. Five women were Caucasian, five women were African American. Two women were Asian. One woman was of Hispanic descent, another women was a Native Hawaiian. All women gave written informed consent, and the study protocol was approved by the Committee of Human Research of the University of California, San Francisco.
HR-pQCT - Image acquisition
Using the patient standard in vivo protocol provided by the manufacturer [20, 27] (60kVP, 900 µA, 100 ms integration time), ultadistal lower extremities were scanned with an HR-pQCT device (XtremeCT; SCANCO Medical AG, Brüttisellen, Switzerland). Unless the subject reported a history of local fracture or amputation, the left lower leg was imaged. Otherwise, the right leg was examined. The extremity were secured and stabilized by a carbon fiber cast and inserted in the scanner. For definition of the scan region, an antero-posterior scout view was taken and a reference line was placed on the tibial joint surface. Located at a fixed off-set 22.5 mm proximal from the tibial endplate, the volume of interest (VOI; 9 mm long; 110 slices) was placed in the ultradistal tibia. Per scan, 750 projections were acquired; the field of view (FOV; 12.6 mm) was reconstructed across a 1536×1536 matrix (isotropic nominal resolution of 82 µm voxels). Each scan took approximately 3 minutes, the effective dose was 3µSv.
MDCT – Image acquisition
We acquired ECG-gated cardiac multidetector computed tomographies (MDCT) on a 16-slice scanner (Siemens Somatom 16, Siemens, Forchheim, Germany). Scans were performed in cranio-caudal direction with patients placed in supine position. ECG electrodes were placed outside the field of view of the planed examination. Scans were performed during inspiratory breath hold with prospective electrocardiogram (ECG)-triggering set at 75% of the RR interval. No contrast material was injected. Scan time per patient was 4–5 seconds, the radiation dose was about 3mSv.
Quantification of Lower Leg Arterial Calcifications (LLAC)
Based on the specific aim of providing a software tool for the quantification of lower leg arterial calcifications captured by HR-pQCT imaging, one of the authors (MAZ) developed a dedicated, semi-automated computer algorithm implemented in IPL (Image Processing Language). Prior to software-based evaluations, images were visually inspected to confirm an image quality better than motion grade 4 [28] and to determine the presence or absence of arterial calcifications. Lower leg arterial calcifications were defined as linear or tubular hyperdensity zones of circular, semi-circular, or crescent-like shape. We required that such calcifications corresponded to the anatomical territory of the anterior tibial artery, the posterior tibial artery, interosseous branches or smaller intramuscular or subcutaneous arterioles. Cutaneous calcifications (typically spot-like) or other non-vascular (e.g. post-traumatic) soft tissue calcifications that did not meet these criteria and were confirmed as negative by an adjudicating board certified radiologist (TML) were not included. The algorithm was only applied to scans in which vascular calcifications were identified.
To be more comparable with cardiac MDCT images (spatial resolution ~250–300 µm) and for the purpose of noise reduction, HR-pQCT images containing vascular calcifications were downscaled from 82 µm to a voxel size of 246 µm (by binning 3×3×3 voxels to one). Based on object size and x-ray attenuation, the outer contours of tibia and fibula were automatically detected by the algorithm. A single operator verified the contours and made corrections if needed (Figure 1). Tibia and fibula volumes were suppressed by inversion, leaving the remaining scan volume ready for the next analytical steps. Subsequently, the bone-free images were gauss-filtered. In the next analytical step, we applied a dual-threshold seed-point segmentation to detect hyperdense image zones corresponding to calcified, non-skeletal tissue [29]. Algorithm-wise, such hyperdense focal zones were defined to consist of voxels with an attenuation of 300 HU (100 mgHA/cm3) or more connected to at least one image voxel with a value of 500 HU (170 mgHA/cm3) or larger. The derived volumes were dilated by one voxel and the resulting masks were checked by the operator and corrected if needed. The operator did not interfere with the automatically provided ‘vascular’ masks unless they were either clearly enlarged by adjacent skeletal motion artifacts, were caused by cutaneous or non-vascular soft tissue calcifications, or focal pixel artifacts (Figure 2). Subsequently, the approved vascular calcification contours were applied to the unfiltered, downscaled images. All resulting voxels > 300 HU were summed to provide the total volume of vascular calcifications. Finally, lower leg arterial calcification (LLAC) mass was calculated as follows: Total volume of vascular calcifications [mm3] × mean calcification density [mgHA/cm3]/1000. In the absence of vascular calcifications LLAC was recorded as zero mgHA. Typical postprocessing time per LLAC assessment ranged between 10 and 20 minutes, depending on the need for manual adjustments.
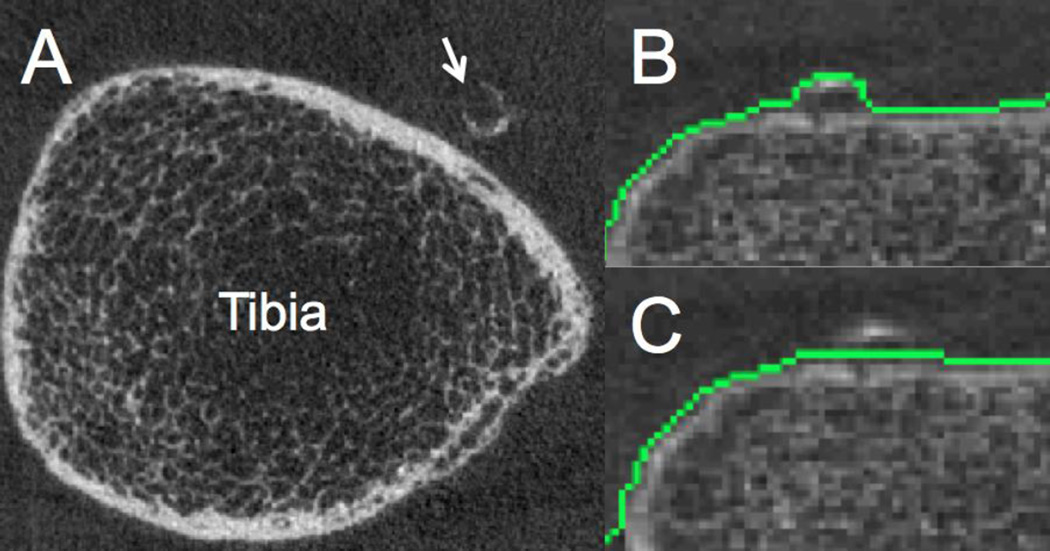
Figure 1. Lower leg arterial calcifications (LLAC) depicted by HR-pQCT.
A) Anterior tibial artery calcification (arrow). B) Automatic detection of bone contours may include calcifications of the juxtaposed anterior tibial artery, requiring manual correction prior to quantitative LLAC assessment (C).
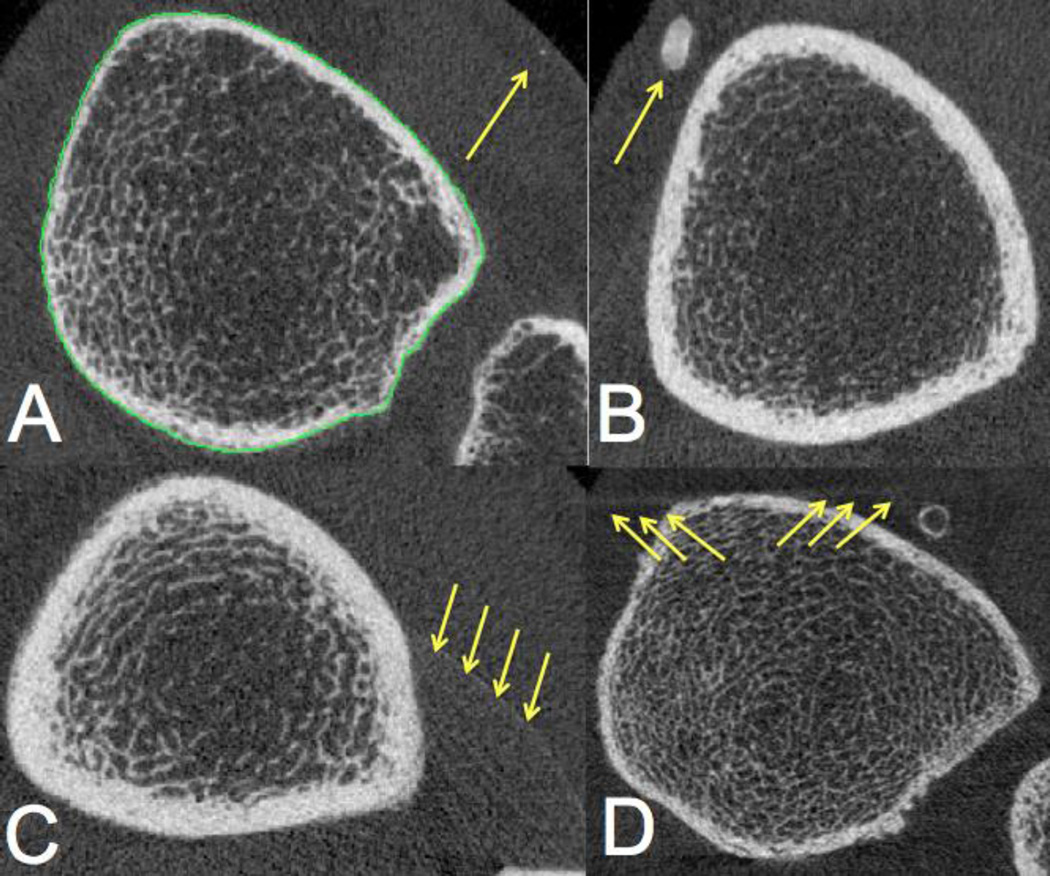
Figure 2. Non-vascular, high-density regions that should be excluded from quantitative analysis of vascular calcifications.
Examples of findings that can mimic arterial calcifications are shown below and highlighted with arrows A) intra-cutaneous spotted calcifications, B) non-vascular, most likely post-traumatic soft tissue calcification, C) focal pixel artifact, D) skeletal motion artifacts.
To determine intra-reader agreement, a single operator analyzed all n=14 scans of the reliability dataset three times. To assess inter-reader agreement, a second reader ran the LLAC analyses in the reliability dataset once.
Quantification of Coronary Arterial Calcifications (CAC)
A single, experienced technician evaluated CAC with a commercially available software package (Syngo CaScore, Siemens Healthcare, Forchheim, Germany) and determined Agatston score [30] for the left main artery (LMA), the left anterior descending artery (LAD), the left circumflex artery (LCX), and the right coronary artery (RCA). Individual artery scores were summed to obtain total CAC that was used for comparison with LLAC. Agatston score is considered the most established quantitative parameter for CAC assessment and is typically used for CT-based cardiovascular risk screening [31].
Statistical analysis
Descriptive statistics were used to describe clinical subject characteristics as shown in Table 1. To evaluate validity of the LLAC measure, we used Spearman correlation coefficients to assess agreement between lower leg arterial calcifications (LLAC) as determined by HR-pQCT and coronary artery calcifications (CAC) as measured by Agatston Score from cardiac MDCT. Bearing in mind important pathophysiologic differences in the formation of CAC and LLAC limiting the value of CAC as a gold standard, we used additional clinical parameters (age, time on dialysis, type-2 diabetes, history of stroke and myocardial infarction) for extended method validation. Specifically, we used Spearman correlation coefficients to describe the association of LLAC with age and time on dialysis. Further, we applied Wilcoxon-Rank-Sum tests to compare differences in LLAC between patients with and without type-2 diabetes mellitus, history of stroke, or history of myocardial infarction.
Table 1. Age, body mass index (BMI), history of type-2 diabetes mellitus, stroke, myocardial infarction, time on dialysis, lower leg arterial calcifications (LLAC) and coronary calcifications (CAC) of study participants of validity and reliability study.
Medians, 25th and 75th percentile values are given.
| Validity Study (n=46) |
Reliability Study (n=14) |
|
|---|---|---|
| Age (years) | 59.0 [51.0 – 68.0] | 62.5 [59.0 – 67.5] |
| BMI (kg/m2) | 24.7 [22.0 – 26.8] | 27.8 [24.6 – 30.7] |
| Type-2 Diabetes mellitus (%) | 35 % | 64 % |
| History of Stroke (%) | 13 % | 0 % |
| History of Myocardial Infarction (%) | 15 % | 0 % |
| Time on chronic dialysis (Months) | 31.5 [11.0 – 68.5] | 0 [0.0 – 0.0] |
| LLAC (mg HA) | 6.65 [0.08 – 24.40] | 0.78 [0.35 – 6.88] |
| CAC (Agatston Score) | 266.3 [15.88 – 1877.28] | Not measured |
To determine reliability, we calculated intra-operator agreement by applying two-way, random, intra-class correlation (ICC) to analysis-re-analysis triplets (3 × n=14) obtained by a single reader. To test inter-reader agreement of the LLAC measure, the last re-analysis run of the first operator was compared with a single run (n=14) of a second operator and expressed by ICC. PASW Statistics 18.0 Statistical Database Software (IBM, Armonk, NY) was used for data analysis. The level of statistical significance was set at p < 0.05.
Results
Validity Study
Medians, 25th and 75th percentile of age, body mass index, lower leg arterial calcifications (LLAC), coronary artery calcifications (CAC) and history of stroke, myocardial infarction and type-2 diabetes mellitus are given in table 1. In addition to end-stage renal failure, 35% of participants of the validity study had type-2 diabetes mellitus. 13% had suffered a stroke, 15% had a history of myocardial infarction. Median time on dialysis was 31.5 months.
76% of participants (35/46) had lower leg arterial calcifications that were visible and quantifiable by HR-pQCT. In 24% of all HR-pQCT scans (11/46 patients), there were no detectable vascular calcifications. 24% of participants (11/46) displayed a low Agatston score (0–10 HU). 35% of participants (16/46) exhibited intermediate amounts of coronary calcifications (Agatston Score 11–400 HU). 41% (19/46) had extensive calcifications as expressed by an Agatston Score > 400 HU.
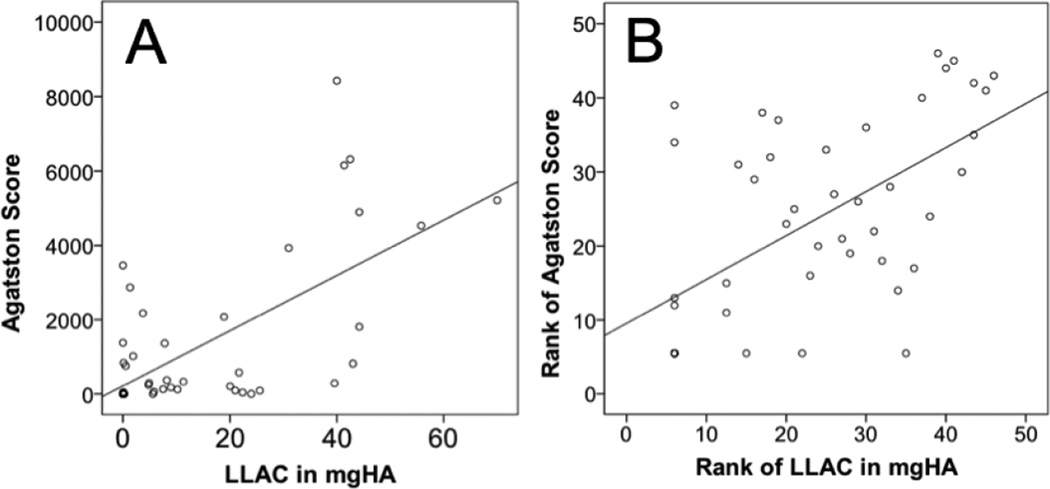
We found significant positive correlations between LLAC by HR-pQCT and CAC by Agatston score (rho = 0.6; p < 0.01). An unranked scatter plots showing individual LLAC and CAC values and a ranked scatter plot for this comparison visualizing the spearman coefficient are given in Figure 3.
Figure 3. Validity study comparing lower leg arterial calcifications (LLAC) and coronary artery calcifications (CAC).
A) Unranked scatter plot showing LLAC by HR-pQCT and CAC as measured by MDCT and Agatston score in n=46 patients. B) Ranked scatter plot of LLAC and CAC visualizing the Spearman correlation coefficient (rho=0.6; p<0.001).
Dialysis patients suffering from type-2 diabetes mellitus had significantly greater median LLAC (20.5 mgHA) than dialysis patients without a history of diabetes (0.9 mgHA; p=0.006). Likewise, participants with a history of stroke had greater median LLAC (26.6 mgHA) than without (5.3 mgHA). Interestingly, neither age nor time on dialysis was significantly different between patients with and without diabetes or stroke. History of myocardial infarction was not associated with significant differences in LLAC. LLAC was positively correlated with time on dialysis (rho=0.337, p=0.029), there was a positive trend towards an association of LLAC with age (rho=0.289, p=0.053).
Reliability Study
All fourteen patients of the reliability study had lower leg arterial calcifications. Subject characteristics are given in table 1. CAC was not measured in the reliability study.
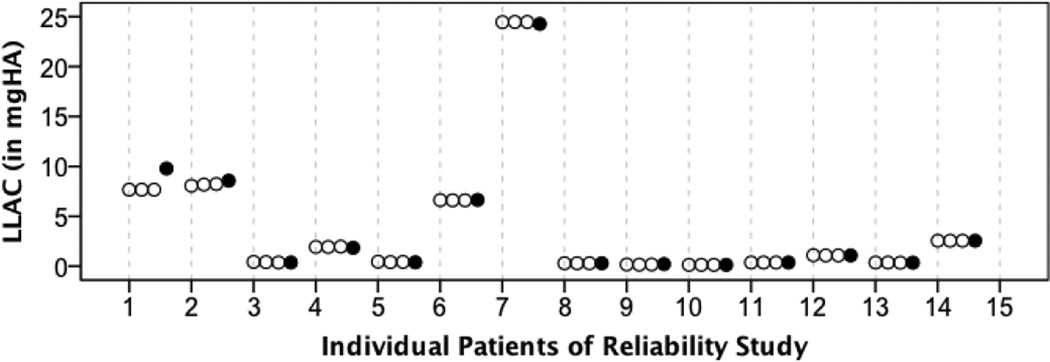
For intra-operator agreement of the LLAC measure, we obtained an intraclass correlation coefficient (ICC) of 0.999 [95% CI = 0.998–1.000]. ICC for inter-operator agreement was ICC=0.998 [95% CI=0.994–0.999]. Figure 4 visualizes three individual LLAC measurements obtained by repeated image analysis by a single operator and one measurement obtained by a second operator.
Figure 4. Reliability study.
Visualization of lower leg arterial calcification (LLAC) values obtained by repeated image analysis by a single operator (white bullets) and a second operator (black bullets).
Discussion
Motivated by the growing interest of the scientific community in the pathophysiologic links between vascular health and bone health, and the absence of a quantitative tool for the assessment of vascular calcifications detected in HR-pQCT studies, we have developed a dedicated technique for this purpose. In the present study, we have demonstrated methodical feasibility, basic clinical validity, and algorithm stability as expressed by high intra- and inter-operator reliability.
We found that in patients on chronic hemodialysis, lower leg arterial calcifications as assessed by HR-pQCT correlated significantly with coronary artery calcifications as assessed by cardiac MDCT and Agatston scoring. However, bearing in mind that the formation of arterial calcifications certainly differs between lower limbs and coronary arteries with respect to the pathophysiologic processes involved, we included additional variables for initial clinical validation of the LLAC measure. With dialysis patients exhibiting substantial, long-standing disturbances in renal function and mineral metabolism, the positive association found for LLAC and time on dialysis is coherent with clinical presentation and the disproportionally high risk of cardiovascular disease in dialysis patients. Along those lines, it seems of particular interest that dialysis patients with type-2 diabetes exhibited extraordinarily high LLAC and that LLAC levels of participants with a history of stroke differed significantly from those without. While these findings seem of major clinical relevance and should therefore certainly be followed up in separate studies, we would like to stress that the present, cross-sectional investigation was primarily intended for basic clinical method validation and that conclusions regarding LLAC and cardiovascular events should therefore be drawn with some caution.
Due to a disproportionally high prevalence of vascular calcifications in patients on chronic dialysis, the specific enrollment of subjects with end-stage renal disease allowed us to compare lower leg arterial calcifications and coronary artery calcifications across a wide quantitative range. In fact, almost a third of participants of the validity study displayed no calcifications in HR-pQCT and either no or very low coronary artery calcifications. About a third exhibited mild to moderate coronary calcifications and the remaining third showed severe arterial calcifications of the heart, correlating well with the novel LLAC measure.
In addition to a very favorable radiation dose profile of HR-pQCT (< 4µSv) when compared with MDCT (~3mSv), the option of a simultaneous, high-resolution assessment of bone mineral density, bone microarchitecture, estimates bone strength and a cardiovascular surrogate parameter appears interesting both in the light of musculoskeletal and cardiovascular research questions.
Interpreting the validity data from a technical point of view, it should to be recognized that HR-pQCT has been primarily built for the visualization and non-invasive quantification of bone microstructure of the ultradistal extremities in research settings. HR-pQCT scanners and clinical MDCT scanners differ with regard to their hardware, X-ray spectrum and contrast-tonoise-ratio. Both methods yield different absolute x-ray attenuation values for e.g. bone or calcified arteries. While cardiac calcium scoring tools typically use attenuation thresholds ≥130 HU to classify voxels as calcifications, this definition was not practical for HR-pQCT. In HR-pQCT, we worked with a higher threshold to define calcifications (300 HU/100 mgHA/ccm). The calibration of the HR-pQCT system to a hydroxy-apatite equivalent density scale also provided independence of Hounsfield units.
The detection of vascular calcifications was based on a dual hysteresis thresholding technique. We have chosen this dual threshold strategy for two main reasons; first to suppress noise from HR-pQCT images and secondly to target even very small regions of increased focal density. Image downscaling was aimed at accounting for differences in contrast-to-noise ratio and additional scanner-specific differences such as spatial resolution/voxel size.
In line with rigorous quality control being generally required by all quantitative imaging approaches, critical review of HR-pQCT scans is a crucial step that precedes the actual image processing: Semi-quantitative motion grading is obligatory to assure reproducible HR-pQCT results. Because the algorithm presented in this study can only measure calcification mass but is not able to decide if vascular calcifications are actually present or absent, HR-pQCT operators need to be trained for this purpose. Adjudication readings with a radiologist should be performed if the HR-pQCT operator feels insecure about calling a calcification vascular. Such feedback should assure that non-vascular soft tissue calcifications are correctly excluded from the analysis (e.g. post-traumatic changes).
Our study certainly had some limitations. Although, almost a third of participants exhibited no or low coronary calcifications and no peripheral vascular calcifications, it has to be stressed that CKD patients are certainly not an ideal representation of the general population due to their disproportionally high cardiovascular risk. The performance and methodical relevance of our quantitative tool therefore remains to be tested in a larger study population consisting of patients with normal or only slightly impaired kidney function. However, we speculate that validity and reliability of LLAC should be basically comparable between CKD and non-CKD patients. From a technical perspective, it has to be acknowledged that vessel motion could certainly have an impact on the precision of our measurements. While cardiac CT was performed with ECG-triggering, we did not specifically account for tibial vessel motion. It therefore remains unclear how much variability in the LLAC measure was introduced by patient-specific differences in heart rate, blood pressure, and vessel motion. The small scan volume of HR-pQCT is another technical caveat of our technique and extensions of scan length might be worth considering in future studies. Subsequent reproducibility experiments should include the effects of patient repositioning in between scans.
In summary, we have demonstrated basic validity and excellent reliability of our novel quantitative method for the assessment of lower leg arterial calcifications by HR-pQCT. We conclude from our study that vascular calcifications of the ultradistal lower extremities can be measured by HR-pQCT, which should facilitate quantitative, patient-oriented research on osteo-vascular interactions. The relevance of LLAC assessment for cardiovascular risk screening should be tested in dedicated prospective studies.
Highlights.
Lower leg arterial calcifications (LLAC) are quantifiable by HR-pQCT
Validity: LLAC and coronary artery calcifications (CAC) were highly correlated
Validity: Significant positive correlations of LLAC and time on dialysis in patients with end-stage renal failure. Moreover, participants with diabetes or stroke had greater LLAC than those without.
Excellent intra- and inter-operator reliability of the semi-automated LLAC algorithm
Acknowledgements
This work was supported by Erwin-Schrödinger Grant No. J-3079 (Austrian Science Fund; to JMP), NIH RC1 AR058405 (to TML). We would also like to thank, Stephanie Boutroy (Université de Lyon), Douglas Kiel (Hebrew SeniorLife and Harvard Medical School) and Mary Bouxsein (Beth Israel Deaconess Medical Center and Harvard Medical School) for critical input. Thanks to Silvia Kiss and Julia Deutschmann (Medical University of Vienna) for technical support, and Claudia Schueller-Weidekamm (Medical University of Vienna) for managing and co-ordinating the Viennese HR-pQCT facility.
Funding: Erwin-Schrödinger Grant No. J-3079 (Austrian Science Fund; to JMP); NIH RC1 AR058405
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Disclosures
Dr. Patsch none
Dr. Zulliger M.A. Zulliger is a former employee of SCANCO Medical AG. He has no other financial interests (ownership, stock options, or similar) in the company.
Dr. Vilayphiou N. Vilayphiou is a current employee of SCANCO Medical AG. He has as no other financial interests (ownership, stock options, or similar) in the company.
Dr. Samelson none
Dr. Cejka none
Dr. Diarra none
Dr. Edelhauser none
Dr. Weber none
A. Burghardt none
Prof. Link none
Prof. Loewe none
Contributor Information
Janina M. Patsch, Email: janina.patsch@meduniwien.ac.at.
Martin A. Zulliger, Email: mzulliger@scanco.ch.
Nicolas Vilayphou, Email: nvilayphiou@scanco.ch.
Elizabeth J. Samelson, Email: samelson@hsl.harvard.edu.
Daniel Cejka, Email: daniel.cejka@meduniwien.ac.at.
Danielle Diarra, Email: danielle.diarra@meduniwien.ac.at.
Gundula Berzaczy, Email: gundula.berzaczy@meduniwien.ac.at.
Andrew J. Burghardt, Email: andrew.burghardt@ucsf.edu.
Thomas M. Link, Email: thomas.link@ucsf.edu.
Michael Weber, Email: michael.weber@meduniwien.ac.at.
Christian Loewe, Email: christian.loewe@meduniwien.ac.at.
References
- 1.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC. Cardiovascular imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 3.Figueiredo CP, Rajamannan NM, Lopes JB, Caparbo VF, Takayama L, Kuroishi ME, Oliveira IS, Menezes PR, Scazufca M, Bonfa E, Pereira RM. Serum phosphate and hip bone mineral density as additional factors for high vascular calcification scores in a community-dwelling: the Sao Paulo Ageing & Health Study (SPAH) Bone. 2013;52:354–359. doi: 10.1016/j.bone.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 5.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcified Tissue International. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 6.Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 7.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 8.Szulc P, Samelson EJ, Sornay-Rendu E, Chapurlat R, Kiel DP. Severity of aortic calcification is positively associated with vertebral fracture in older men-a densitometry study in the STRAMBO cohort. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24:1177–1184. doi: 10.1007/s00198-012-2101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szulc P, Samelson EJ, Kiel DP, Delmas PD. Increased bone resorption is associated with increased risk of cardiovascular events in men: the MINOS study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:2023–2031. doi: 10.1359/JBMR.090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Confavreux CB, Szulc P, Casey R, Boutroy S, Varennes A, Vilayphiou N, Goudable J, Chapurlat RD. Higher serum osteocalcin is associated with lower abdominal aortic calcification progression and longer 10-year survival in elderly men of the MINOS cohort. J Clin Endocrinol Metab. 2013;98:1084–1092. doi: 10.1210/jc.2012-3426. [DOI] [PubMed] [Google Scholar]
- 11.Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S. Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation. 2012;125:2772–2781. doi: 10.1161/CIRCULATIONAHA.112.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amelio P, Isaia G, Isaia GC. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Journal of endocrinological investigation. 2009;32:6–9. [PubMed] [Google Scholar]
- 13.Cannata-Andia JB, Roman-Garcia P, Hruska K. The connections between vascular calcification and bone health. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:3429–3436. doi: 10.1093/ndt/gfr591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, Hawa G, Chapurlat R, Szulc P. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012;97:E575–E583. doi: 10.1210/jc.2011-2836. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 16.Anagnostis P, Karagiannis A, Kakafika AI, Tziomalos K, Athyros VG, Mikhailidis DP. Atherosclerosis and osteoporosis: age-dependent degenerative processes or related entities? Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20:197–207. doi: 10.1007/s00198-008-0648-5. [DOI] [PubMed] [Google Scholar]
- 17.London G, Coyne D, Hruska K, Malluche HH, Martin KJ. The new kidney disease: improving global outcomes (KDIGO) guidelines - expert clinical focus on bone and vascular calcification. Clin Nephrol. 2010;74:423–432. [PMC free article] [PubMed] [Google Scholar]
- 18.Schousboe JT, Wilson KE, Kiel DP. Detection of abdominal aortic calcification with lateral spine imaging using DXA. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2006;9:302–308. doi: 10.1016/j.jocd.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Chow JT, Khosla S, Melton LJ, 3rd, Atkinson EJ, Camp JJ, Kearns AE. Abdominal aortic calcification, BMD, and bone microstructure: a population-based study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:1601–1612. doi: 10.1359/JBMR.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. The Journal of clinical endocrinology and metabolism. 2005;90:6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 21.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 22.Cohen A, Dempster DW, Muller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:263–273. doi: 10.1007/s00198-009-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehrli FW, Ford JC, Haddad JG. Osteoporosis: clinical assessment with quantitative MR imaging in diagnosis. Radiology. 1995;196:631–641. doi: 10.1148/radiology.196.3.7644622. [DOI] [PubMed] [Google Scholar]
- 24.Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:5045–5055. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patsch JM, Burghardt AJ, Kazakia G, Majumdar S. Noninvasive imaging of bone microarchitecture. Annals of the New York Academy of Sciences. 2011;1240:77–87. doi: 10.1111/j.1749-6632.2011.06282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type-2 diabetic postmenopausal women with fragility fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., 3rd Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: Impact of image quality on measures of bone density and micro-architecture. Bone. 2011 doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Burghardt AJ, Kazakia GJ, Majumdar S. A local adaptive threshold strategy for high resolution peripheral quantitative computed tomography of trabecular bone. Ann Biomed Eng. 2007;35:1678–1686. doi: 10.1007/s10439-007-9344-4. [DOI] [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Lee J. Coronary artery calcium scoring and its impact on the clinical practice in the era of multidetector CT. Int J Cardiovasc Imaging. 2011;27(Suppl 1):9–25. doi: 10.1007/s10554-011-9964-5. [DOI] [PubMed] [Google Scholar]