Abstract
Plasmodium knowlesi causes severe and fatal malaria in Malaysia. Microscopic misdiagnosis is common and may delay appropriate treatment. P. knowlesi can cross-react with “species-specific” parasite lactate dehydrogenase (pLDH) monoclonal antibodies used in rapid diagnostic tests (RDTs) to detect P. falciparum and P. vivax. At one tertiary-care hospital and two district hospitals in Sabah, we prospectively evaluated two combination RDTs for malaria diagnosis by using both a pan-Plasmodium-pLDH (pan-pLDH)/P. falciparum-specific-pLDH (Pf-pLDH) RDT (OptiMAL-IT) and a non-P. falciparum VOM-pLDH/Pf-HRP2 RDT (CareStart). Differential cross-reactivity among these combinations was hypothesized to differentiate P. knowlesi from other Plasmodium monoinfections. Among 323 patients with PCR-confirmed P. knowlesi (n = 193), P. falciparum (n = 93), and P. vivax (n = 37) monoinfections, the VOM-pLDH individual component had the highest sensitivity for nonsevere (35%; 95% confidence interval [CI], 27 to 43%) and severe (92%; CI, 81 to 100%) P. knowlesi malaria. CareStart demonstrated a P. knowlesi sensitivity of 42% (CI, 34 to 49%) and specificity of 74% (CI, 65 to 82%), a P. vivax sensitivity of 83% (CI, 66 to 93%) and specificity of 71% (CI, 65 to 76%), and a P. falciparum sensitivity of 97% (CI, 90 to 99%) and specificity of 99% (CI, 97 to 100%). OptiMAL-IT demonstrated a P. knowlesi sensitivity of 32% (CI, 25 to 39%) and specificity of 21% (CI, 15 to 29%), a P. vivax sensitivity of 60% (CI, 42 to 75%) and specificity of 97% (CI, 94 to 99%), and a P. falciparum sensitivity of 82% (CI, 72 to 89%) and specificity of 39% (CI, 33 to 46%). The combination of CareStart plus OptiMAL-IT for P. knowlesi using predefined criteria gave a sensitivity of 25% (CI, 19 to 32%) and specificity of 97% (CI, 92 to 99%). Combining two RDT combinations was highly specific for P. knowlesi malaria diagnosis; however, sensitivity was poor. The specificity of pLDH RDTs was decreased for P. vivax and P. falciparum because of P. knowlesi cross-reactivity and cautions against their use alone in areas where P. knowlesi malaria is endemic. Sensitive P. knowlesi-specific RDTs and/or alternative molecular diagnostic tools are needed in areas where P. knowlesi malaria is endemic.
INTRODUCTION
Human malaria due to the simian parasite Plasmodium knowlesi has now been reported in all countries throughout Southeast Asia except Laos and East Timor (1–9). In Malaysian Borneo, P. knowlesi is the most common cause of human malaria (10, 11) and commonly causes severe disease, with fatal cases also reported (12–16). Early diagnosis of P. knowlesi infection is important; with its 24-h life cycle, it can rapidly cause high parasitemia and is three times as likely as P. falciparum to cause severe malaria (15). Microscopy remains the gold standard for malaria diagnosis across Asia. However, microscopic diagnosis of P. knowlesi remains problematic, since the species is nearly indistinguishable from P. malariae (17) and is frequently confused with P. falciparum and P. vivax (18). Misdiagnosis of P. knowlesi has important clinical implications, including inappropriate treatment in regions without unified treatment strategies, failure to administer antihypnozoite treatment for P. vivax malaria (18), and failure to recognize patients at risk of severe disease (16). Public health surveillance of P. knowlesi is also compromised by inaccurate microscopic reporting.
Malaria rapid diagnostic tests (RDTs) using immunochromatographic capture techniques provide an alternative method of diagnosis and have been increasingly deployed in areas where malaria is endemic. The tests can be conducted by staff with minimal training, and results are rapidly available. In the most recent WHO round 4 malaria RDT product testing results, composite test positivity had considerably increased, with the best-performing tests for P. falciparum and P. vivax approaching 100% even at a parasite count of 200/μl (19). These RDTs utilize monoclonal antibodies targeting specific antigens such as P. falciparum histidine-rich protein 2 (Pf-HRP2) or Plasmodium genus- or species-specific parasite lactate dehydrogenase (pLDH). Although no P. knowlesi-specific monoclonal antibody has been developed, P. knowlesi has been shown to cross-react with P. falciparum- and P. vivax-“specific” pLDH (20).
Several case reports of returned travelers with PCR-confirmed P. knowlesi monoinfection have demonstrated this cross-reactivity, with Plasmodium genus pLDH-, P. falciparum pLDH-, and P. vivax pLDH-based RDTs all yielding positive results but with poor sensitivity at low parasite counts (21–24). In a previous prospective evaluation of RDTs for P. knowlesi malaria, a pan-pLDH-based RDT demonstrated a moderate overall sensitivity for P. knowlesi of 74% (95/129; 95% confidence interval [CI], 65 to 80%), which improved for pretreatment samples (88%; 30/34; CI, 73 to 95%) and for severe disease (95%; 36/38; CI, 83 to 99%) (25). Neither this test nor a more poorly performing aldolase-based RDT also evaluated in that study approached a sensitivity of 100% for parasitemias of >100/μl, which was previously defined as necessary for use as a clinically sufficient malaria diagnostic tool (26, 27). Moreover, neither RDT was able to distinguish P. knowlesi from P. vivax.
As P. knowlesi cross-reacts with a proportion of monoclonal antibodies targeting both P. falciparum and P. vivax pLDHs but does not cross-react with Pf-HRP2, we and others (20) have hypothesized that a combination of RDTs containing these test components may be used for differentiating P. knowlesi, P. falciparum, and P. vivax malaria infections (Table 1). Current commercial RDTs do not routinely specify the exact epitopes present on the monoclonal antibodies used to target Plasmodium species pLDHs and whether they are shared (20), meaning that it is not possible to ascertain the potential P. knowlesi-binding capacity of previously untested pLDH-based RDTs. We therefore prospectively evaluated the sensitivity and specificity of a combination of two RDTs for the diagnosis of P. knowlesi malaria.
TABLE 1.
Predefined clinical specificity reference for RDT component combinations
| Parasite | Combined specificityd of: |
|||
|---|---|---|---|---|
| CareStart |
OptiMAL-IT |
|||
| VOM-pLDH | Pf-HRP2 | Pan-pLDH | Pf-pLDH | |
| P. knowlesia,b | ± | − | ± | + |
| P. falciparumc | − | + | ± | ± |
| P. vivax | + | − | + | − |
| P. malariae | + | − | + | − |
| P. knowlesi-P. vivax | + | − | + | + |
| P. knowlesi-P. falciparum | + | + | + | + |
| P. falciparum-P. vivax | + | + | + | + |
P. knowlesi monoinfection or mixed P. knowlesi-P. vivax infection cannot be differentiated.
VOM-pLDH and pan-pLDH are not required to differentiate P. knowlesi from P. falciparum or P. vivax monoinfection.
Pf-HRP2 is highly specific for P. falciparum; therefore, a positive Pf-HRP2 result indicates P. falciparum infection regardless of the OptiMAL-IT result.
Symbols: +, specific; −, not specific; ±, equivocal.
MATERIALS AND METHODS
Study sites and referral system.
This study was conducted at three hospitals in Sabah, Malaysian Borneo: Kudat District Hospital (KDH), Kota Marudu District Hospital (KMH); and Queen Elizabeth Hospital (QEH), the corresponding adult tertiary-care referral center in the state capital Kota Kinabalu. QEH services a catchment area along the northwestern coast of Sabah with a population of 1.14 million people in six districts. Malaria patients from this area are either admitted to their primary district hospital or referred to QEH with an accompanying pretreatment blood film and whole-blood sample if they meet state transfer guidelines. These include a thick blood film reported as 4+ (indicating >10 parasites/high-power microscopy field) or any evidence of severe malaria, with treatment prior to transfer. Malaria patients from the local Kota Kinabalu area are admitted directly to QEH.
Subjects.
All of the patients presenting to QEH from September 2011 to July 2013 or to KDH and KMH from October 2012 until July 2013 with a microscopic diagnosis of malaria were eligible for inclusion in this study. This included 14 adults with severe (n = 3) or nonsevere (n = 11) malaria previously reported (15, 18). District sites were included to allow pretreatment evaluation of children >1 year of age and adults in a primary-care setting where, in contrast to a tertiary-care referral hospital, malaria cases are more often nonsevere and have lower parasite counts (12, 28). All of the participants were nonpregnant with no major comorbidities and had not previously been enrolled in this study. On the basis of PCR testing, patients who were negative (n = 19), did not have a result (n = 6), or had mixed infections with Plasmodium species (n = 3) were retrospectively excluded. Malaria severity was defined by modified 2010 WHO criteria (15, 29). Written informed consent was provided by patients or their relatives. Approvals were obtained from the Ethics Committees of the Malaysian Ministry of Health and the Menzies School of Health Research.
RDT selection.
On the basis of the WHO round 1 and 2 malaria RDT product test results available at the time of study design (30) (Table 2), RDTs were chosen according to the combined potential specificity of the individual test components for P. knowlesi malaria (Table 1). The test components with the highest reported sensitivities for P. falciparum-specific and non-P. falciparum pLDHs were selected. They were OptiMAL-IT (DiaMed AG), which detects P. falciparum-specific and pan-Plasmodium sp. pLDHs, and CareStart Malaria (Pf/VOM) Combo (Access Bio Inc.), which detects the Pf-HRP2 antigen and non-P. falciparum pan-pLDH.
TABLE 2.
Test positivity in WHO RDT product testing rounds 1 and 2a
| Test | % Test positivity with: |
|||
|---|---|---|---|---|
| 200 parasites/μl |
2,000 or 5,000 parasites/μl |
|||
| P. falciparum | P. vivax | P. falciparum | P. vivax | |
| Pf-HRP2/VOM-pLDH (CareStart) | 89 | 80 | 100 | 100 |
| Pf-pLDH/pan-pLDH (OptiMAL-IT) | 37 | 95 | 96 | 100 |
Data are from reference 30.
Despite potential positive cross-reactivity with Pf-LDH, it may be possible to differentiate a P. knowlesi monoinfection from a P. falciparum monoinfection by using its lack of reactivity with the Pf-HRP2 component. It may be possible to differentiate a P. knowlesi monoinfection from a P. vivax monoinfection by using the lack of reactivity of the latter with Pf-LDH. While this RDT combination will not distinguish a P. knowlesi monoinfection from a mixed P. knowlesi-P. vivax infection, the latter appears rare in Malaysian Borneo, accounting for 5/387 (1.3%) (15) and 1/188 (0.5%) (14) PCR-confirmed malaria cases in two recent studies.
Study procedures.
Demographic and clinical information was recorded on standardized case record forms. Pretreatment venous blood was collected in a labeled EDTA or citrate-theophylline-adenosine-dipyridamole tube. RDTs were transported directly to the study sites by registered courier from the manufacturer in the United States (CareStart) and through a local distributer (OptiMAL-IT). RDTs were subsequently stored out of direct sunlight in monitored, air-conditioned research laboratories at 22°C. Batch numbers and expiration dates were documented. RDTs were taken to the patient enrollment area, where the temperature and humidity did not exceed 32°C and 74%, respectively. All of the RDTs were packaged and sealed individually with desiccant and used immediately after opening, in accordance with the manufacturers' instructions. Results were recorded by one of two research laboratory technicians blinded to the microscopy diagnosis, with regular cross-checking by the study clinician to ensure consistent reporting. Interobserver agreement was tested at the district sites, with a research staff member reading the test first, followed 5 min later by a second person blinded to the initial reading. In the event of discordant results, a third person also blinded read the test, with the final result being the most common reading. Results were recorded as follows: negative, no clearly visible line; 1, faint line; 2, line darker than 1 but lighter than the control; 3, same as the control line; 4, line darker than the control. Thick and thin blood films were prepared on enrollment and examined by microscopists at referring district hospitals or at QEH, with slides later cross-checked by an experienced research microscopist. Parasite density was quantified by the research microscopist using pretreatment slides and reported as the number of parasites per 200 leukocytes or per 1,000 erythrocytes and converted to the number of parasites per microliter by using the patient's leukocyte count or hematocrit, respectively. Where pretreatment slides were unavailable (13/327 [4%]), referring hospital microscopy was used and the grades 1+ to 4+ were converted into numbers of parasites per microliter by using the relevant median parasite density. Parasite DNA was extracted, and PCR was performed as previously described for P. falciparum, P. vivax, P. ovale, and P. malariae (31) or P. knowlesi (32) detection.
Statistical analysis.
Data were analyzed by using STATA, version 12 (StataCorp LP, College Station, TX). Intergroup differences were compared by using the Kruskal-Wallis test for nonnormally distributed continuous variables and a χ2 test for categorical variables. The variables measured included the numbers of true positives, true negatives, false positives, and false negatives. PCR results were used as the gold standard, with Plasmodium species monoinfections compared against all of the other PCR-positive Plasmodium species. Test sensitivity was defined as true positives/(true positives + false negatives), and test specificity was defined as true negatives/(true negatives + false positives). Ninety-five-percent confidence intervals (CIs) were estimated by Wilson's method. Logistic regression was used to assess the relationship between sensitivity and parasite count and also the usefulness of RDT band intensity as a predictor of severity. Interobserver agreement between the first and second readings was measured by using the kappa coefficient. Spearman's correlation coefficient was used to assess the association between RDT band intensity and the parasite count.
RESULTS
Baseline demographic and clinical features.
From October 2012 until July 2013, RDTs were conducted for 193, 93, 37, and 3 patients with PCR-confirmed P. knowlesi, P. falciparum, P. vivax, and P. malariae monoinfections, respectively. This included 321 patients (P. knowlesi, n = 189; P. falciparum, n = 92; P. vivax, n = 37; P. malariae, n = 3) tested with the pan-pLDH/Pf-pLDH (OptiMAL-IT) test. The VOM-pLDH/Pf-HRP2 (CareStart) test was performed for 301 patients (P. knowlesi, n = 178; P. falciparum, n = 85; P. vivax, n = 35; P. malariae, n = 3). Their baseline demographics are shown in Table 3. Patients with P. knowlesi malaria were older than those with P. falciparum or P. vivax malaria (median ages, 40, 30, and 27 years, respectively; P < 0.001) and more likely to be male (81, 73, and 68%, respectively; P = 0.031). The majority of the malaria patients were enrolled at the QEH tertiary-care study site for all of the Plasmodium species. P. knowlesi patients enrolled at district hospitals were younger (median age, 33 versus 44 years; P = 0.001), were less likely to have severe malaria (5 versus 22%; P = 0.001), and had lower median parasite counts (1,436 versus 3,802/μl; P = 0.001) than those enrolled at the tertiary-care hospital. For nonsevere malaria, P. knowlesi malaria patients had a lower median parasite count (1,701/μl) than P. falciparum malaria patients (P = 0.033) but not P. vivax malaria patients (P = 0.918).
TABLE 3.
Baseline demographics, clinical severity, and parasitemia of enrolled subjects
| Species (no. of patients) or parameter | No. (%) of patients |
Median age, yr (IQRa) [range] |
No. (%) of males |
No. (%) of patients with severe malaria |
Median no. of parasites/μl (IQR) [range] |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertiary-care Hospital | District hospital | Total | Tertiary-care hospital | District hospital | Total | Tertiary-care hospital | District hospital | Total | Tertiary-care hospital | District hospital | All patients | Nonsevere malaria | Severe malaria | Tertiary-care hospital | District hospital | |
| P. knowlesi (193) | 114 (59) | 79 (41) | 40 (26–51) [3–94] | 44 (30–52) [16–81] | 33 (20–48) [3–72] | 157 (81) | 97 (85) | 60 (75) | 29 (15) | 25 (22) | 4 (5) | 2301 (672–641464) [30–641464] | 1701 (493–7573) [30–90683] | 56784 (5300–144968) [979–641464] | 3802 (1152–18442) [88–641464] | 1436 (320–7515) [30–143184] |
| P. falciparum (93) | 91 (98) | 2 (2) | 30 (21–44) [13–75] | 30 (21–44) [13–75] | 43 (15–71) [15–71] | 68 (73) | 68 (75) | 0 | 16 (17) | 16 (100) | 0 | 5832 (1464–19000) [26–606108] | 4386 (1464–17208) [26–247897] | 17912 (6554–106070) [33–606108] | 6254 (1464–21972) [26–606108] | 570 (33–1107) [33–1107] |
| P. vivax (37) | 23 (62) | 14 (38) | 27 (11–39) [1–62] | 31 (27–42) [15–62] | 11 (4–12) [1–39] | 25 (68) | 18 (78) | 7 (50) | 3 (8) | 3 (100) | 0 | 3366 (371–13593) [76–60288] | 3004 (371–12544) [76–60288] | 4453 (4025–19520) [4025–19520] | 3381 (1332–6581) [210–19520] | 3004 (600–12544) [76–60288] |
| P. malariae (3) | 1 (33) | 2 (66) | 21 (13–25) [13–25] | 25 | 17 (13–21) [13–21] | 1 (33) | 0 | 675 (77–1212) [77–1212] | 675 (77–1212) [77–1212] | 1212 (1212–1212) [1212–1212] | 376 (77–675) [77–675] | |||||
| P value for: | ||||||||||||||||
| P. knowlesi vs P. falciparum | 0.006 | <0.001 | 0.52 | 0.133 | 0.064 | 0.019 | 0.623 | 0.329 | 0.009 | 0.033 | 0.009 | 0.308 | 0.388 | |||
| P. knowlesi vs P. vivax | <0.001 | 0.023 | <0.001 | 0.069 | 0.418 | 0.058 | 0.271 | 0.337 | 0.395 | 0.918 | 0.483 | 0.165 | 0.342 | 0.417 | ||
IQR, interquartile range.
Microscopy.
Among 193 patients with PCR-confirmed P. knowlesi infection, only 33 (17%) were accurately reported as P. knowlesi or “P. malariae/?P. knowlesi” by hospital microscopists, while 109 (56%) were reported as P. malariae (morphologically indistinguishable from P. knowlesi and the default reporting nomenclature from previous state guidelines). Considered as a single group, this gave a sensitivity of 74% (CI, 67 to 80%) and a specificity of 89% (CI, 82 to 94%) for P. knowlesi. Our dedicated research microscopist correctly identified 158 of 193 P. knowlesi blood films (sensitivity, 83% [CI, 76 to 88%]; specificity, 93% [CI, 87 to 96%]).
RDT sensitivity.
The VOM-pLDH test component had the highest sensitivity (77/178; 43%; CI, 36 to 51%) for P. knowlesi malaria overall but still performed poorly (Table 4). For P. vivax, despite a median parasite count of 3,366/μl, the VOM-pLDH test performance (29/35; 83% sensitivity; CI, 70 to 96%) was more in line with the reported WHO composite test positivity of 80% at 200 parasites/μl than the reported 100% with 2,000 to 5,000 parasites/μl (30). For P. falciparum, three false-positive VOM-pLDH results were recorded, with parasite counts ranging from 9,079 to 21,972/μl. For the PCR-confirmed P. malariae cases, VOM-pLDH demonstrated test positivity (2/3; 67%; CI, 9 to 99%); however, the small numbers did not allow meaningful evaluation. The pan-pLDH test component was insufficiently sensitive for all of the species, with sensitivities of 30% (CI, 24 to 37%), 68% (CI, 52 to 83%), 78% (CI, 70 to 87%), and 67% (CI, 0 to 100%) for P. knowlesi, P. vivax, P. falciparum, and P. malariae, respectively. The Pf-pLDH test also demonstrated poor sensitivity for P. knowlesi (55/190; 29%; CI, 22 to 35%) and suboptimal stand-alone performance for P. falciparum (74/92; 80%; CI, 72 to 89%), despite a median P. falciparum parasitemia of 5,832/μl. The overall sensitivity of the OptiMAL-IT RDT (i.e., a positive Pf-pLDH and/or pan-pLDH test result) for the detection of P. knowlesi (32% [CI, 25 to 39%]) was only a marginal improvement on the individual test components. The CareStart overall sensitivity for P. knowlesi of 42% (CI, 34 to 49%) was slightly less than the VOM-pLDH standalone sensitivity of 43% because of four false-positive Pf-HRP2 results. The sensitivities of the VOM-pLDH, pan-pLDH, and Pf-pLDH tests for P. knowlesi malaria were all lower at the district sites than at the tertiary-care site (35 versus 49% [P = 0.061], 13 versus 42% [P < 0.0001], and 13 versus 40% [P < 0.001], respectively).
TABLE 4.
Sensitivity and specificity of RDTs and their components for each Plasmodium species
| Test and parameter | Proportion, % (CI) |
P value | |||
|---|---|---|---|---|---|
| P. knowlesi | P. vivax | P. falciparum | P. malariae | ||
| CareStart | |||||
| VOM-pLDH, sensitivity | 77/178, 43 (36–51) | 29/35, 83 (70–96) | 3/85, 4 (0–8)d | 2/3, 67 (0–100) | <0.0001a |
| Pf-HRP2, sensitivity | 4/183, 2 (0–4)d | 1/35, 3 (0–9)d | 86/87, 99 (97–100) | 0/3, 0 | |
| OptiMAL-IT | |||||
| Pan-pLDH, sensitivity | 57/189, 30 (24–37) | 25/37, 68 (52–83) | 72/92, 78 (70–87) | 2/3, 67 (0–100) | <0.0001b |
| Pf-pLDH, sensitivity | 55/190, 29 (22–35) | 5/37, 14 (2–25)d | 74/92, 80 (72–89) | 0/3, 0 | <0.0001c |
| CareStart (VOM-pLDH + Pf-HRP2) | |||||
| Sensitivity | 74/178, 42 (34–49) | 29/35, 83 (66–93) | 82/85, 97 (90–99) | 2/3, 67 (9–99) | |
| Specificity | 91/123, 74 (65–82) | 189/266, 71 (65–76) | 214/216, 99 (97–100) | 194/298, 35 (30–41) | |
| OptiMAL-IT (pan-pLDH + Pf-pLDH) | |||||
| Sensitivity | 61/191, 32 (25–39) | 22/37, 60 (42–75) | 75/92, 82 (72–89) | 2/3, 67 (9–99) | |
| Specificity | 28/132, 21 (15–29) | 276/285, 97 (94–99) | 141/231, 39 (33–46) | 290/319, 91 (87–94) | |
P. knowlesi compared against P. vivax only.
P. knowlesi compared against other Plasmodium species.
P. knowlesi compared against P. falciparum only.
False positives.
Sensitivity and parasitemia.
Increasing P. knowlesi parasitemia correlated with test sensitivity for VOM-pLDH (P < 0.001), pan-pLDH (P = 0.001), and Pf-pLDH (P = 0.004) (Fig. 1A and B). Thirty-one percent of the P. knowlesi patients had a parasite count of <1,000/μl, and in this group only 12% had a positive VOM-pLDH test. Among the 27% of P. knowlesi patients with a parasite count of >10,000/μl, 81% (39/48) had a positive VOM-pLDH test result, which increased to 100% (13/13) for those with a parasite count of >100,000/μl. Despite the lowest recorded parasite count for a positive VOM-pLDH test result being 50/μl, the median positive result was 7,089/μl and the highest negative result was 40,807/μl. For both pan-pLDH and Pf-pLDH, the lowest P. knowlesi parasitemia detected was 26/μl and the highest was 641,464/μl. The highest negative result for P. knowlesi with both pan-pLDH and Pf-pLDH was 425,784/μl. The relationship between test positivity and parasitemia was also apparent for P. falciparum with the pan-pLDH (P = 0.048) and Pf-pLDH (P = 0.045) tests. In contrast, P. vivax had a smaller range of parasite counts (76 to 60,288/μl) and no statistically significant relationship between parasitemia and the sensitivity of any of the different test components.
FIG 1.
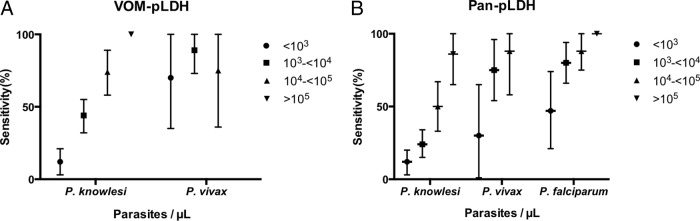
(A) Sensitivity of the VOM-pLDH component of the CareStart RDT by parasite count for P knowlesi and P. vivax infections. Vertical lines represent CIs. (B) Sensitivity of the pan-pLDH component of the OptiMAL-IT RDT by parasite count for P knowlesi, P. vivax, and P. falciparum infections. Vertical lines represent CIs.
Sensitivity and clinical severity.
The VOM-pLDH test had a higher sensitivity among severe P. knowlesi malaria patients (24/26; 92%; CI, 81 to 100%) than the pan-pLDH (20/28; 71%; CI, 54 to 89%; P = 0.049) and Pf-pLDH (17/28; 61%; CI, 41 to 80%; P = 0.006) test components. For P. vivax, both the VOM-pLDH and pan-pLDH tests of all three severe-malaria patients were positive. All of the severe P. falciparum malaria patients tested with Pf-HRP2 were positive (14/14; 100%); however, only 14/16 (86%; CI, 69 to 100%) and 13/16 (81%; CI, 60 to 100%) had positive Pf-LDH and pan-pLDH test results, respectively.
Line intensity.
The intensity of the test line was strongly associated with an increasing parasite count for P. knowlesi malaria with all of the test components, with Spearman correlation coefficients of 0.616 (P < 0.001), 0.408 (P < 0.001), and 0.376 (P < 0.001) for VOM-pLDH, pan-pLDH, and Pf-pLDH, respectively (Fig. 2). For positive VOM-pLDH tests, the highest line intensity reading of 4+ did not independently predict severe P. knowlesi malaria (P = 0.595).
FIG 2.
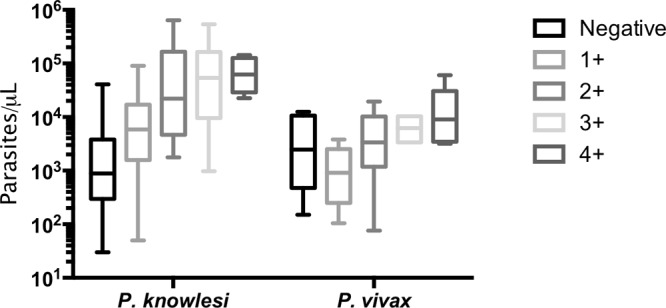
Parasite counts by VOM-pLDH line intensity for P knowlesi and P. vivax infections. Horizontal lines indicate medians, boxes indicate interquartile ranges, and vertical lines indicate ranges.
Interobserver agreement.
Both pan-pLDH/Pf-LDH (n = 95) and VOM-pLDH/Pf-HRP2 (n = 89) demonstrated excellent interobserver agreement, with kappa values of >0.9 for all of the tests. No tests were repeated at the district hospital sites; however, this was not documented at the tertiary-care site.
Combined test specificity and sensitivity.
Individual test component specificity for P. knowlesi is not clinically useful, as the individual test components cannot differentiate P. knowlesi from other Plasmodium species. However, a combination of the tests could theoretically distinguish a P. knowlesi monoinfection from a P. vivax, P. falciparum, or P. malariae monoinfection (Table 1). The use of this predefined RDT combination to diagnose PCR-confirmed P. knowlesi-infected patients yielded 45/181 positive results, with a sensitivity for P. knowlesi diagnosis lower than that of hospital microscopy (25% [CI, 19 to 32%] versus 74% [CI, 67 to 80%]; P < 0.001) but a higher specificity (96% [CI, 91 to 99%] versus 89% [CI, 82 to 94%]; P = 0.030) (Table 5). The sensitivity of the RDT combination for P. knowlesi diagnosis was also lower at the district hospitals (10% [CI, 5 to 19%]) than at the tertiary-care hospital (36% [CI, 26 to 46%]; P < 0.001).
TABLE 5.
Performance characteristics of combined use of CareStart and OptiMAL-IT RDTs compared with those of microscopy for the diagnosis of P. knowlesi malaria
| Method and result | No. of patients with: |
% Sensitivity (CI) | % Specificity (CI) | % PPVb (CI) | % NPVc (CI) | Likelihood ratio |
||
|---|---|---|---|---|---|---|---|---|
| PCR-confirmed P. knowlesi | PCR-confirmed other Plasmodium species | Positive test | Negative test | |||||
| RDTsa | ||||||||
| Positive | 45 | 5 | 25 (19–32) | 96 (91–99) | 90 (78–97) | 47 (40–53) | 6.17 | 0.78 |
| Negative | 136 | 119 | ||||||
| Microscopy | ||||||||
| Positive | 143 | 15 | 74 (67–80) | 89 (82–94) | 91 (85–95) | 70 (63–77) | 6.57 | 0.29 |
| Negative | 50 | 118 | ||||||
Equivocal result for VOM-pLDH, negative result for Pf-HRP2, equivocal result for pan-pLDH, and positive result for Pf-pLDH.
PPV, positive predictive value.
NPV, negative predictive value.
DISCUSSION
P. knowlesi cross-reactivity was demonstrated with all of the pLDH-based test components evaluated in this study; however, the sensitivities of each test component and of the RDTs overall (32 and 42% for OptiMAL-IT and CareStart, respectively) were insufficient for the diagnosis of P. knowlesi malaria. Binding affinity appeared to be strongly related to parasitemia, with test sensitivity particularly poor at lower parasite counts. P. knowlesi patients, including both adults and children, with nonsevere malaria have lower parasitemias than those with nonsevere P. falciparum malaria, with P. knowlesi parasitemias particularly low at the district level. The lack of test sensitivity for these patients is highlighted by the finding that the median parasitemia detected by the VOM-pLDH test (7,089 parasites/μl) far exceeds the median parasitemia among nonsevere P. knowlesi patients presenting to a district hospital (1,040 parasites/μl) or a tertiary-care hospital (2,133 parasites/μl). Although the VOM-pLDH test was positive among all of the patients with P. knowlesi parasite counts of >100,000/μl and among 92% of the patients with severe P. knowlesi malaria, false-negative results occurred among patients with parasite counts of up to 40,807/μl. Given that the risk of severe disease with P. knowlesi is 11-fold higher with a parasitemia of >20,000/μl (15), false-negative VOM-pLDH test results with parasite counts above this level are clearly unsatisfactory.
The insufficient sensitivity of each of the RDTs evaluated in the present study for P. knowlesi diagnosis is consistent with the lack of sensitivity demonstrated in other recent series evaluating RDT performance in hospital settings in countries where P. knowlesi malaria is endemic. These include tests incorporating the following antibody components: pan-pLDH (First-Response, 74% sensitivity) (25), Pf-pLDH/pan-pLDH (OptiMAL-IT, 71% sensitivity with fresh isolates) (33), Pv-pLDH/pan-pLDH (Paramax-3, 40% sensitivity with fresh isolates) (33), and pan-Plasmodium aldolase (ParaHit, 23% sensitivity with fresh isolates [25]; BinaxNOW, 29% sensitivity with fresh isolates [33]).
The cross-reactivity of P. knowlesi in RDTs utilizing “species-specific” P. falciparum or P. vivax pLDH monoclonal antibodies means that their specificity for these other species is affected. This has important implications for the use of RDTs to diagnose P. falciparum and P. vivax infections in areas of Southeast Asia where P. knowlesi is also prevalent. We demonstrate here that the previously reported specificities of the OptiMAL-IT RDT elsewhere in Southeast Asia of 94.7% for P. falciparum (with or without other species) and 96.5% for non-P. falciparum species (34) are not applicable to areas with higher P. knowlesi endemicity. The OptiMAL-IT RDT, in particular, had a much lower specificity for P. falciparum of 39%. The CareStart RDT specificity of 71% for P. vivax was also lower than would be expected in areas where P. knowlesi is not endemic.
A limitation of the RDTs used in this study was that even when they were used in combination they could not differentiate between P. knowlesi and mixed P. knowlesi-P. vivax infections, which would have implications for radical P. vivax cure. In Malaysian Borneo, the prevalence of mixed P. knowlesi-P. vivax infections appears to be low, with only one reported PCR-confirmed case in Sarawak (14) and 5/387 (1.3%) in our recent prospective study in Sabah (15). A separate study in Sabah reported 83 P. knowlesi-P. vivax mixed infections (35) but used earlier PCR primers that can include a false-positive P. vivax result for true P. knowlesi monoinfections (32). In other areas where P. knowlesi malaria is endemic, such as Thailand, India, and Myanmar, mixed P. knowlesi-P. vivax infections have been reported, with prevalences ranging from <1 to 9% (3, 36, 37). Comparing specificity against controls without malaria may also have assisted in ascertaining whether false-positive results may have been interpretation or reporting errors rather than real cross-reactivity between pLDH monoclonal antibodies directed at other Plasmodium species. While it is recommended that RDT evaluations be conducted against the gold standard, microscopy (38), the known difficulties in microscopic diagnosis and reporting of P. knowlesi (18) meant that the RDT sensitivities were analyzed by using PCR results.
We hypothesized that the use of a combination of pLDH and HRP2 tests may make it possible to differentiate P. knowlesi from other Plasmodium monoinfections. While the RDT combination did give a high specificity of 96% for P. knowlesi against other Plasmodium monoinfections, the low overall sensitivities prevent these RDTs from replacing standard microscopy as a diagnostic tool for P. knowlesi in this region. The cost of using two commercial RDTs following positive microscopy to improve specificity is also not feasible in many settings. For P. falciparum, RDTs containing an HRP2 component that does not cross-react with P. knowlesi should therefore be used. The ideal RDT combination would also contain a P. vivax-specific pLDH monoclonal antibody that does not cross-react with P. knowlesi and would therefore enable accurate diagnosis of P. vivax in order to guide the need for radical primaquine therapy. Other, more sensitive, molecular diagnostic tools, such as loop-mediated isothermal PCR (LAMP) targeting the AMA-1 or beta-tubulin P. knowlesi gene, have demonstrated test detection limits as low as 10 to 102 copies, respectively, with reported sensitivities and specificities of 100% (39, 40). The sensitivity of LAMP may be ideal at a district hospital level in Malaysia, given that the majority of P. knowlesi patients present with very low parasite counts. LAMP is also at least as sensitive and specific as nested PCR (40), with the added advantage of being able to be conducted with less training and equipment and at isothermal temperatures. However, the benefit of improving the sensitivity of immunochromatographic RDTs for use in more remote clinics with declining rates of malaria infection due to other Plasmodium species and parallel deskilling of health clinic level microscopists should encourage the development of P. knowlesi-specific monoclonal antibodies. Improved diagnosis of P. knowlesi would assist in the collection of prevalence data to guide public health strategies for this emerging and potentially fatal infection.
ACKNOWLEDGMENTS
We thank the following clinical research staff members, who enrolled the patients, conducted the RDTs, and read the blood slides: Rita Wong, Beatrice Wong, Anne Wee, Ferryanto Chalfein, Wilhelmina Nevir, Sitti Saimah binti Sakam, Siti Norfazizawantie Redzuan, Ema Estiana binti Ishak, Salwah binti Hamit, and Kamariah binti Asmda. We also thank the Director General of Health, Malaysia, for permission to publish this study.
This study was funded by the Malaysian Ministry of Health (grant BP00500420) and the Australian National Health and Medical Research Council (grants 1037304 and 1045156, fellowships [N.M.A., T.W.Y.], and scholarships [B.E.B., M.J.G.]).
Footnotes
Published ahead of print 2 April 2014
REFERENCES
- 1.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017–1024. 10.1016/S0140-6736(04)15836-4 [DOI] [PubMed] [Google Scholar]
- 2.Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. 2004. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg. Infect. Dis. 10:2211–2213. 10.3201/eid1012.040293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang N, Chang Q, Sun X, Lu H, Yin J, Zhang Z, Wahlgren M, Chen Q. 2010. Co-infections with Plasmodium knowlesi and other malaria parasites, Myanmar. Emerg. Infect. Dis. 16:1476–1478. 10.3201/eid1609.100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khim N, Siv S, Kim S, Mueller T, Fleischmann E, Singh B, Divis PCS, Steenkeste N, Duval L, Bouchier C, Duong S, Ariey F, Ménard D. 2011. Plasmodium knowlesi infection in humans, Cambodia, 2007-2010. Emerg. Infect. Dis. 17:1900–1902. 10.3201/eid1710.110355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee KS, Singh B. 2008. Human infections with Plasmodium knowlesi, the Philippines. Emerg. Infect. Dis. 14:811–813. 10.3201/eid1405.071407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S. 2011. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, southern Vietnam. Emerg. Infect. Dis. 17:1232–1239. 10.3201/eid1707.101551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, Tu TM, Loh JP, Leo YS. 2008. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg. Infect. Dis. 14:814–816. 10.3201/eid1405.070863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figtree M, Lee R, Bain L, Kennedy T, Mackertich S, Urban M, Cheng Q, Hudson BJ. 2010. Plasmodium knowlesi in human, Indonesian Borneo. Emerg. Infect. Dis. 16:672–674. 10.3201/eid1604.091624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CE, Adeeba K, Freigang G. 2010. Human Plasmodium knowlesi infections in Klang Valley, Peninsula Malaysia: a case series. Med. J. Malaysia 65:63–65 [PubMed] [Google Scholar]
- 10.William T, Rahman HA, Jelip J, Ibrahim MY, Menon J, Grigg MJ, Yeo TW, Anstey NM, Barber BE. 2013. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax malaria in Sabah, Malaysia. PLoS Negl. Trop. Dis. 7:e2026. 10.1371/journal.pntd.0002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.William T. 2013. Artesunate-mefloquine vs. chloroquine in patients with acute uncomplicated Plasmodium vivax: a randomized open label trial in Sabah, Malaysia. APMEN V: Vivax Working Group Meeting, 5 March 2013, Bali, Indonesia [Google Scholar]
- 12.William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Frederick C, Jelip J, Anstey NM, Yeo TW. 2011. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg. Infect. Dis. 17:1248–1255. 10.3201/eid1707.101017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165–171. 10.1086/524888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneshvar C, Davis TME, Cox-Singh J, Rafa'ee MZ, Zakaria SK, Divis PCS, Singh B. 2009. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin. Infect. Dis. 49:852–860. 10.1086/605439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, Anstey NM, Yeo TW. 2013. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin. Infect. Dis. 56:383–397. 10.1093/cid/cis902 [DOI] [PubMed] [Google Scholar]
- 16.Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. 2012. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar. J. 11:284. 10.1186/1475-2875-11-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowles R, Das Gupta BM. 1932. A study of monkey-malaria and its experimental transmission to man. Ind. Med. Gaz. 67:301–321 [PMC free article] [PubMed] [Google Scholar]
- 18.Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. 2013. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 12:8. 10.1186/1475-2875-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. 2013. Malaria rapid diagnostic test performance. results of WHO product testing of malaria RDTs: round 4 (2012). WHO, Geneva, Switzerland [Google Scholar]
- 20.McCutchan TF, Piper RC, Makler MT. 2008. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg. Infect. Dis. 14:1750–1752. 10.3201/eid1411.080480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ta Tang T-H, Salas A, Ali-Tammam M, Martinez MDC, Lanza M, Arroyo E, Rubio JM. 2010. First case of detection of Plasmodium knowlesi in Spain by real time PCR in a traveller from Southeast Asia. Malar. J. 9:219. 10.1186/1475-2875-9-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hellemond JJ, Rutten M, Koelewijn R, Zeeman AM, Verweij JJ, Wismans PJ, Kocken CH, van Genderen PJ. 2009. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerg. Infect. Dis. 15:1478–1480. 10.3201/eid1509.090358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. 2009. Cross-reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitol. Int. 58:300–302. 10.1016/j.parint.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 24.Tanizaki R, Ujiie M, Kato Y, Iwagami M, Hashimoto A, Kutsuna S, Takeshita N, Hayakawa K, Kanagawa S, Kano S, Ohmagari N. 2013. First case of Plasmodium knowlesi infection in a Japanese traveller returning from Malaysia. Malar. J. 12:128. 10.1186/1475-2875-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. 2013. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. J. Clin. Microbiol. 51:1118–1123. 10.1128/JCM.03285-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moody A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66–78. 10.1128/CMR.15.1.66-78.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. 2000. New perspectives: malaria diagnosis. Report of a joint WHO/USAID informal consultation 25–27 October 1999. WHO, Geneva, Switzerland [Google Scholar]
- 28.Barber BE, William T, Dhararaj P, Anderios F, Grigg MJ, Yeo TW, Anstey NM. 2012. Epidemiology of Plasmodium knowlesi malaria in north-east Sabah, Malaysia: family clusters and wide age distribution. Malar. J. 11:401. 10.1186/1475-2875-11-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. 2010. WHO malaria treatment guidelines. WHO, Geneva, Switzerland [Google Scholar]
- 30.WHO. 2010. Malaria rapid diagnostic test performance—summary results of WHO product testing of malaria RDTs: rounds 1 & 2 (2008-2009). WHO, Geneva, Switzerland [Google Scholar]
- 31.Padley D, Moody AH, Chiodini PL, Saldanha J. 2003. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann. Trop. Med. Parasitol. 97:131–137. 10.1179/000349803125002977 [DOI] [PubMed] [Google Scholar]
- 32.Imwong M, Tanomsing N, Pukrittayakamee S, Day NP, White NJ, Snounou G. 2009. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J. Clin. Microbiol. 47:4173–4175. 10.1128/JCM.00811-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster D, Cox-Singh J, Mohamad DS, Krishna S, Chin PP, Singh B. 2014. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar. J. 13:60. 10.1186/1475-2875-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashley EA, Touabi M, Ahrer M, Hutagalung R, Htun K, Luchavez J, Dureza C, Proux S, Leimanis M, Lwin M, Koscalova A, Comte E, Hamade P, Page A-L, Nosten F, Guerin PJ. 2009. Evaluation of three parasite lactate dehydrogenase-based rapid diagnostic tests for the diagnosis of falciparum and vivax malaria. Malar. J. 8:241. 10.1186/1475-2875-8-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naing DKS, Anderios F, Lin Z. 2011. Geographic and ethnic distribution of P. knowlesi infection in Sabah, Malaysia. Int. J. Collab. Res. Intern. Med. Public Health 3:391–400 [Google Scholar]
- 36.Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S. 2009. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J. Infect. Dis. 199:1143–1150. 10.1086/597414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyagi RK, Das MK, Singh SS, Sharma YD. 2013. Discordance in drug resistance-associated mutation patterns in marker genes of Plasmodium falciparum and Plasmodium knowlesi during coinfections. J. Antimicrob. Chemother. 68:1081–1088. 10.1093/jac/dks508 [DOI] [PubMed] [Google Scholar]
- 38.Bell D, Peeling RW, WHO-Regional Office for the Western Pacific/TDR 2006. Evaluation of rapid diagnostic tests: malaria. Nat. Rev. Microbiol. 4(9 Suppl):S34–S38. 10.1038/nrmicro1524 [DOI] [PubMed] [Google Scholar]
- 39.Lau Y-L, Fong M-Y, Mahmud R, Chang P-Y, Palaeya V, Cheong F-W, Chin L-C, Anthony CN, Al-Mekhlafi AM, Chen Y. 2011. Specific, sensitive and rapid detection of human plasmodium knowlesi infection by loop-mediated isothermal amplification (LAMP) in blood samples. Malar. J. 10:197. 10.1186/1475-2875-10-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iseki H, Kawai S, Takahashi N, Hirai M, Tanabe K, Yokoyama N, Igarashi I. 2010. Evaluation of a loop-mediated isothermal amplification method as a tool for diagnosis of infection by the zoonotic simian malaria parasite Plasmodium knowlesi. J. Clin. Microbiol. 48:2509–2514. 10.1128/JCM.00331-10 [DOI] [PMC free article] [PubMed] [Google Scholar]


